 |
| Click to enlarge |
The slide reinforces an important theme of Provectus' Dr. Eric Wachter, PhD's November 14th presentation (as part of the 3Q16 business update call): there is considerable interest and clinical activity in melanoma, and while he believes there is no doubt about the relevance of PV-10, cutting through the crowd to the front of the pack will require continued effort on Provectus' part. See, for example, the slides below from his presentation:
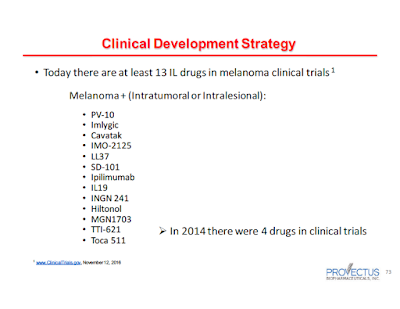 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
Interestingly, however, Viralytics' combination therapy above yielded no complete responses in its Best irRC Overall Response (see the SITC poster here), and did not use RECIST 1.1 in its tumor response measurement. Median doses of CVA21 and pembrolizumab (for the ten patients noted above) were 8 (range 6-11) and 6 (3-11), respectively. CVA21, like T-Vec, has to be delivered often for its effect to manifest, weak or weaker (than PV-10's immunologic signalling) as it is.
Viralytics previously established a collaboration with Merck & Co. in November 2015 to combine CVA21 and pembro in either advanced stage non-small cell lung cancer (NSCLC) or metastatic bladder cancer. In June 2016 the parties initiated a Phase 1b study, one-site (Australia) program for NSCLC where CVA21 would be delivered intravenously (three different dosing levels of CVA21), and not intralesionally or intratumorally.
In his November 21st article "Viralytics' anticancer virus aces checkpoint inhibitor combo trials," FierceBiotech's Phil Taylor provides or references several examples of funded or acquired oncolytic virus companies:
- 2011: Amgen's acquisition of BioVex (U.S.), and thus T-Vec (Imlygic) (formerly OncoVEX) ($1 billion: $425 million upfront and a $575 million earn out), which was approved in 2015,
- 2016: Bristol-Myers' license deal with PsiOxus Therapeutics for $10 million upfront,
- 2016: Celgene's investment in Oncorus' $57 million Serie A financing round,
- 2016: Germany's Boehringer Ingelheim's [option to buy] deal with Austria's ViraTherapeutics for up to $236 million.
Edison Investment Research's Dennis Hulme and Lala Gregorek's November 21st equity research note on Viralytics entitled "Cavatak data continue to impress" presents the valuation rationale below:
 |
| Click to enlarge. |
Interestingly, again, a Viralytics abstract/poster at SITC 2016, "O14 Phase I/II CANON study: oncolytic immunotherapy for the treatment of non-muscle invasive bladder (NMIBC) cancer using intravesical Coxsackievirus A21," noted:
"Notable changes in immune cell infiltrates and expression of PD-L1 within the CVA21-treated NMIBC tissue were also observed. Increased urinary levels of the chemokine, HMGB1, was observed in six of eleven patients following exposure to CVA21."Moffitt Cancer Center noted increased HMGB1 levels in sera of melanoma patients after intralesional PV-10 treatment.
 |
| Click to enlarge. Image source |
Oncolytic, as a label or category, can be somewhat deceiving.
Intralesional (IL) oncolytic virus (e.g., T-Vec, CVA21, HF10, etc.) is different than IL chemical small molecule (i.e., PV-10), both of which might be referred to as oncolytic immunotherapy.
Oncolytic virus immunotherapy however delivered (e.g., intralesionally/intratumorally, intravenously) is different from ablative immunotherapy (i.e., PV-10).
Takeaway: There's a real opportunity for Provectus and PV-10. There is no doubt about the relevance of PV-10, but cutting through the crowd to the front of the pack will require continued effort on the company's part.
No comments:
Post a Comment