News items from July 2013 to May 2014.
"...over existing therapies on one or more clinically significant endpoints." (May 28, 2014)
What was the FDA referencing when it noted existing therapies in its letter response to Provectus? Specifically: "does not indicate that the drug may/the preliminary clinical data do not demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints." The endpoints appear to be pain, bleeding, and significant infection. But what are the existing therapies?
For locally cutaneous advanced melanoma, one of the populations highlighted in the letter, see Risks and Opportunities (May 27, 2014) below, melanoma symptoms appear to include bleeding and pain. See, for example, here and here. Melanoma surgery's complications can include pain, bleeding and infection. See here and here.
According to NCCH Guidelines (January 2014 version), primary treatments for Stage III melanoma, which I presume encompasses and/or is encompassed by locally advanced cutaneous melanoma are shown below:
Dr. Argarwala presented slides at the 10th European Association of Dermato-Oncology (EADO) congress that included two slides detailing Moffitt Cancer Center clinical study results from the center's AACR 2014 poster (the abstract is here), which has not yet been released (it should be released after ASCO 2014). The first slide presented pre- and 7-to-14 day post-treatment immunohistochemistry slides of injected and non-injected (bystander) tumor samples. Strikingly, the post-treatment slides did not show melA (melanoma)-expressed material. The second slide presented T and NKT cell populations (i.e., CD3+, CD4+, CD8+, NKT) pre-, 7-to-14 day post- and 21-to-28 day post-treatment. Populations spiked at the 7-14 day measurement before returning to what appeared to be pre-treatment levels at the 21-28 day measurement (within statistically significant p levels: 0.008, 0.023, 0.008, 0.01, respectively).
Risks and Opportunities (May 27, 2014)
Provectus' stock resumed trading today on the NYSE MKT, falling to $0.75 on trading volume of more than 15 million shares. The stock closed at $2.02 on May 22nd.
Additionally, a risk requiring monitoring materialized when the NYSE MKT announced it was reviewing the continued listing of Provectus' stock on the exchange in its NYSE MKT Reviewing the Continued Listing of the Common Stock of Provectus Biopharmaceuticals, Inc. press release. Should the company receive a listing deficiency letter from the NYSE MKT, there presumably would be a period of time (e.g., several weeks) for management to submit a plan of compliance outlining how they intend to regain compliance by some date (e.g., some number of months to quarters thereafter). This of course bears watching, where the risks or punitive aspects of delisting manifest themselves among other things as less and inconsistent liquidity, certain constituencies not being able to easily buy the stock, and higher costs of capital.
The company issued press release Provectus Biopharmaceuticals Inc. to Hold Conference Call Tuesday, June 3, 2014 (and filed this associated 8-K) noting they would:
Agreement? Then What? (May 26, 2014)
If, as I think the FDA letter response indicates, the Agency and Provectus appear to have finally agreed on endpoints for this new class of drug, a local intralesional agent that treats local-regional disease based on local endpoints, then "what's next," the outline of the path forward for melanoma, very likely has to be an additional study to collect more information to conclusively establish the link between complete response (tumor ablation) and symptom control. A key question for me, then, is to what would a successful study lead (and the associated, estimated time and expense); the position to file its new drug application ("NDA"), or a further study to put Provectus in a position to file its NDA?
What's Next? (May 25, 2014)
In the company's Provectus Biopharmaceuticals Inc. Reaffirms Its Commitment to Bringing PV-10 to Market Notwithstanding FDA Decision on Breakthrough Therapy Designation press release Provectus noted "We are carefully weighing the advice of the Agency and will provide an outline of the path forward for melanoma in our poster presentation June 2nd at ASCO."
The outline and detail of the development program going forward for melanoma will provide a perspective of cost, time, and potential path(s) to market for investors, and begin to re-establish stock market expectations. The FDA's letter certainly conveys the headline negative of no breakthrough therapy designation ("BTD").
It convey's two positives:
"...over existing therapies on one or more clinically significant endpoints." (May 28, 2014)
What was the FDA referencing when it noted existing therapies in its letter response to Provectus? Specifically: "does not indicate that the drug may/the preliminary clinical data do not demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints." The endpoints appear to be pain, bleeding, and significant infection. But what are the existing therapies?
For locally cutaneous advanced melanoma, one of the populations highlighted in the letter, see Risks and Opportunities (May 27, 2014) below, melanoma symptoms appear to include bleeding and pain. See, for example, here and here. Melanoma surgery's complications can include pain, bleeding and infection. See here and here.
According to NCCH Guidelines (January 2014 version), primary treatments for Stage III melanoma, which I presume encompasses and/or is encompassed by locally advanced cutaneous melanoma are shown below:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Moffitt noted in its ASCO 2014 abstract, "[t]reatment with IL PV-10 led to [pathologic complete response (pCR)] in the post-treatment biopsies of both PV10-injected and uninjected study lesions in 4 of the 8 patients, and all 8 exhibited at least partial regression of the injected lesion." As I wrote in my What PV-10 is doing is “unprecedented” blog post, PV-10 is "...under investigation as a non-surgical option to induce tumor regression of cutaneous neoplasms" by the cancer center.
I recall Eric saying on the May 23rd conference call he thought complete response (tumor ablation) is tantamount to symptom control (i.e., because the tumor went away in its entirety during the company's metastatic melanoma Phase 2 trial, referencing the subgroup of patients utilized for Provectus' failed breakthrough therapy designation application, there was no pain, bleeding or infection when the tumor went away). Intraslesional injection of PV-10 into a tumor does not have the complications or challenges melanoma surgery presents in terms of pain, bleeding and infection.
Moffitt found from its feasibility study that in and around the injected lesions there was no viable tumor tissue, and there was healthy tissue around the margins (there also were no tumor-infiltrating lymphocytes because the injected tumor went away faster than the originally planned for 7-14 follow-up period). This tumor tissue disappearance was confirmed by pCR (i.e., by immunohistochemistry and pahology, and of course not measurement).
What are the existing therapies to which the Agency refers in its letter to Provectus? If the FDA is just asking for some more data (as some regulatory experts who have opined suggest), how would Eric design such a study or trial (e.g., patient number, expense, time, single arm or double arm, comparator, etc.) to provide the Agency with it? And where would this study lead (e.g., a bridge to an NDA filing, a bridge to a pivotal trial that hopefully would lead to an NDA filing, etc.)?
So, for a local-regionally focused population utilizing local endpoints, PV-10 [with no treatment limitations] vs. surgery, maybe?
- PV-10: Injection, complete response/tumor ablation within a few days, pathology-confirmed disappearance of melanoma cells, no tissue destruction, outpatient setting, no pain, bleeding or infection, etc.
- Surgery: Excision, rescision, tissue removal, more tissue removal just to make sure, tissue destruction, no guarantee the surgeon got all of the melanoma cells, pain, bleeding and/or infection post-surgery, etc.
These and many other questions require answers from Eric and Provectus.
Risks and Opportunities (May 27, 2014)
Provectus' stock resumed trading today on the NYSE MKT, falling to $0.75 on trading volume of more than 15 million shares. The stock closed at $2.02 on May 22nd.
Additionally, a risk requiring monitoring materialized when the NYSE MKT announced it was reviewing the continued listing of Provectus' stock on the exchange in its NYSE MKT Reviewing the Continued Listing of the Common Stock of Provectus Biopharmaceuticals, Inc. press release. Should the company receive a listing deficiency letter from the NYSE MKT, there presumably would be a period of time (e.g., several weeks) for management to submit a plan of compliance outlining how they intend to regain compliance by some date (e.g., some number of months to quarters thereafter). This of course bears watching, where the risks or punitive aspects of delisting manifest themselves among other things as less and inconsistent liquidity, certain constituencies not being able to easily buy the stock, and higher costs of capital.
The company issued press release Provectus Biopharmaceuticals Inc. to Hold Conference Call Tuesday, June 3, 2014 (and filed this associated 8-K) noting they would:
- Update the market on the scientific content of the two poster presentations at ASCO, and
- Lay out their plans for "securing FDA approval for the use of PV-10 in the treatment of cutaneous melanoma."
As I wrote in my The FDA Response blog post, there seemed to be clear recognition by the Agency of the applicability of local/intralesional agent PV-10 for the treatment of locally advanced cutaneous melanoma, which the FDA deemed a serious or life-threatening disease or condition by virtue of: "We have reviewed your request and while we have determined that treatment of “locally advanced cutaneous melanoma” meets the criteria for a serious or life-threatening disease or condition, the preliminary clinical evidence you submitted does not indicate that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints." {Bold emphasis is mine}
In a respect, this is a population.
In a respect, this is a population.
Additionally, the FDA also seemed to acknolwedge the drug works, notably in malignant melanoma by virtue of: "The preliminary clinical data provided in your request for Breakthrough Therapy designation are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma; however, the preliminary clinical data do not demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints." {Bold emphasis is mine}
Again, in a respect, this is a population too, notably one discussed in NCCN guidelines (a 2013 version is here, the picture below is from a 2014 version):
Again, in a respect, this is a population too, notably one discussed in NCCN guidelines (a 2013 version is here, the picture below is from a 2014 version):
 |
| Click to enlarge. |
This contrast of populations, with overlap and no overlap is interesting. There is no mention of recurrent or recurrence in one population, and mention in another. One population is later stage (and perhaps out of solutions) than another. Management and in particular Eric's comments on the June 3rd conference call to elucidate what's next in the company's regulatory path for melanoma will be key to assessing risk and opportunity going forward.
Agreement? Then What? (May 26, 2014)
If, as I think the FDA letter response indicates, the Agency and Provectus appear to have finally agreed on endpoints for this new class of drug, a local intralesional agent that treats local-regional disease based on local endpoints, then "what's next," the outline of the path forward for melanoma, very likely has to be an additional study to collect more information to conclusively establish the link between complete response (tumor ablation) and symptom control. A key question for me, then, is to what would a successful study lead (and the associated, estimated time and expense); the position to file its new drug application ("NDA"), or a further study to put Provectus in a position to file its NDA?
What's Next? (May 25, 2014)
In the company's Provectus Biopharmaceuticals Inc. Reaffirms Its Commitment to Bringing PV-10 to Market Notwithstanding FDA Decision on Breakthrough Therapy Designation press release Provectus noted "We are carefully weighing the advice of the Agency and will provide an outline of the path forward for melanoma in our poster presentation June 2nd at ASCO."
The outline and detail of the development program going forward for melanoma will provide a perspective of cost, time, and potential path(s) to market for investors, and begin to re-establish stock market expectations. The FDA's letter certainly conveys the headline negative of no breakthrough therapy designation ("BTD").
It convey's two positives:
- "...we have determined that treatment of "locally advanced cutaneous melanoma" meets the criteria for a serious or life-threatening disease of condition...", and
- "The preliminary clinical data provided in your request for Breakthrough Therapy designation are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma..."
A more detailed negative in the letter is:
- "This determination is based on the paucity of data on endpoints indicative of clinical benefit (e.g., pain, infection, significant bleeding) and our inability to determine the clinical significance of the reduction in the size in one to 10 target lesions in patients with locally advanced melanoma, who may have additional untreated cutaneous, subcutaneous, or visceral sites of disease."
A positive interpretation of the above is that while the data Provectus submitted (the 54 patients on which the BTD application was based had no untreated disease with the exception of 1-2 untreated bystander lesions in 26 of these, and these bystander lesions were monitored over the study interval, and all were Stage III and monitored during the study interval for presence or onset of visceral disease) began to establish a connection or link (as the company noted in its May 23rd press release) between complete response and symptom-based endpoints (e.g., pain, infection, significant bleeding), it did not do so conclusively.
I think, specifically:
- "...the paucity of data on endpoints indicative of clinical benefit (e.g., pain, infection, significant bleeding)..." refers to not enough information on these endpoints, despite what the company provided in regard to pain from baseline, and
- "...our inability to determine the clinical significance of the reduction in the size in one to 10 target lesions..." refers to not enough information to establish that complete response was tantamount to symptom control.
As a result, "what's next" very likely is guided by the presumed need for a clinical trial to conclusively establish the connection/link/correlation between complete response (tumor ablation) and symptom control.
"...patients responded even after being refractory to the latest drugs including ipilimumab, anti PD-1 and/or vemurafenib" (May 21, 2014)
PV-10 data for break through therapy designation application highlighted by ASCO by ecancer reporter Janet Fricker, notably Eric's quotes:
- “These sub-analyses show PV-10- injection results are maximized when all lesions get treated. What we’re seeing here is a remarkable response in a heavily pre treated or refractory patient population with locally advanced cutaneous melanoma who have unmet medical needs since, unlike patients with more advanced disease, limited treatment options are available.”
- “These results support a premise that not only is PV-10 safe and effective, but unlike many other intralesional therapies, requires limited injections. This could prove highly advantageous for both patients and clinicians.”
- “What’s really powerful about these results is that patients responded even after being refractory to the latest drugs including ipilimumab, anti PD-1 and/or vemurafenib.”
Provectus added a new strategic advisory board member from Bayer Healthcare, a subsidiary of Bayer AG: Provectus Biopharmaceuticals Inc. Appoints Jacob M. Plotsker to Strategic Advisory Board.
The company issued a response to a negative Seeking Alpha article: Provectus Biopharmaceuticals, Inc. Refutes Inaccuracies in Seeking Alpha Article.
As I wrote in my Judgement Day(s) post, how can a local agent treat a systemic disease? There is growing evidence local delivery, rather than systemic, better leverages the cancerous tumor microenvironment. The tumor microenvironment matters because of heterogeneity within it, and the opportunity for robust, diverse antigen expression or presentation to more effectively harness the immune system. The granting of breakthrough therapy designation to PV-10 for locally advanced cutaneous melanoma (i.e., recurrent, in-transit or satellite melanoma that has not yet spread from the skin to distant sites) should go a very long way in changing the perception and reality of how cancer is and should be treated. Judgement day no. 2 comes by way of a decision on breakthrough therapy designation (on PV-10 as a monotherapy for earlier stages of melanoma) as it relates to treating cancer to deny, prevent or forestall the spread of metastatic disease. Different endpoints are appropriate and necessary for local-regional versus metastatic disease to better measure and determine success in both.
Moffitt Cancer Center is expected to address at ASCO 2014 their work on combining PV-10 with certain checkpoint inhibitors for metastatic melanoma (presumably pre-clinical work). Clinical combination studies for late stage disease by Provectus was very briefly noted at EADO in early-May, either one of Bristol's {ipilimumab [anti-CTLA-4] or nivolumab [anti-PD-1]} or Merck's MK-3475/pembrolizumab [anti-PD-1].
 |
| Click to enlarge. |
In a Form 4 filing Provectus principal Scott acquired ~25,000 shares of common stock through option exercises (a $0.95 exercise price).
PH-10 Update (May 20, 2014)
From Provectus' Product Pipeline webpage, "Full Phase 2c study report submitted to FDA Feb 2014." H/t, a shareholder.
 |
| Click to enlarge. |
The company edited the website's landing page.
 |
| Click to enlarge. |
Press release: Provectus Biopharmaceuticals to Celebrate NYSE MKT Listing with Opening Bell Ceremony. The associated 8-K filing is here.
Article: Child’s Illness Steers a CFO’s Career, CFO.com, an article about Provectus CFO/COO Peter Culpepper
Article: Local start-up Provectus now listed on NYSE MKT, Teknovation.biz, a web site sponsored by Pershing Yoakley & Associates (a firm that includes Provectus shareholders)
Worth (May 17, 2014)
I revised the blog's Worth tab to reflect the change in my per share estimation of worth that results from an increase in fully diluted shares outstanding since I originally wrote it (i.e., from ~200 million as at 3Q13 to ~250 million as at 1Q14).
Provectus is worth $120 per share (a $100-140 range). At the time of an acquisition approximately 250 million fully diluted shares outstanding should imply a $30 billion worth.
Worth may not or may never be share price, or market capitalization.
Worth of $120 per share or a total of $30 billion is my estimate of the company’s intrinsic value on a net present value (“NPV”) basis, an amount I believe could accrue to Provectus shareholders upon an M&A transaction and over time through an earn out.NYSE Bell Ringing (May 17, 2014)
It would appear Provectus management will be ringing the New York Stock Exchange's ("NYSE's") bell on Thursday, May 22nd. From Oak Ridge National Laboratory (~1990s) to Photogen Technologies (NASDAQ: PHGN c. 2000) to a reverse merger into a public shell, and life as an over-the-counter stock (early-2000s to 2014), to the NYSE MKT (May 16th), the old American Stock Exchange since purchased by NYSE Euronext in 2008, to standing on the dais of the NYSE to ring the bell (May 22nd, apparently), an exchange tradition for more than 140 years.
The company launched a redesign of its website today.
 |
| Click to enlarge. |
Updated 5/19/14: The website was changed to better reflect the current status of the drug's BTD application.
PV-10, a broad spectrum kill mechanism for cancer (May 14, 2014)
Thoughts on PV-10's ASCO 2014 abstracts...
Moffitt's "Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions."
- "Six of 8 patients had metastatic disease refractory to previous ipilimumab, anti-PD-1 and/or vemurafenib therapy. Four of these 6 patients exhibited pCR to PV10 in both the injected and uninjected lesions." PV-10 generated a complete response where other drugs failed to do so.
- The definition of pCR, or pathologic Complete Response, can vary, but a good starting point is no evidence of invasive and noninvasive residual tumor tissue in the skin (and in some cases, based on the definition, the lymph nodes).
- "PV-10 increases tumor-specific T cell responses as well as to establish the interaction of intralesional PV-10 with combination checkpoint protein inhibition." As expected, Moffitt has been combining PV-10 with other checkpoint inhibitors.
Provectus' "Efficacy of intralesional Rose Bengal in patients receiving injection of all existing melanoma in phase II study PV-10-MM-02."
- "In these pts with all disease injected plus 26 pts with uninjected disease limited to bystanders (i.e. 54 pts with all disease monitored), CR was achieved in 232 of the 363 injected lesions (64% CR): 121 lesions required a single injection for CR, 84 required 2 injections, 22 required 3 injections and 5 required 4 injections. Additionally, 10 of 28 uninjected bystander lesions achieved CR." Complete response. "...if you inject PV-10 into melanoma tumors, the tumors go away." (Craig Dees).
- "Recurrent locoregional melanoma can be a source of persistent morbidity, including disfigurement frequently accompanied with pain, ulceration, bleeding and infection. The high rate of symptom control in refractory patients, manifest in CR of injected lesions after minimal intervention, is the basis for a breakthrough therapy application based on the 28 patient “all treated” subgroup." The linkage between symptom control and complete response.
The company's accompanying press release Provectus Biopharmaceuticals' PV-10 Data Show Exceptional Complete Response Rates in Refractory Melanoma Patients: "Dees continued, "The duration of response and the bystander response reported in the phase 2 trial was beyond the scope of simple tumor ablation. Last year Moffitt published data from mice showing increased anti-tumor T cell responses, and this year at ASCO they have bridged that nonclinical finding to the same phenomenon in man. It is unprecedented for a small molecule ablative agent to have this kind of immune system activity detectable in peripheral blood of patients. The 1-2 punch from PV-10 (rapidly reducing tumor burden and producing immune system stimulation) is presumably the underlying driver of these durable complete responses in patients with cutaneous melanoma."
PV-10 Abstracts at ASCO 2014 (May 14, 2014)
Abstract #1 (Provectus): "Efficacy of intralesional Rose Bengal in patients receiving injection of all existing melanoma in phase II study PV-10-MM-02." Related company press release, April 1.
Background: The safety and efficacy of intralesional (IL) treatment of refractory cutaneous melanoma with rose bengal disodium (PV-10) was evaluated in an 80 patient international, multicenter, single arm phase II trial (NCT00521053). Overall, PV-10 was well tolerated and 41 of 80 ITT patients (pts) met the primary endpoint of objective response (CR+PR) in their injected target lesions (51% ORR CI 40-63%, 26% CR). Methods: Refractory pts (median of 6 previous interventions, 6.3 cm median sum lesion diameter in biopsy confirmed melanoma) received PV-10 into up to 20 cutaneous and subcutaneous lesions up to 4 times over a 16-week period and were followed for 52 weeks. Best overall response rate (BORR) was assessed by RECIST in up to 10 injected target lesions. Secondary endpoints included assessment of duration of response, BORR of untreated bystander lesions, overall survival and adverse events.Confidence intervals for response rates were based on the exact cumulative probabilities of the binomial distribution (95% confidence intervals). Results: In the subgroup of 28 pts who received PV-10 into all existing melanoma lesions (i.e., no uninjected lesions), ORR by-patient was 71% (CI 51-87%) with 50% CR (CI 31-69%). In these pts with all disease injected plus 26 pts with uninjected disease limited to bystanders (i.e. 54 pts with all disease monitored), CR was achieved in 232 of the 363 injected lesions (64% CR): 121 lesions required a single injection for CR, 84 required 2 injections, 22 required 3 injections and 5 required 4 injections. Additionally, 10 of 28 uninjected bystander lesions achieved CR. Conclusions: Recurrent locoregional melanoma can be a source of persistent morbidity, including disfigurement frequently accompanied with pain, ulceration, bleeding and infection. The high rate of symptom control in refractory patients, manifest in CR of injected lesions after minimal intervention, is the basis for a breakthrough therapy application based on the 28 patient “all treated” subgroup. Although the primary ablative effect is responsible for CR in injected tumors, durability of response and bystander response implicate an immunologic mechanism of action secondary to ablation.Abstract #2 (Moffitt Cancer Center): "Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions." Related company PR, April 2. Jeffrey Weber is a co-author.
| Click to enlarge. |
Background: Intralesional (IL) therapy is under investigation to treat dermal and subcutaneous metastatic cancer. In our murine model, IL injection of PV-10 (10% Rose Bengal) induced regression of injected and uninjected “bystander” melanomas. We observed a consistent increase in anti-tumor T cell responses following IL PV-10 in the mouse model, supporting an immune-based mechanism by which PV-10 mediates regression of uninjected “bystander” tumors. Methods: We translated these findings into a pilot clinical trial that enrolled 8 patients with dermal and/or subcutaneous metastatic melanoma. Two study lesions in each patient were sampled by biopsy pre-treatment; one of the two lesions was injected with IL PV-10, then both residual sites were completely excised. We compared tumors before and after treatment with H&E staining to determine pathologic complete response (pCR), and we confirmed results with MelanA immunohistochemistry. Peripheral blood mononuclear cells (PBMC) before and after IL PV-10 were phenotyped for activation markers by flow cytometry. Results: Treatment with IL PV-10 led to pCR in the post-treatment biopsies of both PV10-injected and uninjected study lesions in 4 of the 8 patients, and all 8 exhibited at least partial regression of the injected lesion. IL PV-10 was associated with an increase in circulating cytotoxic CD3+/CD8+ T cells (paired t test, p=0.008). Pre and post PV-10 treated PBMC from one patient were re-stimulated with autologous tumor in vitro. Compared to pre-treatment, PV-10 treatment produced an increase in tumor-specific interferon-gamma release by ELISA. Six of 8 patients had metastatic disease refractory to previous ipilimumab, anti-PD-1 and/or vemurafenib therapy. Four of these 6 patients exhibited pCR to PV10 in both the injected and uninjected lesions. Conclusions: IL PV-10 treatment can lead to systemic anti-melanoma immunity and pCR in injected and uninjected lesions including treatment-refractory tumors. Further studies are ongoing to determine the mechanism by which PV-10 increases tumor-specific T cell responses as well as to establish the interaction of intralesional PV-10 with combination checkpoint protein inhibition."...if you inject PV-10 into melanoma tumors, the tumors go away." (May 13, 2014)
Updated 5/14/14: Following a request from management to do so, I removed this entry. I will re-post it on June 2nd. In the interim, see EADO Slides (May 8, 2014).
The company will up list its ticker and stock to the NYSE MKT starting Friday, May 16th. See the related SEC filing here (Form 8-A12B, Registration for Listing of a Security on a National Exchange Form). The press release is here.
Updated: The related 8-K filing is here: "On May 13, 2014, the NYSE MKT LLC informed Provectus Biopharmaceuticals, Inc. (the “Company”) that it had approved the listing of the Company’s common stock on the NYSE MKT. The Company’s common stock is anticipated to cease trading on the OTCQB and commence trading on the NYSE MKT on May 16, 2014, under the Company’s existing ticker symbol “PVCT.”"
It Takes Two [to make a market] (May 12, 2014)
Short interest for the period ending April 30th (~1.75 million shares) increased for the third consecutive period by more than 350% over the low set for the period ending March 14th (~385,000). The prior low was ~375,000 for the period ending December 15, 2011. Although the period closing share price stayed roughly the same, $2.39 for April 30th v. $2.42 for March 14th, daily traded volume (according to the website from where short interest comes) fell about 60%. Reported short interest remains a small percentage of float. The nearly 5 times increase over a six-week period is curious, particularly given the potential of three possible, positive, upcoming events: an up-listing of the stock to a major stock exchange, a breakthrough therapy designation decision ("BTD"), and ASCO [abstracts].
 |
| Click to enlarge. |
 |
| The May 12th updated "Message from the CEO" page of Provectus' website. Click to enlarge. |
Several changes in language in this filing (ending March 31, 2014) compared to the last, which was Provectus' 10-K annual filing (ending December 31, 2013). See New SEC filing (May 8, 2014) below. From the Management’s Discussion and Analysis of Financial Condition and Results of Operations section starting on page 15:
- "We believe our continued development of PV-10 with existing funds will yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of solid tumor indications due to its unique immuno-chemoablation mechanism of action." {Bold and underlined emphasis is mine} The previous language or word was "recurrences," lending further credence to a potential broadening of an eventual label for PV-10 for earlier stage disease (i.e., locoregional).
- "This may include the potential for breakthrough therapy designation for PV-10 to treat locally advanced cutaneous melanoma and an accelerated approval path for PV-10 to treat this indication." {Bold and underlined emphasis is mine} The previous language or phrase was "locally advanced recurrent melanoma," which re-emphasizes the above point.
There also were the sentences, "Furthermore, our financial position and corporate governance are such that we meet the relevant listing requirements of both NYSE MKT and NASDAQ. We plan to list on either exchange as appropriate in the near future," which many believe point to a very near-term up listing of the stock to one of these major stock exchanges, very likely the NYSE MKT, triggered by the filing of this 10-Q.
 |
| Click to enlarge. |
Dr. Agarwala's EADO slides are available here; see Agarwala at EADO (May 7, 2014) below. The circulated slides, via Provectus News (the company's e-mail distribution list), did not include two slides detailing some Moffitt Cancer Center clinical study results (presumably so as not to steal or undermine Moffitt's ASCO thunder).
One of the missing slides presented pre- and 7-to-14 day post-treatment immunohistochemistry slides of injected and non-injected (bystander) tumor samples. Strikingly, the post-treatment slides did not show any visible (at least, none that I could see) melA (melanoma)-expressed material.
The second removed slide presented T and NKT cell populations (i.e., CD3+, CD4+, CD8+, NKT) pre-, 7-to-14 day post- and 21-to-28 day post-treatment. Populations spiked at the 7-14 day measurement before returning to what appears to be pre-treatment levels at the 21-28 day measurement (within statistically significant p levels: 0.008, 0.023, 0.008, 0.01, respectively).
New SEC filing (May 8, 2014)
Provectus filed its quarterly 10-Q (ending March 31st). Monthly cash burn appears to be roughly the same quarter-over-quarter at ~$950,000. Quarter-/year-end cash and cash equivalents were $16.7 million. There are several other items of note (and which will form the basis for a subsequent blog post).
For now, here's one: Pharmaceutical preparations for the quarter was $326,410, compared to $258,756 in 3Q13 and $310,160 for all of 2013. At a cost of $30 (based on previous management commentary) to $50 (my increasing production cost "just because") per single use 100 mL vial, the 1Q preparation expense could suggest the production of 6,000 (at $50) to 11,000 (at $30) units. The expense in 2013's third quarter (July-September) may have been related to production runs for Chemistry, Manufacturing and Controls ("CMC") purposes. What would be the reason for 2014's first quarter's production, preparation for commercialization?
Agarwala at EADO (May 7, 2014)
Provectus principal investigator and St. Luke's physician Dr. Sanjiv Agarwala presented PV-10 data and provided associated commentary during a session at the 10th European Association of Dermato-Oncology (EADO) congress. From a slide entitled Bystander Response:
- Bystander response strongly correlated with response of injected lesions
- Objective response of injected lesions accompanied by 61% OR in bystander lesions (56% CR)
- Failure to achieve an objective respone accompanied by 18% OR in bystander lesions
- Difference in outcomes highly significant (P=0.023)
- Bystander response in visceral lesions similar to that in cutaneous lesions
I read the second main bullet as the effect on tumors or lesions inside the body and far away from the injection site(s), thus inaccessible and very likely metastasized were impacted by the immune response harnessed by PV-10 in much the same way as nearby [to the injection site(s)] bystander lesions on the skin (cutaneous).
From a slide entitled Locoregional Blistering:
From a slide entitled Locoregional Blistering:
- Correlated with response of injected lesions
- Presents within 1-7 days (not necessarily first dose, nor on repeat dosing)
- Recovery within 28 days
- Steroids and/or antibiotics have been used for management
- Consistent with an immune-mediated process (no clinical data on etiology)
- Timing is consistent with observed T-cell and NKT data
Etiology means the cause of a disease or abnormal condition (i.e., why whatever is happening, regarding this local immune effect caused or generated by local injection or administration of PV-10), still is unknown.
From a slide entitled Summary:
- PV-10 is a simple, minimally invasive intervention with few contra-indications
- CR is over 50% of injected lesions
- Nonclinical and clinical data implicate PV-10 ablation as an immune cell effector
- T-cell activation and dendritic cell infiltration occur within first week of ablation
- Blistering regquires further study to determine etiology
- Regulatory clarity for Stage III disease expected in May
- Commencement of combination studies in Stage IV disease in second half of 2014
"[C]ombination studies in Stage IV disease" likely refers to combining PV-10 with either ipilimumab (less likely), an anti-CTLA4 agent, or one of the anti-PD-1 agents. I wonder whether this has something to do with Moffitt Cancer Center as a possible primary or secondary clinical site, Dr. Jeff Weber, M.D., Ph.D. as a possible lead or secondary principal investigator, and either nivolumab (Bristol Myers' anti-PD-1 agent) or pembrolizumab (MK-3475) (Merck's) as the combined agent.
Chicago (May 6, 2014)
I plan on attending some Provectus-related events at this year's ASCO annual meeting. I was last there in 2010 when the company provided an overview of ASCO participation by Dr. Sanjiv Agarwala, and a clinical program overview by Eric. Several shareholders with whom I've built relationships over time were in attendance.
 |
| Agarwala's concluding presentation slide |
Sanofi U.S. (the North American affiliate of Sanofi SA) executive, Brendan O'Brien, joined Pfizer and Boehringer Ingelheim executives on Provectus' Strategic Advisory Board. Sanofi was the fifth largest global pharmaceutical company by revenue in 2013; the top ten were Johnson & Johnson, Novartis, Roche, Pfizer, Sanofi, GlaxoSmithKline, Merck, Bayer HealthCare, AstraZeneca and Eli Lilly. Prior to Sanofi, O'Brien was a Pfizer executive.
Missing (May 3, 2014)
 |
| Click to enlarge. |
Having entered into the Controlled Equity Offering sales agreement with Cantor Fitzgerald & Co. ("Cantor") this week (see my Preparing to list (launch) post), one would think Cantor would initiate research coverage of Provectus following the up-listing of ticker onto the NYSE MKT. Cantor's biotechnology research universe is here and below.
 |
| Click on the table to enlarge it. |
April Blog Stats (May 1, 2014)
Blog readership both rose and fell from March 2014 depending on the statistic. I wrote 25 blog posts (5) and news items (20), versus 32 the previous month (8 and 25, respectively). April month-over-month was:
- -8% for # of unique visitors (3,105 v. 3,371),
- +5% for # of page views (35,171 v. 33,377),
- +4% for # of visits (15,096 v. 14,558),
- -6% for # of U.S. cities [from where visitors came] (926 v. 984),
- -36% for # of world cities (174 v. 274), and
- -33% for # of countries (50 v. 75).
 |
| Click on the graph to enlarge it. |
 |
| Click on the graph to enlarge it. |
New Melanoma Therapies Are Ready for Primetime: Sanjiv Agarwala, George S. Mack of The Life Sciences Report, April 29, 2014. Disclosure: 2) The following companies mentioned in the interview are sponsors of Streetwise Reports: Provectus Biopharmaceuticals Inc. H/t: investordummy
"TLSR: I'm noting that Provectus has an expanded access (compassionate use) protocol going on with PV-10. A lot of companies, especially small companies, might not want to dirty up their new drug application or final label by including late- or end-stage patients. I think this shows a lot of confidence in the program.
SA: I agree. We have an expanded access program available for patients who might benefit from this therapy. Obviously, I would only use that protocol for patients who have tried and exhausted most other options, because this is still an investigational drug.
TLSR: Have there been any interesting responses that you've seen in these expanded access patients?
SA: There have been. What we've seen in the expanded access program pretty much reproduces what we've seen in the Phase 2 trial. It's a smaller number of patients—I think we've had nine patients at my center so far. I don't know the data from the other centers as yet. But what we've seen with our expanded-access patients gives us confidence that our data from the Phase 2 study are robust."Extremely Clinically Cost Effective (April 27, 2014)
 |
| Click on the figure to enlarge it. |
Provectus issued press release Provectus Biopharmaceuticals, Inc. Retains Roberti+White, LLC as Public Policy Consultants today.
Hiring of Roberti+White ("R+W") appears to be another step the company is taking to prep for the eventual commercialization of PV-10, much like I wrote in Chemistry, Manufacturing and Controls (April 11, 2014) below about production of both drug substance and product.
It also is good to see management get in front of the potential (and undoubtedly forthcoming) public policy implications and discussions surrounding a drug like PV-10 that is so extremely clinically cost effective.
 |
| Click on the figure to enlarge it. |
I don't think the PR says anything one way or the other about the prospects for breakthrough therapy designation ("BTD") and/or or a pre- or post approval bridging study. Although, it strikes me as another example of management's confidence about the regulatory approval process. Hiring R+W as a key advisor in/to the drug commercialization process speaks, I think, to management's prepping for if/when the drug is approved (i.e., ultimately achieves accelerated or outright [regular] approval). Not waiting to react to the drug commercialization process until after BTD and an FDA decision on approval steps means minimizing the number of days of lost sales. Drug company financial analysts, in ginning up their net present value ("NPV") calculations, obviously would establish a launch date as part of their assumptions to the NPV calculation. A sooner, rather than later, assumed launch date has real return on investment implications for Provectus shareholders.
He should wake up (April 25, 2014)
"The future of immunotherapy will be dramatic, explained Mitch Gold, previously the CEO at Dendreon, now executive chairman of Alpine Biosciences. He stressed the necessity of working toward more effective, less toxic, and easier-to-administer therapies — and noted that the path to achieving this dream is as yet undefined." {Bold emphasis is mine} Cancer immunotherapy’s untapped potential inspires optimism, Dr. Sabrina Richards and Andrea Detter
 |
| Click to enlarge the figure |
 |
| Click to enlarge the table |
New SEC Filing (April 18, 2014)
Provectus raised approximately $3.4 million using Network 1 Financial as a placement agent. Thursday's Form D filing is here. While the fund raising likely was done at a premium to the then share price, no information regarding warrant coverage was provided by management. As at mid-March, when the company filed its 10-K, cash on the balance sheet probably was about $16.5 million (assumes a monthly cash burn rate in 1Q14 of approximately $953K, the same as 4Q13).
This April raise appears to be related to (and facilitated) a potential listing of the stock on the New York Stock Exchange ("NYSE") MKT (the old American Stock Exchange or AMEX). While the NASDAQ required of the company a share price over $3 for five consecutive days, the NYSE MKT appears to have been less stringent on share price (e.g., $2 for one day) but possibly more stringent on other aspects of its listing standards. In particular, I think the company raised money, in part or in whole, to address the required shareholders' equity threshold.
As at June 30, 2013, with a share price at or around $0.65, warrant liability ("WL") on the balance sheet was $2.6 million and stockholders' equity ("SE") was $6.4 million. As at September 30, with a share price at or around $0.77 (and greater price volatility), WL was $3.5 million and SE was $8.7 million. As at December 31 and a $2.41 share price, WL was $12.9 million and SE was $6.6 million. It's possible the raise (net of placement agent fees and expenses), adjusting for another month of cash burn (i.e., through mid-April) and option exercises (and what if any cash from warrant exercises), may have been required to meet the $4 million SE threshold, particularly if higher share prices and price volatility than those measured at 12/31/13 were used for warrant liability calculations.
"...it’s a bona fide immunological response" (April 17, 2014)
PV-10 decreases melanoma cells in tumours by ecancer reporter Janet Fricker:
"The investigators presented data on biopsies sampled from injected tumours and uninjected bystander tumours taken from eight melanoma patients seven to 14 days after PV-10 injection.
The studies showed that immunohistochemical staining of biopsy specimens for mel A (an immunohistochemical marker of melanoma viability) demonstrated the complete disappearance of viable melanoma cells in both the injected and bystander tumours.
“The study shows that there are plausible immunological processes that explain the bystander response observed in patients. This isn’t a spurious event, it’s a bona fide immunological response,” said Eric Wachter, the Chief Technology Officer at Provectus, who developed the drug.
Studies are now underway in an additional seven patients to take biopsies and blood samples at more frequent time intervals after PV-10 injection to elucidate the pathways more clearly." {Bold and underlined emphasis is mine}
Catching on to Craig about a decade later: "Intratumoral Immunization: A New Paradigm for Cancer Therapy" (April 17, 2014)
Intratumoral Immunization: A New Paradigm for Cancer Therapy. Clin Cancer Res April 1, 2014 20; 1747. "Immune cell infiltration in the tumor microenvironment is of prognostic and therapeutic import. These immune cell subsets can be heterogeneous and are composed of mature antigen-presenting cells, helper and effector cytotoxic T cells, toleragenic dendritic cells, tumor-associated macrophages, and regulatory T cells, among other cell types. With the development of novel drugs that target the immune system rather than the cancer cells, the tumor immune microenvironment is not only prognostic for overall patient outcome, but also predictive for likelihood of response to these immune-targeted therapies. Such therapies aim to reverse the cancer immunotolerance and trigger an effective antitumor immune response. Two major families of immunostimulatory drugs are currently in clinical development: pattern recognition receptor agonists (PRRago) and immunostimulatory monoclonal antibodies (ISmAb). Despite their immune-targeted design, these agents have so far been developed clinically as if they were typical anticancer drugs. Here, we review the limitations of this conventional approach, specifically addressing the shortcomings of the usual schedules of intravenous infusions every 2 or 3 weeks. If the new modalities of immunotherapy target specific immune cells within the tumor microenvironment, it might be preferable to deliver them locally into the tumor rather than systemically. There is preclinical and clinical evidence that a therapeutic systemic antitumor immune response can be generated upon intratumoral immunomodulation. Moreover, preclinical results have shown that therapeutic synergy can be obtained by combining PRRagos and ISmAbs to the local tumor site." {Bold emphasis is mine} Immunostimulatory monoclonal antibodies include ipilimumab, nivolumab, lambrolizumab, etc.
ASCO 2014 (April 17, 2014)
American Society of Clinical Oncology ("ASCO") abstract titles are released next week.
 |
| Click on the figure to enlarge it. |
See Moffitt & Dr. Jeff Weber (April 14, 2014) below. Weber's debate with Ross centered on the benefit or lack thereof of intralesional (“IL”) therapies for heavily diseased patients (heavy tumor burden) with visceral metastases.
According to HemOnc Today, "Weber notes that in an age of increasingly effective systemic therapies for stage IV melanoma, it would be difficult to rationalize the use of intralesional therapy alone in more than select subgroups of patients with dermal metastases only. Furthermore, Weber asserts that without significant benefit in PFS and OS, local regression is an insufficient substitute to validate continued interest in current intralesional therapies as a monotherapy."
Viewing Weber's video short I linked to above, one hears (paraphrasing, or noting):
- He has 25 years of experience with IL therapies. He observed that while they have some local benefit or affect, IL therapies are extremely unlikely to have significant systemic benefit.
- The issue at stake in the debate was whether there is a significant systemic role; that is, whether intralesional therapies impact systemic disease. The issue, as it relates to framing the debate, was that patients who die of melanoma die of distant metastatic disease; they do not die from locoregional, cutaneous, dermal or soft tissue disease.
- Amgen's T-Vec trial, while well done, showed modest impact on systemic metastases. He noted T-Vec's distant response of non-injected visceral metastases was 16%, versus 76% for BRAF/MEK drug combinations, a modest 10-15% for Ipilimumab but that has shown 20% 5-year survival for predominantly Stage IV M1c disease, and PD-1 and PD-L1 antibodies that have shown 30-40% response rates and 16.8-month survival for predominantly M1c disease.
- He is hard pressed to see a systemic role for IL therapies like T-Vec in patients with significant disease burden and visceral M1c disease. Stages IIIB-C and IV M1a patients could be treated with IL therapy. To expect, however, significant clinical and biologic systemic benefit (affect) with any IL therapy as we now know them is very low. {Underlined emphasis is mine}
Weber’s HemOnc conference presentation is available at this link. Several things about his comments and presentation (and as they relate to Provectus and PV-10) are interesting. First, his focus in the debate as it related to the use and utility of IL therapies was for patients with significant disease burden and visceral M1c disease, a small fraction of the total melanoma market.
Second, without significant benefit in survival-based endpoints like progression free survival (“PFS”) and overall survival (“OS”), objective response or OR (e.g., complete response, partial response) has not translated for IL therapies into success for Stage IV M1c patients, and more broadly Stage IV in most cases. Systemic disease requires the use of survival-based endpoints. OS is a metastatic melanoma label.
You may recall Dr. Andtbacka's comparison table of IL therapies and their Phase 2 results for metastatic melanoma. Although Vical’s Allovectin-7 and Amgen's T-Vec achieved notable OR results in their Phase 2 trials, comparably successful OS results were not achieved in their Phase 3 trials.
 |
| Click on the figure to enlarge it. |
Allovectin-7's Phase 2 trial comprised 53% Stage 3 patients and 47% Stage 4, while T-Vec's Phase 2 trial comprised 20% Stage 3 patients and 80% Stage 4. PV-10's Phase 2 trial comprised 77.5% Stage 3 patients and 22.5% Stage 4. Although Provectus' Phase 2 trial included some patients with systemic and visceral disease, patient selection was focused on those where PV-10 might achieve loco-regional disease control and thus forestall or prevent metastatic disease.
T-Vec's Phase 3 trial patient staging comprised 30% Stage IIIB-C and 70% Stage IV (27% M1a, 21% M1b and 22% M1c 22%). Provectus' contemplated pivotal Phase 3 trial (under special protocol assessment) would have been comprised of 100% Stage IIIB-C patients. T-Vec's trial used survival-based endpoints of durable response rate (primary) and OS (survival). Provectus' Phase 3 trial design would have used PFS.
T-Vec stumbled through a trial designed to prove, by virtue of survival-based endpoints, its value for treating systemic disease. PV-10 is being evaluated by the FDA using tumor-based endpoints (i.e., a primary endpoint of complete response) for its potential to achieve loco-regional disease control, and forestall or prevent the onset of systemic disease.
Systemic disease v. loco-regional disease. Survival-based endpoints vs. tumor-based endpoints. OS/PFS vs. CR.
Third, I was struck by Weber's “…as we now know them [IL therapies]…” comment. The phrase seems to me very interesting and a tad intriguing in light of Moffitt’s historical, presented and published, yet to be presented and published, and rumored pre-clinical and clinical work on PV-10. Weber's HemOnc presentation (see slide #4 below, for example) omitted mention and reference to PV-10.
 |
| Click on the figure to enlarge it. |
Provectus Biopharmaceuticals Signs Agreement with China's Tririver Capital (April 14th)
The company engaged an investment banking firm in China to help identify distribution and joint venture partners for PV-10. According to Tririver's website, "Starting from 2010, every year we topped the market by both the number of bio-healthcare companies we advised and the number of transactions we closed." As of this writing the last updated news items on the Chinese firm's website is from October 2013 (the website doesn't look like it has been updated in 2014), and there is no mention of their relationship with Provectus.
Immunohistochemical Staining (April 15, 2014)
One of the more interesting items in Provectus' April 7th press release Induction of Systemic Immunity Following Treatment of Tumors with PV-10 Reported by Moffitt Cancer Center Researchers at American Association for Cancer Research Annual Meeting was Moffitt's Dr. Pilon-Thomas, Ph.D.'s comment "...biopsies of patient tumors collected just 7-14 days after PV-10 injection no longer contained viable tumor tissue." This observation was "...evidenced by pathologic evaluation confirmed with immunohistochemical staining of biopsy specimens for melA (a marker of melanoma)." Immunohistochemical staining of biopsies, pre- and post-surgery is to diagnostically determine what and where cancer cells exist pre- and post surgery, for example. Surgeons presumably over-cut or over-excise to ensure they have clean margins around the tumor or lesion area. But, in the case of cancer therapies (i.e., not including surgery), how often does one come across the situation of no tumor tissue or cells in target lesion areas? Wouldn't there be some residual tumor tissue, particularly for partially responding tumors (as opposed to ones exhibiting complete responses)? In the case of Moffitt's study, there was no viable tissue, in both injected and non-injected ("bystander) lesions.
Moffitt & Dr. Jeff Weber (April 14, 2014)
The HemOnc Today Melanoma and Cutaneous Malignancies Conference, which concluded Saturday in New York City, is the second of five medical conferences where PV-10 data will be presented and/or profiled. Conference director and Provectus principal investigator Dr. Sanjiv Agarwala, M.D. said PV-10 would be an integral part of Dr. Robert Andtbacka, M.D.'s Session 4: Local and Regional Therapy. Moffitt Cancer Center's Dr. Jeffrey Weber, M.D., Ph.D., a medical oncologist. Weber debated M.D. Anderson's Dr. Merrick Ross, M.D., a surgical oncologist, on the topic of the current role of systemic intralesional therapies as a monotherapy. ASCO abstract/presentation titles, and presumably authors, become available as early as April 21st. It will be interesting to see if Weber is listed as an author. Thus far we only know the poster, Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions, is authored by "Dr. Amod Sarnaik, MD and colleagues."
Market Maker (April 14, 2014)
Market making firm Cantor Fitzgerald has paid multiple visits to and spent multiple hours on the blog.
Chemistry, Manufacturing and Controls (April 11, 2014)
Chemistry, Manufacturing and Controls ("CMC") is an important aspect of regulatory interactions that comprise the drug approval process, and a key technical section of the new drug application or NDA. The FDA recognizes CMC development parallels clinical investigations.
 |
| Guidance on CMC for Phase 1 and Phases 2/3 Investigational New Drug Applications, 2011 |
Provectus has produced drug substance and drug product with the the new synthesis process, which meets International Conference on Harmonisation ("ICH") guidelines for the manufacture of active pharmaceutical ingredient ("API") suitable for Phase 3 clinical trial material and commercial pharmaceutical use. This synthesis patent is important because while PV-10's API rose bengal can be purchased in bulk from chemical supply companies like Sigma Aldrich, this version would not be up to modern pharmaceutical standards and the drug itself (a 10% solution of rose bengal) cannot be so acquired.
Peter notes "PV-10 is a sterile, non-pyrogenic, high-purity concentrated solution of rose bengal manufactured specifically for Provectus to modern pharmaceutical standards, under current good manufacturing practice (cGMP), by specialty contract manufacturers...Neither the drug substance nor the drug product are available for third-party purchase from any commercial source and both are of markedly higher purity than commercial dye-grade material..." Drug substance, in this case, is rose bengal synthesized or manufactured according to ICH guidelines. Drug product is PV-10 that of course utilizes this ICH-synthesized rose bengal.
With a breakthrough designation therapy decision pending, as I wrote in my blog post The company PV-10 keeps, the decision tree looks to be one of the FDA either (a) requiring the company to conduct a bridging study in order to file a new drug application ("NDA") for PV-10 -- "...before...we have approval to sell PV-10..." -- or (b) permitting the study to be a post-marketing requirement/commitment -- "...after we have approval to sell PV-10...". As such, what work has management done, or is doing, in regards to CMC, both with the FDA and as good business planning and preparation?
Should an approval be in the offing, does Provectus have the ability to scale up production (i.e., to ensure sufficient supply to meet demand for the drug)? With lengthy recent visits to the blog of two chemical supply companies, I am curious about the company's roster of contract manufacturers (to produce both drug substance and product) for ensuring supply reliability and diversity. Although I don't believe PV-10 would be commercialized without a partner or more likely an acquirer, the drug's cost structure (i.e., $30 or thereabouts for a 100 mL single use vial that would sufficiently treat a Stage 0-III melanoma patient) establishing master supply agreements with multiple capable vendors clearly places Provectus in the position to commercialize PV-10 if management so chooses.
"Trial design endpoints should be tailored to the strength of your drug" (April 5, 2014)
Endpoints matter. A lot. At their best they provide information to the FDA, patients and their physicians, the drug company, and investors (both long and short) about the utility of the drug being tested. They matter because they establish the framework and specific metrics required by (negotiated with) the FDA to be met or exceeded for trial success along the way to drug approval. They also matter because they set the bar for the drug to clear. I believe management, through press releases on December 18, 2013 and January 24 and March 24, 2014 (together with other straightforward due diligence), has communicated the following:
- PV-10 is being considered for approval for local (non-metastatic) disease.
- Local (including local regional or loco-regional) disease represents more than 95% of the addressable melanoma market (see, for example, Recurrent Loco-Regionally Advanced Melanoma Market > Metastatic Melanoma Market (January 25, 2014) below).
- Metastatic melanoma ("MM"), a dreadful patients diagnosis, makes up a few percentage points of the market, but has no true, longterm solutions for those afflicted.
- While Provectus pursued MM Phase 1 and 2 clinical trials in the past to arrive at this point, the playing field changed during the December 16, 2013 meeting with the FDA from metastatic to local disease.
- In doing so, the FDA moved away from an MM pathway with survival-based endpoints for PV-10 to the initial pathway to approval of local disease with tumor-based endpoints.
- It would appear the Agency also agreed there is no suitable drug trial comparator to PV-10, from at least two perspectives:
- Drug delivery or administration: There are no approved injectibles, and
- Indication stage: "Standard of care" or primary treatment for Stages 0 to III is surgery. Tumor excision for Stages 0 to II. Excision, lymph node dissection, or various other treatments for Stage III.
- Because of this change, from metastatic disease to local (or non-metastatic) disease, and because of the drug's tissue sparing ability, PV-10 can challenge surgery as a primary, main or initial treatment.
- To the extent it will take time until all oncologists reach for PV-10 first, the drug should be the primary, main or initial neo-adjuvant or adjuvant therapy used. Adjuvant therapy is "[a]dditional cancer treatment given after the primary treatment to lower the risk that the cancer will come back."
- Because of this change, from metastatic to local disease, the primary endpoint now is complete response or CR. Secondary endpoints appear to be symptomatic treatment effects (e.g., pain, infection, bleeding).
- While there should not be a traditional, Phase 3, randomized control trial using survival-based endpoints, there may be a so-called bridging study, which management is saying shareholders should not be worried about.
- The bridging study could occur before or after approval (i.e., before or after a new drug application or NDA filing). See my blog post The company PV-10 keeps.
- The study should be a single arm trial with CR as the primary endpoint, and symptomatic treatment effects as secondary endpoints.
- The trial should use administration protocol that enables PV-10 to be injected as much and as many times as needed until the tumor is destroyed. The drug has demonstrated its ability in Phase 1 and 2 trials to generate substantial CR results despite injection limits. The data, particularly that from the compassionate use program, would suggest study injection protocols (i.e., no limits) would enable the endpoint to met.
- The study would also serve as a registration trial for the drug to be approved and licensed for sale in Australia, Europe, China and India, with trial sites enrolling and treating patients in each of these countries. "Some countries require clinical trials being conducted in that country to be registered, other do not require it, but often strongly encourage it."
- Management believes it has sufficient cash on hand to complete this study.
St. Luke's and Provectus principal investigator Dr. Agarwala is presenting PV-10 data and presumably providing associated commentary in a session at the 10th European Association of Dermato-Oncology (EADO) congress. Note the company PV-10 keeps.
 |
| Click on the table to enlarge it. |
 |
| Click on the table to enlarge it. |
Provectus issued a press release regarding a poster presentation by Moffitt Cancer Center at ASCO:
 |
| Click on the table to enlarge it. |
Today's PR also noted Provectus's recent breakthrough therapy application submission, the sixth such mention.
Conferences, Conferences, Conferences -- Update (April 1, 2014)
Provectus issued two press releases regarding presentations at HemOnc Today in New York in mid-April and ASCO in Chicago at the end of May:
- Provectus Biopharmaceuticals' PV-10 Data to Be Presented at the HemOnc Today Melanoma and Cutaneous Malignancies Conference and
- Provectus Biopharmaceuticals' PV-10 Data to Be Presented at the American Society of Clinical Oncology (ASCO) Annual Meeting.
 |
| Click on the table to enlarge it. |
- HemOnc Today: "Provectus has recently submitted an application to the FDA for breakthrough therapy designation for PV-10 based on the results from its Phase 2 clinical study," and
- ASCO: "Provectus has recently submitted an application to the FDA for breakthrough therapy designation for PV-10 based on the results from its Phase 2 clinical study related to cutaneous melanoma and is researching its efficacy for other indications."
These mentions bring the total number of times BTD submission has been publicly mentioned to 5: two 8-Ks (March 21st & 24th) and three PRs (March 24th, April 1st).
March and Calendar First Quarter Blog Stats (April 1, 2014)
Blog readership both rose and fell from February 2014 depending on the statistic. March month-over-month was:
- +4% for # of unique visitors (3,371 v. 3,242),
- -5% for # of page views (33,377 v. 35,012),
- -1% for # of visits (14,558 v. 14,699),
- -5% for # of U.S. cities [from where visitors came] (984 v. 1,034),
- +48% for # of world cities (274 v. 185), and
- +36% for # of countries (75 v. 55).
 |
| Click on the graph to enlarge it. |
- +173% for # of unique visitors (12,529 v. 4,591),
- +258% for # of page views (139,792 v. 39,016),
- +184% for # of visits (59,813 v. 21,033),
- +94% for # of U.S. cities [from where visitors came] (2,199 v. 1,132),
- +97% for # of world cities (557 v. 283), and
- +23% for # of countries (103 v. 84).
 |
| Click on the graph to enlarge it. |
The adjuvant melanoma indication noted by the Citigroup analyst refers to this: "Used in this context, adjuvant therapy means treatment intended to prevent tumor recurrence in disease-free patients. Typically this is done in patients presenting with advanced stage disease who have a high risk of recurrence after treatment. The endpoint is improved long-term and disease-free survival." (wisdom source: a shareholder who is an internist with patients include those afflicted with melanoma. See @bradpalm1).
More: "[The analyst]...likely referenced Stage 3b and 3c patients because they have the highest chance for future recurrence following surgical resection of the tumor and associated lymph nodes. These are patients with no evidence of distant metastases on presentation, but by the nature of their extensive local disease (treated by wide excision surgical resection including lymph nodes) they’re obviously at high risk for future recurrence because of the possibility that early micrometastases weren’t detected by diagnostic imaging and pathologic sampling." (ibid)
Bristol-Myers search to expand Yervoy's label would now appear to be moving closer to or overlapping that of Provectus and PV-10. According to the analyst, this expansion is worth $3 billion a year.
| Click on the table to enlarge it. |
According to Citgroup says Yervoy (ipilimumab) has no competition in melanoma for at least 5 years. Strange. Not Bristol-Myers' PD-1 drug candidate nivolumab as a monotherapy. Not Merck's PD-1 agent and breakthrough therapy recipient lambrolizumab as a monotherapy. Not nivolumab as a combination therapy. Not lambrolizumab as a combination therapy. You get the idea.
Interestingly, the analyst notes that Yervoy should demonstrate a material improvement over IFN alhpa, the "current and meager gold standard." And: "[t]he anticipated clinical benefit of Yervoy will likely offset the expected immune related adverse event profile for Yervoy, even with prolonged usage." Isn't "meager gold standard" an oxymoron? Doesn't "offset" mean "a consideration or amount that diminishes or balances the effect of a contrary one?" Thus, at best, the outcome is neutral; at worst, still negative. And yet, it's a $3 billion opportunity. Left-side of mouth, meet right side of mouth.
No Competition (March 29, 2014)
@TomSilver39 tweeted last week "Citi:Yervoy (Phase III/Adjuvant Melanoma) Expected to Show a Significant Improvement in Relapse Free Survival," referring to a research note put out by Citigroup.
 |
| Source: Citigroup, via @TomSilver39 |
Expert Advice (March 26, 2014)
@TomSilver39 tweeted today "Jefferies: Expert Bullish on Checkpoint Inhibitors as a Class, Sees Potential in Both PD-1 & PD-L1," referring to a research note put out by investment bank Jefferies.
 |
| Source: Jefferies, via @TomSilver39 |
- The ability to destroy the tumor with targeted modalities. PD-1 and PDL-1 agents do not destroy tumors with meaningful success. They mostly shrink them. PV-10 destroys tumors in which it is injected by rapid ablation. Craig Dees: "...if you inject PV-10 into melanoma tumors, the tumors go away." Moffitt Cancer Center about PV-10: "Single Injection May Revolutionize Melanoma Treatment..."
- Antigen presentation that can lead to the maturation of dendritic cells. Moffitt: "Further preclinical translational testing has shown that treatment of murine B16 cells with PV-10 leads to release of HMGB1, a soluble Damage Associated Molecule Pattern (DAMP) that is important for activation of dendritic cells (DCs). In the murine B16 melanoma model, there is a significant increase in the number of DCs infiltrating the tumor-draining lymph nodes after IL injection of PV-10. These findings suggest that PV-10 treatment leads to the release of DC activating factors and DC recruitment."
- CD8/CD4 activation by targeting PD-1, PDL-1, or OX40. Moffitt about PV-10: "Flow cytometric analysis demonstrated that CD8+ T cells were producing IFN-γ..."
Enhancing the immune response by inhibiting the mechanisms that the body uses to "turn the immune system off."How about not being bigger than Mother Nature? How about not inhibiting anything? How about just increasing anti-tumor T cell responses, and inducing systemic anti-tumor immunity after tumor ablation with PV-10?
By the way, there's that safety thing again...Kohrt: OXO-40 inhibitors can be highly toxic.
Last week we heard Representative Henry Waxman (D-CA) sent a letter to Gilead's CEO "...asking him to justify the price of Hepatitis C drug Sovaldi...Predictably, it has also ignited a debate about very high drug prices (and high stock prices)."
On Monday FierceBiotech's Damian Garde wrote Who's going to win pharma's immunotherapy gold rush?, discussing promising immunotherapies for advanced melanoma, analysts' sky-high sales estimates for the new drug class, and fast-growing pricing pressures. His article followed the Wall Street Journal's Hester Plumridge's article Drug Firms Focus on Advanced Melanoma. About the WSJ piece Garde writes "...Citi told the newspaper that PD-1 treatments could cost as much as $240,000 once they make it to market, far outstripping the costs of current vanguard therapies like Bristol-Myers' Yervoy."
Today FierceBiotech's Tracy Staton writes in her article ASCO plans cost-benefit scorecard for cancer meds as prices grow 'unsustainable,' "ASCO has launched an effort to rate cancer drugs not only on their benefits and side effects, but prices as well."
I think these data points continue a discussion that, while somewhat quiet today, should get much louder with time. The discussion is multi-faceted, with almost equal parts qualitative (e.g., patient care, patient pain, patient benefit) and quantitative (e.g., complete response, tumor destruction or shrinkage, overall survival, progression free survival, durable response, treatment cost, etc.).
Provectus and PV-10 are not in this discussion, and cannot be until the drug is approved or, at a minimum, is much closer to approval than it is today.
Drug A may be "better" than Drug B, but how do you make this determination in the vector space of safety, efficacy and treatment cost? Assume each of safety, efficacy and treatment cost is an axis (i.e., x, y and z). Each axis originates at the origin (0,0,0) and extends outward to higher and higher values. For safety, perhaps the range is not safe (0) to very safe (a high number), or no to mild to moderate to severe side effects (the opposite axis construction). For efficacy, perhaps the range is not efficacious (0) to very efficacious or cure (a high number). For cost, perhaps the range is no cost (0, unlikely, but allow me to extend the example) to very expensive (a high number).
ASCO's drug's cost-benefit analysis or any other undoubtedly will be difficult. My very nascent approach is to set aside safety as a given. There should be no reason to accept there are severe side effects (or material ones), philosophically and as a design matter, when a starting point could and should be to presume side effects should be de minimis if not non-existent (or at worst, extremely and honestly manageable; manageable means they don't cause other problems, not manageable from a physician's perspective). From there, I would suggest using a financial measure of risk, like Sharpe Ratio, to establish a measure of efficacy ("return") per unit cost of treatment ("risk").
See % CR per thousand dollars of treatment cost (February 6, 2014) below. Take a drug or drug candidate's complete response percent success and divide by the cost (per $1,000) for a course of treatment to arrive at this makeshift or hypothetical calculation of pharmaceutical alpha (alpha in a money management sense, where the manager or drug delivers real value). I understand tumor shrinkage (beta) is good. Tumor destruction (alpha) is better. I also understand issues of toxicity, adverse events (immunological or otherwise), recurrence, resistance, etc. are not considered. I updated the table to take into account the potential pricing of PD-1 treatments.
 |
| Click on the table to enlarge it. |
New SEC filing: Form 4 (March 24, 2014)
In a Form 4 filing today Provectus principal Wachter acquired ~640,000 shares of common stock through option exercises ($0.75, $0.93 and $0.95 exercise prices).
Patent Protection Strategy (March 22, 2014)
From a Big Pharma commercial marketing perspective it would seem there are at least four key risks as it relates to PV-10's intellectual property ("IP") protection:
- Eventual generics competition when patents go off-license (i.e., the patent life runway ends),
- Certain geographic jurisdictions negating patents and their associated IP,
- Regional partners who may wish to produce it themselves irrespective of what license agreement might previously be signed and in place, and
- Non-licensed regional and/or other worldwide partners who might want to produce it themselves.
Generics competition
PV-10's progeny or relatives, other halogenated xanthenes like PV-12, etc., could further extend the portfolio's patent cliff well beyond 2031, or may provide a better subsequent therapeutic treatment to PV-10 should (when?) the drug goes generic.
Jurisdictional negation
Negation of patents in places like in India and elsewhere in the so-called developing world sensitive to cancer treatment costs should be mitigated or eliminated altogether by pricing affordable treatments (potentially as we are seeing in the U.S and Europe). Recall that it costs in the tens of dollars to produce a single-use 100 mL vial of PV-10, and price points might be in the range of $5,000 to $20,000-30,000 per vial (which, given the drug's efficacy, is tantamount to per treatment). At some price point reasonable and agreeable to both parties, the acquiring Big Pharma and respective international government (e.g., India), governments then should respect the patents.
Regional partners, and Non-partnered pharmaceutical companies at large
Widely available by barrel or tanker full, low cost but very high value, but requiring special synthesis in regards to impurities in the raw material, there is the risk of regional partners, regional competitors or pharmaceutical companies at large mostly theoretically but not impossibly practically trying to circumvent Provectus' synthesis patent Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes. This circumvention would be to target the company's trade secrets component of its IP protection strategy. Doing so should fall in the realm of nefarious or illegal activity (e.g., like trying to steal the Coca Cola formula), but once the genie's out of the bottle, small comfort to Provectus and shareholders pre-acquisition and the acquiring Big Pharma post.
Combination therapy patent application Combination of Local and Systemic Immunomodulative Therapies for Enhanced Treatment of Cancer, should eliminate the risk of trade secrets fracture through the combination (pardon the pun) on the combination patent and the associated clinical data set (for both safety and per indication). The dataset safety component would require, I think, at least 300 people to have been treated and per indication use (i.e., label) efficacy data. I believe the former has been attained. The latter, I think, would grow step-by-step as Provectus and/or its acquirer progressed the drug through the regulatory approval path to broaden and deepen its use.
Combination therapy patent application Combination of Local and Systemic Immunomodulative Therapies for Enhanced Treatment of Cancer, should eliminate the risk of trade secrets fracture through the combination (pardon the pun) on the combination patent and the associated clinical data set (for both safety and per indication). The dataset safety component would require, I think, at least 300 people to have been treated and per indication use (i.e., label) efficacy data. I believe the former has been attained. The latter, I think, would grow step-by-step as Provectus and/or its acquirer progressed the drug through the regulatory approval path to broaden and deepen its use.
Non-licensed regional entities
Presumably the regional partner and/or worldwide acquirer can police smaller players trying to produce the drug themselves, utilizing among other things the above-mentioned combination patent & clinical dataset tool.
New SEC filing (March 21, 2014)
Provectus filed an 8-K filing to note submission of its breakthrough therapy designation application.
Updated 3/22/14: H/t same shareholder. Members of the company's scientific and strategic advisory boards presumably would have advisory board and consulting agreements in place (a non-Provectus example is here), and presumably receive compensation that would include both cash and warrants. Pfizer's Eagle, Miglani and the rest of these advisory board members thus would be shareholders of the company by virtue of their [presumed] warrant holdings, and investors for Regulation FD purposes. So, if management told Eagle and other board members about Provectus' BTD application submission, the SEC would require an 8-K filing.
Updated 3/22/14: H/t a shareholder. Provectus may have filed the 8-K, regarding submission of the BTD application, because management disclosed it to someone (some entity) for some reason (necessitating a Reg FD disclosure). If so, who and why?
Updated: I must admit to some surprise about this evening's 8-K filing that Provectus "...submitted via overnight courier for Monday, March, 24, 2014 delivery an application to the Food and Drug Administration for Breakthrough Therapy Designation (“BTD”) for its oncology drug PV-10 for the treatment of melanoma." I would have thought the company's January 24th press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes was sufficient: "...Provectus will submit data from its Phase 2 study in a formal breakthrough therapy designation (BTD) request this quarter, and should receive a decision within 60 days of receipt of that request." Searching other 8-K filings for the phrase "breakthrough therapy designation," I was unable to find another example of a company 8-King the submission of its BTD application. I presume there was both a process and a rationale behind management's decision, although it seems management is certain they'll receive BTD (one wouldn't effectively PR a submission with a known decision timeframe, thereby setting market expectations, without believing one wouldn't disappoint).
FY2014 CDER Breakthrough Therapy Requests as of March 07, 2014 indicates FDA performance is 100%, a "[p]ercent where action was taken within 60 days of receipt of the request for breakthrough
therapy designation." An Agency mailroom receipt time/date stamp of March 24th and 100% performance would suggest a decision on or before Friday, May 23rd.
Event risk here includes a positive (management would say 100%; FDA statistics through March 7th would show 30%) or negative (0% vs. 70%) decision, as well as a late (0 [above, here] to 3.26%) or early one (there are no readily available statistics on the average or range of timeframes taken by the FDA to render a decision): positive & early, positive & late, negative & early, and negative & late.
The Party Gets Bigger (March 20, 2014)
A Boehringer Ingelheim executive joined two Pfizer executives on Provectus' Strategic Advisory Board. While not as large a company, balance sheet-wise, as Pfizer, Johnson & Johnson, Amgen or Bayer, Boehringer is sizable with a strong interest in oncology. It closed its 2012 fiscal (calendar) year with more than 17 billion euros ($23 billion give or take at current exchange rates) in assets. It is reasonable to think Boehringer could have interest in acquiring Provectus, and the ability too as M&A financing is cheap and abundant (and their balance sheet and income statement likely could afford it). It seems to me the addition of Dr. Chalil came together quickly, and may have surprised the Pfizer executives. I think it was a good decision by management to begin expanding the board's relationships and, candidly, politely conveying to Pfizer that there indeed are other pharmaceutical companies interested in Provectus. There are rumors of additional forthcoming adds to the strategic advisory board, some of which I'm sure Pfizer is aware of and some perhaps they are not.
"Strategic Advisory Board" (March 18, 2014)
Provectus retitled its corporate advisory board to the/its strategic advisory board, swapping "strategic" for "corporate." It looks like the change was made last month, and is manifested on the website and in the corporate presentation (see below).
 |
| February 13th |
 |
| February 24th |
Expected best-in-class clinical benefit (March 18, 2014)
The title of this news blurb comes from the 10-K: "We believe our continued development of PV-10 with existing funds will yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of solid tumor recurrences due to its unique immuno-chemoablation mechanism of action."
Ironically, shortly after, Amgen press released the T-Vec abstract and presentation from the Society of Surgical Oncology's Cancer Symposium. See blog post "...one of...biotech's top prospects." In doing further due diligence on the topic, I found Melanoma: Engineered Herpes Virus Effective in Treating Local and Distant Lesions from January 24, 2014's Oncology Times:
Caution from Vernon Sondak
Following the presentation, Vernon Sondak, MD, Chief of the Division of Cutaneous Oncology at H. Lee Moffitt Cancer Center, strongly advised during the question-and-answer period that the study results must be interpreted in light of the design of the study and patient inclusion criteria, including the endpoint, the control arm, patient selection, and the choice of patients with or without uninjected lesions: “All of these factors are so critical in, first of all, getting a successful trial and then subsequently figuring out where this kind of therapy fits into the management algorithm for our patients,” he cautioned.
Responding, [Howard L. Kaufman, MD, until recently Director of Rush University Cancer Center and now Associate Director for Clinical Sciences at Rutgers Cancer Institute of New Jersey] thanked him for the comment and added that designing such studies has been a challenge. Particularly in light of what researchers have been learning about immunotherapy in the past two years and delayed kinetics of the response, “it makes it very challenging to pick an endpoint that we could really use as a surrogate of overall survival before we enter into these large Phase III studies,” he said. He mentioned that he has done work looking at durable responses, which appear to be a reasonable surrogate endpoint for overall survival given that many of the patients who responded in this trial had initially progressed before going on to have durable responses.
Answering another question from the audience, Kaufman said that prior therapy with T-VEC did not appear to change the response to subsequent ipilimumab.Provectus' January 24 press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes seems to suggest management may have convinced the FDA (or put differently, the Agency now may agree) tumor-based endpoints are more appropriate or suitable for evaluating the response to treatment for locally advanced melanoma and, thus, more appropriate for PV-10, rather than survival-based endpoints. Moffitt's Sondak's comments above, Amgen's appearance of copying Provectus' own tumor study, growing focus on local treatment for cancer immunotherapy, etc. point to this shift. I imagine this -- moving from survival-based endpoints to tumor-based ones -- is or will be a precedent. When you read the January 24th press release, particularly in the context of the company's ECC 2013 presentation, you can't help but think the new primary endpoint could be complete response, while secondary endpoints could be disease symptoms like pain, infection and/or significant bleeding.
While T-Vec may have some utility in treatment algorithms, its lack of ability to generate robust complete response figures places it a distant second to PV-10.
One wonders if, more broadly speaking, the company eventually will make the case for these new endpoints or metrics for PV-10 for solid tumor cancers.
Conferences, Conferences, Conferences -- Update (March 17, 2014)
PV-10 was mentioned at the Society of Surgical Oncology Cancer Symposium as part of MD Anderson's Dr. Ross' presentation.
 |
| Click to enlarge the table. |
I noted the following that requires follow-up:
- "This study is potentially pivotal since it would be powered to enable accelerated approval under the auspices of a proposed Breakthrough Therapy Designation request for PV-10 to treat primary liver cancer,"
- "We are evaluating potential for further development of PV-10 to treat recurrent breast cancer based on the published data provided by Moffitt as well as interest to address this important indication,"
- "We believe our continued development of PV-10 with existing funds will yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of solid tumor recurrences due to its unique immuno-chemoablation mechanism of action,"
- "Likewise, we believe our development of PH-10 with existing funds will yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of inflammatory dermatoses due to its unique non-steroidal anti-inflammatory mechanism of action," and
- "We believe regulatory clarity, including one or more breakthrough therapy designations, is determined by specifying the expected approval pathways of both PV-10 and PH-10."
Provectus filed its annual 10-K (ending December 31). Monthly cash burn appears to have increased to ~$954,000 +37% quarter-over-quarter. Quarter-/year-end cash and cash equivalents were $15.6 million. Of the $7.3 million in gross proceeds raised by management in the fourth quarter, $6 million was mentioned (disclosed) in the third quarter 10-Q filing. See New SEC filing (November 12, 2013) below. As I now see it (see Fundraising? (November 14, 2013) below), the company raised more than $14 million in Q3 and early-to-mid-Q4.
There are several other clinically-oriented items of note (and which will form the basis for a subsequent blog post). Additionally, management settled the compensation lawsuit (the “Shareholder Derivative Lawsuit” as referenced in the 10-K), subject to approval by the court. See the bottom of page 25 and top of page 26.
Nexavar (Sorafenib) (March 12, 2014)
St. Luke's liver trial press release, see New Press Release by St. Luke’s University Health Network (March 10, 2014) below, and Bayer's press release this week Phase III Trial of Sorafenib as Adjuvant Therapy for Liver Cancer Did Not Meet Primary Endpoint reminded me of the potential PV-10 has to dramatically disrupt the liver cancer market from the perspective of pharmaceutical companies selling into it. The addressable market for liver cancer, and cancers metastatic to the liver, is of course huge (particularly in Asia). I think the therapeutic standard of care is sorafenib, or Nexavar, co-developed and co-marketed by Onyxx Pharmaceuticals (now part of Amgen) and Bayer. Global Nexavar sales were critical to Onyxx, less so to Bayer, and much smaller to Amgen.
 |
| Click to enlarge the figure. |
Any future degradation in (diminishment of) sales will of course reduce earnings (adjusted for the drug's cost of sales) and, thus, market cap. As an example, I believe Bayer currently has an approximately 80 billion euro market capitalization, a 26'ish price-to-earnings ratio, and maybe a 60% operating margin for Nexavar. Every €100 million decrease in sales could result in a loss of about 1.5 billion euros in market cap.
One explanation of Amgen's rationale to acquire the company: "Don’t forget the perfectly sensible strategic rationale behind Amgen’s decision to buy Onyx. The big biotech gains the proteasome inhibitor Kyprolis (carfilzomib) for multiple myeloma, and Nexavar (sorafenib) partnered with Bayer, for liver and kidney cancer, drugs that are already on the market in the U.S. and will immediately contribute to Amgen’s top-line. The company needs new drugs to fill a revenue gap. Before the acquisition it was expecting that sales would be lower in 2015 than in 2013. The addition of Kyprolis fills that void – and it gives Amgen some street cred in oncology, a therapeutic area it has targeted for future growth, though its marketed cancer portfolio mainly includes supportive care products, not cancer interventions. Onyx serves as a leg up for Amgen as it looks to establish itself as a major oncology innovator and bring forward a pipeline of oncology drugs it has cobbled together partly through acquisitions" (Source: The In Vivo Blog, 2013 M&A Of The Year Nominee: Amgen/Onyx, December 2013).
But, can Nexavar sales be expanded through more uses of the drug for liver and kidney cancers (e.g., Could be used for all lines of therapy (e.g., adjuvant, 1st, 2nd and 3rd line, combination)? Nexavar is, after all, the standard of care for hepatocellular carcinoma ("HCC"). Nexavar's failed Phase 3 trial (for use as an adjuvant therapy) might not suggest so. NCCN guidelines for HCC follow.
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
Yet, there is a role for locoregional therapy.
 |
| Click to enlarge the figure. |
What struck me about St. Luke's press release was Agarwala's reference to "...patients with other types of cancer who have exhausted standard treatment options." Provectus' initial pathway to approval (see the company's January 24th press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes) is as the first local agent for recurrent locoregionally advanced melanoma. In the Phase 2 trial these patients were refractory to a median of 6 treatments or therapies (ranging from 0 to 19); that is, many, most or all of them probably had exhausted standard treatment options. Eventually, PV-10 will be used for treatment naive patients with locoregionally advanced melanoma.
PV-10 as a treatment option for HCC patients has the potential to eventually blow a hole in Bayer's earnings, and make the rationale of Amgen's purchase of Onyxx's primarily dependent on Kyprolis in the near- to medium-term. It would make sense to me, if I was an executive of either company, to begin speaking with Provectus; Bayer more urgently than Amgen.
Short Interest Falls Again (March 11, 2014)
 |
| Click to enlarge the figure. |
Short interest fell to about 423K for the two-week period ending February 28th (0.3% of float, per Yahoo! Finance's assumption of ~128MM shares), a number last seen in 2011 (May and before, October).
New Press Release by St. Luke’s University Health Network (March 10, 2014)
St. Luke’s Participates in Interventional Clinical Trial to Evaluate the Benefits of the Tested Melanoma Drug PV-10 in Patients with Late-Stage Liver Cancer
"“Since PV-10 has cleared Phase II testing for melanoma due to its proven effectiveness, it was time to evaluate the drug to see if it could benefit patients with other types of cancer who have exhausted standard treatment options,” says Dr. Agarwala." Agarwala was a principal investigator ("PI") in the company's melanoma trials, and was a PI in Delcath's liver trials for melanoma cancers metastatic to the liver. See PV-10 & HCC/Cancer metastatic to the liver (February 5, 2014) and Liver Trial Update on ClinicalTrials.gov (February 27, 2014) below.
Phase 2 Recurrent Breast Cancer Trial Prep Underway? (March 6, 2014)
Hat tip: A vigilant fellow shareholder. Thank you.
New Press Release (March 6, 2014)
PV-10 Immune Mechanism Data to Be Presented at the American Association for Cancer Research Annual Meeting. "Moffitt Cancer Center Poster Presentation on April 6, 2014."
Compare Provectus' 2014 PR about Moffitt's AACR abstract/poster presentation with the company's 2013 one. And, think about what has occurred, at the company, with the FDA and at Moffitt, in the intervening 12 months or so.
2013: Immunology Data on PV-10 to Be Presented at the American Association for Cancer Research Annual Meeting. "Moffitt Cancer Center Poster Presentation on April 8, 2013"
Craig's 2013 press release quote: "The researchers at Moffitt are providing valuable insights into how PV-10 harnesses the immune system in specific response to the injected tumor. Although the study being presented was done in murine models of melanoma and breast cancer, the currently open clinical trial at Moffitt is designed to investigate this mechanism in melanoma patients. We are pleased to enhance our understanding of intralesional PV-10 and its potential to produce a systemic benefit in cancer patients."
Provectus routinely communicates progress updates through its corporate website presentation, the latest version of which may be found here. Several changes were made to the March 5th version (the current update), compared to the February 27th version (the prior update).
The liver trial continues to enroll patients. And correcting the number of trial patients from 24 to 36, reflecting up to 24 patients treated with PV-10 ("Expansion Cohort 1") and up to 12 patients treated with PV-10 and sorafenib ("Expansion Cohort 2"). Re-read the company's September 2012 press release Provectus Expands Protocol for Phase 1 Liver Cancer Study in the context of Provectus' European Cancer Congress 2013 poster presentation that presented exploratory analyses of metastatic melanoma Phase 2 clinical trial data as lesions and numbers of lesions (rather than just patients and number of patients): "In both expansion cohorts, subjects will receive PV-10 treatment of a single hepatic tumor. Subjects with multiple injectable tumors will be eligible for re-enrollment for treatment of additional tumors if PV-10 is well-tolerated."
In the MM Phase 2 trial, responses of individual lesions were studied and detailed, almost as if each lesion and its outcome was equivalent or nearly equivalent to a patient, particularly in regards to demonstrating, "...via the self-evident ablative effect, that intralesional PV-10 provides a viable strategy to maintain long-term locoregional control of melanoma in patients whose cutaneous and subcutaneous melanoma has recurred."
It is conceivable full cohort numbers may not be necessary to aggregate data and submit a BTD application, but rather only a sufficient number of treated tumors.
The wrap-up (in 2014) of Moffitt's Phase 1 feasibility study. This study elucidated PV-10's mechanism of immune response in humans, the first work of which was revealed in the cancer center's AACR 2014 abstract.
Further work on treating recurrent breast carcinoma and cancers metastatic to the liver. This reads a little confusing [to me]. The company completed its Phase 1 breast cancer trial, and treated a colorectal cancer metastatic to the liver in its Phase 1 liver trial. Do both indications represent candidates for breakthrough therapy designations ("BTDs") on the basis of an initial BTD award for locally advanced melanoma, an understanding of PV-10's dual mechanisms of action ("MOAs") [rapid ablation, systemic immunity], and existing and/or additional data from the breast and liver trials? Or, perhaps, Peter merely was noting additional fundamental research may be occurring in these and other indications.
The possible wrap-up (in 2014) of research into PH-10's unique lack of toxicity by the "leading research facility." Management is overdue on an update of their efforts and work to progress along PH-10's regulatory approval path, which includes of course the rumored work by Rockefeller University to elucidate PH-10's MOA (much in the same way Moffitt Cancer Center did for PV-10's dual MOAs). What there is to say probably requires some additional time, and likely will be detailed in the forthcoming 2014 CEO letter. The annual letter historically was published between early-March and late-May from 2010-2012; prior to this (i.e., 2009 and before), the annual overview was released in January. Since this year's update should address Provectus' oncology plans for the remainder of 2014, it would seem reasonable to publish the letter after a BTD decision.
Cowen Group: Oncology Immunotherapies Differentiated on Efficacy and Durability (March 4, 2014)
Source: Tom Silver @TomSilver39.
Key points:
Dr. Agarwala's HemOnc Today conference on April 10th and 11th has a more detailed program. Of note is Friday's session on Local and Regional Therapy, moderated by Dr. Andtbacka.
Moffitt's Dr. Weber, a medical oncologist, presumably will argue the role for local agents like PV-10 (and T-Vec) when treating systemic disease is in combination with other systemic therapies, especially for heavily diseased or tumor burdened patients. MD Anderson's Dr. Ross, a surgical oncologist, should argue for the use of certain local therapies (like PV-10 and T-Vec) pre- and/or post-surgery (a presentation made by Ross in February may be found here). Management has often commented that surgical oncologists were earlier adopters than their medical counterparts. Moffitt's first presentation on PV-10 was made at the 2013 annual meeting of the Society of Surgical Oncology. This should be an interesting session because one could view each key opinion leader's respective viewpoint as appropriate in context (and, potentially (likely), non-overlapping in terms of patient populations for PV-10 therapy).
Compassionate Use Program (March 2, 2014)
A new site was added as a named location for the compassionate use program, Sharp Memorial Hospital in San Diego, California, which also is a liver cancer trial site. The updated information on ClinicalTrials.gov can be found here.
New Journal Article: Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages (March 1, 2014)
Journal of Immunotoxicology: "Rose Bengal (RB) has been used as a safe agent in clinical diagnosis. In addition, it is used as a photodynamic sensitizer for removing microorganisms and cancer cells. Recently, its preferential toxicity after direct exposure to cancer cells was proven. The present study focuses on anti-cancer and anti-inflammatory activities of RB. The toxicity of RB against AGS gastric cancer and NIH 3T3 fibroblast cell lines was studied using an MTT assay. Patterns of any cell death among the AGS cells were defined using Annexin-V and PI staining. In addition, the effect of RB on nitric oxide (NO) and prostaglandin E2 (PGE2) production induced by lipopolysaccha-ride in J774A.1 macrophages was determined. Modulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expressions in the macrophages was also evaluated by Western blots. The results showed that AGS cells exhibited significant concentration-dependent decreases in growth in response to RB; these cells showed a greater growth inhibition than did non-malignant 3T3 cells, suggesting that anti-growth activity of RB could be cell-specific. Moreover, AGS cells exposed to RB exhibited a significant increase in apoptosis; only at high RB doses did the cells display significant levels of necrosis. While RB also caused a modest decrease in the growth of J774A.1 macrophages, the cells displayed remarkable decreases in NO production and iNOS expression without significant concurrent modulation in PGE2 production or COX-2 expression. The data from this study appears to suggest that RB differentially impacts on transformed cell lines, preferentially suppresses growth of a gastric cancer cell line through induction of apoptosis, and induces changes in cells that could reflect potential anti-inflammatory effects that might be induced in situ."
Based on 2008 global statistics, gastric (stomach) cancer is one of the higher ranked indications for both new cases and deaths.
February Blog Stats (March 1, 2014)
Blog readership fell from January 2014, but increased over December 2013. February month-over-month was -59% for # of unique visitors (3,242 v. 7,892), -51% for # of page views (35,012 v. 71,402) and -52% for # of visits (14,699 v. 30,556).
February-over-December was +19% for # of unique visitors (3,242 v. 2,720), +74% for # of page views (35,012 v. 20,085) and +46% for # of visits (14,699 v. 10,040).
New SEC filings: Form 4 (February 28, 2014)
In Form 4 filings here (Tim) and here (Peter) today, Provectus principals Scott and Culpepper acquired 300,000 and ~190,000 shares of common stock, respectively, through stock option exercises ($1.10 exercise price).
Liver Trial Update on ClinicalTrials.gov (February 27, 2014)
Provectus' liver trial (HCC, and cancers metastatic to the liver) now is recruiting patients at St Luke's University Health Network, Dr. Agarwala's clinical trial-focused medical practice. Dr. Agarwala, a principal investigator for the company's metastatic melanoma trials, likely is addressing patients with melanoma-related cancer metastatic to the liver, like ocular melanoma. See also PV-10 & HCC/Cancer metastatic to the liver (February 5, 2014) below.
Anti-Melanoma Immunity (February 26, 2014)
I find Moffitt Cancer Center's switch from [a systemic anti-tumor] immune response to [anti-melanoma] immunity, in regard to their work on the systemic properties and benefits of PV-10, compelling and, in some ways, intriguing. See Moffitt @ AACR 2014 (February 19, 2014) below.
* To be clear, management has never described PV-10 as a cancer vaccine, nor am I making any inference about PV-10 being a vaccine. Rather, I'm trying to understand the context of the use of the word "immunity" by Moffitt as it relates to PV-10, a local immunotherapy with systemic properties and benefits. Notions of immunity, immunization, in situ vaccination, etc. feel, to me, vaccinesque in terms of the treatment goal and result. Immunotherapies stimulate immune responses, and their use inspires the possibilities of long (durable responses) fat (high impact percentages) survival tails. And even though we hope immunotherapies encourage long-term survival in cancer patients, we don't associate immunity with the likes of anti-CTLA-4, PD-1 and PD-L-1 agents.
From Ribas et al: "Immunotherapy strategies have the notorious ability to induce a low percentage but highly durable tumor responses, resulting in a plateau in the tail of the survival curve. Targeted therapy blocking driver oncogenes in melanoma induces rapid tumor responses but most are not durable, resulting in an early improvement in the survival curve but unclear beneficial effects on the tail of the curve."
Moffitt appears to have evolved its thinking about PV-10 from inducing [a systemic anti-tumor] immune response to inducing [anti-melanoma] immunity, which would seem a broader and bolder statement. Doesn't immunity suggest a much, much thicker survival tail, because recurrence then would not happen (post-PV-10 injection), or happen very infrequently?
"Cancer recurrence is defined as the return of cancer after treatment and after a period of time during which the cancer cannot be detected. (The length of time is not clearly defined.) The same cancer may come back where it first started or somewhere else in the body. For example, prostate cancer may return in the area of the prostate gland (even if the gland was removed), or it may come back in the bones. In either case it’s a prostate cancer recurrence" (Source: What is cancer recurrence?, American Cancer Society). It would seem to me no cancer recurrence (i.e., immunity from cancer [melanoma]) suggests the potential for much, much thicker survival tails.
Clinical Benefit (February 20, 2014)
More recently, an item from Provectus' January 24th press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes struck me, when thinking about it in the context of several recent accelerated approvals ("AAs"): the company's use of the phrase "clinical benefit." First, one quote by Craig: "We're grateful that our work with the Agency, in this and in our previous meetings, to identify a strategy for demonstration of clinical benefit in recurrent patients is bearing fruit."
Second, a comment from the company regarding their meeting with the FDA: "The Agency agreed with Provectus that treatment of cutaneous and subcutaneous tumors in patients with locally advanced cutaneous melanoma (i.e., recurrent, in-transit or satellite melanoma that has not yet spread from the skin to distant sites) could provide clinical benefit to such patients, particularly if the measured objective responses in patients' disease correlated to a demonstrated treatment effect on one or more symptoms of their disease (e.g., pain, infection or significant bleeding)."
In reading about these approvals, such as Northera (February 18th) for neurogenic orthostatic hypotension (non-oncology), Imbruvica (February 12th) for chronic lymphocytic leukemia (oncology), and Mekinist in combination with Tafinlar (January ) for advanced melanoma (oncology), the Agency approved the drug or drugs in question under the AA program, which allows for approval of a drug to treat a serious disease based on:
Moffitt @ AACR 2014 (February 19, 2014)
At the 2013 annual meeting of the American Association for Cancer Research ("AACR"), Moffitt's poster's abstract was entitled Intralesional injection with PV-10 induces a systemic anti-tumor immune response in murine models of breast cancer and melanoma. It concluded "In total, these studies support the induction of tumor-specific T cell-mediated immunity after single treatment with IL-PV-10 in multiple histologic subtypes. The immune mechanism of PV-10 ablation in cutaneous melanoma patients is currently under investigation at our institution." Moffitt essentially validated that PV-10 produced (induced) an immune response in mice (murine) studies for melanoma and breast cancer.
This should be the title of Moffitt's abstract (and poster presentation) at AACR 2014: Induction of anti-melanoma immunity after intralesional ablative therapy. The full abstract will be available next month. I'd hazard the guess Moffitt will discuss PV-10 produces anti-melanoma immunity following injection. "Immune response" has become "immunity."
"A zen approach to cancer...one drug could deal with all cancers because all cancers will have antigens..." (February 18, 2014)
An opinion courtesy of an internal medicine physician and shareholder: "I ran across this interview with Dr. Jim Allison, the discoverer of ipilimumab, that’s worth a very close listen. It provides a good history about the origins of tumor immunotherapy, T-cell activation and his discovery of the role of CTLA-4 that puts current interest in checkpoint inhibition and tumor vaccines in great perspective. It also puts the timing of possible IL [intralesional] PV-10 intervention into better context, especially from an initial T-cell priming point of view.
Looking at the intervention timeline chart below the interview (obviously somewhat outdated) one could envision a therapeutic protocol where IL PV-10 (along with GM-CSF [Granulocyte-Macrophage Colony-Stimulating Factor] for additional DC [dendritic cell] recruitment) is administered several hours prior to anti-CTLA-4 and anti-PD1 infusion as initial therapy. Repeat PV-10 dosing should likely precede additional PD-1 and CTLA-4 treatments to potentiate the long-term T-cell response and the “vaccine response.” Obviously other variables here can be modified, but this chart is a good conceptual start.
This is brilliant. The relevance of utilizing PV-10 as the immunological front-end of a therapeutic T-cell cascade perpetuated these checkpoint inhibitors is self-explanatory."
Change (February 17, 2014)
Provectus made a change to the company's website presentation:
Time to market (February 14, 2014)
Time to market for agents with breakthrough therapy designation ("BTD") status compared to without BTD (Source: Tom Silver, @TomSilver39).
Pfizer's "Immuno-Oncology" Strategy (February 13, 2014)
2Q13 Earnings conference call (July 30, 2013): "Tremelimumab, we did retain particularly the rights to use it for vaccines. And I shared with you some enthusiasm about our cancer vaccines platform. And I can inform you that you do include tremelimumab as one of the options for adjuvant effect on cancer vaccine. So that was a really good way for us to plan when we did that retention." Pfizer's answer in response to a question from Citigroup analyst Andrew Baum about tremelimumab.
4Q13 Earnings conference call (January 28, 2014): "In the vaccines area, you were right. We retained use for tremelimumab, particularly I would like to underline that antibody is part of the platform we are exploring together with unique prostate vaccine antigens and a very comprehensive delivery method of the vaccine to boost immune response. Our initial focus is on prostate cancer and we aspire to be in human studies by 2015. And at that time point, you will actually see a number of Pfizer cancer immunotherapy approaches move forward based on a panel of different monitorable antibodies." Pfizer's answer in response to a question from Baum about tremelimumab.
HemOnc Today (February 12, 2014)
More than likely, PV-10 will be discussed at the above conference organized by Provectus principal investigator Dr. Sanjiv Agarwala, MD (medical oncologist). The drug has been discussed here for the last several years, typically by Dr. Robert Andtbacka, MD (surgical oncologist, University of Utah, Huntsman Cancer Institute), who has been a principal investigator on T-Vec (formerly OncoVEX) and Allovectin-7 clinical trials. He usually pulls out the the intralesional therapy comparison slide below.
PV-10 should be discussed under the second topic of the session, Other Intralesional Therapies in Development, and perhaps in the third, Case Challenge: What is the Role of Systemic Intralesional Therapy?
Journal Publication (February 11, 2014)
Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma, Journal of Surgical Oncology, by Provectus principal investigator Dr. Merrick I. Ross, MD, Department of Surgical Oncology, Melanoma Section, MD Anderson.
Abstract: Rose Bengal is a novel injectable agent that has been evaluated as a rational treatment strategy for melanoma patients with recurrent unresectable local/regional metastases accessible for intralesional injection. PV-10 (10% Rose Bengal) has completed phase 1 and phase 2 multi-center clinical trials demonstrating significant local/regional disease control and also responses in non-injected regional bystander lesions and distant metastases. The published results of studies that have assessed PV-10 are presented, and the rationale for combining its use with recently approved immunotherapeutic agents is discussed.
Article: Summary & Future Directions: Patients with locally and regionally recurrent melanoma, with or without low volume distant metastases, represent a heterogeneous population with management issues that are complex and somewhat unique. Local disease control and prevention of distant disease progression are the relevant treatment goals, but no accepted standard of care exists. Many therapeutic options, including clinical trials, are available. Because of easy access to the local/regional disease, intralesional agents have been studied, but only recently with the rigor of formal prospective trials. IL PV‐10, is well tolerated, easy to administer, and based on Phase 1 and 2 data has an established and excellent safety profile, and results in meaningful responses in injected lesions and untreated bystander lesions. IL PV‐10 compares favorably with the published experience with the vast majority of other injectable agents in terms of side effects and local disease control. Moreover, the documented and corroborated bystander response offers a unique feature not shared by the other injectable agents mentioned previously. While a couple of the newer IL agents, such as allovectin‐7 and T‐VEC (formally known as Oncovex) have recently completed phase 3 evaluation and have demonstrated bystander effects, the bystander response observed with PV‐10 appears to be more frequent (Table I).
Conferences, Conferences, Conferences (February 11, 2014)
I think PV-10, and pre-clinical and clinical work related to the compound, may be mentioned at 5 to 7 medical and scientific conferences from March to July (see below). I will update the table as information is confirmed. Currently, only Moffitt's Sondak's slot at the 4th European Post-Chicago Melanoma/Skin Cancer Meeting can be confirmed.
Moffitt @ ASCO 2014? (February 10, 2014)
Moffitt Cancer Center's Dr. Vernon Sondak will present PV-10 material on Friday, June 27th at the 4th European Post-Chicago Melanoma/Skin Cancer Meeting in Munich, Germany (see below). Note the company PV-10 will keep in the session: Bristol-Myers Squibb's Yervoy and Nivolumab, Merck's MK-3475, GlaxoSmithKline's MAGE-A3, and Amgen's T-Vec.
% CR per thousand dollars of treatment cost (February 6, 2014)
Take a drug or drug candidate's complete response percent success and divide by the cost (per $1,000) for a course of treatment to arrive at this makeshift or hypothetical calculation of pharmaceutical alpha. I understand tumor shrinkage (beta) is good. Tumor destruction (alpha) is better. I also understand issues of toxicity, adverse events (immunological or otherwise), recurrence, resistance, etc. are not considered.
PV-10 & HCC/Cancer metastatic to the liver (February 5, 2014)
The company made several revisions to the expanded liver cancer Phase 1 trial, which can be seen side-by-side here (thank you to a blog reader for the heads up). I also noticed a third site, St Luke's University Health Network (not yet recruiting) in Bethlehem, Pennsylvania, and principal investigator, Dr. Sanjiv Agarwala, were added. Agarwala was a PI for Provectus' metastatic melanoma Phase 2 trial. His medical practice is focused on clinical trials, and he previously was involved in Delcath Systems' Phase 3 clinical trial for the treatment of inoperable metastatic melanoma in the liver. Agarwala more than likely will focus on melanoma mets to the liver (cutaneous, mucosal or ocular).
A number of these mets to the liver indications are so-called small addressable markets, but opportunities nonetheless, like ocular melanoma, which is a $100MM annual market in the U.S. according to a January 2013 Delcath Systems investor presentation. Ocular melanoma metastasis to the liver probably fits the breakthrough therapy designation well, given the language of a BTD drug: "a drug that is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies." There are several directed treatments for this indication, such as resection, immuno-embolization/chemo-embolization, radio-embolization, and percutaneous hepatic perfusion (using the chemotherapy drug Melphalan), as well as a range of systemic treatments (e.g., DTIC, TMZ, immunotherapy, targeted therapy). Hitting a mets liver tumor with PV-10 could be much more successful than other available therapies. For example, a 2003 hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver study of 19 patients yielded a 10% CR (and 52% PR). Provectus' limited liver cancer Phase 1 trial of 5 HCC and 1 colorectal met patients achieved substantial ablation, sustained regression and no disease at the then 9-15 month check-up; however, no further update or detailed analysis of data has been provided.
PV-10's Complete Response (CR) In Context (February 4, 2014)
The December and January that were:
Zero, Nada, Zilch, Zip, Goose Egg... (February 3, 2014)
Pfizer Breast Cancer Drug Succeeds In Mid-Stage Trial: "The oral drug, palbociclib, is considered one of Pfizer's most valuable products in development. Analysts have said it could have annual sales of more than $5 billion if successful." Palbociclib (PD 0332991) received breakthrough therapy designation in April 2013 for the potential treatment of patients with breast cancer. Note the complete response in the poster presentation of results below:
Note: Provectus completed a recurrent breast cancer Phase 1 trial in 2008 ("a classic ascending dose study") for which management has not revealed what if any efficacy results were achieved. The point of the above News entry is to highlight the inability of Big Pharma to kill tumors -- “We are pretty good at shrinking tumors, but not good at getting rid of them. Immune therapy is a way to begin to approach that.” -- Merck senior vice president Gary Gilliland (from an article by Robert Langreth on May 12, 2013)
Opinion (February 2, 2014)
An internal medicine physician and shareholder (who I know of) provided the following opinion, which you also may read on Yahoo! Finance's Provectus chat board today under PVCT's path to approval:
Readership of the blog set new highs for January. 7,892 unique visitors (30,556 visits and 71,402 page views) came from 50 U.S. states and the District of Columbia, 1,802 U.S. cities, 87 other countries and 366 international cities.
A New Drug Class (January 30, 2014)
New Press Release by St. Luke’s University Health Network (March 10, 2014)
St. Luke’s Participates in Interventional Clinical Trial to Evaluate the Benefits of the Tested Melanoma Drug PV-10 in Patients with Late-Stage Liver Cancer
"“Since PV-10 has cleared Phase II testing for melanoma due to its proven effectiveness, it was time to evaluate the drug to see if it could benefit patients with other types of cancer who have exhausted standard treatment options,” says Dr. Agarwala." Agarwala was a principal investigator ("PI") in the company's melanoma trials, and was a PI in Delcath's liver trials for melanoma cancers metastatic to the liver. See PV-10 & HCC/Cancer metastatic to the liver (February 5, 2014) and Liver Trial Update on ClinicalTrials.gov (February 27, 2014) below.
Phase 2 Recurrent Breast Cancer Trial Prep Underway? (March 6, 2014)
 |
| March 6th |
 |
| February 27th |
New Press Release (March 6, 2014)
PV-10 Immune Mechanism Data to Be Presented at the American Association for Cancer Research Annual Meeting. "Moffitt Cancer Center Poster Presentation on April 6, 2014."
Compare Provectus' 2014 PR about Moffitt's AACR abstract/poster presentation with the company's 2013 one. And, think about what has occurred, at the company, with the FDA and at Moffitt, in the intervening 12 months or so.
2013: Immunology Data on PV-10 to Be Presented at the American Association for Cancer Research Annual Meeting. "Moffitt Cancer Center Poster Presentation on April 8, 2013"
Craig's 2013 press release quote: "The researchers at Moffitt are providing valuable insights into how PV-10 harnesses the immune system in specific response to the injected tumor. Although the study being presented was done in murine models of melanoma and breast cancer, the currently open clinical trial at Moffitt is designed to investigate this mechanism in melanoma patients. We are pleased to enhance our understanding of intralesional PV-10 and its potential to produce a systemic benefit in cancer patients."
- Moffitt has played a key role in elucidating PV-10's mechanism of action from several perspectives including: (i) the cancer center's ability to reproduce (achieve the same outcome) and repeat (achieve the same outcome in mice and humans) Craig's work, (ii) understanding and articulating the whys and hows of the MOAs (as third parties, and confirming Craig's explanations and suppositions), (iii) providing experimental procedure rigor and process equal to or greater than Craig's, and (iv) lending their reputation to and support of the outcomes.
- From "researchers" to "translational medicine team:" Bench-to-bedside, "[t]ranslational medicine...is a discipline within biomedical and public health research that aims to improve the health of individuals and the community by “translating” findings into diagnostic tools, medicines, procedures, policies and education."
- "corroboration:" Reproducibility & repeatability. In this case, repeatability; what Moffitt found to happen in mice they found in humans.
- "recurrent locoregionally advanced melanoma:" Provectus' January 24th press release quoted Craig as saying: "This meeting with the FDA is another significant step forward in streamlining the pathway to initial U.S. approval of PV-10 as the first local agent for recurrent locoregionally advanced melanoma."
- "virtually unprecedented:" From a medical science perspective, it is amazing a small molecule like PV-10 has such immunological properties.
- "better explain the consistent “bystander response”:" From local benefit to systemic benefit, which explanations to boot.
- "More data:" At AACR, at ASCO, at EADO's Post-Chicago, ...
Provectus routinely communicates progress updates through its corporate website presentation, the latest version of which may be found here. Several changes were made to the March 5th version (the current update), compared to the February 27th version (the prior update).
The liver trial continues to enroll patients. And correcting the number of trial patients from 24 to 36, reflecting up to 24 patients treated with PV-10 ("Expansion Cohort 1") and up to 12 patients treated with PV-10 and sorafenib ("Expansion Cohort 2"). Re-read the company's September 2012 press release Provectus Expands Protocol for Phase 1 Liver Cancer Study in the context of Provectus' European Cancer Congress 2013 poster presentation that presented exploratory analyses of metastatic melanoma Phase 2 clinical trial data as lesions and numbers of lesions (rather than just patients and number of patients): "In both expansion cohorts, subjects will receive PV-10 treatment of a single hepatic tumor. Subjects with multiple injectable tumors will be eligible for re-enrollment for treatment of additional tumors if PV-10 is well-tolerated."
In the MM Phase 2 trial, responses of individual lesions were studied and detailed, almost as if each lesion and its outcome was equivalent or nearly equivalent to a patient, particularly in regards to demonstrating, "...via the self-evident ablative effect, that intralesional PV-10 provides a viable strategy to maintain long-term locoregional control of melanoma in patients whose cutaneous and subcutaneous melanoma has recurred."
 |
| March 5th |
 |
| February 27th |
The wrap-up (in 2014) of Moffitt's Phase 1 feasibility study. This study elucidated PV-10's mechanism of immune response in humans, the first work of which was revealed in the cancer center's AACR 2014 abstract.
 |
| March 5th |
 |
| February 27th |
 |
| March 5th |
 |
| February 27th |
 |
| March 5th |
 |
| February 27th |
Source: Tom Silver @TomSilver39.
 |
| Click to enlarge the figure. |
- Differentiation of immunotherapies based on efficacy and durability in multiple tumor types; multiple tumor types does not mean multiple indication, but rather multiple antigens (i.e., different tumors may contain different antigens depending on the make-up of the tumor),
- Safety is important,
- Could be used for all lines of therapy (e.g., adjuvant, 1st, 2nd and 3rd line),
- Immunotherapies in the adjuvant setting have the greatest potential to yield cures; it would make sense the earlier you use them the better because the immune system is not too compromised or compromised much less by tumor burden,
- CAR T-cell therapies have the potential to be paradigm shifting alone, presumably because the idea behind "CART" is to effect multiple tumor types (i.e., express more than one antigen to the T-cell through re-engineering ex vivo), and
- Combination, combination, combination.
Dr. Agarwala's HemOnc Today conference on April 10th and 11th has a more detailed program. Of note is Friday's session on Local and Regional Therapy, moderated by Dr. Andtbacka.
 |
| Click to enlarge the figure. |
 |
| 2013 NCCN Guidelines for Melanoma: Click to enlarge the figure |
 |
| 2009 NCCN Guidelines for Melanoma: Click to enlarge the figure |
A new site was added as a named location for the compassionate use program, Sharp Memorial Hospital in San Diego, California, which also is a liver cancer trial site. The updated information on ClinicalTrials.gov can be found here.
New Journal Article: Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages (March 1, 2014)
Journal of Immunotoxicology: "Rose Bengal (RB) has been used as a safe agent in clinical diagnosis. In addition, it is used as a photodynamic sensitizer for removing microorganisms and cancer cells. Recently, its preferential toxicity after direct exposure to cancer cells was proven. The present study focuses on anti-cancer and anti-inflammatory activities of RB. The toxicity of RB against AGS gastric cancer and NIH 3T3 fibroblast cell lines was studied using an MTT assay. Patterns of any cell death among the AGS cells were defined using Annexin-V and PI staining. In addition, the effect of RB on nitric oxide (NO) and prostaglandin E2 (PGE2) production induced by lipopolysaccha-ride in J774A.1 macrophages was determined. Modulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expressions in the macrophages was also evaluated by Western blots. The results showed that AGS cells exhibited significant concentration-dependent decreases in growth in response to RB; these cells showed a greater growth inhibition than did non-malignant 3T3 cells, suggesting that anti-growth activity of RB could be cell-specific. Moreover, AGS cells exposed to RB exhibited a significant increase in apoptosis; only at high RB doses did the cells display significant levels of necrosis. While RB also caused a modest decrease in the growth of J774A.1 macrophages, the cells displayed remarkable decreases in NO production and iNOS expression without significant concurrent modulation in PGE2 production or COX-2 expression. The data from this study appears to suggest that RB differentially impacts on transformed cell lines, preferentially suppresses growth of a gastric cancer cell line through induction of apoptosis, and induces changes in cells that could reflect potential anti-inflammatory effects that might be induced in situ."
Based on 2008 global statistics, gastric (stomach) cancer is one of the higher ranked indications for both new cases and deaths.
 |
| Click to enlarge the figure. Global Cancer Statistics, CA Cancer J Clin. 2011 Mar-Apr;61(2):69-90. doi: 10.3322/caac.20107. |
Blog readership fell from January 2014, but increased over December 2013. February month-over-month was -59% for # of unique visitors (3,242 v. 7,892), -51% for # of page views (35,012 v. 71,402) and -52% for # of visits (14,699 v. 30,556).
 |
| Click to enlarge the figure |
 |
| Click to enlarge the figure |
New SEC filings: Form 4 (February 28, 2014)
In Form 4 filings here (Tim) and here (Peter) today, Provectus principals Scott and Culpepper acquired 300,000 and ~190,000 shares of common stock, respectively, through stock option exercises ($1.10 exercise price).
Liver Trial Update on ClinicalTrials.gov (February 27, 2014)
Provectus' liver trial (HCC, and cancers metastatic to the liver) now is recruiting patients at St Luke's University Health Network, Dr. Agarwala's clinical trial-focused medical practice. Dr. Agarwala, a principal investigator for the company's metastatic melanoma trials, likely is addressing patients with melanoma-related cancer metastatic to the liver, like ocular melanoma. See also PV-10 & HCC/Cancer metastatic to the liver (February 5, 2014) below.
Anti-Melanoma Immunity (February 26, 2014)
I find Moffitt Cancer Center's switch from [a systemic anti-tumor] immune response to [anti-melanoma] immunity, in regard to their work on the systemic properties and benefits of PV-10, compelling and, in some ways, intriguing. See Moffitt @ AACR 2014 (February 19, 2014) below.
 |
| Immunity (medical), Wikipedia |
"The first clinical description of immunity which arose from a specific disease causing organism is probably Kitab fi al-jadari wa-al-hasbah (A Treatise on Smallpox and Measles, translated 1848) written by the Islamic physician Al-Razi in the 9th century. In the treatise, Al Razi describes the clinical presentation of smallpox and measles and goes on to indicate that that exposure to these specific agents confers lasting immunity (although he does not use this term). The first scientist who developed full theory of immunity was Ilya Mechnikov after he revealed phagocytosis in 1882. With Louis Pasteur’s germ theory of disease, the fledgling science of immunology began to explain how bacteria caused disease, and how, following infection, the human body gained the ability to resist further infections.
The birth of active immunotherapy may have begun with Mithridates VI of Pontus. To induce active immunity for snake venom, he recommended using a method similar to modern toxoid serum therapy, by drinking the blood of animals which fed on venomous snakes. According to Jean de Maleissye, Mithridates assumed that animals feeding on venomous snakes acquired some detoxifying property in their bodies, and their blood must contain attenuated or transformed components of the snake venom. The action of those components might be strengthening the body to resist against the venom instead of exerting toxic effect. Mithridates reasoned that, by drinking the blood of these animals, he could acquire the similar resistance to the snake venom as the animals feeding on the snakes. Similarly, he sought to harden himself against poison, and took daily sub-lethal to build tolerance. Mithridates is also said to have fashioned a 'universal antidote' to protect him from all earthly poisons. For nearly 2000 years, poisons were thought to be the proximate cause of disease, and a complicated mixture of ingredients, called Mithridate, was used to cure poisoning during the Renaissance. An updated version of this cure, Theriacum Andromachi, was used well into the 19th century. In 1888 Emile Roux and Alexandre Yersin isolated diphtheria toxin, and following the 1890 discovery by Behring and Kitasato of antitoxin based immunity to diphtheria and tetanus, the antitoxin became the first major success of modern therapeutic Immunology.
In Europe, the induction of active immunity emerged in an attempt to contain smallpox. Immunization, however, had existed in various forms for at least a thousand years. The earliest use of immunization is unknown, however, around 1000 A.D. the Chinese began practicing a form of immunization by drying and inhaling powders derived from the crusts of smallpox lesions. Around the fifteenth century in India, the Ottoman Empire, and east Africa, the practice of variolation (poking the skin with powdered material derived from smallpox crusts) became quite common. Variolation was introduced to the west in the early 18th century by Lady Mary Wortley Montagu. In 1796, Edward Jenner introduced the far safer method of inoculation with the cowpox virus, a non-fatal virus that also induced immunity to smallpox. The success and general acceptance of Jenner's procedure would later drive the general nature of vaccination developed by Pasteur and others towards the end of the 19th century." (Source: Immunity medical, Wikipedia)In speaking with cancer researchers, I understand a cancer immunotherapy to be any treatment that boosts an immune response. Cancer vaccines* are a form of immunotherapy. "Immunotherapy is a generic term, meaning anything that stimulates an immune response with the goal of achieving a therapeutic effect. A vaccine is a specific agent aimed at stimulating an immune response, often by introducing a single protein or even a single peptide antigen for the immune system to react against. If that particular target antigen is not present or not important, then the immune reaction is irrelevant." A cancer vaccine specifically induces an immune response against a defined tumor. In Moffitt's murine model work (some of which was presented at AACR 2013), direct injection of PV-10 into melanoma (i.e., a melanoma tumor) induced an immune response against melanoma, while injection of PV-10 into skin (i.e., not into a tumor, but merely into the skin) did not. This is an example of in situ vaccination; injection into a melanoma tumor leads to an immune response, while injection merely into the skin does not. "In situ vaccination means that the stimulation of the immune system is occurring against the tumor in its natural state, and hence is going to be directed at whatever antigens that particular tumor expresses. So the likelihood of a relevant immune reaction and hence an effective immunotherapy effect, is potentially much higher."
* To be clear, management has never described PV-10 as a cancer vaccine, nor am I making any inference about PV-10 being a vaccine. Rather, I'm trying to understand the context of the use of the word "immunity" by Moffitt as it relates to PV-10, a local immunotherapy with systemic properties and benefits. Notions of immunity, immunization, in situ vaccination, etc. feel, to me, vaccinesque in terms of the treatment goal and result. Immunotherapies stimulate immune responses, and their use inspires the possibilities of long (durable responses) fat (high impact percentages) survival tails. And even though we hope immunotherapies encourage long-term survival in cancer patients, we don't associate immunity with the likes of anti-CTLA-4, PD-1 and PD-L-1 agents.
 |
| Effects of immunotherapy and targeted therapy on melanoma survival curves Clin Cancer Res. Jan 15, 2012; 18(2): 336–341. |
Moffitt appears to have evolved its thinking about PV-10 from inducing [a systemic anti-tumor] immune response to inducing [anti-melanoma] immunity, which would seem a broader and bolder statement. Doesn't immunity suggest a much, much thicker survival tail, because recurrence then would not happen (post-PV-10 injection), or happen very infrequently?
"Cancer recurrence is defined as the return of cancer after treatment and after a period of time during which the cancer cannot be detected. (The length of time is not clearly defined.) The same cancer may come back where it first started or somewhere else in the body. For example, prostate cancer may return in the area of the prostate gland (even if the gland was removed), or it may come back in the bones. In either case it’s a prostate cancer recurrence" (Source: What is cancer recurrence?, American Cancer Society). It would seem to me no cancer recurrence (i.e., immunity from cancer [melanoma]) suggests the potential for much, much thicker survival tails.
Clinical Benefit (February 20, 2014)
More recently, an item from Provectus' January 24th press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes struck me, when thinking about it in the context of several recent accelerated approvals ("AAs"): the company's use of the phrase "clinical benefit." First, one quote by Craig: "We're grateful that our work with the Agency, in this and in our previous meetings, to identify a strategy for demonstration of clinical benefit in recurrent patients is bearing fruit."
Second, a comment from the company regarding their meeting with the FDA: "The Agency agreed with Provectus that treatment of cutaneous and subcutaneous tumors in patients with locally advanced cutaneous melanoma (i.e., recurrent, in-transit or satellite melanoma that has not yet spread from the skin to distant sites) could provide clinical benefit to such patients, particularly if the measured objective responses in patients' disease correlated to a demonstrated treatment effect on one or more symptoms of their disease (e.g., pain, infection or significant bleeding)."
In reading about these approvals, such as Northera (February 18th) for neurogenic orthostatic hypotension (non-oncology), Imbruvica (February 12th) for chronic lymphocytic leukemia (oncology), and Mekinist in combination with Tafinlar (January ) for advanced melanoma (oncology), the Agency approved the drug or drugs in question under the AA program, which allows for approval of a drug to treat a serious disease based on:
- For Northera, "...clinical data showing that the drug has an effect on an intermediate clinical measure...that is reasonably likely to predict the outcome of ultimate interest...,"
- For Imbruvica, "...a surrogate or intermediate endpoint that is reasonably likely to predict clinical benefit...," and
- For Mekinist and Taflinar, "...clinical data showing that the drug has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients."
I'm being repetitive above, but re-visiting Provectus' January 24th press release, according to management, the FDA agreed "...the treatment of cutaneous and subcutaneous tumors in patients with locally advanced cutaneous melanoma...could provide clinical benefit to such patients, particularly if the measured objective responses in patients' disease correlated to a demonstrated treatment effect on one or more symptoms of their disease."
Craig continued: "Measurement of tumor shrinkage via objective response criteria has been considered direct clinical benefit in drug approvals for other skin cancers and we believe a similar case can be made for PV-10 in locally advanced cutaneous melanoma. As advised by the Agency, we will submit data from the 28 patients in our Phase 2 study who had all existing disease treated in a formal BTD request this quarter, and should receive a decision within 60 days of receipt of that request."
Clinical benefit is tumor shrinkage (via objective response). Is (are) the surrogate endpoint(s) "one or more symptoms of their disease (e.g., pain, infection or significant bleeding)?" I think it (they) is (are). The FDA is as much or more of a legal animal as it is a medical, clinical or scientific one. Precedents (of past accelerated approvals) matter, and I think Eric is making (or has made) the case for accelerated approval on the basis of certain precedents, together with data, clinical benefit, etc.
Where does the breakthrough therapy designation ("BTD") fit in? I think it might be described as the umbrella under which the actual pathway to approval lies, whether that is accelerated approval with a post-marketing requirement of a "bridging study," or something else. When thinking about the language of BTD, we read "a drug that is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies."
A serious condition: "These patients suffer with troublesome, disfiguring disease that can persist for many years before presenting at distant sites. Our meeting with the Agency established the parameters for submission of a BTD request tailored to addressing the pressing needs of these patients" (Jan. 24th PR).
Over available therapies: The current National Comprehensive Cancer Network ("NCCN") guidelines for melanoma list clinical trial as the recommendation for patients with recurrent locoregionally advanced melanoma (i.e., where resection is not curative). PV-10 > available therapies.
First things first. Provectus needs to achieve and secure BTD for PV-10.
At the 2013 annual meeting of the American Association for Cancer Research ("AACR"), Moffitt's poster's abstract was entitled Intralesional injection with PV-10 induces a systemic anti-tumor immune response in murine models of breast cancer and melanoma. It concluded "In total, these studies support the induction of tumor-specific T cell-mediated immunity after single treatment with IL-PV-10 in multiple histologic subtypes. The immune mechanism of PV-10 ablation in cutaneous melanoma patients is currently under investigation at our institution." Moffitt essentially validated that PV-10 produced (induced) an immune response in mice (murine) studies for melanoma and breast cancer.
This should be the title of Moffitt's abstract (and poster presentation) at AACR 2014: Induction of anti-melanoma immunity after intralesional ablative therapy. The full abstract will be available next month. I'd hazard the guess Moffitt will discuss PV-10 produces anti-melanoma immunity following injection. "Immune response" has become "immunity."
 |
| Click on the table to enlarge it. |
An opinion courtesy of an internal medicine physician and shareholder: "I ran across this interview with Dr. Jim Allison, the discoverer of ipilimumab, that’s worth a very close listen. It provides a good history about the origins of tumor immunotherapy, T-cell activation and his discovery of the role of CTLA-4 that puts current interest in checkpoint inhibition and tumor vaccines in great perspective. It also puts the timing of possible IL [intralesional] PV-10 intervention into better context, especially from an initial T-cell priming point of view.
Looking at the intervention timeline chart below the interview (obviously somewhat outdated) one could envision a therapeutic protocol where IL PV-10 (along with GM-CSF [Granulocyte-Macrophage Colony-Stimulating Factor] for additional DC [dendritic cell] recruitment) is administered several hours prior to anti-CTLA-4 and anti-PD1 infusion as initial therapy. Repeat PV-10 dosing should likely precede additional PD-1 and CTLA-4 treatments to potentiate the long-term T-cell response and the “vaccine response.” Obviously other variables here can be modified, but this chart is a good conceptual start.
This is brilliant. The relevance of utilizing PV-10 as the immunological front-end of a therapeutic T-cell cascade perpetuated these checkpoint inhibitors is self-explanatory."
 |
| Click on the figure to enlarge it. |
Provectus made a change to the company's website presentation:
 |
| January 2014: Click to enlarge the figure. |
 |
| February 13, 2014: Click to enlarge the figure. |
Time to market for agents with breakthrough therapy designation ("BTD") status compared to without BTD (Source: Tom Silver, @TomSilver39).
 |
| Click to enlarge the figure. |
2Q13 Earnings conference call (July 30, 2013): "Tremelimumab, we did retain particularly the rights to use it for vaccines. And I shared with you some enthusiasm about our cancer vaccines platform. And I can inform you that you do include tremelimumab as one of the options for adjuvant effect on cancer vaccine. So that was a really good way for us to plan when we did that retention." Pfizer's answer in response to a question from Citigroup analyst Andrew Baum about tremelimumab.
4Q13 Earnings conference call (January 28, 2014): "In the vaccines area, you were right. We retained use for tremelimumab, particularly I would like to underline that antibody is part of the platform we are exploring together with unique prostate vaccine antigens and a very comprehensive delivery method of the vaccine to boost immune response. Our initial focus is on prostate cancer and we aspire to be in human studies by 2015. And at that time point, you will actually see a number of Pfizer cancer immunotherapy approaches move forward based on a panel of different monitorable antibodies." Pfizer's answer in response to a question from Baum about tremelimumab.
HemOnc Today (February 12, 2014)
More than likely, PV-10 will be discussed at the above conference organized by Provectus principal investigator Dr. Sanjiv Agarwala, MD (medical oncologist). The drug has been discussed here for the last several years, typically by Dr. Robert Andtbacka, MD (surgical oncologist, University of Utah, Huntsman Cancer Institute), who has been a principal investigator on T-Vec (formerly OncoVEX) and Allovectin-7 clinical trials. He usually pulls out the the intralesional therapy comparison slide below.
 |
| @PropThinker's Undervaluation of Vical’s Full Pipeline Allows A Cheap Bet on Cancer Trial (and $PVCT) |
Journal Publication (February 11, 2014)
Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma, Journal of Surgical Oncology, by Provectus principal investigator Dr. Merrick I. Ross, MD, Department of Surgical Oncology, Melanoma Section, MD Anderson.
Abstract: Rose Bengal is a novel injectable agent that has been evaluated as a rational treatment strategy for melanoma patients with recurrent unresectable local/regional metastases accessible for intralesional injection. PV-10 (10% Rose Bengal) has completed phase 1 and phase 2 multi-center clinical trials demonstrating significant local/regional disease control and also responses in non-injected regional bystander lesions and distant metastases. The published results of studies that have assessed PV-10 are presented, and the rationale for combining its use with recently approved immunotherapeutic agents is discussed.
Article: Summary & Future Directions: Patients with locally and regionally recurrent melanoma, with or without low volume distant metastases, represent a heterogeneous population with management issues that are complex and somewhat unique. Local disease control and prevention of distant disease progression are the relevant treatment goals, but no accepted standard of care exists. Many therapeutic options, including clinical trials, are available. Because of easy access to the local/regional disease, intralesional agents have been studied, but only recently with the rigor of formal prospective trials. IL PV‐10, is well tolerated, easy to administer, and based on Phase 1 and 2 data has an established and excellent safety profile, and results in meaningful responses in injected lesions and untreated bystander lesions. IL PV‐10 compares favorably with the published experience with the vast majority of other injectable agents in terms of side effects and local disease control. Moreover, the documented and corroborated bystander response offers a unique feature not shared by the other injectable agents mentioned previously. While a couple of the newer IL agents, such as allovectin‐7 and T‐VEC (formally known as Oncovex) have recently completed phase 3 evaluation and have demonstrated bystander effects, the bystander response observed with PV‐10 appears to be more frequent (Table I).
Further study of PV‐10 is certainly warranted either in the context of a phase 3 trial, or as a phase 1/2 study in combination with the checkpoint blocking agents or vaccines. The goal of these phase1/2 trials would be to document the safety of these novel combinations and demonstrate enhanced local/regional response and disease control as well as an augmentation of the systemic anti‐tumor responses. The success of such trials would further advance the melanoma treatment landscape. [Bold emphasis is mine.]
I think PV-10, and pre-clinical and clinical work related to the compound, may be mentioned at 5 to 7 medical and scientific conferences from March to July (see below). I will update the table as information is confirmed. Currently, only Moffitt's Sondak's slot at the 4th European Post-Chicago Melanoma/Skin Cancer Meeting can be confirmed.
 |
| Click on the table to enlarge it. |
Moffitt Cancer Center's Dr. Vernon Sondak will present PV-10 material on Friday, June 27th at the 4th European Post-Chicago Melanoma/Skin Cancer Meeting in Munich, Germany (see below). Note the company PV-10 will keep in the session: Bristol-Myers Squibb's Yervoy and Nivolumab, Merck's MK-3475, GlaxoSmithKline's MAGE-A3, and Amgen's T-Vec.
Results and Interpretations of ASCO Presentations 2014? Is Moffitt presenting PV-10 work at ASCO?
% CR per thousand dollars of treatment cost (February 6, 2014)
Take a drug or drug candidate's complete response percent success and divide by the cost (per $1,000) for a course of treatment to arrive at this makeshift or hypothetical calculation of pharmaceutical alpha. I understand tumor shrinkage (beta) is good. Tumor destruction (alpha) is better. I also understand issues of toxicity, adverse events (immunological or otherwise), recurrence, resistance, etc. are not considered.
 |
| Click on the table to enlarge it. |
The company made several revisions to the expanded liver cancer Phase 1 trial, which can be seen side-by-side here (thank you to a blog reader for the heads up). I also noticed a third site, St Luke's University Health Network (not yet recruiting) in Bethlehem, Pennsylvania, and principal investigator, Dr. Sanjiv Agarwala, were added. Agarwala was a PI for Provectus' metastatic melanoma Phase 2 trial. His medical practice is focused on clinical trials, and he previously was involved in Delcath Systems' Phase 3 clinical trial for the treatment of inoperable metastatic melanoma in the liver. Agarwala more than likely will focus on melanoma mets to the liver (cutaneous, mucosal or ocular).
A number of these mets to the liver indications are so-called small addressable markets, but opportunities nonetheless, like ocular melanoma, which is a $100MM annual market in the U.S. according to a January 2013 Delcath Systems investor presentation. Ocular melanoma metastasis to the liver probably fits the breakthrough therapy designation well, given the language of a BTD drug: "a drug that is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies." There are several directed treatments for this indication, such as resection, immuno-embolization/chemo-embolization, radio-embolization, and percutaneous hepatic perfusion (using the chemotherapy drug Melphalan), as well as a range of systemic treatments (e.g., DTIC, TMZ, immunotherapy, targeted therapy). Hitting a mets liver tumor with PV-10 could be much more successful than other available therapies. For example, a 2003 hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver study of 19 patients yielded a 10% CR (and 52% PR). Provectus' limited liver cancer Phase 1 trial of 5 HCC and 1 colorectal met patients achieved substantial ablation, sustained regression and no disease at the then 9-15 month check-up; however, no further update or detailed analysis of data has been provided.
PV-10's Complete Response (CR) In Context (February 4, 2014)
 |
| Click to enlarge the table. |
 |
| Click to enlarge the figure. |
Pfizer Breast Cancer Drug Succeeds In Mid-Stage Trial: "The oral drug, palbociclib, is considered one of Pfizer's most valuable products in development. Analysts have said it could have annual sales of more than $5 billion if successful." Palbociclib (PD 0332991) received breakthrough therapy designation in April 2013 for the potential treatment of patients with breast cancer. Note the complete response in the poster presentation of results below:
 |
| Preliminary results of a randomized Phase 2 study of Pd 0332991 Presented at 2011 San Antonio Breast Cancer Symposium, December 6-10, 2011, San Antonio, Texas |
Opinion (February 2, 2014)
An internal medicine physician and shareholder (who I know of) provided the following opinion, which you also may read on Yahoo! Finance's Provectus chat board today under PVCT's path to approval:
IMO, PVCT's recent PR clarifying their path to licensure through submission of a broader BTD has three additional interesting comments. Apart from the FDA now guiding PVCT away from undertaking a Phase III SPA trial (which would've been a Randomized Controlled Trial of 180 patients with Stage IIIB and IIIC melanoma followed for 30 months with Progression Free Survival (PFS) serving as the primary endpoint), they now are signaling that existing Phase II data for locally advanced cutaneous melanoma (Stage IIC and Stage III disease) may be compelling enough to approve an expedited approval path for PV-10. 28 of the 80 intent to treat (ITT) patients in Phase II had a 71% an objective response rate (ORR) which includes those with a complete response and partial response (tumors shrunk by at least 30%) when ALL observable tumors were injected. This will be how patients will be realistically treated in the future as opposed to previous protocols which limited the number of lesions treated, the frequency of injections and required the observation of untreated lesions for the bystander effect. A 71% ORR may even underestimate the response in Phase IV trials.
Secondly, it might not be fully appreciated, but the current management of all stages of malignant melanoma is the surgical removal of the tumor and lymph nodes following by chemotherapy and possible radiation, particularly in Stages III and IV. Per the PR statement “These data show that if a tumor is accessible to PV-10 injection, the drug is likely to destroy that tumor. If approved, PV-10 would be the first tissue-sparing local therapy for recurrent melanoma….Dees further stated, "Non-specific local treatments that temporarily reduce tumor burden, such as surgery and radiation, are the most commonly used cancer therapies today.” Thus initial treatment with PV-10 would be a profound breakthrough in patients whose early stage disease is initially managed with invasive surgical intervention
Lastly, the statement “Furthermore, we believe our clinical and immunologic mechanism data show that it may be possible to delay or prevent melanoma metastasis to distant sites." is pretty dramatic in the context of current chemotherapeutic management of all cancers. The possibility that PV-10 may act as a “cancer vaccine” to prevent distant metastases has implications way beyond the treatment of locally advanced melanomaJanuary Blog Stats (February 1, 2014)
Readership of the blog set new highs for January. 7,892 unique visitors (30,556 visits and 71,402 page views) came from 50 U.S. states and the District of Columbia, 1,802 U.S. cities, 87 other countries and 366 international cities.
 |
| Click to enlarge the figure. |
"Company ABC files patents for combinations because it (a) prevents other companies from controlling the marketing of ABC's drug in such combinations and (b) provides, in some cases, an opportunity for patent extension; for example, if ABC's drug A is combined with company XYZ's drug X as a new product. The primary value is (a) above, which allows a marketing push to expand application to other indications without relying on a third party to detail the market.
Thus, company ABC would (should) file a combination therapy patent for the combination of drug A with drugs (or classes of agents) B, C, D...n (i.e., math speak for all drugs). A good example of such a combination therapy patent is WO 2010/014784 A2, BMS' Combination of Anti-CTLA4 Antibody with Diverse Therapeutic Regimens for the Synergistic Treatment of Proliferative Diseases patent.
Now, what happens if ABC neglects to include drug {n+1}?
Nothing, because generic disclosure and claims should extend coverage to {n+1}, or at least prevent another the company that developed {n+1} from claiming the (A, {n+1}) combination due to obviousness. The {n+1} owner has trouble claiming (A, {n+1}) due to this obviousness, unless they can show a highly unanticipated advantage. So, it is generally possible for company ABC to add drug {n+1} later, as a continuation of the original patent application, while it is hard for the {n+1} owner to do this due to the obviousness issue.
Omitting drug {n+1} potentially is relevant if {n+1} is unrelated to B...n; for example, drug {n+1} belongs to a new class of agent or has a highly unanticipated advantage."
Immunomodulatory drugs, another class of drug and also an approved class, include anti-CTLA-4 (such as Bristol-Meyers' approved ipilimumab/Yervoy), -PD-1 (Bristol-Myers' nivolumab and Merck's lambrolizumab) and -PD-L-1 agents.
The company's January 24, 2014 press release Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes notes "Treatment of "Locally Advanced Cutaneous Melanoma" to be Path to Initial Licensure" as a byline. Assuming a path to approval, eventual or ultimate approval of the drug should also mean approval of a new drug class, which further speaks to this innovation.
rev·o·lu·tion·ize (January 28, 2014)
"Moffitt Cancer Center researchers have laid the groundwork for a revolutionary new combination therapy for the treatment of advanced melanoma – melanoma that cannot be removed surgically or has spread to other areas of the body. The newly FDA-approved therapy, Mekinist (trametinib) in combination with Tafinlar (dabrafenib), is one of the biggest advancements in melanoma treatment in the past 30 years.
“Melanoma is the most aggressive type of skin cancer and the leading cause of death from skin disease,” said Jeffrey S. Weber, M.D., Ph.D., director of Moffitt’s Melanoma Research Center of Excellence. “This new combination therapy is a huge step in the right direction for the treatment of melanoma, and our researchers played a large role in bringing this treatment option to patients.”"
August 22, 2013: Single Injection May Revolutionize Melanoma Treatment, Moffitt Study Shows [Bold emphasis is mine]
"A new study at Moffitt Cancer Center could offer hope to people with melanoma, the deadliest form of skin cancer. Researchers are investigating whether an injectable known as PV-10 can shrink tumors and reduce the spread of cancer. PV-10 is a solution developed from Rose Bengal, a water-soluble dye commonly used to stain damaged cells in the eye. Early clinical trials show PV-10 can boost immune response in melanoma tumors, as well as the blood stream.
“Various injection therapies for melanoma have been examined over the past 40 years, but few have shown the promising results we are seeing with PV-10,” said Shari Pilon-Thomas, Ph.D., assistant member of Moffitt’s Immunology Program."
 |
| Click to the spreadsheet to enlarge it |
PVCT's market capitalization is $298 million. Its 1-week price change was -24%. Its 52-week price change was +258%. This information was derived from Yahoo! Finance. Herper's "...screen includes biotechnology and medical companies traded on the New York Stock Exchange, the Nasdaq or the American Stock Exchange that had market capitalizations of more than $300 million as of a week ago. The data come from Interactive Data and Thomson Reuters Fundamentals via FactSet Research Systems." Bold and underlined emphasis is mine.
Provectus's PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes
"Treatment of “Locally Advanced Cutaneous Melanoma” to be Path to Initial Licensure. Phase 2 Data to be Submitted in Formal BTD Request This Quarter with FDA Response Expected Within 60 Days of Receipt."
Issued: January 24, 2014. The link to the press release is here, and to the associated 8-K filing here.
Adam Feuerstein: "The Obsolescence of Provectus' Skin Cancer Drug Means Current Speculative Run Ends Badly" (January 23, 2014)
On Thursday, during the trading day, Adam Feuerstein published an article about Provectus entitled The Obsolescence of Provectus' Skin Cancer Drug Means Current Speculative Run Ends Badly. He began more actively tweeting about the company on Monday, a market holiday here in the U.S. (Martin Luther King Day). The article cast Provectus and PV-10 in a negative light. The company's stock sold off heavily (down to $1.85 per share) on immense volume (more than 30 million shares). As of this writing, nothing regarding my investment thesis for Provectus has changed. I have not sold any of the shares we have accumulated thus far.
Updated 1/24/14: Provectus responded to the article by writing a letter to editor of TheStreet.com, filing an associated 8-K in the process.
G-Force (January 22, 2014)
The blog had its first 1K+ visitor day on January 21st: 2,016 visits, 1,194 unique visitors, 5,046 page views, 00:04:45 average visit duration, 43.34% bounce rate, and 25.85% new visits. 45 U.S. states and 589 cities. Rest of the world: 33 countries & 67 cities.
 We wait for Provectus management to reveal regulatory guidance from the FDA. Presumably, they know what they got, and what they have to do to get what they want. I imagine they did what needed to be done. So, when will they say what they got? Much remains to be communicated about further advancing the company's lead compounds to shareholders beyond the result(s) of final FDA meeting minutes:
We wait for Provectus management to reveal regulatory guidance from the FDA. Presumably, they know what they got, and what they have to do to get what they want. I imagine they did what needed to be done. So, when will they say what they got? Much remains to be communicated about further advancing the company's lead compounds to shareholders beyond the result(s) of final FDA meeting minutes:- The promised publication of Provectus' metastatic melanoma Phase 2 clinical trial results, notably duration of response,
- The addition of more liver trial sites to the 2 previously announced, and the number of patients enrolled and treated thus far in the company's expanded metastatic liver cancer Phase 1 trial,
- The announcement of a recently published paper discussing PV-10's orthogonality to other drugs; that is, its lack of drug-drug interaction in combination with other compounds, which will be germane to an eventual liver cancer Phase 2/3 trial comparing sorafenib and sorafenib + PV-10,
- An update on Rockefeller University's work on PH-10's lack of toxicity and the dermatology drug's mechanism of action ("MOA"), much in the same way Moffitt Cancer Center elucidated PV-10's MOA, and
- Speaking of Moffitt, an update on the cancer center's further pre-clinical and clinical translational work on PV-10, such as for use on other indications beyond their public statements on melanoma and breast cancer, and in combination with other therapies like anti-CTLA-4/PD-1/PD-L-1 agents.
Provectus' stock achieved several firsts today, as the company's market capitalization (per Yahoo! Finance) reached nearly $560 million with a $3.99 closing share price. The market cap is remarkable, as a standalone number and also given that aside from the company's December 18th press release Provectus Type C Meeting With FDA Oncology Division Held December 16, 2013 Provectus has not released real news since, depending on your viewpoint, a key patent via its October 24th PR Provectus Awarded PV-10 Patent by U.S. Patent and Trademark Office or Phase 2 clinical trial data viewed a different way via the company's September 30th PR Exploratory Data Analyses of Intralesional PV-10 Clinical Phase 2 Study Results Presented at ECC 2013 Demonstrate Effective Locoregional Disease Control and Support Systemic Immunologic Activity in Refractory Metastatic Melanoma.
Today marked the highest daily volume traded.
 |
| Click the table to enlarge it. |
 |
| Click the table to enlarge it. |
 |
| Click the table to enlarge it. |
 |
| Click the table to enlarge it. |
 |
| January 21st |
The synthesis patent "...details a new process for the manufacture of Rose Bengal and related iodinated xanthenes in high purity, and was allowed in June 2013. The patent covers the process under which pharmaceutical grade Rose Bengal and related xanthenes are produced, reducing the formation of certain previously unknown transhalogenated impurities that currently exist in commercial grade Rose Bengal in uncontrolled amounts. The requirement to identify and control related substances is in accordance with International Conference on Harmonisation (ICH) guidelines for manufacture of API suitable for phase 3 clinical trial material and commercial pharmaceutical use." (PR here)
The third patent above complements the synthesis patent for "...manufacturing Rose Bengal for pharmaceutical use and Rose Bengal analogs covered in [an] earlier patent." (PR here)
Unique visitors to the blog reached nearly 5,200 on a 1-month (31 days) trailing basis (December 21, 2013-January 20, 2014): 20,146 visits, 5,197 unique visitors, 44,644 page views, 00:03:41 average visit duration, 48.48% bounce rate, and 22.71% % new visits. The blog surpassed 3,300 visitors on a 1-month trailing basis on January 7, 2014. See Sustained Awareness (January 8, 2014) below.
Equity Research Coverage (January 21, 2014)
The company has had insubstantial historical research coverage from the likes of Rodman & Renshaw, Maxim Group, Roth Capital, Stonegate Securities, etc. in the past. Provectus' website currently indicates only coverage from Dr. Echo He, MD, PhD at Maxim. Visitors to the blog in recent days, including today, have included Deutchse Bank and Stifel Nicolaus. I'm curious if these visits relate to exploring equity research coverage of the company.
"Advancing A Winning Front In The War Against Cancer" (January 20, 2014)
More changes to the website presentation dated January 20, 2014.
 |
| January 20th |
 |
| January 16th |
 |
| January 20th |
 |
| January 8th |
 |
| January 20th |
 |
| January 16th |
 |
| January 20th |
 |
| January 16th |
In recent weeks, especially over the last several days, a number of financial services institutions visited the blog. They come for their own reasons. Visitors range from broker-dealers to investment banks to asset management firms. They may visit once (thus far), a few times, or in the case of some seem to squat on the blog:
- Asset management firms American Century Investments and T. Rowe Price,
- Investment banks Credit Suisse, Deutsche Bank, J.P. Morgan, Bank of America Merrill Lynch, Morgan Stanley and Citibank/Citigroup,
- Prime broker BTIG,
- Trading firm Susquehanna International Group,
- Brokerage firms and financial advisers Wells Fargo, Charles Schwab, Ameriprise Financial, and Raymond James Morgan Keegan.
- Broker-dealer Aegis Capital.
Moving towards approval. Label getting bigger. (January 16, 2014)
For folks not versed in all things Provectus, it's hard to understand why one has to regularly review the company's website presentation for information and knowledge. Management routinely updates this material. Reading the January 16th version that was revised and posted today, there is old and "new" news. The old news is the company is speaking with the FDA regarding the approval of the drug.
 |
| January 16, 2014 |
 |
| January 14, 2014 |
 |
| January 16, 2014 |
 |
| January 14, 2014 |
New SEC filing (January 15, 2014)
Provectus filed an 8-K in regards to the company's ongoing interaction with the FDA following Provectus' December 16th meeting (conference call) with the Agency: "Reference is made to...a Type C meeting with the FDA Division of Oncology Products 2 on December 16, 2013 to discuss the Company’s oncology drug, PV-10. Subsequently, the Company took the opportunity to provide input into the documentation of meeting minute notes. It is the opinion of the Company that this may have delayed the process of receiving the final meeting minute notes. Therefore, the Company will communicate further guidance from the FDA once the meeting minute notes have been received, which is expected shortly. It is also the Company's opinion that the delay has no bearing on the FDA's decision regarding the efficacy of PV-10 but is rather a matter of observing governmental processes. This delay also has no bearing on the Company's discussions and negotiations with parties outside the FDA's jurisdiction." Bold and underlined emphasis is mine.
Having provided the market a date, January 15th (in the December 18th PR), by which they expected to communicate regulatory guidance, management appears to have indicated through today's filing the delay is (was) due to the FDA and company having to reconcile meeting minute notes, which really boils down to finalizing an agreement between Provectus and the Agency regarding the pathway to approval of PV-10.
Paying Attention (January 15, 2014)
To say folks, the markets, whoever, are paying attention to Provectus' situation is a bit of an understatement, particularly relative to the size of the crowd (or, more to the point, lack thereof) just a few months ago. We see it everywhere: the company's website, this blog, Twitter, Provectus stock boards, the share price, daily trading volume, etc. 14 days into January the blog registered new visitation and readership records for 4 of 8 stats I track.
A lot of folks new or relatively new to the company, drug and stock should pay attention to what management says, doesn't say, etc. That's really how long-time shareholders who pay attention to the company have come to discern the potential path to commercialization and monetization.
For example, there will be no Phase 3 trial. How do I know that? I don't, but [I think] I have a good sense it won't when management removes the slide below (last included in the January 8, 2014 version of Provectus' website presentation). The January 8th version had 31 slides while the January 14th version had 30 slides (of which the below is not one of them).
For example, the drug will be approved within sone reasonable time frame. How do I know that? I don't, but [I think] I have a good sense it will when management adjusts the slide below. Compare the January 8th version with the January 14th version.
The company advertised the arrival of FDA minutes as January 15th in its December 18th press release. Clarity, in management's own words, may not necessarily arrive via a Provectus PR on the 15th, or 16th or even 17th. It may arrive as late as next week. I sure hope it doesn't, but I'll not sweat the additional time it may take. Shortly, perhaps very shortly, I expect the paradigm shift that is PV-10 will be heralded as the local therapy that can (and has been actually or effectively approved to) treat a systemic disease.
More Awareness = More Scrutiny = A Good Thing (January 14, 2014)
The blog set another record for daily visitation and readership on January 13th: 716 unique visitors, 1,112 visits and 2,069 page views.
This week obviously has great significance for the company: regulatory clarity. Provectus also may comment on its current cash balance given the high likelihood of a large number of warrant exercises resulting in a substantial inflow of cash. I think my number of around $17 million, projected on December 31, 2013 (see below), is now higher. It's also possible we get some further update on liver trial progress, which would be a nice follow-up to Orthogonality below and should be relevant when the company submits its breakthrough therapy application for liver cancer (the pivotal trial likely would be a Phase 2/3 RCT of sorafenib (Nexavar) vs. sorafenib + PV-10 suitable/designed for accelerated approval).
Orthogonality (January 13, 2014)
In mathematics, orthogonality is the relation of two lines at right angles to one another (perpendicularity). Drug-drug interactions refers to the interaction between drugs.
In vitro inhibition of human liver cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) enzymes by rose bengal: system-dependent effects on inhibitory potential, a paper's whose authors include Eric and, I believe, Provectus' Jamie (Singer): "...the risk of rose bengal to cause clinically relevant drug-drug interactions is likely low, particularly when administered to cancer patients on an intermittent schedule. PubMed link is here. The article addresses PV-10 and orthogonality in general, and the lack of drug-drug interaction of PV-10 in combination with sorafenib in particular.
A Short or A Short Squeeze, or Much Ado About Nothing (January 13, 2014)
I understand Fidelity regularly calls at least a few Provectus shareholders to inquire about loaning their PVCT shares to cover short interests. Assume I take that (which I received after writing my January 11th post) at face value. I wrote my post Some Size of Short Likely to be Visited upon Provectus Next Week after hearing from a very large shareholder who I speak to regularly and who said to me he was called by Fidelity for the first time on this topic. I originally had titled my post "Some Size of Short Squeeze or Short..." because [without enough understanding of the process and administration of shorting equities; some of my investment industry experience derives from trading various currency and interest rate products] I thought the call (and I assumed others were called) indicated a party (parties) wishing to either cover existing short interests and/or initiate new ones. After calling some Wall Street-type or experienced traders currently holding Provectus shares (I believe them when they say they are committed longs, assuming of course no change in their respective investment theses) to provide me feedback. The consensus was shorts potentially initiating new positions rather than closing existing ones. Ultimately, I don't know for sure what can or will happen next week in relation to last week's calls, and they don't know for sure either. Their first thoughts were not to consider shorting activity related to warrant exercises and sales of common shares. I thought it was worthwhile then, and still do now, however, to let blog readers know of this information, and my view (right as it may be next week, or outright wrong). As I wrote in an update to the post, "...potential or actual shorting does not make me nervous. I'm comfortable with my investment thesis, which likely is a much longer-termed one than prospective short-oriented traders would have."
Telling Blog Stats: "More?" (January 12, 2014)
Blog readership, on a weekly basis, reached new highs for the period January 5th to 11th. For the week, the number of unique visitors was 1,553 (+36% over the last high of 1,138 for December 22nd to 28th). Page views were 10,194 (+50%, 6,818, prior week). Visits were 4,496 (+45%, 3,101, prior week).
Moffitt (January 11, 2014)
Moffitt Cancer Center extended the estimated study completion date of its Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study from March 2014 to December 2014. While some might say that is a bad thing, I think extending the date fits well within the narrative (rumors) of more and broadening study of PV-10 by Moffitt and unprecedented spectacular-ness of Center results. After all, this is the same organization that heralded PV-10 in August 2013 as: Single Injection May Revolutionize Melanoma Treatment, Moffitt Study Shows. One tumor, one injection, one nothing ("One shot, one kill").
Short Interest Rises (January 10, 2014)
Sustained Awareness (January 8, 2014)
Setting aside December 2013 blog stats for visitation and readership for a moment, through 7+ days of January 2014 (through 6 pm ET on 1/8) blog stats set nearly all new record highs (see below).
Unique visitors to the blog surpassed 3,300 on a 1-month (31 days) trailing basis (December 8, 2013-January 7, 2014): 12,453 visits, 3,311 unique visitors, 26,770 page views, 00:03:21 average visit duration, 49.26% bounce rate, and 23.19% % new visits.
Blog reader/Shareholder E-mail (January 7, 2014)
"I am a newbie to buying stocks online. About a month ago I started researching biotech companies, as I wanted my first investment to be something I feel passionate about, specifically a new cancer drug. I have seen my share of family and friends die from cancer, and in my opinion, their treatments made them needlessly suffer. So my search was a wild goose chase until I came upon Provectus. It was a perfect match for my criteria- a drug that is able to treat many types of cancers and doesn't cost an arm and a leg, and most of all does no harm. Plus a management team that have all their ducks in a row. Your blog was extremely helpful, as it went above and beyond my DD, and I thank you for this. I now own a part of this company and their amazing story! This leads me to the purpose of contacting you. As a graphic artist I couldn't help myself when I read what Tim said about the Tennessee welcoming sign. So in my artistic way I want to contribute a little something to your blog. Feel free to use it if you choose."
In my November 5, 2013 post Expect More I had written: "I have spent time trying to understand and record certain history of Craig, Tim and Eric, as ORNL turned into Photogen Technologies turned into Provectus Pharmaceuticals. How did they approach and solve the root of a problem of global importance called cancer? Specificity (at a cellular level). Safety. Route of delivery. A thoughtful choice of Rose Bengal, when it came to the long game of regulatory approval, and drug development expense.
In time, I hope to blog about this history. For now, as the story goes, Craig rushed to the Knoxville airport to test their hypothesis by passing a vial containing a solution of Rose Bengal through an X-ray scanner. In doing so, they not only successfully tested the hypothesis but reduced their idea to practice in the process, showing it worked. As he retrieved the vial and walked away from the machine toward his car he had abandoned in front of the airport, Craig said he felt giddy because he knew he was holding the cure for cancer in his hands. If I have this correct, Tim is said to have remarked he wanted the phrase "cancer was cured here" adorning Welcome to Tennessee signs along those portions of highway crossing state lines. He should get his wish."
I'd like to thank the blog reader and fellow shareholder from Fort Plain, New York for the e-mail and the very cool Tim Scott-inspired graphic.
Volume (January 3, 2014)
One of the impressive aspects of the stock's performance in December and thus far in January has been its daily traded volume. According to the OTC Bulletin Board website, Provectus traded more than 40 million shares in December, compared with 8 million in November (the prior month), nearly 15 million in September (when the stock popped to an intraday high of $1.14 per share) and less than 5 million in January (to begin 2013).
Thus far in January 2014 the stock has traded nearly 7 million shares: 2.4 million on the 2nd and 4.3 million today (per Yahoo! Finance).
CUSIP Watch (January 3, 2014)
No CUSIP change [at least for our holdings] as of this writing.
Interesting (January 3, 2014)
A pre-market tweet today. >$3 today?
Get Shorty in a Big Way (January 3, 2014)
It is conceivable the CUSIP change, which resulted from the company's name change from Provectus Pharmaceuticals to Provectus Biopharmaceuticals (currently is in process), might have been as much about combating naked shorts and reflecting upcoming business developments as it was about better future branding. No more convertible preferred stock, a new CUSIP, some words about the regulatory process, etc., and the stock's price and daily traded volume run since the company's December 17th and 18th press releases have been strong: more than a 100% increase in the share price and [taken at face value from Yahoo! Finance] more than 25 million cumulative shares traded as of this writing.
We're #3 (January 2, 2014)
Google Provectus, and you'll find the blog as the number three search return, all without the use or benefit of search engine optimization. Ahead of it are the company's website and its Yahoo! Finance stock quote.
Strong. Getting Stronger. (December 31, 2013)
Provectus ended its third calendar quarter (3Q13) with about $8.3MM cash on hand. Assume a cash burn rate for the fourth quarter of about $2.1MM (roughly consistent with the prior two quarters; the first quarter came in under $3MM). I speculate the company may have raised an additional $1-2MM in early-October based on awkward wording in the third quarter filing, although I may be wrong about this. The company had about 30MM warrants outstanding as at 12/31/12.
Provectus issued at least about 36MM warrants in 2013 for consultant services and related to fund raising (net of forfeited warrants).
All warrants should be in-the-money, with exercise prices below $2 per share and the vast majority (perhaps 50MM or thereabouts) having a $1 exercise price. Warrants are undoubtedly being exercised. Some are fully exercised; that is, for a holder of one $1 warrant, they pay Provectus $1 in exchange for one share of common stock that they may hold or subsequently sell. Some are exercised on a cashless basis, where the holder receives a fraction of one common stock share for each warrant exercised depending on the then share price. My guess as to cashless exercise is below.
With likely and potential warrant exercises since the share price surge, how much cash might Provectus have on hand as at 12/31/13?
It all depends on the number of warrants exercised. 10MM, 20MM, 30MM, ...?
Projected Cash at December 31, 2013: $17MM? More? Less? The longer the share price remains well above the warrants' exercise prices (i.e., primarily above $1 per share), the more likely the warrants are going to be exercised. This potentially significant influx of cash onto Provectus' balance sheet very likely provides for future clinical trials (and other key operational expenses) without having to raise additional money.
Provectus Biopharmaceuticals (December 29, 2013)
To say folks, the markets, whoever, are paying attention to Provectus' situation is a bit of an understatement, particularly relative to the size of the crowd (or, more to the point, lack thereof) just a few months ago. We see it everywhere: the company's website, this blog, Twitter, Provectus stock boards, the share price, daily trading volume, etc. 14 days into January the blog registered new visitation and readership records for 4 of 8 stats I track.
 |
| Click to enlarge the table. |
For example, there will be no Phase 3 trial. How do I know that? I don't, but [I think] I have a good sense it won't when management removes the slide below (last included in the January 8, 2014 version of Provectus' website presentation). The January 8th version had 31 slides while the January 14th version had 30 slides (of which the below is not one of them).
For example, the drug will be approved within sone reasonable time frame. How do I know that? I don't, but [I think] I have a good sense it will when management adjusts the slide below. Compare the January 8th version with the January 14th version.
 |
| January 8th |
 |
| January 14th. |
More Awareness = More Scrutiny = A Good Thing (January 14, 2014)
The blog set another record for daily visitation and readership on January 13th: 716 unique visitors, 1,112 visits and 2,069 page views.
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
Orthogonality (January 13, 2014)
In mathematics, orthogonality is the relation of two lines at right angles to one another (perpendicularity). Drug-drug interactions refers to the interaction between drugs.
In vitro inhibition of human liver cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) enzymes by rose bengal: system-dependent effects on inhibitory potential, a paper's whose authors include Eric and, I believe, Provectus' Jamie (Singer): "...the risk of rose bengal to cause clinically relevant drug-drug interactions is likely low, particularly when administered to cancer patients on an intermittent schedule. PubMed link is here. The article addresses PV-10 and orthogonality in general, and the lack of drug-drug interaction of PV-10 in combination with sorafenib in particular.
A Short or A Short Squeeze, or Much Ado About Nothing (January 13, 2014)
I understand Fidelity regularly calls at least a few Provectus shareholders to inquire about loaning their PVCT shares to cover short interests. Assume I take that (which I received after writing my January 11th post) at face value. I wrote my post Some Size of Short Likely to be Visited upon Provectus Next Week after hearing from a very large shareholder who I speak to regularly and who said to me he was called by Fidelity for the first time on this topic. I originally had titled my post "Some Size of Short Squeeze or Short..." because [without enough understanding of the process and administration of shorting equities; some of my investment industry experience derives from trading various currency and interest rate products] I thought the call (and I assumed others were called) indicated a party (parties) wishing to either cover existing short interests and/or initiate new ones. After calling some Wall Street-type or experienced traders currently holding Provectus shares (I believe them when they say they are committed longs, assuming of course no change in their respective investment theses) to provide me feedback. The consensus was shorts potentially initiating new positions rather than closing existing ones. Ultimately, I don't know for sure what can or will happen next week in relation to last week's calls, and they don't know for sure either. Their first thoughts were not to consider shorting activity related to warrant exercises and sales of common shares. I thought it was worthwhile then, and still do now, however, to let blog readers know of this information, and my view (right as it may be next week, or outright wrong). As I wrote in an update to the post, "...potential or actual shorting does not make me nervous. I'm comfortable with my investment thesis, which likely is a much longer-termed one than prospective short-oriented traders would have."
Telling Blog Stats: "More?" (January 12, 2014)
Blog readership, on a weekly basis, reached new highs for the period January 5th to 11th. For the week, the number of unique visitors was 1,553 (+36% over the last high of 1,138 for December 22nd to 28th). Page views were 10,194 (+50%, 6,818, prior week). Visits were 4,496 (+45%, 3,101, prior week).
 |
| Click to enlarge the figure. |
Moffitt Cancer Center extended the estimated study completion date of its Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study from March 2014 to December 2014. While some might say that is a bad thing, I think extending the date fits well within the narrative (rumors) of more and broadening study of PV-10 by Moffitt and unprecedented spectacular-ness of Center results. After all, this is the same organization that heralded PV-10 in August 2013 as: Single Injection May Revolutionize Melanoma Treatment, Moffitt Study Shows. One tumor, one injection, one nothing ("One shot, one kill").
Short Interest Rises (January 10, 2014)
 |
| Click to enlarge the figure. |
Setting aside December 2013 blog stats for visitation and readership for a moment, through 7+ days of January 2014 (through 6 pm ET on 1/8) blog stats set nearly all new record highs (see below).
 |
| Click to enlarge the table. |
Blog reader/Shareholder E-mail (January 7, 2014)
"I am a newbie to buying stocks online. About a month ago I started researching biotech companies, as I wanted my first investment to be something I feel passionate about, specifically a new cancer drug. I have seen my share of family and friends die from cancer, and in my opinion, their treatments made them needlessly suffer. So my search was a wild goose chase until I came upon Provectus. It was a perfect match for my criteria- a drug that is able to treat many types of cancers and doesn't cost an arm and a leg, and most of all does no harm. Plus a management team that have all their ducks in a row. Your blog was extremely helpful, as it went above and beyond my DD, and I thank you for this. I now own a part of this company and their amazing story! This leads me to the purpose of contacting you. As a graphic artist I couldn't help myself when I read what Tim said about the Tennessee welcoming sign. So in my artistic way I want to contribute a little something to your blog. Feel free to use it if you choose."
In my November 5, 2013 post Expect More I had written: "I have spent time trying to understand and record certain history of Craig, Tim and Eric, as ORNL turned into Photogen Technologies turned into Provectus Pharmaceuticals. How did they approach and solve the root of a problem of global importance called cancer? Specificity (at a cellular level). Safety. Route of delivery. A thoughtful choice of Rose Bengal, when it came to the long game of regulatory approval, and drug development expense.
In time, I hope to blog about this history. For now, as the story goes, Craig rushed to the Knoxville airport to test their hypothesis by passing a vial containing a solution of Rose Bengal through an X-ray scanner. In doing so, they not only successfully tested the hypothesis but reduced their idea to practice in the process, showing it worked. As he retrieved the vial and walked away from the machine toward his car he had abandoned in front of the airport, Craig said he felt giddy because he knew he was holding the cure for cancer in his hands. If I have this correct, Tim is said to have remarked he wanted the phrase "cancer was cured here" adorning Welcome to Tennessee signs along those portions of highway crossing state lines. He should get his wish."
I'd like to thank the blog reader and fellow shareholder from Fort Plain, New York for the e-mail and the very cool Tim Scott-inspired graphic.
Volume (January 3, 2014)
One of the impressive aspects of the stock's performance in December and thus far in January has been its daily traded volume. According to the OTC Bulletin Board website, Provectus traded more than 40 million shares in December, compared with 8 million in November (the prior month), nearly 15 million in September (when the stock popped to an intraday high of $1.14 per share) and less than 5 million in January (to begin 2013).
 |
| Click to enlarge the figure. |
CUSIP Watch (January 3, 2014)
No CUSIP change [at least for our holdings] as of this writing.
Interesting (January 3, 2014)
A pre-market tweet today. >$3 today?
Get Shorty in a Big Way (January 3, 2014)
It is conceivable the CUSIP change, which resulted from the company's name change from Provectus Pharmaceuticals to Provectus Biopharmaceuticals (currently is in process), might have been as much about combating naked shorts and reflecting upcoming business developments as it was about better future branding. No more convertible preferred stock, a new CUSIP, some words about the regulatory process, etc., and the stock's price and daily traded volume run since the company's December 17th and 18th press releases have been strong: more than a 100% increase in the share price and [taken at face value from Yahoo! Finance] more than 25 million cumulative shares traded as of this writing.
We're #3 (January 2, 2014)
Google Provectus, and you'll find the blog as the number three search return, all without the use or benefit of search engine optimization. Ahead of it are the company's website and its Yahoo! Finance stock quote.
Strong. Getting Stronger. (December 31, 2013)
Provectus ended its third calendar quarter (3Q13) with about $8.3MM cash on hand. Assume a cash burn rate for the fourth quarter of about $2.1MM (roughly consistent with the prior two quarters; the first quarter came in under $3MM). I speculate the company may have raised an additional $1-2MM in early-October based on awkward wording in the third quarter filing, although I may be wrong about this. The company had about 30MM warrants outstanding as at 12/31/12.
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
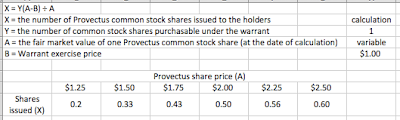 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
Projected Cash at December 31, 2013: $17MM? More? Less? The longer the share price remains well above the warrants' exercise prices (i.e., primarily above $1 per share), the more likely the warrants are going to be exercised. This potentially significant influx of cash onto Provectus' balance sheet very likely provides for future clinical trials (and other key operational expenses) without having to raise additional money.
Provectus Biopharmaceuticals (December 29, 2013)
Best Performing Biotech Stocks (December 28, 2013)
Because of the stock's presence on the OTC, Provectus often if not always is excluded from a variety of different conversations in the mass/mainstream and business media. PVCT is like the He-Who-Must-Not-Be-Named of biotech stocks. Yet, this is the "system," where recognition comes from being on a major stock exchange (i.e., NYSE, NASDAQ, AMEX) and having at least a small cap valuation (i.e., greater than $300 million). A journalist would not have won by writing about the company before now, and at least until both "requirements" above are met.
PVCT's market value (capitalization) has increased to $305 million. Its 1-week price change was +48.2%. Its 52-week price change was +303.7%. This information was derived from Yahoo! Finance (see below).
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
Metrics aside, think about how far the stock has come, irrespective of your optimism ("finally!"), pragmatism ("too far too fast!") or skepticism ("pump-and-dump!"). With regard to Herper's column for last week, PVCT would rank #1 (+48.2%) on the 1-week list, and just outside (+303.7%) the 52-week list.
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
With a couple of hours left in the day (December 26th), the blog experienced the largest amount of visitation and readership (e.g., 480 unique visitors, 35% new visits, etc.) since I began writing in November 2011, and concurrent with a share price that closed yesterday a shade under $2.50, a level not seen since September 2007. I've long thought the blog is a digital measure of (and, perhaps, even proxy for) company and drug awareness among investors (and some non-investors). December's explosion in readership may be a telling and informative indicator as we close out 2013 and launch into 2014.
 |
| Click to enlarge the figure. |
90 days above $2. 5 days above $3. A NASDAQ listing for PVCT. (While not expecting it, I still wonder why it isn't 5 days above $2). December has been an incredible month for the share price. A 212% one-month return. A 66% 5-day return. Within sight of a major stock exchange listing. Eight of the top ten highest daily volume days have been in December, including the top six. The last three days ranked #5 (23rd), #2 (24th) and #3 (26th), respectively.
Provectus stock is mostly if not "nearly exclusively" held by retail investors. Many, perhaps most of them, should be holding profitable positions by now, having previously been underwater for quite a while. There also are a considerable number of warrants that are in-the-money. Retail-investor-sales-of-stock and warrant-conversions-subsequently-sold could slow the stock's rise to list on the NASDAQ. Once there, however, management believes life sciences specialist funds (and other generalist funds) to whom they have spoken over time will initiate positions in advance of a January 15th "regulatory clarity event" (assuming the company sticks to the date it inserted into its December 18th press release, specificity it hasn't really practiced in the past). It's not unreasonable to think the share price will rise in advance of January 15th because of so-called "buy the rumor" activity (where the traditional follow-up practice then would be "sell the news"). Should Dees et al. obtain accelerated approval ("AA") from the FDA (a breakthrough therapy designation would mean a longer timeline to commercialization), and potentially specifics about an AA timeline (or at least enabling folks to infer such), buying the rumor should continue as buying the news.
Ready for Market? (December 26, 2013)
In recent presentations, NYSSA's Life Sciences Conference in October and the LD Micro Conference in December, Craig mentioned Provectus spent more than a million dollars on several production runs to satisfy, among other things, a New Drug Application ("NDA") component/requirement. The product from these runs apparently is ready-to-ship. Two questions arise for me about this information and Craig's comments.
First, the Quality Control Chemistry, Manufacturing and Controls ("CMC") portion of the NDA review is an important one. It focuses on production batches, and not just clinical trial batches. Taken in the context of the company's December 18th PR Provectus Type C Meeting With FDA Oncology Division Held December 16, 2013, a release that mentioned breakthrough therapy designation ("BTD") and accelerated approval ("AA"), made no mention of a Phase 3 trial, and specifically mentioned an FDA-related event date (by or before when meeting minutes would arrive), how close is Provectus to getting PV-10 approved? I'm not suggesting January 15th is an approval date; rather, I think on that date we'll learn just exactly how long, or short, PV-10's "time-line to commercialization" is. In a late-cycle meeting with the FDA weeks prior to the approval of ibrutinib, the Agency "...inquired regarding [Pharmacyclics'] marketing plans whether there is sufficient supply to meet the demand for the drug...The Applicant assured that the Agency of adequate supplies for the drug."
Second, in China, for example, a local trial is necessary for approval, and [from comments made at the LD Micro Conference] Provectus has been in discussions with that country's regulatory authority in that regard. A trial is a prelude to/requirement of in country drug approval. In India drugs may be approved without "...clinical trials on local population to test their safety and efficacy." Taken in the context of discussions with prospective regional transaction partners, how close is the company to receiving term sheets for geographic deals in China and India? Production levels batches, if that indeed is what Craig is referencing (and I think he is), would suggest a deal (or deals) are very close.
For a company that is judicious about how it allocates and spends drug development money, more than a million dollars of product cost (indicating tens of thousands of per patient treatments worth $100MM of sales, give or take) spent in somewhat short order. Why? Management must have reasonable confidence about something(s) to incur this expense.
Why Big Pharma Will Buy Provectus in 2014 (December 26, 2013)
As an investor I wrote in my September 2013 investment letter: “PV-10, a novel oncology compound being developed by Knoxville, Tennessee-based Provectus Pharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCMKTS: PVCT), exemplifies innovation over incrementalism, meaningful over marginal, productized technology over hypothetical, and changing the world over accepting the status quo, with not an insignificant amount of serendipity over contrivance. In sum, these form the quintessential essence of a paradigm shift in the treatment of cancer.
This is where my investment thesis begins and ends: a novel drug compound with a pristine safety profile, a treatment well tolerated by and easily administered to patients, a ready made product inexpensively produced at scale, and a vast addressable market of unmet need that should be fully and very profitably met over time.
My thesis comprises compelling clinical, regulatory, business and stock value propositions in a pharmaceutical industry ravenous for safe and effective oncology solutions, with the prospect of annual market growth rates exceeding other therapeutic areas, that following approval(s) should deliver a lucrative monetization for shareholders.”
I have very high expectations for the company’s monetization. While a portion of this monetization should come from higher share prices, the vast majority should arrive when Big Pharma acquires the company.
Although a seller (management, shareholders) needs a buyer (Pfizer or another Big Pharma company), more importantly the buyer needs to really need Provectus so as to generate a transaction whose valuation is commensurate with PV-10's (and PH-10’s) innovation.
PV-10’s value proposition, and thus its potential to create immense value for its eventual acquirer/owner, is remarkably simple and comprehensive: safety, efficacy, multi-indication viability, use, cost, and pricing. To be clear, the drug is:
- Very safe,
- Very effective, both loco-regionally (local-regionally) and systemically,
- Highly applicable to solid tumor cancers (and, with time to more fully demonstrate, very likely applicable to soft tissue and blood cancers too),
- Beyond safety and efficacy, highly useful as a cancer treatment because of its tissue sparing benefit (with time to more fully demonstrate, use before, instead of, and after surgery, as well as in combination with other therapies to further enhance effectiveness),
- Very inexpensive to manufacture, store and ship,
- Highly flexible in its pricing because of its low development cost, and
- Well protected from an intellectual property (“IP”) perspective.
Interestingly, while PV-10’s compelling value proposition and vast potential for value creation very lucratively would accrue to its acquirer, its potency bodes darkly for those companies who lose out on an auction process for Provectus. Unlike anti-CTLA-4, -PD-1, -PDL-1, -etc. agents where relatives reside with different Big Pharma companies, PV-10 is sufficiently unique so as not to have molecularly similar peers. As a result, only one Big Pharma will possess it and, by virtue of strong IP kung fu, its relatives.
As PV-10 use proliferates, and begins its march towards pervasive use, think of the resultant pharmaceutical industry fracturing as a very profitable hedge fund pair trade:
- Long acquirer, short Roche,
- Long acquirer, short GlaxoSmithKline,
- Long acquirer, short Novartis,
- Long acquirer, short Sanofi Aventis
- Long acquirer, short Merck & Co.
- Long acquirer, short Eli-Lilly,
- Long acquirer, short Bristol-Myers Squibb,
- Long acquirer, short Abbott Laboratories, and
- …well, you get the idea.
People feel. Joy. Excitement. Satisfaction. Anticipation.
People demonstrate. Resolve. Grit. Perseverance.
People solve problems. They innovate. People build companies. They may succeed, but sometimes, more often than not, they fail. Rarely, people shift paradigms. Small molecule drugs like PV-10 and PH-10 alone don't change the world. The people that innovated them do.
When people celebrate, perhaps even drink champagne, and emote, those emotions speak volumes louder than a press release ever could about an outcome.
Even More Telling Blog Stats: "Let's Get It Started" (December 24, 2013)
And...runnin' runnin', and runnin' runnin', and runnin' runnin', and runnin' runnin', and runnin' runnin', and runnin' runnin', and runnin' runnin', and runnin' runnin', and...
Blog readership, on a weekly basis, reached a third consecutive new high for the period December 15th to 21st. For the week, the number of unique visitors was 799 (+4% over the last high of 771 for the prior week). Page views were 4,953 (+27%, 3,888, prior week). Visits were 2,439 (+19%, 2,046, prior week).
 |
| Click to enlarge the figure. |
The share price closed today at $1.84 per share. It was $0.57 on December 21, 2012.
According to an April 2012 article, New Nasdaq $2 / $3 Initial Price Listing Standards, "...a security may qualify for listing on the Nasdaq Capital Market if:
- $3/share price -- for at least five consecutive business days prior to approval, the security has a minimum closing price of at least $3 per share and the issuer has either:
- Equity Standard: (A) stockholders' equity of at least $5M; (B) market value of publicly held shares of at least $15M; and (C) a two year operating history; or
- Net Income Standard: (A) net income from continuing operations of $750,000 in the most recently completed fiscal year or in two of the three most recently completed fiscal years; (B) stockholders' equity of at least $4M; and (C) market value of publicly held shares of at least $5 million; or
- $2/share price -- for at least five consecutive business days prior to approval, the security has a minimum closing price of at least $2 per share and the issuer has (A) market value of listed securities of at least $50M; (B) stockholders' equity of at least $4M; and (C) market value of publicly held shares of at least $15M.
- Net tangible assets in excess of $2M if it has been in continuous operation for at least three years;
- Net tangible assets in excess of $5M if it has been in continuous operation for less than three years; or
- Average revenue of at least $6M for the last three years."
According to Peter, the company would fall under the $3 initial price listing standard (although it would appear Provectus meets the $2 standard, assuming the share price exceed $2).
Growing Awareness (December 21, 2013)
Unique visitors to the blog surpassed 1,900 on a 1-month (31 days) trailing basis (November 20-December 20): 7,704 visits, 1,924 unique visitors, 15,144 page views, 00:02:58 average visit duration, 55.85% bounce rate, and 20.60% % new visits.
Prescient (December 19, 2013)
| Click to enlarge the figure. |
"Recently, advances in the molecular understanding of how the immune system can be modulated to fight melanoma, and of the oncogenic driver mutations that underlie melanoma cells, are leading to dramatic changes in how the field regards standard treatment options for patients with advanced melanoma. As melanoma oncologists, we now need to change our paradigm of therapy for the first time, and consider disease biology in relation to new agents that have shown improvement in overall survival for patients with advanced-stage melanoma. First, 2 clinical trials evaluating the immune-modulating antibody ipilimumab (previously MDX010) have shown a statistically significant improvement in survival, one in previously treated patients with metastatic melanoma compared with treatment with a peptide vaccine, and the other in first-line therapy in combination with dacarbazine compared with single-agent dacarbazine. These data led to the approval of ipilimumab by the FDA in March of 2011, the first new agent in 13 years for melanoma and the first ever based on a positive impact on overall survival. Soon thereafter, a randomized clinical trial showed that the BRAF inhibitor vemurafenib (previously PLX4032/RG7204) improved both survival and interval to progression in first-line therapy compared with dacarbazine, leading to the FDA approval in August 2011. Vemurafenib had previously shown unprecedented high response rates in phase I and II testing in patients with BRAFV600 mutant metastatic melanoma. Similarly, high response rates have been observed in the phase I trial of another specific BRAF inhibitor, dabrafenib (previously GSK2118436; ref. 9; 50%–80% objective response rates in both cases). Given these changes in the standard-of-care therapies for metastatic melanoma with new agents with shown effects on overall survival, it is likely that in the next several years it will be harder to successfully show an additional benefit in overall survival of new agents compared with the recently approved ones. Therefore, the field of melanoma drug development is faced again with the question of which, if any, surrogate endpoints could be considered sufficient to show antitumor efficacy and clinical benefit in future pivotal clinical trials...
Overall survival is not the only clinically meaningful endpoint for a new agent in metastatic melanoma. It is hard to think that physicians would decide not to prescribe BRAF inhibitors (or other agents with similar reproducible high and rapid response rates in molecularly defined subsets of patients) for appropriate patients with bulky and symptomatic disease even if they did not show a prolongation of overall survival in a large cohort of patients followed for a long period of time. On the basis of these considerations, it is clear that overall survival in phase III randomized clinical trials can no longer be considered the only relevant clinical endpoint for new drug development in advanced melanoma. It will continue to be the preferred endpoint if the new agent has a mechanism of action significantly different from the emerging new standards (CTLA-4 and BRAF inhibitors), as long as the new agent does not provide strongly suggestive evidence of paradigm shifting antitumor activity in early single-arm clinical trials.Bold and underlined emphasis is mine.
 |
| Provectus ECC 2012 Poster: Click to enlarge the figure. |
Provectus' PR today included mentions of "breakthrough therapy designation" ("BTD") and "accelerated approval" ("AA") There was, however, no mention of "a Phase 3 trial" or "a special protocol assessment" ("SPA"). As much as the company [it seemed to me] told the market PV-10 was "up for" either BTD (a categorization or "gold star" providing more and faster interaction with Agency personnel, and not a pathway onto itself) or AA, [it also seemed to me] management told the market there was no need for or consideration of a Phase 3 trial.
New SEC filing (December 18, 2013)
Provectus filed an 8-K in regards to today's PR about management's Type C meeting with the FDA.
New press release (December 18, 2013)
Provectus Type C Meeting With FDA Oncology Division Held December 16, 2013
"Under FDA rules, the agency should issue official minutes to the Company within 30 days after such a meeting; in this case by January 15, 2014. The minutes will clarify the available regulatory paths and, therefore, allow the Company to better estimate a time-line to commercialization of PV-10."
Issued: December 18, 2013. The link to the press release is here.
India before China? (December 17, 2013)
In naming Bob Miglani, of South Asian (specifically Indian) descent, to the corporate advisory board ("CAB"), Provectus' PR talked a lot about India.
 |
| Click to enlarge the figure. |
New SEC filing (December 17, 2013)
Provectus filed an 8-K in regards to the voting outcomes for the two proposals at the center of December 16th's special shareholder meeting. Shareholders approved both the name change (from Provectus Pharmaceuticals, Inc. to Provectus Biopharmaceuticals, Inc.) and reincorporation (from Nevada to Delaware).
Knoxville News Sentinel - Provectus alters name, continues FDA talks (December 17, 2013)
"As Provectus Pharmaceuticals continues discussions with the Food and Drug Administration about fast-tracking its cancer drug, stockholders in a special meeting on Monday voted to approve altering the company's name."
Published: December 17, 2013. The link to the article is here.
Corporate Advisory Board (December 15, 2013)
Board member David Darst from OrbiMed's private equity (venture capital) side appears to have left the firm to become CEO of Rgenix, a pre-clinical biotechnology company. I wonder whether his departure removes any potential conflict, however small, from one of OrbiMed's portfolio companies, Eddingpharm (Domain Associates also is an investor), entering into an Asia-based regional transaction with Provectus
More Telling Blog Stats: "It's Getting Crowded" (December 15, 2013)
Blog readership, on a weekly basis, reached new highs for the period December 8th to 14th. For the week, the number of unique visitors was 770 (+42% over the last high of 544 for the prior week). Page views were 3,888 (+23%, 3,153, prior week). Visits were 2,046 (+25%, 1,632, prior week).
 |
| Click to enlarge the figure. |
There are many example from which to draw information about the Investigational New Drug ("IND") and New Drug Application ("NDA") process.
 |
| Click to enlarge the figure (source) |
E.g., Approval of Boehringer Ingelheim Pharmaceuticals' afatinib as first-line treatment for metastatic non-small cell lung cancer with common EGFR mutations. Approved in July 2013, Boehringer had one EOP2 meeting (October 2008) before three Pre-NDA meetings (December 2009, December 2011 and October 2012), the NDA filing (November 2012), NDA acceptance (January 2013) and a late-cycle meeting (May 2013).
Provectus had three EOP2 meetings (April 2010, March 2011 and October 2011). It is December 2013. What meetings have management (Eric, and his regulatory team) had with the FDA since then, and their types, names and characterizations?
I'm looking forward to hearing (reading) what Provectus management says about "regulatory clarity" prior to year-end, as it should provide a hint, or more than a hint, of where they are or might be, if at all, in an NDA review process.
The Week in Review (December 13, 2013)
 |
| Click to enlarge the figure. |
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep. (Stopping by Woods on a Snowy Evening by Robert Frost)
Board of Directors Membership (December 13, 2013)
It was rumored at least one candidate (independent or outside director) has been identified as a possible new Provectus board member. If the individual is added to the BOD, it likely would be carried out in early-Q1 2014.
The New Drug Application ("NDA") (December 11, 2013)
In my On the precipice of… post I categorized potential "menus of choices" for clarity (e.g., Outright or Accelerated Approval for Pop 1, Breakthrough Therapy Designation for Pop 1 and Pop n, Breakthrough Therapy Designation for Melanoma). I felt unsatisfied by the impreciseness of this work, however, because it did not do a good enough job of elucidating the FDA process (to gain approval for PV-10) in which management is engaged.
 |
| Click to enlarge the figure. |
Is the NDA being touched up in preparation for filing? If so, the next step would be to file it with the FDA (a key milestone). Some companies issue press releases when they file their NDAs. Some do not. Preparing-to-file could be a possibility.
Has the NDA been filed? If so, the subsequent outcome (a major milestone) could be FDA acceptance of it. Following filing, the FDA would complete its review and determine whether Provectus' NDA was sufficiently complete to permit a substantive review. In accordance with 21 CFR 314.101(a), if ultimately acceptable, the company's NDA would be considered filed 60 days after the date the Agency received the application. The Agency might grant a Priority Review classification for it. The FDA also would establish a user fee goal date (i.e., a PDUFA date). Companies issue PRs when their NDAs are accepted. A filed NDA could be a possibility. An accepted-by-the-FDA NDA likely is not, as Provectus undoubtedly would have issued a PR if it had been.
Has the NDA been approved? Following acceptance of the NDA, the Agency and the applicant/sponsor engage in collaborative discussions with the goal of and endeavoring to work towards the approval of the drug in question.
 |
| Click to enlarge the figure. |
I will blog about my NDA-related work in greater detail as it relates to Provectus in due course. For the moment, my summary thought is the company is (has to be) engaged at some phase of the NDA review process for PV-10/Pop 1, whether gearing up (post-FDA concurrence) to file an NDA, or waiting to have their NDA accepted by the Agency (and potentially granted Priority Review, possibly together with Breakthrough Therapy Designation).
Telling Blog Stats: "We're Not Alone" (December 8, 2013)
Blog readership, on a weekly basis, reached new highs for the period December 1st to 7th. For the week, the number of unique visitors was 544 (the last high was 502 for the week September 15th to 21st). Page views were 3,153 (3,058, November 17th to 23rd). Visits were 1,632 (1,619, September 15th to 21st).
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
Last month, in my PV-10 v. Standard of Care post, I wrote a parameter of the contemplated pivotal melanoma Phase 3 RCT had changed during the summer, after the 8th World Congress of Melanoma (Hamburg, Germany) in July. This change was communicated via the website presentation, the version from November 20th is below:
 |
| Click to enlarge the figure. |
In making a data-rich case for, very likely outright approval and then (if unsuccessful) accelerated approval, it would seem reasonable management also would endeavor to utilize the same data to make the "anti-case" for why an RCT (and, thus, the SPA) is obsolete. Even if the eventual choice is a single arm trial, such an outcome [in my view] is significant recognition by the FDA.
Investor Conference (December 1, 2013)
Craig is presenting at the 6th Annual LD Micro Conference in Los Angeles, California on December 4th at 10:30 am PST.
A Thought About Upcoming Regulatory Clarity (December 1, 2013)
In my November 30th On the precipice of… post I laid out what I think are possible regulatory clarity scenarios. Clarity of any sort is necessary. I don't really see any bad outcomes, although there could be an indifferent one. It seems to me the leading (likely, but not certain?) scenario is OA for melanoma Pop 1 and BTD for Pop 2. To be balanced, manage risk and gird expectations, that very positive outcome may be replaced by a less to much less positive one; I write this, but still think the entrails tell us management expects OA & BTD.
November Blog Stats (December 1, 2013)
Blog readership was down overall in November. The number of unique visitors was 1,120, down 12% month-over-month ("MoM"). Page views were up 9% MoM at 9,834 (9,038, the previous record high in October). Visits were down 11% MoM at 5,177. I posted 5 times in November.
 |
| Click to enlarge the figure. |
 |
| Click to enlarge the figure. |
Provectus may add pharmaceutical and other industry executives to its board of directors and corporate advisory board, in addition to a Fortune 50 company executive, who has been considered for several months for the advisory board.
More populations: PV-10 vs. Surgery (November 27, 2013)
In a recent article in CancerWatch, Vol. 22 Oct. 2013, 155-156, Provectus principal investigator Dr. Sanjiv Agarwala, a medical oncologist, speculated on potential uses of PV-10 by surgical oncologists: "His surgical colleagues...are interested in the possibility of presurgical PV-10 injections turning unresectable lesions into resectable ones and/or stimulating the immune system to lower the odds of recurrence."
Unresectable cancer is a tumor that cannot be completely removed by surgery. A cancer may be unresectable because the tumor is too large. An injection or multiple injections of PV-10 into the tumor (the approach is determined by tumor volume, which then determines the number of injections per ml of PV-10 to delivery the necessary volume of the drug) could (should) reduce the size of the tumor to a point where the surgeon feels it is appropriate to resect or excise it.
An oncologist concerned about his or her patient's cancer recurrence risk (say, for melanoma) would inject PV-10 prior to surgery so as to harness the drug's immune system stimulative effect (which, of course, derives from its chemoablative effect). At least for now, chemoablation requires intralesional delivery (i.e., injection into the lesion) for the immune effect to occur. Injection following resection or excision would not provide a viable tumor or lesion into which to deliver (inject) PV-10.
PV-10 is very safe and very efficacious, and has multi-indication viability. It is also has a compelling pre-surgical tissue sparing proposition. Diseased (cancerous) tissue is eliminated, while healthy tissue is spared. Dead tissue eventually is discharged and replaced (like in patient picture below from the company's website presentation).
 |
| Click to enlarge the figure. |
While tissue sparing is an important consideration for patients afflicted with melanoma, it also is an important consideration for those with breast and prostate cancer. PV-10's value proposition for melanoma appropriately and directly translates to these other cancers.
Populations (November 27, 2013)
In one of the ECC 2013 articles about Provectus in which Dr. Agarwala was quoted, he said about PV-10: "First, it might be used in patients who are refractory to all other therapies and who have injectable disease. Second, it might be used in patients who have injectable lesions but who are not eligible for systemic therapy (e.g., the elderly or those with comorbidities). Third, it might be used in combination with other therapies (e.g., ipilimumab or nivolumab or both)."
In seeking accelerated (now outright) approval for patients who are refractory to all other therapies and who have injectable disease, the second use above would be included in (i.e., is a part of) the aforementioned "label."
Short Interest Falls (November 26, 2013)
 |
| Click to enlarge the figure. |
Peter could be traveling to China to meet with Hisun-Pfizer Pharmaceutical, the Chinese joint venture between Pfizer and Zhejiang Hisun Pharmaceutical Co., and Sinopharm Group Company, the largest pharmaceutical company in the country (headquartered in Beijing) and jointly owned by China National Pharmaceutical Group and Shanghai Fosun Pharmaceutical. A regional license transaction for China may have turned into a horserace between Hisun-Pfizer and Sinopharm, with Eddingpharm running just behind the co-leaders.
 |
| Click to enlarge the figure. |
The Connecting the Dots...Provectus Pharmaceuticals blog turned two yesterday. Key readership statistics essentially doubled: Unique visitors increased 114% in year two over year 1 (a total of 9,733 from November 17, 2012 to November 16, 2013 v. 4,455 from November 16, 2011 to November 16, 2012), visits increased 118% (64,8882 v. 30,289), and page views increased 97% (89,406 v. 45,303).
 |
| November 16, 2011 to November 16, 2012: Click on the figure to enlarge it. |
 |
| November 17, 2012 to November 16, 2013: Click on the figure to enlarge it. |
Provectus' patent application Combination of Local and Systemic Immunomodulative Therapies for Enhanced Treatment of Cancer, the company's joint application with Pfizer (and Dr. Craig Eagle), appears to have completed a key procedural step in making its way through the approval process. The World Intellectual Property Organization's website notes the result of the International Preliminary Report on Patentability Chapter I (October 2013).
Interesting SITC Abstract (November 15, 2013)
I previously wrote several blog posts on or related to the topic of Korn et al.'s 2008 meta-analysis of 42 MM phase 2 metastatic melanoma studies (70 arms and 2,100 patients): Overall Survival, PV-10 vs. OncoVEX, Allovectin-7, and Blog Reader Comment. I wanted to compare what OS data was available for PV-10 at the time to other studies, fully understanding the caveats of comparing trials and comparisons themselves. Comparisons provide useful information, but of course one does not digest them in a vacuum. All three intralesional therapies above, PV-10, OncoVEX (now T-Vec) and Allovectin-7 placed well outside the solid blue lines below, which are 95% confidence bounds. The dotted line is the overall 1-year survival rate (25%) or the overall 6-month PFS rate (15%).
 |
| Click on the figure to enlarge it. |
I read an interesting abstract at the 2013 Society for Immunotherapy of Cancer ("SITC") Annual Meeting, co-authored by Provectus principal investigator Brendon Coventry: Mathematical modeling of immune kinetics in advanced cancer through meta-analyses of complete response rates: immune synchronisation emerges as the likely key determinant of clinical response. The authors concluded: "Mathematical analyses of over 130 clinical trials, inclusive of over 8000 patients, has demonstrated that the CR rate for patients treated with widely divergent therapies is remarkably fixed at between 5 and 10%. The probability of this occurring by chance alone is extremely close to zero, and is both scientifically and clinically implausible." The authors are aware of PV-10's large CR rate, which is clearly outside the norm. Re-reading Korn et al.'s paper, it also is interesting to note similar ceilings for overall survival (OS) and progression free survival (PFS).
When trying to comprehend PV-10's remarkable efficacy (effectiveness), it's useful to consider the drug's mechanism of action, and Rose Bengal's physical chemistry basis.
Fundraising? (November 14, 2013)
As I noted below in New SEC filing (November 12, 2013), I still struggle with the underlined language in the 3rd quarter 10-Q below: "By managing variable cash expenses due to minimal fixed costs, we believe our cash and cash equivalents on hand at September 30, 2013, together with approximately $6 million received in the three months ending December 31, 2013 due to sales of common stock and warrants, will be sufficient to meet our current and planned operating needs until 2015 without consideration being given to additional cash inflows that might occur from the exercise of existing warrants or future sales of equity securities, although we may, in our sole discretion, direct Alpha Capital Anstalt (“Investor”) to purchase up to $30,000,000 of our common stock per an existing agreement with Investor."
While mostly similar to prior language used by management in their customary annual and quarterly financial reporting, it is not the same, which leads me to believe the company took in some additional dollars (amount = X) in the 4th quarter (i.e., October). If so, these details would be reported in Provectus' FY13 (fiscal year 2013) 10-K filing and not available until mid-March 2014. I reviewed language use variants in prior filings related to "...will be sufficient to meet our current and planned operating needs..." (e.g., until, well into), quarter-end cash balances, proceeds raised very early or in the quarter immediately following, etc., and compared that dataset to this quarter's filing's data points (e.g., "until 2015," $8.3 million quarter-end balance, etc.). Far from a calculation that produces a precise result, I wonder if X = $2-3MM (and the issuance of 6.7-10 million more shares and warrants). If so, it suggests a higher accommodative BDO-required monthly burn rate, which might mean Provectus' accountants, after reviewing what management may or expects to carry out on a forward basis, increased their required 12-month cash-on-hand figure. I cannot be sure of this, of course, but this increase would be consistent with management's bullish statements in the 3rd quarter filing and the associated prospectus supplement.
Regulatory Process (November 13, 2013)
h/t A shareholder:
Shareholder: Did the government shutdown affect the 60-day "clock" of the BTD programs?
FDA: No, it did not.
Shareholder: Does the clock begin the day you receive the application? [Could]...the 60-day "clock"...extend much longer...?
FDA: The clock begins from the stamp date of receipt, so the timeline is no longer than 60 days.Depending on the exact receipt of the company's BTD application, likely sometime between October 1st and 15th, we might expect to hear an outcome by, before or between December 2nd and 16th (60 days after October 1st is November 30th, a Saturday, while the same period following October 15th is December 14th, another Saturday).
A Note of Interest (November 13, 2013)
h/t A shareholder: The FDA granted accelerated approval to Pharmacyclics' Ibrutinib for the treatment of patients with mantle cell lymphoma ("MCL") who have received at least one prior therapy. Ibrutinib was granted breakthrough therapy designation ("BTD") for refractory MCL in February 2013; the drug was the second to receive BTD, for which the company received the designation three times (MCL, Waldenstrom’s Macroglobulinemia, and Chronic or Small Lymphocytic Leukemia). According to the FDA (bold emphasis is mine):
On a conference call, Pharmacyclics confirmed the FDA had split off the MCL NDA application from the CLL application." Management declined to speculate on the reason behind the split. Analysts suggested that potential causes could include the volume of data submitted or a different risk/benefit profile...""The approval was based on the results of a multi-center, international, single-arm trial enrolling 111 patients with previously treated mantle cell lymphoma. The primary endpoint was overall response rate (ORR). The efficacy results demonstrated a 66% ORR (95% CI 56.2, 74.5). Seventeen percent of patients achieved complete responses, and 49% achieved partial responses. The median response duration was 17.5 months (95% CI 15.8, not reached). Safety was evaluated in the 111 patients with previously treated MCL who received ibrutinib 560 mg daily. The most common adverse reactions reported in the clinical trial (occurring in greater than or equal to 20% of patients) were thrombocytopenia, diarrhea, neutropenia, anemia, fatigue, musculoskeletal pain, peripheral edema, upper respiratory infection, nausea, bruising, dyspnea, constipation, rash, abdominal pain, vomiting, and decreased appetite. Five percent of patients had grade 3 or higher bleeding events (subdural hematoma, gastrointestinal bleeding, and hematuria). Bleeding events, including bruising of any grade, occurred in 48% of patients. Treatment-emergent grade 3 or 4 cytopenias occurred in 41% of patients. Twenty-five percent had grade 3 or higher infections."
New SEC filing (November 12, 2013)
Provectus filed its third quarter 10-Q (ending September 30). Monthly cash burn increased to ~$700,000, +6% quarter-over-quarter. Quarter-end cash and cash equivalents were $8.3 million. Management (Craig and Peter) appear to indicate no new money was raised beyond the tranches closed on September 20th and 26th, as detailed in the 8-K filed on September 26th.
Wording in the current 10-Q seemed awkward: "By managing variable cash expenses due to minimal fixed costs, we believe our cash and cash equivalents on hand at September 30, 2013, together with approximately $6 million received in the three months ending December 31, 2013 due to sales of common stock and warrants, will be sufficient to meet our current and planned operating needs until 2015 without consideration being given to additional cash inflows that might occur from the exercise of existing warrants or future sales of equity securities, although we may, in our sole discretion, direct Alpha Capital Anstalt (“Investor”) to purchase up to $30,000,000 of our common stock per an existing agreement with Investor." Bold emphasis is mine; "awkward" language is underlined.
The notion here would be that although the Maxim Group and Network 1 Financial tranches closed on September 20th and 26th, respectively, the money was received by Provectus on or after October 1st, and thus after the third quarter and in the fourth quarter. I may have been off in my prior calculation related to Fundraising (September 26, 2013) below (say, by about $2 million gross of fees), when I tried to piece together exactly how much was raised because of the manner in which the Network 1 raise was communicated in the 8-K filing. Using a $4.6 million end of second quarter cash figure, a $2.1 million third quarter cash burn, and a $6.3 million net of fee third quarter raise, one arrives at an $8.9 million end of third quarter cash figure, which compares to the actual quarter-end figure of $8.3 million. I'm not seeing my error or how to foot this correctly at the moment, but I'm "in the ballpark."
Filed: November 12, 2013. The link to the filing is here.
Notable Corporate Website Presentation Change (November 12, 2013)
 |
| Click on the figure to enlarge it. |
 |
| Click on the figure to enlarge it. |
Regional License Interest? (November 11, 2013)
A number of global and regional pharmaceutical companies, and/or their affiliates, medical centers and universities have visited the blog since its inception. Sometimes visits are brief, and possibly or likely accidental. Sometimes, they are regular, and/or include ones relatively long in duration. I wonder if Sinopharm, China's largest pharmaceutical company, has entered the regional transaction license picture. Peter may have met with Sinopharm during his February/March trip. It seems Chinese pharmaceutical companies are primarily focused on liver cancer; global locations of the highest incidences of liver cancer include Eastern and South-Eastern Asia (China and Chinese Taipei rank in the top 10; see prior embedded link). On a previous version of Provectus' website presentation, management estimated the China market for melanoma, lung, bladder, and hepatocellular carcinoma as a $30 billion opportunity (for which I presume numerous companies would compete). If Sinopharm now is interested, I also wonder if it might potentially challenge Hisun-Pfizer Pharmaceutical's rumored interest in a geographic transaction focused on liver cancer.
Notable rumor (November 10, 2013)
Most, if not nearly all, of the convertible preferred stock Provectus issued in 2010 and 2013, ~1.5 million and 3.4 million outstanding, respectively, as at the company's June 30th 10-Q, may have been sold. If true, this would remove a noticeable weight burdening the share price for some time, and potentially enable recent buying interest to buoy the stock going forward.
Medical conference: Society for Immunotherapy of Cancer (SITC) Annual Meeting, November 7-10 (November 6 2013)
Provectus is a "Bronze" sponsor of the event. The link to the preliminary program is here. The link to the updated schedule is here.
New press release (November 5 2013)
Provectus to Hold Special Meeting of Stockholders December 16, 2013, in Knoxville, Tennessee
Provectus Pharmaceuticals to Change Name to Provectus Biopharmaceuticals, Inc., Reincorporate in Delaware.
Issued: November 5, 2013. The link to the press release is here.
New SEC filing (November 5, 2013)
Schedule 14A: The company has proposed at special meeting of stockholders for December 16 to vote on proposals to change its name from Provectus Pharmaceuticals, Inc. to Provectus Biopharmaceuticals, Inc., and reincorporate from Nevada to Delaware. The link to the filing is here.
Knoxville News Sentinel - Locally developed cancer drug shows 'promising results' (November 4, 2013)
"Armed with early positive results from tests underway at a National Cancer Insitute-affiliated research center, Provectus Pharmaceuticals is in talks with the Food and Drug Admnistration about the next steps to take to push its tumor-destroying drug to market."
Published: November 4, 2013 (online). The link to the article is here (subscription required).
October Blog Stats (November 1, 2013)
Readership of the blog set a few new highs for October. The number of unique visitors was 1,270, down 10% month-over-month ("MoM"). The number of U.S. states from where readers came was 46 (Previous high, Several different months: 42). The number of countries was 44 (January: 44). Page views were up 14% MoM at 9,038 (September: 7,927, when I posted 9 times). Visits were down 4% MoM at 5,791 (March: 6,365, when I posted 56 times). I posted 6 times in October.
 |
| Click to enlarge the figure. |
Multiple sources appear to confirm Provectus has submitted its final special protocol assessment ("SPA") application and a breakthrough therapy designation ("BTD") application to the FDA. While there is no consensus on the timing of the aforementioned submissions, shortly or sometime after October 1st would be a reasonable guess.
Business Development (October 25, 2013)
Craig is in New York this week presenting at the NYSSA Life Sciences Conference on Wednesday, October 30. Peter is in Chicago meeting with shareholders on the same day.
Short Interest at 2% (October 24, 2013)
 |
| Click to enlarge the figure. |
Provectus Awarded PV-10 Patent by U.S. Patent and Trademark Office
Issued: October 24, 2013 The link to the press release is here. The patent (see the news item immediately below) was issued on October 15, 2013.
This patent provides coverage of the medicament PV-10, and is similar to claims previously issued in Europe and a number of other international jurisdictions. It is a so-called "daughter case," or continuation in part, of USP 7,648,695, issued in January 2010 and which covered an analog of PV-10. As a core patent, it's issuance by the US PTO was deemed material, necessitating the 8-K filing. The link to the filing is here.
New patent issued (October 15, 2013)
Provectus was issued a new patent by the USPTO: Medicaments for chemotherapeutic treatment of disease. This patent is related to patents Methods for high energy phototherapeutics (December 2001), Intracorporeal medicaments for photodynamic treatment of disease (June 2008), and Medicaments for chemotherapeutic treatment of disease (January 2010).
Issued: October 15, 2013. The link to the patent is here.
Business Development (October 13, 2013)
Peter and Eric are in New York this week. The 68th Annual Alfred E. Smith Memorial Foundation Dinner is on October 17. Alfred E. Smith IV is a Provectus board member.
Seeking Alpha - Provectus Pharmaceuticals Up 17% In A Month; Potential Still Huge (October 7, 2013)
"The high potential value of PV-10 to major pharmaceutical companies in need of blockbuster drugs is currently ignored by the Market. I agree with Provectus Management that, with a bit more regulatory clarity, it becomes very likely the Market will no longer be able to remain oblivious."
Published: October 7, 2013. The link to the article is here.
Cancer Intelligence / ecancer News - PV-10 in Metastatic Melanoma: blistering predicts good outcomes (October 4, 2013)
"Now we have the mechanistic explanation for our bystander effect, that we’re inducing a highly-specific T cell mediated response," said Wachter. Data on the immunological mechanism of action, he added, suggested that PV-10 might be particularly relevant for patients with inhomogeneous clonal populations.
Published: October 4, 2013. The link to the article is here.
FirstWord Pharma - Intralesional PV-10 Shows High Response Rate in Advanced Melanoma, Evidence of Systemic Effects (October 4, 2013)
"Beyond this, many of my surgical colleagues are interested in the idea of using PV-10 to stimulate the immune system before surgery to potentially lower the odds of recurrence."
Published: October 4, 2013. The link to the article is here.
Blog Stats Through Q3 2013 (October 3, 2013)
From inception in November 2011 through September 30, 2013, the Blog hosted 12,500 unique visitors, received 86,600 visits, had 120,900 page views and recorded an average visit duration of nearly two-and-a-half-minutes from 2,300 U.S. and international cities in all 50 U.S. states and nearly 100 countries. Monthly readership averaged 1,020 unique visitors year-to-date (950 trailing twelve months), 5,630 visits (5,240) and 7,520 page views (7,140) from 425 U.S. and international cities (385) in 40 U.S. states (40) and 90 countries (85).
PharmiWeb.com - Metastatic Melanoma: blistering in PV-10 treatment predicts good outcome (October 2, 2013)
"Injecting cutaneous lesions in Stage III-IV melanoma patients refractory to other treatments with PV-10 provides a viable strategy to maintain long-term locoregional control, concluded the final analysis of an open label single arm phase 2 trial presented at the 2013 European Cancer Congress, 27 September to 1 October, Amsterdam, The Netherlands."
Published: October 2, 2013. The link to the article is here.
Seeking Alpha - Provectus Finds New Oncology Idea In An Old Product (October 1, 2013)
Provectus Finds New Oncology Idea In An Old Product by George S. Mack, who conducts interviews for The Life Sciences Report (he did not interview Peter for which Provectus paid TLSR).
"Yes, we've been demonstrating proof of concept and even curing cancer in mice for 50 years. It just doesn't seem to work out in humans. However, Provectus Pharmaceuticals Inc. (PVCT.OB) did pilot studies in humans with advanced melanoma more than a decade ago with its PV-10 (10% rose bengal disodium), and the compound performed what looked like miracle cures in some patients. Well, now the mice are telling us how it works."
Published: October 1, 2013
BioWorld Today: "Exciting Findings from ECCO 2013" (October 1, 2013)
ImmunoGen (IMGN) for a Roche TH3RESA Phase 3 trial of Kadcylafor the treatment of advanced HER2-positive breast cancer that had progressed despite prior treatment with at least two HER2-targeted medicines. Halozyme Therapeutics (HALO) for a Phase 1b trial of PEGPH20 in combination with gemcitabine for the treatment of patients with stage IV metastatic pancreatic cancer. Amgen (AMGN) for a pivotal Phase 3 trial evaluating talimogene laherparepvec in patients with unresected stage IIIB, IIIC or IV melanoma.
September Blog Stats (October 1, 2013)
Readership of the blog, first launched in November 2011, set new highs for September. The number of unique visitors was 1,405 (last high in August: 1,182), up 19% month-over-month ("MoM"). The number of U.S. cities from where readers came was 450 (August: 414). The number of international or non-U.S. cities was 123 (June: 103). The total number of cities was 573 (August: 497). Page views were up 7% MoM at 7,927 (June: 8,578, when I posted 59 times). Visits were up 4% MoM at 6,048 (March: 6,365, when I posted 56 times). I posted 9 times in September.
New press release (September 30, 2013)
Exploratory Data Analyses of Intralesional PV-10 Clinical Phase 2 Study Results Presented at ECC 2013 Demonstrate Effective Locoregional Disease Control and Support Systemic Immunologic Activity in Refractory Metastatic Melanoma
Issued: September 30, 2013. The link to the press release is here.
ECCO 2013 (September 29, 2013)
I located Provectus' ECCO 2013 poster online here.Mounting evidence of systemic immunologic activity:Magnitude and duration of response inversely related to untreated tumor burden. Blistering may be indicative of nascent local immunologic response. Regression of untreated bystander lesions strongly correlated with OR of target lesions. Regression or stasis of untreated Visceral Metastases observed in patients with OR of Target Lesions. 44% 1-year survival in Stage IV M1c subjects. Conclusion: PV-10 is well tolerated, eliciting rapid, durable response in injected tumors. Exceptional outcome when all or substantially all lesions are injected. Robust control of refractory tumor demonstrated across all study sites.
Fundraising (September 26, 2013)
Provectus raised about $5.2 million through Maxim Group and Network 1 Financial, closing the Maxim tranche of $2.7 million on September 20 and Network 1's $2.5 million tranche on September 26 (which was an extension of a raise the latter did in Q2 when it raised $2.6 million). Money was raised at $0.75 per common share, the placement unit for which also included 150% warrant coverage at a $1.00 exercise price (i.e., 1.5 warrants per share of common stock purchased). More filing details are here.
New SEC filing (September 25, 2013)
In a Form 4 filing, Provectus board member Jan Koe purchased 100,000 shares of common stock (and received 150,000 warrants with a $1.00 exercise price) that appears to be the stub piece/part of Network 1's Q2 private placement (see page 10 of the 10-Q).
Filed: September 24, 2013
Management Interview (September 19, 2013)
Peter was interviewed by The Life Sciences Report.
Interviewed: September 19, 2013
Management Interview (September 19, 2013)
Peter was interviewed by The Wall Street Transcript.
Interviewed: September 13, 2013
Corporate Website Presentation Updated (September 19, 2013)
The presentation was last updated August 20, 2013. Relevant? There is a date change, but it does not appear much if anything actually was changed in the presentation itself.
"Updated:" September 17, 2013
Blog Reader Find: Publication (September 18, 2013)
Isolated limb infusion chemotherapy for melanoma: an overview of early experience at the Adelaide Melanoma Unit
"ILI chemotherapy could be combined with approved or experimental systemic therapies, such as interleukin-2, standard chemotherapy, radiotherapy, or serine–threonine protein kinase B-Raf inhibitory antibodies (B-Raf), Cytotoxic T lymphocyte associated antigen-4 (CTLA-4), mitogen-activated protein kinase kinase (MEK) Programmed cell Death Receptor-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1) inhibitor therapy, or local intra-lesional therapies such as coxsackie virus or rose bengal, and be practice-changing. ILI using non-cytotoxic agents, perhaps including some of those above, might be explored."
Published: August 2013
New press release & SEC filing (September 13, 2013)
Provectus Novel Synthesis Patent Issued by United States Patent and Trademark Office
Issued: September 13, 2013 The link to the press release is here.
As a core patent, it's issuance by the US PTO was deemed material, which necessitated the 8-K filing. The link to the filing is here.
New press release (September 13, 2013)
Provectus to Present Additional Results in 80 Patient International Multicenter Phase 2 Clinical Trial in Metastatic Melanoma at European Cancer Congress 2013
Issued: September 12, 2013. The link to the press release is here.
New patent issued (September 10, 2013)
Provectus was issued a new patent by the USPTO: Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes. This is a key patent for the company.
Issued: September 10, 2013. The link to the patent is here.
Third-Party Murine Model Work (September 7, 2013)
It appears murine model work by an east coast university, apparently paid for by the institution, may soon begin (has begun?) on bladder cancer. If veracious, this study would build on Craig's initial work that displayed efficacy success and included addressing (solving for) the effective delivery of PV-10 to/in this organ.
Seeking Alpha Article - Provectus Pharmaceuticals: Small Cap, Huge Upside (September 7, 2013)
Provectus Pharmaceuticals: Small Cap, Huge Upside by Trust Intelligence
"Given that PV-10 has an outstanding safety profile, no debilitating treatment side effects, and has been effective in treating solid tumors that normally have a high mortality rate, the remarkably low market capitalization makes the investment premise obvious."
Published: September 6, 2013
Blog Reader Find: Publication (September 3, 2013)
Direct toxicity of Rose Bengal in MCF-7 cell line: Role of apoptosis
Abstract: Current therapies for breast cancer are often limited by short-term efficacy due to the emergence of drug resistance. In view of this, there is much interest in the identification of new agents for the treatment of breast cancer. Rose Bengal (RB) has been used as a photosensitiser in photodynamic treatment. In the present study, we investigated the direct cytotoxic and proapoptotic effects of RB, not as a photosensitiser, in MCF-4 cells. Cell viability was quantitated by MTT assay. Apoptotic cells were determined using PI staining of DNA fragmentation by flow cytometry. Bax protein expression was studied by western blotting. ROS was measured using DCF-DA by flow cytometry analysis. The result showed RB decreased cell viability in MCF-4 cells in a concentration- and time-dependent manner. RB induced a sub-G3 peak in flow cytometry histogram of treated cells indicating apoptosis is involved in this toxicity. In Western blot analysis, Bax expression significantly increased in RB-treated cells. RB could also increase ROS production in MCF-4 cells but antioxidant GSH could not decrease the toxicity indicating this toxicity was independent of ROS production. Thus RB exerts proapoptotic effects in a MCF-4 cells and could be considered as a potential chemotherapeutic agent in breast cancer.
Published: April 2013
Blog Reader Find: Publication (September 3, 2013)
Study the anti-cancer and anti-inflammatory properties of Rose Bengal in vitro
Abstract: Rose Bengal (RB) has been used as a safe agent in clinical diagnosis. In addition, it is used as a photodynamic sensitizer for removing microorganisms and cancer cells. Recently, its preferential toxicity after direct exposure to cancer cells was proven. The present study focuses on anti-cancer and anti-inflammatory activities of RB. Toxicity of RB was studied against AGS gastric cancer and NIH 3T3 fibroblast cell lines using MTT assay. The pattern of cell death was defined using annexin-V and PI staining method. Moreover, the effect of treatment with RB on nitric oxide (NO) and prostaglandin E2 (PGE2) production induced by lipopolysaccharide in J774A.1 macrophages was determined. Its modulation on inducible nitric oxide synthase (iNOS) and cyclooxegenase-2 (COX-2) expressions was evaluated using western blot analysis. AGS cells exhibited significant concentration-dependent decrease of growth in response to RB however it showed lower growth inhibition in non-malignant cells suggesting that its anti-cancer activity might be tumor cell specific. Moreover, AGS cells exposed to RB presented a significant increase in apoptosis. RB caused a remarkable decrease in the production of NO and expression of iNOS without any significant modulation of PGE2 production and COX-2 expression. This study suggests that RB preferentially suppress gastric cancer cell growth through the induction of apoptosis, mitigating the further examination of RB in animal models of gastric cancer. In this study, for the first time, we also showed anti-inflammatory effect of RB.
Published: September 2013
Blog Reader Find: Educational & Scientific Meeting (August 29, 2013)
Update for Clinicians on Diagnosis and Treatment of Melanoma and Other Cutaneous Malignancies, sponsored by Physicians' Education Resource and Moffitt Cancer Center, held at Moffitt, and supported by Delcath Systems, Genentech and Provectus. Event co-chairs are Dr. Vernon Sondak and Dr. Jeffrey Weber. Read the brochure here.
Event date: September 28, 2013.
FierceBiotechResearch: Single injection could provide new treatment for melanoma (August 27, 2013)
A new study by researchers at the Moffitt Cancer Center in Tampa, FL, provides promising evidence that an injectable solution called PV-10 could be a potential new treatment for battling melanoma, the deadliest form of skin cancer. Read more: Single injection could provide new treatment for melanoma
Published: August 26, 2013
New SEC filings (August 22, 2013)
Stock option grants were made to members of the board of directors.
Filed: August 21, 2013. The links to the filings are here: Koe, Smith, McMasters, Scottand Dees.
Blog Reader Find: Publication (August 18, 2013)
Sonodynamic excitation of Rose Bengal for eradication of gram-positive and gram-negative bacteria.
Abstract: Photodynamic antimicrobial chemotherapy based on photosensitizers activated by illumination is limited by poor penetration of visible light through skin and tissues. In order to overcome this problem, Rose Bengal was excited in the dark by 28 kHz ultrasound and was applied for inactivation of bacteria. It is demonstrated, for the first time, that the sonodynamic technique is effective for eradication of gram-positive Staphylococcus aureus and gram-negative Escherichia coli. The net sonodynamic effect was calculated as a 3-4 log10 reduction in bacteria concentration, depending on the cell and the Rose Bengal concentration and the treatment time. Sonodynamic treatment may become a novel and effective form of antimicrobial therapy and can be used for low-temperature sterilization of medical instruments and surgical accessories.
Published: December 2012
Competitor (August 12, 2013)
Intralesional therapy agent Allovectin-7, of Vical (NASDAQ: VICL), failed to meet its primary endpoint of objective response rate or secondary endpoint of overall survival of the drug's pivotal metastatic melanoma ("MM") Phase 3 trial. Earlier this year, preliminary results of Amgen's talimogene laherparepvec (formerly OncoVEX) met the primary endpoint of durable response rate. MM Phase 2 response rates for Allovectin-7 and T-Vec/OncoVex have been compared, together with IL-2 and BCG, to PV-10, whose response rates for injected lesions, non-injected lesions and non-injected systemic lesions were dramatically higher than Allovectin-7 and T-Vec.
New SEC filing (August 9, 2013)
Provectus filed its second quarter 10-Q (ending June 30). Monthly cash burn dropped to ~$665,000, -35% quarter-over-quarter. As expected, the company raised $2.6 million via a Network 1 Financial private placement. Quarter-end cash and cash equivalents were $4.6 million. Provectus guided, in my view rather declaratively or definitively, about PV-10 license transactions (regional and/or global) pending regulatory clarity.
Filed: August 8, 2013. The link to the filing is here.
New Liver Trial Sites (August 8, 2013)
Several weeks ago, I learned the second site for the expanded Phase 1 liver cancer study, The Southeastern Center for Digestive Disorders & Pancreatic Cancer in Tampa, Florida, had yet to recruit patients (n.b. neither had the first site, Sharp Memorial Hospital in San Diego, California). Two more sites have been added, with a third in process, bringing the total number of sites to five. A sixth site, in Shanghai, China, should be opened following Eric's visit to the location.
New press release (August 6, 2013)
Research on Mechanism of Action of Rose Bengal Presented at 8th World Congress of Melanoma
Issued: August 6, 2013. The link to the press release is here.
Fundraising? (August 5, 2013)
It's possible, perhaps likely, a Network 1 Financial-like small financing (i.e., several million dollars) is in the offing. While Network 1 has been Provectus' placement agent of choice, management may go direct to certain existing and/or prospective investors. The company's Q2 filing should be available this week. As of March, Provectus noted $5 million of current cash (cash burn appears to be about $1 million per month). Last month, the company entered into a $30 million financing vehicle (not a fundraising) with Alpha Capital Anstaltfor BDO "going concern" purposes. A small fundraising, if/when done, as management previously has maintained, would have been mandated by the accountants.
Principal Investigators (August 5, 2013)
Company principal investigator ("PI") and internationally-recognized melanoma expert Professor John Thompson was named Australia's New South Wales' top cancer researcher of the year, receiving the Premier's Award for Outstanding Cancer Researcher.
Business Development & Regulatory Affairs (July 31, 2013)
Eric is in New York this week, too.
It appears regulatory affairs resources have been shifted to or directed toward PH-10 work, a positive sign if some or all of PH-10-related milestone goals in May's Annual CEO Letter are to be achieved. This resource re-allocation, if true, also provides insight into the status of the data/application preparatory portion of PV-10's regulatory process.
Business Development (July 29, 2013)
Peter is in New York this week.
New CUP site (July 28, 2013)
A new CUP site, not listed on the ClinicalTrials.gov website, treated a neuroendocrine tumor ("NET") that had metastasized to the liver, a new indication.
Corporate advisory board change (July 27, 2013)
Corporate advisory board ("CAB") member and former member of the company's board of directors ("BOD") member Stuart Fuchs no longer is on the CAB. Fuchs joined the BOD in January 2003, resigning in July 2011 when he joined the CAB.
New SEC filing (July 26, 2013)
In an 8-K filing, Provectus entered into an equity line of credit (at-the-market ("ATM") offering-like vehicle) with existing shareholder Konrad Ackermann's Alpha Capital Anstalt. The term of the agreement is 30 months, and replaces a similar, unused, 30-month agreement the company had entered into with Lincoln Park Capital in December 2010(the link to this purchase agreement is here). Management said this fundraising vehicle, which they also communicated they have no intention of using, maintains the going concern opinion of BDO USA, the company's accounting firm.
Filed: July 26, 2013
New CUP site (July 26, 2013)
A new compassionate use program ("CUP") site, not listed onthe ClinicalTrials.gov website, treated ocular melanoma metastasis to the liver, a new indication.
New press release (July 25, 2013)
PV-10’s Systemic Tumor-Specific Immune Response is Subject of Research Article Published in PLOS ONE.
Issued: July 25, 2013. The link to the press release is here.
New Moffitt Cancer Center publication (July 21, 2013)
In publishing their recent paper in PLoS One, Moffitt changed the target journal from a very high profile immunology periodical. According to Moffitt's Dr. Pilon-Thomas, the benefits of PLoS One's open access for disseminating this work outweighed the benefits of publishing it in the more recognized immunology journal.
New patent issued (July 23, 2013)
Provectus was issued a new patent by the USPTO: Intracorporeal medicaments for high energy phototherapeutic treatment of disease.
New Moffitt Cancer Center publication (July 21, 2013)
Citation: Toomey P, Kodumudi K, Weber A, Kuhn L, Moore E, et al. (2013) Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer. PLoS ONE 8(7): e68561.
Published: July 17, 2013. The link to the paper is here.







































No comments:
Post a Comment