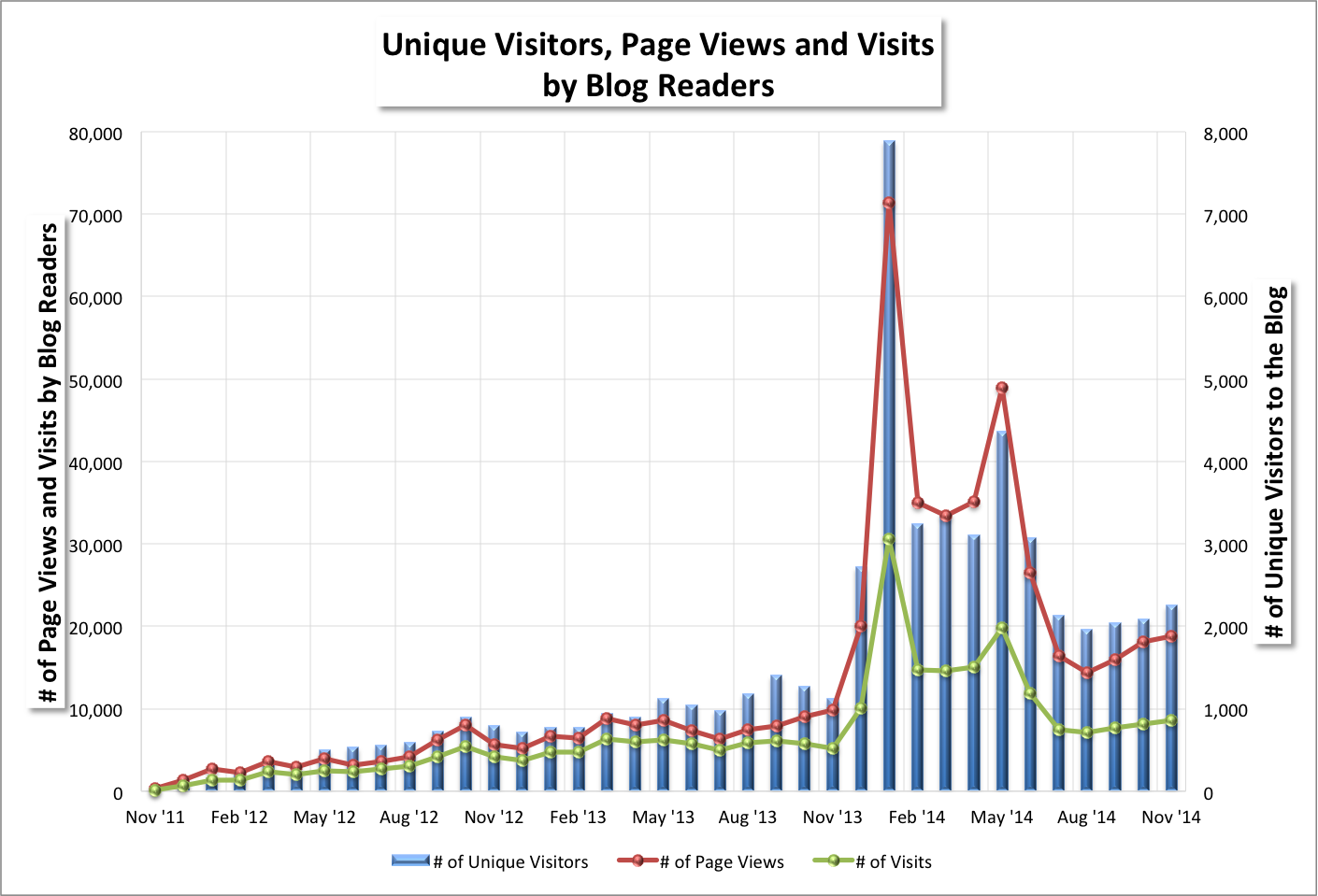
⏏ Blog readership mostly rose from October depending on the statistic. I wrote 22 blog posts (7) and news items (15) in November, versus 22 during the previous month (5 and 17, respectively). November month-over-month changes were:
- +8% for the number of unique visitors (2,255 v. 2,085),
- +4% for page views (18,851 v. 18,106),
- +5% for visits (8,591 v. 8,173),
- -10% for U.S. cities [from where visitors came] (584 v. 649),
- -16% for world cities (137 v. 163), and
- No change for countries (51 v. 51).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
⏏ Roche's Genentech's Dr. Daniel Chen, M.D., Ph.D., PD-L1 Global Development Leader,
noted in May 2014 that:
"One theory about immunotherapies is that they could work for everyone, with any type of cancer. The data to date show that this isn’t the case. Even though everyone has an immune system, not all patients will respond to the same medicine in the same way.
Our immunotherapy program at Genentech has a large biomarker and diagnostic focus so we can find those who are most likely to experience a meaningful benefit.
But what about those who do respond, but not well enough?
Perhaps those people with a small to moderate response should be candidates for combination trials. Some immunotherapies may be more effective if they are combined with different types of medicines, including chemotherapies, personalized medicines and even other immunotherapies.
In this way, biomarkers for cancer immunotherapy don’t just tell us who will or will not respond. Rather, they could help guide treatment strategies involving one or more medicines."
I recently asked Eric about this. He commented (paraphrasing):
Biomarker (i.e., targeted) therapies have proven quite successful in a number of cases, such as vemurafenib (trade name Zelboraf) and imatinib (trade name Gleevec). But these generally have limited time before escape* occurs since they target one or a few mutations, and cancer cells, which are generally highly adept at mutating, mutate to circumvent the therapy.
Our understanding of immune targets relevant to cancer is still very primitive along with our ability to devise drugs specific to unique targets. Part of the problem is that "unique" targets are generally conserved**, just as molecular pathways are, in other kinds of cells and thus we get "off-target" effects (e.g., cholitis, secondary tumors, etc).
Craig would say that PV-10 works by training our very sophisticated immune system to recognize complex patterns of targets that are expressed but sequestered by cancers. This is a bit like grabbing a very carefully selected handful of biomarkers and deploying them simultaneously.
* "Time before escape" is akin to survival time.
** "Conserved" means similar or identical.
⏏ Peter updated Provectus' website to include information about the number and diversity of people and vendors working for the company. See
About Us, Management, and scroll to the bottom of the page. It is a competent first step that provides some useful, basic insight into company operations.
 |
| Click to enlarge. |
Of the 50 FTE figure in the bottom left hand corner, Eric's consultants and contract labor (see "consulting and contract labor" in Provectus' quarterly and annual SEC filings) comprise 36 FTEs (not including himself). The balance of 14 FTEs comprise Provectus' four principals and employees, and 10 FTEs that Peter characterizes as the full-time equivalents of the 134 people servicing and supporting his "corporate infrastructure."
⏏ I recently asked Peter about the possible impact of a potential approval (i.e., a speculative PDUFA date of April 2015) of Amgen's intralesional oncology agent
talimogene laherparepvec ("T-Vec") as a monotherapy for metastatic melanoma. He commented (paraphrasing):
People seem to like the idea of intralesional agents becoming appropriate to treat disease, and it is believed both T-Vec and PV-10 are helping to establish the relevancy of and build the intralesional agent category. People like having options as well, so two intralesional agents are better than one, in general. No matter what happens with T-Vec as a monotherapy, it is already believed T-Vec used in combination with ipilimumab is synergistic, and further builds the intralesional agent category.
⏏ According to Neuroscience For Kids, the blood-brain barrier is semi-permeable, allowing some materials to cross, but preventing others from crossing. Further:
"More than 100 years ago it was discovered that if blue dye was injected into the bloodstream of an animal, that tissues of the whole body EXCEPT the brain and spinal cord would turn blue. To explain this, scientists thought that a "Blood-Brain-Barrier" (BBB) which prevents materials from the blood from entering the brain existed."
According to BrainFacts.org:
"The brain is the only organ known to have its own security system, a network of blood vessels that allows the entry of essential nutrients while blocking other substances. Unfortunately, this barrier is so effective at protecting against the passage of foreign substances that it often prevents life-saving drugs from being able to repair the injured or diseased brain."
Very small amounts of rose bengal cross the blood-brain barrier. See, for example, Table 3 of Klaassen's
Pharmacokinetics of rose bengal in the rat, rabbit, dog and guinea pig (October 1976).
 |
| Click to enlarge. |
Some patients in Provectus' melanoma Phase 2 trial had visceral disease, including brain metastases. How many patients with brain mets is unclear. Provectus has publicly discussed one such patient at medical conferences (Subject 0907 with Stage IV M1c disease), noting "“Near complete resolution” of pulmonary nodules observed at Week 12" during an ASCO 2010 clinical development update presentation.
If the brain is on the other side of the blood-brain barrier (and the immune system is on the other, so to speak), and PV-10 is injected into visible lesions on the skin, how were these brain tumors (nodules) positively impacted?
Blood Brain Barrier, Margaret Reece, Ph.D. (November 2013):
"The blood brain barrier is designed to exclude both pathogens and the cells of the immune system. It also excludes large proteins including immune system antibodies. It is only after viruses and bacteria are able to trigger a breakdown of the blood brain barrier that immune system responders gain entrance. And, when it occurs, it seldom turns out well."
The small amounts of rose bengal that may permeate the barrier are very unlikely to be able to reduce or destroy tumors, and the drug was not systemically administered (just injected into the skin at the location of a cutaneous or subcutaneous lesion). As Craig would say, Mother Nature's immune system knows how to get to brain tumors without destroying the normal brain tissue. But how?
"The brain has often been considered an immunologically privileged organ. This was based on early studies that found few antigen-presenting cells in the central nervous system. In addition, there was a perceived lack of a lymphatic system within the brain to carry immunogenic material in the central nervous system to lymph nodes where a humoral immune response could be initiated. And finally, the presence of the blood-brain barrier (BBB) was thought to prevent the entry of immune cells from the peripheral circulation into the brain. However, there is increasing evidence to suggest that the brain is under immunological surveillance." (Miller, Immunobiology of the blood-brain barrier, December 1999)
.jpeg)





No comments:
Post a Comment