Category Builder (December 30, 2014)
The December edition of Cancer Watch contains an article entitled Promising Strategy for Advanced Melanoma: Intralesional Therapies Plus Systemic Immunotherapies that describes the use of Amgen's talimogene laherparepvec ("T-Vec") and Provectus' PV-10 in combination with systemic immunotherapies (i.e., anti-CTLA-4, -PD-1 and -PD-L1 agents). M.D. Anderson oncologist, T-Vec principal investigator ("PI") and PV-10 PI Dr. Merrick Ross, M.D. was quoted in the article:
"Chemotherapies and BRAF inhibitors, said Merrick Ross, MD, MD Anderson Cancer Center, Houston, TX, probably cause oncolysis by inducing apoptosis. This programmed cell death may not cause the tumor to express antigens in a way that is useful to the immune system. Viral vectors and PV-10, on the other hand, actually rupture the tumor in a manner whereby the antigens are expressed and still intact. “So the type of oncolysis may be very relevant. The tumor ablative agents could be synergistic with ipilimumab. It would make sense because they act at different places in the immune system.”
The oncolytic immunotherapies, including an oncolytic virus such as T-VEC or the chemical ablator, PV-10, actually rupture the tumor in a manner that acts like a multivalent vaccine, releasing antigens potentially specific to the tumor—even including ones that were “hidden” to the immune system when the tumor was intact, Dr. Ross said." {Underlined emphasis is mine}Huntsman Cancer Center's Dr. Robert Andtbacka has described T-Vec and PV-10, among other therapies (such as Vical's Allovectin-7 and Viralytics' Coxsackievirus A21 (or CAVATAK), as intralesional therapies or intralesional injectibles. Andtbacka has been a T-Vec, Allovectin and Coxsackievirus A21 PI.
 |
| Click to enlarge. Andtbacka, 2014 |
 |
| Click to enlarge. Agarwala, edited from Andtbacka, 2014 |
 |
| Click to enlarge. Andtbacka, 2013 |
 |
| Click to enlarge. Andtbacka, 2012 |
 |
| Click to enlarge. Amgen, 2014 |
Consider Memorial Sloan Kettering Cancer Center's checkpoint blockade key opinion leader Dr. Jedd Wolchok, M.D., Ph.D.'s December 2014 Nature article (co-author) Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer. Oncoloytic viruses typically are associated with oncolytic immunotherapies, although Ross described PV-10 as an OI:
"Identification of the immune suppressive mechanisms active within the tumor microenvironment led to development of immunotherapeutic strategies aiming to reverse the immunosuppression and to enhance the function of tumor-infiltrating lymphocytes. Of those, cancer therapy with antibodies targeting the immune costimulatory and coinhibitory receptors has demonstrated significant promise in the recent years, with multiple antibodies entering clinical testing. The responses to these agents, however, have not been universal and have not been observed in all cancer types, calling for identification of appropriate predictive biomarkers and development of combinatorial strategies. Pre-existing immune infiltration in tumors has been demonstrated to have a strong association with response to immunotherapies, with the type I interferon (IFN) pathway emerging as a key player in tumor innate immune recognition and activation of adaptive immunity. These findings provide a rationale for evaluation of strategies targeting the type I IFN pathway as a means to enhance tumor immune recognition and infiltration, which could potentially make them susceptible to therapeutics targeting the cosignaling receptors. To this end in particular, oncolytic viruses (OVs) have been demonstrated to enhance tumor recognition by the immune system through multiple mechanisms, which include upregulation of major histocompatibility complex and costimulatory molecules on cancer cells, immunogenic cell death and antigen release, and activation of the type I IFN pathway. Evidence is now emerging that combination therapies using OVs and agents targeting immune cosignaling receptors such as 4-1BB, PD-1, and CTLA-4 may work in concert to enhance antitumor immunity and therapeutic efficacy. Our evolving understanding of the interplay between OVs and the immune system demonstrates that the virus-induced antitumor immune responses can be harnessed to drive the efficacy of the agents targeting cosignaling receptors and provides a strong rationale for integration of such therapies in clinic." {Underlined emphasis is mine}T-Vec's progress to date (e.g., presumed PDUFA date, clinical combination therapy data, etc.) and comparison to PV-10 should be helpful for the category of intralesional therapies/oncolytic immunotherapies.
 |
| Click to enlarge. T cells in the peripheral blood mononuclear cell ("PBMC") of melanoma patients |
Like Watching Sausage Being Made (December 29, 2014)
When asked about the process Eric engaged in regarding the submission of the protocol for the upcoming pivotal melanoma Phase 3 trial as well as the questions about eligibility requirements, Peter said Eric and his clinical advisory team did not believe it was appropriate to ask those questions other than in the context of providing the protocol itself. The questions could not be properly framed without the FDA also having the benefit of reviewing the more than 100-page protocol submission itself. So, in order to loosen the eligibility requirements, Peter said Eric needed to submit the protocol, which is applicable and works globally as it stands now, and then modify or amend it as necessary and appropriate pending subsequent discussion with the Agency.
When I pressed Peter further on the topic, he said the two-step process was standard practice; that is, it was viewed as appropriate to handle pivotal protocol submissions in the manner Eric in fact did, and to handle operational aspects of the protocol with questions in conjunction with the protocol submission itself. In Provectus' Provectus Biopharmaceuticals to Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment press release, Eric said:
"When we submitted the protocol to the Agency in November, we included a brief list of questions about certain operational aspects of the protocol, in particular regarding eligibility requirements relevant to maximizing the speed of enrollment of patients in the study. This is standard practice for a pivotal submission. The FDA has subsequently indicated that a formal meeting is appropriate to assure that these questions are addressed in a timely and documented manner. We hope the meeting will occur in January or early February 2015." {Underlined emphasis is mine}IP & China (December 26, 2014)
Provectus' Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9-xanthen]-3-one (Rose Bengal) and Related Xanthenes, which [according to the company] "...covers the process under which pharmaceutical grade Rose Bengal and related xanthenes are produced, reducing the formation of certain previously unknown transhalogenated impurities that currently exist in commercial grade Rose Bengal in uncontrolled amounts" and is "...in accordance with International Conference on Harmonisation (ICH) guidelines for manufacture of API suitable for clinical trial material and commercial pharmaceutical use" was filed as an application with the State Intellectual Property Office of the People's Republic of China ("SIPO") in September 2010, the same time as it was filed with the U.S. Patent and Trademark Office ("PTO"). You can search the SIPO site here. I previously found the necessary SIPO links to support this news item but cannot provide them as of this writing, as they seem to no longer work. The PTO published the synthesis patent application in March 2011 (about 6 months after filing). SIPO published it in July 2012, about 16 months after it was published by PTO (22 months after filing). In the case of India, the application was filed in March 2012 (about 18 months after the U.S. filing) and published in May 2013 (about 14 months after filing it in India).
According to Eric (paraphrasing), Provectus files Patent Cooperation Treaty ("PCT") versions of the company's PTO patent applications simultaneously in designated jurisdictions within their respective PCT deadlines. Such filings may be made months after the U.S. case is filed. This allows management to obtain initial input from U.S. examination before the process commences in earnest in other jurisdictions, and to fine tune claims before international prosecution starts. Additionally, Provectus also typically elects to file first in certain key jurisdictions the company has learned are both important and rigorous, which allows management to further refine their international claims before proceeding with other jurisdictions. In the case of India, the PCT deadline is 31 months.
It took about 36 months from filing for the PTO to approve the synthesis patent application. 52 months after filing this patent application with SIPO could suggest an approval timeframe of December 2014 or shortly thereafter.
Two sources of data on China and India per capital Gross Domestic Product ("GDP") are here, and below.
 |
| Click to enlarge. Source link is here. |
Provectus issued a press release and filed an associated 8-K Monday regarding the eligibility requirements of the protocol of its upcoming pivotal melanoma Phase 3 trial. According to Eric, in the PR:
"When we submitted the protocol to the Agency in November, we included a brief list of questions about certain operational aspects of the protocol, in particular regarding eligibility requirements relevant to maximizing the speed of enrollment of patients in the study. This is standard practice for a pivotal submission. The FDA has subsequently indicated that a formal meeting is appropriate to assure that these questions are addressed in a timely and documented manner. We hope the meeting will occur in January or early February 2015." {Underlined emphasis is mine.}It would appear Provectus would like to discuss [with the FDA] loosening one or more inclusion and/or exclusion criteria, which if successful could increase the trial's enrollment rate above management's baseline expectation. One such inclusion criteria probably is:
- #8, "Failed, did not tolerate, or not a candidate for (due to co-morbidities, pre-existing autoimmune disease or drug unavailability) treatment with ipilimumab or another immune checkpoint inhibitor," where the goal(s) could be to remove "Failed" and/or broaden the definition of "not a candidate for."
- #4, "At least 2 cutaneous Target Lesions > 5 mm in longest diameter. Target Lesions should be at least 10 mm from any other lesion"
- #5, "No lesion > 30 mm in longest diameter; and no more than 20 lesions"
- #5, "Calculated required PV-10 dose ≤ 15 mL (based on total tumor burden)," and/or
- #9, "Not a candidate for treatment with vemurafenib, dabrafenib or trametinib (i.e., BRAF V600E wild-type)."
The "risk-reward" of Eric's desire to seek these operational improvements in the trial seems to me to be (i) a "downside" of a 45-day delay with no improvements, and (ii) an "upside" of a late-January/early-February trial start with certain improvements that may increase, substantially or otherwise, the rate of enrollment over that of (i).
Using the 30-45 day review period from the November 6th Provectus Biopharmaceuticals Submits PV-10 Phase 3 Melanoma Protocol to FDA as a guide, the delayed trial (delayed based on management's prior guidance) could be green-lighted, change(s) or no change(s) to inclusion/exclusion criteria, somewhere between January 17, 2015 and February 5th.
Chuck (December 22, 2014)
As I introduced in my Let Me Tell You A Story About Pfizer blog post, the addition of the first Pfizer executive to Provectus' strategic advisory board ("SAB") was clearly stated in August 2011's Dr. Craig Eagle of Pfizer Joins Provectus Pharmaceuticals's Corporate Advisory Board press release.
The announcements of the second and third Pfizer executives, however, were seemingly tacked on to their respective PRs: December 2013's Provectus Announces Name Change to Provectus Biopharmaceuticals, Inc. and Reincorporates in Delaware and December 2014's Provectus Biopharmaceuticals To Sponsor American Association of Physicians of Indian Origin. It seems straightforward to have issued these last two releases as "Bob Miglani of Pfizer Joins Provectus Biopharmaceuticals's Corporate Advisory Board" and "Deanna Angello of Pfizer Joins Provectus Pharmaceuticals's Strategic Advisory Board" (note: the board's name changed from Corporate to Strategic).
The two sides of the trade could be this: Either Provectus management botched the releases that followed Dr. Eagle's ("Side 1") or Pfizer did not consent to an Eagle-style Provectus PR for either or both of Miglani and Angello ("Side 2"). Either Provectus recruited Eagle, Miglani and/or Angello to the SAB ("Side 1") or Pfizer wanted one or more of them there ("Side 2").
When I spoke with SAB member Dr. Joseph Chalil, M.D. of Boehringer Ingelheim over the summer, he said (paraphrasing) he'd advise any emerging company like Provectus to develop, implement and execute a non-U.S.-centric go-to-market strategy parallel to and in combination with a U.S.-centric one. See Moffitt at SITC, Officially (September 23, 2014) below. Dr. Chalil also said he'd further advise biotechnology companies approaching their Phase 3 trials to look beyond the trial itself to the potential launch of their respective drug candidates (i.e., begin assembling a product launch team and developing a launch plan). Angello's skill set would seem to also lend itself to a role on such a team for PV-10.
There undoubtedly are people like Angello at other pharmaceutical companies around the world (i.e., men and women with comparable skill sets and experiences) for Provectus management to seek out and leverage their product launch knowledge for the company's benefit and preparedness. Why did Provectus produce (or agree to produce) Miglani and Angello's press releases in the way they did? The benefit of not including Pfizer's name in the releases' headline seems to accrue more to the Big Pharma company than to Provectus.
PH-10 Mechanism of Action Study (December 22, 2014)
H/t a blog reader and shareholder: Provectus' clinical study protocol for mechanism of action work on PH-10 now is up on ClinicalTrials.gov: A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10.
Updated. Clinical sites for this 30-patient study include:
- International Dermatology Research (Miami, Florida): Current studies and trials run/be running may be found here (65 of them) --,
- University of North Carolina School of Medicine (Chapel Hill, North Carolina), and
- DermResearch (Austin, Texas): Current studies and trials run/be running may be found here (109 of them).
The Decision-Plus interview of Peter -- see Peter (December 18, 2014) below -- had its pros and cons. The venue has no notable brand (as far as I can tell), interviewer Christian Charlot was not well informed, and Peter's replies were neither crisp nor replete with specifics. On the plus side, Peter's efforts to shed more light on and better inform shareholders (and investors at large) about Provectus' clinical development program and process are a welcome change from Eric's historical circumspection. In particular, his comments that (paraphrasing) the FDA had never seen data [from the company's melanoma Phase 2 trial] this good and the Agency had suggested Provectus submit an application for breakthrough therapy designation based on that data were very interesting.
Updated 12/21/14. Another interesting comment from Peter's interview was his mention of the treatment of any kind of tumor on (cutaneous) or under (subcutaneous) the skin in Provectus' compassionate use program (expanded access protocol), irrespective of the type of cancer.
I would have liked him to be more specific when saying (paraphrasing) very well known research organizations throughout the world are using Rose Bengal and the company's PV-10 formulation to show why drug is working so well. Who else besides Moffitt Cancer Center has conducted research on PV-10, and is considered a "very well known research organization?"
It was interesting Peter said Provectus tried to shortcut the process (presumably the regulatory approval path/process), and that management thought they could to do so by working with the FDA, by submitting the breakthrough therapy designation application. I thought it was a good reminder for Peter to say a regional transaction contract in China (presumably with Sinopharm) could be assigned to Provectus' eventual acquirer. I did chuckle by the number of times Peter referenced and said the name Pfizer.
Parallel Processing (December 19, 2014)
In my post entitled "These murine studies support combination therapy with IL PV-10 and co-inhibitory blockade" I wrote Peter noted in the company's third quarter 10-Q: "We also have begun to consider co-development transactions with one or more pharmaceutical or biotech companies to combine PV-10 with immunology agents such as those referred to as immune checkpoint inhibitors...Furthermore, the strategy of the Company for the benefit of stockholders is a series of partnerships followed by an acquisition of the Company along the lines of Celgene-Abraxis, although there can be no assurance that such partnerships or acquisition will occur. An interim transaction could be a co-development deal like Roche-NewLink, Bristol-Celldex or AstraZeneca-Incyte."
I researched the small biotechnology companies Peter referenced in the above mentioned post, and the time between (a) publishing pre-clinical combination therapy work (major conference presentation or peer-reviewed journal article) and (b) striking a relationship or transaction related to combination therapy with a Big Pharma company. It is a very limited dataset, of course:
- Celldex: 6 months (1 collaboration [trial]),
- Incyte: 3-6 months (3 [trials]), and
- NewLink: 6 months (1 [out-license]).
Building on this, I also considered the time between (c) announcing a collaboration, (d) announcing approval of the investigational new drug ("IND") application for the combination therapy clinical trial, and (e) starting the trial. See, for example, Advaxis Announces FDA Acceptance of Its Investigational New Drug Application to Commence Clinical Trials of ADXS-PSA in Combination With Merck's KEYTRUDA(R) (pembrolizumab) for Prostate Cancer. Here, Advaxis announced its collaboration with Merck in August 2014. The IND was accepted in December 2014 (4 months later). Patient enrollment is expected in the first quarter of 2015 (3 months after IND acceptance).
Provectus published pre-clinical combination therapy work at SITC in November 2014. Using the very limited dataset above in order to estimate a timeframe for Provectus and a Big Pharma partner, we might expect:
- A Big Pharma combination therapy collaboration somewhere between February and May 2015,
- An IND acceptance between June and September, and
- A clinical trial start (i.e., a Phase 1b) in 2Q15 or late-3Q15/early-1Q16.
In order to generate relevant systemic data as quickly as possible, it would make sense for Provectus to move forward on their own combination trial (i.e., a Phase 1b trial of 20+ patients) as soon as they can, leveraging their cost structure knowledge related to treating metastatic melanoma patients, key relationships who understand treatment of and have treated late-stage melanoma patients with combination therapies, and understanding of medical reimbursement of the much more expensive approved late-stage checkpoint blockade therapeutics.
So, one might think:
- The per patient cost for a PV-10 combination trial should be more expensive than the per patient cost for a PV-10 monotherapy trial. Say, $50K per patient, or a total cost of about $1MM assuming 20 patients,
- The principal investigator/clinical site could be Dr. Jeffrey Weber, M.D., Ph.D. of Moffitt Cancer Center, and
- The checkpoint blockade candidate would be either Yervoy (CTLA-4) or Keytruda (PD-1).
Starting a combination trial of their own shouldn't prevent Provectus and/or dissuade a Big Pharma company from eventually establishing a combination therapy collaboration, which might take longer to develop and commence.
American Association of Physicians of Indian Origin (December 17, 2014)
Provectus issued a press release and filed an associated 8-K regarding its upcoming sponsorship of the activities of the American Association of Physicians of Indian Origin ("AAPI"), whose mission is "...a forum to facilitate and enable Indian American Physicians to excel in patient care, teaching and research and to pursue their aspirations in professional and community affairs."
Most recently AAPI had been advocating for the appointment of Dr. Vivek Murthy, M.D. as U.S. Surgeon General (see also The Times of India's Indian-American doctors pitch for Dr Vivek Murthy's confirmation).
Provectus strategic advisory board member and Boehringer Ingelheim executive Dr. Joseph Chalil, M.D. received AAPI's New York Presidential award ("outstanding contributions to healthcare") in 2013.
PR quotes included:
Peter (his first Provectus PR quote, as far as I can recall): "We will hit the ground running with AAPI by attending their Global Healthcare Summit (GHS) in Mumbai, India, starting January 2, 2015. At this GHS, we expect around 800 delegates to attend. Between 150 and 200 will be from the USA, and some from the United Kingdom, Canada, Middle East and Australia. In addition, AAPI will have a live extension to its members in the USA who are unable to attend, as well a live telecast to an estimated 50,000 doctors around India. We will inform this huge group of doctors from around the globe about the progress we have made with PV-10 to date, what we are planning in the anticipated phase 3 clinical trial, and what we have learned about PV-10 for indications other than melanoma."
AAPI President, Dr. Ravi Jahagirdar, M.D.: "We are very happy to have Provectus as an official sponsor of AAPI and to enjoy their participation at our coming meetings in Mumbai and Orlando in 2015. The Indian diaspora offers a unique, global network of physicians who can increase the communication of medical developments like the clinical results shown thus far for PV-10. Moreover, we have close professional, educational and family ties to India itself, a nation of 1 billion. We believe this introduction of Provectus to the healthcare industry in India will be well received, since PV-10 has many features that make it well suited for local conditions."Updated 12/18/14. H/t a shareholder and blog reader: Two Provectus strategic advisory board members, Boehringer Ingelheim's Chalil and Pfizer's Bob Miglani, will participate in AAPI's Global Healthcare Summit (see below).
 |
| Click to enlarge. |
Eric, Peter and St. Luke's Dr. Sanjiv Argawala were in Australia last week for meetings with medical advisors to the company, and other key opinion leaders in melanoma. These meetings coincided with the Annual Scientific Meeting of the Clinical Oncology Society of Australia (December 2nd to 4th) in Melbourne.
A month earlier, at the 2nd National Melanoma Conference in Perth, global key opinion leader and Provectus principal investigator Dr. John Thompson, M.D., made a presentation entitled The important, continuing roles of surgery and loco-regional treatments in melanoma management in the era of more effective systemic therapies, noting (among other things):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
A good read on the topic of checkpoint inhibitors comes from Paul Rennert's SugarCone Biotech Blog's post entitled Last Week’s Immune Checkpoint Papers In Nature Are Complicated! He draws two key conclusions; both CD8+ T-cells and IFN-γ (interferon-gamma) generation/production are good.
 |
| Click to enlarge. |
...clinical data on 8 melanoma patients that demonstrated significant decreases in melanoma cells in injected tumors and uninjected bystander tumors 7-14 days after PV-10 injection as evidenced by pathologic evaluation confirmed with immunohistochemical staining of biopsy specimens for melA (a marker of melanoma). The researchers showed that these changes in tumors were accompanied by increased populations of CD3+, CD4+ and CD8+ T cells along with NKT cells in peripheral blood. T cells from one patient were purified and exhibited increased interferon-gamma expression when exposed to the patient's pre-treatment melanoma cells. {Underlined emphasis is mine}A graph of T-cells in peripheral blood mononuclear cells ("PBMC") of melanoma patients is below:
 |
| Click to enlarge. |
 |
| Click to enlarge. Original poster here. Orange ovals added by me. |
 |
| Image source. |
Rose bengal's 140-year history includes use as an industrial dye, food dye, staining solution, diagnostic agent, and therapeutic agent.
One of Provectus' key intellectual property assets is its synthesis patent, Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9-xanthen]-3-one (Rose Bengal) and Related Xanthenes, which protects the process of producing pharmaceutical grade rose bengal (i.e., PV-10) and related xanthenes. This means Provectus would receive money for any medical use of rose bengal and its relatives. The family of halogenated xanthenes, of which rose bengal is a member, requires the halogen molecule (i.e., flourine [F], chlorine [Cl], bromine [Br] and iodine [I]) to be swapped out. Rose bengal, which has chlorine atoms in it, is shown below.
 |
| Click to enlarge. Wikipedia. |
Abstract: The abnormal aggregation of β-amyloid (Aβ) peptides in the brain is a major pathological hallmark of Alzheimer's disease (AD). The suppression (or alteration) of Aβ aggregation is considered to be an attractive therapeutic intervention for treating AD. We report on visible light-induced inhibition of Aβ aggregation by xanthene dyes, which are widely used as biomolecule tracers and imaging markers for live cells. Among many xanthene dyes, rose bengal (RB) under green LED illumination exhibited a much stronger inhibition effect upon photo-excitation on Aβ aggregation than RB under dark conditions. We found that RB possesses high binding affinity to Aβ; it exhibits a remarkable red shift and a strong enhancement of fluorescence emission in the presence of Aβ. Photo-excited RB interfered with an early step in the pathway of Aβ self-assembly and suppressed the conformational transition of Aβ monomers into β-sheet-rich structures. Photo-excited RB is not only effective in the inhibition of Aβ aggregation, but also in the reduction of Aβ-induced cytotoxicity.According to the Alzheimer's Association (U.S.):
"In Alzheimer’s disease, brain cells that process, store and retrieve information degenerate and die. Although scientists do not yet know the underlying cause of this destruction, they have identified several possible culprits.
One prime suspect is a microscopic protein fragment called beta-amyloid (BAY-tuh AM-uhloyd). Some researchers believe flaws in processes governing production, accumulation, or disposal of beta-amyloid are the primary cause of Alzheimer’s. This theory is called “the amyloid hypothesis.”" (Source)According to the Alzheimer's Society (U.K.):
"Beta-amyloid plaques in the brain are thought to trigger the progression of Alzheimer's disease." (Source)There are important caveats, such as the Korean work was done in vitro, which means the work was done with cells outside their normal biological context. And, translating this work into a potential clinical approach may prove difficult because of the limited penetration of green light.
Provectus ultimately figured out light activation was not key to triggering rose bengal's therapeutic benefit, but rather increasing its administered quantity. Perhaps the same might be germane to the very early and pre-clinical Alzheimer’s work above.
Nevertheless, rose bengal continues to demonstrate its novelty.
Updated (12/4/14): From a December 2nd article entitled Shedding Light on Alzheimer's Treatment by David Bradley in ChemicalViews.
"Light-activation of the well known xanthene dye, rose Bengal, might lead to new insights and approaches to halting the aggregation of errant amyloid proteins in the brain which are closely associated with the lethal neurodegenerative disorder Alzheimer's disease (AD). Researchers from South Korea will report details of the findings in the January 2015 issue of the journal Biomaterials."Interferon-gamma (November 30, 2014)
H/t [for the SITC article below] an internist, shareholder and blog reader: In their murine model work to date, Moffitt Cancer Center showed PV-10, individually (July 2013) and in combination with the three main categories of checkpoint inhibitors [anti-CTLA-4s, anti-PD-1s, anti-PD-L1s] (November 2014), generated notable (enhanced) tumor-specific interferon ("IFN")-gamma production (i.e., melanoma, breast, and colorectal in their experiments).
In a November 2014 SITC journal publication article entitled PD-L1 and MHC-I expression in 19 human tumor cell lines and modulation by interferon-gamma treatment, Grenga et al. note:
"Tumor cells express different percentage of PD-L1 and MHC-I in their surface. In most of the cells analyzed, both molecules are increased by exposure to IFN-g. Based on these observations, immunotherapies aiming to increase IFN-g in the tumor microenvironment, such as therapeutic vaccines or T cell adoptive transfer, can facilitate immune recognition of tumor cells by an increase of MHC-I on the surface of tumor cells. On the other hand, the increased PD-L1 expression in the tumor can be an ideal target for anti-PD-L1 antibody treatment."Time management (November 28, 2014)
When Provectus issued its November 6th press release Provectus Biopharmaceuticals Submits PV-10 Phase 3 Melanoma Protocol to FDA, which included "The FDA is expected to review the submission and comment on the proposed study population, clinical endpoints, and statistical analyses within 30 to 45 days," I was reminded of the company's January 18, 2012 press release Provectus Receives Guidance From FDA On Pathway to Approval for Phase 3 Trial of PV-10 For Metastatic Melanoma, which included "The Company intends to pursue the SPA path, which would represent an agreement from the FDA that the Phase 3 study design endpoints, statistical analyses and other components of the planned clinical trials are acceptable to support approval of the product."
Management has previously said there was no requirement to wait [to enroll] following submission of the melanoma Phase 3 trial protocol. Eric's process presumably encouraged him to incorporate as many required and contributive trial endpoints as possible. See, for example, Duration of Complete Response (November 11, 2014) below. Then, his process also might have encouraged him to wait for the above mentioned review period to pass in order to weigh any significant and/or general comments the FDA might have had about the protocol. See my blog post It's Eric's process. Does his process also include seeking a special protocol assessment ("SPA") for the trial protocol itself to achieve agreement with the Agency? {I'm not thinking here that Eric would hold up the start of the trial in order to obtain the/an SPA; rather, I'd think he'd start the trial, potentially pursuing the SPA thereafter.}
The Asia Melanoma Group (November 26, 2014)
Should a Chinese clinical site be part of Provectus' upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma, Peking University Cancer Hospital (a.k.a Beijing Cancer Hospital, Beijing Institute for Cancer Research, and Peking University School of Oncology) may well be that site, and Professor and Dr. Jun Guo, M.D., Ph.D (Vice President of Clinical Oncology, Peking University; Deputy Director, Beijing Institute for Cancer Research; Director of Department of Melanoma & Renal Cancer, Peking University School of Oncology) its lead investigator.
St. Luke's University Health Network's and Provectus' lead melanoma Phase 2 trial investigator Dr. Sanjiv Agarwala spoke at Dr. Guo's October 17th-19th Beijing melanoma conference (2014 Beijing International Melanoma Congress), which was co-chaired by Dana-Farber/Harvard Cancer Center's Professor and Dr. Keith Flaherty, M.D. See 2014 Beijing International Melanoma Congress (October 7, 2014) below.
Dr. Guo is the head of the Asia Melanoma Group, the establishment of which was announced at above mentioned Beijing conference. See China Daily article New group offers hope to melanoma sufferers:
The new group consists of 17 leading melanoma experts from countries including China, Singapore, Korea and Hong Kong. "Given Asian patients' differences in genetic background and disease types to Europeans, it is high time Asian experts stand together to form a group that will break down barriers in research on melanoma," said Guo Jun, deputy director of the Beijing Cancer Hospital and head of the newly-established group. The incidence of melanoma is higher among white people than Asians but Asian people have a much higher probability of mucous melanoma than Caucasians, which usually has a poorer prognosis than melanoma on the skin, Guo explained.Dr. Guo also is a member of Melanoma International Foundation's Scientific Advisory Board, which is co-chaired by Dr. Flaherty.
Capital Formation (November 21, 2014)
There appear to be data that point to Provectus taking down more to all of the $15 million Network 1 Financial private placement. See Fundraising (October 3, 2014). Through November 5th, the company had raised gross proceeds of $7.3 million, or about half of the placement. If/when so, and given certain assumptions outlined in the table below, Provectus could have sufficient capital for company accounting firm BDO and New York Stock Exchange requirements through May 2015 (i.e., about a $1 million a month for an 18-month operating runway at this future snapshot in time), coincidentally to when Sinopharm and Provectus extended their memorandum of understanding. See Sinopharm A-THINK Pharmaceutical Co. (November 14, 2014).
 |
| Click to enlarge. |
"...we continue to point out that we have funding essentially for 18 months. So now we’re saying we have funding well until 2016. If you notice in our comments in this call, we made reference to the fact that we want to in our interaction with External Auditors BDO and New York Exchange to share we are capitalized adequately at all times. So what that means is we believe it is appropriate to maintain the cash on hand. So the run rate continues to build out, where we need to make, we need to offset cash burn either with non-dilutive forms of financing which speaks to our efforts to secure visional license transactions or through financing [indiscernible] we stated in the 10-Q with that -- a private placement."Does this imply the China license transaction and a potential India one are "a long way off?" As I replied to the shareholder who asked me this question, I don't believe so. While I continue to disagree with Craig and Peter's approach to capital formation (and have said so to them while listening to their explanations and and justifications), including the latest, I do believe they are trying to balance third party requirements for cash minimums in the moment with consummating the right deal(s), as Provectus views it and which of course take time to craft and complete.
More of the call transcript:
"...and this is where in my comments, that’s what I am saying, that we are continuing to work with BDO and New York Exchange to maintain cash on hand. This is not an exact science as some people heard me say. There is no -- it's a great topic -- exactly how much cash at the end of each quarter, but it's very appropriate to always have the necessary run rate or enough cash and hand so that we can continue to say, that we have more than a 12 month period [indiscernible] for always an 18-month which speaks to the understanding the…"
I would imagine the parties may have mostly if not entirely moved beyond business and transaction terms and conditions of a China deal, and potentially mostly beyond due diligence, to focus on the regulatory pathway for PV-10 in the country. Time and dollars, I suppose, will tell.
Co-inhibitors' Hamburger Helper to PV-10's Hamburger (November 19, 2014)
I'm still struck by Moffitt Cancer Center's Dr. Shari Pilon-Thomas' recent quote "The spirit of our study was to determine whether combining PV-10 with a checkpoint inhibitor would enhance the systematic immune responses of the initial injection of PV-10" (see my blog post Provectus notebook).
It would seem to me Moffitt (in their work presented at SITC 2014) investigated how and how much co-inhibitory agents (i.e., anti-PD-L1s, anti-PD-1s, anti-CTLA-4s) could improve the systemic benefit of PV-10.
Checkpoint inhibitors are often described as releasing 'the "molecular brakes" that cancer cells apply to the body's immune system.' PV-10 is both accelerator and brake, capable of both stimulating and inhibiting the immune system. It may well be the additional braking provided by co-inhibitory agents ultimately is more beneficial to late-stage patients (than checkpoint inhibitor treatment alone).
Consider a recent editorial in The New England Journal of Medicine by Dr. Vassiliki A. Boussiotis, M.D., Ph.D. entitled Somatic Mutations and Immunotherapy Outcome with CTLA-4 Blockade in Melanoma:
Despite the long debate about the ability of T cells to destroy tumors, the unprecedented recent success of immunotherapy in malignant disorders has provided evidence that the patient's endogenous immune system can be altered to attack established tumors. A major hurdle in tumor immunotherapy is the fact that mechanisms of self-tolerance that prevent autoimmunity also impair T-cell responses against tumors, which do not differ substantially from self. Blockade of the major checkpoint inhibitors cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) has resulted in durable responses in many patients. However, why other patients have only transient responses or no responses at all remains unclear. It is also unclear how patients should be identified as appropriate candidates for immunotherapy.
...[T]hese data provide convincing evidence that in order for a CTLA-4 checkpoint blockade to mediate clinical benefit, T cells must be activated in the context of tumor-associated antigens. {Underlined emphasis is mine}H/t a physician, shareholder and blog reader:
If part of PV-10’s initial response is to increase IFN-γ levels, which in turn induces tumor cells to coat themselves with checkpoint proteins and try to hide from recently stimulated T-cells, then the synchronous use of checkpoint inhibitors at this juncture would counter this tumor response and allow these T-cells to better do their job.A bridging study to an NDA filing (November 16, 2014)
Several readers commented to me that my blog post "This determination is based on the paucity of data..." lacked an effective conclusion, particularly as it related to whether or not the company was on the right regulatory path. Thank you for the feedback. Let me try again, and hopefully be better this time.
By trying to describe what the company surely believed was a seminal meeting with the FDA on December 16, 2013, and noting subsequent activities, events and information via company press releases from there (January 2014) through breakthrough therapy designation application submission and its denial (May) to finalizing of the melanoma Phase 3 trial protocol subject to an 30 to 45-day FDA review period (November), I had hoped to show the trial was akin to a bridging study to an new drug application.
For the outset the company has sought to expedite the approval path for PV-10. While they historically have been unsuccessful in speeding up the drug's regulatory path to market, the path they've endeavored to forge has been the same, which is to advocate the earlier treatment of disease.
The January 2014 PR, reflecting the outcome of the December 2013 meeting with the Agency, clearly implied (I think) no Phase 3 trial with overall survival as a primary endpoint was necessary or needed.
It's my view the two arms of the upcoming pivotal trial are essentially the same. Yet another study, this time by Bristol-Myers, shows a very predictable progression-free survival ("PFS") outcome for systemic chemotherapy (2.2 months with a 95% confidence interval of 2.1 to 2.4 months -- or ~9.5 weeks with a CI of ~9-10 weeks). Patients in Provectus' trial could cross over after at least one 4-week cycle of chemotherapy and disease progression -- it is possible most if not all control arm patients would progress after 1-2 cycles but before 3 cycles (after 4-8 weeks but before 12 weeks, which also is the first assessment period).
It's also my view that measuring overall survival is not particularly important (nor overly relevant) for the trial. Rather, I think the goal of the study, and what is much more important and relevant, is to collect more of the data the FDA wanted as part of Provectus' BTD application (i.e., data that would allow patient symptoms to be conclusively correlated with objective response).
Finally, it is my view the data generated early on from Provectus' Phase 3 trial -- PFS, patient-reported outcomes, crossovers, adverse events, etc. -- should allow its independent data monitoring committee ("DMC") to clearly and swiftly observe is conclusively efficacious, safe, humane and thus necessary to recommend stopping the trial early.
One then would think, expect and hope the Agency would concur with the DMC's recommendation and its rationale.
Sinopharm A-THINK Pharmaceutical Co. (November 14, 2014)
Provectus and Sinopharm extended their memorandum of understanding ("MOU") today until May 16, 2015, announced via the issuance of a Provectus press release and the filing of an associated 8-K. The original MOU was entered into on August 15 (the associated press release is here). Provectus' November 14th PR quotes Sinopharm A-THINK's CEO Dr. Zhidan Jia, who is named on a patent application filed in August 2014 with the U.S. Patent & Trademark Office (Novel histone deacetylase inhibitor of benzamides and use thereof). Assignees of this patent are Shanghai Institute of Pharmaceutical Industry (the precursor of China State Institute of Pharmaceutical Industry) and A-THINK. Some wording in today's PR was awkwardly composed. "The Company stated" should have read "Sinopharm A-THINK stated:"
The Company stated that it is hopeful that a contract will be finalized in the coming weeks, and this extension illustrates that there is sufficient interest on both sides to continue to work out the details.Provectus and its media/investor relations firm Porter, LeVay & Rose both confirmed "The Company" in the PR referred to Sinopharm.
Institutional Ownership (November 14, 2014)
Provectus shares held by institutions who made SEC form 13F filings and shares held as a percent of those outstanding significantly increased during the quarter ending September 30, 2014.
 |
| Click to enlarge. |
Melanoma Phase 3 trial protocol up on ClinicalTrials.gov (November 12, 2014)
Provectus' Phase 3 trial protocol for locally advanced cutaneous melanoma is up on www.clinicaltrials.gov. The link is here.
-- "Subjects in the comparator arm who have completed at least 1 cycle of dacarbazine or temozolomide and who meet the study protocol definition of disease progression but do not have evidence of distant cutaneous, subcutaneous, active nodal or visceral metastases will be eligible to enter the crossover portion of the study and receive PV-10." {Underlined emphasis is mine}
Updated (11/13/14): Provectus issued a press release and filed an associated 8-K regarding the appearance of its melanoma Phase 3 trial protocol appearing on the clinicaltrials.gov website. Endpoints include:
Primary:
- Progression-free survival (PFS)
Secondary:
- Complete response rate (CRR),
- Duration of complete response [see Duration of Complete Response (November 11, 2014) below],
- Change in total symptom score from baseline using the patient reported Skindex-16 instrument,
- Overall survival (OS), and
- Number of participants with adverse events.
A very poor quality transcript of Provectus' Q3 2014 results/earnings call is available from Seeking Alpha. Of particular note to me was one comment at the beginning of Eric's prepared remarks:
Thank you, Pete and thanks to everyone for joining us today. Obviously, the biggest news today is that we have submitted our Phase 3 protocol to the FDA and I’d like to cover that with you now. We have had multiple interactions with the FDA since our Type C meeting with the division of oncology products on December 16, 2013. These contacts have helped us craft and refine our submission. In particular these exchanges provided the rational for the PV-10 Phase 3 randomized control trial in patients with local events for cutaneous melanoma. This Phase 3 randomized control trial of PV-10 in patients with locally advanced cutaneous melanoma will assess response to PV-10 versus that a systemic chemotherapy in patients who have disease limited to cutaneous and subcutaneous sites and we have failed or are ineligible for systemic immunotherapy.
Progression free survival and complete response rate will be assessed using standard criteria that is [indiscernible]. Duration complete response, overall survival and effects on melanoma symptoms such as pain, bleeding, inflammation and infection will also be assessed.
We will also collect comprehensive data on pain medication use. So we now pay debt to replace in proper context. We are working our CROs and clinical sites to begin patient recruitment this year as we’ve stated previously on earlier conferences call and in corporate communications throughout the year. I won’t go into much details about the study design as that will be available shortly on the clinical trials.gov Web site. I’m sure it’s of interest to you. So we’ll announce that when it’s publically available. {Underlined emphasis is mine.}(A) Endpoints, or items to be assessed, from the second paragraph of Eric's comments, appear to be:
- Progression-free survival ("PFS"),
- Complete response ("CR") rate,
- Duration [of] complete response,
- Overall survival,
- Effects on melanoma symptoms, and
- Pain medication use.
- PFS,
- CR rate,
- [Change in] Patient-reported outcomes, and
- Overall survival.
Is duration of CR (B3) a new endpoint or item on which management will collect data during the trial, or did Eric misspeak? I imagine we should definitively know when the protocol appears on www.clinicaltrials.gov. In the interim, I'm going to lean towards the former, particularly when the comment emanated from prepared remarks, and because I think it (the "new endpoint") speaks to a much larger, more macro point about PV-10 and its pivotal Phase 3 trial indication/potential label of locally advanced cutaneous melanoma.
PFS is a measure (in months) of the duration or length of CR plus partial response ("PR"). Duration of CR would seem to extract CR from PFS.
I have written often about Provectus management's approach to cancer, which is to treat earlier stages of the disease (thoughtfully, intelligently, without blind or perfunctory use of surgery) before it has spread uncontrollably by effectively "defeating" or controlling local-regional disease to deny, prevent or forestall the metastatic spread of cancer.
The company's peer-reviewed publication of Provectus melanoma Phase 2 trial data noted: "Future studies will comprehensively assess the effect of PV-10 on PFS to document potential longer-term benefits of locoregional disease control." Under news item Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma (October 28, 2014) I wrote: I think the upcoming pivotal Phase 3 trial (and potentially other studies the company and/or its acquirer may run) should help elucidate how PV-10 can forestall or deny the expansion of melanoma (and, later and hopefully, other solid tumor cancers) to a metastatic and/or visceral spread. This conclusion should help establish the basis for earlier and much earlier use of the drug.
Under news item PV-10 is tantamount to surgery? (September 5, 2014) I wrote: Provectus plans to undertake a project of gathering data across relevant treatments that show when disease (melanoma) is ablated in Stage III it is forestalled from getting to Stage IV, with the expectation of publishing this analysis in a peer-reviewed publication. Advocating more broadly (than for PV-10 itself) that appropriate local treatment is relevant and effective for longer-term patient benefit would seem to be a thoughtful approach to take in parallel with Provectus PV-10-specific clinical development program.
If one is trying to make the case to the FDA, physicians and oncologists that treating PV-10 in appropriate Stage III melanoma patients indeed can forestall or deny the spread of the disease, partial response doesn't cut it. One presumably not only has to show a high rate of complete response, but I imagine also show the complete response achieved has a long duration (i.e., the tumor goes away, and it goes away for a long-time or forever).
Management's (Peter's, Eric's) prepared remarks were fine, providing useful information as illustrated above. As public company managers, they could (should) have handled Q&A in both substance and delivery much better than they did.
Hypothesis: Something stimulatory + Something inhibitory = Cure (November 9, 2014)
One of the most interesting tweets (#SITC2014) from the annual meeting of the Society for Immunotherapy of Cancer ("SITC") was from @BeyondTheCodon about comments from a presentation by Dr. Brendan Curti, M.D. of the Earle A. Chiles Research Institute (EACRI):
 |
| Click to enlarge. |
In September, one of the SITC presentation's co-authors and Moffitt Cancer Center assistant professor and researcher, Dr. Shari Pilon-Thomas, Ph.D., co-authored an online OncLive article entitled Immunotherapy Combined With Chemotherapy for Pancreatic Cancer: A Game Changer? (see blog post Treating Cancer). In it Dr. Pilon-Thomas and her fellow authors write:
Of note, the immune system’s involvement in cancer development and progression has sparked much interest in recent years. The model of the cancer-immunity cycle suggests an interplay of immune-suppression and immune-stimulation. In normal individuals, a state of immunosurveillance is in place. However, within the tumor microenvironment, inhibitory signals and immunosuppressive cells are present and tip the scale in favor of immune suppression. {Underlined emphasis is mine}
Continued: The idea of the cancer-immunity cycle proposes that, for a cancer immune response to be generated, the net balance between immune stimulation versus immune suppression must be tipped in favor of the former. Studies in various cancers have suggested that tumors evade the immunogenic process mostly by factors that promote immunosuppression. {Underlined emphasis is mine}The balance between co-stimulation and co-inhibition is described by Inman et al.: "If sufficient co-stimulation is provided in the presence of adequate tumor-associated antigenic stimulation, the immune system will act against tumor antigen and, thus, destroy early tumors before they become fully established. Contrarily, if co-inhibitory signaling dominates, the immune system will be tolerized to tumor antigens, and the tumor will be permitted to grow unfettered and unmolested by the immune system. If neither co-stimulatory nor co-inhibitory signals dominate, the adaptive immune system may remain in a tenuous state of equilibrium, militating against tumor outgrowth with varying degrees of success." Generalizing (or simplifying):
- If co-stimulation > co-inhibition, the immune system can act decisively against cancer,
- If co-inhibition > co-stimulation, cancer overwhelms the immune system and renders it ineffective or useless, and
- If co-stimulation = co-inhibition (that is, some sort of equilibrium state), the immune system wages battles against cancer to varying degrees of success with potentially no ultimate resolution to the war itself.
 |
| Click to enlarge. Figure 2 (above) of [Daniel] Chen et al.'s article. |
Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, whereas inhibitors shown in red help keep the process in check and reduce immune activity and/or prevent autoimmunity.When Curti references "checkpoint inhib" (checkpoint inhibitors, co-inhibitors, coins, etc.), he's pointing to inhibitory agents, such as PD-1s and PD-L1s. When he references "costim" (co-stimulators, costims, etc.), I think he may be broadly referring to the green stimulatory factors in Step 3 below (rather than, say, the stimulatory factors in Steps 1 and 2).
 |
| Click to enlarge. Figure 2 (above) of [Daniel] Chen et al.'s article, modified by me. |
 |
| Click to enlarge |
Pairing stimulation and inhibition underscores, I think, Curti's "hopeful hypothesis." Costims, like OX40 and CD27 (and others), may prove difficult partners because of their toxicity (let alone in combination with toxic checkpoint inhibitors).
Moffitt's SITC abstract of PV-10 introduced the result of increased tumor regression (response) and improved survival (goal) by pairing the intralesional agent with each category of checkpoint inhibitor. One might characterize PV-10 as the ultimate costim, but probably not in the typical sense of what most people understood agents in this category (i.e., costim, co-stimulatory, co-stimulation, Step 3) to be.
H/t a physician (internist), shareholder and blog reader: "...a thought here (and one that Big Pharma may be absorbing): pre-treatment of tumors with PV-10 raises this critical number [the number proposed by Heery above] to nearly 90-100%."
All of the above relates to late-stage cancer patients, of course.
NK cells & PV-10 (November 7, 2014)
At the 2014 annual meeting of the American Association of Cancer Research ("AACR") in April, Moffitt Cancer Center presented their poster Induction of anti-melanoma immunity after intralesional ablative therapy (abstract only available). According to a Provectus press release at the time:
The Moffitt researchers presented clinical data on 8 melanoma patients that demonstrated significant decreases in melanoma cells in injected tumors and uninjected bystander tumors 7-14 days after PV-10 injection as evidenced by pathologic evaluation confirmed with immunohistochemical staining of biopsy specimens for melA (a marker of melanoma). The researchers showed that these changes in tumors were accompanied by increased populations of CD3+, CD4+ and CD8+ T cells along with NKT cells in peripheral blood. T cells from one patient were purified and exhibited increased interferon-gamma expression when exposed to the patient's pre-treatment melanoma cells.PV-10 generates both local and systemic immune responses. The local response was highlighted in Provectus' European Cancer Congress 2013 poster Locoregional Disease Control in Metastatic Melanoma: Exploratory Analyses From Phase 2 Testing of Intralesional Rose Bengal as locoregional blistering ("Blistering may be indicative of a nascent local immunologic response").
Courtesy of a physician (internist), shareholder and blog reader, today:
There’s been some very interesting presentations thus far at SITC, in particular a very interesting presentation by Dr. Bill Murphy of University of California at Davis regarding the biology of NK cells (my leading candidate for PV-10’s local immune mechanism of action)...[B]asically Murphy suggests that “NK cells role in cancer is not ideal without some manipulation because they’re not designed to target cancer.” Further, “NK subsets evolved mainly for control of viral illnesses because viruses were greatest threat to humans” (per @ChrisHeery here and here).
I found this to be a fascinating thought, from an evolutionary point of view as well (since we probably didnt typically die of cancers hundreds and thousands of years ago), and wondered whether a reason PV-10 works so well is that it somehow is able to uniquely manipulate and thus harness NK cells to rapidly attack and destroy local tumor cells following intralesional injection. If so, this could be a wonderful explanation that not only fits with Moffitt’s preliminary observations regarding PV-10's mechanism of action, but help deconstruct and explain now NK cells are evolving to defend ourselves from not only viruses, but cancers as well. The conclusions of the article Melanoma progression is associated with NK cell exhaustion (Pires et al., SITC 2014) also fits these thoughts: "These data demonstrate that NK cells become progressively exhausted in the context of melanoma progression and that Tim-3 blockade possibly earlier in disease may have some benefit on innate immune function. Finally, our data suggest that soluble MICA is a potential prognostic marker which may contribute to the NK cell exhaustion through its interactions with NKG2D."
Further, the synergistic combination mentioned in Murphy's article* is a proxy, of sorts, for the combination of PV-10 on the front-end and checkpoint inhibitors on the back-end that we’re so excited about. In a sense, CD40 (as well as the front end use of sargramostim or T-VEC) acts to stimulate and mobilize critical dendritic cells just as PV-10 does while IL2, which we used to use frequently in melanoma patients with variable success, is analogous to the new checkpoint inhibitors currently being discussed and presented in every conference. In a sense this “older” combination acts as a proof of concept for combination therapies using newer agents. This just makes sense to me in the context of what we’'ve been talking about these past few months.* Synergistic anti-tumor responses against metastatic cancer using antibodies to CD40 and IL2: coordination of dendritic cell and T cell arms of the immune response
Notepad (November 6, 2014)
➢ The company filed its fiscal third quarter (which is the same as a calendar third quarter)10-Q today, issued a press release about its third quarter results, and filed an associated 8-K. Monthly cash burn increased by about 2% quarter-over-quarter to ~$1.2 million (the second quarter figure was ~$1.1 million). Quarter-end cash and cash equivalents were ~$17.8 million (~$18.1 million). 3Q14's cash burn of ~$3.5 million rate is about 40% higher than the late-spring guidance of a forward run-rate of $2.5 million per quarter (management said they were looking at a 12-month expense runway of less than $10 million on the June 3rd conference call). See also New SEC filing (August 8, 2014) below, and my blog post 2014 Annual CEO Letter (pt. 1). The above mentioned burn rate guidance would have included salaries, overhead, PV-10 and PH-10 mechanism of action study costs, liver study costs (expanded Phase 1), FDA regulatory affair consulting costs, etc. -- generally speaking, matter of course or normal operating costs.
➢ Today, Provectus also issued a press release and filed an associated 8-K (the same 8-K as above) regarding its submission of the company's protocol to the FDA for its upcoming, pivotal Phase 3 trial for locally advanced cutaneous melanoma. The PR also noted "Provectus believes details of the protocol will be available publicly on www.clinicaltrials.gov within the next few days," and "The FDA is expected to review the submission and comment on the proposed study population, clinical endpoints, and statistical analyses within 30 to 45 days." On Provectus' third quarter investor conference call, management reiterated guidance the trial would commence (i.e., begin enrolling patients, presumably) this year.
➢ The company's president and co-innovator of PV-10, Dr. Tim Scott, Ph.D., exercised about 97,000 options to buy underlying common stock ($0.64 strike price), and filed an associated Form 4 on October 31st.
◉ Returning to the 10-Q, Provectus raised money as part of the Network 1 Financial-led financing/private placement I first highlighted in early October -- see Fundraising (October 3, 2014) below -- in what appears to be two tranches: (i) a gross amount of $3.6 million in 3Q14, possibly towards the end of the month:
During the three months ended September 30, 2014, the Company received subscriptions, in the aggregate, for 3,586,300 shares of common stock and five year warrants to purchase 1,793,150 shares of common stock for an aggregate of $3,586,300. Investors will receive five year fully vested warrants to purchase up to 50% of the number of shares purchased by the investors in the offering. The warrants have an exercise price of $1.25 per share. The purchase price for each share of common stock together with the warrants is $1.00. The Company plans to use the proceeds for working capital and other general corporate purposes. Network 1 Financial Securities, Inc. is serving as placement agent for the offering. In connection with the offering, the Company paid $466,219 and issued five year fully vested warrants to purchase 358,630 shares of common stock with an exercise price of $1.25 to Network 1 Financial Securities, Inc., which represents 10% of the total number of shares of common stock subscribed for by investors solicited by Network 1 Financial Securities, Inc.And (ii) a gross amount of $3.7 million in 4Q14 (specifically, November 5th):
On November 5, 2014, in connection with the Company’s private offering of up to $15 million of common stock and warrants to accredited investors as described in Note 4(g), the Company accepted subscriptions, in the aggregate, for 3,702,600 shares of common stock and five year warrants to purchase 1,851,300 shares of common stock. Investors received five year fully vested warrants to purchase up to 50% of the number of shares purchased by the investors in the offering. The warrants have an exercise price of $1.25 per share. The purchase price for each share of common stock together with the warrants was $1.00...In connection with the offering, the Company paid $370,260 and issued five year fully vested warrants to purchase 370,260 shares of common stock with an exercise price of $1.25 to Network 1 Financial Securities, Inc., which represents 10% of the total number of shares of common stock sold to investors solicited by Network 1 Financial Securities, Inc.If I am correct with the above, the current cash balance should be about $20 million.
 |
| Click to enlarge. |
◉ In regard to the actual commencement of the melanoma Phase 3 trial, I imagine the first patients could be enrolled before year-end, but the bulk of the early enrollment may well occur at the beginning of 2015. Listening to Eric, it sounded like he was going to wait until the end of the 30-45 period for any general or significant comments from the FDA. I don't believe Eric is as concerned with how and when the trial begins as he is with how and when it ends (i.e., perfecting trial design, interim analysis, and the potential for the trial to be stopped early). I imagine most investors only heard, and thus focused on, management's guidance interim analysis results could be available by the end of 2015, earlier if enrollment is faster and thus better than expected. I think the potential exists for the trial's independent data monitoring committee to recommend early stoppage given the likelihood of the separation of the PV-10 and comparator progression-free survival curves. See How much is enough, and when? (September 16, 2014) below.
 |
| Click to enlarge. |
We also have begun to consider co-development transactions with one or more pharmaceutical or biotech companies to combine PV-10 with immunology agents such as those referred to as immune checkpoint inhibitors...Furthermore, the strategy of the Company for the benefit of stockholders is a series of partnerships followed by an acquisition of the Company along the lines of Celgene-Abraxis, although there can be no assurance that such partnerships or acquisition will occur. An interim transaction could be a co-development deal like Roche-NewLink, Bristol-Celldex or AstraZeneca-Incyte.Although I would prefer Peter to be "more institutional" and "less retail" with these kinds of communications, and corporate communications (among other things) in general, I nevertheless understand why he illustrates certain matters, such as co-development deal, in this way to Provectus' nearly exclusive retail shareholder base. I think his general point in utilizing examples like Roche-NewLink, Bristol-Celldex and AstraZeneca-Incyte is to convey to shareholders there are several potential ways in which Provectus could partner with another company for combining PV-10 with the partner's drug compound. A nuanced take, in terms of partnering options and opportunities, is:
- "We could sell the entire company" (NewLink sold-off/licensed-off their respective drug to Roche, but applied to Provectus that has only one oncology drug) -- very doubtful, since the economics of any co-development deal are very unlikely to approach the price threshold Provectus management has set for the company,
- "We might do an exclusive deal" (Celldex's exclusivity for Bristol-Myers' PD-1), or
- "We might do a non-exclusive deal" (Incyte's non-exclusive relationship for AstraZeneca's PD-L1)?
Dr. Derek Lowe, Ph.D (chemistry) authors the blog In the Pipeline, a consistently good read. In his October 31st post Nivolumab Racks Up Another Success he wrote:
The entire PD-1 therapeutic area looks to change the oncology landscape, because we're just barely getting into what's possible with different approaches and drug combinations. And that's just part of the immunotherapy efforts. Small molecules are going to be a part of this, but they're not going to be in the lead, unless any of you know of reliable small-molecule ways to modulate the appropriate immune pathways, which is no small challenge. {Bold and underlined emphasis is mine.}"PV-10 is a small molecule ablative agent that rapidly destroys tumors when injected into them (i.e., intralesional injection). It has the ability to selectively cross the cell membrane of cancer cells, and concentrates in structures within each cell called lysosomes (lysosomes contain enzymes involved in digestion of foreign materials within the cell and in destruction of the cell at the end of its lifecycle). PV-10 accumulation causes rupture of these lysosomes, leading to release of their contents and thereby producing rapid cell lysis (or "autolysis", self destruction)." {Bold and underlined emphasis is mine.}
PV-10's mechanism of action and immune response are underscored by the agent's physical chemistry.
Moffitt Cancer Center should present pre-clinical data at SITC 2014 that builds on "the previously reported effects of PV-10 ablation leading to a systemic, tumor-specific T-cell mediated anti-tumor response." We have to wait and see... In the meantime, Moffitt's Dr. Shari Pilon-Thomas, in PV-10 in Metastatic Melanoma: Rapid Responses Led Phase 3 (Walter Alexander, October 31st):
"We think that when you inject PV-10 into a tumor, it destroys the tumor, releasing tumor fragments that are then taken up by immune cells. The immune cells travel to the lymph nodes where they 'educate' or activate T-cells, which can in turn travel anywhere in the body."October Blog Stats (November 1, 2014)
Blog readership mostly rose from September 2014 depending on the statistic. I wrote 22 blog posts (5) and news items (17) in October versus 16 the previous month (7 and 9, respectively). October blog readership stats month-over-month changes were:
- +2% for # of unique visitors (2,085 v. 2,042),
- +14% for # of page views (18,106 v. 15,918),
- +6% for # of visits (8,173 v. 7,741),
- -4% for # of U.S. cities [from where visitors came] (649 v. 701),
- +17% for # of world cities (163 v. 139), and
- +6% for # of countries (51 v. 48).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
We think we know or act like we know a lot more about the immune system than we actually know, and what we do not know is much greater than what we actually know.
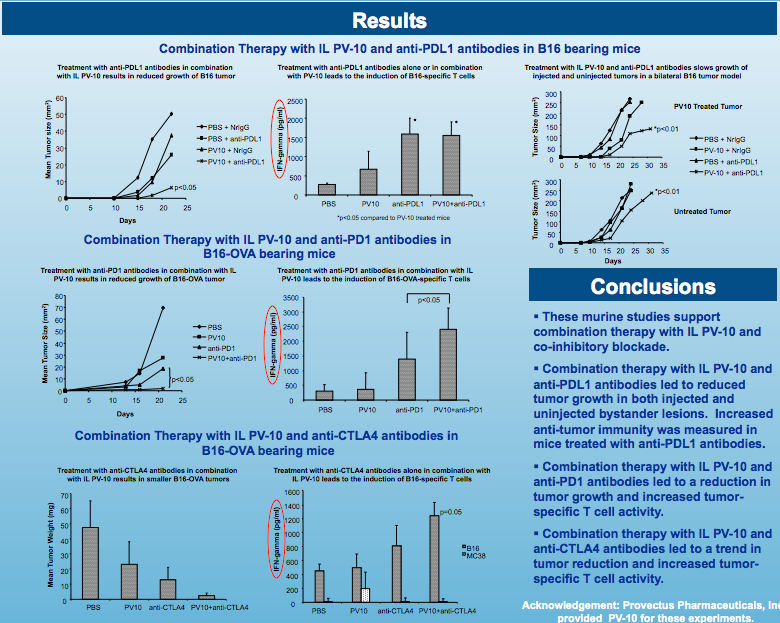
In their continuing work to figure out what PV-10 is, what it can do, and how it does what it does, Moffitt Cancer Center (Dr. Shari Pilon-Thomas, Ph.D. et al.) will present their poster Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma at the 29th annual meeting of the Society for Immunotherapy of Cancer ("SITC") on November 8th during Saturday's poster viewing session, and during SITC's Presidential Reception later that evening*. In looking forward to Moffitt's SITC presentation of some results of their research (preclinical/murine model) work, I am reminded of their poster presentation at the 2013 annual meeting of the American Association for Cancer Research ("AACR"), Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma, and the follow-up associated peer-reviewed PLOS One publication Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer of a few months later. Breast cancer? The April AACR 2013 poster and July PLOS 2013 paper covered what I think are translational research items (where translational research refers to, "...in the sense used here, is about translating progress in basic research into products and procedures that benefit patients"): efficacy of PV-10 in an MT-901 breast cancer model, efficacy of PV-10 in a B16 melanoma model, and the adoptive transfer of T cells. Moffitt concluded the paper by writing: "These studies have demonstrated that intralesional PV-10, in addition to reducing the growth of a directly injected tumor, leads to the induction of a robust anti-tumor T cell response and supports the use of PV-10 to induce systemic anti-tumor immunity for the treatment of metastatic melanoma and breast cancer."
Moffitt previously mentioned their translational research work on the combination of PV-10 and checkpoint protein inhibition at ASCO 2014: "Further studies are ongoing to determine the mechanism by which PV-10 increases tumor-specific T cell responses as well as to establish the interaction of intralesional PV-10 with combination checkpoint protein inhibition"). Checkpoint protein inhibition should refer to a checkpoint protein inhibitor like ipilimumab, an anti-CTLA-4 agent. Chen & Mellman (2013) note that "...immune checkpoint proteins, such as CTLA4,...can inhibit the development of an active immune response by acting primarily at the level of T cell development and proliferation (step 3)."
Moffitt's SITC paper addresses co-inhibitory blockade, which I think at least encompasses CTLA-4 as well as PD-1 and PD-L1 agents. Recall Chen & Mellman also note: "...immune rheostat (“immunostat”) factors, such as PD-L1, ... can have an inhibitory function that primarily acts to modulate active immune responses in the tumor bed (step 7)."
See Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma (October 28, 2014) below.
But I think co-inhibitory blockade also may speak to the balance between co-stimulation and co-inhibition (see blog post Co-stimulatory & Co-inhibitory), which then could further unveil PV-10's potential versatility, and thus it's potential synergy with multiple agents in multiple categories (e.g., checkpoint protein inhibitors, co-stimulatory agents ["co-stims"**], co-inhibitory agents ["co-ins"], etc.). Recall Moffitt's Dr. Weber's June 2014 reply to my question of what parameters one would use to assess the utility of an agent or compound as an immune system primer: "Simply its ability to synergize with the immune agent in question in terms of clinical effect when given prior to the second agent."
Which, finally, brings me back to Moffitt, breast cancer, PV-10's potential versatility, and it's potential synergy with another therapeutic agent. Interferon regulatory factors ("IRFs") express in breast cancer (e.g., a dated article is here, etc.). Is PV-10 synergistic with IRF-oriented/focused agents, and thus could it make such a combination better for the treatment of breast cancer?
* I imagine Moffitt's poster is an award winner: "Presidential Reception: Saturday, November 8 | 6:00 pm – 7:30 pm: As the culmination of the SITC 29th Annual Meeting, the Presidential Reception will honor all award winners and feature poster presentations"
** Novartis acquired a privately-held biotechnology start-up company called CoStim Pharmaceuticals earlier this year.
A gateway into China? (October 30, 2014)
 |
| Click to enlarge. H/t a blog reader & shareholder. Screenshot source. |
Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma (October 28, 2014)
Provectus issued a press release and filed an associated 8-K to announce its peer-reviewed publication of its metastatic melanoma Phase 2 trial data in the the Annals of Surgical Oncology (a direct link to the article may be found here). The article's abstract concluded:
"Intralesional PV-10 yielded durable local control with high rates of complete response. Toxicity was confined predominantly to the injection site. Cutaneous bystander tumor regression is consistent with an immunologic response secondary to ablation. This intralesional approach for local disease control could be complementary to current and investigational treatments for melanoma."The data itself has been presented at six ASCO and ESMO/ECCO conferences since 2009 (see my "The few injections needed in this study bode well for patient compliance with PV-10 treatment" blog post), starting with interim results (ASCO 2009 & 2010), followed by final results (ESMO 2012), and concluding with a sub-group analysis of patients who had all of their disease treated (i.e., all of their lesions injected with PV-10) [ECCO 2013, ASCO 2014 and ESMO 2014]. The paper ended with the two paragraphs below:
Three patients from this study experienced unexpectedly positive responses upon subsequent radiotherapy of previously injected, uninjected, and new lesions. This suggests that the combination of PV-10 with other treatments may have merit in advanced-stage disease with substantial tumor burden inaccessible to injection. In particular, the tumor-specific immune stimulation resulting from PV-10 ablation is potentially additive or synergistic with nonspecific immunotherapies.
In summary, intralesional PV-10 elicited robust and durable tumor regression in refractory cutaneous and subcutaneous melanoma with transient locoregional toxicity. The primary ablative effect of PV-10 reduced the size of injected tumors quickly, while regression of uninjected bystander lesions is consistent with a secondary immune response. These data suggest that PV-10 has utility in the management of melanoma patients with injectable cutaneous and subcutaneous disease. Future studies will comprehensively assess the effect of PV-10 on PFS to document potential longer-term benefits of locoregional disease control. {Underlined emphasis is mine}I found the following interesting:
- In particular, the tumor-specific immune stimulation resulting from PV-10 ablation is potentially additive or synergistic with nonspecific immunotherapies. I imagine non-specific immunotherapies refers to, among other therapeutics and categories of agents, the immune checkpoint blockaders, whether "...immune checkpoint proteins, such as CTLA4, [which] can inhibit the development of an active immune response by acting primarily at the level of T cell development and proliferation (step 3)....[or]...immune rheostat (“immunostat”) factors, such as PD-L1, [which] can have an inhibitory function that primarily acts to modulate active immune responses in the tumor bed (step 7)." PV-10's uniquely high specificity should be very complimentary to the blockaders' general non-specificity.
- The primary ablative effect of PV-10 reduced the size of injected tumors quickly, while regression of uninjected bystander lesions is consistent with a secondary immune response. This continues the discussion of the drug as an immuno-oncology asset (i.e., one that enables/harnesses/interacts with the immune system and generates/encourages a response), but the louder voice on this should be Moffitt at SITC 2014, and
- Future studies will comprehensively assess the effect of PV-10 on PFS to document potential longer-term benefits of locoregional disease control. I think the upcoming pivotal Phase 3 trial (and potentially other studies the company and/or its acquirer may run) should help elucidate how PV-10 can forestall or deny the expansion of melanoma (and, later and hopefully, other solid tumor cancers) to a metastatic and/or visceral spread. This conclusion should help establish the basis for earlier and much earlier use of the drug.
A column out today in The Australian newspaper (published by News Corp Australia) entitled Food dye threatens cancer biotechs’ business model notes:
There’s strengthening evidence rose bengal — a red food dye that has been around for more than a century — has a similar vaccine effect. Rose bengal sells for about $8 a gram and when diluted to a 10 per cent formulation equates to a few cents a dose. As a water-soluble xanthene dye, it has been used in liver function studies and is still used by ophthalmologists as a stain to detect eye damage.
“It’s something very simple and very cheap, and its shining the light on the immunology mechanism in the cancer patient,’’ says University of Melbourne research fellow Martin Ashdown.Two observations: First, while the expense of manufacturing a 5 mL vial of PV-10 is de minimis (very small, and very likely <$100 per vial using the new synthesis process) relative to its likely treatment cost of, say, $100,000 per course, the message should be the drug is extremely clinically cost effective. The graphs below assume a $30,000 treatment cost; however, Provectus' eventual acquirer should set PV-10's price. Nevertheless, Rose Bengal is a very cost effective active pharmaceutical ingredient ("API").
 |
| Click to enlarge. |
Icarus Consultants recently blogged a post entitled Immuno-Oncology drives Pharma Licensing Strategy.
 |
| Click to enlarge. |
In the case of PV-10, despite Moffitt Cancer Center's previous published works on the matter...
March 2012 (Society of Surgical Oncology) -- Intralesional Injection of Melanoma with Rose Bengal Induces Regression of Untreated Synchronous Melanoma In a Murine Model: PV-10 has shown promise in treating metastatic melanoma in previous clinical trials. PV-10 has led to objective responses of both injected and uninjected tumors, suggesting a systemic effect. These murine studies confirm that PV-10 chemoablation results in both a direct effect on injected melanoma lesions as well as a systemic response that leads to regression of synchronous lung metastases. Intralesional PV-10 treatment leads to the induction of tumor specific immunity.
April 2013 (American Association for Cancer Research) -- Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma: These murine studies confirm that PV-10 chemoablation results in both a direct effect on injected lesions as well as a systemic response that leads to regression of uninjected subcutaneous and lung lesions. Intralesional PV-10 treatment leads to the induction of tumor-specific immunity.
July 2013 (PLoS One) -- Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer: These studies have demonstrated that intralesional PV-10, in addition to reducing the growth of a directly injected tumor, leads to the induction of a robust anti-tumor T cell response and supports the use of PV-10 to induce systemic anti-tumor immunity for the treatment of metastatic melanoma and breast cancer.
April 2014 (American Association for Cancer Research) -- Induction of anti-melanoma immunity after intralesional ablative therapy: These findings suggest that PV-10 treatment leads to the release of DC activating factors and DC recruitment. Further studies to determine the role of PV-10 on T cell activation are ongoing. In sum, these clinical and preclinical results increase our understanding of the cytotoxic and immunological mechanisms that may play a role in systemic immunity induced by PV-10 tumor ablation.
June 2014 (American Society of Clinical Oncology) -- Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions: IL PV-10 treatment can lead to systemic anti-melanoma immunity and pCR in injected and uninjected lesions including treatment-refractory tumors. Further studies are ongoing to determine the mechanism by which PV-10 increases tumor-specific T cell responses as well as to establish the interaction of intralesional PV-10 with combination checkpoint protein inhibition....there remains a desire for much more information (i.e., "hard data") about PV-10's immunologic properties. It's not clear to me the broader pharma/biotech community recognizes, let alone appreciates, the drug's immunologic abilities and capabilities, and may not consider PV-10 an immuno-oncology asset
Provectus management seems very cognizant and confident of the opportunity the annual meeting of the Society for Immunotherapy of Cancer ("SITC") in early-November may afford them to establish the drug's immunologic bona fides, by virtue of Moffitt's work to elucidate PV-10's ability to prime and activate the immune system.
Provectus' October 15th press release: Dr. Craig Dees, Ph.D., CEO of Provectus, said, "Dr. Pilon-Thomas and her team at Moffitt have been doing very interesting work assessing the potential combinations of PV-10 with immune checkpoint inhibitors for melanoma. Their work may be an important step forward as part of our corporate strategy for addressing unmet need in late stage melanoma patients and in other uses of PV-10."Many will be attending SITC, such as Big Pharma (and Icarus Consultants).
| Click to enlarge. |
'“Very Impressive Data” for Rose Bengal 10% in Intralesional Melanoma' (October 21, 2014)
Source: P&T TV
ehealth radio network (October 19, 2014)
H/t a share holder and blog reader (thank you): Pink is the Color for Breast Cancer Cure with Peter Culpepper of Provectus Pharmaceuticals (10/18/14). Clink the link to listen to the interview.
New Institutional Ownership (October 17, 2014)
H/t a share holder and blog reader (thank you): Vanguard Group (as of 9/30/14)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
I imagine Provectus and St. Luke's Dr. Sanjiv Agarwala, M.D. will be present/participate in some fashion.
 |
| Click to enlarge. Saturday, November 15th. |
Provectus issued a press release and made an associated SEC 8-K filing today related to establishing a contractual relationship with INC Research, a contract research organization ("CRO") to conduct an FDA due diligence audit of Provectus' regulatory documents for PH-10 and PV-10. This kind of audit, and the larger relationship with INC, would, among other things, (i) assist institutional review boards ("IRBs") and principal investigators at clinical trial sites to follow the protocol of the upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma "to the letter," (ii) prepare for a new drug application filing, and (iii) facilitate the due diligence process of a license and/or or M&A transaction. In addition, employing an organization like INC places a supportive, accountable and expandable resource framework around Eric, who is responsible for Provectus' clinical development program.
Tidying Up (October 12, 2014)
The company made Form 4 SEC filings on October 6th related to management and its June 6th Stipulated Settlement Agreement and Mutual Release, which derived from a shareholder lawsuit over executive compensation: here (Dees), here (Scott) and here (Culpepper). The filings finalize these principals' forfeiture of half of the stock options granted to them in each of 2010 and 2011 (i.e., 450-475K per year).
Provectus issued a press release and made an associated SEC filing on October 8th regarding PV-10 clinical data being presented by St. Luke's Cancer Center's Dr. Sanjiv Agarwala, M.D. at the III Eurasian Melanoma and Skin Cancers Forum in Suzdal, Russia. (the presentation is titled "Intralesional Therapy: Focus on PV-10). From a 2013 article from India's The Economic Times: "[t]he Russian pharmaceutical market is estimated at around USD 20 billion and is growing at a CAGR of around 11 per cent. The retail/out-of-pocket market contributes around 66 per cent to the total market." Pharmstandard is the largest domestic pharmaceutical company in Russia.
Dr. Agarwala has been a principal investigator in a clinical trial of each intralesional agent regularly profiled by the University of Utah Health Care system' Huntsman Cancer Institute's Dr. Robert Andtbacka (at Dr. Agarwala's annual HemOnc Today Melanoma and Cutaneous Malignancies meeting).
 |
| Click to enlarge. Comparison of Phase 2 clinical trials. |
Drs. Andtbacka and Agarwala appear to be named investigators on Vical's Allovectin-7's Phase 3 trial. It is not clear to me whether Dr. Andtbacka was the/a lead investigator.
I believe Dr. Agarwala has presented all six Provectus ASCO and ESMO posters (see blog post "The few injections needed in this study bode well for patient compliance with PV-10 treatment"), and should be the global/lead investigator for the company's upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma. In addition to being a clinical trial site for Provectus' melanoma trial, St Luke's Hospital & Health Network is a compassionate use program site for PV-10, and an interventional clinical site for Provectus' liver trial.
ERT (October 9, 2014)
Provectus' vendor ERT issued a press release today regarding their work for/with the company on its upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma, and patient reported outcomes ("PROs").
“By applying scientific rigor to the implementation of established PRO measures, they are not only striving to meet regulatory guidelines, they are putting the patient at the heart of clinical research. We applaud Provectus for placing such importance on understanding the patient perspective in oncology drug development, and we are delighted to work with them on this program.”Eric first introduced ERT on Provectus' ESMO 2014 poster in late-September.
 |
| Click to enlarge. |
Prescription Drug User Fee Act Patient-Focused Drug Development; Request for Comments (October 8, 2014)
"The Food and Drug Administration (FDA) is announcing an opportunity for public comment related to FDA's patient-focused drug development initiative. This initiative is being conducted to fulfill FDA performance commitments made as part of the fifth authorization of the Prescription Drug User Fee Act (PDUFA V). This effort provides for a more systematic approach under PDUFA V for obtaining the patient perspective on disease severity and currently available treatments for a set of disease areas. FDA is publishing a preliminary list of nominated disease areas for consideration in patient-focused drug development meetings during fiscal years (FYs) 2016-2017. The public is invited to comment on this preliminary list through a public docket." Source link. {Underlined emphasis is mine}
 |
| Click to enlarge. |
Updated: The specificity of this FDA guidance for the nomination of unresectable loco-regional melanoma is a positive (Provectus' upcoming pivotal Phase 3 trial is for unresectable locally advanced cutaneous melanoma [i.e., loco-regional disease]). The timeframe of a couple of years from now for possible public meetings, however, seems to diminish immediacy. Additionally, the rationale behind these particular diseases appear somewhat obscure (e.g., pruritis, sarcopenia, etc.), begging the question of what was the criteria for inclusion. A number of them do not appear to have any specific clinical urgency.
2014 Beijing International Melanoma Congress (October 7, 2014)
Provectus issued a press release and made an associated SEC filing today regarding PV-10 clinical data being presented by St. Luke's Cancer Center's Dr. Sanjiv Agarwala, M.D. at the 2014 Beijing International Melanoma Congress (the presentation is titled "Current Perspectives on Intralesional Therapies;" see below).
 |
| Click to enlarge. |
 |
| Cover |
 |
| Chair and International Faculty |
 |
| Click to enlarge. |
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
(Medscape: How has this conference and the quality of Chinese cancer research evolved since 1997, when CSCO held its first annual meeting?) The Chinese have made huge investments, at both the government and private-sector levels, in support of biomedical research in China. The level of research and the quality of cancer care at many of the major centers are at the same level as that in the United States or Western Europe. The support of specialized cancer programs, especially at major cancer centers and academic centers, and in the infrastructure for conducting clinical trials and database management, has established China as a major player on the world stage in oncology research. As cancer care and research become organized more and more across China -- a country of 1 billion people -- Chinese researchers have the advantage of a gigantic population of cancer patients who are eligible for clinical research programs, and the potential data from these trials can influence the whole field of cancer research. High-quality clinical research and translational research in China has shown very significant advances in the last 10-15 years. Take CSCO, for example, which started as a meeting small enough to include all the attendees in 1 photograph, and this year the attendance will exceed 15,000.
(Medscape: With growing emphasis on the cooperation among societies and more numerous international trials, cancer research is being done on more of a collaborative basis than ever before.) For sure. We are experiencing a globalization of healthcare and biomedical research. With the accessibility of information on the Internet and the ability to travel more easily, we are beginning to share our knowledge at a global level and utilize information that benefits all of us worldwide. For example, the Chinese have one of the largest concentrations of patients with acral lentiginous melanoma, which we see infrequently in the United States. We can learn from their experience with this disease and apply data from their well-conducted trials about those patients that may be relevant to our practice in the United States and in other countries.
(Medscape: Is such research currently being pursued?) Absolutely. In presentations by the Chinese at this meeting, we heard sophisticated translational research and biomarker analyses on melanoma. China will no doubt be a major player in oncology research. With the large volume of patients in China and an increasing ability to conduct collaborative studies across institutions, they can conduct bigger trials among some populations more efficiently than clinical trialists are able to do in other parts of the world.
(Medscape: The ability to conduct trials in an expeditious manner may be attributed in part to lack of regulation, which can affect the reliability of their research. Can we trust their data?) Each government must assess whether the information collected in China's clinical trials conforms to their basic rules of reporting results of clinical trials involving drugs, devices, or diagnostics that are being considered for approval in each country. This includes policies involving conflicts of interest, informed consent, statistical methodologies, and quality of data collection. The regulatory bodies of each country will need to decide. The way things are going, drugs approved for use in the United States will increasingly have an origin of science in countries outside of the United States. In addition, physicians will have to make a decision as to whether the genetic background of their individual patients and the metabolism of these drugs in patients of different ethnicities are similar enough to the patients in the countries where these trials were conducted. For example, pegylated interferon for resected stage III melanoma was approved by the US Food and Drug Administration (FDA) largely on the basis of a trial that was conducted entirely in Europe. Thus, clinical trials conducted elsewhere in the world will increasingly be used in applications for FDA approval in the United States because of international collaborative studies conducted across nations. Of course, we have to assume that the causes of cancer and the genetic background for the metabolism of drugs in patients from different nations are the same as those of US patients, something that we don't presently know to be true. Nevertheless, this is going to be an increasing reality, because it is more difficult to do some types of cancer clinical trials in the United States. As another example, the Australians, who have the largest population with melanoma in the world, have organized their centers to conduct well-designed clinical trials in large numbers of melanoma patients. As a consequence, some aspects of our melanoma management in the United States rely on the research done in Australia.
(Medscape: That seems practical, if Australia has the largest population with melanoma.) Until somebody comes up with something that disproves the applicability of Australian results to Americans or Europeans, then we should accept them at face value but assess the methodology, the safety, and the ethics in the conduct of the clinical trials as part of our acceptance of their results from other countries.Fundraising -- Updated (October 4, 2014)
Peter discussed his views about what I wrote in Fundraising (October 3, 2014) below with me.
A Network 1 Financial-placed financing to meet an NYSE continued listing requirement: He said, paraphrasing, Provectus would have had (and will have) to make public any transaction the company does (e.g., financing, license deal, co-development, etc.) in accordance with SEC requirements. If Provectus decides to do anything in conjunction with the NYSE, he would file almost immediately, as he as done in the past with historical company financings.
My take: Peter appears to routinely check-in with the NYSE as a matter of course, but more of late it seems as the company's stock entered a share price range that might generate concerns about potential de-listing. And while there is a difference between the desired comfort level of the NYSE, and the fundamentals and fundamental opportunity of Provectus, it's clear Peter is focused on insuring the is and remains compliant with the NYSE.Network 1 vs. Cantor Fitzgerald: Peter said, paraphrasing, Provectus did not have to do anything with Network 1, and it could do something with Cantor whenever the company wanted. The company is good (cash-wise) until 2016, as noted in Provectus' corporate presentation on the website; however, NYSE requirements demand constant attention.
My take: It is not clear to me whether the NYSE has requested Peter to do something, or whether he is just being proactive and preparatory as a result of recent routine conversations by seeking investment commitments. I had heard investors approached by Network 1 were told they had two weeks to make a decision. I cannot confirm this to be wholly true (was there, for example a closing date on the subscription agreement?). If so, I'd be of the opinion, for the time being, that Network 1's outreach comprised only hard circling of interest to participate in the placement (i.e., commitments, not funding), and thus an option Peter may or may not exercise if and when he finds himself under an NYSE requirement to do so. This possible $5 million raise, or whatever it turns out to be, should be a non-event in the the context of what Provectus hopes to accomplish in the business of drug development and patient care in the field of oncology.
As for why Network 1 at all, Peter did not directly address the topic nor did I seek an answer at this time. It is what it is, but any frustration I have with his vendor choice should be taken in the context of my views of the path forward, and my focus on management's execution of their plan.Possible China and/or India transactions: Peter said, paraphrasing, such activity is independent of his routine interaction with the NYSE. He believes deals could happen this quarter, as he previously and publicly stated.
My take: I agree the key point is to insure NYSE compliance. If a fundraising ultimately is carried out and, as I wrote below, is limited to around $5 million, the rationale of meeting the requirements of the NYSE to continue listing of the PVCT ticker may be both veracious and appropriate. Insuring NYSE compliance is a matter for the moment in the moment. As such, consummating a regional transaction or two should be unrelated to NYSE compliance activities. If the raise ends up being much larger, however, I think it is legitimate to question the probability of a transaction or two in the near-term, and seek further comment about management's business development focus in order to agitate accordingly.I plan to speak further with Peter next week, and expect him to expand on the items above as well as on other topics.
Fundraising (October 3, 2014)
It sounds like Provectus is undertaking a Network 1 Financial-placed financing. From what I can gather, glean, ascertain, understand, speculate, etc.:
- Apparent rationale: Meet a New York Stock Exchange continued listing requirement
- Possible amount: $5 million
- Possible offering price per share: $1.00
- Possible warrants: 50%, or 1 warrant for every two shares purchased ($1.25 exercise price, 5-year term, cashless exercise)
- Why this amount of money? Why now? The NYSE MKT has both quantitative and qualitative standards and policies with respect to continued listing: "The Rules of the Exchange provides that the Board of Directors may, in its discretion, at any time, and without notice, suspend dealings in, or may remove any security from, listing or unlisted trading privileges. The Exchange, as a matter of policy, will consider the suspension of trading in, or removal from listing or unlisted trading of, any security when, in the opinion of the Exchange: (a) the financial condition and/or operating results of the issuer appear to be unsatisfactory..." Provectus ended June 30th with $18.1 million (see New SEC filing (August 8, 2014) below), having burned about $1.1 million per month in 2Q14. Assuming at least this burn rate, rather than Peter's projected ~$833K per month figure (discussed on the May/June conference calls), the company might have ended September 30th with about $14.8 million. Is this figure, or the overall setting, an unsatisfactory financial condition?
- Why Network 1, again? Is Cantor Fitzgerald not a less expensive alternative? I would imagine the fees charged Provectus by Network 1 for the placement are consistent with past raises: a 10% commission of the aggregate purchase price of the securities sold ($5 million => $500,000), 3% of the offering proceeds as non-accountable expenses ($5 million => $150,000), and cashless warrants equal to 10-15% of total units sold (with a $1.25 exercise price). In contrast, according to the Controlled Equity Offering Sales Agreement that Provectus entered into with Cantor Fitzgerald on April 30th, the Company would pay Cantor a commission of 3% of the gross sales price per share sold. Wouldn't a Cantor transaction be cheaper?
- Is Cantor not a more effective and supportive alternative for the stock itself? The clients put into these Provectus placements by Network 1 seem to be ordinary people (the money itself appears to be recycled, generally speaking, from one placement to another by selling the common stock purchased from placement N to purchase with subsequent proceeds the common stock of placement N+1 while retaining the warrants from both placements). Network 1 merely raises money for Provectus, and expensively at that. The firm has no equity research coverage function or capability, no Wall Street voice, and is about as far from a sponsor as you can get (Network 1's Damon Testaverde previously said to me it was a good thing Provectus' drug was a 10 because management was a 2). See Going it alone (October 2, 2014) below). Cantor is said to have a strong healthcare investment banking and equity research practice that includes numerous prominent life sciences specialist firms and funds as clients. Wouldn't Cantor have provided Provectus much needed institutional investors and sponsors, and ultimately supportive Street voices?
- Is a China and/or India regional license transaction in question? If Network 1's placement is limited to $5 million, the purported rationale of meeting an NYSE continued listing requirement is more likely to be veracious, and there might be less doubt of a deal. If the raise ends up being much larger, one could question the potential for near-term transactions.
A blog reader asked me on Twitter about the lack of Provectus' equity research coverage (Q: Why can't the company pickup analyst coverage?). As of this writing the company lists only Maxim Group on its Analyst Coverage webpage (a screenshot of the page is to the right). As of September 15th, Provectus is listed in Maxim's coverage universe under analyst Dr. Echo He, Ph.D., M.D.*
Equity research coverage of so-called microcap companies ($50-$300 million market capitalization) is a quid pro quo business with the investment banking firms that typically undertake research (not that coverage for higher capitalization companies isn't the same or similar, but perhaps the relationship between investment banks and issuers is less cozy than in the 1990s and 2000s). The investment bank might help raise money for the company through a placement, for example, and at some point would initiate or continue research coverage of the latter.
In Provectus' situation, the company channels nearly all of their fund raising through "investment bank" Network 1 Financial, which does not provide research coverage. This near exclusivity with Network 1 translates into no real opportunity for another firm, especially those that actually undertake research, to get involved. The lack of incentive or promise of work that ultimately funds the research side of the business would mean no coverage for a company like Provectus. Maxim did get some fund raising business in the past (Provectus also used the firm to lead its failed PVCTP "IPO" in 2H2012), and that presumably accounted for He's coverage, which was last updated for corporate clients in February of this year. Additionally, because Network 1 merely raises money for Provectus and has no equity research voice (in reality no substantive voice at all), there is no one in the financial services or investment communities who could or would pound the proverbial table in support of the management team and stock, as is the case with other public companies.
Beyond the above, I also think there is no incentive (or meaningful risk-reward) for real investment banks with research groups, whether boutique, small or large, to initiate coverage on Provectus because the company's principals are likely viewed as outliers in how they have approached (broadly speaking) the path to monetization. It would not be worth the risk to an equity research analyst to cover the company until there is enough regulatory, commercial and stock market validation to do so, by which time it's well beyond a given to say so.
* This Maxim's webpage's URL appears [to me] to indicate a possible date of April 2014. According to a June 19th The Life Sciences Report article, Dr. He is referred to as formerly being with Maxim, although her LinkedIn profile notes she still is with the firm.
September Blog Stats (October 1, 2014)
Blog readership mostly rose from August 2014 depending on the statistic. I wrote 16 blog posts (7) and news items (9) in September versus 20 the previous month (4 and 16, respectively). September blog readership stats month-over-month changes were:
- +4% for # of unique visitors (2,042 v. 1,968),
- +11% for # of page views (15,918 v. 14,362),
- +9% for # of visits (7,741 v. 7,107),
- +5% for # of U.S. cities [from where visitors came] (701 v. 670),
- -5% for # of world cities (139 v. 146), and
- -6% for # of countries (48 v. 51).
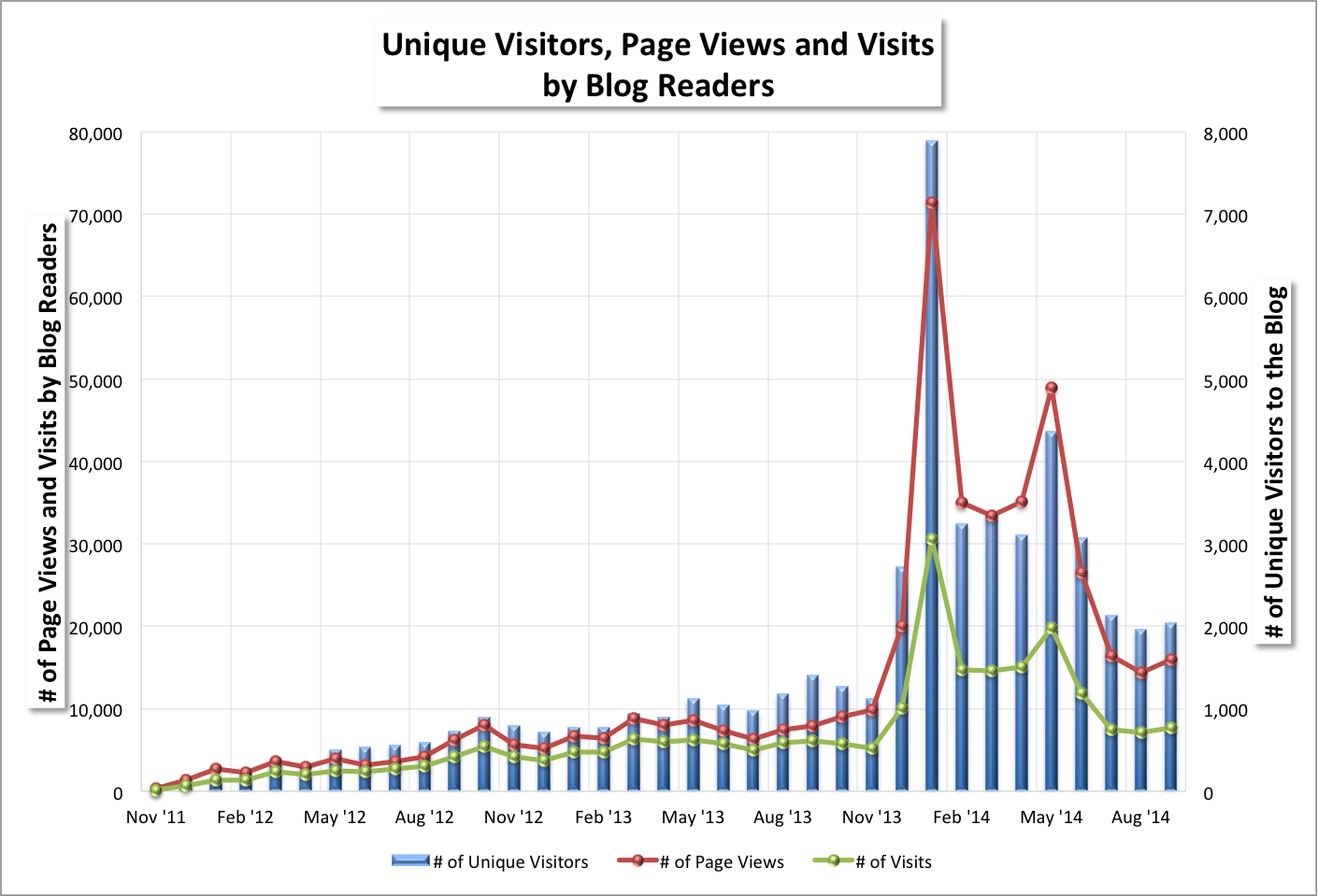 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. From the blog's Google Analytics page (daily measurement: September 2014). |
 |
| Click to enlarge. From the blog's Google Analytics page (daily measurement: Since inception). |
Quarterly statistics are updated as of September 30, 2014, and may be found on the blog's Blog Readership Statistics page.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. From the blog's Google Analytics page (daily measurement: Q3 2014). |
Provectus issued a press release today to announce a team of Moffitt Cancer Center researchers led by Dr. Shari Pilon-Thomas, Ph.D. would present data at the annual meeting of the Society for Immunotherapy of Cancer 29th: "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma." The associated 8-K filing is here. Craig's quote in the PR was:
"Medical oncologists are interested in potential combinations of PV-10 with immune checkpoint inhibitors for patients with significant tumor burden inaccessible to intralesional injection. Dr. Pilon-Thomas's work is an important step forward as part of our corporate strategy for addressing unmet need in late stage melanoma patients." {Underlined emphasis is mine}Co-inhibitory blockade, which, as I wrote about in my blog post Co-stimulatory & Co-inhibitory, should represent different inhibitor factors, such as PD-1, PD-L1, IDO, LAG-3, etc. (see Figure 2 of Chen et al., or the right hand side of the illustration below), and thus different therapies, such as anti-PD-1, anti-PD-L1, and IDO inhibitors.
 |
| Click to enlarge. |
- Anti-CTLA-4 agent ipilimumab [Bristol-Myers] (maybe tremelimumab [AstraZeneca/Medimmune]),
- Anti-PD-1s pembrolizumab (Keytruda) [Merck] and nivolumab [Bristol-Myers],
- Anti-PD-L1s MPDL3280A [Roche/Genentech] and MEDI‐4736 [AstraZeneca/Medimmune], and
- Perhaps others?
 |
| Click to enlarge. |
Another key value driver of monetization is a regional license transaction or two. When I spoke with Provectus Strategic Advisory Board Member Dr. Joseph Chalil, M.D. of Boehringer Ingelheim over the summer, he said (paraphrasing) the advice he'd give to any emerging company like Provectus would be to develop, implement and execute an emerging markets (and thus non-U.S.-centric) business ("go-to-market") strategy parallel to and in combination with U.S. centric and developed world one. He of course has been assisting Provectus in their endeavors to strike a deal in China and/or India. In such context, it is interesting to note Craig's quote in the PR announcing the addition of Chris Kaplan, the new SAB member. See New Strategic Advisory Board Member (September 22, 2014).
"Chris has a formidable resume and proven track record of success in pharmaceutical sales and marketing. His experience in international business is clearly going to be an asset to Provectus, and I expect his dedication to healthcare innovation and delivery to be equally valuable."Where drug spending is limited by GDP per capita, emerging markets -- less than a third of the addressable market but 85% of the population -- need cost effective solutions.
 |
| Click to enlarge. |
New Strategic Advisory Board Member (September 22, 2014)
Provectus added a new strategic advisory board member ("SAB"), Chris Kaplan, President of Cajetan (presumably his personal management consulting vehicle) and formerly of Boehringer Ingelheim (Senior Vice President and Chief Marketing Officer), Novartis and Bristol-Myers. The associated 8-K filing is here.
Of the SAB members the company has added from Big Pharma...
- Dr. Craig Eagle, M.D. (Pfizer, 2011),
- Bob Miglani (Pfizer, 2013),
- Dr. Joseph Chalil, M.D. (Boehringer Ingelheim, 2014),
- Brendan O'Brien (Sanofi, 2014),
- Jacob Plotsker (Bayer, 2014), and
- Kaplan (formerly Boehringer Ingelheim, 2014)
Moffitt @ SITC (September 19, 2014)
The title of Moffitt's presentation at the 2014 annual meeting of the Society for Immunotherapy of Cancer is below.
 |
| Click to enlarge. |
Sinopharm (September 18, 2014)
KV Aromatics signs $10 mn contract with China's Sinopharm (September 17) [India's The Economic Times newspaper]
KV Aromatics Pvt Ltd today said it has signed an annual agreement of $10 million (about Rs 61 crore) with China's Sinopharm. The agreement is part of the visiting Chinese delegation to the country. Chinese President Xi Jinping is on a 3-day visit to India. This is the second year running that KV Aromatics Pvt Ltd, which supplies natural essential oils, mint products, aroma fine chemicals and herbal products, has bagged the contract, the company said in a statement. KV Aromatics Director Aman Agarwal said: "This year also we have been awarded with a single business contract worth $10 million by our esteemed client, from Beijing, China." Elaborating on the company it has signed agreement, Agarwal said Sinopharm is a Fortune 500 company owned by Central Government of China with revenues of $33.27 billion. The signing ceremony was attended by senior government officials and industrialists of India and China, the company added. {Underlined emphasis is mine}Sinopharm: Operating Leverage, MS Raises TP (September 16) [Barron's]
It is not hard to see China’s pharmaceuticals industry will be a booming business, with the Chinese demographics aging and living longer. Last week, the pharmaceuticals were the top performers even though the aggregate stock market fell. Morgan Stanley raised its price target for China’s largest drug distributor Sinopharm (1099.HK) from 28 Hong Kong dollars to HK$34 today, on better margins. The new price target implies 21 times Morgan Stanley’s 2014 estimates. What does Morgan Stanley like about Sinopharm? First, Sinopharm is the largest drug distributor in China, which allows it to explore economies of scale and operating leverage as it gets even bigger. Second – and importantly – it is the only drug distributor owned by the central government, which means any conducive policies will flow through Sinopharm first. {Underlined emphasis is mine}Should Provectus consummate a regional license transaction with Sinopharm for "[the People's Republic of] China territory," it will be interesting to see and evaluate the deal terms. There are few recent precedent transactions for which financial details are available. For example, in September 2014 Eddingpharm signed a license deal with Ablynx for the latter's investigational rheumatoid arthritis drug.
Ablynx will receive an upfront of 2 million euros ($2.6 million), payable in two tranches, and is entitled to receive development and commercial milestone payments plus tiered, double-digit royalties of up to 20%, based on annual net sales of ozoralizumab generated by Eddingpharm in Greater China.In January 2013 Celsion entered into an agreement with Zhejiang Hisun Pharmaceutical Company for the former's liver cancer therapy (only $5 million was paid before Celsion's pivotal Phase 3 trial hepatocellular carcinoma failed, and the deal was terminated):
A credit of $10 million from the two payments ($5 million for the technology development agreement and $5 million for the exclusive option) toward a non-refundable upfront license payment of $25 million due to Celsion at signing of the definitive license agreement. $55 million in upfront milestone and regulatory milestone payments within the next 18 months. $45 million in milestone payments for reaching certain sales targets. Escalating double-digit royalties on net sales of ThermoDox in the Greater China Territory.Sinopharm officials reportedly will visit the U.S. later this month to meet with representatives from Provectus, MD Anderson Cancer Center, Moffitt Cancer Center, and St. Luke's University Hospital Network, as well as other individuals and organizations.
How much is enough, and when? (September 16, 2014)
In Provectus' upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma, the primary endpoint will be progression-free survival ("PFS") using RECIST 1.1, with secondary endpoints of complete response rate ("CRR") using RECIST 1.1, overall survival ("OS"), and patient-reported outcomes. The comparator is systemic chemotherapy: dacarbazine ("DTIC") or temozolomide ("TMZ"). These trial parameters were first presented at the American Society of Clinical Oncology annual meeting on the company's poster Efficacy of intralesional rose bengal in patients receiving injection in all existing melanoma in phase II study PV-10-MM-02.
PFS for DTIC/TMZ for Stage III/IV melanoma patients appears to be well documented in clinical trial literature, demonstrating a sharp drop-off around the 1.5 to 2.5-month mark (i.e., 1.5 to 2.5 months after treatment began). Examples of this include:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Table from Provectus' ECCO 2013 poster presentation. |
Provectus reported a mean PFS of more than 9.7 months for the sub-group of Stage III patients in the Phase 2 trial (under modified RECIST); this sub-group encompassed the so-called sub-sub-group of patients with all disease treated. See the company's 2012 European Society for Medical Oncology poster Immuno-chemoablation of metastatic melanoma with intralesional rose bengal. Patients had their lesions (disease) initially injected at t = 0 and then again, if needed, at 8, 12 and 16 weeks. When Provectus simulated Phase 2 data for Stage III patients under RECIST 1.1 by deeming any increase in tumor burden from nadir of more than 20% to be PD (i.e., if a patient had CR at week 8 followed by any local recurrence at week 12 or 16 they would be deemed to have progressed), median PFS for Stage 3 patients was 3.7 months. One way to look at this median PFS for this sub-group is that PV-10 injection lasted approximately for the duration of the treatment interval -- a PFS figure of 3.7 months (95% confidence interval ["CI"] of 2.9 to 4.5 months) vs. a treatment interval of 3.7 months (16 weeks ⅹ [12 months ÷ 52 weeks]). If the treatment interval was longer, it would seem reasonable to postulate median PFS would be longer as well, until at some point maximum PFS was obtained based on the timeframe of observance.
In the upcoming Phase 3 trial Provectus will measure PFS as the trial's primary endpoint, utilize RECIST 1.1 to measure it, and inject patients every two weeks until CR or PD is achieved (i.e., the duration of the treatment interval will be until one of the two outcomes is achieved). It then could be reasonable to project a PV-10 arm PFS of 12 months for a 12-month observance period, 9 months for a 9-month period, 6 months for a 6-month period or 3 months for 3-month period -- where, at best, the PFS and its confidence interval are the same line, or, at worst, the CI is a narrow band around the PV-10 PFS figure, which itself would be a horizontal line at 1.0 or 100%. Thus, patients with all of their disease treated by PV-10 would never progress, while patients in the control arm (those treated with DTIC or TMZ) would suffer progression as historically demonstrated through prior use and over many previous clinical trials.
 |
| Click to enlarge. |
Why Keytruda's approval is a good thing for PV-10 (September 8, 2014)
The FDA approved Merck's [anti-]PD-1 agent pembrolizumab (trade name Keytruda) for late-stage or metastatic melanoma. Keytruda's approval and Bristol-Myers' likely eventual approval [in the U.S.] for PD-1 agent nivolumab are good for PV-10, according to Provectus.
- KOLs see PD-1s as treating the bulk of late stage cancer.
Key opinion leaders ("KOLs") see PD-1s, which fall under the category of checkpoint protein inhibitors that also include CTLA-4 and PD-L-1 agents, as an improvement over CTLA-4 agents like approved ipilimumab (trade name Yervoy) and tremelimumab. KOLs will use PD-1s to treat as many indications as they can scientifically support. Abstracts of Merck oncology-sponsored pembrolizumab studies being presented at ESMO 2014 in late-September, for example, include bladder and gastric cancer, advanced melanoma, non-small cell lung cancer ("NSCLC"), and head and neck cancer.
- Generally speaking, PD-1s are preferable to CTLA-4s for late-stage cancer because the former are less toxic, more efficacious and have shown greater multi-indication viability than the latter.
- Due to cost, combining PV-10 with an approved drug is preferable to combining it with an investigational one.
It is preferable to combine with an approved drug like Keytruda, rather than unapproved [in the U.S. for melanoma] nivolumab, because approved compounds in a combination study would be reimbursed (the investigational one would not), just like it would have been preferable to combine with Yervoy before Ketruda was approved. Thus, a contemplated combination study of PV-10 and Keytruda would see the latter reimbursed by insurance companies.
The question begs, since Yervoy was approved in 2011, why Provectus did not combine PV-10 with it sooner. The answer appears to be because the scientific rationale or support to do so (notwithstanding the company's AACR 2013 work) was not available until literally this year; that is, rationale and support for both PD-1s and CTLA-4s. KOLs knew the rationale existed but without relevant data, these combinations were not viewed as appropriate by the medical community. Furthermore KOLs have known, before and since Yervoy's approval, that PD-1s were coming and expected to be approved, and much prefered Provectus combine PV-10 with them versus CTLA-4s due to inherent concerns about the unexpected consequences of CTLA-4 treatment (i.e., primarily toxicity concerns). It also has been viewed as much more valid to combine the newer version of checkpoint protein inhibitors (PD-1s) versus the earlier version (CTLA-4s).
- There is room to improve Keytruda's clinical relevance for late-stage cancer.
Although Keytruda and nivolumab are notable improvements over Yervoy, KOLs believe there is room to improve their clinical relevance through combination with other approved and investigational drugs. Results of the combination study of Yervoy and Amgen's talimogene laherparepvec ("T-Vec") in late-stage melanoma presented at ASCO 2014, for example, showed both greater lesion destruction and increased immunological signaling than the individual therapies achieved by themselves.
PV-10, which has shown greater tumor destruction and immunological signaling than T-Vec, should be the "...perfect front end for immunologic back ends like Keytruda (pembrolizumab) for combination treatment" when ultimately combined with the PD-1 in a clinical setting.
- Moffitt Cancer Center has combined PV-10 with PD-1s in murine model work.
Given the center's comments thus far about PV-10 (see blog post Moffitt), and (ii) its clinical experience with PD-1s and PV-10 in context (see, for example, Moffitt's PV-10-related ASCO 2014 abstract/poster presentation, and the center's recent Keytruda press release), it is not to hard to conjecture the combination of PV-10 and Keytruda should much more clinically relevant to late-stage and viscerally-spread melanoma than Keytruda alone.Changing treatment landscapes recognizing more solutions (most especially combinations) are needed, the benefit of reimbursed drugs versus non-reimbursed one, and market recognition of utilizing a co-development partner versus a "go-it-alone" approach without adequate scientific and medical justification are all important considerations.
PV-10 is tantamount to surgery? (September 5, 2014)
I've previously written on the blog in various posts and news items at various times that PV-10 is akin to surgery, or perhaps better put is "surgery plus," because of the investigational drug's key characteristics (i.e., clinical value proposition):
- Potentially as or more effective, as manifested in PV-10's ablative ability to generate a complete response sufficiently complete that not only is the tumor visibly completely destroyed but pathological complete response can be achieved (Moffitt, ASCO 2014), and occult tumor cells, "disease that is not detected by standard pathological techniques," are destroyed (Moffitt, Post-Chicago Meeting on Skin Cancer 2014) too,
- Potentially as or more safe, as manifested in the drug's ability to be given very frequently (passing through and excreted from the body,
- Potentially as or more easily administered, as manifested in PV-10's ability to be delivered via injection in an outpatient setting by a suitably trained physician or nurse practitioner, and
- Potentially more compassionate because healthy tissue is spared, as manifested in the drug's ability to prevent the creation or resultant craters in the skin as destroyed diseased tissue is appropriately removed or excreted from the body.
Merck's Keytruda, its anti-PD-1 one agent pembrolizumab, was approved this week for late-stage or metastatic melanoma after other therapies have failed. The Big Pharma messaged the drug's positioning in melanoma diagnosis and the treatment process:
 |
| Click to enlarge. Keytruda Cancer Drug Approval Is A Triumph For Merck: R&D Chief Roger Perlmutter On What's Next |
Keytruda is another tool for the medical oncologist's kit to treat late stage or metastatic melanoma. Since 2011 four other drugs have been approved for this disease stage: ipilimumab and vemurafenib in 2011, and dabrafenib and trametinib in 2013. The silent masses who make up the bulk of patients with melanoma (and the much larger addressable market), however, remain without effective options:
 |
| Click to enlarge. SEER Stat Fact Sheets: Melanoma of the Skin, National Cancer Institute |
Interestingly, and I suppose not the least bit ironically, there does not appear to be medical literature that tabulates and documents that if you stop melanoma at Stage III (when, generally speaking, if you effectively and comprehensively treat the patient you can save him or her) it won't progress to Stage IV. You may recall on the blog's PVCT page I wrote about effective local-regional disease "defeat" or control to deny, prevent or forestall the metastatic spread of cancer:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Potential innovational cancer drug treatment progress like PV-10 still seems to boil down to having to show that effective local treatment (where the treatment does not have to be systemic) can be a good thing. Provectus plans to undertake a project of gathering data across relevant treatments that show when disease (melanoma) is ablated in Stage III it is forestalled from getting to Stage IV, with the expectation of publishing this analysis in a peer-reviewed publication. Advocating more broadly (than for PV-10 itself) that appropriate local treatment is relevant and effective for longer-term patient benefit would seem to be a thoughtful approach to take in parallel with Provectus PV-10-specific clinical development program.
Blog readership fell up to about 13% from July 2014 depending on the statistic. I wrote 20 blog posts (4) and news items (16) in August versus 14 the previous month (4 and 10, respectively). August blog readerships stats month-over-month changes were:
- -8% for # of unique visitors (1,968 v. 2,130),
- -13% for # of page views (14,362 v. 16,429),
- -5% for # of visits (7,107 v. 7,451),
- -12% for # of U.S. cities [from where visitors came] (670 v. 761),
- -4% for # of world cities (146 v. 152), and
- -0% for # of countries (51 v. 51).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Colorectal cancer is "...the second leading cause of cancer-related deaths in the United States, and more than half of patients with this disease will eventually die of tumor metastasis. It is estimated that in the U.S. there are ~75,000 new cases for men and ~70,000 new cases for women annually (Siegel et al., 2012)." Interestingly, lots of our immune system resides in our guts. "The crucial position of the gastrointestinal system is testified by the huge amount of immune cells that reside within it. Indeed, gut-associated lymphoid tissue (GALT) is the prominent part of mucosal-associated lymphoid tissue (MALT) and represents almost 70% of the entire immune system; moreover, about 80% of plasma cells [mainly immunoglobulin A (IgA)-bearing cells] reside in GALT."
This type of cancer has so far proved resistant to immunotherapy.* According to one researcher, colorectal cancer tumors may not send out suppressors like melanoma tumors, where anti-CTLA-4 and PD-1 agents have had the majority of their success.
According to Provectus materials (as of this writing, slides numbered 20 and 24 of the company website presentation), the company has successfully addressed colorectal cancer in pre-clinical and clinical settings with PV-10. It may be the drug harnesses immunological signaling networks involved in colorectal cancer growth, and potentially better optimizes interactions between tumor cells and their surrounding stroma. To this end, after melanoma, primary liver cancer and possibly/potentially breast cancer, the company could further clinical development of PV-10 and colorectal cancer with a Midwest cancer center through a contractual relationship Provectus appears to have entered into with the center in June.
* The author notes ipilimumab and nivolumab, which are being tested in A Study of Nivolumab and Nivolumab Plus Ipilimumab in Recurrent and Metastatic Colon Cancer (CheckMate 142).
Seeking Alpha, and this blog (August 26, 2014)
A Seeking Alpha ("SA") editor reached out to me via e-mail last Thursday:
SA Editor: "I was reading your blog on Provectus and we'd like to publish your latest two blog posts (here and here) as articles on your Seeking Alpha account. Would you be ok with this? As you're probably aware, publishing these as articles on Seeking Alpha would be a great way to get exposure for your writing. I'm happy to get them posted for you if you'd like. Please let me know."He was referring to my August 10th Juxtaposition and August 17th Immune Surveillance posts. I followed-up:
Me: "I'd certainly be okay with it; however, would you please include in the disclosure piece that presumably would accompany each article that Seeking Alpha asked me to publish the posts as articles? As you know I stopped publishing on SA in October 2013."As context to the transparency/disclosure request of SA above, I occasionally placed copies of selected blog posts on SA as Instablog posts (noted under the blog's Disclosure tab). I stopped doing this in October 2013 because posting selectively or infrequently at SA provided a less than complete picture of my writings on this topic to their readers, and I did not (nor do I currently) have the time or inclination to post both there and here. When I first thought about contributing to SA a couple of years ago (as a way to test my investment thesis), I submitted a second version of my Why I’m Long Provectus investment letter to SA in June 2012 for publication. SA turned me down because, according to them, Provectus stock traded over-the-counter at the time. SA accepted the third version in September 2013.
Continuing, the editor replied:
SA Editor: "We normally wouldn't add a disclosure noting our interaction with you via e-mail. The only disclosure we would need is whether you are long or short the stock and whether you have a business relationship with the company (Provectus) or are being paid by any third party to write your articles. Can you please let me know if you are long/short and if you have any business relationship or are being paid by a third party to write so I an include the proper disclosure on the articles?"I had not contemplated returning to SA but thought that if I did, it would make sense for the site to disclose their interest in my writing so as to explain to readers why I returned (and under what circumstances). Continuing:
Me: "I understand SA's perfunctory disclosure (I filled it out when I published my first article on your all's site in September 2013, Why I'm Long Provectus Pharmaceuticals). If I can't get the added disclosure noting our interaction via e-mail, then I'm afraid I'll have to pass on agreeing to publish my latest posts as articles."
SA Editor: "Can you elaborate on why you want us to tell readers that we reached out to you about your blog posts? We regularly reach out to thousands of bloggers about their content."
Me: "It just is/would be a matter of transparency. I noted in my final Instablog post I no longer would post on SA. I would imagine readers should know why I returned to write more articles, since I had no previous intention of being an SA contributor until you reached out to me."The editor's reply of "Sorry, but I'm unfamiliar with why you decided to not write on SA..." did not further this discussion. As a result I thanked him, moved on: "No worries [Name]. Thanks again for reaching out[,]" and won't be publishing on SA; this blog is a sufficient platform.
Cancer in China: Breast, Lung, Liver (August 25, 2014)
Provectus garnered some Asia news coverage of their memorandum of understanding ("MOU") with Sinopharm from PharmAsia: Sinopharm Starts Talks With Provectus Over Anticancer.
According to Wang et al. (2012), based on estimated incidence data from the GLOBOCAN 2008 database, "[i]n China, lung cancer was the predominant type of cancer detected in males; in females, breast cancer was the main type of cancer." According to Zeng et al. (2014), "Breast cancer is the most common cancer among women in China for several years... The age-specific incidence rate of female breast cancer was relatively low before 25 years old, but dramatically increased after then. The incidence rate reached peak at the age group of 55-59 years, and then gradually decreased." Liver cancer ranked fourth for women and second for men (based on age-standardized incidence rates, or ASRw, world population standard). According to a February 2014 report by the World Health Organization, "[d]espite high rates of smoking, China's rate of lung cancer is actually lower than that of most European nations. Death rates in China from stomach and liver cancer, however, are the highest in the world."
The Sinopharm-Provdectus MOU said "parties recognized the achievements gained during PV-10's clinical trial on melanoma, breast cancer and cancer of liver, plus the systematic effect on human immune efficacy, and PV-10 could be applied to other indications such as lung cancer, pancreatic cancer, prostate cancer and kidney cancer." I imagine this sentence references or makes reference to clinical and pre-clinical data in Provectus' electronic data room Sinopharm would or should have reviewed as part of their initial due diligence prior to signing the MOU. Provectus historically has referenced multi-indication clinical and pre-clinical data in their corporate website presentation, an August 8th version of which is below (orange rectangles were added by me). I believe the company has pre-clinical kidney cancer data, and [I think] the website presentation has not been updated to reflect it.
 |
| Click to enlarge. |
The therapeutic implications of immunogenic cell death ("ICD") in/to cancer treatment has grown clearer in recent year. See, for example, Therapeutic implications of immunogenic cell death in human cancer, Palombo et al. (2014).
ICD is characterized by the exposure and/or release (secretion) of damage-associated molecular patterns ("DAMPs"). Important DAMPs include calreticulin ("CRT"), heat-shock proteins ("HSPs") -- namely HSP70 and HSP90, amphoterin ("HMGB1"), and ATP.
At AACR 2014 Moffitt Cancer Center noted about their murine model work with PV-10 that "...treatment of murine B16 cells with PV-10 leads to release of HMGB1, a soluble Damage Associated Molecule Pattern (DAMP) that is important for activation of dendritic cells (DCs)." {Underlined emphasis is mine}
In cell line work (cell lines ▸ murine models ▸ human studies) published this week on PLoS One entitled Rose Bengal Acetate PhotoDynamic Therapy (RBAc-PDT) Induces Exposure and Release of Damage-Associated Molecular Patterns (DAMPs) in Human HeLa Cells, Panzarini et al. (2014) noted that ATP, HSP70, HSP90, HMGB1 and CRT DAMPS were exposed and/or released after treatment of these cell lines with RBAc-PDT. {Underlined emphasis is mine}
By better understanding how and why ICD occurs, researchers and biopharmaceutical companies can presumably develop or refine more targeted and effective anticancer therapies. Kroemer et al. (2013) in their paper Immunogenic Cell Death in Cancer Therapy, for example, postulate ICD "constitutes a prominent pathway for the activation of the immune system against cancer, which in turn determines the long-term success of anticancer therapies." Rose Bengal seems to induce the exposure and/or release of all key types of DAMPs, the molecules that elicit ICD, which may be why Moffitt's Dr. Jeffrey Weber, M.D., Ph.D. said "PV-10 might offer the perfect way to prime the immune system."
Moving Time? (August 20, 2014)
Following up on my news item Provectus & China (August 18, 2014) below, and my initial thoughts and questions about the company's MOU with Sinopharm, it is interesting the Chinese pharmaceutical group moved now (by virtue of their agreeing to/signing a memorandum of understanding with Provectus). I wrote in June 2013's For $PVCT, it's the FDA's move blog post about Peter's Provectus' game theory "rules:" No one moves unless they have to move. Everyone moves when somebody moves.
"No one, not Big Pharma, not regional players, not life sciences investors and, not least of all, not Provectus management has to move until the FDA moves. Then, I think, expect and suspect, everyone moves."One could argue the FDA moved by compelling the company to undertake a pivotal Phase 3 trial for locally advanced unresectable cutaneous melanoma, and Eric agreed to move now because he believes he has the right patient population, endpoints and drug supply, and can adequately predict the outcome of the trial. The same could be said for a pivotal liver Phase 2/3 trial, where the company may well have cemented a framework ultimately agreeable to the FDA and Eric on an initial pathway to approval for this indication.
As of this writing the blog's most popular post is December 2011's Words Have Meaning, where I wrote:
"I believe words are nothing more than vehicles for meaning. By writing this, I am referring to the company's press releases. In developing a relationship with the management team, I believe this is very much the case at Provectus: The words they write tell us things, meaningful things."Take for example Provectus' January 2014 press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes, where the company spoke directly to and quoted directly from minutes of management's December 16, 2013 Type C meeting with the FDA. Irrespective of the outcome of the subsequent breakthrough therapy designation application, [I believe] management told its audience what the FDA thought and said regarding PV-10, its relevancy and applicability, and pathway to initial approval. Management wrote in the PR:
"The Agency agreed with Provectus that treatment of cutaneous and subcutaneous tumors in patients with locally advanced cutaneous melanoma (i.e., recurrent, in-transit or satellite melanoma that has not yet spread from the skin to distant sites) could provide clinical benefit to such patients, particularly if the measured objective responses in patients' disease correlated to a demonstrated treatment effect on one or more symptoms of their disease (e.g., pain, infection or significant bleeding)."Several months later, in the FDA's BTD denial letter, the Agency wrote:
"We have reviewed your request and while we have determined that treatment of “locally advanced cutaneous melanoma” meets the criteria for a serious or life-threatening disease or condition, the preliminary clinical evidence you submitted does not indicate that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints...The preliminary clinical data provided in your request for Breakthrough Therapy designation are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma; however, the preliminary clinical data do not demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints."As such Provectus' press release Provectus Signs Memorandum of Understanding with Sinopharm-China State Institute of Pharmaceutical Industry, and Sinopharm A-THINK Pharmaceutical Co., Ltd. provided an opportunity for management to speak, in their own unique way, about progress to shareholders.
- PR byline: Negotiations on Licensing PV-10 in China Opened. As the company previously said on its conference calls, Provectus had been negotiating with several Chinese companies (rumored to include Eddingpharm and Hisun-Pfizer Pharmaceuticals). The MOU presumably includes a ninety-day period of exclusivity (a binding but non-material provision) to consummate a transaction (by replacing the MOU with a definitive agreement) with Sinopharm that has an out for the Chinese company and Provectus (one month prior written notice) should the former not get what it wants or the latter gets something better from another potential partner. While Provectus likely will go with Sinopharm under the assumption a regional transaction in China is achieved by the company, it's very possible the MOU PR could or would elicit additional MOUs from other potential Chinese partners in this process.
- The key component of the MOU provides that "Sinopharm-CSIPI and Sinopharm A-THINK desire to obtain an exclusive license to commercialize PV-10 within [the People's Republic of] China territory, and PVCT is willing to grant such license to Sinopharm." Provectus probably characterized this as the MOU's key component because, in the absence of the actual transaction itself, the signed MOU and this statement represent the closest thing to commercial validation the company has achieved thus far from a global pharmaceutical entity*.
- The MOU provides that "the parties agreed that PV-10 is an advanced innovative drug representing the oncology research trend worldwide, [which] will provide extensive economic and social benefits to [the Chinese] market." I think this is language inserted into the MOU by Sinopharm, and I doubt Provectus wrote or communicated this point of view. MOUs contain statements that the parties agree to this, this and that, where typically one party contributes a statement the other party agrees to (by virtue of signing the MOU).
- In addition, the MOU says that all "parties recognized the achievements gained during PV-10's clinical trial on melanoma, breast cancer and cancer of liver, plus the systematic effect on human immune efficacy, and PV-10 could be applied to other indications such as lung cancer, pancreatic cancer, prostate cancer and kidney cancer." I find this particular sentence intriguing because it may allude to more and new clinical and pre-clinical data available to prospective regional and worldwide partners in Provectus' electronic data room -- and that the market is not yet aware of -- and which (particularly data from Provectus' expanded Phase 1 liver trial) may have spurred Sinopharm to move. What data, given the indications mentioned in the sentence, are there in the data room?
Now that the Chinese have moved, who else will?
* From Sinopharm's website's overview page of the company:
Since 2005 SINOPHARM Group has consecutively posted the top sales numbers among China’s commercial pharmaceutical enterprises, and continued to have sales of over RMB 100 billion in the past few years. Its market capitalization ranked fourth worldwide in the pharmaceutical distribution industry. It has established partnerships with the world's leading pharmaceutical companies such as Pfizer, MSD, Bayer, IPSEN, SANKYO, Baxter, etc. SINOPHARM Group has more than 300 wholly owned and controlled subsidiaries (including two domestic listed companies - Sinopharm shares and Shenzhen Accord Pharmaceutical Co., Ltd). Its distribution net covers 180 cities in 31 provinces. 11 regional DCs (Distribution Centers) are located in major cities in the PRC – Beijing, Shanghai, Tianjin, Guangzhou, Shenyang, Changsha, Taiyuan, Wuhan, Nanning, Yangzhou and Shijiazhuang. New DCs are under construction in Urumqi, Zhenzhou, Chengdu, Shijiazhuang and other cities.
In addition to its nationwide distribution network, Sinopharm Group is built on China National Pharmaceutical Group’s powerful pharmaceutical R & D platform and owns 9 industrial pharmaceutical companies. Among these companies is Shenzhen Zhijun Pharmaceutical Co., Ltd., which produces antibiotics, anticancer drugs, cardiovascular drugs, medication, and various health care products. In terms of retail pharmacies, the Guoda Drugstore, which belongs to the SINOPHARM Group, is the top pharmaceutical retail operator in China's pharmaceutical in terms of sales volume. As one of only 3 licensed nationwide anesthetic distributors in China, the Group now accounts for the vast majority of the market share in the industry.Provectus & China (August 18, 2014)
Provectus entered into a memorandum of understanding ("MOU") with two subsidiaries/related parties/affiliates of Sinopharm Group ("Sinopharm"), China State Institute of Pharmaceutical Industry ("CSIPI") and A-THINK Pharmaceutical Co ("A-THINK"). The company issued a press release today and also filed an associated 8-K.
Sinopharm is China's largest state-owned pharmaceutical group, "China’s largest distributor and leading supply-chain solution provider for pharmaceutical and healthcare products," ranked 357th on the 2014 Fortune Global 500 list with revenues of $33.3 billion, ranked 23rd on Genetic Engineering &
Biotechnology News' 2014 Annual Top 25 Biotechnology Companies with a market capitalization of $7.89 billion (HK$61.13 billion), and one of two Chinese state owned enterprises ("SOE") targeted for privatization.
Sinopharm is majority owned by China National Pharmaceutical Group Corporation ("CNPGC") via Sinopharm Industrial Investment Co.
 |
| Click to enlarge. Sinopharm 2013 Annual Report. |
CSIPI was formed from the merger "of the Shanghai Institute of Pharmaceutical Industry (SIPI) and other institutes and companies:"
"China State Institute of Pharmaceutical Industry (hereinafter short for CSIPI) was originated from Shanghai Institute of Pharmaceutical Industry which was founded in 1957. Shanghai Institute of Pharmaceutical Industry used to be the scientific research and technology innovation base in our pharmaceutical industry. In 2000, it was listed in central enterprises and was subordinated to State-Owned Assets Supervision and Administration Commission of the State Council (SASAC). In April 2010, according to the requirement of “building a central enterprise healthcare industry platform” presented by SASAC, Shanghai Institute of Pharmaceutical Industry, Sinopharm Group and China National Biotic Group were recombined and the new Sinopharm was formed." (CSIPI website)A-THINK is 75% owned by Sinopharm.
 |
| Click to enlarge. Sinopharm 2013 Annual Report. |
Several initial thoughts and questions come to mind:
- Partnering with Sinopharm hopefully (by virtue of its SOE origin and size) should provide local enforcement of Provectus drug product and intellectual property,
- It's good Provectus wants to produce drug product in the U.S. and ship it to China (PV-10's storage and shipping costs should be negligible relative to its contemplated treatment cost/price in China); however, I wonder if that may be a stumbling block to an eventual transaction,
- What will the deal and/or operational structure look like should a transaction be consummated (i.e., a traditional license agreement with upfront, milestone and royalty payments, or a joint venture, or something in between),
- How would the Provectus-Sinopharm MOU influence discussions with other potential Chinese pharmaceutical company partners?, and
- How would the MOU influence discussions with potential Indian pharmaceutical company partners?
Provectus' stock ownership by institutional investors, hedges funds, etc. (as a percent of shares outstanding, and shares owned) historically has been small to negligible. Using 13F filings as a gauge of ownership, Provectus shares held by institutions who filed this SEC form and shares held as a percent of those outstanding significantly increased during the quarter ending June 30, 2014. On an absolute basis ownership still is negligible, but it certainly bears watching in quarters to come.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. 13Fs filed 3/31/14. |
 |
| Click to enlarge. 13Fs filed 12/31/13. |
 |
| Click to enlarge. 13Fs filed 6/30/13. |
In my Juxtaposition blog post I wrote I don't think Eric cares as much about when the trial starts as he does about how and when the trial ends. His upfront work to ensure the solidity of the trial design, and the process of carrying it out from start to finish, is of course very important.
Eric designed into Provectus' Phase 3 trial for locally advanced, unresectable (unresected) cutaneous melanoma enrollment of 210 patients to observe 105 progression free survival ("PFS") events, with an interim analysis planned at the observance of either 52 or 53 events (I don't know whether to round up or down from 52.5). The trial's primary endpoint, PFS, presumably is defined as the time from randomization to progression of disease ("PD") by RECIST version 1.1 criteria, as determined by the investigator, or death for any reason in the absence of documented PD. These two measurement review periods (after 105 PFS have occurred, and after 52-53), conducted by the trial's data monitoring committee ("DMC"), are pre-specified, which means Eric would have allocated alpha for them in the trial's statistical analysis plan ("SAP").
 |
| Click to enlarge. "Efficacy of intralesional rose bengal in patients receiving injection in all existing melanoma in phase II study PV-10-MM-02" American Society of Clinical Oncology, June 2014 |
In other words one has to adjust or incorporate into trial statistical significance the act and therefore subsequent penalty on said statistics of peering into the data before trial completion. The more one looks the more one has to penalize oneself (or one's trial stats' significance). The greater the effect size (i.e., the better the investigation drug is likely to be compared to the control drug) the easier it would be to statistically absorb more data looks.
As described in the portion of Provectus' ASCO 2014 poster illustrated above, the company also will conduct outcome assessments starting at week 12, and then every 12 weeks thereafter (e.g., week 24, week 36, etc.) for up to 18 months, and then every 6 months until disease progression, death or study closure. Such assessments, presumably of both arms, should address safety, and very possibly efficacy (i.e., PFS). These measurement review periods too, I think, are or could be pre-specified (but they may not be).
Thus, there may exist the possibility for the DMC to weigh in on how the trial is progressing as early as the 12th week following the start of the trial (assuming sufficient patient numbers), and how much better (or not) PV-10 is doing versus the control drug (dacarbazine), assuming the DMC reviews both safety and PFS data. If the trial begins accruing the week of October 1st, week 12 would be the week of December 24th. If, for example, accrual starts November 15th, week 12 would be February 7th.
Early study termination, whether after week 12 or following the interim analysis, is not without risk or lacking in certainty. "It has been well-recognized that overestimation of the true treatment effect can occur in trials with multiple data looks that stop early for efficacy (Ellenberg SS et al., JAMA, 2010)." This risk could be mitigated if PV-10 PFS data more than soundly beats dacarbazine results (assuming sufficient patient numbers), and Provectus also can demonstrate a benefit on quality-of-life factors (i.e., patient reported outcomes) to show the PFS data is not merely a random high. Overall survival benefit would require time to elapse for such benefit to be measured and characterized.
Potential New FDA Advisors (August 11, 2014)
It would appear the regulatory consulting firm Eric noted on last Thursday's conference call Provectus is contemplating bringing on board is Washington D.C.'s Greenleaf Health. Firm professionals have senior FDA leadership experience. Eric discussed the firm in a non-specific way saying:
I want to startup my remarks by emphasizing that our primary focus is on managing the FDA regulatory process. Provectus is contemplating adding to its consulting advisors a group to help us validate our efforts with the agency. This group is a full-service regulatory consulting firm that provides strategic guidance to companies like Provectus regulated by the Food and Drug Administration in developing innovative solutions to pressing public health challenges around the globe. {Underlined emphasis is mine}Greenleaf's description of itself includes:
Greenleaf Health is a full service regulatory consulting firm that provides strategic guidance to companies regulated by the Food and Drug Administration that are developing innovative solutions to pressing public health challenges around the globe.New SEC filing (August 8, 2014)
Provectus filed its second quarter 10-Q (ending June 30th) on Thursday. Monthly cash burn increased 20% quarter-over-quarter to ~$1.1 million. Quarter-end cash and cash equivalents were ~$18.1 million. It would appear the company had a much higher burn in the quarter's April and May months while appearing to bring June's burn in-line with guidance of a forward run-rate of $2.5 million per quarter (~$833K per month), presumably starting that month (management said they were looking at a 12-month expense runway of less than $10 million on the June 3rd conference call).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
The quarterly filing also contained an exhibit describing in more detail the settlement agreement over historical executive compensation. Monies, whether the full amount per principal of $2.24 million (times 4) or the reduced amount after certain credit of $1.12 million per principal (times 4), would be paid back within 5 years. The principals will pay a minimum of $200K per person per year, which could start this quarter (I do not think management will pay anything other than the minimum annual amount, which appears to be almost "garnished" from their annual salary). As a result, net monies (i.e., total monies owned less minimum annual payments) realistically should not be counted on to meaningfully mitigate expenses over the next 12 to 24 months. A repayment of $800K per year is about one month of monthly burn or, put another way, reduces monthly burn by $67K per month.
Single shot of PV-10 or two, usually vs. How many? (August 3, 2014)
You may recall the title of Moffitt Cancer Center's August 2013 press release Single injection may revolutionize melanoma treatment. The statement derived from Moffitt's "...initial study, researchers injected a single dose of PV-10 into mice with melanoma. The result was a significant reduction in the skin cancer lesions, as well as a sizable reduction in melanoma tumors that had spread to the lungs."
In Provectus' metastatic melanoma Phase 2 trial's subgroup All Disease Followed (N=54, out of 80), comprised of All Lesions Treated (N=28) and Bystanders Untreated (N=26), a majority of lesions of lesions achieved a complete response after one or two injections. See below (Provectus' ASCO 2014 poster).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Items from Moffitt poster Induction of anti-melanoma immunity after intralesional ablative therapy are available here and below.
 |
| Click to enlarge |
 |
| Click to enlarge. |
From: Ipilimumab update by Michele Maio, Medical Oncology and Immunotherapy, Department of Oncology, University Hospital of Siena, Istituto Toscano Tumori, Siena, Italy at the 10th EADO Congress, Vilnius, Lithuania, May 7-10, 2014 (where Provectus principal investigator Dr. Sanjiv Agarwala also presented in the same session)
 |
| Click to enlarge. |
H/t @TECHNICALGUY111: "Note on $PVCT..Has lowest weekly volume since 8/19/13 which is just before the start of the last run."
 |
| Click to enlarge. |
For the first time since at least 2005, Provectus' board of directors did not award employee directors, currently Drs. Craig Dees, Ph.D. and Tim Scott, Ph.D., stock option grants. Outside directors Dr. Kelly McMasters, M.D., Ph.D., Al Smith and Jan Koe received theirs of 50,000 options each.
July Blog Stats (August 1, 2014)
Blog readership fell about 15-40% from June 2014 depending on the statistic. I wrote 14 blog posts (4) and news items (10) in June versus 18 the previous month (6 and 12, respectively). July blog readerships stats month-over-month changes were:
- -31% for # of unique visitors (2,130 v. 3,070),
- -38% for # of page views (16,429 v. 26,505),
- -37% for # of visits (7,451 v. 11,884),
- -22% for # of U.S. cities [from where visitors came] (761 v. 974),
- -28% for # of world cities (152 v. 212), and
- -15% for # of countries (51 v. 60).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Provectus updated its corporate web presentation today (versus the previous July 28th version) to note "Cash on hand supports planned operations until 2016" (versus the previous "Cash on hand supports planned operations until 2015"). See below.
 |
| Click to enlarge. July 31st version. |
 |
| Click to enlarge. July 28th version. |
Pathologic complete response: No viable melanoma cells (July 31, 2014)
For locally advanced melanoma:
Surgery (resection) + Adjuvant inteferon alpha + Ipilimumab (4 times) + Vemurafenib + Trametinib + Dabrafenib + Surgery (resection, graft)
 |
| Click to enlarge. Source: https://twitter.com/cancerdocNYC/status/494875311570288641 The above patient did not receive PV-10. |
Background: Intralesional (IL) therapy is under investigation to treat dermal and subcutaneous metastatic cancer. In our murine model, IL injection of PV-10 (10% Rose Bengal) induced regression of injected and uninjected “bystander” melanomas. We observed a consistent increase in anti-tumor T cell responses following IL PV-10 in the mouse model, supporting an immune-based mechanism by which PV-10 mediates regression of uninjected “bystander” tumors. Methods: We translated these findings into a pilot clinical trial that enrolled 8 patients with dermal and/or subcutaneous metastatic melanoma. Two study lesions in each patient were sampled by biopsy pre-treatment; one of the two lesions was injected with IL PV-10, then both residual sites were completely excised. We compared tumors before and after treatment with H&E staining to determine pathologic complete response (pCR), and we confirmed results with MelanA immunohistochemistry. Peripheral blood mononuclear cells (PBMC) before and after IL PV-10 were phenotyped for activation markers by flow cytometry. Results: Treatment with IL PV-10 led to pCR in the post-treatment biopsies of both PV10-injected and uninjected study lesions in 4 of the 8 patients, and all 8 exhibited at least partial regression of the injected lesion. IL PV-10 was associated with an increase in circulating cytotoxic CD3+/CD8+ T cells (paired t test, p=0.008). Pre and post PV-10 treated PBMC from one patient were re-stimulated with autologous tumor in vitro. Compared to pre-treatment, PV-10 treatment produced an increase in tumor-specific interferon-gamma release by ELISA. Six of 8 patients had metastatic disease refractory to previous ipilimumab, anti-PD-1 and/or vemurafenib therapy. Four of these 6 patients exhibited pCR to PV10 in both the injected and uninjected lesions. Conclusions: IL PV-10 treatment can lead to systemic anti-melanoma immunity and pCR in injected and uninjected lesions including treatment-refractory tumors. Further studies are ongoing to determine the mechanism by which PV-10 increases tumor-specific T cell responses as well as to establish the interaction of intralesional PV-10 with combination checkpoint protein inhibition. Underlined emphasis is mine.
“Ironically, the original aim of the trial to assess tumour-infiltrating lymphocytes was thwarted when biopsies of patient tumours collected just seven to 10 days after PV-10 injection no longer contained viable tumour tissue,” Dr. Shari Pilon-Thomas, Moffitt Cancer CenterVarious (July 30, 2014)
A new article about PV-10: Intralesional Injections Trigger Immune Responses in Melanoma by Caroline Helwick in The ASCO Post, Volume 5, Issue 12 (July 25, 2014). The article reviews (i) Amgen’s intralesional agent T-Vec’s Phase 3 OPTiM study of Stage IIIB and IV melanoma, (ii) a Phase 1 trial combining T-Vec and Bristol-Myer’s ipilimumab, and (iii) two Provectus trials. Of note were Moffitt Cancer Center’s Dr. Amod Sarnaik, M.D.'s quotes about the center’s Phase 1 human feasibility study involving PV-10:
“[Intralesional] PV-10 can induce regression of injected and uninjected metastatic melanoma, enhance tumor-specific reactivity in circulating T cells, and lead to responses in treatment-refractory tumors,” and
“After PV-10 injection, we saw a significant increase in circulating T cells, including CD3-positive and cytotoxic CD8-positive cells. This suggests an immunologic-mediated antitumor response is engendered by PV-10. We are hoping to undertake combination trials that combine PV-10 with promising systemic immunotherapies.”An associated article to the one above by the same author is Expert Point of View: Axel Hauschild, MD by Caroline Helwick in The ASCO Post, Volume 5, Issue 12 (July 25, 2014). The author writes about Hauschild:
“In general, he maintained that the overall and complete response rates are impressive, especially the regression of untreated lesions, ie, the bystander effect, as is the lack of toxicity associated with intralesional injections. However, the current studies use unconventional response criteria, which makes interpretation difficult, and they are evaluating a highly selected subset of stage IIIB/IV patients (those with injectable lesions and no visceral metastasis), whose outcomes will be better.”In my blog news item Evolving Utility of Intralesional Therapy for Melanoma (July 2, 2014) below, I wrote that a critic of Provectus commented on PV-10 following ASCO 2014: "At the ASCO meeting, Dr. Axel Hauschild gave an oral presentation and review of intralesional therapies in which he described the response criteria used by Provectus in the PV-10 phase II study as "unusual." The PV-10 study also enrolled "highly selected" melanoma patients, Hauschild said." Dr. Sarah Weiss wrote an article on June 4th entitled Is There a Role for Intralesional Therapy in Melanoma? (sign-up required) that reviewed and summarized Hauschild's presentation. Hauschild referenced OncoSec's (IL-12 + electroporation's) melanoma Phase 2 trial as using a modification to RECIST (soft tissue or MR sonography to characterize response) and having a "highly selected group of patients." The critic incorrectly quoted or paraphrased Dr. Hauschild's references to PV-10 when in fact it was a completely different study Hauschild was discussing that the critic quoted. Helwick's July 25th article reveals what Hauschild meant when he said "highly selected group of patients." He meant “stage IIIB/IV patients (those with injectable lesions and no visceral metastasis).” OncoSec's Phase 2 trial enrolled subjects with Stage IIIB, IIIC and IV M1a disease. Provectus enrolled a broader array of patients (see below), although the company is pursuing an initial pathway to approval for unresectable locally advanced cutaneous melanoma (with a Phase 3 trial that should primarily if not exclusively recruit Stage IIIB-C patients).
 |
| Click to enlarge. |
“The major questions are: How strong is the systemic response from an intralesional treatment? And what is the size and localization of responding metastases, ie, skin vs visceral organs?” he asked. “I have only seen a tiny 1-cm nodule in the lung respond; I have not seen major responses in visceral organs. The vast majority of stage IV melanoma patients have M1c disease.” Dr. Hauschild also emphasized that with the growing numbers of drugs for unresectable advanced melanoma, intralesional therapy has some competition. “The most interesting of these studies is the one combining T-VEC with ipilimumab, which showed some benefit in M1c patients. Maybe this is a good compromise, and the setting where we need to move ahead…. The future lies in combinational approaches with drugs from the new melanoma landscape.””Sarnaik's quotes would suggest the combination of PV-10 with Bristol-Meyer’s nivolumab and/or Merck’s pembrolizumab. In keeping with my blog news item My Melanoma Expert is Better Than Your Melanoma Expert... (July 29, 2014) below, I would not be too surprised if Dr. Hauschild finds a Phase 3 PV-10 monotherapy trial interesting enough to be a principal investigator and for his German hospital to be a clinical site.
I would expect and hope Peter characterizes the company’s upcoming conference call on August 7th as an earnings call to typically and traditionally discuss financial results for the second quarter and other topics, and to provide a question & answer session for those who call-in. Of course I presume the call is an earnings one because it should be conducted after the 10-Q is filed (according to Peter's comments from the last conference call), and would seem generally in line and keeping with its status on a major stock exchange. In the aftermath of the FDA's denial of Provectus’ breakthrough therapy designation application ("BTD") the company held three investor conference calls on May 23rd, June 3rd and June 19th to discuss the Agency's BTD denial letter, the company's path forward, and the outline of the upcoming Phase 3 trial for unresectable locally advanced cutaneous melanoma. Although the earnings descriptor is a misnomer for a development stage (pre-revenue, pre-profit) company like Provectus, the August 7th call should be branded like those of other public companies undertaking these kinds of regular quarterly communications. Because management did not conduct these calls when the company traded over-the-counter, existing and prospective investors currently may not view the August 7th call as a quarterly earnings call that companies on major stock exchanges carry out to report historical information and provide real-time replies to questions regarding prospective events and items (with future such calls regularly and perfunctorily scheduled and held).
MD Anderson noted Provectus' compassionate use program, a surgical oncology clinical trial (program) in which the institution is involved, in its August 2014 Melanoma Horizons newsletter.
 |
| Click to enlarge. |
My Melanoma Expert is Better Than Your Melanoma Expert... (July 29, 2014)
...a.k.a, my quarterback is better than your quarterback, etc. (with tongue firmly planted in cheek).
In her OncLive article Immunotherapy Advances in Melanoma Icahn School of Medicine at Mount Sinai's Assistant Professor of Medicine, Hematology and Medical Oncology, and of Dermatology's Dr. Yvonne Saenger, M.D. began:
"Melanoma care has fortunately undergone a whirlwind of changes over the past several years. Novel immunotherapies are perhaps the most exciting recent development in cancer care because patients can enjoy long-term benefit from these treatments, meaning that they are possibly “cured.” Thus, it is now considered possible to rid the body of cancer using immunotherapy in a significant fraction of patients with advanced melanoma. Immunotherapy can be very toxic, but it lacks the characteristic poisoning effects of chemotherapy and has the appeal of offering a more “natural” solution to cancer as it builds on the body’s own innate defense mechanisms to cause tumor rejection. Over the coming decades, the challenge will be to expand the minority currently curable with immunotherapy and also to introduce immunotherapies earlier in the disease course to prevent systemic spread in the first place."She discussed CTLA-4:
"While the initial data for CTLA-4 blockade showed efficacy not far superior to interleukin-2, follow-up studies have shown impressive rates of survival following CTLA-4 therapy, with 4-year survival rates of approximately 40% in previously untreated patients treated with high-dose CTLA-4 blockade on clinical studies. These data suggest that, while most patients do not experience immediate tumor shrinkage, tumors may respond later in the treatment course, resulting in improved survival. The CTLA- 4–blocking antibody ipilimumab (Yervoy) was approved for the treatment of melanoma in March 2011."Then, Dr. Saenger discussed PD-1/PD-L1:
"The success of CTLA-4 blockade spurred further research, resulting in the discovery of a second clinical target, PD-1. PD-1, similar to CTLA-4, is expressed by activated T cells, and it appears to be induced by chronic inflammation. Similar to CTLA-4, PD-1 signaling leads to inactivity and sluggishness of T cells. Thus, CTLA-4 and PD-1 are described as “checkpoints” in immune activation. One way to describe the role of PD-1 is that the immune system might decide to tone down a prolonged immune response, as it appears unlikely that the foreign entity will be successfully eliminated and the attack becomes a waste of resources. Thus, PD-1 expression is high on T cells during chronic viral infections and in T cells infiltrating tumors...Thus, interrupting the PD-1/ PD-L1 interaction appears to be more effective than CTLA-4 blockade, and will likely be the cornerstone of future therapy for melanoma."Followed by T-Vec:
"The immune checkpoint inhibitor approach has yielded amazing results in melanoma, but other strategies are also under active investigation and nearing fruition. For example, oncolytic viruses are receiving increased interest. Talimogene laherparepvec (T-VEC) is the most advanced in clinical trials...Oncolytic viruses are attractive therapeutic options because they are derived from genuine pathogens and therefore are more potent mediators of immune activation than conventional vaccines....It is highly likely that intralesional therapies such as T-VEC will be part of future melanoma therapy and may enhance the activity of immune checkpoint blockade."Dr. Saenger finished with biomarkers:
"One challenge in immunotherapy is to develop accurate techniques to measure immune activation. In early stage melanomas, it is known that the immune system can infiltrate into the tumors and control their growth. Recently, our group has proposed a biomarker to predict recurrence-free survival and overall survival in patients with stage II/III melanoma at high risk of recurrence. This biomarker is based on the expression of immune genes and patients with higher expression of immune genes have better clinical outcomes. Similarly, we have found that survival in patients treated with CTLA-4 blockade can be predicted based on patterns of gene expression in blood. Genomic biomarkers based on analysis of blood and tumor tissue are likely to play a role in the future for prognostication and for selection of immunotherapy options to be used in specific patients."She concluded with:
"In summary, tremendous progress has been made in immunotherapy for melanoma over the past 5 years. A diagnosis of metastatic melanoma is no longer the death sentence it once was. The future of melanoma therapy is likely to include combinations of different immune therapies, including immune checkpoint blockade and intralesional treatments, such as T-VEC. These immune therapies may also in the future be combined with targeted treatments, such as BRAF inhibitors, and/or conventional therapies designed to break down the tumor mass, such as irradiation. Further new targets for immune activation, including LAG-3, CD40, GITR, and OX-40, are on the horizon as part of combination strategies."The takeaways from Dr. Saenger's article (really, from her concluding remarks), like much of the opinion on melanoma and cancer immunotherapy specifically, and cancer and immunotherapy generally, are:
- Progress has been and continues to be made towards defeating cancer (i.e., "at worst," more, better patient options; at best, the/a cure is in the distance or within sight; somewhere in the middle, we know much more now than we did before),
- The likely approach to treating late stage and visceral melanoma is with combinations of treatments and therapies: one or more of {conventional therapies, targeted therapies, immunotherapies}, and
- There's are a role in late stage treatment for intralesional treatments (like T-Vec).
Of course, Moffitt Cancer Center's Jeffrey Weber, who is a consultant to and/or participated in advisory boards and received honoraria from Provectus (and Bristol-Myers and Merck, among others like Amgen, AstraZeneca, Genentech, Novartis and GlaxoSmithKline), recently said post-ASCO 2014 "PV-10 might offer the perfect way to prime the immune system." Priming the immune system would be analogous or equivalent to Dr. Saenger's enhancing the activity of immune checkpoint blockade. Weber also recently said post-ASCO 2014 "Now there are some interesting drugs, and T-Vec is one of them. I see this as a niche drug that would be best used to prime the immune system and follow up with a drug such as pembrolizumab, nivolumab, ipilimumab, or a combination of those. After priming the immune system, you then boost it with the checkpoint protein inhibitors."
University of Utah's Huntsman Cancer Institute's Dr. Robert Andtbacka, M.D., who was a principal investigator in three intralesional agent clinical trials -- Amgen's (BioVex's) T-vec (OncoVex), Vical's Allovectin-7 and Viralytic's Coxsackievirus Type A21 -- would show PV-10 may be better than T-Vec based on Phase 2 trial data when it comes to response rates (a 2012 version of his presentation is below; see slide 21 in the link). Dr. Andtbacka is a consultant to Amgen (BioVex), and does not appear to have receive money from Provectus. I think it's a good risk-reward bet that if you spoke to Dr. Andtbacka, privately after you paid his consulting fee (a number of hundred dollars per hour) or if he chose not to charge you, he'd say PV-10 was better than T-Vec. Is my melanoma expert better than yours?
 |
| Click to enlarge. |
Combining PV-10 with another drug potentially presents another pathway to approval; for example, combining PV-10 with an anti-PD-1 agent for metastatic melanoma (i.e., late stage and visceral disease: Stage IV M1a-c). One of the rationales presumably for combining therapies for treating late stage cancer patients, using the cancer immunity cycle as a foundation, is that different drugs target distinct steps of the cycle (see below: 50th ASCO Annual Meeting, Chicago, Roche Analyst Event, Sunday, 1 June 2014).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Puzanov et al.'s work on the combination of Bristol-Myers' ipilimumab (ipi) and Amgen's intralesional agent (and PV-10 competitor) talimogene laherparepvec (T-Vec) demonstrated preliminary efficacy of 24% complete response (CR), 41% objective response rate (CR + partial response [PR]) and 76% disease control (CR + PR + stable disease or SD). This study also measured immune response (i.e., CD8+ T cell populations), which was presented at ASCO 2014 (see below). The y-axis for Total CD8 is 10^2 and 10^3 cells per microLiter. Repeat injections of T-Vec and multiple administrations of ipi generate the immune response.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
In my 2014 Annual CEO Letter (pt. 1) blog post I wrote that while my analysis was rough one could make a reasonable argument Provectus could have enough money to reach and subsequently provide an interim analysis of its melanoma Phase 3 trial (for unresectable locally advanced cutaneous melanoma) without a further injection of capital. The graph endeavoring to make this point is below.
 |
| Click to enlarge. |
Provectus' June 12th 8-K detailing the settlement of the shareholder derivative lawsuit noted a date of July 24th for a hearing on the terms of the proposed settlement. Depending on how much is paid back by management (i.e., ~$4.5 million or ~$9 million), the additional cash should provide further runway for the company to reach the trial's interim readout and make its case to the Agency for filing the NDA. Below, for example, I assume these monies are paid back in 3Q14. Without consideration of the melanoma Phase 3 trial, the projected cash balance would look something like the below. At a projected $2.5 million average quarterly burn, Provectus would approach its accounting firm BDO LLC's minimum cash threshold figure of about $4 million in the 1Q16 timeframe, assuming repayment of the lower amount (the higher payment obviously provides at least an additional quarter of runway).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Oncolytic Immunotherapy PV-10-style, aka 2014 Annual CEO Letter pt. 2 (July 12, 2014)
It was interesting to read this sentence in Provectus management's annual CEO letter for 2014, and one particular phrase: "When we completed data collection two years ago, we knew we had a promising oncolytic agent with consistent response in injected tumors, but we also had intriguing data on the bystander effect that implicated a secondary immunologic mechanism of action." Underlined emphasis is mine. Oncolytic is used in the sense of "pertaining to the destruction of tumor cells."
Oncolytic immunotherapy is an en vogue (e.g., fashionable, popular) industry term. Before I continue with this news item post, PV-10 is an oncolytic agent. Amgen's talimogene laherparepvec ("T-Vec") is an oncolytic virus. An oncolytic agent is an agent that preferentially kills cancer cells (S1). An oncolytic virus is a virus that preferentially infects and kills cancer cells (S2). I modified sentence S2 from Wikipedia (to produce S1), adjusting for agent (in this case, merely a compound or drug or small molecule) rather than virus, which in the case of T-Vec was engineered from the herpes simplex 1 virus (italicized emphasis is mine).
Note, for example, a recent review article entitled "Going viral with cancer immunotherapy", and published in Nature Reviews Cancer:
"Recent clinical data have emphatically shown the capacity of our immune systems to eradicate even advanced cancers. Although oncolytic viruses (OVs) were originally designed to function as tumour-lysing therapeutics, they have now been clinically shown to initiate systemic antitumour immune responses. Cell signalling pathways that are activated and promote the growth of tumour cells also favour the growth and replication of viruses within the cancer. The ability to engineer OVs that express immune-stimulating 'cargo', the induction of immunogenic tumour cell death by OVs and the selective targeting of OVs to tumour beds suggests that they are the ideal reagents to enhance antitumour immune responses. Coupling of OV therapy with tumour antigen vaccination, immune checkpoint inhibitors and adoptive cell therapy seems to be ready to converge towards a new generation of multimodal therapeutics to improve outcomes for cancer patients."Note Amgen's marketing of T-Vec as an oncolytic immunotherapy, an innovative area of research exploring the potential of a modified virus to induce a tumor specific immune response.
"Find it: Oncolytic Immunotherapy (OI) is an innovative area of cancer research that aims to use a modified virus to induce tumor cell lysis, through selective replication, and activate T cells for a specific, systemic immune response."
"Fight it: Oncolytic viruses may not only cause direct tumor cell destruction through oncolysis, but may also have the potential to induce a systemic immune response that targets specific tumor cells throughout the body."A further Amgen word on oncolytic immunotherapy:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
The company received a positive response to its prior application under Rule 24b-2 of a request for confidential treatment of information excluded from exhibit 99.2 of its May 23rd 8-K filing (i.e., the breakthrough therapy designation denial letter from the FDA), and made an associated SEC filing today. Specifically, when management filed the letter as part of the above mentioned 8-K, they redacted the name and contact information (i.e., phone number) of an FDA staffer: If you have any questions, contact ***, Regulatory Health Project Manager, at ***. Italicized emphasis is mine; redacted items are represented by ***. At the time Provectus noted on the exhibit that "*** Indicates a portion of this document has been omitted and filed separately with the Secretary of the United States Securities and Exchange Commission pursuant to Provectus Biopharmaceutical, Inc.’s, application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934." Examples of similar language in the correspondence between the Agency and other companies may be found here and here.
Provectus issued an 8-K filing today related to today's letter to shareholders.
The company's COO/CFO, Peter Culpepper, exercised 100,000 options to buy underlying common stock ($1.25 strike price), and filed an associated Form 4 on July 1st.
Provectus made an 8-K filing on June 19th related to its conference call of the same day and an associated press release.
Evolving Utility of Intralesional Therapy for Melanoma (July 2, 2014)
"At the ASCO meeting, Dr. Axel Hauschild gave an oral presentation and review of intralesional therapies in which he described the response criteria used by Provectus in the PV-10 phase II study as "unusual." The PV-10 study also enrolled "highly selected" melanoma patients, Hauschild said."During the above mentioned oral presentation, a poster highlights session entitled Evolving Utility of Intralesional Therapy for Melanoma, Dr. Hauschild reviewed five posters:
- Abstract #9025, IL-12 + electroporation (OncoSec Medical): Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma,
- #9026, T-Vec (Amgen): Patterns of durable response with intralesional talimogene laherparepvec (T-VEC): Results from a phase III trial in patients with stage IIIb-IV melanoma,
- #9027, PV-10 (Provectus): Efficacy of intralesional Rose Bengal in patients receiving injection of all existing melanoma in phase II study PV-10-MM-02,
- #9028, PV-10 (Moffitt Cancer Center): Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions, and
- #9029, T-Vec + ipi (Amgen+Bristol Myers): Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma.
"One study looked at IL-12 given intralesionally by electroporation, which involves injection of the plasmid into tumors. Electrodes are then inserted and create a more porous environment to allow for distribution of the drug. IL-12 is thought to upregulate interferon gamma. Nine out of 28 patients were found to have objective responses and three patients had a complete response in the lesion. Additionally, 13 out of 22 patients demonstrated regression of non-injected lesions.
One limitation seen in this intralesional therapy was that the standard RECIST criteria were difficult to use for measuring response. For example, very small lesions may be difficult to assess using RECIST criteria, despite the fact that a clinical response might be noted. It was proposed that another way to characterize response in these lesions other than using RECIST criteria would be to use soft tissue sonography or MR sonography. The strength of IL-12 intralesional therapy was the very low toxicity reported—mainly consisting of injection site pain and inflammation. This was a very small study with a highly selected group of patients and without a control arm, which makes it difficult to draw conclusions from it. However, the reported overall response rates were reasonable; several patients had a complete response; and some of the untreated lesions demonstrated regression." {Underlined emphasis is mine}Hauschild referenced OncoSec's (IL-12 + electroporation's) melanoma Phase 2 trial as using a modification to RECIST (soft tissue or MR sonography to characterize response) and having a "highly selected group of patients."
Dr. Weiss next summarized Hauschild's comments about PV-10 (nos. 9027 and 9028):
"Another intralesional therapy known as PV-10 was looked at in a phase II study of 80 patients with cutaneous melanoma who had a mean age of 70 years old and who were refractory to a median of six prior therapeutic interventions. PV-10 is an intralesional injection that accumulates in tumor lysosomes and results in lysosome rupture. In 57 evaluable patients, there was a 71% overall response rate with a time to response of 1.8 months. It was also noted that melanin A expression decreased after treatment with intralesional PV-10."Provectus' melanoma Phase 2 trial used modified RECIST, and the patient population had a median age of 70 and were a "fairly refractory group of patients" (i.e., the median number of prior treatments was 6). Nevertheless, the critic incorrectly quoted or paraphrased Dr. Hauschild's references to PV-10 when in fact it was a completely different study Hauschild was discussing that the critic quoted.
 |
| Click to enlarge. |
"Dr. Hauschild raised several interesting conclusions from the current debate regarding the utility of intralesional therapy in melanoma. Because intralesional therapy appears to have virtually no significant systemic toxicities and has been shown to have complete responses in some patients, this type of therapy may be considered in combination with other standard therapies, but its role as a single agent is not clear...
Furthermore, the goals of treatment need to be clarified. Would improved quality of life from resolution of a skin lesion be enough to provoke clinicians to use intralesional therapy? His definition of response rates, and how to measure and assess responses also needs to be addressed, as discussed above. Finally, more randomized studies, with active control arms, are needed. It would be difficult to show that single-agent intralesional therapy would demonstrate a progression-free survival or overall survival advantage over BRAF inhibitors or anti-PD-1 immunotherapy, but combination therapy should be further studied."Moffitt Cancer Center's Dr. Vernon Sondak recently concluded (see Intralesional Therapy for Melanoma (June 29, 2014) below):
- Among the many agents currently being evaluated for use as intralesional therapy for melanoma, PV-10 possess favorable safety and handling properties and appears to induce rapid ablation of injected lesions to a degree similar to or possibly even better than other agents
- Preclinical data suggest PV-10 would be a good candidate to evaluate in conjunction with available systemic therapies and new agents in development
- The optimal path for development and registration of PV-10 and other intralesional therapies is still unclear and needs to be better defined
Meanwhile, Provectus plans to embark on what appears to be a pivotal Phase 3 trial for unresected locally advanced cutaneous melanoma in 3Q14 using standard RECIST 1.1 for primary endpoint progression free survival and secondary endpoint complete response rate.
June Blog Stats (July 1, 2014)
Blog readership fell about 10-45% from May 2014 depending on the statistic. I wrote 18 blog posts (6) and news items (12) in June versus 37 the previous month (9 and 28, respectively). June blog readerships stats month-over-month changes were:
- -30% for # of unique visitors (3,070 v. 4,365),
- -46% for # of page views (26,505 v. 48,963),
- -40% for # of visits (11,884 v. 19,839),
- -20% for # of U.S. cities [from where visitors came] (974 v. 1,220),
- -11% for # of world cities (212 v. 239), and
- -9% for # of countries (60 v. 66).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Intralesional Therapy for Melanoma (June 29, 2014)
More from Moffitt Cancer Center's Dr. Sondak's presentation on intralesional therapy for melanoma and PV-10 at at the 4th European Post-Chicago Melanoma Meeting:
Potential Applications*
- Ablation of locally recurrent melanoma
- Defined as within 2 cm of the primary site
- AJCC stage poorly defined
- Ablation of dermal or subcutaneous regionally recurrent ("in transit") melanoma
- Defined as >2 cm from the primary stite and not beyond the first regional nodal basin
- Stage IIIB or IIIC depending on nodal status
- Ablation of dermal or subcutaneous metastatic melanoma beyond the nodal basis
- Stage IV M1a disease
- Induction of necrosis/antigen release and presentation in dermal or subcutaneous metastatic melanoma in patients with multifocal stage IV disease prior to or concomitant with systemic immunotherapy
- "Consolidation" by ablation of residual dermal or subcutaneous metastatic melanoma in patients with multifocal stage IV disease after treatment with systemic therapy
- Elimination of resistant clones
- Conversion from partial to complete response
- Simple to store, handle and use and reuse
- Modest local toxicity and minimal to no systemic toxicity
- Rapid and complete induction of necrosis/antigen release in injected lesions
- Excellent healing of the injected site after tumor necrosis
- Reliable and reproducible induction of regional and systemic immune effects capable of destroying occult tumor cells, "bystander lesions" and distant metastatic lesions regardless of prior treatments
- Among the many agents currently being evaluated for use as intralesional therapy for melanoma, PV-10 possess favorable safety and handling properties and appears to induce rapid ablation of injected lesions to a degree similar to or possibly even better than other agents
- Preclinical data suggest PV-10 would be a good candidate to evaluate in conjunction with available systemic therapies and new agents in development
- The optimal path for development and registration of PV-10 and other intralesional therapies is still unclear and needs to be better defined
** Slide header: Intralesional Therapy for Melanoma with PV-10
Why Priming the Immune System Matters (June 28, 2014)
"The immune system can be leveraged to fight cancer via four broad, overlapping strategies, comprising priming/boosting of the immune system, T-cell modulation, reducing immunosuppression in the tumour microenvironment and enhancing adaptive immunity." These strategies, related as they are, boil down [it would appear] to harnessing the immune system's various components in a safe, effective, long-lasting and, as it relates to the specific immunotherapy or treatment (or combination(s) of treatments) employed, cost effective way for the benefit of the patient and, it seems growing day by day, the medical insurance system.
Priming the immune system matters for both early and late stage cancer patients. For late stage to heavily tumor burdened, priming should make checkpoint inhibitors of today and their progeny of tomorrow more effective and thus more relevant (and likely less expensive as a regimen, which is would be comprised of dosing amounts and schedules). "...[I]mmunotherapies may have efficacy in patients with early-stage disease and low tumour burdens, enabling the immune system to eradicate primary tumours and micrometastases and protect against recurrence."
PV-10's use as an immune system primer, where the drug is capable [as a result of or deriving from the rapid tumor ablation it causes shortly after injection] which of course still requires clinical trial evaluation and validation, may turn out to be more catalytic to Provectus' acquisition, and more contributory to the eventual cumulative worth of the drug. I think Moffitt Cancer Center and Dr. Jeffrey Weber, M.D., Ph.D. are important to this realization (and eventual hoped for monetization).
Weber has been consistent in his approach to and thoughts of PV-10, which clearly have evolved over time. It has been said he was a skeptic (as presumably individuals in his role typically are) when Moffitt and Provectus first engaged over PV-10 sometime in 2011. Later, his skepticism appears to have turned into growing interest. In September 2013 saying: "I think the drug has some serious potential as an immune modulator, and would be very interesting to combine with checkpoint inhibitors." It would seem his view of combination was to improve the effectiveness of checkpoint inhibitor use and treatment. I think the realization of Big Pharma, oncologists, researchers, equity research analysts and investors is mono-therapeutic applications of checkpoint inhibitors like Bristol Myers Yervoy (ipilimumab) and anti-PD-1 agent nivolumab, Merck's anti-PD-1 agent pembrolizumab, and even Roche's anti-PD-L1 agent MPDL3280A for late stage patients is small, or at least much smaller than first thought.
During medical oncologist's Weber's April 2014 HemOnc Today Melanoma and Cutaneous Malignancies Conference "debate" with surgical oncologist and MD Anderson physician Dr. Merrick Ross, a proponent of PV-10, his focus as it related to the use and utility of intralesional ("IL") therapies was for patients with significant disease burden and visceral M1c disease. See Debating Systemic Intralesional Therapies (April 16, 2014) under the blog's Archived News tab. Weber said to expect, however, significant clinical and biologic systemic benefit (affect) with any IL therapy as we now know them is very low. He went on to list as these IL therapies that we now know below:
 |
| Click to enlarge. |
As noted in the news item "We are at the beginning of a revolution in immunotherapy..." (June 21, 2014) below -- where the researcher had noted [as a result of vacine usage] "...tumors had become immunogenic, which means the immune cells surrounding the tumor now had the ability to attack the cancer cells." PV-10 may make non-immunogenic tumors immunogenic, and already immunogenic tumors more so. The article I referenced below noted that "...tumors had become immunogenic because the ratio of "effector T cells" to "regulatory T cells" had increased." Moffitt showed at AARC 2014 a statistically significant increase in CD3+, CD4+ and CD8+ T cells as well as NKT cells in peripheral blood following injection of PV-10 (i.e., the mean and confidence intervals of these cell populations pre- and post-PV-10 injection did not overlap, with p values all below 0.05 -- 0.008, 0.023, 0.008 and 0.01, respectively).
The utility or value of an agent or compound as an immune system primer is assessed on its ability to synergize with the immune agent in question (e.g., another immunomodulatory agent, a checkpoint inhibitor) and make more the combination more clinically effective when given prior to the second agent. That is, the primer is given first. As an aside, radiotherapy may be considered an immune system primer, and could be combined with a cancer immunotherapy (first radiation, second immunotherapy).
Interestingly, in a recent article entitled Invisibility Cloak for Immune Cells, the author wrote: "The immune system also includes natural killer cells (NK cells), which recognize and eliminate tumor or virus-infected cells. NK cells therefore combat the body’s own stressed cells to prevent them from becoming a potential hazard. However, this bears its risks. Other immune cells, the specific killer cells, which are also known as CD8+ T cells and multiply prodigiously and mature in response to an infection, can also exhibit stress symptoms and thereby potentially end up on the NK cells’ hit list."
The researchers the author further noted "...discovered this using mice, which they infected with two model viruses. If the animals’ CD8+ T cells lacked these interferon receptors, not only did the NK cells eliminate the virus-infected cells, but also the immune cells that were supposed take action, thereby weakening the antiviral immune response." The cell population amounts moved in different directions.
As a result of their Phase 1 feasibility study of PV-10 in humans Moffitt showed CD8+ T cells and NKT cells both increased in population (on a statistically significant basis, p=0.008 and p=0.01, respectively) following PV-10 injection.
Effect Size Matters (June 24, 2014)
Effect size is a measure of strength (of something over something else). In the context of a clinical trial where the responses of two groups (a control group and a test drug one) are being compared, the difference in response between that of the control group and that of the test drug group is known as the effect size.
Should the control and test (treatment) groups be close in response, and thus the test drug has a small effect (i.e., it is a less efficacious agent), a large or larger number of patients are needed in order for the test drug to distinguish itself; that is, for the confidence interval (say 90% or 95% interval) of the test drug to not overlap or run into the confidence interval of the control. Conversely, if the test drug is very effective, a large effect size may anticipated, and thus a relatively small number of trial patients are needed; that is, the confidence interval of the test arm, in this case, may be large without overlapping the confidence interval of the control arm. The fact Provectus only is utilizing 210 patients in its pivotal Phase 3 trial signals this study assumes a relatively large effect size for PV-10 (i.e., PV-10 is very efficacious). Generally speaking, the more effective a drug is the less patients would be needed (without taking into account other factors that might influence clinical trial design) in a trial.
More effective, less patients needed. Less effective, more patients needed.
Consider the spreadsheet table below, which quantifies the above discussion. In order to generate the same difference of patients helped (64 is between 62-67), the 15% "effective" drug requires a trial of 750-800 patients (774 to be precise) . The 30% "effective" drug, which is better than the 15% drug, needs 350 patients to achieve the same difference of 64 patients. The 50% "effective drug, which is better still, needs an even smaller number of patients in its trial (200).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
From 300 (below):
 |
| Click to enlarge. Chemoablation of Metastatic Melanoma with PV-10 4th Interdisciplinary Melanoma & Skin Cancer Centres Meeting November 2010 |
 |
| Click to enlarge. Efficacy of intralesional rose bengal in patients receiving injection in all existing melanoma in phase II study PV-10-MM-02 ASCO 2014 |
 |
| Click to enlarge. OPTiM Trial in Stage IIIb/c and IV Advanced Melanoma 2009/2010 |
"We are at the beginning of a revolution in immunotherapy..." (June 21, 2014)
Johns Hopkins University's Dr. Elizabeth Jaffee, M.D. said in her January keynote lecture at the 2014 Gastrointestinal Cancers Symposium that “We are at the beginning of a revolution in immunotherapy...Technologically, the advances that have been made are of the same magnitude of technical advances as Apple brought to communications.”
 |
| Come Sail Away, Styx |
Eric conveyed a key viewpoint in his comments on the call. Paraphrasing, he said you do not start clinical trials unless you can predict their outcomes. This statement reinforced for me that, notwithstanding the special protocol assessment ("SPA") the company secured in 2012/2013 for a pivotal metastatic melanoma Phase 3 trial, he was not convinced that trial would have been successful given the then parameters of [primarily, I think] endpoints and endpoint definitions, [and secondarily] patient population, dosing schedule, and PV-10 product supply. See What does the fool say? (June 18, 2014) below. Now, he believes he has a trial design (e.g., patient population, endpoints and endpoint definitions, dosing schedule, product supply) that should lead to a successful outcome (potentially, I believe, a material probability of the trial being stopped early).
While I have to yet to post my financial analysis and projections related to estimated operating costs (that include other clinical development programs) and pivotal Phase 3 trial expenses, it appears a base case trial cost could range from $4 to $5 million, or $20,000 to 25,000 per patient (assuming 210 U.S., Australian and Western European patients). This is lower than the approximately $38,000 per patient for Provectus' 80-patient metastatic melanoma Phase 2 trial (see p. 23 here), which included PV-10 drug substance and drug product cost that are not necessary for or applicable to carrying out the Phase 3 trial, various inefficiencies that management says have been streamlined in regards to the pivotal trial, and certain R&D overhead that was attributed to Phase 2 trial costs whereas such overhead now is incorporated in operating costs. Total and per patient costs may be lower still depending on the proportion of Indian and/or Chinese patients in the trial and the amount of such cost borne by the respective regional pharmaceutical partner (i.e., $3 million, say, for 210 patients based on certain assumptions). Since cash outflow lags expense recognition, trial expenses would be spread out over time because of this and as a function of the enrollment rate. Additionally, the number of patients for Provectus to reach its interim assessment period is of course not 210 but likely around half of this. This would appear to bolster management's guidance they have sufficient cash on hand to reach and communicate the pivotal trial's interim analysis.
It would appear Provectus can start preparing the trial as soon as the protocol is filed (i.e., the FDA just needs the protocol to be filed). There are, however, likely to be administrative deals with various initial sites, contract research organizations ("CROs") and the independent data monitoring committee that would need to be completed before the trial could operationally commence. Compassionate use program or expanded access sites ("CUP") should be able to come on-line more quickly because they already have PV-10 supply and are used to protocol requirements similar to the PV-10 Phase 3 trial arm. Eric clearly established the industry and clinical relevance of the trial, as well as the relevance and appropriateness of the patient population, primary and secondary endpoints, and dosing schedule of PV-10 (he also noted the company's preparedness to have PV-10 under the new patented formulation readily available for trial use at the seven existing CUP sites: St. Luke's Hospital and Health Network (Bethlehem, PA), M.D. Anderson Cancer Center (Houston, TX), University of Louisville (Louisville, KY), Sharp Memorial Hospital (San Diego, CA), Melanoma Institute Australia (Sydney, Australia), Princess Alexandra Hospital (Brisbane, Australia) and Royal Adelaide Hospital (Adelaide, Australia). H. Lee Moffitt Cancer Center & Research Institute (Tampa, FL) undoubtedly will be a site (as Eric mentioned, enrollment rate assumptions included modeling of Moffitt's experience from its Phase 1 feasibility study). It will be interesting to see what additional U.S. sites would be added, such as Memorial Sloan Kettering Cancer Center (New York, NY), UCLA's Jonsson Comprehensive Cancer Center (Los Angeles, CA), etc. It also will be interesting to see what Western Europe, Indian and/or Chinese sites could be in the mix. For example, Dr. Axel Hauschild, M.D., Ph.D may be an investigator and University Hospital Schleswig-Holstein (Kiel, Germany) may be a site. It also would appear India and/or China site participation in the pivotal Phase 3 trial is sufficient for registration and approval of the drug (assuming a successful trial of course) in those geographies.
Getting back to Dr. Jaffee likening "...the revolution in immunotherapy to the same excitement as the Beatles brought to music, or the same magnitude of technology advances made by Apple," I think the pharmaceutical industry is catching on to what PV-10 already has begun to show.
 |
| Click to enlarge. Dr. Elizabeth M. Jaffee Discusses Future Directions in Immunotherapy Treatment for GI Cancers in Friday Keynote (January 2014) |
 |
| Click to enlarge. Dr. Elizabeth M. Jaffee Discusses Future Directions in Immunotherapy Treatment for GI Cancers in Friday Keynote (January 2014) |
Helping patients generate more T-cells makes other therapies more effective and, frankly, more relevant. Recall that Moffitt's ASCO 2014 poster noted about PV-10 that "[o]ne to two weeks after injection significant increases were detected in peripheral blood T-cells, including CD8+ (p=0.03), CD4+ (p=0.06), CD3+ (p=0.03), and NKT (p=0.05)." PV-10's immunologic activity is surprisingly robust, which probably is why Moffitt's Dr. Jeffrey Weber said "PV-10 might offer the perfect way to prime the immune system."
Interestingly, Dr. Jaffee's pancreatic cancer work with GVAX -- irradiated tumor cell lines engineered to recruit immune cells by expressing GM-CSF (so, in the case of Dr. Jaffee's work, the cell lines were pancreatic ones) -- generated neither complete nor partial responses in tumors, yet immunotherapy combination (in this case a live but attenuated Listeria monocytogenes strain) improved overall survival. The vaccine appears to have made the immunotherapy and its use more relevant and effective.
The Phase 1b study of the combination of Amgen's T-Vec (an intralesional agent) and Bristol-Myers' ipilimumab (a systemic immunotherapy and immunomodulatory agent) generated an overall response rate ("ORR," CR+PR) of 41% (24% CR or complete response, 18% PR or partial response), and 35% SD or stable disease. By itself ipilimumab in its Phase 3 trials generated ORRs of 5-11%. In its Phase 2 trial T-Vec generated an ORR of 28% and CR of 20%. This presumably led researchers to conclude "[i]n consideration with published reports, these data, although preliminary, suggest higher CR and OR rates than either agent alone and earlier responses after ipi initiation during T-VEC+ipi than with ipi alone."
For all disease treated, PV-10 achieved a 71% ORR -- 50% CR and 21% PR -- and total disease control was 82% (which would be, for the T-Vec + ipi trial above, 24% + 18% + 35%, or 77% total disease control). Moffitt in its Phase 1 feasibility study was able to achieve a 100% ORR in injected lesions (50% CR and 50% PR). I think Dr. Weber knows just how good PV-10 can be in combination with systemic immunotherapies.
What does the fool say? (June 18, 2014)
Motley Fool author Sean Williams wrote an article earlier this week that included a mention of Provectus. He said:
"Wholly clinical-stage biopharmaceutical company Provectus Biopharmaceuticals only debuted on the NYSE Arca exchange a month ago, moving from the over-the-counter boards, but it has garnered a lifetime's worth of criticism in that short time.
The primary allure for optimists in Provectus is its clinical-stage melanoma drug PV-10. According to data released in October 2012 from Provectus' midstage study, 51% of patients injected with PV-10 had an objective response: 26% with a partial response and 25% with a complete response (no trace of cancer detected). Including those who exhibited stable disease, total disease control was 69%. These results were incredibly promising, and PV-10, as expected, was much more effective in patients when their melanoma was discovered in stage three rather than stage four. Median overall survival for stage three patients was just beyond a year, while it was 7.3 months for stage four patients.
So if the data is so good, why did Provectus plunge as much as 90% at one point over just a few days last month? The evident answer to that question, and the reason for the daily collection of possible class action lawsuits against the company, is its failure to garner the breakthrough therapy designation from the Food and Drug Administration. This denial essentially means that PV-10's only way to market is through a costly and long phase 3 study.
However, what really has short-sellers salivating and other investors like myself who are on the sidelines watching this story develop confused, is the unexplained delay between the company's phase 2 study and the beginning of phase 3 enrollment. Provectus has announced that it plans to discuss how to implement its PV-10 phase 3 study this week during a conference call; nonetheless, it has been years since the company reported initial PV-10 data, yet management has taken no action to advance the drug. Honestly, it's just a bit weird that management has waited this long, especially considering how positive the data turned out to be.
It's obvious that the FDA and Provectus haven't been on the same wavelength before, which allows me to remain skeptical of this company until I see patients being enrolled and dosed."The data the author references comes from Provectus' poster presentation Immuno-Chemoablation of Metastatic Melanoma with Intralesional Rose Bengal (PV-10) at the ESMO (European Society for Medical Oncology) 2012 Congress. Results were even more promising when the company conducted a subgroup analysis of the Phase 2 trial for patients for whom all of their melanoma disease was treated rather than the above mentioned intent to treat or ITT population (presented at the European Cancer Congress 2013 (ECCO 17- ESMO-38 - ESTRO 32) in September 2013): 71% of patients injected with PV-10 had an objective response rate ("ORR"): 50% with a complete response ("CR") and 21% with a partial response ("PR"). Including those who exhibited stable disease ("SD"), total disease control was 82%.
It does seem heretofore like management has not been on the same wavelength as the FDA (as that wavelength seems to have shifted over time) until the company's December 16, 2013 meeting with the Agency (the subsequent press release regarding meeting minutes is here), when it first appeared the FDA was willing to entertain the application of PV-10 for loco-regional disease and an applicable label (i.e., locally advanced cutaneous melanoma), which management had been seeking but previously could not establish with the Agency.
Locally advanced cutaneous melanoma is not metastatic melanoma.
"Melanoma that occurs on the skin, called cutaneous melanoma, is the most common type of melanoma. This type of melanoma occurs in all parts of the skin, including the soles of feet, on the palms of the hand, in between toes and fingers, and underneath the finger and toe nails" (Source: Cutaneous Melanoma, or Melanoma of the Skin, Melanoma Research Foundation). {Bold and underlined emphasis is mine}
"The incidence of primary cutaneous melanoma has been increasing dramatically for several decades" (Source: Guidelines of Care for the Management of Primary Cutaneous Melanoma, a work group of the American Academy of Dermatology, 2011).
"For patients with primary cutaneous melanoma, the term "locoregional metastases" includes local recurrences, in transit and satellite metastases, and regional lymph node metastases" (Source: Cutaneous melanoma: Management of in transit metastases, UpToDate, 2013-2014). {Underlined emphasis is mine}
Vical began its pivotal melanoma Phase 3 trial for intralesional ("IL") agent Allovectin-7 (Stage III/IV patients) in 2006. This trial utilized ORR (i.e., CR + PR) at > 24 weeks as the primary endpoint. Overall survival ("OS") was a secondary endpoint. BioVex (acquired in 2011 by Amgen) began its pivotal melanoma Phase 3 trial for IL agent OncoVEX GM-CSF/talimogene laherparepvec (also Stage III/IV patients) in 2009. This trial utilized durable response rate ("DRR"), "the rate of CR or PR lasting continuously for 6 or more months," as the primary endpoint. OS was a secondary endpoint. Provectus began its melanoma Phase 2 trial of Stage III/IV patients in 2007 utilizing a trial design focused on ORR (the Phase 1 trial began in 2005).
 |
| Clinical Development at Provectus, March 2009 |
"Cutaneous melanoma (CM) is considered to have a high malignant potency. Metastatic spread may arise from very small tumors" (Source: Time Course and Pattern of Metastasis of Cutaneous Melanoma Differ between Men and Women, PLoS One, 2012).
For all disease treated, PV-10 achieved a 71% ORR -- 50% CR and 21% PR -- and total disease control was 82%.
Allovectin-7's Phase 2 trial comprised 53% Stage 3 patients and 47% Stage 4, while T-Vec's Phase 2 trial comprised 20% Stage 3 patients and 80% Stage 4. PV-10's Phase 2 trial comprised 77.5% Stage 3 patients and 22.5% Stage 4. Although Provectus' Phase 2 trial included some patients with systemic and visceral disease, patient selection was focused on those where PV-10 might achieve loco-regional disease control and thus forestall or prevent metastatic disease. Typically the associated patient staging would be Stage IIIB-C and IVM1a. T-Vec's Phase 3 trial patient staging, however, comprised 30% Stage IIIB-C and 70% Stage IV (27% M1a, 21% M1b and 22% M1c 22%).
The approval of Bristol-Myers' ipilimumab (Yervoy) for unresectable or metastatic melanoma in 2011 seems to have shifted the melanoma playing field towards OS and away from ORR. Ipilimumab achieved ORRs of 5-11% in trials where the vast majority of patients (>70%) had Stage IV M1c disease (i.e., visceral). Although "a measurable response to therapy (SD < PR < CR) that is durable and leads to an increase in PFS, which in turn allows a significant increase in OS," management could not convince the FDA non-OS endpoints were more suitable to ascertain success at achieving local-regional disease control or defeat.
In 2012 (and 2013 when the company appears to have been granted a special protocol assessment by the FDA for a pivotal Phase 3 trial) Provectus seemed to inch the ball forward towards more suitable primary endpoints with progression free survival ("PFS") and "progression defined to include increased tumor burden, advent of active nodal disease, onset of distant metastasis and symptomatic deterioration based on validated instruments." But, as Eric said on the May 23rd conference call regarding work with the Agency subsequent to this: "And what it shows is that as our understanding of PV-10 has matured over time and as the melanoma landscape has evolved over time, our interaction with the Agency has improved over time in that we now are in a position that the Agency has helped us to define indications that they believe our drug shows potential value and have helped us to define types of end points that are vastly different than perhaps progression-free survival in the proposed phase three randomized control trial from the third type [B] meeting, which would have used DTIC as a comparator. It's impossible to run that study [under] the current climate. We wouldn't enroll patients with the appropriate, uh, extent of disease burden." See my blog post "Why did it take four years...to arrive at this point?"
Subject to confirmation on tomorrow's conference call, the contemplated pivotal Phase 3 trial's primary endpoint appears to be PFS with progression defined (via RECIST 1.1) for the PV-10 arm and the systemic chemotherapy comparator arm as achieving either CR (the target lesion goes away) or PD (aka progressive disease, where the target lesion has increased in diameter by at least 20%). This would seem to be a better and more applicable endpoint and endpoint definition for PV-10 to strive. OS remains a secondary endpoint.
Now, finally, are the FDA and management on the same wavelength as it relates to suitable primary endpoints to measure local-regional disease control for cutaneous melanoma (rather than overall survival ones typically utilized for metastatic melanoma)? Provectus has persisted in seeking appropriate endpoints and a trial design that provide the potential for a higher probability of a successful outcome in a continuously changing landscape of melanoma disease treatment. It's understandable after a long and winding road the Foolish author and others would wait until the presumed pivotal cutaneous melanoma Phase 3 trial commences before reassessing their investment decision in Provectus.
Various (June 16, 2014)
Why did the FDA denial (of breakthrough therapy designation) letter contain "For further information regarding Fast Track, refer to the draft guidance noted in the above paragraph?"
In the same letter, having "determined that treatment of “locally advanced cutaneous melanoma” meets the criteria for a serious or life-threatening disease or condition," is the preceding a label-like description? Having noted "preliminary clinical data provided...are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma, is the preceding not a specification of applicability to an NCCN Guideline population (or clinical/pathologic stage) (page ME-8)?
The company moved closer to a settlement of the compensation lawsuit, filing an 8-K last week (a settlement was entered into subject to court approval and a settlement hearing). The previous communication on the topic came from the 10-K filed in March.
Defining Melanoma, and Other Thoughts from Union Square* (June 14, 2014)
Opposing views to one's trading or investment thesis are worthwhile to read (hear) and review in order to understand the other side of the trade. They can reinforce or expand on a bull or bear's existing views of thesis wrongness (e.g., see section But what if I’m wrong? in under the blog's Why I'm Long Provectus Biopharmaceuticals tab). They can encourage or force consideration of new wrongness or potential risks, short- and/or long-term. They also can help re-focus on or remind one of key aspects of a bullish (if they're bearish) or bearish (if they're bullish) viewpoint. The latest bearish article on Provectus is here.
I'm struck by two themes or lines of thinking it does and can foster. First, despite multiple uses of the word melanoma, one walks away with the impression the disease is homogeneous in its striking of victims, and that it no longer is a serious or life-threatening disease or condition; that current approved and investigational drugs, aside from PV-10, have it handled. Second, it did help to remind of the potential critical importance and the implications therein of immune priming for the therapeutic treatment of solid tumor cancers.
Melanoma like other cancers can be categorized into different stages and sub-stages, which describe the location of the disease and the virility of its spread (e.g., Stages 0 (in situ), IA, IB, II, III, III (in transit), IV (metastatic), etc.). There are other ways to describe the different stages and levels of disease severity, and the number of people afflicted with it. For example, localized melanoma confined to primary site represented 84% of cases, 9% were regional melanoma spread to regional lymph nodes, and distant melanoma where cancer had metastasized was 4% (unknown or unstaged determination was 3%); see SEER 18 2004-2010, All Races, Both Sexes by SEER Summary Stage 2000. I think the article meant for melanoma to mean advanced melanoma or metastatic melanoma. The melanoma tumor or lesion microenvironment is much more heterogeneous than that of other solid tumor cancers. Treating advanced and metastatic melanoma remains a challenge. Moffitt Cancer Center's Jeffrey Weber recently commented on a presentation by Dr. Steven Rosenberg, M.D., Ph.D., Chief of Surgery at the National Cancer Institute saying "Checkpoint inhibitors are quickly becoming the standard of care for metastatic melanoma, but 50 to 60% percent of patients do not benefit from these agents." He went on to say (paraphrasing) patients who are refractory to these therapies have few, if any, other treatment options. Treating advanced or metastatic melanoma or melanoma in an adjuvant setting, which in the case of an ipilimumab (Yervoy) trial referred to completely resected high-risk Stage II and III melanoma patients, also can be very expensive situations. At the dose and administration schedule used in the aforementioned Yervoy trial, "...this amounts to a price tag in excess of $500,000 for the first year and over $1 million for the full three-year course of therapy." Weber estimated "...the full regimen used in the trial would cost as much as $1.5 million for a single patient..." The cost of a cancer treatment vs. its value was a topic of discussion at the 2014 annual meeting of the American Society of Clinical Oncology ("ASCO"). A treatment dose of Bristol-Myers Yervoy can cost $120,000, while Merck's MK-3475 (pembrolizumab) may cost $240,000, and Bristol's nivolumab might be somewhere in between (for sources, see Revisiting % CR per thousand dollars of treatment cost, a.k.a. ASCO's "...cold-eyed advice on a raft of cancer treatments" (March 26, 2014) under the blog's Archived News tab) A article about advanced melanoma worth reading is Tales from ASCO: Q&A with MRA’s Chief Science Officer Louise Perkins.
For metastatic melanoma immune priming through combinations of drugs as well as combinations of approaches could present effective solutions for patients with very late stage disease refractory to prior agents and therapies. Amgen and Bristol-Myers Squibb are conducting a study of T-Vec combined with ipilumumab, and Amgen and Merck are planning a study of T-Vec combined with pembrolizumab (Did Hauschild misspeak again?). I wrote about T-Vec limitations below in PV-10 & Amgen's Talimogene Laherparepvec (June 9, 2014). More broadly speaking, could immune priming using an intralesional agent ("IL") present opportunity across the spectrum of cancer treatment stages and solutions? For example, consider the potential for an IL agent to be administered before surgery to in one instance facilitate some amount of transition of unresectable to resectable. The addressable market simply might be (the number of therapeutic treatments) times (the number of applicable solid tumor cancers) x (the cost or price of the IL agent utilized with each treatment).
As for Provectus, management's conference call on June 19th and answers await.
* In a Starbucks. My venti Americano (espresso with my half-and-half, please) just ran out...
PV-10 & Amgen's Talimogene Laherparepvec (June 9, 2014)
PV-10 and T-Vec (talimogene laherparepvec) are investigational intralesional ("IL") therapies that have shown evidence of bystander responses, as are/were coxsackievirus A21 and velimogene aliplasmid (aka Allovectin-7), as discussed by medical doctors Howard Kaufman (Rutgers Cancer Institute of New Jersey), Merrick Ross (MD Anderson), and Jeffrey Sosman (Vanderbilt-Ingram Cancer Center) at a not-an-official-event of ASCO 2014 entitled Intralesional Therapy for Unresectable Melanoma: Current Approaches and Novel Treatment Options.
T-Vec completed its pivotal melanoma Phase 3 trial and a melanoma Phase 1b combination study with Bristol-Myers' ipilimumab (i.e., combination safety and tolerability). Planned metastatic melanoma combination trials include a randomized Phase 2 study of T-Vec & ipi and a Phase 1b/2 study of T-Vec & Merck's MK-3475. Allovectin-7 failed its pivotal Phase 3 trial. PV-10 should commence its pivotal Phase 3 trial in 3Q14, and coxsackievirus A21 is a Phase 2 drug.
As I wrote in my "IL PV-10 may be rationally combined with systemic immunotherapy for the treatment of metastatic melanoma" blog post, Dr. Axel Hauschild, M.D., Ph.D. (University Hospital Schleswig-Holstein), a member of OncoSec's Melanoma Advisory Board, believes (paraphrasing) there is a role for IL agents in combination with other agents to treat metastatic melanoma because they have no systemic toxicity, have high CR rates, and are good candidates for combinatorial use due to immune priming.
Complete response ("CR") rates for both patients and treated (injected) lesions are presented below for three IL agents: Amgen's T-Vec, OncoSec's IL-12 EP, and Provectus' PV-10. Keeping Hauschild's comments in mind, treated lesion CRs ranged from 45% (IL-12 EP) to 74% (PV-10). As an aside, patient CRs ranged from 11% (IL-12 EP) to 50% (PV-10).
 |
| Click to enlarge |
Provectus' management believe it is critical to treat as many accessible lesions with PV-10 as possible. The more lesions you inject, the more of them go away (i.e., PV-10's high complete response results from the drug's very effective and rapid ablation, the first of its two-step mechanism of action ["MOA"]) -- a very important aspect of the likely pivotal melanoma Phase 3 trial protocol (i.e., inject all accessible lesions until they achieve complete response) -- and the more lesions you inject, they believe, the more you expose the immune system to as many antigens (fragments resulting from rapid tumor ablation caused by PV-10 injection) in context (i.e., PV-10's immunologic activity, the second of the two-step MOA). Given the very high amount of heterogeneity of a melanoma tumor microenvironment, this exposure should increase with each treated lesion. The more lesions are injected, the greater the potential for even more antigens to be expressed in context to the immune system. The company's Phase 1 and 2 trial protocols limited injection flexibility; however, a subgroup of the Phase 2 trial's patients (28 of 80) had all disease treated (i.e., all lesions injected).
I think its safe to say, in PV-10's case, more is definitely better. More injection volume (predicated on tumor size). More injected tumors (all accessible ones).
Why didn't Amgen researchers and clinicians treat all lesions in T-Vec's pivotal Phase 3 trial? They couldn't because the company obtained T-Vec (formerly OncoVEX) by buying BioVex. The protocol of the pivotal Phase 3 trial had already been finalized by BioVex prior to the company's acquisition by Amgen. BioVex management probably didn't think along the same lines as Provectus management regarding using an intralesional agent to prime the immune system by injecting as many tumors as possible in order to prime it as optimally as possible. Perhaps the planned T-Vec combination studies will include having all accessible lesions injected in their respective protocols.
It also may be that T-Vec's ability to prime the immune system simply is not as robust as PV-10's. Untreated (or bystander) lesion CRs ranged from 22% (T-Vec) to 54% (PV-10); IL-12 EP had none. Patient CRs ranged from 6% (T-Vec) to 23% (PV-10); IL-12 EP again had none. The N for PV-10 here of 28 is different from the N above of 26 (i.e., different patients).
 |
| Click to enlarge |
Regulatory affairs experts with seasoned FDA dealings, like Provectus' vendor Roberti+White, are better placed to appropriately and accurately assess the impact of the Agency's new May 2014 Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics, particularly on the breakthrough therapy designation ("BTD"). Prior guidance, I believe from June 2013, may be found here. Did Provectus and other companies get caught up in changes in guidance, specifically [in the company's case] as they related to BTD? Did Provectus's anticipated BTD outcome expected on or around May 23rd get waylaid as a result of guidance changes that resulted in the Agency's May document? It doesn't matter now and I don't believe going forward (before, and at the time, I wondered what would come with BTD), although it may provide some helpful insight.
For example, it is interesting to note the following change to clinically significant endpoints for BTD. In May [2014], guidance was "An effect on an established surrogate endpoint that typically would be used to support traditional approval" (page 13) while from June 2013 it was "An effect on an established surrogate endpoint" (page 12) {underlined emphasis is mine}.
Provectus management has long sought accelerated options and paths to regulatory approval, which has manifested itself as much lower capital spending on clinical development than many other companies and situations. Unfortunately, capital formation -- the strategy and tactics for/to which require all management team members to contribute in their respective positional roles and incorporate their respective operational responsibilities -- thus far has been far from optimal.
Nevertheless, the past is the past. Risk-reward going forward is attractive to me. It appears Eric now may have (subject to filing a final protocol that would be acceptable to/accepted by the FDA) a direct, straightforward, unambiguous and very attainable path sufficient for the approval of PV-10 for locally cutaneous advanced melanoma.
The Path [for Melanoma] (June 7, 2014)
 |
| The Path |
The path is a pivotal Phase 3 trial, where pivotal is a study design that would provide sufficient evidence necessary for drug approval.
The trial design/protocol has its pros (e.g., standard endpoints like progression free and overall survival ["PFS" and "OS," respectively] assessed using standard RECIST 1.1 criteria, which is the current version of RECIST), and there appear to be both great opportunity (e.g., a patient population with very limited treatment options, the potential for trial stoppage [early or otherwise]) and challenges (e.g., the patient enrollment rate to reach accrual goals, sufficient cash balances necessary through trial completion and not just through an interim data readout) in conducting it.
Eric provided an initial estimate of n=210 for the trial's patient number; a two-to-one randomization means about 140 patients in the PV-10 arm and about 70 patients in the control arm. I think this low number is notable (particularly in the context of a 90% powered trial). The trial protocol will have to be filed with and accepted by the FDA (as such, the aforementioned n, while likely, still is preliminary). PV-10's effect factor, it's ability to achieve high levels of complete response (and thus long PFS), can, biostatistically, warrant a lower patient number (i.e., a lower n) than other less effective agents and therapies. Below is a table of selected examples (i.e., based on a cursory review) of patient numbers for metastatic melanoma Phase 3 trials.
 |
| Click to enlarge |
It doesn't appear either Provectus or Moffitt Cancer Center will release their PV-10 posters (abstracts may be found here and here, respectively), as material from them as well as other related or associated study information likely should be published in peer-reviewed publications this year.
Moffitt concluded their Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions ASCO poster with:
- "IL PV-10 can induce regression of injected and uninjected metastatic melanoma
- IL PV-10 can enhance tumor-specific reactivity in circulating T-cells
- IL PV-10 leads to responses in treatment refractory tumors
- IL PV-10 may be rationally combined with systemic immunotherapy for the treatment of metastatic melanoma."
(3) means patients had melanoma tumor that did not respond to other kinds of approved and investigational therapies but that responded to PV-10. "Of the 13 consented patients [in Moffitt's feasibility study], 5 had no previous treatment, 6 received ILI or ipilimumab [sold as Yervoy], and 2 received PD-1 blocking antibody; 6 received two or more prior systemic therapy." ILI refers to isolated limb infusion. Other systemic therapies included vemurafenib (sold as Zelboraf), temozolomide or "TMZ" (an orally administered systemic chemotherapy that will be used as a comparator in the company's upcoming Phase 3 trial), and carbotaxol (another systemic chemotherapy).
(4) should suggest PV-10 may be used in combination with certain other systemic immunotherapies (i.e., anti-CTLA-4 and anti-PD-1 agents), presumably for greater benefit than the individual treatments alone, for patients with metastatic melanoma. This conclusion likely reflects successful murine model work conducted at Moffitt that likely was led by Jeffrey Weber (a co-author of the abstract and poster).
It is about time Eric publishes Provectus' metastatic melanoma Phase 2 trial data (this evolution is a good start). There is a portion of the biotech world for which publishing the data in a reputable oncology journal (e.g., New England Journal of Medicine, Journal of Clinical Oncology, Nature, British Medical Journal, The Lancet, Lancet Oncology, American Association of Cancer Research publications, etc.) is very meaningful. A good to great choice of journal for the company's PV-10 data (i.e., it must be top to very top tier), would begin a much needed process of repairing management's credibility (i.e., for this item, further establishing the veracity of the data through the peer review process of a very respected/high quality journal).
Blog readership rose about 30-40% from April 2014 depending on the statistic. I wrote 36 blog posts (8) and news items (20) in May versus 25 the previous month (5 and 20, respectively). May blog readerships stats month-over-month changes were:
- +41% for # of unique visitors (4,365 v. 3,105),
- +39% for # of page views (48,963 v. 35,171),
- +31% for # of visits (19,839 v. 15,096),
- +32% for # of U.S. cities [from where visitors came] (1,220 v. 926),
- +37% for # of world cities (239 v. 174), and
- +32% for # of countries (66 v. 50).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
In addition to his discussion (on the May 23rd conference call) of the history of the company's regulatory interactions with the FDA since the end of Provectus' metastatic melanoma Phase 2 trial about four years ago to arrive at this point (see my blog post "Why did it take four years...to arrive at this point?,", "Quote A"), another informative and useful set of comments followed from another question to Eric.
As with the above mentioned post, I edited the transcript to remove "so," "um" and "uh," and made a few other minor verbiage changes to make the material below ("Quote B") more readable.
Quote A is Eric's recounting of the company's historical regulatory interaction with the FDA through the breakthrough therapy designation ("BTD") denial. Quote B provides insight, in my view, into the process of and deliberation on submitting for BTD.The longer version of my answer is that definitely we were very encouraged by the guidance that we received from the Agency in that meeting. That being said I described myself recently as being a professional worrier. Maybe that’s good for someone who is responsible for clinical development in a small company. But, I was worried about being able to conclusively demonstrate a correlation between this high level of objective response, and I’ll use that loosely. What I mean by that is objectively observable response, uh, evidenced by complete responses in patients. All of their disease is gone after PV-10 injections in 50 percent of those patients versus what I knew was very thin data concerning the types of symptoms that the Agency was suggesting should be shown improvement in. We did our best with that study. Looked at, as I mentioned earlier, pain data, and we were able to draw some supporting evidence to show that there is definitely a trend in pain data that matches the trend in objectively observed response of tumors. The Quality of Life EORTC-QL2-C30 instrument, this 30-question questionnaire that’s principally designed for patients with late stage systemic disease, maybe that are taking toxic chemotherapy, I had great concerns that that was not going to be valuable because we had already shown in the full analysis for patients in studies that there were no clear trends evident other that the patients didn’t get worse, and that proved to be correct. There was nothing that we could extract from that particular instrument. It was a measured risk submitting the application. But again as I mentioned earlier, our logic seemed clearer that if we were making the patients' tumors disappear in 50 percent of the patients that was a very large effect size, and that was tantamount to making any symptoms that they might have been suffering from the tumor burden disappear.
Returning to some fundamental questions related to history, and fundamental questions I hope are answered or begun to be answered by Eric on the company's June 3rd conference call (the list below is far from exhaustive):
- Did the FDA guide Provectus to submit a BTD application?
- We also presented a straw man outline for Breakthrough Therapy Designation request and asked for advice on such requests. To our surprise, in the meeting the Agency focused on the [ECC] 2013 subgroup analyses and clinical response evidenced in these patients via clinical photography.
- The longer version of my answer is that definitely we were very encouraged by the guidance that we received from the Agency in that meeting.
- Did the Agency agree certain clinically significant endpoints or endpoints indicative of clinical benefit were appropriate to measure local and intralesional agent PV-10?
- We had a lengthy discussion regarding patients with locally advanced cutaneous melanoma, the need for therapies for these patients, and the types of end points appropriate for demonstration of clinical benefit. Based on our positive impression and discussions from the meeting in the meeting minutes released a month later, we prepared and submitted our Breakthrough Designation application in March [2014].
- And what it shows is that as our understanding of PV-10 has matured over time and as the melanoma landscape has evolved over time, our interaction with the Agency has improved over time in that we now are in a position that the Agency has helped us to define indications that they believe our drug shows potential value and have helped us to define types of end points that are vastly different than perhaps progression-free survival in the proposed phase three randomized control trial from the third type [B] meeting, which would have used DTIC as a comparator.
- Do the above endpoints provide or present a pathway to approval if met or exceeded? To be answered...
- Is a so-called bridging study, i.e., the vehicle to gain more information for the FDA, the final clinical step prior or providing the opportunity to file a new drug application ("NDA"), or is it a step-to-a-step, a pivotal trial that itself then would lead to an NDA filing? To be answered...
The June 3rd conference presents itself as a very critical opportunity for Eric to advance the narrative of supportive FDA and the specifics of an initial pathway to approval for PV-10.