Links to news items from July 2013 to [earlier in] May 2016 may be found on the Archived News page.
Hey batta batta batta swing batta batta (October 19, 2016)
Updated below: 10/26/16.
In tracking (diligencing) Provectus intellectual property portfolio, two of three daughter patent applications -- all continuations of August 2015-awarded patent Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (#9,107,887) -- received final rejection notices from the US PTO; '309 (application #14/748,579) on September 23rd and '318 (#14/748,634) on October 12th. '165 (#14/748,608) remains in process as of October 17th. In trying to break my investment thesis while at the same time openly discussing my ongoing due diligence on Provectus, I believe it's important to highlight both successes and failures, or in this case more work that can and very likely will be done to become successful again.
Updated (10/26/16): '165 (#14/748,608) received a final rejection notice on October 18th.I do not read too much into these final rejections.
The process of prosecuting a patent application is a bit like going to bat in baseball, where there are, in general, three strikes before you are out (i.e., receive a final rejection). The are exceptions, however, such as when an examiner makes an office action "final" earlier if he or she feel the application is not advancing prosecution of the case, or when the applicant resets the strike counter by filing a "request for continued examination" (RCE) (which requires remittance of an additional fee to the US PTO).
- "Request for continued examination: A request for continued examination (RCE) is a request by an applicant for continued prosecution after the patent office has issued a "final" rejection or after prosecution "on the merits" has been closed (for example by a Notice of Allowance (NOA)). An RCE is not considered a continuing patent application - rather, prosecution of the pending application is reopened. The inventor pays an additional filing fee and continues to argue his case with the patent examiner." (source: "Continuing patent application" Wikipedia page
As a recipient of over 30 U.S. letters patent, Provectus CTO Dr. Eric Wachter, PhD has been at bat many times, struck out many times, but appears to have rarely failed to gain coverage of an invention.
Updated below: 10/18/16.
 |
| Click to enlarge. Image source |
Intralesional PV-10 should be discussed:
 |
| Click to enlarge |
- Dr. Jun Guo, MD, PhD, Peking University Cancer Hospital (China),
- Dr. Georgina Long, MD, Unversity of Syndey/Melanoma Institute of Australia (Austalia),
- Dr. Sanjiv Agarwala, MD, Temple University School of Medicine/St. Luke's (USA),
- Dr. Merrick Ross, MD, MD Anderson (USA),
- Dr. Axel Hauschild, MD, University Hospital, Schleswig-Holstein (Germany),
- Dr. Robert Andtbacka, MD, University of Utah/Huntsman Cancer Institute (USA), and
- Dr. Grant McArthur, MD, Peter MacCallum Cancer Center (Australia).
This or That redux. InvestorVillage poster pvct-whale (aka pvct in cleveland, PVCT Fixer) (newer discussion board [8/16-], old board [11/14-8/16]) listed purported catalysts and news items, and provided commentary too. I think it is worthwhile to review that post in the context of my table under This or That (October 15, 2016) below.
- 'Allegedly', 2 pretty serious suitors for ph-10 offering upfront money. Their ph10 consultant and their industry thought leaders going through the 'process'. What are the names of the serious suitors (e.g., has one of them been here/there before)?
- Liver by end of year. Has a decision been made to present expanded liver Phase 1 data at ESMO Asia 2016 in December (Singapore) or APASL 2017 in February (2017)? See An Incomplete Story (October 5, 2016) below.
- pediatric combo trial with NYC hospital by end of year. Is it PV-10 as a monotherapy, or in combination with another drug or agent? Is it one hospital or a consortium of institutions? Has preclinical work been conducted? Has drug been shipped?
- If possible pediatric use of PV-10 is true, how absolutely baffling would that be? [partly sarcastic] An unapproved, "local" agent being contemplated for the treatment of systemic disease in children. "Crazy!"
- another Reuters article 'shortly" Why not?
- combo data 1stQ of 2017. I believe the goal is to have data in a form for prospective partners to review by the end of the year, although such timing of course could slip. I imagine data in presentable or poster form would be ready in the new year at the earliest. See An Incomplete Story (October 5, 2016) below.
- 6 more sites coming on line by end of year & interim pushed back 8-12mths from now Timing, subjective as it is because of the [somewhat] subjectivity of the number of events required to trigger an interim analysis of Provectus' pivotal melanoma Phase 3 trial, depends of course on the number of sites and the number of patients each site recruits. See 81 (October 12, 2016) below. Eight to twelve months is a WAG, but then again my "clinical trial math" could be too.
- Peter, Eric, and Tim all ponying up big for the rights offering. Peter selling his gold and putting half his net worth into this next raise. Why wouldn't they, if they did not believe in the drug and its potential (or likely) approvability, which is essential and fundamental to any investment thesis. We heard at the end of the 2000s that melanoma had been solved. Sadly, for patients, this proved not to be true. We hear today that immuno-oncology (IO) is the key to beating cancer. But, as Dr. Jim Allison, PhD observed at the Vatican Conference on Regenerative Medicine in April, we need to make big advances to move from occasional cures to routine cures. This will take multiple tools. Provectus CTO Dr. Eric Wachter, PhD has invested almost 20 years in one tool, so I believe his position is clear. [Company COO and interim CEO] Peter and his gold...what a strange cat :)
- Network1 says they have the solution and that they can fix this company. Mngt shakeup, and get their people on the board so they can control it. Oh and wants Peter to put up the ip as collateral for this next raise in case the company goes under. Have you met or spoken to Damon Testaverde (or, in the case of his son Keith, received an email from him...) or Bill Hemming, among other Network 1 folks? I suppose we could be who we are, unless we work hard to be who we think and hope it's better, more productive, more contributory, more collaborative, and more fun to be. It is unlikely brown shoe stock brokers would have a meaningful, substantive and potentially successful solution to fix Provectus, to the extent some parts of it require modifications, additions and changes. But I can't be too mad at Damon and his crowd, or Maxim or whomever... Peter and the independent directors employed them after all in roles and capacities when better decisions and choices clearly could have been made and implement.
Will you be there at the end, and why?
When it absolutely, positively has to be there... (October 17, 2016)
Updated below: 10/17/16.
H/t a regular hatter (AL): More blocking and tackling [work] to advance the PV-10 trademark:
Updated (10/17/16): Explanation: To validate the trademark pending application for "PV-10," it was necessary to show use of the mark in commerce. In this case, the example illustrates a shipping container used for transport of vials of PV-10 from one party to another. To stanch any conclusion that "in commerce" meant Provectus was selling PV-10, this could include non-commercial examples. The above is an example of the label used for shipment of PV-10 to any recipient (e.g., clinical site, testing laboratory, etc).
Updated below: 10/17/16.
H/t a regular hatter (AL): More blocking and tackling [work] to advance the PV-10 trademark:
- October 14th: Trademark Snap Shot Amendment & Mail Processing Stylesheet, and
- September 26th: Specimen
 |
| Click to enlarge. |
 |
| Image source |
Updated below: 10/15/16, 10/16/16 {twice}, 10/17/16 {thrice} and 10/18/16.
While persevere in this evening's press release could be viewed as the ridiculous use of a word with a negative connotation in this context (dedicated might have been better messaging), Provectus' COO and interim CEO Peter Culpepper made an interesting comment: "...we intend to be active in our communication with stockholders up to and including our quarterly investor conference call in November..." I would speculate the call might held during the second week of November (the week of the 7th); the special shareholder meeting is on the following week on the 14th.
Does one buy common shares now ("this"), or wait until the rights offering in December to buy by participating ("that"), or both? What's the value proposition or risk-reward of this versus that? I believe, like others, that the answers depend on what if any news comes out between now and the deal's pricing, and thus the potential impact on the share price as a result. Does one buy before the supposed news, or wait and see what if anything materializes before deciding to buy?
A very real challenge to Peter's intent to be "active" in company communications to stockholders, however, will be Provectus CTO Dr. Eric Wachter, PhD, and what Eric feels comfortable saying on the call about clinical development program progress -- outside of expected or possible newsflow in the coming weeks and before the end of the year. That is, Eric is much more likely for information to be made public by others before he is willing or understanding enough to provide prior guidance.
All that truly is known in regards to timing (SITC 2016, November 9th to 13th) is Moffitt Cancer Center's poster presentation of murine model work combining PV-10 and chemotherapy (standard of care gemcitabine, I speculate) for pancreatic cancer. I imagine management's eventual decision, presumably for 2017, is whether to initiate an early-stage clinical trial for this tumor type (and thus whatever cancer indication is appropriate).
Updated (10/15/16).1: I had shared an October 7th article from The Cancer Letter entitled "With 20 Agents, 803 Trials, and 166,736 Patient Slots, Is Pharma Investing Too Heavily in PD-1 Drug Development?" (author: Laura Brawley) with Eric.
Provectus is not doing the same thing everyone else is doing: e.g., a small molecule, no biosafety restrictions, no cryogenic cold chain, no treatment over and over and over, etc. I can appreciate it must be more than a little frustrating to Eric that he isn't pursuing an oncolytic virus, which probably would be easier* -- but then he knows fully well he'd have to give up on the potential to actually change the standard of care in cancer treatment.
* I'm jesting here, or being sarcastic, in some regards.
Updated (10/16/16).2: Returning to the core thought underlying this news entry, Peter's very real challenge regarding Eric's current handling of data and, I believe, desire not to publicly communicate such data (and, as importantly, rationale [among other things] for generating it) quicker, as I noted above, all that is known in regards to timing is Moffitt's poster presentation of preclinical work combining PV-10 and chemotherapy for pancreatic cancer. I speculate (guess) away below, however (the below is very much a work in progress):
Updated (10/16/16).3: Data drive deals. Given the issues, perhaps among others, of (i) the complexity of pertinent dataset and (ii) Eric's desire for rigorous scientific validation, can he prepare the various datasets in a form and format for the company to achieve critical deal, collaboration, relationship or transactions milestones? In some ways the answer to this question drives the answers to those I first asked: e.g., does one buy common shares now, or wait until the rights offering?
Updated (10/15/16).1: I had shared an October 7th article from The Cancer Letter entitled "With 20 Agents, 803 Trials, and 166,736 Patient Slots, Is Pharma Investing Too Heavily in PD-1 Drug Development?" (author: Laura Brawley) with Eric.
What could possibly be wrong with putting all eggs in one basket? But this is indeed a common theme in the industry. Once an approach is validated “everyone” follows it. I was struck at ESMO by the number of studies in the intralesional space, just in melanoma. This is to a smaller extent an example where Imlygic’s approval has “validated” the concept and makes it more attractive for companies to pursue.Consider oncolytic virus companies Takara Bio (Japan, agent: HF10, trial protocol) and Genelux (San Diego & Germany, GL-ONC1, trial protocol). These examples illustrate Eric's point (I'd put it another way) about the biopharmaceutical industry being filled with followers and me toos.
Clearly these sponsors are attempting to duplicate the success of Imlygic with equivalent products. The study designs shown at ESMO for these products were all remarkably similar, down to the details of administration (dose schedule).It's hard not to address the 800 lb gorilla in this room, which is why all of these oncolytic viruses (e.g., herpes, coxsackie, vaccinia, etc.) need to be given (a) so often and (b) for so long. They are purported to replicate and spread, and are detected throughout the patient. They also produce "flu-like" symptoms, which presumably means they are causing active infections. But, in contrast, prophylactic vaccines do not require 12 to 20 courses, and exposure to the wild types of these viruses that typically leads to disease on minimal initial exposure.
Provectus is not doing the same thing everyone else is doing: e.g., a small molecule, no biosafety restrictions, no cryogenic cold chain, no treatment over and over and over, etc. I can appreciate it must be more than a little frustrating to Eric that he isn't pursuing an oncolytic virus, which probably would be easier* -- but then he knows fully well he'd have to give up on the potential to actually change the standard of care in cancer treatment.
* I'm jesting here, or being sarcastic, in some regards.
Updated (10/16/16).2: Returning to the core thought underlying this news entry, Peter's very real challenge regarding Eric's current handling of data and, I believe, desire not to publicly communicate such data (and, as importantly, rationale [among other things] for generating it) quicker, as I noted above, all that is known in regards to timing is Moffitt's poster presentation of preclinical work combining PV-10 and chemotherapy for pancreatic cancer. I speculate (guess) away below, however (the below is very much a work in progress):
 |
| Click to enlarge. |
Two key opportunities potentially/possibly present themselves this quarter. First, one could presume a PH-10 license deal requires a successful end-of-Phase 2 (EOP2) meeting with the FDA. For when is the meeting scheduled? Second, a multi-indication collaboration between a Big Pharma and its immune checkpoint inhibitor, and Provectus and PV-10, presumably requires (a) preliminary data from the Phase 1b study of PV-10 and pembrolizumab for advanced melanoma and (b) some or whatever data from the Provectus' immune biomarker work. When could Eric provide this information to prospective partners?
Of course, if management pulls one or both of the above off, would there be a need for the rights offering?
Updated (10/17/16).4: [i] I am grateful to a shareholder who shared [what I nerdily and rightfully refer to as] due diligence materials he or she recently gathered. My perspective along this journey has long been influenced by fellow retail shareholders who are smart and intelligent, are outsiders and not insiders, and who may or may not know how the game is played or be able (or choose not) to play it, but always have been willing to share data, information and knowledge in hopes that collectively we are better prepared to survive and potentially (hopefully) prosper (i.e., garner a return on capital, rather than a return of a fraction of capital) from the "game." Yet, without such input and insight, we truly are left at the mercies of those who have more information (I tell my students Wall Street isn't necessarily smarter that Main Street, but typically or usually better at information arbitrage than the rest of us). I have encouraged him or her to share their information with the newer InvestorVillage Provectus stock discussion board.
[iii] It is clear some folks are taking sides, so to speak. I don't believe Network 1 believes Provectus will achieve any key bridge events (my terminology, not theirs) before a rights offering is done.
[iv] A new Merck & Co. location visited the blogging, reading (among other things) September 5, 2015 blog post Trendwatching, and exiting via the linked picture below.
[v] Terminology. The end of the valuation and transactional bridge should the outcome of the pivotal melanoma Phase 3 trial (one could reasonably argue several aspects of it, like a data monitoring committee pre-call, an interim data readout, preliminary full data, etc.). Bridge events would include a PH-10 license, a co-development collaboration combining PV-10 with another checkpoint inhibitor, etc.
Updated (10/17/16).5: [a] As I noted below under An Incomplete Story (October 5, 2016), InvestorVillage Warlord is a good recent follow. For this news entry update, see here and here. In his latter post Warlord wrote "No bp has seen the ph-10 Moa because it has to go to FDA first. The top researchers and management has seen this and it should be big when it gets out. But bp needs to wait." In my best IV poster STARLIGHT66 impression, I am led to believe prospective partners have seen mechanism of action data.
[b] Another round of PH-10 rumors surfaced today regarding intellectual property (IP). Speaking of STARLIGHT66, he engaged in such here. In doing deals where IP is important, let alone critical, addressing the topic [in detail] as part of a transaction often is done after a "go" business decision (as opposed to "no go") has been made. So, it is likely upfront, milestone and royalty payment discussions already have been had.
[c] I noted under Friday (September 23, 2016) the rumor that mechanism of action data is/has been available for prospective partners (and that Seth Orlow has been doing the rounds with it). Some days thereafter STARLIGHT66 posted (and later deleted his post) regarding [some] prospective deal terms in reply to IV poster canis_star; it was something to the effect of a $30-40 million upfront payment and a $1B deal value. With his (STARLIGHT66's) follow-up post regarding "all or nothing at all" on the IP, which clearly undervalues the company (i.e., oncology + dermatology* vs. dermatology only), it strikes me folks could be beginning to price Provectus.
* If am to read STARLIGHT66 properly.
[d] Visitor. Wachtell, Lipton, Rosen & Katz recently visited the blog. The law firm has been Pfizer's outside M&A counsel (e.g., Allergan, Anacor, Medivation).
Clinical Trial Status Update: Melanoma P3 (October 13, 2016)Of course, if management pulls one or both of the above off, would there be a need for the rights offering?
Updated (10/17/16).4: [i] I am grateful to a shareholder who shared [what I nerdily and rightfully refer to as] due diligence materials he or she recently gathered. My perspective along this journey has long been influenced by fellow retail shareholders who are smart and intelligent, are outsiders and not insiders, and who may or may not know how the game is played or be able (or choose not) to play it, but always have been willing to share data, information and knowledge in hopes that collectively we are better prepared to survive and potentially (hopefully) prosper (i.e., garner a return on capital, rather than a return of a fraction of capital) from the "game." Yet, without such input and insight, we truly are left at the mercies of those who have more information (I tell my students Wall Street isn't necessarily smarter that Main Street, but typically or usually better at information arbitrage than the rest of us). I have encouraged him or her to share their information with the newer InvestorVillage Provectus stock discussion board.
Updated (10/18/17).7: InvestorVillage poster pacificnorthwest1 posted the due diligence material I referred to above, her notes of a conversation with a Maxim Group (and former Network 1) stock broker historically involved with Provectus.[ii] In response to InvestorVillage poster leave_the_gun, I implicitly assumed a third possibility, of buying after the rights offering, was part and parcel of the second possibility of participating in the offering itself -- because (appealing to the efficient markets hypothesis, in context and FWIW) I assumed one would get as good a price in the deal as after it (where I assumed the stock market wouldn't dramatically over or under react to the transaction). leave_the_gun appropriately corrects me in that some existing or prospective shareholders would want to see, as a further datapoint, how the stock market would reacts (ingests information) to the rights offering pricing.
[iii] It is clear some folks are taking sides, so to speak. I don't believe Network 1 believes Provectus will achieve any key bridge events (my terminology, not theirs) before a rights offering is done.
[iv] A new Merck & Co. location visited the blogging, reading (among other things) September 5, 2015 blog post Trendwatching, and exiting via the linked picture below.
 |
| Click to enlarge. Visitor exit link |
Updated (10/17/16).5: [a] As I noted below under An Incomplete Story (October 5, 2016), InvestorVillage Warlord is a good recent follow. For this news entry update, see here and here. In his latter post Warlord wrote "No bp has seen the ph-10 Moa because it has to go to FDA first. The top researchers and management has seen this and it should be big when it gets out. But bp needs to wait." In my best IV poster STARLIGHT66 impression, I am led to believe prospective partners have seen mechanism of action data.
[b] Another round of PH-10 rumors surfaced today regarding intellectual property (IP). Speaking of STARLIGHT66, he engaged in such here. In doing deals where IP is important, let alone critical, addressing the topic [in detail] as part of a transaction often is done after a "go" business decision (as opposed to "no go") has been made. So, it is likely upfront, milestone and royalty payment discussions already have been had.
[c] I noted under Friday (September 23, 2016) the rumor that mechanism of action data is/has been available for prospective partners (and that Seth Orlow has been doing the rounds with it). Some days thereafter STARLIGHT66 posted (and later deleted his post) regarding [some] prospective deal terms in reply to IV poster canis_star; it was something to the effect of a $30-40 million upfront payment and a $1B deal value. With his (STARLIGHT66's) follow-up post regarding "all or nothing at all" on the IP, which clearly undervalues the company (i.e., oncology + dermatology* vs. dermatology only), it strikes me folks could be beginning to price Provectus.
* If am to read STARLIGHT66 properly.
[d] Visitor. Wachtell, Lipton, Rosen & Katz recently visited the blog. The law firm has been Pfizer's outside M&A counsel (e.g., Allergan, Anacor, Medivation).
Updated (10/17/16).6: Wachtell Lipton last visited September 2015.
Updated below: 10/13/16 {thrice}, 10/14/16 and 10/15/16.
H/t a regular hatter, PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma, new site:
 |
| Click to enlarge |
Updated (10/13/16).2: Thing one. Trading on the NYSE MKT in common stock (PVCT) and warrants (PVCT.WS) was suspended because the common share price dropped below 6 cents.
Updated (10/14/16).4: Provectus issued a press release today related to the above mentioned trading suspension and re-trading of the stock and warrants on another exchange, "Trading in Provectus Biopharmaceuticals Common Stock and Warrants Suspended by NYSE MKT." As of this writing no associated 8-K was filed.
Of note to me were two statements and quotes. First: "In an effort to regain compliance with the listing standards of the NYSE MKT, the Company filed a preliminary proxy statement with the SEC on October 5, 2016 to request that its stockholders approve, at a special meeting of stockholders to be held on Monday, November 14, 2016, at 1:00 p.m. Eastern Time at 265 Brookview Centre Way, Suite 600, Knoxville, Tennessee 37919, among other items, a reverse stock split, which will be at the discretion of the Company's board of directors to effectuate if the proposal receives the requisite stockholder approval at the special meeting. The reverse stock split, if approved by stockholders and effectuated by the Company's board of directors, will combine each 10 to 50 shares of common stock (with the exact ratio to be determined in the sole discretion of the Company's board of directors) into one new share of common stock, and will increase the price of the Company's common stock accordingly. A reverse stock split will be necessary for the Company to maintain its listing on the NYSE MKT, unless the Company's stock price begins trading at higher levels for a sustainable period of time.
There can be no assurance, however, that the Company's stockholders will approve the reverse stock split. Even if stockholders approve the reverse stock split and the Company effectuates the reverse stock split, the Company may still be subject to delisting if the price of its common stock again falls below $0.06 or fails to rise above $0.20 and the Company is not otherwise able to meet applicable listing requirements of the NYSE MKT."
I would imagine stockholder approval of an increase in the number of authorized shares is more likely than approval of a reverse split. I am hard pressed to believe management wants a reverse split.
Second, "Peter R. Culpepper, Interim CEO and COO of Provectus, stated, "we are committed to persevere in our efforts for both patients and stockholders to win, and we intend to be active in our communication with stockholders up to and including our quarterly investor conference call in November, which is planned to coincide with the filing of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016 with the SEC.""
I'm pleased to know Peter (and presumably Eric) are committed to persevering, like the protagonist in the Unbroken story; however, Louis Zamperini was a former Olympic track star. As such, both persistence and competence, among other important traits, come into play. I certainly do not think Peter and Eric, individually and collectively, have been as competent in each of their respective roles and various responsibilities as they could have been. If we don't seek to examine different ways of doing things that are best practices at best (or better practices than we're currently engaged in at worst), especially if they are in fields or areas where we have no or little experience, we might be persistent but all that crosses the finish line is the withered carcass of underwater stockholders.Thing two. Provectus COO and interim CEO Peter Culpepper is speaking to two groups of shareholders on the East Coast today. Feedback from one such meeting (i.e., folks who I think are purely shareholders, as opposed to the other group who include broker-dealers) was a tad startling. These folks have had a historically poor view of management, including and especially of Peter, who has been their primary interface over the years. Without putting too many words in their collective mouth, it probably is not too far from the truth to say they previously despised him (at least that's my interpretation or read of the situation). Yet, they apparently walked away being impressed with Peter, presumably both in what he said and how he said it. One attendee now appears convinced core management will prevail. Of company CTO Dr. Eric Wachter, PhD (I assume), said individual also now believes he no longer is the wise guy trying to get things done outside of normal FDA guidance.
Updated (10/14/16).4: Peter [apparently] also met with Network 1 folks (management, I believe, and not necessarily clients). There was a rumor circulating today that the firm, presumably through the selling of clients' Provectus holdings, was responsible for driving the share price below the 6-cent listing threshold on Thursday morning. I wouldn't have thought Network 1 would have that those many shares to drop the share price nor the intent to do so. But what do I know?
While the meeting may have been a positive one for either or both parties (Network 1, Peter), I have to wonder how Network 1 can be of help to Provectus. What is Network 1's current motivation to help, assuming of course the rumor of getting clients to previously sell out of their Provectus positions is true? I would have to imagine it is the millions and millions of non-tradeable warrants the firm and its clients still hold. You have to wonder whether (and under what circumstances) they could or would ask Peter for (or demand from him) a repricing of these warrants' exercise prices.
Updated (10/15/16).5: I do not have a favorable opinion of management and employees of Network 1 and Maxim. I have tried over time to coach (I use this term somewhat lightly, and do not mean to be overly presumptuous) Peter in regards to aspects of his role and responsibilities. Eric and he have grown as executives, managers and industry professionals on the dime of past and present retail shareholders. Peter has been, at various times, one or more of inexperienced, naive, lazy, forced, etc. in his use of these firms to fundraise. That he may or may not have been ripped a new one is of no consequence (I appreciate him taking one for the "team") if he does not further use or kowtow to Network 1.
As an aside, I have been a paid or volunteer board member, board observer, advisory board member, and committee member for nearly 20 privately held companies and nonprofits over the years. Speaking of potential new orifices, I currently sit on the audit and finance committee of one of the largest (sadly) non-profits of its kind in the country (to provide some domain expertise the committee previously was lacking). It has an annual budget far in excess of Provectus'. I am humbled every quarter by a fellow committee member's intelligence, pragmatism, detail-orientedness, persistence and outspokenness (when appropriate) when reviewing entity materials and discussing them with management (sometimes I am almost humiliated by the degree of her competency, and find myself always grateful to her for the learning experience).Updated (10/13/16).3: Immune biomarkers. In an August 2016 paper led by an OncoSec-related clinical investigator, Dr. Adil Daud, MD, "Tumor immune profiling predicts response to anti–PD-1 therapy in human melanoma," it was noted that:
"It is becoming increasingly clear that the immune composition in tumors is markedly different from that observed in peripheral blood. Thus, robust biomarkers that predict response to immunotherapy will most likely be derived from tumor tissue."I asked Eric if he needed to address immune biomarkers with tissue samples? The Phase 1b study combining PV-10 and pembrolizumab in patients with advanced (Stage IV) melanoma had as one of its secondary endpoints change in immune biomarkers (peripheral blood mononuclear cells [PBMCs] assessed versus baseline values for changes in T cell populations).
He replied: These two aspects of immune response are not directly related. In our case, markers in blood are upstream indicators of immune activation by PV-10 ablation. In the work you cite, it is being noted that the presence of immune cells in tumors, and certain genetic characteristics of tumors (such as extent of PD-L1 expression) are prognostic for response to checkpoint inhibition. This latter concept is proving highly relevant in lung cancer (n.b., recent differences in the outcomes of Phase 3 studies of anti-PD-1 in lung cancer using different thresholds for PD-L1 expression). For melanoma the jury is at best still out regarding such relevance.
81 (October 12, 2016)
I believe Provectus management, specially company CTO Dr. Eric Wachter, PhD, needs to start an accurate, understandable, honest dialogue with shareholders -- sooner rather than later -- of the status of the company's clinical development program's many parts.
As I noted under Biological basis (October 11, 2016) below, Eric said (on Provectus' August 10th business update call) the triggers for the interim and final interim assessment of efficacy and safety for the company's pivotal melanoma Phase 3 trial were the generation of 81 and 162 events, respectively. The contemplated trial enrollment (N) is 225, so that 81 events for an interim analysis would have been designed out of 113 patients (or half of N).
Since the trial is randomized two-to-one (treatment arm-to-control arm), of the 81 events that are assumed to occur or needed to occur in order for the interim analysis to be triggered, 54 of them would or are needed to fall in the treatment arm and 27 in the control arm. This equivalent or same behavior is the so-called "null hypothesis;" that is, both arms are assumed to behave the same.
The prevalent or consensus thinking is that (i) few-to-no events would occur in the PV-10 treatment arm because there would be no progressions of the injected target lesions, while (ii) many-to-all progressions (events) would occur in the chemotherapy and oncolytic virus (T-Vec) control arm. So, while the null hypothesis (at the outset of the trial) is that the two arms behave the same, the alternative hypothesis (borne out by the trial's outcome) is that the two arms behave very differently (i.e., there are far fewer events in the treatment arm, and far more in the control).
If 27 events are required, the study then must recruit 81 patients, or maybe more, to generate 27 ( you can see I am playing around in absolutes for ease of math).
But does the trial need to register 27 events in the control arm in order to trigger an interim analysis? I don't believe so; however, I think the threshold figure is a subjective decision (that would be made by the study's data monitoring committee (DMC). I point to Eric's comments from the August 10th call:
"And so, we are able to monitor at the terminal end of the study, and if the clinical trial data monitoring committee determines that it's in the best interest to end the study, it could end before we had all of the progression events occurring. I doubt that'll happen. It will be a very unusual circumstance, but it would probably be positive for the drug because it would suggest that a large number of patients weren't progressing because of the majority of the patients in a two-to-one randomized study are from the PV-10 arm, and that probably would imply that the PV-10 patients weren't progressing."As I also noted below, one cannot consider the 27 or less [expected] control arm events (out of 27 patients) in a vacuum, but rather in relation to the number of events generated in the treatment arm -- the [expected] "imbalance."
In the eyes and minds of the DMC, what is the presumably subjective (but with some form of objectivity vis a vis statistical significance) imbalance threshold that would allow them to establish what indeed is in the best interests of the study's patients?
27 control arm events vs. zero or a few treatment arm ones? 20-to-0 or a few? 15-to-0 or a few?
Multiply these numbers by three, and you arrive at the number patients required to be treated (assuming, of course, extreme arm performance): 81, 60 and 45, respectively. I've written about this before; see August 13, 2016 blog post The Untrigger, where I projected enough patients by/in January 2017 (1Q17):
"Rather than hash out or re-hash my work/writing about prescribed event and time triggers, the number of patients needed to be enrolled to hit 81 events, etc., I'd like to state (speculate) the following -- [I believe] there is a trigger Eric does not want to explicitly speak about or affirm, which could/would be an imbalance in the number of events between the treatment and control arms, which could/would cause a statistical and ethical dilemma for the study's IRC. What would an imbalance look like or comprise? In the extreme, or potentially actually, there would be no events in the PV-10 treatment arm, and events of whatever frequency or magnitude in the chemotherapy/oncolytic virus control arm. Takeaway: The pivotal trial does not have to reach 81 events for the interim analysis to be triggered...
The statistical dilemma is having a treatment arm with no or very few events, and a control arm with many, many more (like 0 and 15, or 0 and 20). The ethical dilemma is a treatment arm that works and works very, very well (i.e., no disease progression), and a control arm that demonstrates what everyone already predicted (i.e., everybody progresses).
The question then would be, "how many events in total and across the two arms would be required for the IRC to recognize an imbalance, and thus trigger the interim analysis?" Twenty events in the control arm (and none in the treatment arm)? Thirty? A two-to-one study randomization then would suggest enrollment and treatment of 60 patients. Or 90.
Math, math, math."What leads me to believe some number less than 27 is required has been Eric's insistence on utilizing a handful of clinical trial sites. Notwithstanding gaudy efficacy (albeit more locally oriented than systemic) and near-pristine safety results, breakthrough therapy designation (BTD) was denied on the basis of a paucity of data (not poor data). There was chatter coming from management that some key opinion leaders (KOLs) believed the Phase 3 trial design was unethical because of the inclusion of chemotherapy as the original and initial comparator for this patient, where clinical trial is the preferred approach to treatment. Yet, setting aside the process and time taken to account for the approval of fellow intralesional (IL) T-Vec (for advanced melanoma), 20 months into the study Eric only has six sites recruiting in the U.S. and Australia. Rather than say site activation was/has been "in process" (Provectus COO, and interim CEO Peter Culpepper's words), I believe Eric chose to have a smaller number of high quality sites (sites where he knew experienced investigators could administer PV-10 properly and effectively) to get to his low threshold of control arm events, rather than a larger number of sites to potentially get there faster but with perhaps greater uncertainty over the predicted outcome.
Except that, and this was well established prior to the commencement of the trial, it was going to be a challenge to recruit patients because of the trial design, particularly only being allowed to use as target lesions those cutaneous melanoma/disease locations. In addition, it also appears to me that sites were not providing him with the expected numbers of patients, particularly in Australia. And all he needed was a "few" patients whose outcome was reasonably well predicted in both arms to generate the information he needed to drop into the data module of the new drug application that was ready to go...
Not that patients were not being recruited. Not that something was "wrong" with the trial protocol. Not that he "couldn't" get sites up and running. Not that something, somewhere was "wrong."
Rather, after having his butt handed to him Eric wanted to him when he thought BTD was a done deal (and, likely now in hindsight, with a [this] pivotal trial as what would come with it), he wanted to be certain the pivotal trial would win, will win. That he chose a certain way, but that way was going slower than expected even if the outcome was assured (in his view). This approach, which I can't say I wholly disagree with, of trying very hard to be certain and risking the perfect being the enemy of the good or really good, is denigrated when you say nothing, or say it cryptically or poorly.
And when you let time elapse. The merits of your approach are denigrated by your silence and the passage of time.
There truly may be things that indeed are wrong, like, you know, checkpoint inhibitors are going to cure melanoma so there's no need for PV-10... But here's some math that could be more right than wrong (at least I believe so):
- Let's assume Provectus' pivotal melanoma Phase 3 trial has treated about 20 patients (range: say, 15-25) by the end of October among the current six sites recruiting as denoted on CT.gov, and
- Let's also assume sites can recruit one patient per site per month (if not more) as a result of the proposed protocol refinements (subcutaneous lesions now can be target ones), which become effective at month-end when a handful of European Union (and maybe Australian) sites could come online (yes, WAGs) -- to make the math easier, I'm simply saying just add per month the number of sites that are then recruiting to 20.
How long would it take to arrive at 45 (15 events), 60 (20) or 81 (27) patients, from here on out? Again, depending on assumptions and math, three to six months?
Starting a dialogue with shareholders to say some of the above would help, and giving realistic timing that isn't that hard to model with reason also would help too.
I'm led to believe... Should the share price drop below six cents, trading would be suspended until the ticker was moved to over the counter, after which trading would resume. Setting aside administrative matters and process, the ticker would be up-listed after the share price achieved the $0.20-level (although I am unsure for how long, etc.).
So, in addition to giving management and the independent board members discretion over more shares outstanding, which has merit, shareholders are being asked to given them discretion over a reverse split (whether one is necessary or not), which given their lack of experience, knowledge, intelligence and intent might be a bridge too far.
Biological basis (October 11, 2016)
Updated below: 10/11/16.
Provectus issued a press release and made an associated 8-K filing related to potential changes in the clinical trial protocol of its pivotal melanoma Phase 3 study, "Announces Poster Presentation on PV-10 at European Society of Medical Oncology 2016 Congress Now Available Online." The ESMO 2016 trials-in-progress poster is here.
I though the poster's conclusion's focus on PV-10's branding as an ablative immunotherapy was nice.
 |
| Image source |
"In particular, allowing patients with primarily or exclusively subcutaneous melanoma (that is, melanoma below the skin surface) and those with larger tumors should not significantly affect the biological basis for our phase 3 study, but could substantially expand the fraction of patients with locally advanced disease that can participate in the study."Possible refinements are noted at the bottom of the screenshot of the relevant portion of the poster, below:
 |
| Click to enlarge. Image source |
Lesion size. Previously, work by Australian compassionate use program (special access scheme) site Princess Alexandra Hospital noted smaller lesion size (and younger age) were predictors of response; however, investigators were dealing with small lesions: " In the current study, lesion size was also found to be predictive. Of the five patients who achieved a complete response, the average lesion diameter was 3 mm compared to the cohort average size of 6.3 mm." Lesion size of upto 5 cm is comparable to fellow intralesional drug T-Vec/Imlygic's upper lesion size treatment range; prescribing information is here.
Subcutaneous injection. Recurrent, satellite or in-transit locally advanced cutaneous or subcutaneous melanoma metastases comprise AJCC Stage IIIB, IIIC or Stage IV M1a with no active nodal metastases. T-Vec's pivotal Phase 3 melanoma permitted subcutaneous disease to be injected as target lesions (for purposes of determining response). Provectus has allowed subcutaneous disease to be injected from the beginning; however, the proposed change of permitting subcutaneous lesions to be TARGET lesions.
The company had been hesitant to permit subcutaneous lesions as target lesions because study photography would not be particularly compelling for lesions that are not visible on the skin surface. Tumor measurement approach RECIST does not require photography. In this case, radiologic assessment of subcutaneous lesions that are target ones would allow RECIST determination. Provectus expects to permit subcutaneous lesions to be target ones because a significant portion of patients with locally advanced disease have primarily subcutaneous disease. The current criteria appears to unnecessarily exclude patients that have the same biologic characteristics as those currently allowed.
As such, there is no change to the biological basis of the trial. Eric noted to me: "The disease characteristics and natural course are equivalent to these patients vs those eligible under the current criteria. Thus, the biology is the same."
According to Provectus, anecdotally, sites like MD Anderson (Dr. Merrick Ross, MD) (zip code 77030) and Huntsman Cancer Institute (Dr. Robert Andtbacka, MD) (84112) were recruiting 10-20 patients per site per month for IL agent Allovectin-7's pivotal melanoma Phase 3 trial. Target enrollment of that trial was 390, and there were 88 study location.
Takeaways:
- If these potential changes are proposed to and acknowledged (accepted) by the FDA, it would appear to me Provectus certainly has further increased the patient population within Stage IIIB-IV M1a to which PV-10 is applicable, for both faster/greater trial enrollment and label purposes,
- I cannot see an interim data readout analysis by year-end; hence, the indications of a readout in Q2 may (and probably) have merit; see An Incomplete Story (October 5, 2016) below, and
- Taking an optimistic viewpoint, the potential increase, within the category, of patients with measurable disease presents an interesting "twist." More patients with more measurable or target disease would permit more event generation, and thus potentially increase enrollment and increase the rate of event generation (in those patients whose disease progresses in the control arm, and then who would crossover into the treatment arm).
Updated (10/11/16): He was against management before he was for Peter before he was for Eric before he was against Eric? I am "hearing" the potential protocol changes displayed at ESMO 2016 should be implemented, but what do I know...?
CTAs. Eric noted on the ESMO 2016 poster that clinical trial agreements for the pivotal melanoma Phase 3 trial were underway with/at European Union sites, and Russia was added to anticipated geographies, compared to what was on the ASCO 2016 trials-in-progress poster.
Amendments. Multiple amendments to trial protocols, particularly smaller ones and refinements, like one of the ones Eric modeled the Phase 3 trial after seem par for the course, particularly in a fluid (oncology) industry setting.
In-line. H/t InvestorVillage poster canis_star who linked to a melanoma combination therapy poster at ESMO 2016 of an IL agent (CAVATAK, an oncolytic virus) and anti-CTLA-4 drug ipilimumab. The poster included a helpful table comparing other IL agents like T-Vec and HF-10. CAVATAK + ipi produced an in-line response rate with a better safety profile.
 |
| Click to enlarge. Image source |
How many such events would have to occur in the control arm for an interim assessment to be performed? Eric went into a long discussion regarding the null hypothesis (I have written about this extensively in relation to triggers visible and invisible, clinical trial math, etc.), where one assumes both arms -- the control arm, and the treatment arm -- behave the same.
The figure could be anywhere from 0 to 75 (i.e., 225 ÷ 3), but the null predicts it would be about 27 events (81 ÷ 3), since the study is randomized 2:1 and it is assumed one third of the events would occur in the comparator arm (per the null, it is assumed 54 events [81 ÷ 3, then × 2] would occur in the PV-10 arm). If the number of events in the control arm is much less than 27, PV-10 is not faring well, whereas if the number is much greater than 27, things are going well for PV-10.
So, does the company need a trial enrollment of somewhere around 81 patients or so (randomized on a two-to-one basis), or maybe a little to a lot less (like 45-60, a number I've thrown out there before), if there is a large imbalance in events between the two arms), in order for an interim analysis to be conducted? If I speculate enrollment is in the teens to about 20, it would seem clear to me the protocol change to allow subcutaneous target lesions is/was crucial. How long do six current sites plus future sites at some point(s) in time get Eric an additional 25-60 patients? It's just a race to the data and a question of how much is left when the time comes.
I am struck by... (October 10, 2016)
Updated below: 10/10/16 {twice}.
a. Following data releases at ESMO 2016 (it continues until tomorrow), the further drop in Bristol-Myers' share price, which as of this writing was below $50. When I wrote August 30th blog post The Day Big Pharma's Earth Stood Still, which Bristol-Myers locations visited a few times, the price was over $57. The stock last saw $75 on August 4th.
b. The messaging of journalists reporting on the checkpoint inhibitor competition between Merck & Co. and Bristol-Myers. See, for example, Endpoints' John Carroll's October 9th "Merck’s glorious victory in lung cancer marks a Russian winter for Bristol-Myers" and Forbes' Matthew Herper's October 9th "Bristol's Opdivo Disappoints in Lung Cancer, Losing Ground To Rival Merck."
c. Bristol-Myers now saying anti-PD-1/PD-L1 drugs and agents are the same.
 |
| Image source |
 |
| Image source |
e. The lack of separation of non-small cell lung cancer survival curves between Keytruda + chemotherapy, and chemotherapy alone.
f. Merck & Co. having no pivotal (registration) combination therapy trials (aside from utilizing chemotherapy) for Keytruda in gastrointestinal cancer.
g. Non-immunogenic (versus immunogenic) being further divided into immune exclusion and immune ignorance, which correspond to Provectus CTO Dr. Eric Wachter, PhD's points regarding immune suppression and tumor non-immunoactivity. See Kobayashi Maru (October 4, 2016) below.
Updated (10/10/16).1: h. I'm not struck by this, but it's worth noting here.
 |
| Image source |
 |
| Click to enlarge |
 |
| Click to enlarge. Image source |
Updated (10/10/16).2: i. Either or both of independent board members Al Smith and Jan Koe not tapping out by now, given how in over their heads they must find themselves (e.g., not understanding what to do, not knowing what to do, etc.) for the purported experience they bring to the table as directors.
For example, they should (i) instruct Provectus COO and interim CEO Peter Culpepper and CFO John Glass to smartly reduce monthly cash burn (likely much more than Peter has done, an is willing to do or capable of doing), (ii) require Peter and John to create a 6-month (illustrative timeframe) monthly cash burn projection (I don't believe Peter did such a crucial and prudent exercise during his tenure as CFO) that the board then would require Peter/John to update every two weeks or month, (iii) swiftly evaluate (it would not take too long) and then dismiss (as appropriate) several of Peter's lower quality vendors (with eye to replacing them or having Peter do the work), and (iv) assist company CTO Dr. Eric Wachter, PhD with (i.e. wrest control from him) communications of what clinical and preclinical work Provectus is undertaking and when to communicate it. There are several other straightforward things they could do to right the ship as they race towards the data. There also are several shareholders willing to replace Smith and/or Koe to effect these changes.
j. How challenging must it be for the board to deal directly with Eric when you don't know the questions to ask him, and you don't understand what he is saying in the situations when he answers you. The board has deferred to Peter, who continues to defer to Eric in regards to communicating what, and what when. If you don't know or understand what what is, how can do determine what what is important to what degree, and when you might communicate each relevant what?
An Incomplete Story (October 5, 2016)
Updated below: 10/6/16 {thrice}, 10/7/16 and 10/8/16.
Provectus issued a press release and made several SEC filings today related to the company seeking (i) an increase the number of authorized shares of common stock, par value $.001 per share from the current figure of 400 million to 1 billion, (ii) to raise up to $21 million from existing shareholders (record date not yet determined) via the issuance of common stock (price not yet determined) that also would include warrant coverage (amount not yet determined), and to effect a reverse stock split between 1-for-10 and 1-for-50. Filings included an 8-K (of the press release of the above), a proxy filing/Schedule 14A (a shareholder meeting on November 14th) and a registration statement/S-1. There is much to read in the various components of the filings that were made today, and several topics to analyze and address, such as the potential delisting of the company's common stock from the NYSE MKT.
Stepping back for a moment, it would seem simple and straightforward to observe that Provectus has until November 14th (or by when votes comfortably could be made, such as at least 48 hours before the voting deadline) to compellingly convince existing shareholders (of whatever record date the company decides) to buy into/buy up the rights offering through the issuance/generation of news and/or release of data, whatever this item or these items may be. It could not convince non-tradeable warrant holders through several iterations of the warrant exchange program(s). Make the case this time and Provectus could well pave the way to one or more catalysts for itself and the share price. Fail to make the case, and that row becomes exceedingly tough to hoe (to reach its hoped or desired for end).
Updated (10/6/16).1: Some further thoughts this morning, with thank yous to the numerous people to whom I spoke, texted and e-mailed yesterday about all things Provectus:
- Is the global pharmaceutical industry market for oncology setting up well for Rose Bengal and PV-10? I believe so,
- Can Rose Bengal/PV-10 potentially achieve pathologic complete response (ablation) so as to induce a potentially robust immune response in the body (immunotherapy) against cancer? I believe so,
- Thus, can PV-10/Rose Bengal potentially improve, and potentially dramatically so, cancer patient outcomes around the world? I believe so,
- Can these outcomes be achieved across some, many or all solid tumor cancers? I believe so,
- Do data drive deals? Of course,
- Does Provectus have enough data? Not yet,
- Is there a better sense of timing around such and other data? I believe so,
- Have I been wrong about former and current management, and former and current independent board members, in context? Certainly,
- Have I been right about the drug substance (Rose Bengal) and/or drug products (PV-10, PH-10)? Near-certainly yes, and
- Would I support the board and management's recommendations for the proxy vote? Yes, in context; however, the board of directors requires new independent members (as part of a clear transition facilitated by the fundraising and vote) who have proper intent, sufficient competence and constant vigilance to oversee and direct management to and over the finish line for the benefit of shareholders.
It is not yet clear to me whether to buy shares prior to and/or participate in the rights offering, as I believe there should be news flow of one sort or another that may positively influence the details of the offering*. I may do one or the other or both.
Updated (10/6/16).2: More thoughts follow:
- It will take time for the SEC to make Provectus' S-1 effective. I would be surprised if the rights offering is completed sooner than early- to mid-December. As I noted under Kobayashi Maru (October 4, 2016) below, and setting aside the share price-related listing requirement, given enough cash and stockholders' equity at third quarter's end, the fundraising could have been extended towards the end of the audit/audit reporting cycle (i.e., early-December).
- But, Provectus now has a share price-level listing concern. The only benefit for shareholders to agree to (vote for) a reverse split is to mitigate this concern, remain on the NYSE MKT, and not provide a distraction to the company and its story as the quarter unfolds. Otherwise, what's the point of a reverse split?
- Do I really want to allow Provectus management and its independent board members to have discretion and control over more authorized shares and a reverse split decision (as sought in the recently filed proxy)? Each of them, in their own special and respective ways, has demonstrated himself to be lacking as a decision-maker,
- We can argue about most any assumption as it relates to most any analysis; however, I do not believe one can argue about math (or the ideal gas law). For someone pursuing a CFA charter, his analysis does not bode well for passing Level I (a good reply is here). In the end, analysis matters,
- InvestorVillage poster Warlord is, I believe, a Provectus shareholder from/in Denmark, along with other Danish shareholders, and a worthwhile read. At least four helpful and potentially insightful points were made in a recounting of a local meeting with management (ESMO 2016 this week will be held in Copenhagen):
Updated (10/8/16).5: Warlord continues to provide helpful information from the Danish investor meeting.
- The end of the Phase 1b combination therapy trial of PV-10 and pembrolizumab for advanced (solely Stage IV) cancer is [could be] 1Q/2Q17. As an aside, I believe preliminary data will be presented at HemOnc Today Melanoma 2017 in late-March,
 |
| Image source |
Updated (10/7/16).4: On Provectus' August 10th 2Q16 business update call Eric said, in regards to the timing of the availability of interim Phase 1b study results, "Okay, Bill. I will try to address those in the order that they were asked. It’s possible that we would report some initial data from the Phase 1 b portion of the study this year. If that is the case, it would be some form of interim data. It is much more likely that will be in the first half of next year, picking up into the 2017 oncology cycle. If we submitted an abstract today to a major meeting, that wouldn’t be recorded until sometime late in the fourth quarter, so we have to acknowledge that it’s likely that a public readout of those data would not be until sometime in the first half of next year. That is not to say that in the context of confidential discussions with potential corporate partners, there may not be some opportunity to share those data with partners, but that is also, again, pure speculation at this point." Understanding that Eric guided "possible" (by year-end) during the summer, it may well be that he meets this "expectation."
- The interim pivotal melanoma Phase 3 trial analysis is [could be] 2Q3Q17. It would seem to me, as I have recently thought about this topic, that aside from an imbalance of events themselves between the two arms (PV-10; chemotherapy or oncolytic virus), PV-10 could now have to beat fellow intralesional (IL) agent T-Vec on the primary endpoint of progression-free survival (PFS), which takes (requires) time (to elapse), and
- There may be another amendment to the Phase 3 trial protocol to further ease patient enrollment restrictions, and
Updated (10/7/16).4: I'd like to see the detail of these protocol amendments or changes that could substantially speed up enrollment. For now, however, as I reevaluate enrollment rates, my estimate of patients already recruited to this trial may be less than 20 as of "now."
 |
| Click to enlarge |
Updated (10/8/16).5: I agree with InvestorVillage poster STARLIGHT66 regarding the timely communication of potential or prospective protocol changes. Eric very, very likely (undoubtedly) could have communicated this on the August 10th 2Q16 business update call. I continue to believe adult supervision is required for management (because they continue to embarrass themselves as business leaders), and Provectus' independent board members are no adults (although many children are far smarter and more responsible). As an aside, it might be nice if STARLIGHT66 was his usual helpful and informative self, was less cryptic and less Maxim/Network 1'ish, and did not take posts down.
- There is an expectation of certain news flow in advance of the shareholder meeting to approve the proxy vote proposals (November 14th) and the rights offering (early-December).
Updated (10/6/16).3: Some final things I think:
- I think any license deal for PH-10 would have to envision the completion of an end-of-phase 2 meeting with the FDA to address the issue of toxicity, or in PH-10's case the near-complete if not complete lack thereof. I would imagine any pivotal psoriasis Phase 3 trial protocol already has been developed, perhaps such as a variation of the mechanism of action study (A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10) and/or the prior randomized controlled trial (Randomized Study of PH-10 for Psoriasis),
Updated (10/7/16).4: Deal comps on upfront dermatology/psoriasis payments: $115 million, global/US, July 2016 (licensee: AstraZeneca, licensor: Leo Pharma, tralokinumab/brodalumab); $50 million, Europe, July 2016 (Almirall, Sun Pharma, tildrakizumab); $50 million, U.S., March 2016 (Dr. Reddy, Xenoport, XP23829);...$80 million, global, September 2014 (Sun Pharma, Merck, tildrakizumab)
Updated (10/8/16).5: It's not clear to me whether Provectus' expectations have been socialized with prospective licensees (buyers and sellers in potential transactions each have their own expectations of course); however, the point of making a cursory list of comparable (dermatology/psoriasis) transactions' upfront payment component is to potentially note the seriousness of the advisor's consideration of PH-10's possible clinical worth in that market. There could/would be other components, such as regulatory and/or commercial milestone and royalty payments.
- I think it's interesting that Rose Bengal as I-131 was used for evaluating jaundice in children,
- I think it would be very difficult to envision either Al Smith or Jan Koe, both independent Provectus board members, continuing in their roles for much longer if the company is ever to make good on its promise and potential. To different extents, in different situations, each director has been, is, and more than likely would continue to be in over their heads,
- I think a good board member is as much an a@$-kicker of management as he or she is a cheerleader of them,
- I think it would be a great idea for management to hold an investor conference call to discuss the rights offering (e.g., an explanation of the structure and how it could or would be priced, the rationale for undertaking it [as obvious as that sounds, hearing it directly from management is meaningful in my view], potential timing, etc.). It also would be a good idea for the company's CTO Dr. Eric Wachter, PhD to use the same call to provide potentially non-overlapping (with the subsequent 3Q16 business update call) commentary of various aspects of work under his purview,
- As I previously noted, the value proposition of the current rights offering is no different than the prior warrant exchange programs. Management must do their best to make it compelling and a "no brainer" for existing investors to [over-]subscribe. To not do so (understanding that some things are within their control and others are not) is a clear cut case of business malpractice, and
- I think it's just a race to the data and, for existing shareholders like me with a much higher cost basis than the current share price, a question of how much is left when the time comes.
Updated (10/7/16).4: I lied. I have more things I think. I believe there is a decision-making tug of war about presenting the expanded Phase 1 liver data at either ESMO Asia 2016 (Singapore) in December and the Asian Pacific Association for the Study of the Liver (APASL) (Shanghai) in February 2017.
Updated (10/8/16).5: For only the third time (I believe) in the company's too-long history, a third party might issue a press release about Rose Bengal. How crazy is that (given, for example, the joint patent combination therapy portfolio with Pfizer)? Prior releases would have been Moffitt Cancer Center's August 2013 "Single Injection May Revolutionize Melanoma Treatment, Moffitt Study Shows" and Boehringer Ingelheim (China)'s July 2015 "Boehringer Ingelheim and Provectus biopharmaceutical companies signed letters of intent - the two sides will cooperate to promote melanoma and liver cancer research in new drugs in Chinese mainland, Hong Kong and Taiwan."
I believe Provectus is expecting "media awareness," although I think Warlord is being illustrative about the New York Times.
Visitor. Merck & Co. (Kenilworth), via a Merck Europe router (I now am using better geolocation tools), recently visited the blog again, this time traversing September 2016 blog posts like September 26, 2016' SITC 2016: Intralesional injection with Rose Bengal and systemic chemotherapy induces anti-tumor immunity in a murine model of pancreatic cancer.
Updated (10/8/16).5: For only the third time (I believe) in the company's too-long history, a third party might issue a press release about Rose Bengal. How crazy is that (given, for example, the joint patent combination therapy portfolio with Pfizer)? Prior releases would have been Moffitt Cancer Center's August 2013 "Single Injection May Revolutionize Melanoma Treatment, Moffitt Study Shows" and Boehringer Ingelheim (China)'s July 2015 "Boehringer Ingelheim and Provectus biopharmaceutical companies signed letters of intent - the two sides will cooperate to promote melanoma and liver cancer research in new drugs in Chinese mainland, Hong Kong and Taiwan."
I believe Provectus is expecting "media awareness," although I think Warlord is being illustrative about the New York Times.
Visitor. Merck & Co. (Kenilworth), via a Merck Europe router (I now am using better geolocation tools), recently visited the blog again, this time traversing September 2016 blog posts like September 26, 2016' SITC 2016: Intralesional injection with Rose Bengal and systemic chemotherapy induces anti-tumor immunity in a murine model of pancreatic cancer.
 |
| Image source |
- Inhibitor: Pembrolizumab for patients with advanced prostate adenocarcinoma: 13% objective response (no complete responses), measurement by RECIST 1.1; NCT02054806 and
- Stimulator: Pfizer's OX40 for patients with advanced cancer: 4% objective response (no complete responses), measurement by both RECIST and irRECIST; NCT02315066.
Consider Provectus CTO Dr. Eric Wachter, PhD's references to cutaneous and uveal melanoma under Kobayashi Maru (October 4, 2016) below. Consider also the references to these melanoma indications in the October 3rd GEN article by Dr. Jeffrey Buguliskis, PhD entitled "The Future of Immunotherapy Is Not Stopping at the Checkpoints." I followed up with Eric on my original questions to him below. Was he saying, say for uveal melanoma or another suitable example (like cutaneous melanoma), that the primary and potentially PV-10-injectable site could/is immunogenic, while the secondary site such as on the liver could/is non-immunogenic? How much "power" could be gotten from injecting an immunogenic site versus injecting a non-immunogenic one? Has Provectus undertaken any of that kind of analysis? I would imagine one would like to hit both "sets" of tumors, like for cutaneous melanoma, those on the skin and those on the liver, for the best benefit, but I also could imagine the nature and definition of immunogenicity belies the idea of an immune response emanating from the PV-10 injection.
Think about this in regards to uveal melanoma: "Uveal melanoma (UM) is a rare disease that can be deadly in spite of adequate local treatment. Systemic therapy with chemotherapy is usually ineffective and new-targeted therapies have not improved results considerably. The eye creates an immunosuppressive environment in order to protect eyesight. UM cells use similar processes to escape immune surveillance." {source}
Clearly for cutaneous melanoma the mets to the liver are relatively non-responsive to IO agents (PD-1, etc.) whereas the lung mets are highly responsive. As I noted earlier, we are still learning. This is to be determined, several projects are in the works since this is, for obvious reasons, an area of great interest in the oncology community.
canis_star also posted intralesional (IL) agent HF10 + anti-CTLA-4 drug ipilimumab in patients with advanced melanoma (at ESMO 2016); best overall response 39.5% (11.6% complete response) with measurement by irRC (NCT02272855).
Melanoma, IL and Rose Bengal key opinion leader Dr. Sanjiv Agarwala has discussed this pairing, among others.
 |
| Click to enlarge. Image source |
The following table discusses additivity and synergism:
 |
| Click to enlarge |
Prior data for HF10 and ipi had suggested synergism between the two (despite no response for HF10 in melanoma as a monotherapy). That HF10 and ipi are synergistic is consistent with the belief fellow oncolytic virus T-Vec is synergistic with ipi, while only being additive with anti-PD-1 drug pembrolizumab.
Visitor. Roche (Basel, Switzerland) recently visited the blog again, this time traversing October 23, 2014 blog post More of Better > More or Less of Worse, exiting images here and here.
Visitor. Roche (Basel, Switzerland) recently visited the blog again, this time traversing October 23, 2014 blog post More of Better > More or Less of Worse, exiting images here and here.
Kobayashi Maru (October 4, 2016)
Updated below: 10/4/16.
H/t InvestorVillage poster rmgillis for linking to an animation of PV-10's mechanism of action from Provectus' old website (pvct.com; the new website is provectusbio.com). A "screenshot video" of it is below:
Prostate cancer. In an October 3rd TargetedOncology article by Lisa Miller entitled "Prostate Tumors May Not Be Immunologic, Says Expert," I note:
"Perhaps prostate cancer is not an immunologic solid tumor because it is not as hypermutated as other cancer types, Slovin said. A signifcantly higher proportion of somatic mutation frequencies are found in bladder cancer, both lung adenocarcinoma and lung squamous cell carcinoma, and melanoma—the most hypermutated of 27 different cancer types tested.
Even after genomic profiling and various clinical trials, it is still unclear why the few patients with prostate cancer who do respond to immunotherapy treatments stand apart from the majority of patients who do not. However, Slovin noted that there are a few exceptional responders in this area who have durable responses lasting several years.
[Dr. Susan F. Slovin, MD, PhD of Memorial Sloan Kettering Cancer Center] noted that prostate cancer immunotherapy trials have not shown any abscopal effects or T-cell response potentia- tion either, further adding to the expectations that the prostate may not be immunologic. The possibility remains, however, that with a boost to the immunologic signals of the organ with other immunotherapy strategies or combinations, the immune system could indeed respond in time."I asked Provectus CTO Dr. Eric Wachter, PhD about this (slightly edited below for readability).
Three main theories are being applied when IO is less effective than expected: (i) T cell exhaustion, (ii) immune suppression, and (iii) a non-immunoactive tumor. The last often is attributed to low mutation rate. This may be true, but the immune system is very sophisticated so I suspect (iii) is accompanied by one or more of the others. Pfizer's Dr. Craig Eagle, MD teaches that the immune system can be trained to recognize tumor, addressing at least (ii) and (iii). Uveal melanoma (melanoma from the retina) is non-immunogenic when metastatic to the liver. It is unlikely that this is due to mutation load. Similarly, cutaneous melanoma metastases in the liver are relatively non-responsive to IO. So I think Slovin has a point, but I don't know whether it is a major or minor point. We are still learning!What's good for the goose is good for... National Institute for Health and Care Excellence (NICE) guidance for T-Vec, published September 28th:
"Approximately 10-15% of patients have troublesome locally advanced or 3c/M1a disease. Whilst these patients are likely to benefit from the current NICE-approved agents, they are also the group of patients who will benefit from TVEC. Increasing treatment options for patients (ie targeted therapy and immunotherapy) have already been shown to improve survival in advanced melanoma, so it is logical to further expand new treatment options for eligible patients." {my underlined emphasis}Takeaway: Addressable market for PV-10 as a monotherapy upon its approval for locally advanced cutaneous melanoma.
Cash concerns. A quick 'n dirty cash burn analysis I shared with another shareholder..."By the time of the second Maxim raise, around the end of August, I imagine Provectus had around $1.9-2.1 million on hand. They ended June with about $4.9m. The company may have current cash burn rate of about $1.5 million per month (April was exceedingly high at $1.85 million, while May and June averaged about $1.51 million). Provectus raised about $5.4 million (net), giving it around $6 million as of the beginning of October. The NYSE MKT listing threshold is a total stockholders' equity (TSE) of $6 million, which means adding about $4 million to the cash figure at any point in time (i.e., assets minus liabilities, ex-cash). The stockholders equity could be about $10 million (although I need to get a better handle on the impact of the preferred stock on TSE, and as such this analysis doesn't reflect that). We can do the math. It is possible, but how probable is another question, that the company could go through the end of December and still have about $1.5-2 million of cash left and, maybe, a TSE of $5.5-6 million, especially if the company has reduced cash burn."
Shares outstanding. Some people believe the anti-dilution provision of the August Maxim-led raise, when tabulated, would require Provectus to seek additional shares because the amount the company currently has authorized but that are not yet issued is insufficient. I currently do not subscribe to that view, based on Baker Donelson's opinion letter and comments by management on the topic.
Updated (10/4/16): I believe management is being consistent in what they say about the above topic to whomever asks. Provectus of course requires shareholder approval for any in increase the number of authorized of common (and/or other class[es] of) shares. My point, however, was the company's outside counsel provided a legal opinion based on Provectus having enough shares as is. I find it hard to believe (but am open to being wrong) the law firm (irrespective of how aggressive or conservative they are, and they are too conservative in their work for/with Provectus for my tastes) would not have included some kind of caution in said opinion regarding what otherwise is an open-ended share issuance commitment as a function of a certain future share price formula calculation, because there can be no guarantee a share increase at some point in the future, if sought, would receive shareholder approval.
Perhaps a larger issue, for met at least, is over a public discourse of this topic. Existing and prospective shareholders of course must always do their own due diligence...always. You can't [always] trust management. There always is benefit to sunlight, and to proper disclosure as well. Saying some things publicly and possibly other potentially inconsistent things privately, despite useful and insightful analysis from time to time, how much better are you than Maxim and Network 1 Financial?Predictive and Prognostic (October 3, 2016)
Under Catching on to Craig about a decade later: "Intratumoral Immunization: A New Paradigm for Cancer Therapy" (April 17, 2014) on the blog's Archived News I page, I had written:
"Intratumoral Immunization: A New Paradigm for Cancer Therapy. Clin Cancer Res April 1, 2014 20; 1747. "Immune cell infiltration in the tumor microenvironment is of prognostic and therapeutic import. These immune cell subsets can be heterogeneous and are composed of mature antigen-presenting cells, helper and effector cytotoxic T cells, toleragenic dendritic cells, tumor-associated macrophages, and regulatory T cells, among other cell types. With the development of novel drugs that target the immune system rather than the cancer cells, the tumor immune microenvironment is not only prognostic for overall patient outcome, but also predictive for likelihood of response to these immune-targeted therapies. Such therapies aim to reverse the cancer immunotolerance and trigger an effective antitumor immune response. Two major families of immunostimulatory drugs are currently in clinical development: pattern recognition receptor agonists (PRRago) and immunostimulatory monoclonal antibodies (ISmAb). Despite their immune-targeted design, these agents have so far been developed clinically as if they were typical anticancer drugs. Here, we review the limitations of this conventional approach, specifically addressing the shortcomings of the usual schedules of intravenous infusions every 2 or 3 weeks. If the new modalities of immunotherapy target specific immune cells within the tumor microenvironment, it might be preferable to deliver them locally into the tumor rather than systemically. There is preclinical and clinical evidence that a therapeutic systemic antitumor immune response can be generated upon intratumoral immunomodulation. Moreover, preclinical results have shown that therapeutic synergy can be obtained by combining PRRagos and ISmAbs to the local tumor site." {Bold emphasis is mine} Immunostimulatory monoclonal antibodies include ipilimumab, nivolumab, lambrolizumab, etc."My underlined emphasis is above. One of the authors was Stanford's Dr. Holbrook Kohrt, MD, PhD, who died earlier this year.
According to Dr. Nils Brünner, MD of the University of Copenhagen (Denmark) (essentially copied below from his article):
- A prognostic biomarker is a biomarker that provides information on the likely course of the cancer disease in an untreated individual, and
- A predictive biomarker is a biomarker that can be used to identify subpopulations of patients who are most likely to respond to a given therapy. With predictive biomarkers it should be possible to select the therapy with the highest likelihood of efficacy to the individual patient. Thus, predictive biomarkers are the basis for individualized or tailor-made treatment.
"Multiple potential biomarkers of response and resistance to checkpoint inhibition have been identified, Weber continued, pointing to, at baseline, myeloid-derived suppressor cells; high levels of T reg function; inflammatory gene products in serum; T-cell fraction and clonality; gamma interferon gene profile; and neo-antigen numbers. Also, at relapse, potential biomarkers include loss of beta 2 microglobulin and interferon signaling and JAK-STAT mutations.
Weber outlined proposed potential response biomarkers in the pathway of programmed death (PD) blockade: "Several biomarkers -- including high levels of programmed death-ligand 1 [PD-L1] expression, TH1-type chemokines, infiltrating T cells, mutations, low levels of immunosuppressive elements, and epithelial–mesenchymal transition/stem-like features -- may be associated with an active response to PD pathway blockade," he said.
In addition, pretreatment monocytic myeloid-derived suppressor cells are associated with response and overall survival with nivolumab after ipilimumab. Weber and colleagues have also developed a pretreatment patient-selection test to determine the benefits of nivolumab based on serum mass spectra.
Other potential biomarkers include mutation load and the emergence of neo-antigens that are recognized by T cells; TCR clonality of pembrolizumab; and preexisting T-cell infiltration as a biomarker for efficacy of pembrolizumab.
Pretreatment tumor-infiltrating lymphocytes (TILs) may also be effective in determining the use of nivolumab followed by ipilimumab. Responders to this sequential therapy are more likely to have high TIL fraction and clonality compared with non-responders, he said."See dataset iii under A race to the data (September 18, 2016) below.
Visitor. Memorial Sloan Kettering Cancer Center (MSKCC) recently visited the blog (again). As I previously noted, Dr. Paul Chapman, MD has been/is a consultant to Provectus (see Ridiculousness (April 7, 2016) on the blog's Archived News V page). There potentially or possibly are three areas of PV-10 interest/utility for MSKCC.
fas·tid·i·ous (October 2, 2016)
It is exceedingly difficult to get an investigational drug approved, especially an oncology one. According to an analysis by BIO, Biomedtracker and Amplion, "Clinical Development Success Rates 2006-2015," the overall likelihood of approval from Phase I/1 for oncology developmental drug candidates was 5.1% (11.9% for all non-oncology indications; 9.6% for all indications).
 |
| Click to enlarge. Image source. Blue emphasis is mine. |
Here we are, as Provectus shareholders, observing company management endeavoring to undertake a pivotal Phase 3 trial of PV-10 versus chemotherapy or oncolytic viral therapy for the treatment of locally advanced cutaneous (Stage IIIB-IV M1a) melanoma. Other issues and challenges notwithstanding, winning clinical trials (for anyone and any company) is tough.
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
I'm reminded of the immense personal and professional pride a former classmate of mine had (and vividly and excitedly described to me) to be part of the team that brought Cubicin to market; yet, he "only" was part of the new drug application (NDA) team (before eventually heading up business development).
Winning should mean PV-10 becomes first-line treatment for its melanoma indication per NCCN guidelines, where currently recommended or preferred treatment is clinical trial. For those of us who believe our clinical trial math is correct, it's never been about if, but rather when. And we haven't even begun to address applicability, agnosticism, orthogonality, synergy, etc. — because if you cannot get your investigational drug approved, you cannot drive value towards (baseline let alone greater) worth, no matter how much you believe the latter is or could be.
September Blog Readership Stats (October 1, 2016)
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
The Year of the Cold Tumor (September 30, 2016)
Checkpoint inhibitors do not work well on cold tumors; that is, tumors that are non- or less immunogenic, or are immune insensitive (or less immune sensitive) cancers.
I asked Provectus' CTO Dr. Eric Wachter, PhD why Moffitt Cancer Center explored the combination of immunotherapy PV-10 and chemotherapy (which I believe to be gemcitabine) for pancreatic cancer? See September 26, 2016 blog post SITC 2016: Intralesional injection with Rose Bengal and systemic chemotherapy induces anti-tumor immunity in a murine model of pancreatic cancer.
This is clearly an unmet need and progress with immunotherapy in many other tumors has not been replicated with this. The paradigm is the same here as with HCC (a similarly challenging tumor): combining tumor stressors may lead to successful recruitment of functional immunity. I am excited to see that 2016 is shaping up, as Jeff Weber predicted in January regarding melanoma, to be the year where multiple approaches are commonly being tested as ways to break the detente that tumors can establish with a patient’s immune system.
In "The Checkpoint Immunotherapy Revolution," Alexander, P T. 2016 Mar; 41(3): 185–191, the following was attributed to Dr. Jeffrey Weber, MD, PhD said:
"Finally, Dr. Weber pointed to the locally injected or intralesional therapies, such as talimogene laherparepvec (T-VEC), PV-10 (Rose Bengal 10% disodium, Provectus), and coxsackievirus A21 (Cavatak, Viralytics). Not only do they generally share the virtue of being devoid of dose-limiting toxicities, but they also may be able to “prime” the immune response, Dr. Weber said, so that “beyond trying to diminish the Treg and MDSC activity, you could augment T-cell activity, causing an influx of them to the tumor and making a cold tumor hot.”" {my underlined emphasis}As reported last year and this year, anti-PD-1 drug nivolumab (Opdivo) generated 14-16% objective response (7/48 to 35/214; 3 and 2 complete responses, respectively) in hepatocellular carcinoma (HCC). The clinical trial protocol for the study is here. Response rates were measured after six months.
Eric noted on the August 10th 2Q16 business update call:
"We already know that we can destroy HCC with this ablative process and the hypothesis that that should lead to similar signaling, which can have implications for--well, single-agent therapy [unintelligible] HCC, but more importantly for combination with things like anti-PD-1 [unintelligible] . We have already shown that the basic immunology occurs in HCC models, so I would say that--one of the things I’m highly confident in, I’m highly confident that we will show that this same functional immune signaling functions in HCC...
But, we are also looking at conducting the non-clinical studies to show that Chen and Mellman signaling--the seven steps occur in HCC in mice to justify, then, a second-pronged approach, which is particularly relevant for the West, which would be a combination of PV-10 with a checkpoint inhibitor, be that anti-CTLA-4, anti-PD-1, or anti-PD-L1. And so, that work we are starting, and I expect that that would presumably lead to similar development in HCC that we have seen in melanoma, which is a 1b/2 combination, or PV-10 plus a checkpoint inhibitor for HCC."Provectus' expanded liver Phase 1 study protocol is here. While response indeed was observed at 28 days following injection, response and response rates also were evaluated and measured over a much longer period of time (3 mos, 9-15 mos) (so response was not zero).
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
"Immunotherapy Combined With Chemotherapy for Pancreatic Cancer: A Game Changer?" (September 29, 2016)
Provectus issued a press release on Tuesday and filed an associated 8-K regarding more Moffitt Cancer Center's murine model work (pancreatic cancer, PV-10 + chemotherapy), Announces Acceptance of Abstract for Poster Presentation at 31st SITC Annual Meeting. The single "substantive" sentence in the PR was:
"The abstract, "Intralesional injection with Rose Bengal and systemic chemotherapy induces anti-tumor immunity in a murine model of pancreatic cancer," describes research undertaken at Moffitt Cancer Center by a team of scientists led by Shari Pilon-Thomas."Why are (i) this work (by Moffitt), (ii) this combination therapy (of PV-10 and chemotherapy) and (iii) this tumor type (pancreatic cancer) germane?
One of the money comments from the August 10th 2Q16 business update conference call was Eric saying:
"After a long haul, it is now clearly accepted that tumor ablation with PV-10 can lead to stimulation of a useful anti-tumor immune response."That is, PV-10 is an immunotherapy.
I first wrote about this work under Mix & Match (December 29, 2015) on the blog's Archived News IV page. In September 2014 Dr. Pilon-Thomas and others authored OncLive paper "Immunotherapy Combined With Chemotherapy for Pancreatic Cancer: A Game Changer?" The article's abstract says:
"Pancreatic cancer has one of the poorest prognoses out of all human malignancies. Only 20% of patients who present with this disease have surgically resectable tumors at the time of diagnosis, and of these 20% of patients, the vast majority will experience recurrence within the first 2 years. Chemotherapy is the mainstay of treatment. Unfortunately, little improvement has been made in the past few decades with regard to overall survival (OS) with chemotherapy alone. Of note, the immune system’s involvement in cancer development and progression has sparked much interest in recent years. The model of the cancer-immunity cycle suggests an interplay of immune-suppression and immune-stimulation. In normal individuals, a state of immunosurveillance is in place. However, within the tumor microenvironment, inhibitory signals and immunosuppressive cells are present and tip the scale in favor of immune suppression. This enables cancer cells to evade the immune system and allows tumors to grow. Studies in a variety of solid tumors have shown that many chemotherapies, including those used as standards of care in pancreatic cancer, have immune-modulating functions, including inhibition of immune suppression and stimulation of immune function."The paper continues:
"As previously mentioned, single-agent gemcitabine has long since been the first-line standard of care for pancreatic cancer across all stages. Gemcitabine has been found to have multiple effects on the cancer-immunity cycle. It improves immune stimulation by inducing dendritic cell maturation, increasing epitope presentation on tumor cells, and decreasing tumor infiltrative MDSCs. These studies show that gemcitabine also augments the immune response in favor of generating immunity. Therefore, the data lay the foundation for investigating combinations of this chemotherapy with immunotherapy."I noted on September 26, 2016 blog post SITC 2016: Intralesional injection with Rose Bengal and systemic chemotherapy induces anti-tumor immunity in a murine model of pancreatic cancer that I believed the systemic chemotherapy used in Moffitt's work was gemcitabine (Gemzar®), which by now is produced by generics manufacturers.
The paper concludes:
"The cancer-immunity cycle is an ideal model to envision how tumor cells evade immunosurveillance as well as where future modalities may intervene with hopes of potentiating tumor cell death. The cancer-immunity cycle together with the immunemodulating functions of chemotherapies that are used in pancreatic cancer creates a rationale for investigating vaccine-chemotherapy combinations.
Studies to date have suggested benefits of adding immunotherapies to standard chemotherapy regimens. Additional benefits are also suggested by the indication that immunotherapy may render improved chemosensitivity at later dates. In addition, vaccines are often well tolerated with minimal toxicities, which make them a favorable approach. The hope is that we can identify the appropriate combination of vaccine and immune-modulating chemotherapy that will eradicate the disease. There is also likely to be a role for immune checkpoint therapy with inhibitors of PD-1 and PD-L1. Such phase I single-agent studies are currently in progress for pancreatic cancer. The results of studies so far create hope that the combination of chemotherapy with immunotherapy may be a game changer in the treatment of pancreatic cancer.
The path to integrating immunotherapy with chemotherapy will likely be bumpy and afflicted by setbacks, many of which we have already seen. The only hope for changing the paradigm of treating pancreatic cancer is to maintain persistent clinical trials. Any pancreatic cancer patient should be presented with an opportunity to enroll in a trial, or should be referred to a center that offers them. These patients should be given a chance at tomorrow’s drugs as well as an opportunity to contribute to advancing the treatment of this disease."In the intervening period of time, from September 2014, when Dr. Pilon-Thomas wrote the above paper, to May 2016, when her Moffitt team published their PV-10 mechanism of action work entitled "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1," much did not change:
"Immune-based strategies to treat pancreatic cancer, such as immune checkpoint inhibitors, therapeutic vaccines, and combination immunotherapies, are showing promise where other approaches have failed. Immune checkpoint inhibitors, including anti-CTLA4, anti-PD-1, and anti-PD-L1 antibodies, are effective as single agents in immune sensitive cancers like melanoma, but lack efficacy in immune insensitive cancers including pancreatic cancer." Foley et al., "Current progress in immunotherapy for pancreatic cancer," Cancer Lett. 2016 Oct 10;381(1):244-51Moffitt appears to have a found an immunotherapy in PV-10 that not only is an effective single agent in melanoma, an immune sensitive cancer, but also may have efficacy in an immune insensitive cancer like pancreatic cancer -- and thus when combined with chemotherapy may be a game changer in the treatment of pancreatic cancer.
Irony: I-131 for Thyroid Cancer Metastases (September 28, 2016)
See September 28th CancerNetwork article entitled "I-131 for Thyroid Cancer Metastases," which describes work by Bikas et al., "Metformin Attenuates 131I-Induced Decrease in Peripheral Blood Cells in Patients with Differentiated Thyroid Cancer," Thyroid. 2016 Feb;26(2):280-6:
"BACKGROUND:
131I treatment (tx) of differentiated thyroid cancer (DTC) is associated with hematopoietic toxicity. It was hypothesized that metformin could have radioprotective effects on bone-marrow function. The objective was to determine whether metformin prevents 131I-induced changes in complete blood counts (CBC) in patients with DTC...
CONCLUSIONS:
Metformin attenuated the 131I-induced decrease in CBC parameters, and its radioprotective properties were more prominent in WBC. Patients who were taking metformin during 131I tx also experienced a faster recovery in their blood counts, when compared to the control group. Further study is warranted in order to examine if the radioprotective properties of metformin observed in the current study for 131I tx can also apply to other forms of therapeutic chemo- and radiotherapy.""Radioiodine therapy is a nuclear medicine treatment for an overactive thyroid, a condition called hyperthyroidism, and also may be used to treat thyroid cancer. When a small dose of radioactive iodine I-131 (an isotope of iodine that emits radiation) is swallowed, it is absorbed into the bloodstream and concentrated by the thyroid gland, where it begins destroying the gland's cells." {source}
Rose Bengal has been a diagnostic agent for more than100 years. It's original and first medicinal use was an intravenous hepatic diagnostic -- 131I-radiolabeled Rose Bengal/Robengatope® -- and
topical ophthalmic diagnostic (Rosettes®, Minims®).
What it'll take (September 27, 2016)
Updated below: 9/28/16.
Bristol-Myers and Nektar Therapeutics today announced a collaboration to combine the former's anti-PD-1 antibody with the latter's CD122-biased agonist in a number of tumor types and cancer indications. As Provectus seeks a so-called co-development transaction with a Big Pharma partner to explore the combination of PV-10 with an immunomodulatory or targeted agent, I thought the framework of this transaction was useful to examine and review. As an aside, Nektar visited the blog earlier this year. For this blog news item, please consider the following resource materials:
- The Bristol-Myers press release of the relationship (also linked above), "Bristol-Myers Squibb and Nektar Therapeutics Announce Oncology Clinical Collaboration to Evaluate the Combination of Opdivo (nivolumab) and NKTR-214,"
- Nektar's [partial] SEC filing related to the collaboration and relationship, "Nektar Therapeutics (NKTR) Offers Additional Detail on Bristol-Myers Squibb Collaboration,"
- Forbes/Timmerman Report's Luke Timmerman's article on the collaboration, "Bristol-Myers and Nektar Cast Wide Net in Quest to Find the Right Immunotherapy Combo," and
- GEN News Highlights' article on the collaboration, "BMS, Nektar to Assess Opdivo/NKTR-214 Combination in Five Tumor Types."
- a. Could a collaboration be possible? [i.e., industry trends]
- a.1. What is the macro clinical value proposition picture of cancer therapy approaches for end-stage patients?
- a.2. What is the micro clinical value proposition picture of combination therapy make-up for the above patient population?
- b. Could a collaboration be probable? [i.e., Provectus-Big Pharma discussion trends]
- b.1. What data/"data" are required to convince Big Pharma?
- b.2. What terms and conditions are required to convince Provectus?
a.1. Irrespective of the pros, cons and rationale for and of precision or personalized medicine, the larger trend in treating end-/late-stage cancer is the use of combinations and cocktails of therapies and therapeutics. As such, it would seem, biopharmaceutical M&A or licensure to acquire drugs and drug compounds is about combination therapy, sales of monotherapeutic use notwithstanding. Generally speaking, I would point to Amgen/Onyx/Kyprolis (see Adam Feuerstein's "Amgen Multiple Myeloma Drug Kyprolis Fails to Help Newly Diagnosed Patients") and Pfizer's acquisition of Medivation (for when Pfizer explores combinations and permutations of Xtandi).
Takeaway: Combinations and cocktails appears to be driving Big Pharma's biomedical perspective, and thus their business and acquisition strategies. Check.
a.2. Now that the hype over checkpoint inhibitors has died down (i.e., the need to turn cold tumors hot), Big Pharma and others are turning to the combination of an inhibitory agent (antagonist) and a stimulatory one (agonist). But which combination or permutation? One might take as given, for now, that the inhibitory agent is an anti-PD-1/anti-PD-L1 compound; however, at this point in time, the decision is based on whether you, as a Big Pharma, have an inhibitory agent, and then which one(s) (e.g., anti-CTLA-4, -PD-1, PD-L1). "Next gen" inhibitors like LAG-3, TIM-3, etc. are in the pipeline and may or may not be in the clinic, with approval some time away. Thus, I would imagine the key question is "what is the stimulatory agent of choice? As Timmerman noted:
"The smaller company has an experimental drug that is supposed to “press on the gas” of the immune system, which conceptually ought to amplify BMS’s drug nivolumab (Opdivo) that “releases the brakes” by inhibiting a receptor on cells called PD-1."Interestingly, the goal of stimulating T cells in prior collaborations of combinations and cocktails has expanded to the stimulation of NK cells. As GEN noted:
"NKTR-214 is designed to stimulate cancer-killing immune cells by targeting CD122-specific receptors found on the surface of these immune cells, known as CD8+ effector T cells and natural killer (NK) cells, directly in tumors, thus increasing expression of programmed death 1 (PD-1) on these immune cells. Necktar has an ongoing Phase I/II study to evaluate single-agent NKTR-214 in cancer patients."Takeaway: PV-10 upregulates, and thus is a stimulatory agent. It also stimulates, among other things, both T and NK cells. Check.
b.1.i. Setting aside safety and efficacy of the combination or cocktail -- that is, there must be some threshold or floor for these parameters -- it would seem there are a few key gates or "datasets," such as, first, PD-1 expression. As the Bristol-Myers press release noted:
"NKTR-214 is an investigational immuno-stimulatory therapy designed to expand specific cancer-fighting T cells and natural killer (NK) cells directly in the tumor micro-environment and increase expression of PD-1 on these immune cells."Timmerman opined:
"This isn’t the first time scientists have thought about stimulating killer T-cells and Natural Killer cells to fight cancer. Genetically engineered Interleukin-2, sold as Proleukin, has been around for years to try to accomplish a similar goal. It can provide long-lasting remissions for about 10% of patients with melanoma, but it comes with severe toxicities. Many patients can’t tolerate it."Although it's not that simple, as there are other relevant specific components of the immune to address to, like regulatory T cells. As Timmerman noted:
"Nektar is betting that it can overcome the challenges based on new understanding of the biology, Doberstein said. While the old drug stimulates pro-inflammatory pathways, it also stimulates T-regulatory cells that dampen the immune response. It also doesn’t last long in the blood. That means it needs to be dosed at high levels, and at frequent intervals, to achieve its desired effect, Doberstein said. The Nektar drug is supposed to avoid stimulating the T-regulatory pathway and its dampening effect."Takeaway: I believe one of the points that Moffitt Cancer Center's AACR 2016 poster addressed was PD-1 expression by PV-10. So-called Tregs also were addressed. Check, and check.
b.1.ii. Second, it's about multi-indication viability in cold or colder tumor types and cancer indications. The Bristol-Myers/Nektar relationship, per Nektar's SEC filing, would cover five tumor types and seven potential indications. The BMS press release noted, in regards to tumor type:
"The Phase 1/2 clinical trials will evaluate the potential for the combination of Opdivo and NKTR-214 to show improved and sustained efficacy and tolerability above the current standard of care in melanoma, kidney, colorectal, bladder and non-small cell lung cancer patients. An initial dose-escalation trial is underway with Opdivo and NKTR-214."Hot (immunogenic) tumor types presumably are melanoma and non-small cell lung cancer (NSCLC). Bladder? Cold or colder (non- or less immunogenic) types then would be kidney and colorectal. See item no. 6 under Desert > Beach (July 8, 2016) below, which includes two tables of where checkpoints work well, and don't work well.
But, it also would seem to me, the stimulatory partner in the pairing has to demonstrate immunological signalling in multiple tumors types. Nektar has preclinical data in this regard (AACR 2016 and ASCO 2016), and only recently (August 2016) begun an early-stage clinical trial examining its agent in unspecified, locally advanced and metastatic solid tumor cancers -- dose escalation, and the collection of immunological biomarkers in plasma (blood) and tumor samples.
Takeaway: Moffitt Cancer Center and the University of Illinois at Chicago have undertaken preclinical, and in some cases clinical, immunologic work in multiple tumor types: e.g., melanoma, breast cancer, colorectal cancer, pancreatic cancer. Check.
b.1.iii. As I introduced in b.1.ii above, another key gate or dataset is the immune biomarker work, as Timmerman noted:
"BMS and Nektar say they plan to move in parallel on a series of small combo trials for about 250 patients with five different malignancies–melanoma, kidney cancer, colorectal cancer, bladder cancer and non-small cell lung cancer. The studies will collect blood and tumor biopsies before and after treatment to see what difference the drugs are making not just against the tumor itself, but whether they are stimulating the immune system the way they are supposed to, based on previous laboratory and animal experiments. That’s the sort of the information that should tell the companies very quickly whether they are on the right track, and how to prioritize resources for the more rigorous randomized trials necessary to win FDA approval for any new medicine."Question: Does Provectus have enough of this data for Merck, BMS, etc. to answer the questions and generate the information Timmerman poses and to which he points?
b.2.i On the surface, the BMS-Nektar relationship is one of no upfront payment, and shared study costs (contrasted with, for example, BMS' relationship with PsiOxus that included an upfront payment, while costs were shared -- see Church (August 15, 2016) below).
Below the surface, and NKTR-214 is one of several Nektar agents, there are strings attached, as noted in Nektar's SEC filing {my underlined emphasis}:
- "Ownership of, and global commercial rights to, NKTR-214 remain solely with Nektar under the Agreement. If Nektar wishes to license the right to commercialize NKTR-214 in one of certain major market territories prior to September 30, 2018 (the “Exclusivity Expiration Date”), Nektar must first negotiate with BMS, for a period of three months (the “Negotiation Period”), to grant an exclusive license to develop and commercialize NKTR-214 in any of these major market territories. If BMS and Nektar do not reach an agreement for an exclusive license within the Negotiation Period, Nektar will be free to license any right to NKTR-214 to other parties in any territory worldwide except that in the event that Nektar receives a license offer from a third party during a period of 90 calendar days after the end of the Negotiation Period, Nektar will provide BMS ten business days to match the terms of such third-party offer. After the Exclusivity Expiration Date, Nektar is free to license NKTR-214 without any further obligation to BMS.
- Each party grants to the other party a non-exclusive, worldwide (subject to certain exceptions in the case of the license granted by BMS), non-transferable and royalty-free research and development license to such licensing party’s patent rights, technology and regulatory documentation to use its compound solely to the extent necessary to discharge its obligations under the Agreement with respect to the conduct of the Combined Therapy Trials.
- The Agreement also contains certain reciprocal exclusivity provisions that run until the Exclusivity Expiration Date. Nektar agrees not to conduct any preclinical or clinical research with, or grant rights under its proprietary intellectual property or relevant investigational new drug applications to, certain restricted third parties regarding an anti-PD-1 antagonist or anti-PD-L1 antagonist together with the NKTR-214 (a “Restricted NKTR-214 Combination”), and BMS agrees not to conduct any preclinical or clinical research with certain restricted third parties regarding Nivolumab together with an IL2-based CD122 agonist (a “Restricted Nivolumab Combination”). Nektar and BMS remain free to conduct any preclinical or clinical research—involving a Restricted NKTR-214 Combination in the case of Nektar and a Restricted Nivolumab Combination in the case of BMS—on their own or in collaboration with academic or other non-profit entities.
- Subject to termination rights for breach, bankruptcy or a material safety issue/clinical hold, the term of the Agreement will continue in effect until completion by all centers or institutions participating in the Combined Therapy Trials, the delivery of study data to both parties and the completion of any then agreed upon protocol, statistical analysis and bioanalysis plan. In the event a third party merges with or acquires Nektar, Nektar is free to assign or transfer the Agreement without the consent of BMS."
 |
| Click to enlarge. Image source |
immunotherapy (the subsequent tumor-specific immune response). As much as Provectus shareholders might ask themselves what does Big Pharma want in order to enter into a collaboration, they might want to ask what would Provectus want in return (and, thus, on their behalf)?
Some housekeeping. Provectus' ESMO 2016 trials-in-progress poster "1158TiP - Intralesional rose bengal for stage III and IV melanoma" is the same as the company's ASCO 2016 trials-in-progress poster.
Combo #1. Data from the combination of epacadostat (an IDO inhibitor) and pembrolizumab in an ESMO 2015 abstract were made available, "1110PD - Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: Updated phase 1 results from ECHO-202/KEYNOTE-037." Endpoints' John Carroll noted:
"By all means, it’s still early days on outcomes, but in this new package released ahead of ESMO there was an improvement in the objective response data, which hit an ORR of 55% (or 12 out of 22 patients) among all patients, including 5 complete responses (23%) and 7 partial responses (32%). Among treatment-naive patients the ORR was 58% and other tumor types being studied demonstrated ORRs ranging from 27% to 60%, notes RBC.
PFS was not yet hit, though analysts are wondering if that may be revealed with more info at the upcoming conference. Incyte’s shares $INCY surged about 4% on the update.
RBC’s Simos Simeonidis likes what he sees. “(T)he fact that PFS wasn’t reached as of this abstract release, with a median follow-up of 42 weeks (10.5 months), suggests epacadostat+pembro is likely to compare favorably to the current SOC.”
It’s particularly encouraging, he adds, that the safety profile looks better than what’s now available, though it certainly isn’t free of SAEs.
“Though more mature data broken out by specific indication will be necessary to better contextualize potential treatment benefits, this hints that the PFS may be extending beyond 10 months, which would be superior than the low-end 6-month clinical efficacy bar for single-agent pembro and approaching the 11.5-month high-end bar for combo nivo+ipi in melanoma,” writes Jefferies’ Brian Abrahams. {my underlined emphasis}
Half of the patients were Stage IV M1c; I think the remainder were Stage IIIB-IV M1b. See Broader label for the single agent Vs. A combination of agents (June 22, 2016) below.
Combo #2. In another ESMO 2016 abstract, intralesional (IL) drug T-Vec (Imlygic) and anti-CTLA-4 drug ipilimumab (Yervoy) demonstrated synergy for patients with advanced melanoma, "TVEC + ipi doubles the response rate of ipiliumab alone in 173 pt controlled phase 2 study in melanoma, no increased tox (ESMO 2016)."
 |
| Click to enlarge. Image source |
Friday (September 23, 2016)
Updated below: 9/24/16 {twice} and 9/25/16.
SITC. Regular abstract titles for the 31st Annual Meeting & Associated Programs of the Society for Immunotherapy of Cancer (November 9-13, National Harbor, Maryland) should be available on Monday. See dataset v/viii under A race to the data (September 18, 2016) below.
Genentech. The Roche subsidiary visited again this quarter, January 29, 2016 blog post PV-10 (Rose Bengal) and the Cancer Immunity Cycle.
Hokkaido University. The Japanese academic institution visited the blog for nearly eight minutes. As context, average session (visit) duration (any blog visitor) can vary from 2 minutes, 10 seconds [2:10] (this year; image below) to 2:10 (this quarter) to 2:35 (this month)
PH-10. There is a rumor mechanism of action data is/has been available for prospective partners (and that Seth Orlow has been doing the rounds with it). Provectus' dermatology "deal guy" is Dr. Seth Orlow, MD, PhD, Chairman of the Ronald O. Perelman Department of Dermatology at NYU School of Medicine (NYU Langone Medical Center) -- Seth Orlow, M.D., Ph.D., Joins Provectus Pharmaceuticals' Corporate Advisory Board (November 2010). NYU Langone also is where former Moffitt Cancer Center physician, investigator and researcher Dr. Jeffrey Weber, MD, PhD resides. See dataset iv under A race to the data (September 18, 2016) below.
Nevertheless, Peter, over time, has tried to elucidate Eric's preclinical/clinical development programs for PV-10 and PH-10 to shareholders. Peter typically seeks to confirm or validate what he hears from Eric and place it into the greater context of the biopharmaceutical industry. When his salesmanship does not get in the way, we often hear a thoughtful, cogent, communicative executive, who often is much more excited about the therapeutic prospects for Rose Bengal than the average Provectus shareholder. Like Eric (drug development), Peter has grown into a life sciences/biotech executive on the dime and backs of retail shareholders whose support and patience have enabled this management team to bring the drug(s) to market in the right way. One example of this personal development was Peter's excitement over the independent reproduction and thus validation of Rose Bengal's immunologic/immunotherapeutic features first presented at AACR 2013, PV-10 Immunology Data Presented by Moffitt Cancer Center Researchers at the American Association for Cancer Research Annual Meeting. Peter at the time described the April poster and, later, the July peer-reviewed publication "Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma" as "the shot heard round the world." Most shareholders to whom I spoke thought he was referring to an impact on the share price or the perception of the molecule by Big Pharma (perhaps selectively hearing what they wanted to hear), rather than him trying to convey the groundbreaking importance of Moffitt's work as one more step (but a very key one at the time) towards gaining more full recognition of Rose Bengal's therapeutic benefits.
Returning to PH-10, what has Peter said on Provectus' recent business update calls? On the March 16th call he said:
Australia. Eric below:
November 5, 2015 3Q15 business update call: "As we noted previously, our initial focus has been on opening key strategically significant sites headed by influential investigators to establish a critical mass for the program, and we’re beginning to expand from that base. In the U.S. this is a site-by-site process, where each site has a legal and Institutional Review Board, or IRB requirements. In Australia, we’ve used a new national ethics application process, or NEAP, along with standard contracting indemnification template agreements for the first time nationwide. These features are representative of the constantly evolving process for the conduct of clinical trials. We can’t change the ala-cart nature of site start-up in the U.S., but the nationwide approach in Australia is expect to
expedite start-up once the initial site is Brisbane is active."
March 16th 4Q15/CY15 business update call: "As the trial progresses over the course of 2016, we will assess the paths for expedited development, fast track accelerated approval, market approval with the FDA in the U.S. and the Therapeutic Good Administration in Australia. I want to stress here that the disappointment we all shared when the FDA declined to give us breakthrough therapy designation 2014 has no relevance to these accelerated options. The fact is that we will have much more data to bolster [indiscernible] and there is no regulatory penalty incurred because our BT [sp] application was not approved then."
"In regard to the TGA, yes, definitely moving forward with approval in Australia is something that
we have been considering, we are currently considering, and we will continue to consider,
especially given that roughly half of the melanoma patients that received PV-10 throughout the
history of our development have been from Australia. So--and we have a significant base of
data now on patients in Australia."
May 10th 1Q16 business update call: "While all these amendments were designed to enhance patient eligibility and enrollment in this global study and are necessary to adapt to a constantly changing playing field in oncology and the evolving process for starting and executing clinical trials on a global scale, these necessary changes have led to delays in execution of our study. I must, unfortunately, report that we are behind with regard to our initial schedule for reaching
the interim and final analysis triggers for the study. I'm confident that we're--we are implementing the proper study to support licensure of PV-10, but at present estimate that we are approximately six months behind schedule with regard to site start up, patient recruitment, and eventual data readout. What are we doing to address this? First, we've enlisted the support of a very prominent
clinical investigator in Germany to lead the European portion of our study. He joins similarly
selected leads for each of the geographic regions, North America, Oceania, Brazil, and China,
but the study stands. Second, we've been working with our lead site in Australia to ensure that it can fill--fulfill a nationwide regulatory role under the National Ethics Application System, or NEAF, as additional Australian sites join the study."
"Processes for approval in Australia, we are assessing. We've been assessing them for, since inception of our clinical work in Australia. One of the aspects of closing the expanded access protocol is that we now have an opportunity to collate all of the data from that process to be able to actually use it for at least supportive purposes if not some, a better, higher purpose in terms of regulatory approval. So we’ll be continuing to review that opportunity in Australia on an ongoing basis and as the case dictates, we may elect to move forward in Australia on a regulatory stance that’s faster or slower than in the U.S."
August 10th 2Q business update call: "We held our final advisory board meeting for Europe in June and have a motivated team of melanoma investigators at top centers ready for the study. After months of preparation, we expect to begin filing applications for, with local regulatory authorities in Europe later this month. We’re also continuing to work with the investigator community in our traditional
territories of the U.S. and Australia to build on a very solid base of sites that have been rolling
throughout all, all of this."
"So, we're continuing to--as I said in my comments, we're continuing to enroll patients in our
existing centers, at centers in the US and Australia, and expand the study throughout other
regions of the world that we've identified to bring us to that interim time point and eventually
to the final outcome of the study."
Really Exciting (September 22, 2016)
Source article & link: Dr. Saenger on Ongoing Research in Melanoma
#2. Inhibition of macrophages, eh? "Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages," Rabe et al., J Immunotoxicol. 2014 Oct;11(4):367-75.
Grammatical error (September 21, 2016)
Updated below: 9/21/16 and 9/22/16.
According to Biotechnology Industry Organization (BIO), intellectual property (IP) is the lifeblood of the biotechnology industry. With thanks to a fellow shareholder, regular hatter and all around neat person for this item.
Provectus' patent with Pfizer, Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (US PTO #9,107,887; August 2015), notes as its first claim:
Provectus replied that this patent had a grammatical error in the published form (as highlighted above in bold), which was corrected (i.e., it is an inhibitor of down-regulation).
Also, it still is hard to understand how PD-1 and PD-L1 antibodies are “covered” by this '887 patent under “systemic inhibitors of immune system down-regulation,” as Provectus says or insists. While this is descriptive of how such antibodies function, where is the separate claim for them as they have for CTLA-4 antibodies in the second claim and body of the patent? Ironically, the way the first claim seems to be written (although the second and eleventh claims appear to be correct), there’s no inclusion of a “systemic inhibitors of immune system down-regulation” classification of drugs to combine with PV-10.
Anti-CTLA-4 and -PD-1 antibodies are examples of down-regulation class of inhibitors, which function on T cells. Anti-PD-L1 is in the same class, functioning on the target of the T cell (i.e., the cancer cell). While no patent is truly proven until it’s challenged, Provectus believes these classes of agents (i.e., anti-CTLA4, -PD-1 and -PD-L1) were well defined at the time of the invention (i.e., the PV-10 combination therapy patent), and the claims drawn to the classes of agents cover these examples that were known at the time of the invention and any new members of these classes that are subsequently discovered.
I followed up still wondering that “system inhibitor” actually replacing “systemic enhancer” in a very important claim seemed more than grammatical housekeeping. This, and not specifically articulating what other combinations of PV-10 and checkpoint inhibitors are covered by the patent (e.g. PD-1, PD-L1, IDO, etc.) seems like "sloppy work." I say that Provectus shareholders have come to understand Provectus CTO Dr. Eric Wachter, PhD's compulsive nature, which doesn’t make sense in regards to the lack of specificity in the combo patent portfolio (patent #9,107,887; US PTO application nos. 14/748579, 14/748608 and 14/748634).
The specification regarding the systemic inhibitor versus systemic enhancer issue is clear on this matter. The mistake was noted, and corrected without drama after the patent issued. These claims reflect an evolving playing field and are appropriately generic to account for supplanting of CTLA-4 by PD-1 and eventual supplanting of PD-1 by something else.
Thus, the company arrives at the following summation:
CorMedix is "a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of infectious and inflammatory disease," which thought to "unlock additional value for taurolidine" by exploring the agent's potential in cancer (hence the POETIC collaboration):
Note the language in the CorMedix press release about taurolidine's mechanism of action:
Does CorMedix own the therapeutic use of taurolidine in cancer? Probably not. Consider US patent Use of taurolidine to treat tumors, which was/is assigned to Geistlich Pharma Ag. A patent search of "CorMedix." A patent application search of the same turns up one item related to gadolinium. Interestingly, POETIC includes Memorial Sloan Kettering Cancer Center (MSK), which completed a Phase 1 cancer study of taurolidine entitled "Taurolidine in Treating Patients With Recurrent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer" (MSK was the sponsor; National Cancer Institute was a collaborator).
The lack of standing with respect to taurolidine's cancer use likely is why CorMedix is using a "proprietary" formulation of the compound in its collaboration with POETIC. I wonder how "proprietary" proprietary really is with respect to molecular tweaking. In fact, the cell line (in vitro) work, presumably for at least neuroblastoma, POETIC would do with CorMedix's version of taurolidine is predated by Luckert et al. (Department of Pediatric Surgery, University Medical Center Hamburg Eppendorf, Hamburg, Germany), "Taurolidine specifically inhibits growth of neuroblastoma cell lines in vitro," J Pediatr Hematol Oncol. 2014 May;36(4):e219-23.
So, what if CorMedix and POETIC successfully move beyond cell line work to animal studies to, eventually/possibly, human trials; how much of the potential addressable oncology market (adult and pediatrics) for taurolidine is capturable by CorMedix and its shareholders? I would find it hard to say given the land grab and use that already has occurred, and initially think "not much" or "limited," if at all.
Takeaway:
A potential offset to the recent and particularly gross mismanagement of the capital structure side of the business (the August Maxim-led offering) could be the generation, production and/or release of potential datasets between now and year-end.
a. With only six recruiting clinical sites (assuming no additional recruiting Australian sites beyond Princess Alexandra Hospital) and possibly 15-35 treated patients in the pivotal melanoma Phase 3 trial through about mid-September, I'm hard pressed to believe this "dataset" (an interim data readout) could be generated or released this year.
b. I believe a preliminary or interim dataset of the melanoma combination Phase 1b study already is available for prospective Big Pharma partners to see; however, the earliest this data might be publicly discussed could be at HemOnc Today Melanoma 2017 in late-March.
iii. A dataset related to the melanoma combo dataset above, and potentially very supportive of and related to a prospective combination therapy deal, is Provectus' immune biomarker work, which might be completed prior to year-end. I'm unclear as to its eventual presentability (medical conference) or publishability (peer-reviewed journal). I wonder if the University of Illinois at Chicago is carrying out this particular work.
iv. I believe the company is hoping PH-10 mechanism of action data is released by year-end, but of course timing/venue and above all likelihood are TBD/unknown.
v. Preclinical data of some sort could presented at SITC 2016 before mid-November. Regular abstract titles should be available some day this month. Provectus and Moffitt Cancer Center separately have presented data at this conference in 2012 (Provectus), 2014 (Moffitt; combination with co-inhibitory blockade) and 2015 (Moffitt; mechanism of action).
vi. Clinical liver data should be presented at ESMO Asia 2016 around mid-December. Abstract titles might be available in early-November.
vii. I believe Provectus is expecting another "dataset" in 2H16.
viii. I have been trying to connect various comments I've heard over the last couple of weeks regarding one more dataset (preclinical and/or clinical; separate from or included in the above), which could be (x) PV-10 and pancreatic cancer as (y) a monotherapy or in combination with another class of drug, such as a targeted therapy. The company had reported preclinical monotherapy/mechanism of action work on pancreatic cancer in 2012. I then returned to the company's 2013 annual CEO letter that noted:
It took a while, but Provectus (via Moffitt) completed PV-10's circuit of the cancer immunity cycle for melanoma, undertaking expansive and detailed mouse-to-man-to-mouse translational medicine investigation and exploration. The company's CTO Dr. Eric Wachter, PhD mentioned on the 2Q16 business update call that Provectus was exploring similar or comparable immunologic work on PV-10 for hepatocellular carcinoma (HCC). Perhaps he has added pancreatic cancer to the pile.
My mistake. When two Pfizer Internet Protocol (IP) addresses began regularly visiting the blog starting around the 2016 JP Morgan Healthcare Conference in January (one IP address about four times as many as another), I initially had thought (based on an IP address geolocation search at the time) the visitors were from a New York City location. Upon further view, the actual location appears to be Chicago (or thereabouts). Pfizer acquired Lake Forest, Illinois-based, injectables pharmaceutical company Hospira in 2015 (and beginning to divest itself of Hospira’s pumps and devices business this year). Hospira appears to have been slotted under Pfizer Injectables.
As an aside, a Pfizer R&D location (either Cambridge, MA or Hartford, CT) paid visited the blog last week, reading among other things May 23, 2014 blog post Provectus was denied breakthrough therapy designation by the FDA, including the FDA denial letter itself.
Back to the future (September 16, 2016)
Updated below: 9/16/16 {twice}.
In September 14, 2016 blog post Life & Death, and the Biggest Checkbook, I noted (among other things) that:
Updated (9/16/16): In 2006, OncoImmune founders authored a paper entitled "Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity," which discussed the combination of a CTLA-4 compound and an anti-4-1BB compound. 4-1BB is a co-stimulatory agent or agonist (the "front-end") that helps to get the car moving (or helps to step on the gas pedal) — Step 3, Priming and Activation of the Cancer Immunity Cycle below — in the analogy of releasing the brakes of the car by the immune checkpoint inhibitors (the "back-end") — Step 7, Killling of Cancer Cells. See 4. Immune checkpoint inhibitors need help. under More Notes (August 10, 2015) on the blog's Archived IV News page.
Updated below: 9/24/16 {twice} and 9/25/16.
 |
| Image source |
Genentech. The Roche subsidiary visited again this quarter, January 29, 2016 blog post PV-10 (Rose Bengal) and the Cancer Immunity Cycle.
Hokkaido University. The Japanese academic institution visited the blog for nearly eight minutes. As context, average session (visit) duration (any blog visitor) can vary from 2 minutes, 10 seconds [2:10] (this year; image below) to 2:10 (this quarter) to 2:35 (this month)
 |
| Click to enlarge |
Updated (9/24/16).1: Deal guys or gals, generally or broadly speaking (writing), may comprise (i) business leaders and executives undertaking M&A, (ii) VC, PE and other investment professionals, and (iii) investment bankers, lawyers and other transaction specialists representing (i) or (ii) in discussing, negotiating and/or papering of the deal/transaction (e.g., pitch decks, letters of interest/intent, term sheets, due diligence, definitive agreements, etc.).
The deal process (looking at it from Provectus' perspective as the seller), and thus the deal people involved in it, in my view, should comprise, first of all, the company's COO and interim CEO Peter Culpepper and CTO Dr. Eric Wachter, PhD understanding what they have in PH-10 (for this example) as a treatment for inflammatory dermatoses, and thus its potential or possible worth. Among the many problems I have with Provectus independent board members is their cosmetic understanding of Rose Bengal's therapeutic treatment possibilities and potential, and thus their uninformed opinion or view of the molecule's worth.
Second, after developing and determining their perspectives of value and worth, management should establish their position(s) on monetizing value/worth (e.g., more upfront and less back-end, less upfront and more back-end, etc.). These business terms ultimately should be decided by Peter and Eric, who are closest to the ground, and ratified by the independent board members; however, the terms (i.e., maximum, minimum) should be developed, outlined and established in consultation with industry vendors (like Dr. Orlow), who presumably are experts or possess some amount of expertise.
Third, the seller's intermediary or representative (e.g., Dr. Orlow, an investment bank like, um, Maxim, a law firm, etc.) interfaces with prospective buyers to see if they can come to agreement on the business terms (or some facsimile of) sought by management. Sometimes, the seller's executives undertake some-to-a-lot of the heavy lifting, depending on their personal preferences and experiences, and internal resources.
Fourth, the deal or transaction is papered (i.e., definitive and other agreements are negotiated, drafted, reviewed, revised, etc.) and then closed.
The above is a simplification of course, and my goal merely was to articulate the essence of the approach. Is Peter a deal guy? I don't think that's the relevant question. He was/is a business leader and executive, while I am/was a [former] investment professional (albeit with experience seeking [begging for] start-up company investment capital). Peter has not smelled like a deal person to me, primarily because of my frustration with his fundraising approach, decision-making and process, but I don't want to compare my so-called deal sheet with his. I could, however, compare the quality and quantity of my board experience, and passion for being a thoughtful, collaborative, competent board and board committee member of privately held companies and nonprofit organizations with that of Provectus' independent directors, but I digress...
And, in addition, Peter only can undertake his work is he has sufficient material with which to work. Cue Eric...
I believe the proper question to ask is, "can Peter manage the deal process?" I believe he can; however, his process requires perfunctory but non-trivial oversight. My deal experience (formerly through corporate venture capital, and opportunistic private equity/M&A) comprises sourcing, due diligence, investment structuring, negotiations, transaction execution, portfolio company management and securities monetization. I wrote my own term sheets and letters of intent/interest that were blessed by general or outside counsel. I read and reviewed definitive and other agreements prepared by counsel, painful as those experiences were. I have been a buyer (investor), and not a seller (like Peter is now), and there are clear and stark differences between these groups/types of people.
I firmly believe Peter fully understands, in this example, PH-10's treatment potential and possibilities, and the treatment's potential financial worth. I believe he has a good sense of the business terms management (and presumably the board) desire and thus seek, and probably has scoped out maxima and minima vis a vis hoped for and acceptable terms. I think he is using competent and experienced vendors to facilitate a transaction, whether to better understand what additional clinical information prospective licensees seek or to garner deal terms and conditions for consideration; in this case, he is using at least Dr. Orlow, and maybe others for dermatology*. I believe Peter is thoughtful and detailed enough to seek a comprehensive transaction.
It's not that PH-10 suddenly is up for sale now. It's been for sale for quite some time; however, the uniqueness of the molecule — e.g., while PV-10 upregulates, PH-10 was/is thought to down-regulate; PH-10 does not appear to have dose limiting toxicities — has required much more time and data than originally expected to generate an initial pathway to approval, and a real license transaction. Some history:
- PH-10's license seeking history could date back to as early as December 2009 when Provectus produced preliminary Phase 2 data for psoriasis and atopic dermatitis ("As previously announced, our development plans include seeking licensure of PH-10 for the treatment of serious dermatological diseases. Based upon these initial results, we are ramping up these efforts, and are actively seeking to engage a financial advisor to assist us with a proposed licensing transaction."),
- There purportedly was interest from Galderma in early-2010; however, no substantive term sheet (i.e., no upfront payment, mostly or all back-end), apparently, materialized. To get terms and condition that management desired or would accept, "data in context" was needed, which is Peter's current and ineffective way of saying "more data was/is necessary),
- Dr. Orlow was retained in November 2010, Joins Provectus Pharmaceuticals' Corporate Advisory Board,
- More data: The company began to undertake a randomized controlled study of psoriasis in December 2010, Initiates Phase 2c Clinical Trial of PH-10 for Psoriasis,
- Top-line data for the above mentioned Phase 2 study was released in March 2012 ("We expect the data from this randomized study will eventually lead to a term sheet for a proposed licensing agreement which will trigger the engagement of a financial advisor to assist us with that transaction"),
- 2012'ish-2014'ish: Eric is/was distracted by/tied up with PV-10-related matters, and/or appears unable to advance PH-10 past the FDA because of seemingly unanswerable questions (e.g., how can there be dose limits if PH-10 does not cause dose limits)?
- Provectus initiates a mechanism of action (MOA) study for PH-10 in December 2014, Protocol for Phase 2 Study of Mechanism of Action of PH-10 on Immunologic Markers of Psoriasis Now Available Online, to have Rockefeller University and Dr. James Krueger, MD, PhD essentially elucidate for PH-10 what Moffitt Cancer Center did for PV-10,
- Provectus is/was awarded a PH-10-specific patent, "Topical medicaments and methods for photodynamic treatment of disease" (US PTO #8,974,363) in March 2015, and
- The company's PH-10 MOA study appears to have generated observations, conclusions and additional information in the spring/summer of 2016. See Provectus' March 16th 4Q15/CY15 business update call and May 10th 1Q16 business update call.
* In the case of Provectus' letter of intent with Boehringer Ingelheim (China), the company used Maxim Group as a strategic advisor to structure and negotiate the deal/transaction. Provectus currently has engaged Maxim to be its investment banker (deal firm/representative/intermediary) for prospective Indian partners, and [I believe] Israeli ones too. I have told Peter it would be, um, imprudent, to use Maxim for any larger transaction (i.e., the end-game).
Network 1 "negotiated" Provectus' memorandum of understanding with Sinopharm-China State Institute of Pharmaceutical Industry and Sinopharm A-THINK Pharmaceutical. Remind me of the status of that "deal" again.... Peter cannot push on a string.
Updated (9/24/16).2: As much as Eric clearly has been and is the driver of value creation at Provectus — two key areas that come to mind are clinical trial design and intellectual property management — he also has been the impairer of value — e.g., missing his own deadlines, setting expectations he subsequently does not meet, a desire not to want to communicate progress until process completion, etc. Only time will tell whether such value impairment is determined to have been temporary, or concluded to be have been permanent.
I've known for some time that Eric is challenged by communications, whether that means his need to control it, his understanding of its importance to an entity that is a public company, his opinion(s) or stance(s) about when, if at all, to say something (i.e., when the process is complete), his trust, or lack thereof, of colleagues and the board, or his view(s) on the nature of biopharmaceutical industry competition and the need/necesscity for treating much if not everything as proprietary, among other things. None of these are static of course; I'm sure his perspective(s) has (have) evolved over time and with experience, good or bad. I do not doubt that working with Eric has been challenging for Peter (Peter's shortcomings as a biz/corp dev executive notwithstanding).
Yet, in the same breath of saying (or in the same muscle exertion to write) the above, Eric would have been one of two people for whom I would have sought to work (the other being an individual who secured the largest seedling funding in [I believe] DARPA history). Some people have a good perspective of the opportunity and challenge that Eric is and presents. It's been disappointing, for example, to have attributed to him in regards to fundraising (paraphrasing), "Don't pimp my investigators and data" (circa 2012), to have his process for determining what and when to communicate as that of a wine sommelier deciding when to serve a particular vintage of wine (2015) [for which I vomited in my mouth upon hearing] or to be Provectus' sole arbiter of preclinical/clinical development W5H communications. On the one hand, it might not be too surprising given those who are/were a@$clowns (Dees), those lacking in integrity (Scott), those who were/are too busy (McMasters), those who were/are uninformed (Smith), and those who were/are unintelligent (Koe). On the other hand, it also might not be too surprising given those who may have felt they could not push back harder or were kept at arm's length for far too long (Peter).
In regards to PH-10 and MOA study results, I suggested to Eric in March and again in June to consider a communications approach similar to or an appropriate variation of Vitae Pharmaceuticals' communication of its Phase 2a proof-of-concept psoriasis clinical trial's top-line results (March 2016), where Vitae management hosted a conference call to discuss these top-line proof-of-concept results and next steps for their dermatology compound and also made Dr. Krueger (Head of the Laboratory for Investigative Dermatology at Rockefeller University) — whose lab also is conducting Provectus/PV-10's MOA work — available for the call's Q&A. In doing so, I conveyed to Eric that rolling out PH-10 MOA study results, or at the very least communicating process and progress in a respectful manner to Provectus shareholders could be carried out in a manner that not only served a capital markets need for Provectus, but could also be very consistent with how one approaches presentation and/pr publication of biomedical data results in the industry. Viate was acquired by Allergan in September.Eric said on the March 16th 4Q15/CY15 business update call:
"As I mentioned in my remarks, we have completed all of the data collection from the patients. We are in the process of compiling the clinical data. So, this is the numerical data that's collected when a patient visits the clinic.
We are working with a major research university on analyzing skin biopsies that were collected three times during the course of the study for each patient; at the beginning of the study pretreatment, after four weeks of application of vehicle, and then four weeks after application of PH-10.
That work is essentially complete. We are in the process of receiving that this week. And I think that it's possible that we will have a preliminary analysis of that completed this month. Certainly if not this month, early into the next month, the month of April."He then said on the May 10th 1Q business update call:
"...we’ve also been busy on the mechanism of action for topical PH-10 and completed clinical work for our mechanism of action study in psoriasis patients in December. We’re finalizing compilation of clinical data from the study with immunohistopathologic analysis of tissue collected from study participants now also complete. And we are reviewing a data together to assess changes in skin biopsies collected pre- and post-PH-10. The study was designed to allow us to probe possible immunologic, structural, and hyperproliferative changes in psoriatic plaque and detect any evidence of cellular atypia upon application of PH-10. It will also allow us to assess concordance of any such changes with clinical observations in those plaques.
We continue to expect these data to inform decisions on an anticipated request to meet with FDA to assess strategies for advancing the program from Phase II into Phase III including whether any additional clinical or non-clinical safety data are necessary to advance to Phase III."
"We expect to have analysis of PH-10 data set completed this quarter. So by the end of June we haven’t determined yet when and how that will be reported, presumably try to report that in some sort of conference fashion in the second half of the year as is our typically with the case for these type of data leading if it looks favorable publication. I’ve only seen portions of the data as stated in previous conference calls are expected to be quite interesting. What I’ve seen looks quite interesting, but until we’ve done a full analysis, we can’t make any quantitative statements."Updated (9/25/16).3: In dealing with an individual like Eric who (i) is exclusively and solely leading and controlling the key value driving roles, responsibilities and activities of the company, and (ii) may not necessarily fully or wholly embrace the capital markets as it relates to the pursuit of scientific and medical innovation and discovery, anyone could have a challenge to more properly and effectively develop, build and grow a company. Layer onto the opportunity and challenge that is Eric issues of bringing a novel molecule (chemical versus biologic), a novel treatment approach (local versus systemic) and an unfamiliar administration route (intralesional/intratumoral versus oral or intravenous) to market, properly protecting the intellectual property of the asset, dealing with an opaque or less than transparent regulatory agency, being a first-time drug development team, having to deal with an evolving oncology playing field, etc., the notion of headwinds seems understated by far.
Nevertheless, Peter, over time, has tried to elucidate Eric's preclinical/clinical development programs for PV-10 and PH-10 to shareholders. Peter typically seeks to confirm or validate what he hears from Eric and place it into the greater context of the biopharmaceutical industry. When his salesmanship does not get in the way, we often hear a thoughtful, cogent, communicative executive, who often is much more excited about the therapeutic prospects for Rose Bengal than the average Provectus shareholder. Like Eric (drug development), Peter has grown into a life sciences/biotech executive on the dime and backs of retail shareholders whose support and patience have enabled this management team to bring the drug(s) to market in the right way. One example of this personal development was Peter's excitement over the independent reproduction and thus validation of Rose Bengal's immunologic/immunotherapeutic features first presented at AACR 2013, PV-10 Immunology Data Presented by Moffitt Cancer Center Researchers at the American Association for Cancer Research Annual Meeting. Peter at the time described the April poster and, later, the July peer-reviewed publication "Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma" as "the shot heard round the world." Most shareholders to whom I spoke thought he was referring to an impact on the share price or the perception of the molecule by Big Pharma (perhaps selectively hearing what they wanted to hear), rather than him trying to convey the groundbreaking importance of Moffitt's work as one more step (but a very key one at the time) towards gaining more full recognition of Rose Bengal's therapeutic benefits.
Returning to PH-10, what has Peter said on Provectus' recent business update calls? On the March 16th call he said:
"Touching briefly on PH-10, as Eric will provide much more information, I want to make three points about 2016. First, we expect to report on our Phase 2C mechanism results--and mechanism results. Second, we expect to have an end of Phase 2 meeting with the FDA regarding toxicology and further clinical development. And third, we will be seeking a licensing agreement based on the strength of the mechanism in other PH-10 Phase 2 studies."On the May 10th call, he said:
"Turning briefly to our work with PH then, our investigational dermatologic drug, we anticipate reporting on our Phase 2 results. They projected in a Phase 2 meeting with the FDA and toxicology work necessary support--to support PH-10 approval. This will be communicated as soon as we can...
We expect to have analysis of the PH-10 dataset completed this quarter, so by the end of June. We haven’t determined yet when and how that would be reported. We’re, presumably, try to report that in some sort of conference fashion in the second half of the year as is typically the case for these types of data leading if it looks favorable to publication."Finally, on the August 10th "winter is coming" call, Peter said:
"Turning briefly to our work with PH-10, our investigational dermatologic drug, I noted in our last conference call that we anticipate reporting results this year on our Phase 2 mechanism of action study in psoriasis patients. The clinical portion of this work was completed at the end of December, biomarker samples have subsequently been analyzed, and we’ve recently reviewed these data with the team that did these analyses. Eric will comment on this during his remarks and the implications for a projected end of Phase 2 meeting with the FDA and toxicology work necessary support PH-10 approval."
Eric's comments on this call were:
"Turning to PH-10, we are sorting through the immunologic and histopathologic data from our mechanism of action for topical PH-10. I can’t go into detail yet about what we are learning, but in general my assessment is that these results will be as important to PH-10 as the Moffitt work has been to PV-10. When new kinds of therapy come along everyone likes to understand the biologic story underlying the clinical observations, and it appears that this may be a very interesting story that explains observations we’ve made throughout clinical development of the drug. I look forward to sharing details on this with our stakeholders in the next few months. I will note that the observations from this study should play an important role in anticipated discussions with FDA to assess strategies for advancing the program from Phase 2 into Phase 3."Peter's original framing in 1Q16 (March) of the PH-10 expectations management was setting for CY16 were:
- Reporting of the MOA dataset (see dataset iv under A race to the data (September 18, 2016) below), which he continue to guide in 3Q16 still should be expected in CY16.
- Getting together with the FDA for an EOP2 meeting. When is this meeting?, and
- Seeking licensure (whether licensure is achieved is a separate topic). Does the rumor of Dr. Orlow shopping PH-10 with MOA data in hand represent this statement?
"We already know that we can destroy HCC with this ablative process and the hypothesis that that should lead to similar signaling, which can have implications for--well, single-agent therapy [unintelligible] HCC, but more importantly for combination with things like anti-PD-1 [unintelligible] . We have already shown that the basic immunology occurs in HCC models, so I would say that--one of the things I’m highly confident in, I’m highly confident that we will show that this same functional immune signaling functions in HCC."H/t a shareholder and regular hatter: "New Evidence Supports a Key Role of the Immune System in HCC" (September 2016):
"In conclusion, recent studies support the vital importance of immunosuppression in HCC and key molecular components that contribute to hepatocarcinogenesis. While novel immunomodulatory therapies are on the horizon, sorafenib may reduce the immunosuppressive network in HCC. Further work is needed to fully capitalize on the immune system as an anticancer therapy for HCC."Key HCC trials are below:
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
November 5, 2015 3Q15 business update call: "As we noted previously, our initial focus has been on opening key strategically significant sites headed by influential investigators to establish a critical mass for the program, and we’re beginning to expand from that base. In the U.S. this is a site-by-site process, where each site has a legal and Institutional Review Board, or IRB requirements. In Australia, we’ve used a new national ethics application process, or NEAP, along with standard contracting indemnification template agreements for the first time nationwide. These features are representative of the constantly evolving process for the conduct of clinical trials. We can’t change the ala-cart nature of site start-up in the U.S., but the nationwide approach in Australia is expect to
expedite start-up once the initial site is Brisbane is active."
March 16th 4Q15/CY15 business update call: "As the trial progresses over the course of 2016, we will assess the paths for expedited development, fast track accelerated approval, market approval with the FDA in the U.S. and the Therapeutic Good Administration in Australia. I want to stress here that the disappointment we all shared when the FDA declined to give us breakthrough therapy designation 2014 has no relevance to these accelerated options. The fact is that we will have much more data to bolster [indiscernible] and there is no regulatory penalty incurred because our BT [sp] application was not approved then."
"In regard to the TGA, yes, definitely moving forward with approval in Australia is something that
we have been considering, we are currently considering, and we will continue to consider,
especially given that roughly half of the melanoma patients that received PV-10 throughout the
history of our development have been from Australia. So--and we have a significant base of
data now on patients in Australia."
May 10th 1Q16 business update call: "While all these amendments were designed to enhance patient eligibility and enrollment in this global study and are necessary to adapt to a constantly changing playing field in oncology and the evolving process for starting and executing clinical trials on a global scale, these necessary changes have led to delays in execution of our study. I must, unfortunately, report that we are behind with regard to our initial schedule for reaching
the interim and final analysis triggers for the study. I'm confident that we're--we are implementing the proper study to support licensure of PV-10, but at present estimate that we are approximately six months behind schedule with regard to site start up, patient recruitment, and eventual data readout. What are we doing to address this? First, we've enlisted the support of a very prominent
clinical investigator in Germany to lead the European portion of our study. He joins similarly
selected leads for each of the geographic regions, North America, Oceania, Brazil, and China,
but the study stands. Second, we've been working with our lead site in Australia to ensure that it can fill--fulfill a nationwide regulatory role under the National Ethics Application System, or NEAF, as additional Australian sites join the study."
"Processes for approval in Australia, we are assessing. We've been assessing them for, since inception of our clinical work in Australia. One of the aspects of closing the expanded access protocol is that we now have an opportunity to collate all of the data from that process to be able to actually use it for at least supportive purposes if not some, a better, higher purpose in terms of regulatory approval. So we’ll be continuing to review that opportunity in Australia on an ongoing basis and as the case dictates, we may elect to move forward in Australia on a regulatory stance that’s faster or slower than in the U.S."
August 10th 2Q business update call: "We held our final advisory board meeting for Europe in June and have a motivated team of melanoma investigators at top centers ready for the study. After months of preparation, we expect to begin filing applications for, with local regulatory authorities in Europe later this month. We’re also continuing to work with the investigator community in our traditional
territories of the U.S. and Australia to build on a very solid base of sites that have been rolling
throughout all, all of this."
"So, we're continuing to--as I said in my comments, we're continuing to enroll patients in our
existing centers, at centers in the US and Australia, and expand the study throughout other
regions of the world that we've identified to bring us to that interim time point and eventually
to the final outcome of the study."
Really Exciting (September 22, 2016)
Source article & link: Dr. Saenger on Ongoing Research in Melanoma
"There are 2 really exciting areas right now, Saenger says, one of those being locally targeted therapies. For example, regarding the abscopal effect, practitioners can radiate a lesion and generate a systemic immune response. An extension of that method is talimogene laherparepvec (T-VEC; Imlygic), which will likely yield better results than a checkpoint blockade alone. Therefore, she adds, combining it with a local strategy is going to be very important, especially as researchers move beyond melanoma into less immunogenic tumors that have evolved from strategies that exclude the T cells from the tumor bed.
The second area that will gain traction is targeting myeloid cells, Saenger says. Additionally, there are agents where you can combine activation of the T cells and inhibition of the macrophages that look like they will have promise."#1. Local/local-regional/regional therapies...abscopal effect/"bystander" effect? Dr. Yvonne Saenger, MD is a T-Vec clinical investigator. PV-10 > T-Vec (Imlygic).
#2. Inhibition of macrophages, eh? "Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages," Rabe et al., J Immunotoxicol. 2014 Oct;11(4):367-75.
Grammatical error (September 21, 2016)
Updated below: 9/21/16 and 9/22/16.
According to Biotechnology Industry Organization (BIO), intellectual property (IP) is the lifeblood of the biotechnology industry. With thanks to a fellow shareholder, regular hatter and all around neat person for this item.
Provectus' patent with Pfizer, Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (US PTO #9,107,887; August 2015), notes as its first claim:
"1. A method of treatment of a melanoma, or primary or metastatic liver cancer in a human comprising separately administering a therapeutically effective amount of: (1) an intralesional chemoablative pharmaceutical composition to elicit ablation of at least one melanoma, or primary or metastatic liver cancerous tumor; followed by administration of (2) a therapeutically effective amount of a systemic immunomodulatory anticancer agent that is a systemic enhancer of immune system down-regulation or that is a systemic enhancer of immune system up-regulation in a combination therapeutic regimen, wherein said intralesional chemoablative pharmaceutical composition comprises an intralesional (IL) chemoablative agent comprising rose bengal (4,5,6,7-tetrachloro-2',4',5',7'-tetraiodofluorescein) in an appropriate pharmaceutical composition, including a 0.1% (w/v) or higher concentration aqueous solution of rose bengal, or a physiologically acceptable salt of rose bengal, said intralesional chemoablative pharmaceutical composition being administered intralesionally into said at least one melanoma, or primary or metastatic liver cancerous tumor at about 0.1 mL/cc lesion volume to about 2 mL/cc lesion volume." {my bolded and underlined emphasis}Shouldn't “system enhancer” bolded above be “systemic inhibitor?” An inhibitor of immune system down-regulation is a net enhancer. An enhancer of immune system up-regulation is an enhancer too. Why would someone want to enhance an immune system down-regulator, which is how this claim appears to be currently written?
Provectus replied that this patent had a grammatical error in the published form (as highlighted above in bold), which was corrected (i.e., it is an inhibitor of down-regulation).
Also, it still is hard to understand how PD-1 and PD-L1 antibodies are “covered” by this '887 patent under “systemic inhibitors of immune system down-regulation,” as Provectus says or insists. While this is descriptive of how such antibodies function, where is the separate claim for them as they have for CTLA-4 antibodies in the second claim and body of the patent? Ironically, the way the first claim seems to be written (although the second and eleventh claims appear to be correct), there’s no inclusion of a “systemic inhibitors of immune system down-regulation” classification of drugs to combine with PV-10.
Anti-CTLA-4 and -PD-1 antibodies are examples of down-regulation class of inhibitors, which function on T cells. Anti-PD-L1 is in the same class, functioning on the target of the T cell (i.e., the cancer cell). While no patent is truly proven until it’s challenged, Provectus believes these classes of agents (i.e., anti-CTLA4, -PD-1 and -PD-L1) were well defined at the time of the invention (i.e., the PV-10 combination therapy patent), and the claims drawn to the classes of agents cover these examples that were known at the time of the invention and any new members of these classes that are subsequently discovered.
I followed up still wondering that “system inhibitor” actually replacing “systemic enhancer” in a very important claim seemed more than grammatical housekeeping. This, and not specifically articulating what other combinations of PV-10 and checkpoint inhibitors are covered by the patent (e.g. PD-1, PD-L1, IDO, etc.) seems like "sloppy work." I say that Provectus shareholders have come to understand Provectus CTO Dr. Eric Wachter, PhD's compulsive nature, which doesn’t make sense in regards to the lack of specificity in the combo patent portfolio (patent #9,107,887; US PTO application nos. 14/748579, 14/748608 and 14/748634).
The specification regarding the systemic inhibitor versus systemic enhancer issue is clear on this matter. The mistake was noted, and corrected without drama after the patent issued. These claims reflect an evolving playing field and are appropriately generic to account for supplanting of CTLA-4 by PD-1 and eventual supplanting of PD-1 by something else.
Thus, the company arrives at the following summation:
 |
| Click to enlarge |
Updated (9/21/16): Takeaway: The above actually puts Provectus CTO Dr. Eric Wachter, PhD in a good light, by his acknowledging this error (instead of being dismissive or defensive) and then his addressing these subtle issues in a thoughtful and logical manner.
Updated (9/22/16): Endpoints' John Carroll wrote about the next generation of checkpoint inhibitors in "The next checkpoints? Immuno-oncology upstart looks to find new ways to unleash T cells," further writing, "Just don’t ask yet what his first new targets are. This is a hotly competitive field, and iOmx wants to maintain silence on its first programs as the CEO sorts through the first round of partnering interest."
Eric notes the above certainly illustrates the field is very dynamic, and that overly specific patent/patent application claims run the risk of becoming obsolete. It's worth repeating what he said regarding Provectus' combination therapy patent portfolio:Consider the practical example of IP challenges of CorMedix partnering with POETIC (Pediatric Oncology Experimental Therapeutics Investigators Consortium) (September 2016) to explore a proprietary formulation of taurolidine for rare orphan pediatric tumors such as neuroblastoma and osteosarcoma. According to the small molecule's Wikipedia page, taurolidine (molar mass = 284 g/mol, cf. 984 for Rose Bengal) is an antimicrobial that is used to try to prevent infections in catheters.
While no patent is truly proven until it’s challenged, Provectus believes these classes of agents (i.e., anti-CTLA4, -PD-1 and -PD-L1) were well defined at the time of the invention (i.e., the PV-10 combination therapy patent), and the claims drawn to the classes of agents cover these examples that were known at the time of the invention and any new members of these classes that are subsequently discovered.
CorMedix is "a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of infectious and inflammatory disease," which thought to "unlock additional value for taurolidine" by exploring the agent's potential in cancer (hence the POETIC collaboration):
 |
| Click to enlarge. CorMedix presentation, Rodman & Renshaw 18th Annual Global Investment Conference |
"Taurolidine's mechanism of action involves a variety of important steps which include the induction of cellular apoptosis, the inhibition of angiogenesis, the down regulation of pro-inflammatory cytokines, as well as decreasing tumor adherence and an enhancement of immune mediated anti-tumor activity. Our goals are to better elucidate Taurolidine's mechanism effects and leverage its history of safety to maximize its anti-neoplastic potential in an intelligent and efficient manner." {my underlined emphasis}Taurolidine's history with cancer in biomedical literature spans 86 references as of this writing (cf. Rose Bengal's 256).
Does CorMedix own the therapeutic use of taurolidine in cancer? Probably not. Consider US patent Use of taurolidine to treat tumors, which was/is assigned to Geistlich Pharma Ag. A patent search of "CorMedix." A patent application search of the same turns up one item related to gadolinium. Interestingly, POETIC includes Memorial Sloan Kettering Cancer Center (MSK), which completed a Phase 1 cancer study of taurolidine entitled "Taurolidine in Treating Patients With Recurrent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer" (MSK was the sponsor; National Cancer Institute was a collaborator).
The lack of standing with respect to taurolidine's cancer use likely is why CorMedix is using a "proprietary" formulation of the compound in its collaboration with POETIC. I wonder how "proprietary" proprietary really is with respect to molecular tweaking. In fact, the cell line (in vitro) work, presumably for at least neuroblastoma, POETIC would do with CorMedix's version of taurolidine is predated by Luckert et al. (Department of Pediatric Surgery, University Medical Center Hamburg Eppendorf, Hamburg, Germany), "Taurolidine specifically inhibits growth of neuroblastoma cell lines in vitro," J Pediatr Hematol Oncol. 2014 May;36(4):e219-23.
So, what if CorMedix and POETIC successfully move beyond cell line work to animal studies to, eventually/possibly, human trials; how much of the potential addressable oncology market (adult and pediatrics) for taurolidine is capturable by CorMedix and its shareholders? I would find it hard to say given the land grab and use that already has occurred, and initially think "not much" or "limited," if at all.
Takeaway:
- IP, and IP ownership, obviously matter to worth and valuation.
 |
| Image source |
a. With only six recruiting clinical sites (assuming no additional recruiting Australian sites beyond Princess Alexandra Hospital) and possibly 15-35 treated patients in the pivotal melanoma Phase 3 trial through about mid-September, I'm hard pressed to believe this "dataset" (an interim data readout) could be generated or released this year.
b. I believe a preliminary or interim dataset of the melanoma combination Phase 1b study already is available for prospective Big Pharma partners to see; however, the earliest this data might be publicly discussed could be at HemOnc Today Melanoma 2017 in late-March.
iii. A dataset related to the melanoma combo dataset above, and potentially very supportive of and related to a prospective combination therapy deal, is Provectus' immune biomarker work, which might be completed prior to year-end. I'm unclear as to its eventual presentability (medical conference) or publishability (peer-reviewed journal). I wonder if the University of Illinois at Chicago is carrying out this particular work.
iv. I believe the company is hoping PH-10 mechanism of action data is released by year-end, but of course timing/venue and above all likelihood are TBD/unknown.
v. Preclinical data of some sort could presented at SITC 2016 before mid-November. Regular abstract titles should be available some day this month. Provectus and Moffitt Cancer Center separately have presented data at this conference in 2012 (Provectus), 2014 (Moffitt; combination with co-inhibitory blockade) and 2015 (Moffitt; mechanism of action).
vi. Clinical liver data should be presented at ESMO Asia 2016 around mid-December. Abstract titles might be available in early-November.
vii. I believe Provectus is expecting another "dataset" in 2H16.
viii. I have been trying to connect various comments I've heard over the last couple of weeks regarding one more dataset (preclinical and/or clinical; separate from or included in the above), which could be (x) PV-10 and pancreatic cancer as (y) a monotherapy or in combination with another class of drug, such as a targeted therapy. The company had reported preclinical monotherapy/mechanism of action work on pancreatic cancer in 2012. I then returned to the company's 2013 annual CEO letter that noted:
"Another key clinical achievement was the commencement of patient enrollment in the Company's Phase 1 protocol expansion study of PV-10 for liver metastasis, which includes two additional study cohorts to evaluate the safety and efficacy of PV-10 as a mono and combination therapy for patients with hepatocellular carcinoma ("HCC"). The combination therapy will assess PV-10 in patients with HCC who are on a stable dose of sorafenib, a standard treatment for HCC. In addition to the protocol expansion, we recently announced the addition of a second clinical site to this trial. At the Southeastern Center for Digestive Disorders & Pancreatic Cancer at Florida Tampa Hospital, Tampa, Florida, Dr. Alexander S. Rosemurgy, M.D., a widely-respected leading pancreatic cancer surgeon, will serve as principal investigator, which is slated for completion by December 2013." {my underlined emphasis}In the same letter there also was a goal for the upcoming year (2013): "Investigating new oncology indications for PV-10, such as pancreatic cancer." In signing the memorandum of understanding with Sinopharm in 2014 Provectus noted:
"In addition, the MOU says that all "parties recognized the achievements gained during PV-10's clinical trial on melanoma, breast cancer and cancer of liver, plus the systematic effect on human immune efficacy, and PV-10 could be applied to other indications such as lung cancer, pancreatic cancer, prostate cancer and kidney cancer." {my underlined emphasis}It is possible a pancreatic cancer met or more to the liver might be on a new liver poster (vi. above). Maybe Moffitt is undertaking preclinical work on pancreatic cancer (I know, it's a stretch, but they've of course previously conducted PV-10 work for melanoma and breast cancer) because of Dr. Rosemurgy's connection to Moffitt. See, for example, August 2015 Tampa Bay Times article "New treatment takes aim at pancreatic cancer with a targeted assault on the tumor," which does not discuss or reference PV-10 but connects Rosemurgy and Moffitt (and does discuss local therapy for pancreatic cancer). An interesting review paper on pancreatic cancer, "From bench to bedside a comprehensive review of pancreatic cancer immunotherapy," Kunk et al., Journal for ImmunoTherapy of Cancer 2016 4:14 concluded:
"Due to the lack of meaningful clinical benefits of cancer vaccines, the potential positive immunological effect of chemotherapy and radiation therapy, and the promising outcomes of immune checkpoint inhibitors, the focus has shifted towards combining these modalities. Gemcitabine, a standard chemotherapy that is used traditionally to treat pancreatic cancer, has been found to mediate immunological effects such as tumor associated antigen cross presentation by dendritic cells and the induction and expansion of cytotoxic T cells responses in addition to reduce the number of myeloid suppressor cells. Radiation therapy can also increase the immunogenic properties of tumor cells by enhancing MHC class I expression, thereby increasing their vulnerability to CTLs. Another frequent effect of DNA damage inflicted by radiotherapy or chemotherapy is the increase in the expression of death receptors (in particular Fas/CD95 and TNF-related apoptosis-inducing ligand [TRAIL] receptors, enabling lysis of the tumor cells by Fas/CD95 ligand and TRAIL-positive immune effectors. As detailed in Table 3, the majority of ongoing trials investigate a combination strategy of the immunotherapy with chemotherapy, radiation or both. Of interest are multiple trials targeting mesothelin and/or GVAX with chemoradiation and multiple immune checkpoint inhibitors combined with chemotherapy. Our group is currently investigating the immunological effect of the combination of chemoradiation and anti-PD-1 as a neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer compared to neoadjuvant chemoradiation alone (NCT02305186). This neoadjuvant setting will allow investigators to study the effect of combination therapy on the tumor microenvironment. Another promising combination by the Hopkins group combined GVAX with anti-CTLA-4 and demonstrated a 1-year improvement in OS by 20 % compared to GVAX and cyclophosphamide alone."AstraZeneca/MedImmune's anti-PD-L1 agent durvalumab generated low response rates for gastroesophageal, pancreatic and hepatocellular carcinoma tumors.
 |
| Click to enlarge. Screenshot from the ESMO 2014 poster. |
My mistake. When two Pfizer Internet Protocol (IP) addresses began regularly visiting the blog starting around the 2016 JP Morgan Healthcare Conference in January (one IP address about four times as many as another), I initially had thought (based on an IP address geolocation search at the time) the visitors were from a New York City location. Upon further view, the actual location appears to be Chicago (or thereabouts). Pfizer acquired Lake Forest, Illinois-based, injectables pharmaceutical company Hospira in 2015 (and beginning to divest itself of Hospira’s pumps and devices business this year). Hospira appears to have been slotted under Pfizer Injectables.
As an aside, a Pfizer R&D location (either Cambridge, MA or Hartford, CT) paid visited the blog last week, reading among other things May 23, 2014 blog post Provectus was denied breakthrough therapy designation by the FDA, including the FDA denial letter itself.
Back to the future (September 16, 2016)
Updated below: 9/16/16 {twice}.
In September 14, 2016 blog post Life & Death, and the Biggest Checkbook, I noted (among other things) that:
- While Pfizer has consistently been late, or erred along the way, to the immuno-oncology (IO) game, one could argue it does not really matter because the Big Pharma has the biggest checkbook (and marketing departments could well trump R&D ones),
- Pfizer out-licensed (sold) an anti-CTLA-4 compound and ipilimumab (Yervoy®, Bristol-Myers) relative, tremelimumab, to AstraZeneca (MedImmune) five years ago,
- The only approved combination (or cocktail therapy) for advanced melanoma, and a combination being tested in other solid tumor cancer indications, is interchangeable-anti-PD-1 drug nivolumab (Opdivo®, Bristol-Myers) + ipilimumab (anti-CTLA-4),
- A Bernstein analyst recently opined that AstraZeneca's combination of its anti-PD-L1 drug compound durvalumab and tremelimumab (anti-CTLA-4) could challenge Bristol-Myers' checkpoint inhibitor pairing (because, I would imagine, AZN's combination essentially is a copy of Bristol-Myers because while anti-PD-1s are interchangeable, they also are similar to anti-PD-L1 compounds),
- When Pfizer and Provectus originally conceived of a patent portfolio based on the combination of PV-10 and immunomodulatory agents like checkpoint inhibitors (e.g., anti-CTLA-4, anti-PD-1, anti-PD-L1, etc.), Pfizer's only economic claim only was the combination of Provectus' PV-10 and anti-CTLA-4 compound tremelimumab. Complete benefit comes only with the acquisition of the tiny biotech, and
- Pfizer and Provectus recently co-advanced combination therapy intellectual property by virtue of each of them signing a terminal disclaimer for a "jointly owned" combination patent application. See Is Pfizer paying more [IP] attention to Provectus? (September 2, 2016) below.
 |
| Click to enlarge. Image source. Author article |
In August 2015 Reuters (Bill Berkrot) wrote an article entitled "Pfizer, Bristol revive cancer drugs that rev up immune system," which discussed immune system "accelerator" agent 4-1BB (also know as CD137) and highlighted early success by a Stanford physician and researcher Dr. Holbrook Kohrt, MD, PhD. Bill Berkrot wrote February 2016 article "Old red dye shows promise as new cancer foe" about Rose Bengal, PV-10 and Provectus.
In October 14, 2014 blog post The Immune Checkpoint Inhibitor Global 4 (or 5), I noted a combination trial of Merck's pembrolizumab and Pfizer's 4-1BB agent. Under Question: Who owns what? Answer: Not Pfizer. (June 3, 2016) I noted preliminary data from this combination, from ASCO 2016: "Six out of 23 treated patients (26%, 95% CI: 10.2%, 48.4%) had confirmed complete (CR) or partial response (PR) per RECIST 1.1: CR in SCLC (n=1), PRs in RCC (n=2), NSCLC (n=1), H&N (n=1) and anaplastic thyroid (n=1)."
Pfizer's deal with OncoImmune may be everything about combining ONC-392 with Pfizer's 4-1BB agent utomilumab. Drug pricing flexibility derives from having combination components in-house, according to Pfizer (May 2016):
"Speaking at the UBS Healthcare Conference, Ms. Liz Barrett, Pfizer’s Global President and General Manager of Oncology, hit the challenge of cancer drug pricing head on. When talking about combination therapy, she said that as “you are going to have two drugs, three drugs, four drugs, we will have to think about different [pricing] models…” Barrett was pressed on this by the UBS interviewer, Marc Goodman: “I mean each drug costs let’s say $100,000 and you are putting three together, it can’t cost $300,000.” But, she held firm: “No, absolutely.”
Barrett explained that Pfizer PFE -0.59% feels pretty good about its oncology drug pipeline, believing that it is uniquely positioned. “If you’ve got three drugs, be it two immuno-oncology drugs and a targeted therapy and we own those, our ability to price will be an advantage for us… I think we feel really good about our future and where we are is our ability to be able to have access to multiple medicines and be able to be in a unique position to price them.”" {my underlined emphasis}Updated (9/16/16): Closing my thoughts on the above, I'd like to understand if Pfizer's economic interest in Provectus' combination therapy patent portfolio is limited to the combination of PV-10 and anti-CTLA-4 drug compound tremelimumab, or encompasses the entire class of anti-CTLA-4 agents.
Speaking of (writing about) combinations, Provectus informed the pharmaceutical industry it is open to clinical collaborations.
 |
| Click to enlarge. H/t a shareholder; a screenshot from Provectus' Facebook page. |
 |
| Click to enlarge. Provectus' new website. |
T-Vec + pembro (September 12, 2016)
I believe it's accepted PV-10 is a better clinical product (and intralesional agent) -- greater tumor destruction/higher complete responses and stronger immunologic signalling -- than T-Vec/Imlygic (the former also is a far better consumer product than the latter).
Huntsman Cancer Institute's Dr. Robert Andtbacka, MD made comments at the European Post-Chicago Melanoma/Skin Cancer Meeting (June 30th-July 1st):
Interestingly, there is not much difference between the impact of T-Vec as a monotherapy and T-Vec/pembro as a combination therapy on non-injected visceral tumors (100% reduction in lesion area/size: 9% v. 10%). The phase 1b results for the combination of T-Vec and ipi seemed to imply a greater improvement in Stage IV patients for the combo. Perhaps part of the difference is that pembro alone works much better in those patients, and could overlap any patients who would have benefited from T-Vec + ipi vs ipi.
Takeaway: Neither T-Vec + ipi nor T-Vec + pembro may ultimately challenge the already approved combo of ipi + nivo (for advanced melanoma) despite dual checkpoint inhibitors' toxicity.
More Intellectual Property Management (September 11, 2016)
Updated below: 10/12/16.
As I noted under Is Pfizer paying more [IP] attention to Provectus? (September 2, 2016) below, (a) Provectus advanced combo therapy and daughter patent application '309 (PV-10 + targeted cancer therapies) in early-August and (b) both Provectus and Pfizer advanced combo therapy and daughter patent application '318 (PV-10 + immunomodulatory cancer agents) in late-August. In Provectus' reply to the US PTO's non-final rejection of both -- see, for example, September 2, 2016 blog post There's additivity, synergy, and then there's PV-10 (1 + 1 = 3) -- the company's CTO Dr. Eric Wachter, PhD noted (among other things):
As I surfaced this weekend through ongoing due diligence, in early-September, both Provectus and Pfizer advanced the second of their two combo therapy and daughter patent applications, '165 (PV-10 + immunomodulatory cancer agents), which had received a non-final rejection from the US PTO in April.
Takeaway: What appears to be the central focus of Provectus' (and thus Pfizer's) approach and reply [to the US PTO] for '165 advancement is the nature of combination and combining. Is the combination of PV-10 and a checkpoint inhibitor or a targeted therapy the delivery of two separate, so-called compositions? Or can it also be just one? In combination, and as part of a combination regimen.
Updated (10/12/16): The USPTO rejected (final rejection) '309 (September 20th) and '318 (October 11th).
I believe it's accepted PV-10 is a better clinical product (and intralesional agent) -- greater tumor destruction/higher complete responses and stronger immunologic signalling -- than T-Vec/Imlygic (the former also is a far better consumer product than the latter).
Huntsman Cancer Institute's Dr. Robert Andtbacka, MD made comments at the European Post-Chicago Melanoma/Skin Cancer Meeting (June 30th-July 1st):
"An evaluation of responses in a phase 1b trial of T-VEC plus pembrolizumab in unresected stage III or IV melanoma found that in 50 injected lesions, there was a 100% reduction in tumor area from baseline in 70% (n = 35). The same 100% reduction was found in 40% of 20 noninjected nonvisceral lesions (n = 8) and in 10.3% of 29 noninjected visceral lesions (n = 3). “Despite the small number of patients, the first combination trials seem to imply a synergy, with the combinations producing a higher response rate than the sums of the rates for the individual agents. But phase 3 trials will be the true test,” he said."I tabulated clinical data from T-Vec + pembro at both the lesion- and patient-levels, and compared them with previous T-Vec + ipi, and ipi + nivo results, all for patients with advanced melanoma.
 |
| Click to enlarge. |
Takeaway: Neither T-Vec + ipi nor T-Vec + pembro may ultimately challenge the already approved combo of ipi + nivo (for advanced melanoma) despite dual checkpoint inhibitors' toxicity.
More Intellectual Property Management (September 11, 2016)
Updated below: 10/12/16.
As I noted under Is Pfizer paying more [IP] attention to Provectus? (September 2, 2016) below, (a) Provectus advanced combo therapy and daughter patent application '309 (PV-10 + targeted cancer therapies) in early-August and (b) both Provectus and Pfizer advanced combo therapy and daughter patent application '318 (PV-10 + immunomodulatory cancer agents) in late-August. In Provectus' reply to the US PTO's non-final rejection of both -- see, for example, September 2, 2016 blog post There's additivity, synergy, and then there's PV-10 (1 + 1 = 3) -- the company's CTO Dr. Eric Wachter, PhD noted (among other things):
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. |
Takeaway: What appears to be the central focus of Provectus' (and thus Pfizer's) approach and reply [to the US PTO] for '165 advancement is the nature of combination and combining. Is the combination of PV-10 and a checkpoint inhibitor or a targeted therapy the delivery of two separate, so-called compositions? Or can it also be just one? In combination, and as part of a combination regimen.
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
The rejection of '309, related to combinations with targeted therapies, appears to say the claim(s) were obvious.
The rejection of '318 appears to acknowledge combination with approved and late-in-the-pipeline immune checkpoint inhibitors, but does not appear to acknowledge or allow such for newer or "next generation" ones.
 |
| Click to enlarge |
I Want (September 10, 2016)
Updated below: 9/10/16.
 |
| Image source |
Common features of a good pairing would be potential synergy (efficacy), non-overlapping toxicities, better adverse event profiles, etc.
Unique to PV-10, in a pairing, would be its orthogonality to different classes of cancer treatments; that is, PV-10 could be paired with each of radiation, chemotherapy, targeted therapies, and immunotherapies, and enable the pairing to be synergistic. Recall that Provectus' CTO Dr. Eric Wachter, PhD noted in a US PTO filing, see September 2, 2016 blog post There's additivity, synergy, and then there's PV-10 (1 + 1 = 3), that, presumably on the basis of preclinical work, "...no combination has failed to exhibit a synergistic effect."
Let's accept as given that Provectus management are individually and uniquely challenged when it comes to professional, mature, responsible communication. "President" Dr. Tim Scott, PhD has been mute for quite some time. Eric does not like to speak until a process or project is complete, among other fair and not unreasonable disagreements I have with him in regards to communicating what I believe to be substantial and substantive company progress. And COO and interim CEO Peter Culpepper drops hints and breadcrumbs (when he could rely on what he is in rarer moments, which is intelligent, thoughtful and cogent). To end this rant, the management team that got us here Peter also turned the company's stock into a "free-floating financial widget" (until one hopes the same management stops the free-floating); see Capital Structure (September 9, 2016).
In Provectus' September 23, 2015 press release, "Announces Initiation of Phase 1b/2 Clinical Trial to Study PV-10 in Combination with Immune Check Point Inhibitor Pembrolizumab," with a byline of 'Study could be a "Significant Step to Co-Development Transaction."' In this breadcrumb Peter notes:
"This study is both scientifically and commercially important to Provectus. Scientifically, combination therapy in cancer treatment is a rapidly maturing area, where rational combination of agents is replacing the empirical approaches of the past. Commercially, this is the second of three steps that we hope will significantly strengthen our hand in negotiating a co-development transaction with an immunotherapy-focused partner. Our joint patent with Pfizer was the first; this study is the second; and the third is our immune mechanism of action clinical study, which is underway at the Moffitt Cancer Center and which has completed recruitment."Step #1: The initial combo patent with Pfizer (#9,107,887) (the "parent") was awarded in August 2015. Two patent applications (the "daughters") were advanced this summer (following non-final rejections). Step #2: According to Eric (i.e., the last business update call), the company has preliminary, interim, clinical data of the combination for melanoma (publication probably in 1Q17). Step #3: Moffitt's mechanism of action study was published in May. So, nearly one year out Peter, are we there yet?
I previously noted a changes to Provectus' Phase 1b/2 trial protocol of "PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma." See Question: Who owns what? Answer: Not Pfizer. (June 3, 2016) below. The Phase 1b endpoint of Change in immune biomarkers, Peripheral Blood Mononuclear Cells (PBMCs) assessed vs. baseline values for changes in T cell populations was removed from the Phase 2 portion (or whatever pivotal construct it eventually becomes, like a pivotal Phase 3 randomized controlled trial).
Later, I noted there may be an undisclosed immune biomarker study going potentially examining PBMC. See Who you got?! (August 4, 2016) below.
I now think I'm wrong in regards to the kind of biomarkers for which prospective Big Pharma partners are looking. In the August 30th FT.com article "Merck plays long game in precision medicine battle" by David Crow, it was noted:
"The most established explanation is that the drugs work best in sufferers whose tumours have high levels of a substance known as PD-L1 — the biomarker that Merck tested for."And:
" Dr Roger Perlmutter, Merck’s top scientist and the architect of the company’s precision medicine strategy, continued to defend the approach even as Bristol-Myers pulled ahead. “If you use PD-1 therapies very broadly, the worry is you’re depriving patients the opportunity of getting another therapy from which they might benefit,” he told the Financial Times in an interview in June. Dr Perlmutter said early studies had shown that patients with lower levels of PD-L1 in fact do better on chemotherapy, and suggested it would be unfair to give them Keytruda or Opdivo instead."You can find some current, useful information about predictive biomarkers for pembrolizumab here. The Merck author often is quoted in co-development press releases.
More to the point, consider this recent paper, Daud et al., "Tumor immune profiling predicts response to anti–PD-1 therapy in human melanoma," J Clin Invest. 2016;126(9):3447–3452. Dr. Daud, MD is an OncoSec key opinion leader and consultant to the company, and going so far as to own stock in it. There also is an OncoSec employee listed as a co-author (former Chief Medical Officer Dr. Robert Pierce, MD in 2013). Dr. Pierce previously:
"a key member of the global development team behind Merck’s FDA-designated “breakthrough” anti-PD-1 program (MK-3475). Dr. Robert H. Pierce joins OncoSec Medical from Merck Research Labs – Palo Alto (formerly DNAX Research Institute/Schering-Plough Biopharma) where he spent almost seven years leading a 20–person team, dedicated to developing disease-oriented and tissue-based translational medicine platforms. As Executive Director, Dr. Pierce was responsible for contributions to multiple successful IND applications, including critical biomarker development programs such as the anti-PD-L1 immunohistochemistry assay supporting Merck’s MK-3475 trials. In addition, Dr. Pierce was instrumental in designing two Phase 2 anti-PD-1 (MK-3475) oncology studies. Prior to focusing on immunomodulatory receptor (IMR) programs, Dr. Pierce had served as a discovery project team leader for two novel drug candidates."MK-3475 is/was pembrolizumab (after it was lambrolizumab).
OncoSec is a competitor to Provectus from an intralesional/intratumoral delivery perspective, and is routinely mentioned by Dr. Sanjiv Agarwala, MD in his presentations. For example, from his comments at the European Post-Chicago Melanoma/Skin Cancer Meeting (June 30th-July 1st):
"While the list of intralesional therapies is growing rapidly, those closest to approval employ electrochemotherapy (cisplatin, bleomycin), chemical ablation (rose bengal disodium 10% [PV-10]), and oncolytic viruses (herpes simplex virus [HSV], coxsackievirus, and reovirus), with talimogene laherparepvec (T-VEC [Imlygic, Amgen]) among them already approved in the U.S. and Europe. Single-agent clinical trials are ongoing with PV-10 (phase 3), electroporation of interleukin (IL)-12, and coxsackievirus type A21 (CVA21)(Cavatak, Viralytics, Ltd.), and combination trials are ongoing with T-VEC, PV-10, and HF10 (an attenuated, replication-competent HSV)." {Bold emphasis is mine}OncoSec and its Phase 2 study of electroporation of IL-12 (epIL-12) as a monotherapy of course was a point of confusion for Adam Feuerstein; see Knowing then what I know now... (July 21, 2016).
In 2014 OncoSec initiated an investigator-initiated Phase 2 trial combining epIL-12 and pembrolizumab for patients with advanced melanoma. In OncoSec's press release, it was noted that Merck would supply the drug; however, this was not a formal collaboration as there was no quote by Dr. Eric Rubin, MD. Dr. Pierce left OncoSec in June 2016 and now is an independent biotechnology consultant.
Whew! What was my point? Dr. Daud/OncoSec's paper noted:
"It is becoming increasingly clear that the immune composition in tumors is markedly different from that observed in peripheral blood. Thus, robust biomarkers that predict response to immunotherapy will most likely be derived from tumor tissue."And:
"Tumor-infiltrating CTLA-4hiPD-1hi CTLs have been shown to contain the majority of tumor-antigen–specific T cells. These cells are only detectable in tumors (i.e., not peripheral blood) and have been suggested to have an “exhausted” phenotype."Takeaway: Tumor tissue-based, not peripheral blood (or PBMC)-based, immune biomarkers may be what prospective Big Pharma co-development partners (at least for co-inhibitory blockade) [finally] want from Provectus.
Updated (9/10/16): I believe I should have written that Big Pharma may want both peripheral blood- and tumor tissue-based immune biomarkers. And, as if on cue, InvestorVillage poster bradpalm1 (a regular hatter for the blog) wrote:
"For several reasons I believe this narrative is correct and Pete and Eric have been constrained from disclosure by these bigs as this dance has evolved over the last year or so."Yes, immunotherapy would appear to be evolving in front of our eyes, requiring Eric and Peter to evolve [the company's approach to combination therapy, whether co-inhibitory blockade or targeted therapy] as the co-development dance evolve.
Consider this ASCO 2015 poster (Erbitux/EGFR inhibitor, Motolimod/Toll receptor, head and neck cancer) that discusses the biomarker targets or goals from blood, and blood and tissue:
 |
| Click to enlarge. |
Capital Structure (September 9, 2016)
That "longtime" (those holding Provectus common stock prior to, say, June 18, 2015) and longtime shareholders are here (a September 9th closing share price of $0.12) now seems wholly understandable and completely logical, but of course with the full benefit of complete hindsight. Ob/Gyn, fund manager and adjunct Columbia professor Dr. David Sable, MD wrote a Wharton Magazine blog post in May 2013 entitled "The Curse of the Poor Capital Structure," where he opened with:
"...the market has rationally chosen to ignore the value of the underlying assets of the company, instead focusing (correctly) on the stock price as an entity in and of itself."He continued:
"A share of common stock should represent ownership of a percentage of the assets of the issuing company, or a percentage of the discounted future cash flows of the company. But sometimes a share of stock is a just a share of stock: a free-floating financial widget whose value floats merrily along, ignorant of any value added to the company’s assets. In these cases, the company’s management, board of directors and the investment bankers advising the company have inadvertently conspired to weaken the connection between company progress and movements in the share price." {My underlined emphasis}In other words, PVCT, Provectus' common stock that trades on the NYSE MKT, is just a digital entity to be electronically bought and sold each day, having no real relationship to Rose Bengal vast therapeutic worth and the company's inarguable progress towards its eventual unlocking. Underscoring Dr. Sable's perspective, sometimes a share of stock is just a share of stock. Ironically, a Connecting the Dots reader at the time asked me to comment on Dr. Sable's blog post, which I did as May 26, 2013 blog post $PVCT Blog Reader Question/Request.
Provectus' COO and interim CEO would say current management is who brought the company to this clinical development progress point. Inarguable as that might be, current management together with no small help from Provectus' independent board members are who brought the company to this market capitalization point, and the very weakened connection between Provectus' clinical progress and movements in its share price.
Housekeeping items. i) It'll be interesting to confirm (merely for poop and giggles) who said what to InvestorVillage poster pvct-whale: "...a person close to the situation told me it was 'over' for the common shareholders because of this raise" and "...another person equally as close to the situation said that PC and co said they had a few tricks up their sleeves before the end of the year." Is anybody really "close" to this situation? By the way, I believe the operative word is aces, not tricks.
ii) InvestorVillage poster STARLIGHT66 makes a [very] germane point regarding deal [doc] prep. Are we wondering, however, whether the independent board members and Peter (and "President" Dr. Tim Scott, PhD and CTO Dr. Eric Wachter, PhD) were looking at the same deal structure (i.e., convertible preferred stock) but with different terms and for different amounts of money (i.e., May/June versus August)?
If a 60-trading day, volume-weighted average share price measurement period (for anti-dilution/conversion purposes) would have begun back then and "the board knew full well that there was no news or event that would benefit the share price," how much different would the outcome have been? Under the former timeframe (say, the end of June), we would need to see how September plays itself out. The latter timeframe provides until late-November. The overlapping period of these two timeframes is this month.
If Eric and Peter are successful in a timely revealing of whatever tricks Peter believes he has up his sleeves, the connection between company progress and share price movement should strengthen, and allow for PVCT to reflect real and true ownership in the company and not be merely a financial widget.
iii) Top of mind, more PV-10 in the biomedical literature (that I had not yet seen): "Novel melanoma therapy," Hsueh et al., Exp Hematol Oncol. 2015; 5: 23 -- PV-10 under Promising novel agents under development.
iv) Consider melanoma, intralesional (IL) agent and Rose Bengal key opinion leader Dr. Sanjiv Agarwala, MD's comments at the European Post-Chicago Melanoma/Skin Cancer Meeting (June 30th-July 1st):
"“Why would anyone be interested in local treatment of a systemic disease?” Dr. Agarwala began. While the new systemic immunotherapies and targeted therapies are exciting and have raised the bar quite high, “we are hitting a toxicity limit. Secondly, let’s not forget that melanoma is not just a systemic disease.” Three percent to 10% of primary melanomas develop local/in-transit recurrences with a greater than 50% risk of distant disease and death. In addition, the soft tissue and skin metastases that occur frequently in melanoma may be associated with considerable morbidity, which can itself lead to mortality, or the patient can suffer for a long time with local disease that can be very hard to control with the available treatments, including surgery. Furthermore, a significant portion of patients may not be candidates for the aggressive systemic therapies." {My underlined emphasis}v) It would seem to me that seeking co-development now, or more recently, for Provectus is more than just melanoma (despite presumably expected stellar results).
 |
| Click to enlarge. Image source |
See for example, October 2013 FDA document "Paving the Way for Personalized Medicine, FDA’s Role in a New Era of Medical Product Development:"
"The success of personalized medicine depends on the development of accurate and reliable diagnostics and, in some cases, on the identification of predictive biomarkers."More to come.
Despite questionable and debatable, historical, corporate decision-making of Provectus' managers and independent board members, and an unfortunate and frustrating share price and market capitalization unreflective of Rose Bengal's vast therapeutic worth, some of those same managers have made undeniable, profound progress to date in so far as the widespread medical acceptance of PV-10's clinical benefit potential.
How "weird" is it for a global investigational drug (i.e., a drug compound as yet unapproved in any global jurisdiction) to be a standard of care at an Australian medical institution (Princess Alexandra Hospital)?
Consider the melanoma key opinion leader who recently shared that he and colleagues believe PV-10 will be widely used off-label following approval.
Consider a comment of Princess Alexandra's Dr. Tavis Read, MD, who also is seeking a PhD, during his presentation about PV-10 (see PV-10: Consistent and Durable Responses (September 4, 2016) below):
"So, we found that patients often progress for unknown reasons and we've been using this treatment because we think it offers a less invasive treatment approach than surgical excision. All of our patients were treated under local anaesthetic, the majority of which were in the outpatient setting. A number of those were in theater, but most were in the outpatient setting." -- {"This treatment" is PV-10}This is the gap, the vast chasm that lies between Rose Bengal and PV-10's capital markets perception and its clinical value proposition reality.
To [some, many?] traders and investors, Provectus is an "old story," where age or ripeness might obscure or obfuscate a seemingly faster growing clinical opportunity. The "old" story might have been the use of PV-10 for Stage III melanoma (locally advanced cutaneous; in-transit), yet this path to an initial approval (unlocked in 2013/2014) is fundamentally necessary to establish valuation. A newer story finally emerged in 2015; that of liver, with a focus on Asia. The newest story, and one that probably caught Provectus' managers' off guard for a time is the potential for PV-10 to be the ultimate front-end for the vast investment Big Pharma has made in their checkpoint inhibitors.
I thought it germane to revisit Maker et al., "The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses," J Clin Cell Immunol 6:343, 2015, where the authors concluded:
"Rose Bengal has been studied recently in several malignancies as an intralesional therapeutic agent. Experiments evaluating the mechanism of action demonstrate that RB is taken up selectively in the lysozomes of transformed cells. It induces cell death by apoptosis and necrosis. Upon treatment with RB in-vivo, many treated patients and animals have been found to generate a systemic anti-tumor response resulting in growth suppression of untreated lesions. RB-mediated tumor cell death may expose tumor antigens that may otherwise evade immune detection. Upon re-exposure to these antigens, a robust cytotoxic T-cell response has been demonstrated in experimental models. Though most profoundly described in melanoma cells, clearly an immunoreactive malignancy, the effect has been shown to be not limited to one specific type of malignancy. Our current research is establishing the role of RB in generating anti-tumor immune responses in gastrointestinal cancer and liver metastases. Decrease in tumor burden and stimulation of an immune response with PV-10 has been demonstrated in animal models of metastasis, and correlations of these responses in clinical studies is consistent with such results. That PV-10 treatment can potentially increase circulating cytotoxic T-cells, even in patients who were previously treated with immune-activating checkpoint blockade, supports the possibility that RB induced cytotoxicity may activate T-cells that are responsible for the bystander effect on untreated lesions. As such, intralesional therapy with RB may be a promising new mode of therapy to stimulate T-cell mediated anti-tumor immune responses." {My underlined emphasis}Gastrointestinal (GI) cancer "is a term for the group of cancers that affect the digestive system. This includes cancers of the oesophagus, gallbladder, liver, pancreas, stomach, small intestine, bowel (large intestine or colon and rectum), and anus."
Provectus' CTO Dr. Eric Wachter, PhD noted on the company's August 8th business update conference call the activity of the preclinical/clinical development program to combine PV-10 with a checkpoint inhibitor for the treatment of hepatocellular carcinoma (HCC), and thus I imagine or would hope GI cancer more broadly.
Consider Hoon et al., "Current Status and Perspective of Immunotherapy in Gastrointestinal Cancers," J Cancer 2016; 7(12):1599-1604. The authors' abstract notes:
"Cancer immunotherapy is at dawn of the Renaissance after the Medieval Dark Ages. Recent advances of understanding tumor immunology and molecular drug development are leading us to the epoch of cancer immunotherapy. Some types of immunotherapy have shown to provide survival benefit for patients with solid tumors such as malignant melanoma, renal cell carcinoma, or non-small cell lung cancer. Several studies have suggested that immune checkpoint inhibition might be effective in some patients with gastrointestinal cancers. However, the era of cancer immunotherapy in gastrointestinal cancers is still in an inchoate stage. Here we briefly review the current status and perspective of immunotherapeutic approaches in patients with gastrointestinal cancers."Inchoate means "just begun and so not fully formed or developed; rudimentary."
 |
| Click to enlarge. |
"Cancer immunotherapy has shown promising results in various types of cancers. Especially immune checkpoint inhibitors are leading a recent renaissance of immune-mediated anticancer treatments. Early studies have suggested that immune checkpoint inhibition might also be effective in some patients with gastrointestinal cancers. However, the era of cancer immunotherapy in gastrointestinal cancers is in an inchoate stage. The immune system and immunosuppressive mechanisms surrounding GC or CRC are still obscure compared to malignant melanoma, renal cell carcinoma, or NSCLC. To increase efficacy of cancer immunotherapy in gastrointestinal cancers, we need to build more profound understanding of tumor immune system. Knowledge of potential relationships between tumor cells and their microenvironment is also essential in gastrointestinal malignancies." {My underlined emphasis}Survival (September 6, 2016)
Updated below: 9/6/16, 9/7/16 and 9/8/16.
The Princess Alexandra Hospital compassionate use program (CUP) data of in-transit melanoma patients treated with PV-10 (see below) not only provided further validation of the investigational drug's ablative (response) consistency (i.e., complete response, objective response, disease control), but it also provided glimpses of (a) the response's durability and (b) overall survival (OS).
True OS data for PV-10's potential approval and subsequent label can only come from Provectus' pivotal Phase 3 trial, PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma.
It is interesting [to me at least] to compare what data there is against benchmarks of whatever sort if only to better evaluate the potential outcome of the Phase 3 trial, and also to better validate PV-10's clinical value proposition, which for Stage III melanoma patients is -- if all disease is treated (injected) -- to prevent, forestall or stop its spread to Stage IV (i.e., M1b-c).
Setting aside its self-described limitation (i.e., lack of information on disease progression, therapies used, and genetic factors), Song et al.'s 2015 paper, "Overall survival in patients with metastatic melanoma," provides context for both Princess Alexandra's CUP data and Provectus' metastatic melanoma Phase 2 clinical trial, Phase 2 Study of Intralesional PV-10 for Metastatic Melanoma.
 |
| Click to enlarge |
Returning to InvestorVillage (IV) poster mplaut's wonderment of how PV-10's consistently stellar response rates were not reflected in its survival outcomes, perhaps the beginning of an answer for all of us lies in OS rates, rather than what we have for now, OS length. Provectus' Phase 2 trial demonstrated a robust 1-year OS rate compared to that tabulated by Song et al. for patients with Stage III disease; however, the study only calculated OS for this time 12-month timeframe.
It will be up to the pivotal melanoma Phase 3 trial to generate OS data that investigators will study in years to come to validate or refute the study's hypothesis that if all Stage III disease is treated, advancement or spread to Stage IV might well be prevented, forestalled or downright stopped (i.e., much longer OS that otherwise would have been expected, or generated by currently approved or recommended drugs and treatments. Dramatically better 1-, 2-, 3-, 5- and 10-year OS rates might be generated by an effective PV-10 containing or defeating locally advanced cutaneous melanoma; however, investigators would have to collect and tabulate such data over time
Updated (9/7/16).2: Dr. Read at Princess Alexandra communicated to IV poster mplaut that "[t]he full study has been submitted for publication and is not yet available for the public." (H/t, and thank you).Math. I wrote below that Princess Alexandra's median OS would increase should the patients (and thus the data) be reviewed longer or assessed at a later date (and assuming patients lived longer). Generally speaking, I believe that to be true, and my goal merely was to be accurately right (if possible) rather than precisely wrong. I'll use a ludicrous example to make my point, but of course it's an extreme example that is not representative of the actual data set. And, an OS curve of the data is necessary for us to have a full picture and thus understanding of the data.
In the 45-patient study, Read et al. noted a mortality rate of 48.9% (22 ÷ 45); that is, 22 patients had died by/at the time of review while 23 patients still were alive (51.1%). In this data set, ordered from smallest to greatest or smallest to largest, the 23rd point is the median value. Below, I assume 22 patients generate a figure of 5 for "some metric" (i.e., the patients who died) while assigning larger values for the other 23 (i.e., the patients who remained alive). If the values of some metric increases for the latter (the alive group) by, say, 10 (i.e., 10 more months of survival), the median increases (as does the mean). My point simply is that a longer assessment might well show longer survival.
 |
| Click to enlarge |
Updated (9/7/16).2: In additional commentary by Network 1 that has been circulating, the firm also primarily primarily independent board member Jan Koe for turning down what the firm apparently believed was a better deal offered to Provectus than what Maxim undertook.
IV poster pvct-whale wrote, "I was told by NW1 that they were going to offer Mgnt a straight up 1 common and 1 warrant as a deal at .25 and Mgnt didn't take it." IV poster STARLIGHT66 replied, "You are incorrect. The deal done with Maxim albeit "shitty" was done because of a guarantee of $6mm being done. The deal was in reality a Firm Committment offering although listed as a Best Efforts."
It's up to you to blame (if you wish) whoever you wish, but all six individuals of management and the board of directors -- Culpepper, Wachter, Scott, Smith, Koe and McMasters -- individually and collectively presided over a decrease in Provectus' share price of more than 80% between two Maxim-led offerings in just under 15 months. Data don't lie.
$0.80, June 17, 2015; June 18th PR, Announces Proposed Public Offering of Common Stock and Warrants. $0.13, August 30, 2016; August 30th PR, Announces Closing of Public Offering.
Updated (9/8/16).3: My takeaway merely is this in regards to any he said-he said disagreement between Network 1, Koe, Culpepper, etc.: Decision-making, process and execution was lacking all around to varying degrees depending on the management or board member party in question.
On the one hand, as Peter has usually done in regards to fundraising (his typical flippant, hand-to-mouth approach to capital structure despite his protestations [to me] to the contrary), he probably tried to pursue some "shitty" Maxim-based approach earlier for a sufficient, guaranteed amount of money to keep the company in good standing through the end-of-the-year (because winter was coming). Whether the money would have been enough is another topic (there historically has been little-to-no substantive cost control at Provectus, and little-to-no cash and expense forecasting).
If, on the other hand as innuendo suggests, the board involved itself in fundraising decision-making, process and execution, independent members ultimately achieved a "shittier" deal underscored by its tardiness: (a) the painting of going concern issues on the August 9th 10-Q, (b) the portrayal of a challenging fundraising ("best efforts" instead of a "firm commitment"), (c) worse terms, and (d) the same fundraising vendor.
There is a rumor of a substantial amount of the Series B convertible preferred stock being converted into common stock. I would be curious if others could assist in refuting or supporting such contention.Updated (9/6/16).1: CT.gov update. H/t InvestorVillage poster ReturnOfJuggernaut. The ClinicalTrials.gov webpage for Provectus' A Study to Assess PV-10 Chemoablation of Cancer of the Liver was updated to note Vanderbilt-Ingram Cancer Center is recruiting.
 |
| Click to enlarge |
While Moffitt Cancer Center presented preclinical data of PV-10 combined with co-inhibitory blockade (i.e., anti-CTLA-4, -PD1 and -PD-L1) at SITC 2014, "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma" (abstract), Moffitt did not present response rate data to frame what synergy is and could be in such a way to better explain 1 + 1 > 2, much less 1 + 1 = 3.
For reference, see September 2, 2016 blog post There's additivity, synergy, and then there's PV-10 (1 + 1 = 3), and The bar (June 24, 2016) and Additive: 1 + 1 < 2. Synergistic: 1 + 1 > 2 (best case, >> 2) (June 25, 2016) on the blog's Current News page.
PV-10: Consistent and Durable Responses (September 4, 2016)
Updated below: 9/4/16 and 9/5/16 {twice}.
I located the Princess Alexandra Hospital (Australia) site experience clinical data with PV-10 and in-transit melanoma. Response rate results are consistent with prior studies and site experiences of PV-10 administered alone, and with radiation; see the table below and, specifically, the row of the yellow-colored cell for this newish data. This data was presented at The 2016 Royal Australasian College of Surgeons Annual Scientific Congress in May. For background, see "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived V News page. In addition, Princess Alexandra's presentation dataset also included some durability of response and overall survival data.
The abstract of Princess Alexandra is below. The work was presented by Dr. Tavis Read, MD, who also was an investigator in Foote et al.'s investigator initiated Phase 2 study of PV-10 and radiation in patients with advanced melanoma.
Durability and overall survival PV-10 data included:
Updated below: 9/2/16 {thrice}.
Takeaways:
Mr. Pugmire is San Diego-based intellectual property (IP) counsel (potentially among other things) at Pfizer.
'165 (PV-10 + immunomodulatory cancer agents) has not yet been advanced.
Immune System Photo Op (September 1, 2016)
Updated below: 9/1/16 {thrice}.
H/t the photo below, InvestorVillage poster Jack Russel, from left to right: Provectus COO and interim CEO Peter Culpepper, Melbourne, Australia's Peter MacCallum Cancer Centre's Dr. Grant McArthur, MD and [I think] a Provectus vendor [who I believe I know] at the 16th World Congress on Cancers of the Skin/12th Congress of the European Association of Dermato-Oncology in Vienna, Austria.
Pivotal melanoma Phase 3 trial
Checkpoint inhibitor resistance, hypoxia, b-catenin, TGF-beta, HMGB1, whatever... (August 31, 2016)
From my Twitter feed for this project:
Hypoxia -- "deficiency in the amount of oxygen reaching the tissues:"
Updated below: 8/29/16 {twice}.
First, this is about helping the independent members of Provectus' board of directors understand what they have therapeutically in Rose Bengal and PV-10 (and PH-10 as well as halogenated xanthene-based analogs to come).
These board members were too busy, uninterested and/or inexperienced to learn, understand and, thus, project the immense clinical value and pharmacoeconomic proposition the drug holds for cancer patients as an immunotherapy potentially agnostic to solid tumor cancer and that possibly may be safe, effective and affordable for the entire world. I believe they are trying to be and act better, because it's never too late to be and act better (in any situation, let alone this one).
The potential to ablate (destroy) any solid tumor cancer into which it is injected and subsequently potentiate (mount, improve, increase) an immune response for any solid tumor cancer is why I previously wrote the therapeutic worth of the drug substance (Rose Bengal) and product (PV-10) has never been higher.
Second, this is about helping the independent directors better work with and manage core management to strive to maximize the worth of the drug in a [final] transaction.
President, board member and co-founder Dr. Timothy Scott, PhD has not been a real company manager for quite some time, like former Chairman, CEO and a co-founder Dr. Craig Dees, PhD. Core managers are Provectus COO and interim CEO Peter Culpepper and Dr. Eric Wachter, PhD. Dr. Scott needs to relinquish his "role" and "responsibilities" as "President," and remain as a board director.
First, in changing how the independent board members approach working with and appropriately managing (overseeing) core management, these directors should ask and answer for themselves key questions such as but not limited too:
Third, this is about helping (telling) core management to manage the company better.
I do not believe there is a whole lot of subjectivity to what I wrote above. There are clear deficiencies in how Peter and Eric undertake their some of their work (corp/biz dev, clinical development/R&D), and these need to change, starting "now." I'll address detail in a subsequent blog post or new entry.
Generally speaking, I'm not uncomfortable with Eric proceeding with his clinical development program in the way he is doing, nor am I uncomfortable with Peter becoming acting CEO. If Provectus' board could have found a suitable CEO candidate, they would have by this time. But they can't because the company has not "won," and when it does there will be no end to list of accomplished or unaccomplished people interviewing for the position. At that point Peter can move aside, or the questions might be posed, "Do we need a CEO now? Where are we in the timeline?, etc."
I have no doubt Korn Ferry is surfacing up pharma people with solid achievements on their CVs for the board to interview. If I were one of these folks, presumably smart and intelligent, traditionally experienced, hard working, etc., I'd see Provectus as a stepping stone to someplace else. If I were one of them, I'd probably want to move the company to a so-called biotech hub like Boston (Cambridge), San Francisco, etc. and rebrand it, whatever that brand might or could be. If I were one of them, I'd probably want to do a reverse split, recap the company, sell-off Rose Bengal/PV-10/PH-10 for whatever I could get and buy another investigational compound, etc..
If I were one of these Korn Ferry-supplied candidates, I probably would not know what kind of shape my NDA was in, how far along my manufacturing was, what agnostic and orthogonal mean, and/or the pharmacoeconomic Mjolnir that Provectus' investigational drugs can and will be for oncology and dermatology patients around the world, and to the global pharmaceutical industrial complex.
But, Eric and Peter have to act and be better; I think it can be objectively shown they have not too many times for the chances they have been permitted by retail shareholders who have been run over or "murdered" over the last few years. I believe I know what each of them is afraid of. For Peter, it's not being around at the end of the story to tell and write his story. For Eric, it's having someone like a new CEO of the style or lack of substance I wrote above mess with his process, which I think, for the most part, is really good (but can be improved in certain ways).
Fourth, and finally, this is about wanting, committing and never giving up helping the independent directors and core management anyway we can.
I am committed to helping the board and management anyway I can, like I know other shareholders want and are prepared to do, such as better explaining through this blog the immense opportunity for cancer patients of an immunotherapy agnostic to solid tumor cancer.
If the board and core management will not strive to be and act better, starting "now," I will not support, and would more aggressively than I ever have fight against, an increase in the number of shares outstanding if Provectus asks for it (I think they have enough shares to get to where they need to get) and push for the company to be sold instead.
I have offered several members of management and several times (recently and over the last few years) to work for the company for free, locking-up my shares in the process, and merely being reimbursed for reasonable out-of-pocket expenses (in principle, it would be by GSA standards and with no per diem). It's no insult to me to be ignored (and rejected). Just be and act better.
I believe there is substantial asset value in Provectus that of course is not recognized by its current market capitalization. As a former corporate venture capitalist in the service of SAIC employee owners, I was fortunate to garner a lot of experience investing (and generating an return on investment), and in the process undertaking clean and dirty wind downs, asset and stock sales, and winning on investments, where several times we stepped in as the lead investor to drive, achieve consensus about and make accommodations, adjustments and compromises that served everyone (management and shareholders), even if it cost us. It's just work, complex and hard work, and with the potential, initial backdrop or background of drama, before everyone is back on track to fulfilling what we all believe the investigational drug can do for patients and is worth to Big Pharma.
None of what I think and believe could and should be done is drastically out of line with what Eric and Peter are doing. Core management knows fully well the opportunity they have in an immunotherapy agnostic to solid tumor cancer. In February 2013 CancerWatch article entitled "Back to Phase 1: Understanding Systemic Effects of PV-10," Eric said:
Eric has long wanted to see Rose Bengal and PV-10 used therapeutically as pervasively as aspirin. He has done yeoman's work (understanding his challenges and issues, which each of us has) to position the drug in the global oncology space. I can and will give Peter certain kudos (which really, simply be should about "doing your job").
The game is far from over. I could make the case it's just beginning.
Updated (8/29/16): As I've previously written, shareholders of all shapes and size have been helpful, useful and insightful on this project's journey. InvestorVillage poster STARLIGHT66 wrote:
Updated below: 8/27/16.
The clear and present opportunity for cancer patients, existing and prospective Provectus shareholders, and the world at large is the agnosticism of Rose Bengal and PV-10, a 10% solution of Rose Bengal (in saline) administered ideally into all accessible, target, cancerous lesions and tumors (i.e., all disease burden).
Conceptually, agnosticism is one of two key characteristics of Rose Bengal and PV-10 for solid tumor cancers and patients with end-stage disease. As an aside, but also very importantly, the other concept is orthogonality. Agnosticism means PV-10 can ablate any kind of solid tumor cancer, and is building towards being able to mount an immune response irrespective of solid cancer indication. Orthogonality means Provectus' drug product can be combined with several or many different categories or classes of drug or cancer treatment with (a) little-to-no clinically relevant drug-drug or drug-treatment interaction and (b) great potential to be synergistic with these drug classes or treatment types.
Think about agnosticism another way. Bristol-Myers and Merck's anti-PD-1 drugs nivolumab and pembrolizumab, respectively, are being applied to different cancer indications with varying degrees of success and failure (to shrink or destroy tumors) depending on tumor "temperature" (i.e., hot and cold tumors). For example, see 5. under Desert > Beach (July 8, 2016) below.
PV-10 has generated preclinical and clinical data showing it can ablate -- destroy by injection (antitumor cytotoxicity) -- numerous solid tumor cancers such as melanoma, breast cancer, ovarian cancer, hepatocellular carcinoma (HCC), gastric cancer, liver metastases, and sarcoma. Thus, PV-10 is agnostic to solid tumor cancer in regards to destroying tumors into which it is injected. For example, read "The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses," Maker et al., J Clin Cell Immunol. 2015 Aug; 6(4): 343.
The opportunity in front of Provectus is to demonstrate PV-10 is agnostic to solid tumor cancer in regards to mounting an immune response once a cancer tumor is injected. Any kind or type of cancer. For the company to demonstrate its multi-indication viability more so than nivolumab or pembrolizumab, PV-10 must show it can truly potentiate (increase the power, effect or likelihood of) an immune response in different solid tumor cancers. There is some preclinical and clinical data that show PV-10 is an immunotherapy -- that it can destroy via immune response (induction of antitumor immunity) -- in solid tumor cancers such as melanoma, HCC, lung metastases, and gastrointestinal cancer, and liver metastases.
This is the "good" Provectus Biopharmaceuticals.
Updated (8/27/16): The "good" Provectus should see ablative (and small molecule) immunotherapy PV-10 employed in the treatment of all solid tumor cancers before, during and after surgery; in combination with other therapeutic agents and therapies, and after all else fails. Very recent diligence of key opinion leaders would underscore the eventual widespread use of the drug; however, it needs to be approved in at least one setting.
The management of this good company fully understands where the pharmaceutical industry is moving (e.g., end-stage cancer patient treatment with combinations and cocktails using immunotherapies), hears this feedback, and wholly understands the opportunity PV-10 has as a potential or possible agnostic immunotherapy for all solid tumor cancers.
But there is a "bad" Provectus. Recall the good bank/bad bank discussions during the global financial/credit crisis of ~2007-2009:
It is ironic to recall that several years ago Network 1 Financial's Damon Testaverde called Provectus' drug a ten and its management a two. He probably was among the first to espouse what some-to-many investors felt at the time but didn't or couldn't say.
Agnostic immunotherapy PV-10 now has the potential to be (along with the manager's clinical development program that has brought it to where it is today) a fifteen (the good Provectus), while management and the board of directors are some minus number (the bad one).
It's easy to differentiate the good Provectus from the bad, and to observe the gaping chasm between the reality of Rose Bengal and PV-10, and the perception of the company. The concept of a good Provectus and a bad one is simple. A good solution to filling the vacuum, while not complex, is complicated and requires hard work, but I believe shareholders can and should constructively and productively push back, and I think that time starts now.
Immunological “ignition switch” (August 26, 2016)
H/t InvestorVillage poster canis_star for news and link that underscore the substance of this blog news entry:
Combination therapy started a few years ago as throwing poop on the wall to see what sticks. Naturally, it has evolved or advanced to examining the poop before one throws it, to more specifically trying to understand the baseline immunologic signalling that each party in the combination or cocktail brings to the table. Call it addressing both the innate and adaptive immune systems, as noted in Biothera's 2016 press release. Call it synergy. Call it parts of a car (e.g., brakes, gas pedal, engine, gas tank, steering wheel, ignition, etc.). Call it whatever you want.
Eric and Peter understand this (I believe), although the board does not fully but may be getting there slowly.
What is a PAMP (Biothera)? "Pathogen-associated molecular patterns, or PAMPs, are molecules associated with groups of pathogens, that are recognized by cells of the innate immune system."
What is a DAMP (Provectus)? "Damage-associated molecular pattern molecules (DAMPs) also known as danger-associated molecular pattern molecules, are host molecules that can initiate and perpetuate a noninfectious inflammatory response." And, DAMPS are recognized by both the innate and adaptive immune systems; for example, here and here.
Can do you get the deal you want or deserve? Data drive deals. In the press releases above, Biothera and Merck were exploring/going to explore non-small cell lung cancer (NSCLC), advanced melanoma (MM) and/or metastatic triple negative breast cancer (TNBC). What's the list? MM, hepatocellular carcinoma (HCC), NSCLC, colorectal cancer, breast cancer or TNBC, ovarian, prostate, neuroendocrine tumors (NETs)...
Griswoldesque (August 24, 2016)
Updated below: 8/24/16 {thrice}, 8/25/16, 8/26/16 {twice} and 8/27/16.
Provectus filed a prospectus for an as yet undefined sale of Series B convertible preferred stock and warrants (on common stock). My guess, for now:
Using a fully diluted shares outstanding number would yield a more accurate dilution figure (probably 12-16% worst case, on a base of somewhere north of 300 million shares, options and warrants).
Updated (8/24/16).1: Another summer, another Griswold family-esque road trip across America. 2009-2012, 2016: Las Vegas to Fort Lauderdale. 2013: Vegas to Toronto.
Provectus issued a press release in conjunction with the prospectus filing, Announces Proposed Public Offering of Preferred Stock and Warrants. As in the prospectus, no details were provided in the PR.
Provectus' COO and interim CEO noted on the August 10th 2Q16 business update call (my underlined emphasis):
HCC Immunology Data (August 17, 2016)
Updated below: 8/18/16.
On Provectus' 2Q16 business update call, company CTO Dr. Eric Wachter, PhD discussed the different solid tumor cancers for which Provectus had basic-to-advanced immunology data: melanoma, colorectal cancer, breast carcinoma, and hepatocellular carcinoma (HCC). You can begin to see this on the slide below that I developed for the company, which was first displayed at June's The BIO International Convention (Provectus' COO and interim CEO Peter Culpepper delivered the presentation):
Presented posters and published papers are noted in black, solid tumor cancer indications in orange.
Eric also said on the call in regards to HCC (with my underlined emphasis):
I believe Eric has sanctioned some immunology work on HCC in Asia, but I'm unsure whether the work is clinical or preclinical.
Updated (8/18/16): I re-read a paper by the University of Illinois at Chicago (UIC) on PV-10, "The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses," Maker et al., J Clin Cell Immunol. 2015 Aug; 6(4): 343. First, in regards to HCC immunology data, and the similarities Eric noted between melanoma and HCC (2Q16 business update call: "Our next tumor on the radar will be hepatocellular carcinoma because there are challenges in HCC that are comparable to those in melanoma), the authors note:
Hepa 1-6 refers to hepatocellular carcinoma (HCC) or malignant hepatoma. Recall Provectus introduced the notion of PV-10's immuno-ablative outcome (the name it utilized back when) as tantamount to in situ vaccination.
UIC noted its ongoing work with PV-10 in the paper too: "Our current research is establishing the role of RB in generating anti-tumor immune responses in gastrointestinal cancer and liver metastases."
Your Rose Bengal protection [of its therapeutic use] team at work (August 17, 2016)
Tweet image:
The above paper, THE LIBERATION OF ADSORBED SUBSTANCES FROM PROTEINS. A FUNCTION OF THE BILE SALTS: I. PRELIMINARY REPORT, JPET July 1925 vol. 25 no. 6 449-457:
As just a guess, consider "Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer," Rusling et al., Analyst. 2010 Oct; 135(10): 2496–2511 (with my underlined emphasis):
Institutional (August 16, 2016)
Updated below: 8/16/16 {twice}.
13F filings (through today) for the period ending June 30, 2016 showed:
Institutional holdings (i.e., 13-F-based reporting) of publicly traded warrants increased 5.7% QoQ (to 2.86 million). See Institutional (February 16, 2016) on the blog's Archived News V page for the last entry on institutional holdings. As an aside, I revised 1Q16 holdings above to reflect more accurate information since the February 16th blog writing.
Interestingly, of the presumed firms and funds that bought the Maxim-led June 2015 public offering of common stock and tradable warrants, all but Wharton graduate's Ms. Bihua Chen's Cormorant Asset Management retained their original position in both the common stock (PVCT) and warrants (PVCT/WS or PVCT.WS). Millennium management retained their original position in the warrants. Cormorant increased its reported, quarter-end, common stock ownership in 2Q16 by 1.2% QoQ. Sabby Management sold most of its remaining common stock position (~-87% QoQ). It is possible, however, that Cormorant sold some-to-most-to-all of their position in the current quarter (and, particularly, last week).
I'll take this opportunity to further encourage Provectus management and board of directors that the company (both the board and management), who have had no meaningful experience in their professional backgrounds regarding raising money and/or have not known how to raise it, to try to act better going forward.
As management, raising money is not about picking up the phone and calling investment bankers for money or leaving it to them to do your jobs (plural) (and thus merely checking a corporate development box, or perfunctorily if at all helping another person's domain [because to be successful you have to consider that yours is his, and his is yours]). As a board, raising money is not about waiting for management to come to you.
Fundraising of all sorts is about so much more; from understanding and forecasting wants and needs in concert with others, to developing and updating a pitch deck in concert with and with input from others, to considering and speaking to new players in new geographies as much as to those to whom you've already spoken, to establishing and following through with a sufficient time runway to do it that also ensures no one is rushed yet also having everyone act intelligently and promptly, to...
To all manner of folks, ranging from those who may not have highfalutin professional investment experience to those who may have been on both sides of the table (like me), you, management, and you, the board, have historically behaved and acted, in this regard of fundraising (irrespective of your individual and collective cumulative excuses), in an embarrassingly unproficient manner considering the potentially immense clinical value of the drug substance and product you all have been developing.
Please, from here on out, let's roll...
Updated (8/16/16): Irrespective of whether you've taken money from others for a small business, or whether you raised it "professionally" (e.g., venture capital, private equity, initial public offerings, PIPEs [private investment in public equity], etc.), how you approach raising money is instructive about how you might treat or spend money; how you treat or spend money is instructive about how you might raise it.
Updated (8/16/16): Factless is as factless writes (1/2).
PV-10: Consistent and Durable Responses (September 4, 2016)
Updated below: 9/4/16 and 9/5/16 {twice}.
I located the Princess Alexandra Hospital (Australia) site experience clinical data with PV-10 and in-transit melanoma. Response rate results are consistent with prior studies and site experiences of PV-10 administered alone, and with radiation; see the table below and, specifically, the row of the yellow-colored cell for this newish data. This data was presented at The 2016 Royal Australasian College of Surgeons Annual Scientific Congress in May. For background, see "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived V News page. In addition, Princess Alexandra's presentation dataset also included some durability of response and overall survival data.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
- Median follow-up duration: 30.7 months,
- Median duration of complete response (CR): 6.1 months, and
- Median overall survival (OS): 25.5 months.
In this May presentation Dr. Read said Princess Alexandra was planning to establish an in-transit melanoma clinic as of mid-this year.
Updated (9/4/16).1: According to Balch et al., "Final Version of 2009 AJCC Melanoma Staging and Classification Data," J Clin Oncol. 2009 Dec 20; 27(36): 6199–6206, OS differs considerably by stage of melanoma. According to Song et al., "Overall survival in patients with metastatic melanoma," Curr Med Res Opin. 2015 May;31(5):987-91, in an analysis of patient data (N = 1,682) from the Surveillance, Epidemiology, and End Results (SEER) database of unresectable stage IIIB/C and stage IV (M1a, M1b, M1c) melanoma between 2004 and 2009:
Is Pfizer paying more [IP] attention to Provectus? (September 2, 2016)Updated (9/4/16).1: According to Balch et al., "Final Version of 2009 AJCC Melanoma Staging and Classification Data," J Clin Oncol. 2009 Dec 20; 27(36): 6199–6206, OS differs considerably by stage of melanoma. According to Song et al., "Overall survival in patients with metastatic melanoma," Curr Med Res Opin. 2015 May;31(5):987-91, in an analysis of patient data (N = 1,682) from the Surveillance, Epidemiology, and End Results (SEER) database of unresectable stage IIIB/C and stage IV (M1a, M1b, M1c) melanoma between 2004 and 2009:
- "Patients at stage IIIB/IIIC had a median overall survival (OS) of 24.3 months, with a survival rate of 67.2% at 1 year, 42.9% at 2 years, and 32.1% at 3 years," and
- "For patients at stage M1a, the median OS was 22.3 months, 1 year, 2 year, and 3 year survival rates were 64.5%, 40.4%, and 26.4%, respectively."
At the time of Princess Alexandra Hospital's analysis, median OS was 25.5 months, presumably of Stage IIIB-C- and/or Stage IVM1a-diseased-staged patients, compared to ~22-24 months from the Song et al. analysis.
Takeaway: Should later follow-up be conducted of the Australian patients who received PV-10 at Princess Alexandra, median OS would increase -- if patients continued to live, of course -- thus demonstrating even greater survival benefit for patients with in-transit melanoma (Stage IIIB-Stage IVM1a) over the SEER database baseline.
Updated (9/5/16).2: Several slides from Dr. Read's presentation are below.
Note for the slide above: Dr. Read: "The overall survival length interestingly was 49.3 months from the time of their in-transit melanoma diagnosis, which is longer than in some series."
Note for the slide above: Dr. Read: "So this is the really interesting part."
Note for the slide above: Dr. Read: "The median overall survival interestingly was 25.5 months, which although was only, sort of, over a five-year recruitment window started to approach what you would expect reported in the literature."
Updated (9/5/16).3: I believe the OS data from Brisbane, Australia's Princess Alexandra Hospital's (PAH's) 45-patient, special access scheme (compassionate use/expanded access program in the U.S.) experience is (a) very informative in regards to Provectus' ongoing pivotal melanoma Phase 3 trial and (b) far from "marginally better."*
I'll address (a) in a subsequent blog post or news entry. For now, my summary view is that PAH's durability and survival data complete or round out my perspective of PV-10 meeting and beating all of its trials primary and secondary endpoints.
In regards to (b), the additional PAH clinical data reinforce the following attributes of PV-10 and Provectus' CTO Dr. Eric Wachter, PhD's clinical development program:
Takeaway: Should later follow-up be conducted of the Australian patients who received PV-10 at Princess Alexandra, median OS would increase -- if patients continued to live, of course -- thus demonstrating even greater survival benefit for patients with in-transit melanoma (Stage IIIB-Stage IVM1a) over the SEER database baseline.
Updated (9/5/16).2: Several slides from Dr. Read's presentation are below.
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated (9/5/16).3: I believe the OS data from Brisbane, Australia's Princess Alexandra Hospital's (PAH's) 45-patient, special access scheme (compassionate use/expanded access program in the U.S.) experience is (a) very informative in regards to Provectus' ongoing pivotal melanoma Phase 3 trial and (b) far from "marginally better."*
I'll address (a) in a subsequent blog post or news entry. For now, my summary view is that PAH's durability and survival data complete or round out my perspective of PV-10 meeting and beating all of its trials primary and secondary endpoints.
In regards to (b), the additional PAH clinical data reinforce the following attributes of PV-10 and Provectus' CTO Dr. Eric Wachter, PhD's clinical development program:
- Consistency: As shown in the screenshot of the Excel spreadsheet table above, 155 melanoma patients (mostly Stage IIIB-C to IV M1a, but some IV M1b-c) treated with PV-10 in clinical trials and compassionate use settings have generated what appear to be biopharmaceutical industry-leading ("interesting") response rates:
- 26-50% complete response (CR),
- 48-78% [best] overall or objective response (OR) {CR + partial response (PR)}, and
- 68-93% disease control or stability {CR + PR + stable disease or disease stability (SD)}.
Response [rate] does not necessarily translate into progression-free survival (PFS), nor do OR and/or PFS translate into OS. In light of the industry (or at least some of it) acknowledging the criticality of the tumor microenvironment and/or the potential of approaches that make a cancer patient's tumor his or her friend through local-regional access/treatment (e.g., intralesional [IL] agents, radiation), however, the better and more complete the response (the higher the OR and CR rates), presumably the greater potential for success.
- Predictability: First, consistency leads to predictability, so that if I know has happened is likely to happen, not only can I predict with potential, probable or likely success the outcome of "my" clinical trial.
Second, Provectus now has, primarily as a result of Melbourne, Australia's Peter MacCallum Cancer Centre's (PeterMac's) 21-patient special access scheme experience (Lippy et al.), predictors of complete response for PV-10 monotherapy treatment are age (the younger the patient, the better) and lesion size/lesion size (grouped) (the smaller the lesion the better) -- "Younger patients and those with smaller lesions were more likely to respond to treatment,"
- Durability: How do locally- or regionally-administered therapeutics (e.g., IL agents) and therapies (e.g., radiation) compete with systemically-administered drugs and approaches to show efficacy in a systemic disease (cancer)? Physicians, skeptics, Big Pharma, etc. require more information on, among other things, the durability of the agent or treatment; that is, the length of time of the response.
We now have some data, albeit summary stuff barring a published, peer-reviewed paper, on durability (length of time) of CR from PV-10 treatment -- 6.1 months,
- "Survivability" (overall survival): In addition to durability, the industry at large requires OS data. While OS data will be collected as part of Provectus' Phase 3 trial (12-month data was collected as part of the company's Phase 2 trial), PAH's experience of 25.5 months at the time the data was reviewed already was better than many or most comparable datasets, and only has room to get better. Of course, OS data for skeptics [apparently] must be collected in as part of a randomized controlled trial (RCT), and
- Treatment "evolution:" PAH and PeterMac's data, as well as the Phase 1 and 2 melanoma clinical trials were undertaken in a different "time," and it's important to understand that limitation, when IL injection technique sophistication, PV-10 purity, and injection frequency were not as defined as they are now. My underlined emphasis below.
Courtesty of, and with great thanks to regular hatter @bradpalm1: "The first thing that strikes me here is the timeframe of the study which ran from 2008 through 2015. Can’t recall the context of how PVCT was doing early PV-10 trials back in 2008, but it’s certain that the IL injection technique, the purity of PV-10 and the frequency of injections wasn’t as defined as it is now. They also point out the diverse phenotypic characteristics of these lesions (nodular, bulky, macular, palpable, non-palpable) which points to the relative lack of sophistication of the IL technique over this time. For example, how do you know you’re injecting PV-10 into the body of the tumor if it’s non-palpable in the deep subcutaneous fascia or has varying degrees of width/consistency?
They also state that median treatments for each patient was 2 (range 1-4) which seems low for the management of older and previously treated Stage 3/4 patients. Also, if the mortality rate “at time of review” was 48.9% (50% already dead?) then they apparently reached median OS just before the time a decision for a second injection could’ve been made. If so, I don’t know how the median OS could change much although the mean OS would obviously be extended as those patients who received their 2nd, 3rd and 4th injections responded favorably. I think that the best one could say is the results of this study appear consistent with the results from previously early studies, but how efficiently was anyone performing IL injections of PV-10 back starting back in 2008?"Takeaways:
- PV-10's consistent, predictable, durable, "survivable" results pretty much "guarantee" (although there are no guarantees) the success of Provectus' pivotal melanoma Phase 3 trial, and
- Given what Eric and clinicians now know about PV-10 treatment, and with better PV-10, the results of the trial are likely to be better.
"With OS only 25.5 months, this is only marginally better than the overall results of 22-24 months. We are not told when the patients were treated, but the study went on for several years and a good number of the patients were probably treated much more than 2 years ago. Even if the patients are being followed up, and I presume they are, the figures are not likely to improve that much.
To me, this is a disappointing result, but not a disaster, It just makes PV-10 no worse than whatever the other patients received in terms of effectiveness, and probably much better in other terms such as cost and even quality of life.
If I am missing something, please fill me in."
Updated below: 9/2/16 {thrice}.
Takeaways:
- The growing turf war in immuno-oncology (IO), or for immunotherapy combination or cocktail regimens, very likely is for the front-end (i.e., primer, stimulatory agent) of the treatment,
- Immune checkpoint inhibitors (e.g., anti-PD-1s, anti-PD-L1s) are very likely to be interchangeable or insufficiently differentiated from one another,
- Assuming a good enough front-end (primer) to the back-end (checkpoint inhibitor), market differentiation may boil down to, among other parameters, the strength of a Big Pharma's marketing department and combination/cocktail therapy pricing,
- All but one Big Pharma may see their market capitalizations shrink or be permanently impaired if one of them finds a perfect or more perfect primer to pair with their checkpoint inhibitor.
In March 2015 Provectus and Pfizer were awarded (i.e., both were co-assignees) a patent combining PV-10 with other classes and categories of drugs, "Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer" (the "parent"). In June 2015 Provectus filed three other patent applications, "daughters" of the aforementioned parent. Two daughters were co-filed with Pfizer and related to systemic immunomodulatory anticancer agents, here and here. The third daughter filed solely br Provectus related to a systemic targeted anticancer agent, here.
 |
| Click to enlarge. |
According to Provectus management, Pfizer only would gain economic benefit from its "ownership" of the patent(s) if it were to acquire Provectus. That is, it would appear, whoever buys Provectus gains control of and economic benefit from the combination therapy patent(s).
All three daughter applications received a non-final rejection from the US PTO this past spring:
- '165 in April (Pfizer co-),
- '309 in May, and
- '318 in June (Pfizer co-).
In early-August Provectus advanced '309 (PV-10 + targeted cancer therapies) by providing a response to the US PTO's non-final rejection action through revised claims. See a screenshot below from this applications PAIR webpage.
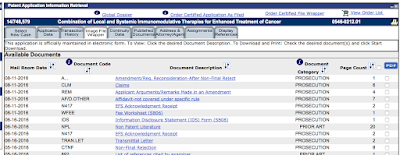 |
| Click to enlarge. |
In late-August Provectus, and it would appear Pfizer, advanced '318 (PV-10 + immunomodulatory cancer agents) by providing a response to the US PTO's non-final rejection action through revised claims and that included a Pfizer-filed terminal disclaimer. See a screenshot below from this applications PAIR webpage.
 |
| Click to enlarge |
Regarding a terminal disclaimer:
"In the obviousness-type double patenting, a first patent or application has at least one claim which, though not identical to any claim of the second patent or application, is an obvious variation and would not be patentable were one citable as prior art against the other. In this situation, there is no effort to patent the identical invention in two separate patents. The potential harm is that by having claims that are obvious variations of each other in two separate patents, the expiration of the first patent results in an improper extension of the patent rights due to the unexpired second patent. The obviousness-type double patenting objection may be overcome by filing a "terminal disclaimer." This is a written statement by the owner stating that the owner has disclaimed the period of the second issued patent that would extend beyond the expiration of the first patent. This terminal disclaimer avoids the double patenting objection only so long as the patents are commonly owned." (Source: "Double Patenting—One Patent per Invention," Arnold B. Silverman)Pfizer's work on '318:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated (9/2/16).1: "What is a terminal disclaimer?," Williamson Intellectual Property Law
"When an inventor already has a patent issued on an invention and files another patent application on essentially the same invention, only making changes deemed obvious, the USPTO will likely require that the applicant sign a binding agreement to limit the second patent, referred to as a “terminal disclaimer.” In general terms, the disclaimer will state that the later patent will expire at the same time as the former one, and will further state that the later patent will be enforceable only if both the first and second patents remain commonly owned. A quick example would be as follows: Patent A is issued and later Patent B is issued to the same inventor with a terminal disclaimer attached. Under these circumstances, Patent B will have the same expiration date as Patent A, even though it was filed later."
And: "Tips For Avoiding Problematic Terminal Disclaimers," Portfolio Media
"The Federal Circuit’s recent decisions in STC.UNM v. Intel Corp.[1] and In re Dinsmore[2] illustrate dangers associated with filing a terminal disclaimer to overcome a nonstatutory double-patenting rejection. In each case, the appellant tried to address the requirement that the terminally disclaimed patent had to be commonly owned with a patent owned by another party in order to be enforceable. Unfortunately, the appellants found that the options for addressing the common ownership requirement are very limited once the patent has issued. In each case, the problematic terminal disclaimer might have been avoided through more careful prosecution.
STC.UNM v. Intel Corp.
In STC.UNM v. Intel Corp., the licensing arm of the University of New Mexico (UNM) sued Intel Corp. for infringement of U.S. Patent No.6,042,998 (’998 patent), which was terminally disclaimed over U.S. Patent No. 5,705,321 (’321 patent). After the suit commenced, discovery revealed that it the ’998 patent was unenforceable because it was not commonly owned with the ’321 patent: Although the patents had inventors in common, UNM owned the ’998 patent solely and the ’321 patent jointly with Sandia Corporation. To remedy this problem, UNM assigned an undivided interest in the ’998 patent to Sandia so that both patents were jointly owned by Sandia and UNM, rendering the ’998 patent enforceable." {my bolded and underlined emphasis}
Potential takeaway: Pfizer may have undertaken the terminal disclaimer filing to make '318 enforceable if and when approved.
Updated (9/2/16).2: Provectus also filed a terminal disclaimer; see below. This joint action would suggest the parties are in conversation at the very least about advancing this IP asset and process.
 |
| Click to enlarge |
Updated (9/2/16).3: The Applicant Arguments/Remarks Made in an Amendment document submitted on August 31st as part of advancing the patent application beyond the non-final rejection (for multiple reasons, including double patenting) noted in regards to the filing of the terminal disclaimers by Provectus and Pfizer:
 |
| Click to enlarge |
 |
| Click to enlarge |
Immune System Photo Op (September 1, 2016)
Updated below: 9/1/16 {thrice}.
H/t the photo below, InvestorVillage poster Jack Russel, from left to right: Provectus COO and interim CEO Peter Culpepper, Melbourne, Australia's Peter MacCallum Cancer Centre's Dr. Grant McArthur, MD and [I think] a Provectus vendor [who I believe I know] at the 16th World Congress on Cancers of the Skin/12th Congress of the European Association of Dermato-Oncology in Vienna, Austria.
 |
| Click to enlarge. Purple arrows are mine. Original picture source |
- Purple arrow (new) pointing down: Noting lead Human Research Ethics Committee (HREC) approval,
- Purple arrow (new) pointing up: Adding Russia as an anticipated trial country.
- Orange arrow (reminder, since ASCO 2016) point left: Innate AND adaptive immune systems (CD8+, CD4+ -- innate; NKT -- both innate and adaptive), and
- Orange arrow (reminder, since ASCO 2016) point right: At least one injectable lesion for Stage IV disease.
Updated (9/1/16).3: H/t the photo below, InvestorVillage poster canis_star, from left to right: Provectus COO and interim CEO Peter Culpepper, Melbourne, NYU Langone Medical Center's Dr. Jeffrey Weber, MD, PhD and a Provectus vendor at WCCS 2016. Dr. Weber had said at ASCO 2014, while working at Moffitt Cancer Center, that "PV-10 might offer the perfect way to prime the immune system."
 |
| Click to enlarge |
Updated (9/1/16).1: There is a rumor that Provectus management cut their annual salaries from $500K per manager to $300K per manager (i.e., a reduction in monthly cash burn of about $50K). If true, the information typically be made available in the 2017 annual proxy statement (Form DEF 14A), and should be apparent in the third quarter financial statements filing (10-Q) in November (upon a quarter-over-quarter analysis of certain income statement items).
Updated (9/1/16).2: I communicated with management on the topic immediately above, and will address this in subsequent blog post or news entry.
More photo ops. On the August 10th 2Q16 business update call, Peter said:
August Blog Readership Stats (September 1, 2016)Updated (9/1/16).2: I communicated with management on the topic immediately above, and will address this in subsequent blog post or news entry.
More photo ops. On the August 10th 2Q16 business update call, Peter said:
BI's a great forward-looking indicator in the way Big Pharma looks at Provectus, in my view. So, what--I'm glad you raised it because BI is one of the top pharmaceutical companies in the world. They're very well-respected in the industry. They're certainly the largest private pharmaceutical company in the world. They are well respected in the industry. They did a tremendous amount of due diligence in the data room before we entered into the formal LOI. They're well-known and well-respected, and they're forward-looking from our standpoint because they're the first company that's actually shown the ability to provide soft dollar costs that we don't see on the balance sheet, but it's invaluable to help us advance PV-10 in a meaningful way. So, in my view, they're like many other potential partners, Merck and Bristol and Pfizer with German Merck and Hoffmann-La Roche and Novartis that are very closely monitoring everything we do."H/t a regular hatter who provided the pictures below that Provectus uploaded to its corporate Facebook page, and who also noted, "in Pete's mind, this is how he sends a message:"
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. Solid tumor cancer indication likely up next. |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
From my Twitter feed for this project:
 |
| Click to enlarge. Tweet image source |
"Targeting hypoxia in cancer therapy," Wilson et al., Nature Reviews Cancer 11, 393-410 (June 2011)
"Abstract
Hypoxia is a feature of most tumours, albeit with variable incidence and severity within a given patient population. It is a negative prognostic and predictive factor owing to its multiple contributions to chemoresistance, radioresistance, angiogenesis, vasculogenesis, invasiveness, metastasis, resistance to cell death, altered metabolism and genomic instability. Given its central role in tumour progression and resistance to therapy, tumour hypoxia might well be considered the best validated target that has yet to be exploited in oncology..."Beta-catenin --
"beta-catenin signaling and cancer," Morin, Bioessays. 1999 Dec; 21(12):1021-30.
"Abstract
Since its discovery as a protein associated with the cytoplasmic region of E-cadherin, beta-catenin has been shown to perform two apparently unrelated functions: it has a crucial role in cell-cell adhesion in addition to a signaling role as a component of the Wnt/wg pathway. Wnt/wg signaling results in beta-catenin accumulation and transcriptional activation of specific target genes during development. It is now apparent that deregulation of beta-catenin signaling is an important event in the genesis of a number of malignancies, such as colon cancer, melanoma, hepatocellular carcinoma, ovarian cancer, endometrial cancer, medulloblastoma pilomatricomas, and prostate cancer..."TGF-beta --
"TGFbeta in Cancer," Massagué, Cell. 2008 Jul 25;134(2):215-30.
"Abstract
The transforming growth factor beta (TGFbeta) signaling pathway is a key player in metazoan biology, and its misregulation can result in tumor development...."HGMB1 -- Moffitt Cancer Center, "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1 [HMGB1]"
- Hypoxia & HMGB1, e.g., "Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9"
- beta-catenin & HMGB1, e.g., beta-catenin is regulated by HMGB1
- TGF-beta & HMGB1, e.g., HMGB1 is regulated by TGF-beta
Hypoxia, Rose Bengal -- See "Overview of Cell Death Mechanisms Induced by Rose Bengal Acetate-Photodynamic Therapy," Panzarini et al., International Journal of Photoenergy, Volume 2011 (2011), Article ID 713726
Rose bengal: No known resistance.
What this is about (August 28, 2016)Rose bengal: No known resistance.
 |
| Click to enlarge. Image source |
Updated below: 8/29/16 {twice}.
First, this is about helping the independent members of Provectus' board of directors understand what they have therapeutically in Rose Bengal and PV-10 (and PH-10 as well as halogenated xanthene-based analogs to come).
These board members were too busy, uninterested and/or inexperienced to learn, understand and, thus, project the immense clinical value and pharmacoeconomic proposition the drug holds for cancer patients as an immunotherapy potentially agnostic to solid tumor cancer and that possibly may be safe, effective and affordable for the entire world. I believe they are trying to be and act better, because it's never too late to be and act better (in any situation, let alone this one).
The potential to ablate (destroy) any solid tumor cancer into which it is injected and subsequently potentiate (mount, improve, increase) an immune response for any solid tumor cancer is why I previously wrote the therapeutic worth of the drug substance (Rose Bengal) and product (PV-10) has never been higher.
Second, this is about helping the independent directors better work with and manage core management to strive to maximize the worth of the drug in a [final] transaction.
President, board member and co-founder Dr. Timothy Scott, PhD has not been a real company manager for quite some time, like former Chairman, CEO and a co-founder Dr. Craig Dees, PhD. Core managers are Provectus COO and interim CEO Peter Culpepper and Dr. Eric Wachter, PhD. Dr. Scott needs to relinquish his "role" and "responsibilities" as "President," and remain as a board director.
First, in changing how the independent board members approach working with and appropriately managing (overseeing) core management, these directors should ask and answer for themselves key questions such as but not limited too:
- How much do you believe Provectus is worth?
- Why?
- How did you arrive at that number?
- What do you believe are the company’s key assets and facets?
- Why?
- How did you arrive at your rationale?
- How do you leverage these assets & facets to arrive at your figure of worth?
- Does your figure match management's? If so, why? If not, why not?
- How does current core management try to get their, your or some intermediate figure?
There is more heavy lifting to do before all of us can sleep, but the opportunity of course still exists for the current situation to be turned into a successful legacy (if that is important to these directors).
Second, in changing their approach, the independent directors should establish different guiding principles to whatever they have had in the past (which, at least to me, has appeared to be pretty much nothing of import, quality or quantity), such as but not limited to:
- Establishing a culture of categorization: “Need to have,” “nice to have,” and “don’t need,”
- Prepare and present short-, medium- and long-term actionable steps; a key goal should be to smartly reduce the monthly burn by as much as possible as quickly as possible
- Establish a culture of regularly substantive communications, and
- Understand how the clinical development program supports the worth of the business
- Evaluate how the business strategy can achieve such worth
- Evaluate how the business tactics implement said strategy
- Establish a culture of accountability and collaboration
- Break down silos
- Healthily challenge and debate the assumptions, strategy, expectations-setting, and performance of the clinical development program
I do not believe there is a whole lot of subjectivity to what I wrote above. There are clear deficiencies in how Peter and Eric undertake their some of their work (corp/biz dev, clinical development/R&D), and these need to change, starting "now." I'll address detail in a subsequent blog post or new entry.
Generally speaking, I'm not uncomfortable with Eric proceeding with his clinical development program in the way he is doing, nor am I uncomfortable with Peter becoming acting CEO. If Provectus' board could have found a suitable CEO candidate, they would have by this time. But they can't because the company has not "won," and when it does there will be no end to list of accomplished or unaccomplished people interviewing for the position. At that point Peter can move aside, or the questions might be posed, "Do we need a CEO now? Where are we in the timeline?, etc."
I have no doubt Korn Ferry is surfacing up pharma people with solid achievements on their CVs for the board to interview. If I were one of these folks, presumably smart and intelligent, traditionally experienced, hard working, etc., I'd see Provectus as a stepping stone to someplace else. If I were one of them, I'd probably want to move the company to a so-called biotech hub like Boston (Cambridge), San Francisco, etc. and rebrand it, whatever that brand might or could be. If I were one of them, I'd probably want to do a reverse split, recap the company, sell-off Rose Bengal/PV-10/PH-10 for whatever I could get and buy another investigational compound, etc..
If I were one of these Korn Ferry-supplied candidates, I probably would not know what kind of shape my NDA was in, how far along my manufacturing was, what agnostic and orthogonal mean, and/or the pharmacoeconomic Mjolnir that Provectus' investigational drugs can and will be for oncology and dermatology patients around the world, and to the global pharmaceutical industrial complex.
But, Eric and Peter have to act and be better; I think it can be objectively shown they have not too many times for the chances they have been permitted by retail shareholders who have been run over or "murdered" over the last few years. I believe I know what each of them is afraid of. For Peter, it's not being around at the end of the story to tell and write his story. For Eric, it's having someone like a new CEO of the style or lack of substance I wrote above mess with his process, which I think, for the most part, is really good (but can be improved in certain ways).
Fourth, and finally, this is about wanting, committing and never giving up helping the independent directors and core management anyway we can.
I am committed to helping the board and management anyway I can, like I know other shareholders want and are prepared to do, such as better explaining through this blog the immense opportunity for cancer patients of an immunotherapy agnostic to solid tumor cancer.
If the board and core management will not strive to be and act better, starting "now," I will not support, and would more aggressively than I ever have fight against, an increase in the number of shares outstanding if Provectus asks for it (I think they have enough shares to get to where they need to get) and push for the company to be sold instead.
I have offered several members of management and several times (recently and over the last few years) to work for the company for free, locking-up my shares in the process, and merely being reimbursed for reasonable out-of-pocket expenses (in principle, it would be by GSA standards and with no per diem). It's no insult to me to be ignored (and rejected). Just be and act better.
I believe there is substantial asset value in Provectus that of course is not recognized by its current market capitalization. As a former corporate venture capitalist in the service of SAIC employee owners, I was fortunate to garner a lot of experience investing (and generating an return on investment), and in the process undertaking clean and dirty wind downs, asset and stock sales, and winning on investments, where several times we stepped in as the lead investor to drive, achieve consensus about and make accommodations, adjustments and compromises that served everyone (management and shareholders), even if it cost us. It's just work, complex and hard work, and with the potential, initial backdrop or background of drama, before everyone is back on track to fulfilling what we all believe the investigational drug can do for patients and is worth to Big Pharma.
None of what I think and believe could and should be done is drastically out of line with what Eric and Peter are doing. Core management knows fully well the opportunity they have in an immunotherapy agnostic to solid tumor cancer. In February 2013 CancerWatch article entitled "Back to Phase 1: Understanding Systemic Effects of PV-10," Eric said:
"“Back in the preclinical days at Provectus, Craig Dees, PhD, theorized that ablation of tumors with PV-10 might lead to unmasking of tumor antigenic material. I don’t think he anticipated that it would work as well as it does.”"Yeah, it seems as if it has worked really, really well.
Eric has long wanted to see Rose Bengal and PV-10 used therapeutically as pervasively as aspirin. He has done yeoman's work (understanding his challenges and issues, which each of us has) to position the drug in the global oncology space. I can and will give Peter certain kudos (which really, simply be should about "doing your job").
The game is far from over. I could make the case it's just beginning.
Updated (8/29/16): As I've previously written, shareholders of all shapes and size have been helpful, useful and insightful on this project's journey. InvestorVillage poster STARLIGHT66 wrote:
"While Culpepper is quite dispensable, Eric is not. There is a possibility, that Eric can become so fed up and disgruntled by "stakeholders" actions that he decides to "pack it in" and leave perhaps even going to a big pharma. One might say that he would never walk away from his years of work and his monetary investment but who knows. Eric is a man of means so I believe money is not a consideration. If pushed to a point of intolerance, he might just say "fuck it" and bail. Don't think that it would happen but with this company never say never. My point is that for all actions there are always potential consequences. Just something to think about."A couple of thoughts:
- Everybody is dispensable, including Eric (a topic I'll address at a later date), and
- With no disrespect intended toward the poster, New York is a long way from Knoxville. Yes, there's always a possibility, anything could happen, never say never, Scholes and Merton could never be wrong (could they?!), etc. I periodically communicate with Eric, but of course where I live is a longer way, and communicate with folks who periodically meet with him. I'm gonna "guess" Eric isn't a quitter. There always are reactions to actions; that is the nature of physics. Diligence is important. Remaining vigilant in doing so on an ongoing basis is as well. Part of building and constantly testing an investment thesis has to be a fundamental incorporation of Eric into it. There are founders (i.e., Dees, Scott), and then there is Eric.
I think STARLIGHT66 wrote it well when he said in a later post, "In many instances, situations such as we find ourselves in are the fodder for creating reputation, heroes and legends."
Updated (8/29/16): InvestorVillage poster pvct-whale wrote:
Eric has had differences of opinion and/or clashed with key opinion leaders (KOLs) and strategic advisory board members (on the clinical side) in the past. He engages as consultants KOLs in oncology from around the world. A few years ago there were group evaluations, differences of opinions and "clashes" regarding pivotal melanoma Phase 3 trial comparators such as ipilimumab because it was approved in 2011.
Another Big Pharma consultant (he has worked for and currently works for Big Pharma, and also currently [I believe] consults to Provectus) suggested that PV-10 be marketed or referred to as a checkpoint inhibitor because the investigational drug kills tumor cells like on Step #7 of Chen and Mellman's Cancer Immunity Cycle. Interestingly, it would appear PV-10 engages both the innate and adaptive immune systems, while checkpoint inhibitors engage only the latter.
The Agnostic (August 27, 2016)Updated (8/29/16): InvestorVillage poster pvct-whale wrote:
"Eric is more the problem than Pete. Although Pete has worn out his welcome too. Most people go right to blaming Pete and he definitely has his share of the blame, but the real problem is Eric. If anyone tells you different then they don't know what they are talking about. I know for a fact that 2 important KOL's have guided Eric in what the FDA wants early on and Eric did not listen and did it his way."STARLIGHT66 wrote in response:
"...I agree with paragraph #1 and know of instances or the same instances as you explained."On the one hand, Eric has been and can be a tremendous challenge insofar as corporate development (fundraising), capital markets (setting and meeting/missing expectations) and communications are concerned. On the other hand, there is the perspective he will be heralded as one of the greatest leaders in biopharma clinical development and IP management in history.
Eric has had differences of opinion and/or clashed with key opinion leaders (KOLs) and strategic advisory board members (on the clinical side) in the past. He engages as consultants KOLs in oncology from around the world. A few years ago there were group evaluations, differences of opinions and "clashes" regarding pivotal melanoma Phase 3 trial comparators such as ipilimumab because it was approved in 2011.
Another Big Pharma consultant (he has worked for and currently works for Big Pharma, and also currently [I believe] consults to Provectus) suggested that PV-10 be marketed or referred to as a checkpoint inhibitor because the investigational drug kills tumor cells like on Step #7 of Chen and Mellman's Cancer Immunity Cycle. Interestingly, it would appear PV-10 engages both the innate and adaptive immune systems, while checkpoint inhibitors engage only the latter.
Updated below: 8/27/16.
 |
| Image source |
Conceptually, agnosticism is one of two key characteristics of Rose Bengal and PV-10 for solid tumor cancers and patients with end-stage disease. As an aside, but also very importantly, the other concept is orthogonality. Agnosticism means PV-10 can ablate any kind of solid tumor cancer, and is building towards being able to mount an immune response irrespective of solid cancer indication. Orthogonality means Provectus' drug product can be combined with several or many different categories or classes of drug or cancer treatment with (a) little-to-no clinically relevant drug-drug or drug-treatment interaction and (b) great potential to be synergistic with these drug classes or treatment types.
Think about agnosticism another way. Bristol-Myers and Merck's anti-PD-1 drugs nivolumab and pembrolizumab, respectively, are being applied to different cancer indications with varying degrees of success and failure (to shrink or destroy tumors) depending on tumor "temperature" (i.e., hot and cold tumors). For example, see 5. under Desert > Beach (July 8, 2016) below.
PV-10 has generated preclinical and clinical data showing it can ablate -- destroy by injection (antitumor cytotoxicity) -- numerous solid tumor cancers such as melanoma, breast cancer, ovarian cancer, hepatocellular carcinoma (HCC), gastric cancer, liver metastases, and sarcoma. Thus, PV-10 is agnostic to solid tumor cancer in regards to destroying tumors into which it is injected. For example, read "The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses," Maker et al., J Clin Cell Immunol. 2015 Aug; 6(4): 343.
The opportunity in front of Provectus is to demonstrate PV-10 is agnostic to solid tumor cancer in regards to mounting an immune response once a cancer tumor is injected. Any kind or type of cancer. For the company to demonstrate its multi-indication viability more so than nivolumab or pembrolizumab, PV-10 must show it can truly potentiate (increase the power, effect or likelihood of) an immune response in different solid tumor cancers. There is some preclinical and clinical data that show PV-10 is an immunotherapy -- that it can destroy via immune response (induction of antitumor immunity) -- in solid tumor cancers such as melanoma, HCC, lung metastases, and gastrointestinal cancer, and liver metastases.
This is the "good" Provectus Biopharmaceuticals.
Updated (8/27/16): The "good" Provectus should see ablative (and small molecule) immunotherapy PV-10 employed in the treatment of all solid tumor cancers before, during and after surgery; in combination with other therapeutic agents and therapies, and after all else fails. Very recent diligence of key opinion leaders would underscore the eventual widespread use of the drug; however, it needs to be approved in at least one setting.
The management of this good company fully understands where the pharmaceutical industry is moving (e.g., end-stage cancer patient treatment with combinations and cocktails using immunotherapies), hears this feedback, and wholly understands the opportunity PV-10 has as a potential or possible agnostic immunotherapy for all solid tumor cancers.
But there is a "bad" Provectus. Recall the good bank/bad bank discussions during the global financial/credit crisis of ~2007-2009:
"“Bad banks” are back. The concept is simple. The bank divides its assets into two categories. Into the bad pile go the illiquid and risky securities that are the bane of the banking system, along with other troubled assets such as nonperforming loans. For good measure, the bank can toss in non-strategic assets from businesses it wants to exit, or assets it simply no longer wants to own as it seeks to lessen risk and deleverage the balance sheet. What are left are the good assets that represent the ongoing business of the core bank.
By segregating the two, the bank keeps the bad assets from contaminating the good. So long as the two are mixed, investors and counterparties are uncertain about the bank’s financial health and performance, impairing its ability to borrow, lend, trade, and raise capital. The bad-bank concept has been used with great success in the past and has today become a valuable solution for banks seeking shelter from the financial crisis." {source: Understanding the bad bank, McKinsey & Co., December 2009}This bad Provectus suffered too long from a severe vacuum of leadership, even as its founders conceived of a very different way to treat cancer (through the patient's tumor[s]), and found a forgotten theradiagnostic that fit the bill so much better than they expected it could. Even as its so-called financial executive made incorrect choices and continues to make weak choices about capital structure (albeit now having to have inexperienced board members contribute poor decisions to the mix), and a board of directors that (politely speaking) previously was asleep now merely is sleep walking.
It is ironic to recall that several years ago Network 1 Financial's Damon Testaverde called Provectus' drug a ten and its management a two. He probably was among the first to espouse what some-to-many investors felt at the time but didn't or couldn't say.
Agnostic immunotherapy PV-10 now has the potential to be (along with the manager's clinical development program that has brought it to where it is today) a fifteen (the good Provectus), while management and the board of directors are some minus number (the bad one).
It's easy to differentiate the good Provectus from the bad, and to observe the gaping chasm between the reality of Rose Bengal and PV-10, and the perception of the company. The concept of a good Provectus and a bad one is simple. A good solution to filling the vacuum, while not complex, is complicated and requires hard work, but I believe shareholders can and should constructively and productively push back, and I think that time starts now.
Immunological “ignition switch” (August 26, 2016)
H/t InvestorVillage poster canis_star for news and link that underscore the substance of this blog news entry:
- August 24, 2016, Biothera Pharmaceuticals Expands Relationship with Merck, Enters Collaboration for Combination Cancer Immunotherapy Trials in Multiple Indications.
Necessarily related news and a link is: December 10, 2015, Biothera Announces Clinical Study to Evaluate Combination Therapy of Imprime PGG and Merck’s Checkpoint Inhibitor Keytruda in Patients with Non-Small Cell Lung Cancer.
I am not focused on the deal terms, such as number of indications, sponsor of the study, supply of pembrolizumab, and lack of disclosure of other collaboration terms because these are not what company COO and interim CEO Peter Culpepper and CTO Dr. Eric Wachter, PhD are seeking. Rather, I focused on the quote in the 2016 Biothera PR that noted (my underlined emphasis):
"Combination therapies with breakthrough medicines such as KEYTRUDA are potentially the next major advance in the treatment of cancer. We believe that Imprime PGG is uniquely suited to complement immune checkpoint inhibitor therapy and meaningfully enhance patient outcomes. The trial will assess safety and efficacy, as well as provide biomarker and pharmacodynamic data that will inform the design of potential Phase 3 pivotal studies."Thank you, Peter (first underlined statement; enhance or improve patient outcomes). Thank you, Eric (second underlined statement; biomarkers, PK, etc.; trial protocols are among a biotechnology company's crown jewels).
In Provectus' September 23, 2015 press release "Announces Initiation of Phase 1b/2 Clinical Trial to Study PV-10 in Combination with Immune Check Point Inhibitor Pembrolizumab" company COO and interim CEO Peter Culpepper noted in regard to the combination of PV-10 and checkpoint inhibitor pembrolizumab:
"This study is both scientifically and commercially important to Provectus. Scientifically, combination therapy in cancer treatment is a rapidly maturing area, where rational combination of agents is replacing the empirical approaches of the past. Commercially, this is the second of three steps that we hope will significantly strengthen our hand in negotiating a co-development transaction with an immunotherapy-focused partner. Our joint patent with Pfizer was the first; this study is the second; and the third is our immune mechanism of action clinical study, which is underway at the Moffitt Cancer Center and which has completed recruitment."The Provectus trial protocol is here.
Combination therapy started a few years ago as throwing poop on the wall to see what sticks. Naturally, it has evolved or advanced to examining the poop before one throws it, to more specifically trying to understand the baseline immunologic signalling that each party in the combination or cocktail brings to the table. Call it addressing both the innate and adaptive immune systems, as noted in Biothera's 2016 press release. Call it synergy. Call it parts of a car (e.g., brakes, gas pedal, engine, gas tank, steering wheel, ignition, etc.). Call it whatever you want.
Eric and Peter understand this (I believe), although the board does not fully but may be getting there slowly.
What is a PAMP (Biothera)? "Pathogen-associated molecular patterns, or PAMPs, are molecules associated with groups of pathogens, that are recognized by cells of the innate immune system."
What is a DAMP (Provectus)? "Damage-associated molecular pattern molecules (DAMPs) also known as danger-associated molecular pattern molecules, are host molecules that can initiate and perpetuate a noninfectious inflammatory response." And, DAMPS are recognized by both the innate and adaptive immune systems; for example, here and here.
Can do you get the deal you want or deserve? Data drive deals. In the press releases above, Biothera and Merck were exploring/going to explore non-small cell lung cancer (NSCLC), advanced melanoma (MM) and/or metastatic triple negative breast cancer (TNBC). What's the list? MM, hepatocellular carcinoma (HCC), NSCLC, colorectal cancer, breast cancer or TNBC, ovarian, prostate, neuroendocrine tumors (NETs)...
Griswoldesque (August 24, 2016)
Updated below: 8/24/16 {thrice}, 8/25/16, 8/26/16 {twice} and 8/27/16.
Provectus filed a prospectus for an as yet undefined sale of Series B convertible preferred stock and warrants (on common stock). My guess, for now:
Using a fully diluted shares outstanding number would yield a more accurate dilution figure (probably 12-16% worst case, on a base of somewhere north of 300 million shares, options and warrants).
 |
| Image source |
Provectus issued a press release in conjunction with the prospectus filing, Announces Proposed Public Offering of Preferred Stock and Warrants. As in the prospectus, no details were provided in the PR.
Provectus' COO and interim CEO noted on the August 10th 2Q16 business update call (my underlined emphasis):
Question: "...I wanted to just ask you what the projected cash need is to get to Eric's projected data read goals.
Peter: "...So, we haven't communicated with any guidance. We will want to make sure at a minimum to $6 million stockholder equity requirement that we have referred to on the New Stock Exchange NKT [sp]. We are at slightly more than $9 million at the end of Q2. So, we certainly want to continue to be above the $6 million in stockholder equity. So, that's a sixth floor that we look at from a cash standpoint. We certainly, as we know from a burn rate perspective, have a certain burn that different people have done a good job in this course. It's clear from the 10Q that we filed yesterday what the burn rate is. I think we want at a minimum get enough cash this quarter to cover a quarter burn."Some thoughts:
- The underlying security of the fundraising is a convertible preferred stock with non-tradeable warrants (on common stock). Prospectus: "There is no established public trading market for the Preferred Stock or the Warrants, and we do not expect a market to develop for the Preferred Stock or the Warrants. We do not intend to apply for listing of the Preferred Stock or Warrants on any securities exchange, and we do not expect that the Preferred Stock or the Warrants will be quoted on the NYSE MKT."
- Contrast the above approach with what I would have imagined the approach would have been in the second quarter, a sale of common stock and tradeable warrants (on common stock). At this point, it is too early to compare what might have been with what will come; however, August 2016 participants of convertible preferred and non-tradable warrants may not dump their holdings as quickly as those who participated in the July 2015 offering (of common stock and tradable warrants).
- Terms of 2016's Series B convertible preferred shares (and warrants) do not appear to be significantly different from 2012's Series A convertible preferred shares (and Series D warrants): e.g., full ratchet anti-dilution price protection, dividend rate, dividend term, etc.
- Provectus expended approximately $5 million in cash during the second quarter; see Quick: 2Q16 (August 9, 2016) below. If the amount raised is about $5 million (and that indeed is the 3Q cash expenditure figure), the company should exceed the stockholders' equity threshold when the financial statement review cycle begins again upon quarter end on September 30th.
Updated (8/24/16).2: The easiest thing would be to wait for tomorrow, or whenever the offering, announced earlier today, prices and closes before opining. Does it close tomorrow or this week, or does it take time?
It does seem odd to me, however, that Provectus' board of directors authorized Peter to pursue this Maxim-led approach, given what happened with the July 2015 Maxim-led public offering. A real director would have pushed back well in advance of the offering or, if ultimately forced for whatever self-justified reason(s) to go through with it, asked Peter to tender his resignation (for both the process and the outcome; yes, I know, Eric...). What compelling case did Peter et al. make to pursue this Maxim pathway?
 |
| Click to enlarge |
The history of the July 2015 offering (of common stock and warrans [on common stock] was this:
- A press release announcing the offering issued on June 18th,
- Pricing on June 19th, a prospectus filed with transaction details and a press release,
- Closing on June 24th and a press release,
- A murdered share price ([allegedly] by the book runner) to facilitate the transaction.
 |
| Click to enlarge |
The history of the failed or terminated October 2012 was this:
- A prospectus filed with no transaction details on September 4th,
- A deal presumably (and presumably intelligently) sought on the basis of some regulatory milestone that never came to pass (i.e., a special protocol assessment for a pivotal melanoma Phase 3 trial),
- Lots of share price volatility, and polite conversations with Maxim retail stock brokers, and
 |
| Click to enlarge. |
It's not clear to me that Maxim had enough time to plan the re-murder of the share price, unless that presciently occurred (in the context of the current offering) on August 10th. I believe that planned murder failed prior to the 6th European Post-Chicago Melanoma/Skin Cancer Meeting. But money has/had to be raised to fund Provectus through the end of the year (or two quarters worth), when several major and/or minor catalysts could well occur.
This August offering starts with Peter once again turning to Maxim (that has repeatedly slapped him with an open hand), independent board members apparently okay with using Maxim, and a prospectus filed with no transaction details. Is this a successful 2012, or a repeat of 2015?
Updated (8/24/16).3: While both Peter and Eric have no credibility in regards to either setting expectation or meeting expectations, simply put, they expect several major and/or minor catalysts or events before year-end (some of which of course could be shunted to next year). Hence what was originally planned as a two-quarter raise in late-spring has become a one-quarter-and-then-we'll-see raise. The catalysts comprise, broadly stated, in no particular order, and that we've heard before:
 |
| Image source |
- More and better media awareness,
- Novel or as yet unexplored use of the oncology drug product by key opinion leaders in their space,
- A pathway to a geographic license in another region,
- Validation of multi-indication cancer combination therapy treatment of PV-10 and an immune checkpoint inhibitor,
- More liver cancer data,
- An imbalance of events in the two arms of the pivotal melanoma Phase 3 trial.
Peter has a considerable amount with which to work. Insofar as a combo co-development relationship goes, I believe prospective partners may feel or require that PV-10 prove it can be a potentiator of immune response across several cancers. I can understand where he is coming from when it comes to a potential (but maybe not probable) interim analysis prior to year-end. What if other Australian clinical sites (i.e., Peter MacCallum, Royal Adelaide, Melanoma Institute Australia -- all compassionate use program/special access scheme sites) are enrolling and treating patients under the auspices of central site Princess Alexandra?
Without consideration of these additional locations:
 |
| Click to enlarge. |
Consideration of these locations:
 |
| Click to enlarge. |
In both cases enrollment rate matters.
Updated (8/25/16).4: Provectus priced its public offering late-yesterday/today, Announces Pricing of Public Offering to Raise $6 Million. As I initially thought (see the initial entry under this blog news item), dilution should be approximately (a) 23% on a (non-fully diluted) total shares outstanding basis and (b) 17% on a [rough analysis] fully diluted basis.
Updated (8/25/16).4: Provectus priced its public offering late-yesterday/today, Announces Pricing of Public Offering to Raise $6 Million. As I initially thought (see the initial entry under this blog news item), dilution should be approximately (a) 23% on a (non-fully diluted) total shares outstanding basis and (b) 17% on a [rough analysis] fully diluted basis.
 |
| Click to enlarge. Rough analysis |
Updated (8/26/16).5: Some retail investors/blog readers were concerned, and some folks from the Network 1 Financial orbit were "warning," about the "toxicity" of the offering's conversion provision or clause (my underlined emphasis below):
"The Preferred Stock is convertible into shares of Common Stock at a conversion price equal to $0.25 per share, subject to adjustment as provided in the Certificate of Designation, at any time at the option of the holder prior to the fifth anniversary of the date of issuance, at which time all shares of outstanding Preferred Stock shall automatically and without any further action by the holder be converted into shares of Common Stock at the then effective conversion price, provided that the holder will be prohibited from converting Preferred Stock into shares of Common Stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the total number of shares of Common Stock then issued and outstanding. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days after such notice to the Company.
The Preferred Stock is subject to full ratchet anti-dilution price protection upon the issuance of equity or equity-linked securities within 60 trading days of the date of issuance of the Preferred Stock at an effective Common Stock purchase price of less than the conversion price then in effect, subject to certain exceptions as provided in the Certificate of Designation. In addition, if the conversion price in effect on the 60th trading day following the date of issuance of the Preferred Stock exceeds 85% of the average of the 45 lowest volume weighted average trading prices of the Common Stock during the period commencing on the date of issuance of the Preferred Stock and ending on the 60th trading day following the date of issuance of the Preferred Stock (as adjusted for stock splits, stock dividends, recapitalizations, reorganizations, reclassification, combinations, reverse stock splits or other similar events during such period) (the “Adjusted Conversion Price”), then the conversion price shall be reset to the Adjusted Conversion Price and shall be further subject to adjustment as provided in the Certificate of Designation. In either case, if a holder of Preferred Stock converts its shares of Preferred Stock prior to any such price reset event, then such holder will receive additional shares of Common Stock equal to the number of shares of Common Stock that would have been issued assuming for such purposes the Adjusted Conversion Price were in effect at such time less the shares issued at the then Conversion Price (subject to being held in abeyance based on beneficial ownership limitations); provided, however, that only the initial purchaser of Preferred Stock and Warrants in the Offering will receive the benefit of such price protection and such issuance of shares of Common Stock upon a price reset event."My thoughts:
- "Full ratchet" anti-dilution is of course the most severe of the different kinds of dilution protection (e.g., weighted average, no price-based dilution) (more information on this, but from a venture capital perspective is here),
- "Full ratchet" protection only applies to the preferred stock, and not the non-tradeable warrants of the offering, unlike in 2012 when the protection applied to both the Series A convertible preferred and the Series D warrants (on the preferred stock),
- There is a timeframe noted above for when the dilution protection dissipates of 60 trading days, which in capital markets parlance is about 3 calendar months (i.e., roughly 20 trading days per month), and
- As noted by InvestorVillage poster STARLIGHT66, the trading/investment style and strategy of the Maxim clients who may have made up some to most to all of the offering was/is to convert their preferred stock holdings into common stock as quickly as possible in order to subsequently sell the common shares as quickly as possible.
It would seem unlikely that preferred stock folks of a "Maxim-type" would be waiting around for the full ratchet to kick in. The approach of these folks, from a style and strategy perspective, is distinct and "event driven," where the event is the offering. Retail investor concerns are appropriate, and diligence is warranted. Network 1 folks' "toxic" comments are more related to their anger of having their clients be cut off at the knees for a second time (where the first time was the July 2015 Maxim-led offering).
Of course, the board of directors and Provectus COO and interim CEO Peter Culpepper could explain why they decided for a second consecutive time to go with Maxim clients that hold the aforementioned investment style and strategy rather than more supportive prospective shareholders. They are out there. You do have to work to bring them on board of course. Once a deal guy or gal, always a deal guy or gal. Never a deal guy or gal, not a deal guy or gal.
Updated (8/26/16).6: In writing some of the above (update.5) I was incorrect, and thus embarrassed myself with the poor quality of the underlying analysis; there are inexcusable excuses (e.g., a cross country road trip, the start of school, etc.). I realize I completely whiffed on my understanding of (or belief that) the offering as being soft- or hard-circled in so far as the money for a fundraising was accounted for (i.e, raised). Now that is stupid.
A "best efforts" fundraising (e.g, a definition is here), or proposed offering (the PR read Announces Pricing of Public Offering to Raise $6 Million), as a label or issue, is similar to the "going concern" label or issue painted over Provectus' second quarter 10-Q filing. As such, the drop in share prices around or after both events makes sense. People don't believe the company can cross the finish line. I realize I also misread or misunderstood InvestorVillage poster STARLIGHT66's post linked above, which (after making a few phone calls) I better get in terms of how much has been raised under the Maxim-led offering to date (i.e., at least a couple of million dollars). He obviously is correct in then writing that we await the closing of the transaction to understand or confirm how much indeed was raised. I also understand Network 1 Financial folks' anger (but not necessarily their rhetoric) presumably because of their belief they had the money Provectus needed. Hence, my comments regarding Maxim clients, style and strategy, etc. in the context of this offering are not correct, and thus not appropriate.
There is the larger issue that I raised above (and that has always been there as many folks know and understand) regarding the board and Peter undertaking competent, thoughtful, effective, coordinated, respectful, singing-from-the-same-page, etc. fundraising, which I will go into more detail in an upcoming blog post, that remains, and I doubt will change (and only and until it does). But for now, some of my analytical work was poor, as noted above.
Updated (8/27/16).7: With the filing of an 8-K (and associated exhibits) on Thursday and a finalized prospectus on Friday, Provectus should have the money it intended to raise through this most recent Maxim-led offering. Making two central assumptions to projecting the runway this cash may provide, (a) $3-4 million in assets (ex-cash and cash equivalents) minus liabilities and (b) $1.2-1.6 million per month in cash burn, total stockholders' equity may require no further fundraising this year, or fundraising prior to year-end.
Updated (8/26/16).6: In writing some of the above (update.5) I was incorrect, and thus embarrassed myself with the poor quality of the underlying analysis; there are inexcusable excuses (e.g., a cross country road trip, the start of school, etc.). I realize I completely whiffed on my understanding of (or belief that) the offering as being soft- or hard-circled in so far as the money for a fundraising was accounted for (i.e, raised). Now that is stupid.
A "best efforts" fundraising (e.g, a definition is here), or proposed offering (the PR read Announces Pricing of Public Offering to Raise $6 Million), as a label or issue, is similar to the "going concern" label or issue painted over Provectus' second quarter 10-Q filing. As such, the drop in share prices around or after both events makes sense. People don't believe the company can cross the finish line. I realize I also misread or misunderstood InvestorVillage poster STARLIGHT66's post linked above, which (after making a few phone calls) I better get in terms of how much has been raised under the Maxim-led offering to date (i.e., at least a couple of million dollars). He obviously is correct in then writing that we await the closing of the transaction to understand or confirm how much indeed was raised. I also understand Network 1 Financial folks' anger (but not necessarily their rhetoric) presumably because of their belief they had the money Provectus needed. Hence, my comments regarding Maxim clients, style and strategy, etc. in the context of this offering are not correct, and thus not appropriate.
There is the larger issue that I raised above (and that has always been there as many folks know and understand) regarding the board and Peter undertaking competent, thoughtful, effective, coordinated, respectful, singing-from-the-same-page, etc. fundraising, which I will go into more detail in an upcoming blog post, that remains, and I doubt will change (and only and until it does). But for now, some of my analytical work was poor, as noted above.
Updated (8/27/16).7: With the filing of an 8-K (and associated exhibits) on Thursday and a finalized prospectus on Friday, Provectus should have the money it intended to raise through this most recent Maxim-led offering. Making two central assumptions to projecting the runway this cash may provide, (a) $3-4 million in assets (ex-cash and cash equivalents) minus liabilities and (b) $1.2-1.6 million per month in cash burn, total stockholders' equity may require no further fundraising this year, or fundraising prior to year-end.
 |
| Click to enlarge. |
Updated below: 8/18/16.
On Provectus' 2Q16 business update call, company CTO Dr. Eric Wachter, PhD discussed the different solid tumor cancers for which Provectus had basic-to-advanced immunology data: melanoma, colorectal cancer, breast carcinoma, and hepatocellular carcinoma (HCC). You can begin to see this on the slide below that I developed for the company, which was first displayed at June's The BIO International Convention (Provectus' COO and interim CEO Peter Culpepper delivered the presentation):
 |
| Click to enlarge. |
Eric also said on the call in regards to HCC (with my underlined emphasis):
"We already know that we can destroy HCC with this ablative process and the hypothesis that that should lead to similar signaling, which can have implications for--well, single-agent therapy [unintelligible] HCC, but more importantly for combination with things like anti-PD-1 [unintelligible]. We have already shown that the basic immunology occurs in HCC models, so I would say that--one of the things I’m highly confident in, I’m highly confident that we will show that this same functional immune signaling functions in HCC."When Eric says the company already has shown basic immunology for HCC with PV-10, I believe he is referring to, in part or in whole (since Eric of course has not disclosed all work he has sanctioned), previous work by former Chairman and CEO and a co-founder Dr. Craig Dees, PhD. Some slides from a 2013 presentation by Craig describe HCC immunology; that is, inject an HCC tumor (the primary tumor) with PV-10 and an uninjected HCC tumor (the secondary tumor) also responds. Contralateral below should mean the other side or flank of the mouse; the primary tumor is on one flank, and the secondary tumor presumably is on the other.
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated (8/18/16): I re-read a paper by the University of Illinois at Chicago (UIC) on PV-10, "The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses," Maker et al., J Clin Cell Immunol. 2015 Aug; 6(4): 343. First, in regards to HCC immunology data, and the similarities Eric noted between melanoma and HCC (2Q16 business update call: "Our next tumor on the radar will be hepatocellular carcinoma because there are challenges in HCC that are comparable to those in melanoma), the authors note:
"Furthermore, in syngeneic orthotopic models of murine hepatocellular carcinoma (HCC), RB similarly induced chemoablation in all treated tumors. Twenty-one to 81 days after RB treatment, when re-challenged with the same tumor, durable immunity was demonstrated in 14/14 animals without measurable tumor formation, whereas B16-F10 melanoma tumors, i.e., non-HCC cells, were able to be established in 13/13 animals [20]. Additionally, immunity to establishment of a new HCC tumor could be created through adoptive transfer of splenocytes from treated animals. This was an interesting finding given that analysis of splenic composition from animals with B16 melanoma that experienced distant lung tumor regression after RB treatment of flank tumors did not demonstrate any difference in the percent of T cells, Tregs, NK cells, B cells, myeloid derived suppressor cells, or macrophages [15]. The authors further demonstrated that bystander lesions disappeared or decreased in size in HCC models, whereas, no bystander tumors were ever observed to resolve in nude mice without a competent T-cell immune system. These experiments established that, similar to melanoma, in immunocompetent mice with orthotopic primary hepatocellular carcinoma flank tumors, an anti-tumor response can be induced by priming the animals with RB treatment of tumors. These experiments raise the possibility that RB induced cytotoxicity exposes antigens and mounts an immune response that may protects animals from additional tumor formation that can be adoptively transferred to other animals using splenocytes."The relevant slides from Craig's presentation above relate to work presented by Provectus at SITC 2012.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Your Rose Bengal protection [of its therapeutic use] team at work (August 17, 2016)
Tweet image:
The above paper, THE LIBERATION OF ADSORBED SUBSTANCES FROM PROTEINS. A FUNCTION OF THE BILE SALTS: I. PRELIMINARY REPORT, JPET July 1925 vol. 25 no. 6 449-457:
"Abstract
Ultrafiltration studies have shown that rose bengal and bromsulphalein, dyestuffs that are excreted in the bile, and bilirubin are in vitro completely bound to the proteins of the blood. Sodium taurocholate exerts a marked activity in the liberation of these substances from the proteins.
Phenolsulphonephthalein circulates in the blood partly bound of the proteins. Bile salts are also capable of freeing the bound portion of this dye, so that in vitro it becomes almost entirely diffusible.
Sodium taurocholate possesses the property of increasing the degree of permeability of semipermeable collodion membranes to dyestuffs.
These properties of the bile salts indicate their physiological function in the liberation from a bound state of substances that are combined with the body proteins, and suggest a further influence upon membrane permeability."Another related paper by Rosenthal, THE COMBINING POWER OF PROTEINS WITH ROSE BENGAL: II. APPLICATION AS A QUANTITATIVE TEST ON THE SPINAL FLUID, Arch Intern Med (Chic). 1926;38(4):527-535:
"In a previous article1 a method was described whereby the adsorption of rose bengal B (tetrachlortetraiodofluorescein) on proteins could easily be determined in a quantitative way. This dye stuff attains its maximum rose color at pH 4.8 or above, but it behaves as an indicator dye and is decolorized on adding acid; dilute aqueous solutions are practically colorless below pH 2.2. If proteins are present the dye will combine with them in the form of its colored salt, and on bringing the acidity of the solution to pH 2.2, that portion of the dye in combination with the proteins retains its color, while the free dye is decolorized. The color that remains on acidifying the solution therefore represents the adsorbed dye and it may be easily estimated by colorimetric comparison with a standard solution of rose bengal of known strength."Consider the below from the above paper:
 |
| Click to enlarge. |
"This critical review evaluates progress toward viable point-of-care protein biomarker measurements for cancer detection and diagnostics. The ability to measure panels of specific, selective cancer biomarker proteins in physicians’ surgeries and clinics has the potential to revolutionize cancer detection, monitoring, and therapy."How relevant are the laws of adsorption as applied to Rose Bengal when combined with protein (or protein particles) for the early detection of cancer biomarkers that might provide predictive or prognostic information for a Rose Bengal-based combination therapy?
Institutional (August 16, 2016)
Updated below: 8/16/16 {twice}.
13F filings (through today) for the period ending June 30, 2016 showed:
- A decrease in institutional share holdings of Provectus: 8.45 million shares (down from 9.03 million as at 3/31/16, or about -8% quarter-over-quarter [QoQ]) and 4% of shares outstanding (not a fully diluted figure) (down from 4.2%), and
- A decrease in the number of filers to 49 (down from 51).
 |
| Click to enlarge |
 |
| Click to enlarge |
Interestingly, of the presumed firms and funds that bought the Maxim-led June 2015 public offering of common stock and tradable warrants, all but Wharton graduate's Ms. Bihua Chen's Cormorant Asset Management retained their original position in both the common stock (PVCT) and warrants (PVCT/WS or PVCT.WS). Millennium management retained their original position in the warrants. Cormorant increased its reported, quarter-end, common stock ownership in 2Q16 by 1.2% QoQ. Sabby Management sold most of its remaining common stock position (~-87% QoQ). It is possible, however, that Cormorant sold some-to-most-to-all of their position in the current quarter (and, particularly, last week).
I'll take this opportunity to further encourage Provectus management and board of directors that the company (both the board and management), who have had no meaningful experience in their professional backgrounds regarding raising money and/or have not known how to raise it, to try to act better going forward.
As management, raising money is not about picking up the phone and calling investment bankers for money or leaving it to them to do your jobs (plural) (and thus merely checking a corporate development box, or perfunctorily if at all helping another person's domain [because to be successful you have to consider that yours is his, and his is yours]). As a board, raising money is not about waiting for management to come to you.
Fundraising of all sorts is about so much more; from understanding and forecasting wants and needs in concert with others, to developing and updating a pitch deck in concert with and with input from others, to considering and speaking to new players in new geographies as much as to those to whom you've already spoken, to establishing and following through with a sufficient time runway to do it that also ensures no one is rushed yet also having everyone act intelligently and promptly, to...
To all manner of folks, ranging from those who may not have highfalutin professional investment experience to those who may have been on both sides of the table (like me), you, management, and you, the board, have historically behaved and acted, in this regard of fundraising (irrespective of your individual and collective cumulative excuses), in an embarrassingly unproficient manner considering the potentially immense clinical value of the drug substance and product you all have been developing.
Please, from here on out, let's roll...
Updated (8/16/16): Irrespective of whether you've taken money from others for a small business, or whether you raised it "professionally" (e.g., venture capital, private equity, initial public offerings, PIPEs [private investment in public equity], etc.), how you approach raising money is instructive about how you might treat or spend money; how you treat or spend money is instructive about how you might raise it.
Updated (8/16/16): Factless is as factless writes (1/2).
 |
| Image source |
Dr. Sally Church, PhD wrote a Biotech Strategy Blog (BSB) post entitled "Beyond T-Vec - a look at oncolytic viral immunotherapy." Dr. Church does not appear to be either an innovator or an early adopter (as labeled on the technology adoption life cycle). Rather, her professional experience, among other things, biases her (which is neither "right" nor "wrong") towards early or late majorities (I'd lean towards early).
I think it is worth paying attention when she begins to opine on a newer or novel category of drug. In the case of oncolytic viruses (OVs) (she does not categorize them explicitly as intralesional or intratumoral presumably because OVs have been explored via both intralesional and intravenous administration), she is commenting as the majorities begin to pick up on what the innovators and early adopters (e.g., Agarwala, Andtbacka, Weber, etc.) have been saying for a while. She is, however, rightfully wanting to know more about durability of responses and survivability, which these innovators and early adopters also have been wanting to see as well.
Two thoughts crystallized quickly [in my head] but only after reading Dr. Church's blog post. The first one is "factors," as represented by the screenshot from her post below, and which is the subject or focus of this blog news entry.
 |
| Click to enlarge. Image source |
At a fundamental level the only features PV-10 and talimogene laherparepvec/T-Vec/Imlygic® have in common are (a) the route of administration (i.e., both drugs are injected into cancerous lesions and tumors) and (b) some of the apparent effects on CD8+ T cells (i.e., both drugs have been shown to increase CD8+ T cells in peripheral blood).
Imlygic's prescribing information/packaging insert notes:
"12.1 Mechanism of Action
IMLYGIC has been genetically modified to replicate within tumors and to produce the immune stimulatory protein GM-CSF. IMLYGIC causes lysis of tumors, followed by release of tumor-derived antigens, which together with virally derived GM-CSF may promote an antitumor immune response. However, the exact mechanism of action is unknown."The need to inject T-Vec into the target (same) lesion or tumor multiple times highlights a key difference between PV-10 and Imlygic (i.e., the direct and immediate effect on injected tumor tissue vs. an unknown mechanism of action on an injected tumor). Provectus has shown that PV-10 requires just 1 to 2 injections -- Thompson et al. (ASO 2015), "Onset of response occurred at a median of 1.9 months, corresponding with the first assessment of response" -- whereas Andtbacka et al. (JCO 2015) reported, "Median duration of treatment in the T-VEC and GM-CSF arms was 23.0 weeks (range, 0.1 to 78.9 weeks) and 10.0 weeks (range, 0.6 to 72.0 weeks), respectively" or 12 cycles of T-Vec.
It will be interesting to see how the OV field plays out now that there are other agents based on different viruses advancing through clinical development, as Dr. Church notes in a table on her post (a screenshot of which is below).
 |
| Click to enlarge. Image source |
Updated (8/16/16): I find following Dr. Church for this project very helpful, notably and primarily for for her Big Pharma mindset perspective, and for her perspectives on clinical trial design.
As an update this entry, first, consider she said Stage III patients see dermatologists rather than [medical] oncologists. Dr. Church is from the U.K. where this may be the approach. Provectus' CTO Dr. Eric Wachter, PhD is of the opinion, which is consistent with my due diligence on the topic, that in the USA and Australia, surgical oncologists traditional see Stage III patients. In Germany (and certain parts of continental Europe), it’s dermato-oncologists; see here, for example. In some parts of the world, it indeed would be dermatologists. But as Eric noted to me, everywhere, Big Pharma mostly still is oriented toward addressing the needs and wishes of medical oncologists.
Second, consider who Dr. Church decided to interview as part of the second and third parts of her BSB series on OVs, the second part being entitled "Can PsiOxus become a world leading immuno-oncolytic virus company?" PsiOxus is a UK-based biotechnology company that intravenously (IV) administers its OV (adenovirus) enadenotucirev (EnAd). Big Pharma, like Dr. Church, is slowly waking up to the evidence that the doorway to potentially making cures routine (rather than expecting occasional cures in this current era as we do now; phraseology source Dr. James Allison, MD, The Vatican regenerative medicine meeting, April 2016), is/is through the tumor.
It would appear that Dr. Church right now may believe IV administration is the appropriate delivery system for OVs (PsiOxus also has examined intralesionally (IL)/intratumorally (IT) delivering its investigation drug compound -- see IV/IT mechanism of action study -- but appears to believe IV is better/more efficacious than IV).
In December 2015 PsiOxus initiated a combination therapy trial for IV OV enadenotucirev and anti-PD-1 immune checkpoint inhibitor pembrolizumab (Keytruda) in certain tumour types, including metastatic colorectal cancer. To date one clinical site is recruiting patients.
In June 2016 Bristol-Myers and PsiOxus "agreed to an exclusive clinical collaboration to assess the safety, tolerability, and preliminary efficacy of the combination" of enadenotucirev and anti-PD-1 immune checkpoint inhibitor nivolumab (Opdivo) (Phase 1 studies, no tumor types as yet mentioned or that I could find). Deal terms included:
"BMS agreed to make a one-time $10 million upfront payment to PsiOxus, while the companies agreed to share development costs. PsiOxus will oversee conducting the Phase I study...
The companies said they also agreed to work exclusively with each other on anti-programmed death-1/anti-programmed death ligand-1 (anti-PD-1/PD-L1) antagonist antibody and enadenotucirev combination regimens, with BMS having a time-limited right of exclusive negotiation for commercial rights to enadenotucirev."Updated (8/19/16): Some existing shareholders may overlook or ignore in their frustration that Provectus' core management and independent members of the company's board of directors are doing the right thing in so far as Provectus COO and interim CEO Peter Culpepper's fifth pillar (of the company's business strategy goes -- "clinical study designs that generate randomized, pivotal, and otherwise significant clinical data to support potential approval of PV- 10 and PH-10 for their respective indications." {The update below should have been part of the one above but an Internet access glitch appears to have split them.}
Updated (8/20/16): To use a historical political phrase merely for effect, Dr. Church was "for" PV-10 (in November 2010, melanoma Phase 2 trial results, Melanoma 2010 Congress, Sydney, Australia) before she was "against" it (in October 2014, following denial of breakthrough therapy designation (BTD) in May).
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
I thought Eric's comments on the 2Q16 business update call regarding some Provectus history was notable:
"And I believe there is a representation of this on the company website, which shows that over the last several years we have worked with colleagues at Moffitt Cancer Center, we have done some work internally, and we have done some work at the University of Illinois-Chicago to show that PV-10 unambiguously triggers that first step, the destruction of tumor. And that event performs all of the expected downstream signaling of the immune system, leading eventually to a functional immune response against an untreated tumor.
So, when we started this work in Australia in the clinic in 2005, we described that as a an bystander response, which was the common terminology at the time. Immuno-oncology was not particularly well regarded at that period. It got to be even less of an important area of investigation as we approached the end of the first decade of the 21st century. And then, it became a very hot area with the approval of anti-CTLA drug Urvay [sp] and subsequent approvals of a number of anti-PD-1s and presumably eventually anti-PD-L1s, all drugs that harness T cells to have a functional--or improve their functional response against tumor tissue.
So, I think that the story is now very well documented in the literature. We have shown that this occurs in [unintelligible] models of melanoma. We have shown this occurs in [unintelligible] models of colorectal carcinoma. We have evidence to show that this happens in [unintelligible] breast carcinoma. And, most importantly, we have shown that key elements of this signaling are occurring in melanoma patients. That was one of the key aspects of that work with Moffitt. I have touted them many times."2005; c. 2010; now.
Despite the industry seemingly moving in Rose Bengal and PV-10's direction -- e.g., co-stimulation, keys, gas pedals, tumor microenvironment, making the tumor your friend, etc. -- it's would've been difficult not to stop paying attention to Provectus. BTD denial for most folks was about the drug not working rather than a paucity of data. See March 29, 2016 blog post Trying to Understand Provectus' Clinical Development Program (e.g., trial design, protocols, data, etc.). Lawsuits and overt bad management behavior certainly don't help. Ineffective communications for whatever reason(s) don't help. I could go on...
There is no reason for anyone who makes a living off of writing about biotechnology companies to talk about Rose Bengal, PV-10 and Provectus. It doesn't "pay" monetarily, but much more importantly reputationally to write about this unique, novel small (but heavy) molecule and its analogs. It's easier to simply go about one's work, and report or write about what's current, what's cleaner, what's understood, what's popular, what's... That's why it was different for Reuters' Bill Berkrot to write his February 2016 piece "Old red dye shows promise as new cancer foe."
Of course, what does that say of Peter and Maxim? Despite using the firm for representation (failed fundraisings, questionable fundraising, Boehringer Ingelheim letter of intent [LOI], India, other), the investment banks equity research folks can't bring themselves to cover the company. The role of equity research analyst (buy side, sell side) is an important role often undertaken by smart, intelligent, principled industrious people. But how far as a company and a core management have you fallen when you pay Maxim handsomely, foolishly and yes, at times, stupidly (i.e, you know what I mean Peter: certain aspects of 2012's failed PVCTP "IPO"), and cannot even get their biotech analysts to write about you. I know Peter accepts the situation for what it is (although acceptance is not an excuse in my view), and likely wouldn't disagree with me that Maxim biotech analysts aren't exactly intellectually honest. See Coverage gap? (January 25, 2016) on the blog's Archived News V page.
The situation Peter accepts is what I opened these last two updates with, "clinical study designs that generate randomized, pivotal, and otherwise significant clinical data to support potential approval of PV- 10 and PH-10 for their respective indications." When Maxim feels it's safe and will pay to support Provectus publicly it will.
Just because Dr. Church may be publicly "against" (dated) Rose Bengal doesn't mean she isn't paying attention or hasn't evolved her view (again). Many folks only want to associate their personal or professional brands with winners, but after the winners have been validated or won (because analysis of challenging, novel, unique situations that pays more, monetarily or reputationally, and is easier and appears more astute in the tail stream of winning-ness). Conversely, it pays to pile on, even if you're not paying attention too closely. The below isn't a big deal, but shouldn't details matter?
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
We still are trying to figure out PV-10's targets, barrier effects limiting optimal delivery, and resistance effects. Thus far we understand Rose Bengal and PV-10 are not designed to rely on a single pathway, receptor or antigen to work (and have no known resistance). Again, they are unique and novel; arguments again could be on the basis of familiarity. That is why Eric talks about Moffitt's seminal PV-10 mouse-to-man-to-mouse work and paper; see May 12, 2016 blog post Moffitt: IL RB in melanoma elicits tumor immunity via activation of DCs by the release of HMGB1.
Dr. Church's perspectives on clinical trial design are insightful, and valuable. What does she think of Provectus' pivotal melanoma Phase 3 trial (and other trial designs for that matter)? Several people observed she visited Moffitt's SITC 2015 poster, "Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1." Her Big Pharma experience at Novartis and with imatinib (Gleevec), a targeted therapy, provide her with a small molecule perspective, albeit a biologic and one that is orally administered.
Much of Moffitt's preclinical and clinical work has supported rational combination approaches for PV-10 and [fill in the blank]. For example, ASCO 2014 (abstract, poster):
“This data provides more and more evidence that you are altering both local and systemic immunity in a positive way,” said Jeffrey Weber, the senior author, from Moffitt Cancer Center. “It also provides a rationale for combination trials of PV-10 with check point protein inhibitors, such as ipilimumab, pembrolizumab and nivolumab. PV-10 might offer the perfect way to prime the immune system.” {Quote source}Eric and Peter understand data drive deals, and the attention of people. Provectus has demonstrated PV-10's ablative agnosticism in multiple solid tumor cancers. The company has shown Rose Bengal's orthogonality to different classes of drugs, but more work could be and is being done here. Provectus is demonstrating PV-10's immunologic agnosticism to multiple solid tumor cancers. Attention should follow.
Forward Guidance (August 14, 2016)
 |
| Image source |
- You say: "If you have been watching the program “Game of Thrones,” you’ll be familiar with the phrase “winter is coming.”"
- You say what you believe you mean, you contradict yourself, or you remove things you may have wanted to say:
- {prepared remarks} "I said in our last call, quote, “this coming November, we will be in an excellent position to discuss the status and timing of data and the effects on our business becomes with evermore advanced clinical data.” Endquote."
- {prepared remarks} "As Eric noted in our last quarterly call, these changes are necessary in an era of rapidly evolving treatment options in oncology, and while they have resulted in some delays from our initial study timelines, we continue to push to have interim data available as soon as possible, possibly before the end of the year." [Original/draft: "So, there are more potential participants based on both their medical condition and on their geography. Our interim data, therefore, should be available before the end of the year."]
- {prepared remarks, original/draft} "At the corporate level, we are making preparations for the commercialization and marketing of PV-10 and PH-10. We will have our interim data in a few months from the phase 3 and from the phase 1b/2 – we believe these will give us the ammunition needed to secure regulatory approval." [{prepared remarks, final} At the corporate level, we are making preparations for the commercialization and marketing of PV-10 and PH-10. Data from the phase 3 and from the phase 1b/2 clinical studies in melanoma should give us the ammunition needed to secure regulatory approval."]
- {Q&A} "One comment, I just have a quick comment to add onto Eric. And that's to say that, to be precise, we said we would have more of a refined understanding we believe to communicate on the November call. I--the actual--I believe it's fair to say from the comments the interim assessment would be by the end of the year at the earliest, but we'll have a more refined assessment for you and for stockholders on the November call. A lot will depend on a lot of the in-play activity that Eric has alluded to that we should have a better handle on communicating more precisely on the November call."
- {prepared remarks} "Our next call will cover the third quarter, and we expect to hold it in November. We expect to have a handle on the timing of our interim results at about that time."
- You say: "In fact, Pete suggested yesterday that rather than giving a prepared set of talking points that we go through a very detailed presentation as a webinar. I resisted that because I don’t know that it would be of interest to most investors."
- You talk in circles. See Eric's replies to questions about the interim analysis of the pivotal melanoma Phase 3 trials.
- You may do objectively suboptimal (perhaps inefficient, inexperienced or bordering on potentially stupid) things in your believed service of the guidance you believe you are giving. {actual stupid is this}.
Lack of good, consistent, competent, responsible (current and historical) forward guidance -- where "good," "consistent," "competent" and "responsible" combine to define commentary that is understandable [to professional investors, say, and thus even to the so-called average investor], directive, instructive, reasonably time-based as possible in context and as appropriate, and that typically bear themselves out -- obviously has hurt the company with almost everyone. Such lack has unnecessarily increased the divergence between most shareholders' perception and the reality of Rose Bengal, PV-10, PH-10 and Rose Bengal analogs' clinical value proposition. I don't see much daylight between my views on worth and the depth and breadth of this clinical value prop. But then again, who am I?
Updated below: 8/13/16.
Tampa, FL's H. Lee Moffitt Cancer Center & Research Institute first undertook preclinical and clinical cancer immunology work on PV-10, and presented results for melanoma and breast cancer, such as preclinical work "Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma" at AACR 2013 (this work was published in PLoS ONE later that year).
Then, the University of Illinois at Chicago undertook immunology work on PV-10, and presented results for colon cancer at SSO 2015, "Intralesional Injection of Rose Bengal Induces an Anti-tumor Immune Response and Potent Tumor Regressions in a Murine Model of Colon Cancer." The immunology research being conducted by the Ajay Maker Laboratory is for gastrointestinal cancer and liver metastases.
Now, we learn Houston, Texas' MD Anderson is conducting immunology work on PV-10 for more and different solid tumor cancers. This also is noted in Provectus' 2Q16 filing:
"We believe that our investigational drugs PV-10 and PH-10 provide us with two products in multiple indications, which have been shown in clinical trials to be safe to treat serious cancers and diseases of the skin, and important immunologic data has been corroborated and characterized by institutions such as Moffitt Cancer Center in Tampa, Florida, M.D. Anderson in Houston, Texas, and the University of Illinois at Chicago."I believe immunology work on PV-10 currently is being done in Asia for hepatocellular carcinoma (HCC). Where and on what else is immunology work being conducted for PV-10?
Updated (8/13/16): I have believed, and also known because I have surfaced previously unknown or undisclosed work several times, for some time that much more work has been going under Eric's preclinical and clinical development program for PV-10, PH-10 and analogs than has been disclosed by Provectus (him) to date. The 10-K provides such hints about other cancer indications. Management used to try to provide such perspective to shareholders via a slide from its historic corporate presentation; for example, below, from 2012:
 |
| Click to enlarge. |
Of course we do not know how good the outcomes have been or are, and I certainly disagree with Eric's approach but may not necessarily disagree completely with his philosophy or ideology or approach but his execution and integration of this into the company's communication plan (i.e., I think he could've had his cake and eaten it too).
Solid cancer indications noted in the 10-K for 2015 (in order as they appear) starting on page 3, Oncology (PV-10), on which Eric may have done more work than he has disclosed to date could include lung, breast, colorectal, prostate, stomach, etc.; these essentially mirror what the company used to show above. I am loathe, however, to include certain others. There is work going on that Eric has not yet deemed appropriate to discuss, and that notion ultimately is the point of this entry.
Quick: 2Q16 (August 9, 2016)
Updated below: 8/10/16 {thrice}, 8/11/16 {thrice}, 8/12/16 and 8/13/16 {twice}.
Provectus issued a press release today and filed an associated 8-K regarding its 2Q16 financial results, "Reports Second Quarter 2016 Financial Results," in conjunction with filing its 10-Q. I plan to update this blog news item as I further review and analyze the filing, and eventually the transcript of the 2Q16 business update conference call to be held tomorrow.
1. There was no material change in (opening) risks and uncertainties quarter-over-quarter (QoQ).
Updated (8/10/16).1: 1Q16 on the left, 2Q16 on the right.
 |
| Click to compare. Tool: Text Compare! |
3. The company's monthly cash burn for the quarter appears to have been $1.6 million, -40% QoQ (the prior quarter-over-quarter change was +62%, at $2.7 million per month). April was approximately $1.76 million, while May and June may have averaged $1.55 million.
Updated (8/10/16).1: See the table below.
 |
| Click to enlarge |
Picking as a reasonable starting point the May 2014 denial of breakthrough therapy designation, COO and interim CEO (and then but now former CFO) Peter Culpepper said he expected a forward monthly burn rate of approximately $1 million a month (i.e., a quarterly cash burn of about $3 million). This projection never materialized. Quarterly cash expenditure as a monthly figure from 2Q14 to 1Q16 ranged from a low of $1.1 million per month (2Q14 total: $3.4 million) to a high of $2.7 million per month (1Q16 total: $8.1 million). The company said it spent approximately $1.8 million in April of 2Q16 (in the 1Q16 quarterly filing). Monthly cash expenditure in May and June were lower, as noted above. There have been the challenges with former Chairman, CEO and a co-founder Dr. Craig Dees, PhD, and the associated direct and indirect costs having to address and deal with his actions and activities. More trials are being run. More travel, media and other expenses are being incurred. In most cases, these increased costs are understandable. There should be the opportunity to considerably reduce monthly cash expenditure, primarily out of G&A but also potentially out of R&D (but only smartly). Burn can be increased with more available and properly raised cash, and a clear, compelling rationale to do so. There also should be the intention and action to sustainably maintain efficient and cost-effective levels going forward. There have been and continue to be clear evidence of inefficient and ineffective spending.
A potential approach to "being better" might include undertaking a review of all income statement items under G&A and R&D; identifying “need to haves,” “nice to haves” and non-essentials; and, cutting all non-essential spending, cutting or temporarily suspending, where appropriate, some, most or all “nice to haves.” Cost areas would include management salaries, travel and travel expenses, certain consultants (and their functional role and responsibilities) reporting to Peter, [upon a deep review and discussion with Provectus CTO Dr. Eric Wachter, PhD] certain consultants (and their functional role and responsibilities) reporting to him (I believe he is, but I don’t necessarily assume he is more efficient and effective with spending), etc. The goal must be to smartly reduce cash burn significantly, and I believe the opportunity (inefficiency or waste) exists and that it can be done.
I would also encourage preparing a rolling 12-month cash burn projection for monthly review with both management and the board of directors. Peter and CFO John Glass must develop this, justify the base plan to the board before it is further refined and presented for approval, and then regularly visited for actuals vs. plan.4. Total stockholders' equity (TSE) was $9.1 million, above the $6 million threshold previously stated by Provectus to maintain its NYSE MKT listing.
Updated (8/10/16).1: Provectus removed language from the prior quarter's filing discussing relevant listing requirements. 1Q16 on the left, 2Q16 on the right.
 |
| Click to enlarge. Tool: Text Compare! |
Updated (8/10/16).2: See various line items below.
 |
| Click to enlarge. |
Updated (8/10/16).3: Presumably because Provectus did not raise money in the second quarter, and because of having to (in my view) customarily and perfunctorily address such matters and on what they impact, forward-looking comments regarding "going concern" were introduced by the CFO. See above and below. 1Q16 on the left, 2Q16 on the right.
 |
| Click to enlarge. Tool: Text Compare! |
I don't believe there currently is a meaningful hurdle to overcome in regards to raising capital via equity in a decent manner (other than to think about how to do it better than in the past), although the parameters and process(es) involved are neither insubstantial nor inconsequential.
I do believe a more germane and very relevant higher hurdle that needs to be overcome is Eric's approach to communications. It's not the drug substance (Rose Bengal) or drug products (PV-10, PH-10, analogs), but management's processes and communications of them in certain cases (and bad actions and activities in others). Years ago, MD Anderson's and PV-10 clinical investigator Dr. Merrick Ross, MD is rumored to have said of Provectus management something to the effect (or actually), "Even these guys can't f@$# it up." {Several proponents of intralesional (IL) therapy, among them Dr. Ross, a surgical oncologist, have long foreseen the roles IL agents could play as monotherapies and in combination with other drug classes and cancer treatments.}
A recovering lawyer (and among other things, a great deal mechanic) for whom I worked as a corporate venture capitalist repeatedly said to me, "Good process can't make a bad deal good, but it can make a good deal great." I believe Eric has good-to-great processes in several areas, but if he communicates irregularly, ineffectively and/or inefficiently, his quiet competence is not appreciated, and thus not reflected in the value of the public company for which he is a key steward.
I communicated to Eric that Provectus management never stopped having to communicate to a variety of investor types or constituencies (e.g., retail, professional, institutional, etc.). I've long understood, as have others, that Craig, President, board member and a co-founder Dr. Tim Scott, PhD, Eric and Peter's inexperience (it's not about just speaking to them, it's the process) speaking to institutional investors (funds of one investment style/strategy or another), life sciences or generalist. I encouraged him (Eric) to contemplate and consider raising his level of communication to the "greatest common denominator" (GCD) (in his view or perception), rather than maintain the "lowest common denominator" (LCD) at which he [for whatever reason] feels he has to communicate.
My reasons for this increase comprised but were not limited to: (a) Understanding and knowing very well that there are many types and kinds of investors; (b) Generally speaking, investors do not have a high level of respect for core management (Eric and Peter), and for that matter, non-core management [Scott] and the board, because of his/their perceived lack of ability or lack of interest in dealing with Wall Street; (c) Wall Street, as a public company, should be acknowledged but not necessarily kowtowed to. Core management's ongoing challenge (until it is not) is that management have a history of essentially dismissing the Street; and (d) There should be absolutely no reason to dumb down communications. Raising the level of communications to the GCD speaks to institutional investors, particularly in the life sciences arena, and also impresses upon retail investors -- many of whom are very smart and intelligent, have hung around and been battered around in the process as PVCT shareholders, and helped Eric and the company be in the clinical development program position Provectus is today (i.e., to have done it the right way) -- the level and detail of sophistication with which Eric and his team does execute. Many retail investors who follow the company and/or own shares very much understand intelligent process and competent execution. If, for whatever legitimate or silly reason or reasons Eric is "afraid" to speak, my counsel would be: Get over it.
So yes, it is possible to f@$% things up. Be competent, and communicate competence competently, and all manner of investor folk then would/will view you as competent.Updated (8/11/16).4: 2Q16 Business Updated Conference Call
Provectus held it's quarterly call yesterday. The prepared remarks can be found here on the company's new website. The call transcript from SeekingAlpha is here. Among other things, I found several items or topics interesting and notable:
- PV-10, an immunotherapy,
- Hepatocellular carcinoma & sorafenib,
- Hepatocellular carcinoma & co-inhibitory blockade,
- Prospective partner interest,
- Clinical trial math,
- PH-10, and
- Core management and the board of directors.
Updated (8/11/16).7: A transcript of the entire call from the company is here, and appears to be [much] better than the SeekingAlpha transcript.
5. Clinical trial math: Eric noted the required (prescribed) number of progression events for the full study and interim analyses of the primary endpoint of progression-free survival (PFS) to be triggered were 162 and 81, respectively. In general, oncology studies require approximately 70% to 80% of patients to incur an event (e.g., adverse event, disease progression, lost to follow-up, other) to trigger an analysis. Provectus' dropout adjustment means the number of events the company projects is at the lower end of this range.
Eric previously has mentioned Provectus' pivotal melanoma Phase 3 trial design was strongly influenced by other pivotal melanoma Phase 3 trials, such as ipilimumab (Yervoy); see "Ipilimumab plus dacarbazine for previously untreated metastatic melanoma," Robert et al., N Engl J Med. 2011 Jun 30;364(26):2517-26. The ipi protocol published in the NEJM notes an overall survival analysis scheduled when 416 events (i.e., deaths) were observed. With a study size was 502 patients, the percentage of prescribed events was 83%. The paper's Statistical Analysis discussion included:
"The target number of events for the primary analysis was 416 deaths, which we estimated would give the study approximately 90% power to detect a 37% increase in median overall survival to 11 months with ipilimumab plus dacarbazine, with a corresponding hazard ratio for death of 0.727, assuming a total sample of 500 patients (250 randomly assigned to each group) and a median survival of 8 months for the patients receiving dacarbazine plus placebo."
In my July 16, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part II, I had re-done my clinical trial math. I could estimate Eric's trial's target number of events for the PFS primary endpoint would give his study a 90% power to detect a 70% increase in median PFS to 2.6 months with PV-10, with a corresponding hazard ratio for progression of 0.588 (my assumption), assuming a total sample of 225 patients (150 randomly assigned to the treatment group, 75 randomly assigned to the control group) and a median PFS of 1.5 months for the patients receiving dacarbazine (I am unsure as yet how to incorporate Imlygic's math). Takeaway: I believe insiders and outsiders (and me too) think the trial should and will win (i.e., meet its primary endpoint), and win convincingly (i.e., healthily beat the primary endpoint and substantially statistically significantly so, and meet its other endpoints too in such a manner as well). The key question, of course, is when will the trial win (i.e., when will the interim analysis be triggered).
Updated (8/11/16).5: 2. Hepatocellular carcinoma & sorafenib, 3. Hepatocellular carcinoma & co-inhibitory blockade
There is a beautiful symmetry to Eric's preclinical/clinical development work in hepatocellular carcinoma (HCC) when you consider the mouse-to-man-to-mouse work to demonstrate PV-10's efficacy and its mechanism of action. First, let me establish a fundamental basis, as Eric said in his prepared remarks (with my underlined emphasis below):
For HCC (like for melanoma), PV-10 could be a single agent or monotherapy, and it could be used in combination with an immune checkpoint inhibitor.Updated (8/11/16).5: 2. Hepatocellular carcinoma & sorafenib, 3. Hepatocellular carcinoma & co-inhibitory blockade
There is a beautiful symmetry to Eric's preclinical/clinical development work in hepatocellular carcinoma (HCC) when you consider the mouse-to-man-to-mouse work to demonstrate PV-10's efficacy and its mechanism of action. First, let me establish a fundamental basis, as Eric said in his prepared remarks (with my underlined emphasis below):
"Between Pete’s comments and my introductory remarks, we’ve stated most of what needs to be mentioned regarding documentation of the immuno-ablative properties of PV-10, and years of work conducted by Moffitt Cancer Center, both in animals and man, was detailed in a pivotal paper published by that team in May. After a long haul, it is now clearly accepted that tumor ablation with PV-10 can lead to stimulation of a useful anti-tumor immune response. We are continuing research activities along this line of reasoning, but at a fundamental level this is probably the most important paper published to date on the drug." Takeaway: PV-10 is an immunotherapy.Now, in the Q&A session he framed HCC as the next cancer up (with my underlined emphasis below):
"Our next tumor on the radar will be hepatocellular carcinoma because there are challenges in HCC that comparable to those in melanoma. We already know that we can destroy HCC with this ablated process and my hypothesis is that that should lead to similar signaling which can have implications for both single agent therapy or lesion PV-10, HCC but more importantly for combination with things like anti-PD1 Keytruda or Opdivo."
The Seeking Alpha call transcript is horrible; nevertheless, Eric then said (with my underlined emphasis below):
"We have already shown that the basic immunology occurs in HCC model. So I will say that among the things that I'm having confident in, I'm highly confident that we will show that we will show the same functional and the signaling functions in HCC and I use that as an opportunity to comment on something that I did mention in my prepared remarks which is we have talked about our HCC strategy for Asia and I made some comments about that during the prepared remarks that we’re looking at is both single agent and in combination. I firmly expect that that will play out as a combination strategy at two levels. First is a successor to our current study where we’re using PV-10 with sorafenib and there is from our interactions with KOLs around Asia there is from our interactions with KOLs around Asia, there is no recent nod to proceed with that to a larger study in Asia and that’s what we expect to do with a work in Hong Kong, Taiwan and Singapore that I alluded to.
But we’re also looking adopting the non-clinical studies to show that that channel enrollment is signaling the seven steps occur in HTC and might to justify them a second pronged approach which is particularly relevant for the U.S. which would be a combination of PV-10 with the checkpoint inhibitor in that anti-CTLA-4, anti-PD1 or anti-PDL1 so that works, we’re starting and I expect that will considerably lead to similar development in HCC that we have seen in melanoma which is 1a2 combination where PV-10 plus checkpoint inhibitor for HCC."Finally, before getting to my points, there was an inadvertent and incorrect pre-release of management's prepared remarks for the call. The correct version later was uploaded, and the portion I want to compare against is below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Here are the points I'd like to make, as you read all of the above:
- Provectus (Eric) appears to be nearing the completion of its (his) early-stage liver study (R&D). The original Phase 1 work comprised 6 patients. The expanded Phase 1 work's first additional cohort would comprise 24 patients. The expanded work's sorafenib cohort would comprise 12 patients. More data from this trial could be presented at ESMO Asia 2016, which runs from December 16th-19th; "We also expect present updated data on the study later this year at a major oncology conference in Asia and to get a companion journal article into the literature,"
- The company (he) appears ready to move forward on exploring HCC in Asia using sorafenib as the standard of care (and apparently not or not necessarily a local ablation technology per the respective Asian region's choice). The clinical trial approach would be a Phase 1b/2 of sorafenib vs. sorafenib + PV-10, which is a combination study of sorts, as Eric noted, but facilitates the approval of PV-10 (where Provectus owns the data, and controls the marketing of its own drug if combined with sorafenib in a combination regimen). This approach already was discussed by the company, and noted on its July 2015 liver posters, such as Barcelona's ESMO GI 2015. An illustrative paper on HCC and sorafenib is "The Role of Sorafenib in Hepatocellular Carcinoma," Gholam, Gastroenterol Hepatol (N Y). 2015 Apr; 11(4): 253–255, and
- Provectus (Eric) will begin some preclinical (murine model) work to combine PV-10 with co-inhibitory blockade (CIB) (i.e., anti-CTLA-4, -PD1, PD-L1) in HCC, as Moffitt Cancer Center did and then presented at SITC 2014. While PV-10 has been discussed as a primer or front-end in combination with CIB for melanoma, there hasn't been any real discussion about combination for HCC other than in a generalized, conceptual, multi-indication co-development "platform" deal with a Big Pharma having a checkpoint inhibitor. As I noted under item no. 6 of Desert > Beach (July 8, 2016), Bristol-Myers' Opdivo and Merck & Co.'s Keytruda have not had much success with HCC, with the former being farther ahead in terms of generating such indication data. I would have to believe Eric is embarking on a process- and mouse-to-man (and maybe back to mouse again if necessary) translational medicine-driven approach for PV-10 and CIB because of direct interest by BMY, MRK and others. It cannot be a random event to add the PV-10/CIB combination prong to his longstanding liver clinical development program, and given limited company resources at this time.
Updated (8/11/16).6: 1. PV-10, an immunotherapy
It bears repeating (see update.5 above), PV-10 is an immunotherapy: "...it is now clearly accepted that tumor ablation with PV-10 can lead to stimulation of a useful anti-tumor immune response."
Updated (8/12/16).7: 6. PH-10
Updated (8/12/16).7: 6. PH-10
I found several statements by Eric on this topic notable (with my underlined emphasis):
{Prepared remark} "Turning to PH-10, we're sorting through the immunologic and histopathologic data from our mechanism of action study of topical PH-10. I, unfortunately, can't go into detail yet about what we're learning, but in general, my assessment is that these results will be as important to PH-10 as the Moffett work has been to PV-10.
When new kinds of therapy come along, everyone likes to understand the biologic story underlying clinical observations, and it appears that this may be a very interesting story that explains observations we've made throughout clinical development of the drug. I look forward to sharing details on this with our stakeholders in the next few months. I will note that the observations from this study should play an important role in anticipated discussions with FDA to assess strategies for advancing the program from Phase I--I'm sorry--from Phase II to Phase III."
{Q&A} "And what's remarkable to me is the way in which the immunologic mechanism data that we've amassed on PV-10 has guided our understanding on how to move the drug from early stage clinical development to late stage clinical development, and we're seeing exactly that same pattern being repeated with PH-10. So, I think from a technology perspective, there's never been a better time for the company."
{Q&A} "So, we have recently met with the investigational team that we have been using that [unintelligible] prominent immunology expert and dermatology in New York to review the immunologic data that we collected in that study. It was actually a fascinating experience because we had some hypotheses about how PH-10 might be working. And this certainly has confirmed some of those hypotheses, and it has opened up some new areas of investigation.
So, we are wrapping up the analysis of what--I think it was interesting he referred to it as the basic analysis data set. In the next weeks we will assess whether there is a way to fast track that information into the public domain. And we’re also moving to conduct a secondary, more detailed analysis of those same samples that we are collecting from those patients. We enrolled between 20 and 30 patients in that study last year.
And we have biopsy specimens that they provided at various time points in the study. The initial analyses of those look very encouraging. We will report on those as soon as possible, and we’re going to conduct, as we said, a more detailed analysis to get a finer point on that mechanism. It does appear that we have potentially a very unique mechanism of action that would be possibly quite valuable."
Updated (8/13/16).9: Initial mechanism of action (MOA) results of PV-10 identified the central role of T cells in clinical observations of immunoablation, the so-called "bystander effect." Initial results on PH-10 appear to implicate a similarly critical process that clarifies clinical observations. Prospective pharma partners (that would license the investigational dermatology drug compound) need to understand how a new therapy fits into approaches for treating disease (and thus how to market it). In both cases (i.e., both PV-10 and PH-10), the MOA studies provide this clarity.
In regards to the basic analysis of PH-10, as Provectus did with PV-10 (and continues to do; see Immunology work; now MD Anderson (August 13, 2016) above), these basic experiments are being followed up by more detailed studies to more fully complete the company's understanding of the finer details of PH-10's MOA (and I presume its immunology as well). Provectus is using existing clinical samples for this, as Eric mentioned on the call (and, as the company did for for PV-10), and undoubtedly would continue to mine greater understanding from these samples.4. Prospective partner interest
On the call Peter said:
"So, in my view, they're like many other potential partners, Merck and Bristol and Pfizer with German Merck and Hoffmann-La Roche and Novartis that are very closely monitoring everything we do."On the call and in the transcript, they're refers to Boehringer Ingelheim. By now even the most casual observer would take what Peter says with copious amounts of salt. There are times, however, and they are by no means infrequent, when Peter is very intelligent, cogent and thoughtful in his remarks. I translate what Peter is saying into Big Pharma is aware of Provectus, has seen or will see published and non-public preclinical and clinical data, and Provectus [in more recent times] regularly meets with Big Pharma.
In regards to Provectus' melanoma combination Phase 1b/2 study, Eric said on the call (with my underlined emphasis):
"It’s possible that we would report some initial data from the Phase 1 b portion of the study this year. If that is the case, it would be some form of interim data. It is much more likely that will be in the first half of next year, picking up into the 2017 oncology cycle. If we submitted an abstract today to a major meeting, that wouldn’t be recorded until sometime late in the fourth quarter, so we have to acknowledge that it’s likely that a public readout of those data would not be until sometime in the first half of next year. That is not to say that in the context of confidential discussions with potential corporate partners, there may not be some opportunity to share those data with partners, but that is also, again, pure speculation at this point."My translation of what Eric is saying is the company hasn't met with Big Pharma yet to share such data, but if and when Provectus management does, said data could/should/would be shared. The more important question [to me] is not (and has never been) whether the data are shared, but whether the data (and other data) can spur a transaction of one sort or another. Management could provide much more insight into why they don't believe the data to date have not moved Big Pharma.
7. Core management and the board of directors
I'll have more to say on this topic in my final update of this blog news item.
Updated {8/13/16}.8: For now, I'll merely touch on the topic of Eric's soapbox (because his philosophy and ideology, and thus approach, should be critical consideration for any shareholder, both pro and con):
"I will use that as an opportunity to jump on a soapbox that I think--I hate to bring this up. But, one of the problems that plagues Provectus--[unintelligible] have got a lot of very interested stakeholders. And occasionally some of those stakeholders do inappropriate things like, for example, they might contact an investigator in a clinical program or a non-clinical program and under some pretense try to probe for inside information. And I consider that to be very unethical. And it’s one of the reasons that we try to protect these outside parties that we work with. And it’s something that I feel very passionate about. We’re not trying to be deceptive. We’re simply trying to protect those individuals until it is no longer possible to do that...We count on them not only to do legitimate, objective, independent work, but to remain motivated and not become irritated in their relationship with Provectus and its various stakeholders."I asked Eric for more insight into the underpinnings of his soapbox (and whether I was a pestilence):
"I have no reason to believe you have conducted yourself in this manner, but we hear regularly from our collaborators about clearly inappropriate entreaties. Many companies in our industry do not disclose their investigators (an alternate means of complying with required CT.com listing is to provide a central contact that screens and routes inquiries as appropriate). I don't like this approach for a number of reasons but certainly appreciate why it's used. We appreciate your openness in your interactions of this type but unfortunately not all stakeholders share your commitment to transparency. Further, if a small percentage of our stakeholders regularly approached our investigative team in a similar open manner this would still be problematic. I assume you've seen a doctor sometime in the past 10 years so you can appreciate that these are exceptionally busy individuals who need to carefully protect their time; answering frequent questions about a company with whom they are collaborating is an irritation that probably doesn't fit into that equation. I hope this adds further insight into the basis of my remarks... I'll pack up my soapbox now."
 |
| Click to enlarge. Image source |
Composition patents are valued by Big Pharma because they afford the broadest scope of protection to a molecule. A patent, and thus a patent portfolio, only is as good (or bad) as its writing and the protected area it encloses (or excludes). Of course, one only truly knows this when the patent (or portfolio) is punched in the nose (legally speaking). Early on, and as the company continues to advance its lead halogenated xanthene through the global regulatory process, Provectus established its "freedom to operate," which should ensure the commercial production, marketing and use of PV-10 does not infringe the intellectual property rights of others, and more importantly, give comfort to Big Pharma upon acquisition that it/they also have freedom to operate post-approval(s).
Composition patents are generally deemed stronger than "method of use" or use patents. In the case of Rose Bengal or PV-10, Provectus has been the first entity to advance the molecule's therapeutic and second medicinal use, where the original and first medicinal use was as a diagnostic. Still, consider:
"Pharmaceutical firms regard strong patent protection as critical to drug development and thus may forego development of small molecules for which they cannot secure strong “product” or “composition-of matter” patents (1). During the time these composition-of-matter patents remain in force, they not only give the patentee the right to exclude others from making and selling the drug for the same purpose as the patentee but also block the marketing of any new use that another party discovers." {Source: "Use Patents Can Be Useful: The Case of Rescued Drugs," 2014}Footnote (1) in the quote above notes:
"E.g., Armitage, supra note 281, at 270; cf. BESSEN & MEURER, supra note 1, at 256–57 (“[P]atents have worked best where boundaries can be staked in verifiable physical characteristics, like small molecules.”); Scherer, supra note 38, at 29, 27–30 (“A plausible argument can be advanced that the [Hatch–Waxman] Act [and patent system] shaped an ideal compromise in terms of stimulating pharmaceutical innovation.”)." {Source: Texas Law Review, Volume 87, Number 3, February 2009, "Unpatentable Drugs and the Standards of Patentability."}CT.gov update (August 8, 2016)
Updated below: 8/9/16.
The ClinicalTrials.gov webpage for Provectus' A Study to Assess PV-10 Chemoablation of Cancer of the Liver was updated to add a new clinical investigator and his site: Vanderbilt-Ingram Cancer Center's (VICC's) Dr. Daniel Brown, MD. The site is not yet recruiting.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
Updated (8/9/16): The ClinicalTrials.gov webpage for Provectus' PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma was updated to add a new clinical investigator and his site: Cedars-Sinai Medical Center's Dr. Richard Essner, MD. The site is not yet recruiting.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
Who you got?! (August 4, 2016)
Updated below: 8/6/16 {thrice} and 8/7/16 {four times}.
This blog news item may read somewhat disjointed, as I try to collect, reflect on and write about some general (and maybe specific) thoughts; there could be several updated entries over time.
A. Who would you back, and what kind of process would you expect from: the guys who wanted to be famous, or the guy who wanted the drug compound to be pervasive in its use?
Updated (8/6/16).3: On Friday Bristol-Myers announced a Phase 3 trial of its anti-PD-1 drug nivolumab/Opdivo failed to meet its endpoints of progression-free survival (PFS) (primary) and, I believe, overall survival (OS) (secondary) for non-small cell lung cancer (NSCLC). The failure appears to be due to choices in the design of the trial:
 |
| Click to enlarge. Image source |
The failure highlighted several items of note to me in regards to PV-10. First, the Bristol-Myers' Opdivo trials for NSCLC, whether the above failed trial of Opdivo as a monotherapy or the ongoing trial of Opdivo and Yervoy as a combination therapy, were/are seeking to be first-line treatments/therapies through their comparison with chemotherapy. I believe Provectus' pivotal melanoma Phase 3 trial, if successful, could position PV-10 as a first-line treatment for locally advanced cutaneous melanoma (e.g., local, satellite and/or in-transit recurrence).
Second, both Bristol-Myers trials were/are using RECIST 1.1 for PFS. PFS in Provectus' pivotal Phase 3 trial is measured using RECIST 1.1.
Third, Bristol-Myers management appeared to provide context to their decision-making by contrasting and comparing Opdivo's use as a monotherapy, and its use in combination with Yervoy:
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
Note the use of Opdivo in both monotherapy and combination therapy roles for the same indication and apparently similar patient populations. Yet, Provectus' two melanoma trials are determined to show the roles of PV-10 as a monotherapy and combination therapy in two distinct patient populations, Stage III-IVM1a and Stage IV, where intralesional agent (IL) key opinion leaders (KOLs) have been asking the same questions: what is the role of IL agents as monotherapies, and what is their role in combination therapies?B. With the patent allowance for the use of halogenated xanthenes in pharmaceutical compositions and as medicaments being awarded on August 23rd (see "a new synthesis patent...will make it very clear that our protection of Rose Bengal for therapeutic use is in fact ours" (June 17, 2016)), could intellectual property protection be conferred until around 2033 (i.e., twenty years from this patent's June 2013 filing or 371(c) date)?
Updated (8/6/16).1: Recall that Provectus' intellectual property (IP) protection, as I wrote, comprises: (i) Second medicinal use (as a therapeutic), (ii) Method of use, Formulation (including trade secrets), (iii) Synthesis (to ICH Guidelines specification), and (iv) Combination (with other cancer treatments). Also remember IP needs to be protected both domestically (i.e., in/for the U.S.) and globally (i.e., in/for the rest of the world), which the company has.
Consider the below in regards to the medicinal chemistry, and the importance of intellectual property to a biotechnology company's value: "[t]he composition of matter form is likely most important in the pharmaceutical industry." I believe the synthesis patents are akin to composition of matter-level IP protection.
 |
| Click to enlarge. Image source |
Consider the challenge Biogen faces with Tecfidera® (dimethyl fumarate). Biogen has method of use protection in the U.S., but not in Germany. Biogen does not have, I believe because of dimethyl fumarate's long history of use, composition of matter protection for the compound. See "Tecfidera®: an approach for repurposing," Spencer, Pharmaceutical Patent Analyst, March 2014 ,Vol. 3, No. 2, Pages 183-198:
 |
| Click to enlarge. Image source |
See also "EU revokes one of the patents on Biogen blockbuster Tecfidera," Eric Palmer, FiercePharma, March 11, 2016:
 |
| Click to enlarge. Image source |
Imagine the protection Provectus now has, or potentially will have: both method of use and composition of matter of both PV-10 and PV-10 analogs.C. A recently collected and connected dot may confirm the clinical use of PV-10 in a previously undisclosed location since 2013, much like Melbourne, Australia's Peter MacCallum Cancer Centre. Is it an undisclosed compassionate use program/special access programme site (and why)? This potential observation would confirm there is some-to-much Provectus' CTO Dr. Eric Wachter, PhD (the guy who wanted/wants PV-10 to be used as pervasively as aspirin) is doing, preclinically and clinically, that has not yet been disclosed by the company.
Updated (8/6/16).1: There was Moffitt Cancer Center before there was Moffitt; i.e., [at early as at least] June 2012's 2nd European Post-Chicago Melanoma Meeting, January 2013 press release. Updated (8/6/16).2: Of course, Eric has utilized Moffitt for a considerable amount of preclinical R&D/work, such as combining PV-10 with different classes and categories of drugs (e.g, targeted therapies, immunotherapies, etc.). Existing and prospective investors, and the stock market and biopharmaceutical industry ecosystem at large, only are made aware of the specific work (or work projects) when they are presented at medical conferences or published in peer-reviewed journals. We do not, however, know what the overarching strategy and tactics of the R&D program has been or is, although I believe we can reasonably speculate as to its goals and potential benefits.
There wasn't PeterMac (oncology, special access scheme, starting in 2010 based on May 2016 RACS annual scientific congress) before there was; e.g., August 2015 corporate presentation: PV-10: Compassionate Use Program [slide], Participating sites (at least one additional not listed). {Could/should be a melanoma pivotal Phase 3 trial site, and a melanoma combination therapy Phase 1b/2 study site}
There hasn't been and currently isn't Rockefeller University (dermatology, mechanism of action, speculated about in May 2013) until there will be; i.e., May 2016 business update conference call: "We are working with a major research university on analyzing skin biopsies that were collected three times during the course of the study for each patient..." (Eric).
There wasn't the University of Illinois at Chicago (UIC) (oncology, mechanism of action, identified in June 2015 but later understood to have started in 2014) until there was; i.e., subsequent June 2015 press release.
Current speculation: There isn't Tom Baker Cancer Centre (later Calgary Cancer Centre) (oncology, special access programme?, possibly identified in August 2015 -- see Notes (August 6, 2015) on the blog's Archived News IV page, starting as early as March 2013?) until there could/will be. {Could be a melanoma pivotal Phase 3 trial site} Updated (8/7/16).4: The same or different? (see below)
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
As an aside, what I believe to be the site of/a site related to the University of Colorado School of Medicine visited the blog this past week; intralesional (IL) agent clinical investigator Dr. Rene Gonzalez, MD is from here. {Could be a melanoma pivotal Phase 3 trial site}
Updated (8/6/16).2: I believe there has been an ongoing and an undisclosed clinical immune biomarker program being undertaken for PV-10 as a monotherapy, and in combination with pembrolizumab. Peripheral blood biomarkers (immune and/or otherwise) can potentially predict the response of a patient to a particular cancer drug or therapy. Updated (8/7/16).4: See below.
 |
| Click to enlarge. Image source |
E. One of the "bio-Twittersphere" cognescenti flipped (his or her view) (my word) on PV-10's clinical progress in 2015? I will be curious if and when he or she will publicly comment so.
Updated (8/7/16).3: I believe I understand the substance and form of equity research coverage. When my CFA Institute Research Challenge teams embrace they are in it to win it (by taking a hedge fund analyst's approach to due diligence, analysis, investment thesis and price target [because you have to have one], rather than the equity research analyst's), they succeed not only in the competition but more importantly in obtaining on their own lessons and experiences for their future careers.
I don't believe investors should overvalue equity coverage, although I'm not convinced one can undervalue it except as a box to be checked by a competent public company management team. As such, some, most or many capital markets participants understand the role equity research coverage plays, whether from the sell-side of the so-called buy-side ("independent") (i.e., quid pro quo). Which is why we have to place into context coverage or lack thereof in regards to Provectus.
On the one hand, Provectus' COO, interim CEO and former CFO Peter Culpepper is/was who he is/was and who he is/was not (which I've discussed with him regularly privately). Eric and he (Peter certainly is not alone in this) essentially cannot resume sell-side equity research coverage, although I appreciate (as far as I can tell, but I can't see that far nor) not buying buy-side coverage. The other hand is the disingenuous one from the ecosystem. Lazy is criticizing the molecule rather than properly, objectively eviscerating each and all of management for certain actions and decisions. Dishonest, from former scientists and researchers, is to say nothing of the molecule (although is it now worse for you by previously having called it "snake oil"). Or, if opinions are your own, why does your firm ask for money? As such, how much is your personal or professional and/or your firm's opinion really worth?
 |
| Click to enlarge. Tweet image sources |
 |
| Click to enlarge. Image source |
Updated (8/7/16).4: Bristol-Myers's NSCLC Phase 3 trial failure, a failure of trial design, since Merck's trial of pembrolizumab/Keytruda in a similar patient population was positive, and pembro/Keytruda and nivolumab/Opdivo are equivalent drugs, highlight for me the importance of trial design, and Eric's grasp and detailed understanding of this of course. Trial protocols are among the crown jewels of a biopharmaceutical company. Clinical trial designing (e.g., patient populations, endpoints, comparators, inclusion and exclusion criterias, etc.), and their outputs (protocols) and outcomes (won or lost trials), matter of course.
First, there is the [output] consideration of whether the generalities and specificities of the trial design is proper such that it actually could or would lead to a pathway to approval (in discussion with the FDA and/or other global regulatory agencies or bodies) if and when successful.
Second, there is [outcome] consideration of whether the trial wins, and wins convincingly to establish the investigational drug's commercial market viability. For IL drug T-Vec/Imlygic, it won its trial pivotal melanoma Phase 3 trial but not convincingly (i.e., the primary endpoint of durable response rate was met but the secondary endpoint of OS was not).
Finally, and third, and specific to PV-10 in regards to trial designing, there is the consideration of designing a trial that addresses the nature and an IL agent, and the treatment goals of the local agent, in the context of the respective trial's hypotheses and that these local agents exist in a systemic world. As such, the reason for why I am curious about/following this bio-Twittersphere cognoscente is his or her comments regarding PV-10's Phase 3 trial protocol/designing/some such thing.F. I believe establishing, or at least framing, worth is important to valuation and valuation expectations, and constructing an investment thesis. Management believes they have threshold proof of concept data establishing PV-10 is agnostic to all solid tumor cancers. From worth, there are several techniques or methodologies for valuing biotechnology companies and assets (e.g., risk adjusted net present value (discounted cash flow), comparables (precedent transactions), peak sales (peak sales multiples), real options, scenario analysis/decision–tree modeling/Monte Carlo simulation, etc.). There's going to be a time and place to discuss worth and valuation. As an aside, traditional (professional) investment management would not consider Provectus a "value investment," nor people considering an investment in the company to be "value investors."
Updated (8/7/16).1: Speaking to the medical science and the clinical value proposition first, and thus worth, valuation and monetization prospects second (and which should naturally follow if the former is sufficiently robust), "value prop" drivers (and thus worth drivers) include but are not limited to (i) agnosticism, (ii) orthogonality and (iii) synergism.
(i), agnosticism, has been clinically demonstrated over time and mostly in the ablative first step of PV-10's two-step approach to fighting cancer, where the second step is the subsequent immune response. Agnosticism, a construct of multi-indication viability, would mean PV-10 is capable of ablating, destroying or shrinking, all manner of solid tumor cancer upon injection irrespective of disease presentation or tumor type. Clinically speaking, primary melanoma and liver cancer (hepatocellular carcinoma or HCC) tumors have been injected. HCC presented as itself, and/or with Hepatitis B, Hepatitis C, cirrhosis, and/or portal hypertension. Secondary liver cancer tumors include colorectal cancer, NSCLC, melanoma, and ovarian. Agnosticism could be shown if and when Eric were to discuss what may have happened to the primary cancer sites of the injected secondary liver cancer mets.
So-called drivers (ii), orthogonality, and (ii), synergism, should be and are on display in Provectus' Phase 1b/2 study of PV-10 and anti-PD-1 pembrolizumab/Keytruda in Stage IV melanoma patients. Orthogonality, a construct of safety, would mean little, de minimis, no clinically relevant drug-drug interaction (by giving both drugs in combination regimen to a patient).
Synergism, a construct of efficacy for combination cancer therapy, would mean one plus one would be greater than or equal to two; in the best case, the "sum" would much greater than two. This construct contrasts with additivity, where one plus one would be less than or equal to two. This topic originally was introduced under The bar (June 24, 2016) and Additive: 1 + 1 < 2. Synergistic: 1 + 1 > 2 (best case, >> 2) (June 25, 2016) below.
I updated MD Anderson's Dr. Merrick Ross, MD's slide no. 160 of ASCO 2016 Melanoma Symposium's "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist" for preliminary combination data of oncolytic virus CVA 21 (Coxsackievirus A21) and ipilimumab/Yervoy in Stage III and IV melanoma patients. The upshot for this latest addition, which Dr. Ross probably didn't include because the data is partial (some of the patients treated, but not all) and preliminary, is that CVA 21 and ipilimumab are synergistic, as are oncolytic virus T-Vec and ipi; however, T-Vec and pembro are potentially additive but not synergistic.
 |
| Click to enlarge. |
Updated (8/7/16).2: The CVA21 + ipi Phase 1b study has several features of note to me, and that are representative of the oncolytic virus/oncolytic immunotherapy candidates in the pipeline behind T-Vec and ablative immunotherapy PV-10 either as a monotherapy or as part of combination therapy. First, it only is a Phase 1b trial design (that still is recruiting patients); that is, there is no contemplation on the CT.gov webpage for the study of a later-stage, follow-up trial. The trial designs of nivo + ipi (approved), T-Vec + ipi, T-Vec + pembro, PV-10 + pembro, etc. were filed more completely.
Second, this CVA21 + ipi study, like T-Vec + ipi and T-Vec + pembro, uses a non-standard criteria to measure tumor response and progression (e.g., PFS, objective response rate); in the case of the Viralytics (the company developing CVA21) work, it is immune-related response criteria irRC-WHO. The trial designs of nivo + ipi (approved) and PV-10 + pembro use RECIST 1.1.
Third, Viralytics' Phase 1b study released preliminary efficacy data on 6 patients (who were assessable, of 11 patients treated). Incyte and Merck & Co. advanced their combination of Incyte’s IDO1 inhibitor epacadostat in combination with Merck’s pembrolizumab in patients with advanced or metastatic melanoma from a Phase 1b study to a pivotal Phase 3 trial on the basis of data from at least 7 patients. Epac + pembro yielded an [objective] response rate of 57% in melanoma. See Broader label for the single agent Vs. A combination of agents (June 22, 2016) below. Note that the program was initially filed as a Phase 1b/2 study (i.e., a more complete trial design) and would use RECIST 1.1. I cannot find any response rate data for epac as a monotherapy; however, one might say, based on clinical data of the combination of epac + ipi that epac + pembro may be more additive or synergistic than epac + ipi -- on the basis of a higher RECIST-based response of the former over the latter.
 |
| Click to enlarge. Image source |
I speculate Eric may have PFS and ORR melanoma combination therapy data suitably sufficient in length of time on at least 5-6 patients (potentially or likely more), and that trial investigators may present the data at SMR 2016. A question, I suppose, could be other than, "Who you got?!" for combination therapy, is (if PV-10 + pembro, and thus + nivo is really synergistic), "Whose world does it rock more?"Evolution in the era of immune checkpoint inhibitors (August 1, 2016)
Tweet:
 |
| Tweet image source |
 |
| Click to enlarge |
"A U.S. cancer institute asserted Sept. 25 in federal district court litigation that two U.S. researchers are co-inventors of cancer immunotherapy patents that are a result of the collegial exchanges of ideas and draft manuscripts with a Japanese researcher (Dana-Farber Cancer Institute, Inc. v. Ono Pharmaceutical Co., Ltd., D. Mass., No. 1:15-cv-13443, filed 9/25/15)."Complaint.
Systemic delivery of Rose Bengal analogs? H/t a regular hatter on the following thoughts about the soon-to-be-awarded daughter synthesis patent Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one(Rose Bengal) and Related Xanthenes.
Specifically, claims 73 and 74:
"73. The medicament of claim 70 further comprising at least one targeting moiety coupled at one or both of positions R.sub.11 or R.sub.12 to compounds of Formula 4 wherein R.sub.1 is independently F, Cl, Br, I, H or C.sub.1-C.sub.4 alkyl; R.sub.2, R.sub.3, R.sub.4 and R.sub.5 are independently Cl, H or I having at least one substituent selected from R.sub.2, R.sub.3, R.sub.4, R.sub.5 as I and at least one as Cl or H; R.sub.6 is independently H or C.sub.1-C.sub.4 alkyl; and all tautomers thereof; atropisomers thereof; closed lactone derivatives thereof; enantiomers thereof; pharmaceutically acceptable salts thereof and mixtures thereof.
74. The medicament of claim 73 wherein said targeting moiety is selected from the group consisting of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), amino acids, proteins, antibodies, ligands, haptens, carbohydrate receptors, carbohydrate complexing agents, lipid receptors, lipid complexing agents, protein receptors, protein complexing agents, chelators, encapsulating vehicles, short-chain aliphatic hydrocarbons, long-chain aliphatic hydrocarbons, aromatic hydrocarbons, aldehydes, ketones, alcohols, esters, amides, amines, nitriles, azides, hydrophilic moieties, hydrophobic moieties and mixtures thereof"Comment: Targeting moieties attached to parent molecule? Before this concept was simply mentioned in the body of patent, but now issued claims? An intravenous formulation of PV-12 (with enhanced lipophilicity?) attached to a targeting ligand in the future? Local intralesional delivery evolving to systemic targeted delivery?
July blog readership statistics.
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated below, again, once more.
Predictors. I believe the Australian single site experiences of Melbourne's Peter MacCallum Cancer Centre and Brisbane's Princess Alexandra Hospital are predictors of, and should be more reflective of, Provectus' melanoma pivotal Phase 3 trial (than the company's metastatic melanoma Phase 2 trial).
There are more similarities between the site experiences and the pivotal trial's protocol, than the Phase 2 and 3 trial protocols. One still has to take certain things into account when adjusting for the differences in the site experiences and Phase 3 trial protocol. In particular, the site experiences collected durability of response, and survivability data, and in the case of Princess Alexandra did so using a traditional tumor measurement approach like RECIST (PeterMac did not note its means of measurement). Recall the pivotal trial's primary endpoint is progression-free survival (PFS), while secondary endpoints include complete response rate (CRR), duration of complete response, and overall survival (OS). PeterMac noted in its peer-reviewed publication that "[a]fter a median follow up of 11.7 months, disease control was achieved in 63% of patients. Five patients (26%) achieved a complete response, another five (26%) patients achieved a partial response, and two patients had stable disease (11%) at the time of last follow-up." Princess Alexandra's data have been presented on a poster, but have not yet been published.
 |
| Click to enlarge |
 |
| Click to enlarge |
Quality over quantity. I know it's obvious, but with Provectus' CTO Dr. Eric Wachter, PhD, I believe a small number of good-to-great clinical trials sites are more useful than a large number of average-to-good sites. For example, see "The art and science of forecasting with clinical informatics" (July 19, 2016). With former Chairman and CEO and a co-founder Dr. Craig Dees, PhD and President Dr. Tim Scott, PhD and a co-founder, I would imagine it was/is the opposite, quantity over quality.
Seeking default, Provectus vs. Dees. A judgement of default was entered against Craig on July 20th (it originally was sought/filed on May 5th and amended on June 3rd). H/t InvestorVillage poster joeytakasugi: A request for an entry of default against Craig's wife was filed in July.
Ito. The more I think about it, the more I wonder if (believe) the catalyst or Eureka moment was Craig et al.'s (Craig's, specifically) reading of Ito et al., "Induction of thyroid tumors in (C57BL/6N x C3H/N)F1 mice by oral administration of 9-3',4',5',6'-tetrachloro-o-carboxyphenyl-6-hydroxy-2,4,5,7-tetraiodo-3-isoxanthone sodium (Food Red 105, Rose Bengal B)." J Natl Cancer Inst 1986 Jul; 77(1):277-81.
The local doorway to the systemic world. H/t @bradpalm1: "Combining Ipilimumab With Local Treatments Improved Survival for Patients With Melanoma" (July 27, 2016):
"We found that adding local peripheral treatments, including external radiotherapy, electrochemotherapy, or internal radiotherapy, to systemic ipilimumab treatment doubled survival chances in our patient cohort and did not increase immune-related side effects...
We were also able to begin to investigate the potential immunologic mechanism underlying the benefit of adding local peripheral treatment to ipilimumab. It seems that local peripheral treatments activate immune cells, which are then able to attack tumors at sites away from the local treatment site."Interesting.
 |
| Click to enlarge. Tweet image source |
“It’s true that I was very positive on Kyndrisa,” Bienaimé says. “The FDA asked us to file. We had priority review, breakthrough designation. We had everything. The signals were very positive. So they do all that, and then they tell you your data were garbage.” He laughs. “Call it a mistake–fine.”Insufficient tumor destruction. Uninjected agonists cannot generate enough tumor destruction to kickstart the antigen cascade. Ben Adams, FierceBiotech, "Q2 clear-out sees AstraZeneca dump gastric cancer, NASH PhIII studies" (July 28, 2016):
"It also dropped a combo trial for its PD-L1 drug durvalumab (which recently gained an FDA "breakthrough" in bladder cancer) and MEDI6383, an OX40 agonist, also targeting solid tumors.
The OX40 agonist has shown antitumor efficacy in preclinical trials and is believed to have the ability to combine effectively with therapeutic cancer vaccines, as well as immuno-oncology targets. A Phase I for MEDI6383 on its own has also been culled."See Precursor (April 15, 2016) under the blog's Archived News V page:
At AACR: A first-in-human phase I dose escalation study of the OX40 agonist MOXR0916 in patients with refractory solid tumors.
"Eleven of 70 pts (16%) have been treated with MOXR0916 for > 6 months (≥9 cycles) with a best response of stable disease per RECIST v1.1."
OX40 is a stimulatory agent found in Step 3 of the Cancer Immunity Cycle.Updated (7/28/16): Visitor. Gilead Sciences (Foster City, CA) visited the blog's Archived News I page (July 2013-May 2014) for a few minutes this evening/afternoon, exiting a link* under the item Chemistry, Manufacturing and Controls (April 11, 2014). The last visit in May (also the Foster City router, but a different IP address) more than likely was a "mis-visit."
* The FDA recognizes CMC development parallels clinical investigations.
Updated (7/29/16):
 |
| Click to enlarge. Time is Eastern (Daylight) Time. |
Also see the table below under PeterMac (April 12, 2016) on the blog's Archived News V page.
 |
| Click to enlarge. |
Expired. Provectus issued a Schedule TO/A (Final Amendment) filing yesterday noting the warrant exchange program expired "at 4:00 p.m., Eastern Time, on July 28, 2016." The last expiration-related filing, for the expiration on March 28th, is here. No warrants were tendered for this second iteration of the program. I compared the language of both expiration-relating filings using Text Compare!, and aside from dates and numbers in context (about 8 million warrants were tendered last time), there do not appear to be material differences in the filings language.
 |
| Click to enlarge. |
Infantile (July 26, 2016)
One of several of management's smart and intelligent perspectives from Provectus' early days (that also continue today), this one in regards to drug approval strategy, was to use Rose Bengal (PV-10, PH-10), a halogenated xanthene, as their lead active pharmaceutical ingredient (investigational drug compounds) because of the API's established safety profile and history as an intravenous hepatic diagnostic (131I-radiolabeled Rose Bengal/Robengatope®) and topical ophthalmic diagnostic (Rosettes®, Minims®). These were Rose Bengal's original and first medicinal uses.
In parallel, and over time, management began to gradually protect the company's intellectual property of (a) therapeutically using and (b) making/manufacturing for therapeutic use all of the halogenated xanthenes (i.e., relatives of Rose Bengal), which might prove to be more efficacious than the lead API/drug compounds but do not yet have established safety profiles. For example, PV-12 is a Rose Bengal analog with the halogen bromine attached to several sites on the parent xanthene molecule, and apparently more potent than PV-10.
 |
| Click to enlarge. Corporate presentation, slide titled Some Clinical Trial Data: Melanoma, Cancers of the Liver |
Rose Bengal (as PV-10 has a half‐life of ~30 minutes in the blood stream, and in Provectus' melanoma and liver cancer clinical trials has demonstrated good and consistent pharmacokinetic (PK) properties in adults.
Rose Bengal's therapeutic use has been formally described and discussed in biomedical literature. There are foreshadowing anecdotes as well, such as Delprat's 1920 observation of his patient that "while observing with interest the injection of this beautifully colored “medicine” he stated that he felt much better and wanted more." See June 9, 2015 blog post He stated that he felt much better and wanted more. Or Ito's 1986 observation of Rose Bengal dose-dependent survival increases/improvements in mice. See June 13, 2015 blog post Reproducibility, the Hallmark of Western Science.
There also is a robust library of biomedical literature experimenting on/with, describing and discussing Rose Bengal's diagnostic applications in adults, and notably in children. See, for example October 15, 2015 blog post Still Standing, or Rose Bengal in children (hepatoblastoma, radiopharmaceutical) (May 6, 2016) on the blog's Archived News V page. In medicine, children are not small adults when it comes to safety, efficacy, dosing, etc. Most of the pediatric literature related to Rose Bengal refers to the API as liver function diagnostic 131I-Rose Bengal. From a safety and PK perspective, it is interesting, available for review, and dates back to at least the 1960s.
1963: White et al., PEDIATRIC APPLICATION OF THE RADIOIODINE (I-131) ROSE BENGAL METHOD IN HEPATIC AND BILIARY SYSTEM DISEASE, Pediatrics. 1963 Aug;32:239-50. (Departments of Radiology, Pediatrics, and Surgery of The Children's Mercy Hospital, Kansas City, Missouri)
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source, 2006-2016 |
 |
| Click to enlarge. Image source, 2014-2016 |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
Knowing then what I know now... (July 21, 2016)
Updated below, again, once more, again.
...I would own more shares at a lower cost basis. Oh, I wish.
In July 19, 2016 blog post It's the small molecule chemical, stupid I reflected on the question of knowing what I know now about how unfamiliar or skeptical the pharmaceutical industry and its ecosystem have become with small chemical molecule-based cancer treatments (let alone ones that are delivered intratumorally, intralesionally or non-surgically locally), how differently may or would I have invested.
It seems to me Provectus' clinical trials must generate data that demonstrates or shows PV-10's ability to provide a systemic clinical impact, effect or benefit; that is, the company must generate data in context.
I believe there is significant curiosity about or interest in Rose Bengal and PV-10 in the oncology world; for example and for what it's worth, see Visitors (April 6, 2016) on the blog's Archived News V page (that is the only metric I directly can view and assess; the rest is rumor or hearsay). I think management has made substantial clinical, regulatory, intellectual property protection, manufacturing and other progress; how fast [or slow] this progress occurred is, of course, up for debate. I think Provectus' assets are worth more than when I first hazarded my guess of this figure in 2014; how much of this worth management can monetize or is capable of monetizing also is up for debate.
Alternative to my original reflection, knowing then what I know now, would I be here (and invested) knowing what I know now? I believe I would.
Updated (7/23/16): Posters on one analog, digital or social medium or another (e.g., existing investors, prospective investors, pumpers, trolls, supporters, critics, neutrals, observers, journalists, commentators, etc.) have been very valuable sources of a wide range of topics, material, opinion and information (whether they know it or not, particularly when combined with information I know of a topic, him or her, their opinion, a potential company action or decision, etc. — sometimes one cannot connect the dots until one or more dots materialize). It has helped me undertake necessary ongoing due diligence, and to remain vigilant. I wrote about this, in part, in January 6, 2012 blog post The Wisdom Of Crowds.
Whether I engage posters or biopharmaceutical industry ecosystem constituents or not (because they go on by themselves unsolicited or without prodding, or others engage them, and I merely observe), we are all "lab rats" that require watching and observing our reactions to trade or investment stimuli — all of this approach and activity is in the service of seeking the answer(s) to the question(s) all traders and investors must ask themselves: "What if I am wrong? What would that scenario look like?" (i.e., the other side of the trade/investment).
Which brings me to fellow lab rat @johnhallnj, who tweeted to me today:
 |
| Click to enlarge. Tweet image sources here, here, here and here. |
 |
| Click to enlarge. Tweet image source from 2016 |
 |
| Click to enlarge. Tweet image source from today |
Because from its inception Provectus has been mostly a retail investor and non-life sciences professional investor story, it's been necessary, in part, to observe the John Halls of the world, thus harnessing that crowd's wisdom, in seeking an ongoing answer to the question: "Does active pharmaceutical ingredient (API) Rose Bengal's and thus pharmaceutical product PV-10's, and PH-10's actual, potential and perceived therapeutic benefits and strengths continue to sufficiently exceed management's actual and perceived weaknesses to potentially generate a sufficient return on investment for the risk incurred on it over time?"
Updated (7/23/16): My fuzzy recollection is that on June 3rd a New York-based Provectus investor was panicked by John's comments about an Adam Feuerstein tweet about the company.
I was flying back from ASCO, and fielded a conference call with John and another investor to discuss the topic (as I walked off the plane and to my car). There was no substance to the discussion with John that day because he merely reacted to a tweet without temporal substance; however, the panicked investor, who already had a low opinion of management, "suddenly" became unsure about the drug (and its ASCO 2014-related clinical trial information) because of his existing view of management.
Ten days later Mr. Feuerstein followed up with an article based on his June 3rd tweet entitled "Biotech Stock Mailbag: Vanda, Navidea, Provectus." At the time, I addressed the issue under Evolving Utility of Intralesional Therapy for Melanoma (July 2, 2014) and Various (July 30, 2014) on the blog's Archived News II page. To recap, Feuerstein said the following about what was "[n]ot good" for Provectus in regards to PV-10, "unusual," "highly selected" melanoma patients, and Dr. Axel Hauschild, MD, PhD:
Two years later, we've learned several things, among them:
Updated below.
This news item will be updated throughout the day. Initially, here are several slides of interest to me from Roche's half-year results 2016 investor update (white-bordered pinky/reddish star emphases are mine):
Updated (7/21/16): The slides from Roche above are interesting to me for several reasons including but not limited to:
Precedents. Clinical trials used to support drug approval are themselves precedents, or form clinical trial "law." Provectus' pivotal melanoma Phase 3 trial was designed after the randomized controlled trial that saw ipilimumab approved in 2011. Princess Alexandra Hospital's work -- see In reverse order (July 19, 2016) below -- was an open-label, single arm, nonrandomized study. Pembrolizumab for advanced melanoma was approved on the basis of a single arm study.
Updated (7/23/16): My fuzzy recollection is that on June 3rd a New York-based Provectus investor was panicked by John's comments about an Adam Feuerstein tweet about the company.
 |
| Click to enlarge. Tweet image source |
Ten days later Mr. Feuerstein followed up with an article based on his June 3rd tweet entitled "Biotech Stock Mailbag: Vanda, Navidea, Provectus." At the time, I addressed the issue under Evolving Utility of Intralesional Therapy for Melanoma (July 2, 2014) and Various (July 30, 2014) on the blog's Archived News II page. To recap, Feuerstein said the following about what was "[n]ot good" for Provectus in regards to PV-10, "unusual," "highly selected" melanoma patients, and Dr. Axel Hauschild, MD, PhD:
"At the ASCO meeting, Dr. Axel Hauschild gave an oral presentation and review of intralesional therapies in which he described the response criteria used by Provectus in the PV-10 phase II study as "unusual." The PV-10 study also enrolled "highly selected" melanoma patients, Hauschild said.
Commenting on the future for intralesional therapies in melanoma, Hauschild, like Weber, recommended combination approaches with immunotherapies like PD-1s. He noted that Amgen (AMGN) and Bristol-Myers Squibb (BMY) are conducting a study of T-Vec combined with nivolumab. The challenge for therapies like PV-10, Hauschild added, was demonstrating a benefit for melanoma patients beyond what's been seen recently with PD-1s and combination therapy already. The just-completed ASCO meeting was buzzing with incredible immunotherapy data in melanoma, so finding a role for intralesional therapies will be difficult, Hauschild said."Two post-ASCO articles about Hauschild and his comments during the "Evolving Utility of Intralesional Therapy for Melanoma" session confirmed the discussant's (Hauschild's) comments attributed by Feuerstein to Provectus and PV-10 instead were made about OncoSec and its IL-12 + electroporation melanoma Phase 2 trial. Interestingly, when called out by another Provectus shareholder (a regular hatter for this blog, and an individual of great insight and perspective as well [an internal medicine doctor who has treated cancer patients and has broad experience with approved and novel cancer treatments; an entrepreneur; an individual familiar with patent strategy, the US PTO patent application process, etc.) about Hauschild, Mr. Feuerstein subsequently deleted his fiery retort.
Two years later, we've learned several things, among them:
- Anti-PD-1 drugs appear to be forming the backbone of cancer treatment, advanced and approved almost by brute force by Big Pharma,
- There may be some challenges ahead for anti-PD-L1 agents,
- Immune checkpoint inhibitors such as anti-CTLA-4, -PD-1 and -PD-L1 drugs and investigational agents are not a panacea, and need help, specifically with addressing the tumor microenvironment (TME) (including immunogenicity, and turning hot tumors hotter, and cold tumors hot),
- There is growing data and belief that the category of intralesional agents, led by approved T-Vec and late-stage trialed PV-10, because of their direct application to TME (among other features and benefits) may be synergistic with co-inhibitory blockade (potentially more so than other pairings), and
- Hauschild is the lead European clinical investigator for Provectus' pivotal melanoma Phase 3 trial of PV-10 as a monotherapy or single agent for patients with Stage IIIB-IVM1a staged or disease. Rhetorical: If Hauschild thought PV-10 was "bad" and Provectus was doing "something untoward" clinically, would he have signed up to participate in the trial?
The takeaway is that for some people, and for some time, some of management's behavior and actions (which Mr. Feuerstein tried to frame, albeit in a less than intellectually honest manner) have overshadowed some very good work by some of management to display a lot of Rose Bengal and PV-10's actual and potential clinical benefit.
Updated (7/24/16): Returning to John's statements:
Recalcitrant (July 21, 2016)Updated (7/24/16): Returning to John's statements:
- "no one will ever fault you for due diligence..." Due diligence is what investors of all stripes should and must do on an ongoing basis for any investment (or, in context, trade),
- "but if you believe you have to stay the course. In the case of $PVCT I need to see P3 data before I even start" I currently stay the course because of my answers to the two questions I posed above. For John, as he later tweeted, more data is required to convince him that, what [I believe] he believes is there indeed is there, despite some of the negative aura of Provectus management,
- "you can have the science nailed but dilution is always more than you can imagine with small Bios" I think most long-time Provectus shareholders who remain positive and constructive on the company (on net) have a good-to-great handle on the science, its therapeutic prospects and its potential clinical benefits. The latter half of this statement is somewhat throwaway. Dilution occurs whether pre-money valuations go up or go down for the/a particular fundraising. The forecast usually (always) is wrong. I've been very surprised, however, at how poor the company's COO and interim CEO Peter Culpepper is/was at the fundraising process. I did not expect him to lack so much respect for it, and to have so little good process notwithstanding the challenges of dealing with Provectus' CTO Dr. Eric Wachter, PhD. I also did not expect the company's board to be so go-along, get-along, so Rip Van Winkle, so lacking in fundamental competence, etc. Yes, "blame" for continual down round valuations should be laid at everyone's feet, not just Peter's. One does what one can to help management, where such help is accepted. The board and Peter have to raise money in the near-term. I can only hope they don't do something stupid, as they repeatedly have in the past, and
- "plenty of Bios I have been too early on, and I will likely be too early on others in the future, to this I say scale in." Yes John, we and I'm sure many others scaled into Provectus ownership. Like physicians from Florida, Wall Streeters from New York City boroughs, soccer/swim dads like myself, and other retail shareholders, we often can be early when investing in emerging companies like Provectus. Is it because we're not paid to be right and on-time as professional portfolio managers are? Is it because we don't have our exit (monetization) already planned or scheduled at the we enter (initiation of a position)? Maybe, like in the case of Provectus, we are all quite capable of developing a thoughtful, cogent investment thesis but at times (particularly in what we believe to be highly innovative and/or disruptive situations) underestimate the challenges involved in building a business, in a highly regulated industry, by a team of outsiders with a pretty good Plan A but no real Plan B until they needed to find and make one, to bring a novel approach to bear, with a really novel technology, during a time of industry-wide innovation, change and upheaval. Crazy stuff...
A lab rat (after reading the above): "I understand where John is coming from. I probably know a fraction of what he knows of the biotech industry. Management's questionable decisions have played a role in everyone who has ever heard or invested in PVCT. Craig's embezzlement does not help at all.
That being stated one cannot deny the potential of PV-10. I do not need to see interim data to "place my bet." I know enough about the P3 trial in conjunction with Moffitt's work and others to see it will dominate TMZ and TVEC. Nor do I need to be an expert to understand PV-10 + Keytruda will have an ORR greater than ~50%. Of course I cannot have a 2-hour discussion to dissect the minute details of the protocols to prove my point.
T-VEC and the checkpoint inhibitors have paved and will pave the way for PV-10 to be a success. As Eric mentioned some time ago, oncology is a moving playing field. Over the last 2 years, per your blog, things have changed for the better for drugs such as PV-10.
All in all, my (our) money is still at risk.
I believe most are on the sidelines not wanting to get burned. We all have heard "news is coming soon". In fact, for the last 6 years, I have been hearing that. It will come.
I believe the tipping point will occur between November (starting with the P1b combo + full liver data) and June (P3 interim). I agree with what John wrote. We need solid guidance on the interim data. I am not 100% convinced Peter can provide interim in or by December. The August CC will set the groundwork for this rest year, but it's the November CC I am really looking forward to.
All in all, the most profitable scenario is to buy as much as one can now, although the risk factor(s) are still present and will be until news comes. But by then you will not be able to get in at 32 cents."
Updated below.
This news item will be updated throughout the day. Initially, here are several slides of interest to me from Roche's half-year results 2016 investor update (white-bordered pinky/reddish star emphases are mine):
Slide 34
 |
| Click to enlarge |
Slide 35
 |
| Click to enlarge |
Slide 67
 |
| Click to enlarge |
Slide 72
 |
| Click to enlarge |
Slide 73
 |
| Click to enlarge |
- Combos, combos, combos,
- Several Roche early-stage combination trial data readouts are scheduled for 2016 or 2017 (Provectus is expected to readout preliminary data from its Phase 1b combination trial of PV-10 and pembrolizumab in 2016),
- While the combination and collaboration of Amgen's intralesional drug T-Vec (Imlygic) and Roche's anti-PD-L1 drug atezolizumab (Tecentriq) was announced in June 2015 (see Desert > Beach (July 8, 2016) below), no trial is listed among Roche's materials above. No trial is listed yet on ClinicalTrials.gov. This absence might give credence to a rumor that Merck & Co. is bogged down in its 660-person Phase 3 trial with Amgen (the trial's sponsor) combining T-Vec and anti-PD-1 drug pembrolizumab (Keytruda).
Provectus vs. Dees. H/t InvestorVillage poster joeytakasugi: A default was entered against former Chairman, CEO and a co-founder Dr. Craig Dees, PhD.
Axel Hauschild. See Stuff (July 12, 2016) below. His disclosures as of December 2015 include the below:
 |
| Click to enlarge. |
Which is best? Locoregional Therapy of Melanoma Metastases (September 2nd) at the 2016 “World Congress on Cancers of the Skin” and “Congress of the European Association of Dermato-Oncology” (EADO) in Vienna, Austria:
 |
| Click to enlarge |
Guessing. My speculation about enrollment in the pivotal melanoma Phase 3 trial:
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. Clinical Program Review, ASCO 2010 |
Initiated? (July 20, 2016)
Updated below, again.
H/t a regular hatter:
Updated (7/21/16): I previously have communicated with the above consultant and others. While I endeavor to not ask Provectus-, Rose Bengal- or PV-10-specific questions, I often query them about their backgrounds, areas of expertise, interest in the molecule, etc. Typically, and often unsolicited, I hear consistent praise of and/or respect for the company's CTO Dr. Eric Wachter, PhD's intelligence, process, and integrity, among other several relevant and germane traits and characteristics. I would imagine it would be highly unusual for Eric's consultants/vendors to not be very good at what they do.
For example, take long-time key consultant Jamie Singer. In regards to Provectus, Jamie is named on at least seven key company parent and daughter patents and patent applications Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes (#8,530,675, #9,273,022 and 13/916,408 [allowed]) and Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (#9,107,887) (cumulatively and visibly [on US PTO websites] thus far, 3 awarded patents, 1 allowed patent, and 3 in-process applications [20150290318, 20150290309 and 20150290165]).
Additionally, as Jamie Singer, she is named on composition of matter patent Phenylalkyl and pyridylalkyl piperazine derivatives (#7,101,886). As Jamie Davis, she is named on five other composition of matter patents: Substituted-3-indolyl-4-piperidino-alkyl heterocycles for the treatment of depression (#7,332,506), Substituted-3-indolyl-4-piperidino-alkyl heterocycles for the treatment of depression (7,148,235), Substituted-3-indolyl-4-piperidino-alkyl heterocycles for the treatment of depression (6,939,870), Alkynyl containing hydroxamic acid compounds as matrix metalloproteinase and TACE inhibitors (6,825,354), and Alkynyl containing hydroxamic acid compounds as matrix metalloproteinase and tace inhibitors (6,340,691).
Updated (7/22/16): In the category of "if I knew then what I know now," I have been very surprised by the weakness of Provectus' corporate communications and messaging, deficits of which include but are not limited to arrogance, opacity, fear, inexperience, incompetence, and bad process. I think my experiential dataset is sufficient (in my opinion) to believe the company could have undertaken communications and messaging in a more intelligent, thoughtful, careful, appropriate, down-the-middle-of-the-fairway manner.
The upshot of how Provectus communicates now or has communicated in the recent past, or how it doesn't or hadn't, is that it has cast a negative perspective in the last several years (communications and messaging weren't always as weak or poor) on Rose Bengal and the oncology and dermatology therapies derived from it, and certain positive processes undertaken by management. I believe there was a time, pre-circa 2010 perhaps, when there was legitimate, intellectually honest curiosity-to-interest in the halogenated xanthene's cancer fighting potential by a good number of constituents of the biopharmaceutical ecosystem. Whatever high ground there may have been certainly has been lost for the time being, as has the potential to juxtapose scientific and clinical differences to recent trainwrecks or more risky therapies. One could argue Rose Bengal and PV-10 are the anti-Theranos because of independent preclinical and clinical reproducibility by multiple third parties of the molecule's mechanism of action in multiple solid tumor cancer indications. One also could argue that Rose Bengal and PV-10 are the anti-Juno Therapeutics because they are highly unlikely to kill patients.
As such, one could gloss over what management says, or what others say of what management is doing. Take for example what I wrote under Network 1, Maxim, China, etc. (January 3, 2016) on the blog's Archived News V page:
In reverse order (July 19, 2016)
It would seem expanded access (compassionate use) programs (CUP) typically follow pivotal trials, and not the other way around. That is, most companies open a CUP as their pivotal Phase 3 trial winds it way to conclusion, rather than open a CUP before they start a pivotal trial (as Provectus did).
Consider a March 2014 Quintiles presentation entitled "Operational Considerations for Running Effective Expanded Access Programs: Spotlight on Oncology" by Rebecca Thompson and Dr. Jean-Louis Merot, MD. Selected slides from the presentation, with my orange purple arrows, follow:
Going back to at least 2009, and maybe earlier, Provectus appears to have bifurcated its overall compassionate use program (CUP). One portion of it was focused on a treatment regimen similar to a potential Phase 3 randomized controlled trial. The other was focused on other solid cutaneous or subcutaneous tumors.
Provectus' CUP (expanded access protocol or program) no longer is available in both the US and Australia. In response to a question on the May 11th 1Q16 business update conference call, the company's CTO Dr. Eric Wachter, PhD said:
Interestingly, as I noted under "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived News V page, Australia's Princess Alexandra Hospital's experience with PV-10 was a prospective, non-randomized, single centre study.
The Princess Alexandra study collected a wide array of interesting endpoints:
The Aussies thought other intralesional therapies were better than T-Vec (July 17, 2016)
A good document to review, in the context of PV-10's potential and eventual approval in Australia is the May 2016 Australian Public Assessment Report for Talimogene Laherparepvec ("T-Vec/Imlygic AusPAR"), a 112-page document. An AusPAR "provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission." The TGA, or Therapeutics Good Administration, is the Australian equivalent of the FDA.
The T-Vec/Imlygic AusPAR has some interesting parts to it. First, consider items First Round Benefit-Risk Assessment and First round recommendation regarding authorisation starting on page 50. In particular: "It is recommended that approval not be granted for use of Imlygic as monotherapy in
melanoma." Reasons included {underlined emphasis is mine}:
Could one of them be, oh I don't know,...PV-10? See, for example, from the FDA's CBER review of T-Vec:
"In our center, the use of [an approved therapy] has steeply decreased with the availability of [an unapproved one]." (July 17, 2016)
Updated below, again.
The takeaway I wanted blog news item Weird (July 15, 2016) immediately below to have is/was associated and encapsulated in the title of this item. See June 1, 2016 blog post Intralesional PV-10 for In-Transit Melanoma—A Single-Center Experience, which discusses paper Lippey et al., "Intralesional PV-10 for in-transit melanoma-A single-center experience," J Surg Oncol, 2016 May 30 about Australia's Peter MacCallum Cancer Centre's compassionate use/expanded access program (special access scheme in Australia) experience with PV-10.
PeterMac, as it is also known, has been in the news recently: e.g., July 15th's Herald Sun's "Cancer treatment: Immunotherapy lab to give Victorian patients access to latest treatments" {underlined emphasis is mine}:
Observation #1a: Brisbane, Queensland's Princess Alexandra Hospital, one of the major hospitals in Brisbane and a teaching hospital, considers PV-10 an existing standard of care at its facility for patients with in-transit melanoma.*
Conclusion #1b: Princess Alexandra considers unapproved PV-10 a better treatment option, where appropriate of course, than approved isolated limb infusion (ILI), which also is a standard of care at the hospital.
Conclusion #1c: Princess Alexandra considers unapproved PV-10 a more cost effective treatment than ILI. Princess Alexandra is a public hospital in single-payer Australia, although healthcare in the country is provided by both private and government institutions. Cost matters.
Observation #2a: Melbourne, Victoria's Peter MacCallum Cancer Centre, Australia’s only public hospital dedicated to cancer treatment, research and education, noted that the use of ILI at PeterMac steeply decreased with the availability of PV-10.
Conclusion #2b: PeterMac authors (Lippey et al.) originally concluded in the abstract of the RACS poster presentation that "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool with an acceptable side effect profile for the management of unresectable in transit and locally recurrent melanoma;" however, the "cost effective treatment tool" phrase was removed from the peer-reviewed paper. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived News V page.
Fact #3: ILI is considered medically necessary, and thus reimbursable therapy for a certain indication or presentation of melanoma in the U.S.; for example, see (past or present) The Centers for Medicare & Medicaid Services (CMS), Excellus BlueCross BlueShield, BlueCross BlueShield of Massachusetts, BlueCross BlueShield of Alabama, etc. A recent example of current use in the US is June 8, 2016's "A limb is saved, a life is changed as Moffitt Cancer Center works to perfect a targeted blast of chemo."
PV-10 use also has been undertaken at Sydney, New South Wales' Sydney Melanoma Unit and the Newcastle Melanoma Unit, and Adelaide, South Australia's Royal Adelaide Hospital. In total, PV-10 has been provided to patients in four of Australia's six states.
* Repeated follow-up emails (for further clarification) to the nurse practitioner contact at Princess Alexandra (for germane clinical trials) -- and who also kindly replied to my original inquiry and said PV-10 is not a standard of care, and that the matter would be addressed -- have gone unanswered. See Correction (April 19, 2016) on the blog's Archived News IV page.
Updated (7/17/16): H/t InvestorVillage poster leave_the_gun for being a reminder for me to re-visit ILI cost under this blog news item (I previously had surfaced the following article under A selection of literature (June 30, 2016) below).
Amgen's Ma et al., "Use Patterns and Costs of Isolated Limb Perfusion and Infusion in the Treatment of Regional Metastatic Melanoma: A Retrospective Database Analysis," Adv Ther. 2016; 33: 282–289 examined the cost of ILI/isolated limb perfusion (ILP):
The authors note the following limitations of their analysis:
ILI for melanoma is a cancer therapy (my lingo) that comprises or requires:
Weird (July 15, 2016)
When have you heard in the biopharma world that an unapproved or investigational cancer drug (PV-10/Rose Bengal) is considered:
Australia, again (July 14, 2016)
1. Provectus issued a press release and made an associated 8-K filing in regards to the company's Aussie subsidiary and office, Establishes Australian Subsidiary. In particular, COO and interim CEO Peter Culpepper noted:
"Intralesional treatment for advanced melanoma: what's on the horizon?" (July 14, 2016)
Updated below.
The source link is here.
Updated (7/13/16): From the same publication, Future Medicine, Dr. Agarwala wrote in 2012: "Intralesional therapy for metastatic melanoma with a focus on PV-10."
In 2015 he wrote: "Rose Bengal for melanoma treatment: will it translate to the clinic?"
Takeaways: Dr. Agarwala, and others (e.g., Andtbacka/Huntsman Cancer Institute, Ross/MD Anderson), going back to at least 2012, identified a role for intralesional (IL) drug compounds as single agents, calling them "[l]ocally ablative with systemic impact." At the time, those agents later in the pipeline were Allovectin-7, OncoVex (later talimogene laherparepvec) and PV-10.
But Agarwala, Andtbacka, Ross and others also foresaw the role of IL drugs in combination with newly approved immunotherapies and targeted therapies (at the time, ipilimumab/anti-CTLA-4, and vemurafenib and dabrafenib/BRAF), with goal of improving their response rates while delivering overall lower toxicities. T-Vec and PV-10 now are the leading IL agents, with others in the pipeline primarily focused on combinatorics like CAVATAK and HF10, both oncolytic viruses like T-Vec (whereas PV-10 is a small chemical molecule or ablative immunotherapy).
Evolution (July 13, 2016)
Provectus issued a press release and made an associated 8-K filing in regards to a presentation by PV-10/Rose Bengal key opinion leader and St. Luke's Health Network's Dr. Sanjiv Agarwala, MD at the 6th European Post-Chicago Melanoma/Skin Cancer Meeting in Munich, Germany on June 30th. Consider the presentations over the last couple of years (e.g., 2014 to 2016) that Dr. Agarwala has made about intralesional therapies and oncolytic agents, and note -- as the treatment landscape (e.g., R&D, preclinical studies, clinical trials, drug approvals, etc.) has changed -- how his presentations (i.e., introductions/conclusions) essentially have delivered the same overall message during this "evolution."
Stuff (July 12, 2016)
Updated below.
1. H/t InvestorVillage poster Warlord who noted Provectus' Phase 1 neuroendocrine tumors (NET) liver mets trial has started recruiting at Australia's The Queen Elizabeth Hospital (Adelaide).
The trial protocol, which was updated on July 8th, was first received by CT.gov on February 23rd; the original study start date was March (2016).
2. Provectus issued a press release and made an associated 8-K filing in regards to an ESMO 2016 poster presentation the company's clinical investigators should make, Announces Acceptance of Abstract for Poster Presentation at European Society for Medical Oncology 2016 Congress. The abstract/poster are entitled "Intralesional Rose Bengal for Stage III and IV Melanoma."
Will there be substantive information on the poster, or will it a trials in progress type like that presented at ASCO 2016, "Intralesional rose bengal for treatment of melanoma?"
Updated (7/19/16): I located more information on the above poster presentation by downloading the latest (or final) ESMO 2016 program. First, as I mostly thought, the poster is a trials-in-progress one about the two melanoma trials Provectus currently is running, like the poster at ASCO 2016. Second, as I mostly thought too, Germany's Dr. Axel Hauschild, MD, PhD is/will be a clinical investigator for one or more of the company's trials; see 10-Q Notes (May 10, 2016) on the blog's Archived News V page:
ASCO 2016:
3. During Provectus' COO and interim CEO Peter Culpepper's video interview (with Wendy Gillette) at the Marcum MicroCap Conference (June 1-2), at around the 58 second mark, Peter says [lower concentration] PH-10 down regulates for dermatology, in contrast to [higher concentration] PV-10 for oncology.
I noted in July 2, 2016 blog post Shapeshifter that concentration influences Rose Bengal's shape transformation (sphering). Intralesionally (intratumorally) injected PV-10 for oncology is a 10% solution of Rose Bengal, while topically applied PH-10 for dermatology is a 0.001% to 0.01% gel of the active pharmaceutical ingredient (API) Rose Bengal.
In regards to PH-10's mechanism of action, Provectus previously speculated "[a]pparent local down-regulating action on aberrant immune expression in skin." See a slide from one of the company's 2014 corporate presentations below.
Has Rockefeller University, the Laboratory for Investigative Dermatology, and Dr. James Krueger, MD, PhD confirmed such down-regulation? On the March 16th 4Q15/CY15 business update conference call Provectus' CTO Dr. Eric Wachter, PhD said:
Do Dr. Jedd Wolchok/Sloan Kettering Understand Immunotherapy's History? (July 12, 2016)
Updated below.
It would appear that Memorial Sloan Kettering Cancer Center (MSKCC) recently (i.e., that is 2015, perhaps earlier) embraced the history of the medical institution's involvement with harnessing the immune system to fight and treat cancer by describing the work of Dr. William Coley, MD, "now considered the “Father of Cancer Immunotherapy,”" on its website. See MSKCC webpage "Immunotherapy: Revolutionizing Cancer Treatment since 1891." Dr. Coley began his career as a bone surgeon at New York Cancer Hospital, which later became part of MSKCC.
When writing about immunotherapy, and usually pointing to Bristol-Myers' anti-CTLA-4 drug ipilimumab (Yervoy) and/or anti-PD-1 drugs pembrolizumab (Merck & Co., Keytruda) and nivolumab (Bristol-Myers, Opdivo), many mainstream media and medical writers and journalists often include in their introduction descriptions of Dr. Coley's work. I think it makes for a good story, as the writers endeavor to link history, and descriptions and lessons from the past, to the present day, and potentially our future.
MSKCC says Dr. Coley's work "paved the way for the modern immunotherapies that are helping patients today."
Route of delivery matters. Dr. Coley's work, and his approach to treatment, comprise two key feature, one of which nearly all who write about him (but not everyone) routinely ignore, conflating his discoveries, observations and conclusions with drugs incapable of delivering what he experimented with in order to seek better patient outcomes.
Coley's approach to the treatment of cancer was composed of (a) a "drug compound," the heated-killed bacteria known as Coley’s toxins whose actions following treatment somehow engaged the immune system (e.g., fever), and (b) the route of administration by injection of the dead bacteria into the patient's tumors.
The key feature of Coley's work that is ignored, conflated, confused or misunderstood: the manner in which the drug, drug compound, biologic or small molecule is delivered. Yervoy, Keytruda and Opdivo are immunotherapies that are intravenously administered to patients; they are not injected into patient tumors.
Google "Wolchok" and "Coley," and thousands of results are returned. MSKCC's Dr. Jeff Wolchok, a medical oncologist, apparently uses Coley's story when he (Dr. Wolchok) explains immunotherapy. Dr. Wolchok, holder of MSKCC's Lloyd J. Old Chair for Clinical Investigation, was a student of Dr. Lloyd Old, MD, who (according to Memorial Sloan Kettering):
The administration of BCG (aka Bacillus Calmette-Guérin) for bladder cancer is intravesical, which means it is "put directly into the bladder through a catheter, instead of being injected into a vein or swallowed." See the illustration below.
Intravesical, like intravenous is not injection into the tumor (i.e., intralesional, intratumoral).
Ironically, the second aspect of Coley's work, route of delivery, was explored in the 1970s with BCG (or BCG immunotherapy) in metastatic melanoma when the drug was it was directly injected into metastatic melanoma lesions limited to the skin; see, for example, "BCG Immunotherapy of Malignant Melanoma: Summary of a Seven-year Experience." Unfortunately, the immunotherapy failed a Phase 3 trial; see 2004 paper "Mature results of a phase III randomized trial of bacillus Calmette–Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I–III melanoma (E1673):"
Consider April 2016's "Germ of an Idea: William Coley's Cancer-Killing Toxins", which more appropriately places Dr. Coley's work into context:
Finally, it is interesting to note that Allison, Wolchok and others submitted a patent application (published in 2014) for the use of an oncolytic virus with immune checkpoint inhibitors via the injection of the virus into tumors.
Updated pivotal melanoma Phase 3 trial protocol (July 11, 2016)
Provectus' CTO Dr. Eric Wachter, PhD appears to have amended the trial protocol faster than expected.
The proposed changes were first presented at ASCO 2016; see Trials in progress (June 9, 2016) below. Side-by-side changes on ClinicalTrials.gov are here. Changes comprise:
1. Official title: "PV-10 Intralesional Injection vs Systemic Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma Without Distant Metastases" was changed to "PV-10 Intralesional Injection vs Systemic Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma"
2. Inclusion criteria:
3. Exclusion criteria:
Two points. First, recall that Australia's PeterMac noted predictors of complete response as age (p = 0.004) and lesion size (0.004, 0.008). The younger, the better. The smaller or lesser (the lesion, the grouping of them), the better. The number of injected lesions and the time from primary diagnosis to treatment were not predictive. The presence of ulceration, blistering, eschar, or pain following injection was predictive of response. See Red Food Dye No. 105 (June 1, 2016) below.
Second, the change of the official title, for me, feels like a refinement of the ultimate label (by removing the phrase "without distant metastases."
On another note, the compassionate use/expanded access program (special access scheme in Australia), as noted previously, was closed.
More optimal (July 10, 2016)
Some thoughts about the article, and some related thoughts include:
Finally, returning to the paper above, the physician authors [in my view] properly reference William Coley's work: "Intratumoral treatment as a form of regional immunotherapy can trace its origin back to 1892 with the description of Coley’s toxin." See also October 15, 2015 blog post Still Standing. Google (for news of) "immunotherapy" and "Coley," and see how many articles invoke Coley and non-injectable immunotherapies (e.g., checkpoint inhibitors); see, for example, yesterday's Allentown Morning Call article "On the cusp of a cure:"
Updated below, again.
For the love of small furry animals (and the occasional large one), would someone save me from this humidity (57%? sure...)?
Potential 2H16 data catalysts (July 6, 2016)
Updated below, again.
1. More liver Phase 1 study results: ESMO 2016, the ESMO 2016 congress, October 7-11.
Updated 7/19/16): Based on the ESMO 2016 program only noting Provectus' melanoma trials in progress poster presentation, liver data are unlikely or will not be presented here.
2. Initial melanoma Phase 1b study results combining PV-10 and pembrolizumab in Stage IV patients: SMR 2016, the 13th International Congress of the Society for Melanoma Research, November 6-9 (SITC 2016 [November 9-13] might also be a venue, but I would lean towards SMR for clinical data).
Updated (7/6/16): Recall Provectus' COO and interim CEO's comments from the May 10th 1Q16 business update conference call: "It is conceivable the interim data will be available prior to one of our upcoming conference calls. And if so, it is possible that we will hold a special update call separate from the filing related call." The 3Q16 filing (and presumably call) would be done during the week of November 7th (Monday), which could suggest SMR rather than SITC.
3. PH-10 mechanism of action study results: Sometime in 2016? On the March 16th 4Q15/CY15 business update conference call Provectus' Dr. Eric Wachter, PhD noted in regards to a question about PH-10 mechanism of action work:
Updated (7/6/16): I'm hard pressed to believe the pivotal melanoma Phase 3 trial interim readout is anything but a 1H17 event (and close to the end of the half than its beginning), despite Eric's comments on the May 10th 1Q16 business update conference call (when discussing the Imlygic-focused protocol changes):
So, from late-summer or early-fall, how long might it take to complete the interim readout, given these ASCO-proposed changes? The proposed changes purportedly would speedup recruitment, but I'll guess another 3-month delay might be in the cards, strictly due to making and filing the changes, which may or may not be addressed by faster enrollment.
A bridge (July 3, 2016)
Updated below: 9/3/16.
Last year at Melanoma Bridge 2015, organized by Fondazione Melanoma Onlus under the auspices of National Cancer Institute “Fondazione G. Pascale" (Naples, Italy), Sidra Medical and Research Center (Doha, Qatar), the Italian Association of Medical Oncology (AIOM) and the Society for Immunotherapy of Cancer (SITC), PV-10/Rose Bengal key opinion leader and St. Luke's Health Network's Dr. Sanjiv Agarwala, MD presented "Overview of intralesional oncolytic therapy" during the News on Immunotherapy session.
The Combination Therapies session contained several studies of which data were presented earlier that year (e.g., T-Vec + pembro at SMR 2015 [Long et al.], epacadostat + pembrolizumab at SMR 2015 [Gajewski et al.]).
This year at Melanoma Bridge, as the program materializes and formalizes, Dr. Agarwala appears to be presenting during the Combination Strategy Session, along with, for example, Dr. Long.
Could Dr. Agarwala present some Phase 1b combination study data of PV-10 and pembrolizumab at an earlier medical conference, like SMR or SITC 2016, and then revisit the topic at Melanoma Bridge 2016? As much as Dr. Georgina Long, MD of Melanoma Institute Australia and the University of Sydney might re-visit data on another combination presented at ASCO 2016, or another conference later in the year but before Melanoma Bridge. See the presentations at ASCO with which Dr. Long was involved: e.g., pembrolizumab + dabrafenib and trametinib, pembro + ipilimumab, T-Vec + pembro, nivolumab + ipi.
Updated (9/3/16): Dr. Agarwala's presentation at Melanoma Bridge 2016 appears to have a name now: "Innovative combination strategies – oncolytic therapy and systemic immunotherapy approaches" (setting aside the digital burp that cause the '–').
In his June 30th presentation at the 6th European Post-Chicago Melanoma/Skin Cancer Meeting in Munich, Germany, Dr. Agarwala referred to intralesional agents as intralesional oncolytic therapies, and in this category included:
I'm going to guess preliminary interim PV-10 + pembrolizumab combination therapy data for advanced melanoma now is ready and available.
Australia (July 1, 2016)
Updated below, again, once more.
"A long time ago in a galaxy far, far away....," specifically November 2010, Provectus met with the Therapeutic Goods Administration purportedly to review a path for approval of PV-10 in Australia. See the associated press release here. At the time, Dr. Eric Wachter, PhD, head of the company's pre-clinical/clinical development program and later name Provectus' CTO presumably authored most if not all of the the words in release, which noted:
About five and half years later (May/June 2016) Provectus establishes an Australian subsidiary. To what end? In An Australian office? (June 16, 2016) below I wrote about, as an example, CAR T company and U.S.-based Bluebird Bio, which established an Australian subsidiary, Bluebird Bio Australia Pty Ltd, in March 2014. Was Bluebird expecting approval in Australia when it set up the sub? I don't believe so. Rather, this company (before or around the time of creating the Australian sub) planned to treat an Australian patient, which occurred later that year ; see its December 2014 press release "bluebird bio Announces Data Demonstrating First Four Patients with β-Thalassemia Major Treated with LentiGlobin™ are Transfusion-Free." I would imagine Bluebird's approach to cancer treatment, unlike Provectus', requires proximate work to extract material (T cells) from a patient before it (the biologic material) is put back into him or her. I cannot imagine this material is shipped back to the U.S. (Cambridge, Massachussetts, or wherever Bluebird's production or lab facility is), and then returned to Australia. I would have to believe such work would be (would have been) done in Australia, requiring staff and lab space, which would generated SGA and/or R&D expense that would be run through the Australian sub, which then would roll-up to the U.S. parent's income statement, balance sheet and statement of cash flows. As such, Bluebird's near-term business goal for establishing its Australian subsidiary was to run expenses through it (and likely use it for liability reasons as well).
Provectus has run trials in Australia in the past and shipped product there. There has not appeared to be a historical clinical trial-based requirement to establish a subsidiary. What is the requirement now? Perhaps, in light of the introduction to this blog news item, a different question to ask is "What is the current requirement for Provectus to obtain approval in Australia?" That is, has the landscape, generally and specifically, changed from 2010 to 2016, such that the "terms" for an early evaluation for marketing approval may be different now?
Updated (7/2/16): H/t a regular hatter who provided the Australian Securities and Investments Commission (ASIC) details for Provectus Biopharmaceuticals Australia Pty Ltd. See three screenshots below (the original document is available as an automatic download using this link)
Trood Pratt & Co. (not Trodd as in the document, but with the same address) is a Sydney-based accounting firm. A screenshot from the firm's May client alert is below:
The Australian subsidiary's office address of 75 Pacific Highway, Suite 7, Waitara, New South Wales, 2077. Various pictures and web items include:
Dr. David Sarson, PhD is the co-contact on Provectus' "A Phase 1 Study of PV-10 Chemoablation of Neuroendocrine Tumours (NET) Metastatic to the Liver" at The Queen Elizabeth Hospital in Woodville, Australia. Dr. Sarson is/has been a [managing] director (owner) of Delpharm Consultants Pty Limited (the website does not work).
"Delpharm Consultants is one of Australia's leading independent consultancies focusing on pharmaceutical development and related regulatory issues."
Is Provectus' Australia subsidiary for cost reimbursement related to the PV-10 Phase 1 NET liver mets, or a pathway to approval for PV-10 in melanoma?
Updated (7/2/16): In a continued dialog on the topic of Australia with the hatter of the hat tip immediately above, he or she noted Delpharm Consultants involvement with Novartis Fluad; see page 187 of "The Australian Immunisation Handbook, 9th Edition" (2008). This piqued my interest to search the TGA's website for "Delpharm Consultants," which returns 12 items.
One item of note to me was a January-June 2012 AusPAR for U.S.-based Osiris Therapeutics' Prochymal that had Delpharm as the sponsor. Prochymal is a stem cell therapy. It received marketing authorization in Canada in May 2012 and marketing consent in New Zealand in June 2012. Osiris sold Prochymal to Australia's Mesoblast in October 2013, which re-labelled the drug MSC-100-IV. The story, good and bad, continues. A November 2015 short thesis is here. The drug was licensed to a Japanese company, JCR Pharmaceuticals, which launched the drug in Japan in February 2016.
From June 28, 2016 TGA webpage "Australian Public Assessment Reports for prescription medicines (AusPARs):"
I'm very much guessing at the following. Consider the verbiage and figure below from "Transparent Regulations for Prescription Medicines: The Australian Way."
There is on the TGA website a "Pre-submission Pilot Evaluation Plan Estimator" that another hat tipper pointed me to. The TGA noted on another webpage, June 29, 2016 "Prescription medicines: pre-submission pilot:"
The Australian subsidiary was incorporated on May 18th, and opened on June 6th. Using these dates would of course change the math of the dates above.
Updated (7/3/16): According to the curriculum vitae (CV) of Princess Alexandra Hospital's Dr. Mark Smithers, MD, Delpharm was a co-sponsor of Provectus' "A Phase 2 Study of Intralesional PV-10 in the Treatment of Metastatic Melanoma."
As the regular hatter contributing to this news item noted, Delpharm is listed in the CV as a sponsor, but the Australian company does is not named on ClinicalTrials.gov (or the Australian-New Zealand equivalent.
June blog readership statistics.
Category vs. Agent (June 30, 2016)
Updated below.
It seems to me that Huntsman Cancer Institute's Dr. Robert Andtbacka, MD might be an intralesional (IL) therapy key opinion leader (KOL), a "category KOL," while Rutgers Cancer Institute of New Jersey's Dr. Howard Kaufman, MD is a talimogene laherparepvec (T-Vec) KOL and St. Luke's University Health Network Dr. Sanjiv Agarwala, MD is a PV-10 (rose bengal) KOL, both agent KOLs.
Furthermore, one or both of Andtbacka and Agarwala might also be IL combination KOLs too, more so than other clinicians who were/are IL agent KOLs (e.g., Kaufman), or KOLs of immunotherapies that could be combined with IL agents (e.g., NYU Langone Medical Center's Weber, Memorial Sloan Kettering Cancer Center's Chapman, UCLA's David Geffen School of Medicine's Ribas, etc.). Agarwala has been discussing the combination of IL agents with immunotherapies (immune checkpoint inhibitors) and targeted therapies (e.g., vemurafenib) as early as 2012. Andtbacka has succinctly described the clinical value proposition of combining IL agents with immune checkpoint inhibitors (see A selection of literature (June 30, 2016) immediately below):
Provectus' metastatic melanoma Phase 2 trial prospectively explored the idea and concept behind the bystander effect because some patients had all of their lesions (disease) treated, and some did not.
BioVex (acquired by Amgen for OncoVEX, or T-Vec) did not initially (prospectively) undertake a bystander effect analysis in the course of its metastatic melanoma Phase 2 trial (from 2008'ish); Kaufman did a post hoc analysis: Kaufman et al., "Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study," J Immunother Cancer. 2016 Mar 15;4:12.
Very carefully "comparing" PV-10 and T-Vec results, one could say (or maybe not):
Updated below.
January 2016: "Use Patterns and Costs of Isolated Limb Perfusion and Infusion in the Treatment of Regional Metastatic Melanoma: A Retrospective Database Analysis" (Amgen paper)
Updated (6/30/16): June 2016 (27th): "Neoadjuvant T-VEC Topic of Phase II Melanoma Study" (Huntsman Cancer Institute, Amgen)
Updated below.
1. I previously wrote about an Australian subsidiary of Provectus, and a potential or possible in-country office. See An Australian office? (June 16, 2016) below, which also re-visited the company's CTO Dr. Eric Wachter's previous comments regarding the eventual wind down of Provectus' compassionate use/expanded access program (called a special access scheme in Australia).
At the April 9, 2015 panel session Provectus held in New York -- see Audiophile (April 19, 2015) and Kick-off (June 22, 2015) on the blog's Archived News III page -- the company said its pivotal melanoma Phase 3 trial would comprise 25 sites in the U.S. and 10 in Australia (not including sites Provectus might or might not have in other countries, such as China, India and Brazil).
On the company's May 7, 2015 1Q15 conference call, in regards to the pivotal melanoma Phase 3 trial, Eric said: "As I've noted previously, we expect enrollment to be approximately one-third from the U.S.; one-third from Australia; and one-third from the rest of the world." See Conference Call, part 3 (May 10, 2015) on the blog's Archived News III page.
On Provectus' May 11th 1Q16 business update conference call, Eric's Eric's plan to address the Phase 3 being behind schedule was:
Updated (6/29/16): H/t regular finders of hats InvestorVillage poster canis_star who observed sponsors of every approved drug in Australia had a registered Australian company (i.e., with a name that included Pty Limited). Reviewing one of Australia's Therapeutic Goods Administration (TGA)' web pages the poster linked to, June 2, 2016's Prescription medicines: registration of new chemical entities in Australia, all sponsors in 2014, 2015 and 2016 were Australian subsidiaries, whether titled with a name that included Pty Ltd or, in the case of Bayer, Australia Ltd (i.e., Bayer Australia Ltd).
I previously address managed entry/access in Australia under blog news item Managed entry agreements/Managed access programmes (April 16, 2016) on the Archived News V page. In 2011, Australia introduced a managed entry scheme for listing new medicines pending the availability of more conclusive evidence of cost-effectiveness to support continued listing at a higher price (source: taken from Wonder et al., "Australian Managed Entry Scheme: A New Manageable Process for the Reimbursement of New Medicines?").
A March 2015 working document entitled "Framework for the Managed Access Programme for submissions to the PBAC" notes {underlined emphasis is mine}:
Is Eric pursuing a managed entry scheme approach in Australia for PV-10 for the treatment of in transit melanoma? Both Peter MacCallum Cancer Centre (PeterMac) in Melbourne and Princess Alexandra Hospital in Brisbane presented abstracts about in transit melanoma patients they treated from 2010-14 and 2005-15, respectively. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived News V page.
2. A visitor from Paris' Université Pierre et Marie Curie (UPMC)/Pierre and Marie Curie University visited the blog earlier today for some time. He or she visited, among other pages, June 13, 2015
blog post Reproducibility, the Hallmark of Western Science.
A 2015 paper including UPMC co-authors was entitled "Trial Watch—Oncolytic viruses and cancer therapy," Pol et al., Oncoimmunology. 2015 Dec 8;5(2):e1117740. eCollection 2016. One of the authors (at UPMC). There is a relationship between UPMC and France's INSERM, both medical research institutions. One of the paper's author is INSERM and Gustave Roussy Cancer Center's Dr./Prof. Laurence Zitvogel, MD, PhD, whose current research includes tumour immunology and immunotherapy.
When you Google "rose bengal" and "Zitvogel," papers by Panzarini et al. come up regarding Rose Bengal Acetate PhotoDynamic Therapy that reference Dr. Zitvogel's work (i.e., published papers) and, for example, immunogenic and tolerogenic cell death.
In Moffitt Cancer Center's PV-10 mechanism of action paper "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1" paper, the authors reference Panzarini (one paper) and Zitvogel (three papers).
What is UPMC's interest in Rose Bengal and PV-10?
3. H/t InvestorVillage poster Area51 for the below, Provectus' lawsuit against former Chairman, CEO and a co-founder Dr. Craig Dees, PhD:
A short perspective (June 27, 2016)
At June 15th, Provectus' short interest as a percent of float was 0.8%. For several different reasons, the company is not relevant to the capital markets.
The top 5 biotechnology firms with the highest short interest are listed in the tweet below, ranging from ~28% to more than 48%.
Despite the discrepancy in short interest figures between the top 5 and Provectus, there should opportunities for traders to make some money in the tens of thousands of dollars from time to time.
Additive: 1 + 1 < 2. Synergistic: 1 + 1 > 2 (best case, >> 2) (June 25, 2016)
Updated below.
Following up on the blog news item immediately below, The bar (June 24, 2016), additive in the context of immuno-oncology combinations might be represented as one plus one is less than or equal to two; that is, efficacy (e.g., response rate) of the combination would be better than the individual efficacies of the pair's components. The sum of the parts is greater than the whole.
A synergistic combination, however, could or should generate efficacy greater than the sum of the individual efficacies, and, in a best case, much greater than the sum; that is, the whole is greater than the sum of the parts.
Taking MD Anderson's Dr. Merrick Ross, MD's combo therapy for melanoma slide -- see ASCO 2016: "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist" (June 22, 2016) -- I tried to illustratively model the difference between additivity and synergism.
My takeaway for this news item is not the simple (above) addition of un-apples-to-un-apples trial response rates that might imply, suggest or show a PV-10 + pembrolizumab combination will generate very notable response rates. I do believe, however, that the potential is there.
More importantly, I return to the question I asked below. Would/will a PV-10 + pembro combination be additive in its results, or synergistic? If the pairing is additive, I'd imagine we see response rates (the Phase 1b study's secondary endpoints are progression-free survival and objective response rate) greater than ~60%.
If the pairing is synergistic, what would/could the response rate be? If the pairing is synergistic, how synergistic could it be?
Updated (6/27/16): If one was to try to compare apples-to-apples — still "sort of," and still very carefully and with upteen caveats — you'd have to consider the following before trying to assess or speculate about the outcome of the Phase 1b study combining PV-10 and pembrolizumab (n.b. I am trying to do the former, and I do have my own thoughts and beliefs about it, without treading on the latter):
The bar (June 24, 2016)
What is the bar for a combination therapy? A tweet from Dr. Vanessa Lucey, PhD/@vmlucey provides:
In particular, note "What is additive vs. synergistic? Are we observing enhanced tumor antitumor immunity?" Consider Moffitt Cancer Center's AACR 2016 abstract conclusion:
Now consider a tweet from Merrilyn Datta/@merrilyn57:
Provectus' CTO Dr. Eric Wachter, PhD's process appears to be transitioning from a Phase 1b combination of two agents (one approved, one investigational) to a randomized Phase 2.
But what could be the bar for perceived success of PV-10 and pembrolizumab? The bar might include an objective response rate of at least ~60% for melanoma (a so-called "solid tumor cancer").
The acronym RRMM above stands for Relapsed/Refractory Multiple Myeloma, a so-called "blood cancer."
Mostly cloudy. A high of 88, and 62% humidity. (June 23, 2016)
Updated below, again.
1. Provectus principals Dr. Timothy Scott, PhD (President), Dr. Eric Wachter, PhD (CTO) and Peter Culpepper (COO, and interim CEO) have a number of stock options that expire today; 1.05 million, 680K and 1 million, respectively. No significant amount of options are available to Dr. Scott and Peter until 2020, and Eric until 2025. If some of these options are exercised, one would imagine Form 4s would be filed by or on Monday, June 27th.
A quick 'n dirty analysis of share ownership by the principals could suggest:
2. A very quick 'n dirty analysis could suggest Peter might raise capital sometime in July, setting aside the current warrant exchange offer/program. For re-evaluation, however, does the threshold have to be crossed on a month-end basis, a quarter-end basis, or on some other time frame measure?
One also wonders if Peter's interaction with the board on the topic of fundraising in the past has been and/or currently is thoughtful and prepared, or last minute, rushed and jams directors, or something in between.
3. H/t a shareholder and periodic hatter in regards to another melanoma combination of NewLink's IDO inhibitor indoximod and co-inhibitory blockade. The Phase 1b results of indoximod/ipilimumab combination in seven patients yielded an objective response rate (ORR) (by RECIST) of 29% (14% complete response [CR]). Compare this result to epacadostat/pembrolizumab's combination of 57% and 29%, respectively. NewLink's Phase 2 study (this company is the sponsor; there is no collaborator) appears to be a non-randomized clinical trial of three arms: indoximod + ipilimumab, indoximod + nivolumab, and indoximod + pembrolizumab.
Updated (6/23/16): H/t the same hatter who noted NewLink presented more recent combination data at ASCO 2016:
4. Of the potential amendments to the pivotal melanoma Phase 3 trial protocol, see Trials in progress (June 9, 2016) below, the third (i.e., an aggregate diameter of target lesions of at least 1 cm [currently 1 to 5 lesions of at least 1 cm in longest diameter]) could be the most important because classic in-transit patients are similar to the example in Lippey et al.'s Figure 1, which observed that (a) the majority of patients Peter MacCallum treated with PV-10 had lesions <1 cm (12 of 19 patients) and (b) the number of lesions for most patients was <10 (13 of 19) (see Table II in Lippey et al.).
5. Huh? 17th Annual International Lung Cancer Congress
Updated below, again.
H/t a regular hatter:
Updated (7/21/16): I previously have communicated with the above consultant and others. While I endeavor to not ask Provectus-, Rose Bengal- or PV-10-specific questions, I often query them about their backgrounds, areas of expertise, interest in the molecule, etc. Typically, and often unsolicited, I hear consistent praise of and/or respect for the company's CTO Dr. Eric Wachter, PhD's intelligence, process, and integrity, among other several relevant and germane traits and characteristics. I would imagine it would be highly unusual for Eric's consultants/vendors to not be very good at what they do.
For example, take long-time key consultant Jamie Singer. In regards to Provectus, Jamie is named on at least seven key company parent and daughter patents and patent applications Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes (#8,530,675, #9,273,022 and 13/916,408 [allowed]) and Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (#9,107,887) (cumulatively and visibly [on US PTO websites] thus far, 3 awarded patents, 1 allowed patent, and 3 in-process applications [20150290318, 20150290309 and 20150290165]).
Additionally, as Jamie Singer, she is named on composition of matter patent Phenylalkyl and pyridylalkyl piperazine derivatives (#7,101,886). As Jamie Davis, she is named on five other composition of matter patents: Substituted-3-indolyl-4-piperidino-alkyl heterocycles for the treatment of depression (#7,332,506), Substituted-3-indolyl-4-piperidino-alkyl heterocycles for the treatment of depression (7,148,235), Substituted-3-indolyl-4-piperidino-alkyl heterocycles for the treatment of depression (6,939,870), Alkynyl containing hydroxamic acid compounds as matrix metalloproteinase and TACE inhibitors (6,825,354), and Alkynyl containing hydroxamic acid compounds as matrix metalloproteinase and tace inhibitors (6,340,691).
Updated (7/22/16): In the category of "if I knew then what I know now," I have been very surprised by the weakness of Provectus' corporate communications and messaging, deficits of which include but are not limited to arrogance, opacity, fear, inexperience, incompetence, and bad process. I think my experiential dataset is sufficient (in my opinion) to believe the company could have undertaken communications and messaging in a more intelligent, thoughtful, careful, appropriate, down-the-middle-of-the-fairway manner.
The upshot of how Provectus communicates now or has communicated in the recent past, or how it doesn't or hadn't, is that it has cast a negative perspective in the last several years (communications and messaging weren't always as weak or poor) on Rose Bengal and the oncology and dermatology therapies derived from it, and certain positive processes undertaken by management. I believe there was a time, pre-circa 2010 perhaps, when there was legitimate, intellectually honest curiosity-to-interest in the halogenated xanthene's cancer fighting potential by a good number of constituents of the biopharmaceutical ecosystem. Whatever high ground there may have been certainly has been lost for the time being, as has the potential to juxtapose scientific and clinical differences to recent trainwrecks or more risky therapies. One could argue Rose Bengal and PV-10 are the anti-Theranos because of independent preclinical and clinical reproducibility by multiple third parties of the molecule's mechanism of action in multiple solid tumor cancer indications. One also could argue that Rose Bengal and PV-10 are the anti-Juno Therapeutics because they are highly unlikely to kill patients.
As such, one could gloss over what management says, or what others say of what management is doing. Take for example what I wrote under Network 1, Maxim, China, etc. (January 3, 2016) on the blog's Archived News V page:
In July 2015, Provectus and Boehringer Ingelheim (BI) China made announcements of their entering into/signing a letter of intent: Provectus Biopharmaceuticals Signs Letter of Intent with Boehringer Ingelheim (China) to Collaborate in Bringing PV-10 to Market in China (Provectus' PR on July 2nd) and Boehringer Ingelheim and Provectus biopharmaceutical company signed a letter of intent - the two sides will cooperate to promote melanoma and liver cancer research new drugs listed in the Chinese mainland, Hong Kong and Taiwan (BI's PR, translated into English, on July 3rd).
On July 10th, Jialing Dai wrote a LinkedIn article entitled Boehringer's Ambition In Liver Cancer. The article's byline read: "Eye On Oncology Market: Boehringer Ingelheim In-Licenses China Rights For A Provectus Late-Stage Oncology Compound." The article was edited by Howard Fields. Dai describes his some of his work as "PharmaDJ is online portal in English to provide tailored services and intelligence of China market for pharma executives. our goal is to build up a bridge to China market for foreign companies doing business in China." In his article Dai wrote:
"PV-10, an injectable formulation of rose bengal, also known as Provecta, is the leading compound of the Knoxville, Tennessee-based company. The firm received orphan-drug designation from the U.S. FDA for treating of hepatocellular carcinoma (HCC) in 2011 and melanoma in 2007. Currently, the drug is in a U.S. Phase III clinical trial for melanoma and Phase I trial for HCC.
“We need to wait on the outcomes of these studies before deciding to file or not with CFDA, but we certainly have an interest in developing this drug in HCC in China, as there is a large unmet medical need,” BI China told PharmaDJ in an interview." {Underlined emphasis is mine}
Dai also noted in his article the below:
"The German pharma giant doesn't have a footprint in the China oncology market so far. However, BI is intent on building its oncology platform in the country, where it filed an approval application in 2013 for its first oncology product, Giotrif (afatinib).
“We are now awaiting CFDA’s decision regarding our application, but hope to have a positive opinion from CFDA very soon so that we can provide Giotrif to NSCLC patients in China,” BI said. Giotrif was approved in the U.S. and Europe in 2013." {Underlined emphasis is mine}"BI China" could be or likely is Stephen Doyle, Vice President and Head of Specialty Care, China, at Boehringer. Who's to say Jialing Dai's reporting is or is not accurate or veracious, or that his organization and he are or are not veracious.
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
It would seem expanded access (compassionate use) programs (CUP) typically follow pivotal trials, and not the other way around. That is, most companies open a CUP as their pivotal Phase 3 trial winds it way to conclusion, rather than open a CUP before they start a pivotal trial (as Provectus did).
Consider a March 2014 Quintiles presentation entitled "Operational Considerations for Running Effective Expanded Access Programs: Spotlight on Oncology" by Rebecca Thompson and Dr. Jean-Louis Merot, MD. Selected slides from the presentation, with my orange purple arrows, follow:
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. Clinical Development at Provectus, March 2009 |
"One of the aspects of closing the expanded access protocol is that we now have an opportunity to collate all of the data from that process to be able to actually use it for at least supportive purposes if not better higher purposes in terms of regulatory approvals."Approvals, plural.
Interestingly, as I noted under "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived News V page, Australia's Princess Alexandra Hospital's experience with PV-10 was a prospective, non-randomized, single centre study.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
- Primary: complete clinical response of treated lesions (determined using RECIST), and
- Secondary: overall response (OR), clinical benefit, time-to-best response, disease-free survival (DFS) and overall survival (OS).
The Aussies thought other intralesional therapies were better than T-Vec (July 17, 2016)
A good document to review, in the context of PV-10's potential and eventual approval in Australia is the May 2016 Australian Public Assessment Report for Talimogene Laherparepvec ("T-Vec/Imlygic AusPAR"), a 112-page document. An AusPAR "provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission." The TGA, or Therapeutics Good Administration, is the Australian equivalent of the FDA.
The T-Vec/Imlygic AusPAR has some interesting parts to it. First, consider items First Round Benefit-Risk Assessment and First round recommendation regarding authorisation starting on page 50. In particular: "It is recommended that approval not be granted for use of Imlygic as monotherapy in
melanoma." Reasons included {underlined emphasis is mine}:
- "Although the modified virus is novel the use of intra-lesional therapies for melanoma is not. Advantages over other forms of intra-lesional therapies or surgical removal have not been shown.
- Its efficacy is modest and restricted to a small subgroup of patients in a first line setting. Some 70 % of patients do not appear to have significant benefit from even prolonged treatments. It appears to have minor effects on visceral metastases.
- Evidence suggests (as monotherapy) it would not be useful as salvage therapy in patients failing existing treatments.
- The need for transport and storage at -80ºC would limit its applicability to large centres.
- Its administration would require trained personnel and facilities. Treatment may be needed over long periods of time at frequent intervals. These factors would likely impact on its cost effectiveness. These issues are likely to be important outside clinical trial settings."
Could one of them be, oh I don't know,...PV-10? See, for example, from the FDA's CBER review of T-Vec:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Or from Sloot et al., 2016:
Second, consider the second round evaluation starting on page 50. For the Second round evaluation of clinical data submitted in response to questions: "After consideration of the responses to clinical questions, the benefits of Imlygic in the proposed usage are unchanged from those identified in the first round evaluation." The second round was completed with a recommendation of (a) monotherapy use restricted to patients with Stage IIIB/C and IV1a melanoma, and (b) combination use with immune checkpoint inhibitors restricted to clinical trials.
 |
| Click to enlarge |
 |
| Click to enlarge |
Amgen originally sought a T-Vec label of melanoma that is regionally or distantly metastatic; the final, amended label was melanoma in patients with un-resectable cutaneous, subcutaneous
or nodal lesions after initial surgery.
"In our center, the use of [an approved therapy] has steeply decreased with the availability of [an unapproved one]." (July 17, 2016)
Updated below, again.
The takeaway I wanted blog news item Weird (July 15, 2016) immediately below to have is/was associated and encapsulated in the title of this item. See June 1, 2016 blog post Intralesional PV-10 for In-Transit Melanoma—A Single-Center Experience, which discusses paper Lippey et al., "Intralesional PV-10 for in-transit melanoma-A single-center experience," J Surg Oncol, 2016 May 30 about Australia's Peter MacCallum Cancer Centre's compassionate use/expanded access program (special access scheme in Australia) experience with PV-10.
PeterMac, as it is also known, has been in the news recently: e.g., July 15th's Herald Sun's "Cancer treatment: Immunotherapy lab to give Victorian patients access to latest treatments" {underlined emphasis is mine}:
"A new world-leading immunotherapy research program is set to place Melbourne at the forefront of the latest global battle against cancer.
A state-of-the-art immunotherapy lab will be opened in the vacant top floor of the $1.1 billion Victorian Comprehensive Cancer Centre, giving Victoria’s cancer patients first access to the biggest breakthroughs in cancer treatment for more than 50 years.
The Andrews Government will announce the program today to coincide with tomorrow’s official opening of the new Peter MacCallum Cancer Centre, which will be attended by US Vice President Joe Biden in his role as chairman of the US-led Moonshot Initiative to promote collaboration between the world’s leading cancer research institutions.
It will see a $6 million, 60-person research lab built as the centrepiece of the VCCC’s 13th floor, which had been earmarked for a 42-bed Peter Mac private hospital until the Premier Daniel Andrews controversially scrapped the plan.
Several other research-based programs will also be announced for the 13th floor today.
The cutting-edge immunotherapy lab will be the largest in the Southern Hemisphere and will bring together for the first time researchers from Peter Mac, the Doherty Institute and the University of Melbourne to accelerate the biggest advance in cancer treatment since the advent of chemotherapy."
 |
| Click to enlarge. Image source |
Conclusion #1b: Princess Alexandra considers unapproved PV-10 a better treatment option, where appropriate of course, than approved isolated limb infusion (ILI), which also is a standard of care at the hospital.
Conclusion #1c: Princess Alexandra considers unapproved PV-10 a more cost effective treatment than ILI. Princess Alexandra is a public hospital in single-payer Australia, although healthcare in the country is provided by both private and government institutions. Cost matters.
Observation #2a: Melbourne, Victoria's Peter MacCallum Cancer Centre, Australia’s only public hospital dedicated to cancer treatment, research and education, noted that the use of ILI at PeterMac steeply decreased with the availability of PV-10.
Conclusion #2b: PeterMac authors (Lippey et al.) originally concluded in the abstract of the RACS poster presentation that "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool with an acceptable side effect profile for the management of unresectable in transit and locally recurrent melanoma;" however, the "cost effective treatment tool" phrase was removed from the peer-reviewed paper. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived News V page.
Fact #3: ILI is considered medically necessary, and thus reimbursable therapy for a certain indication or presentation of melanoma in the U.S.; for example, see (past or present) The Centers for Medicare & Medicaid Services (CMS), Excellus BlueCross BlueShield, BlueCross BlueShield of Massachusetts, BlueCross BlueShield of Alabama, etc. A recent example of current use in the US is June 8, 2016's "A limb is saved, a life is changed as Moffitt Cancer Center works to perfect a targeted blast of chemo."
PV-10 use also has been undertaken at Sydney, New South Wales' Sydney Melanoma Unit and the Newcastle Melanoma Unit, and Adelaide, South Australia's Royal Adelaide Hospital. In total, PV-10 has been provided to patients in four of Australia's six states.
* Repeated follow-up emails (for further clarification) to the nurse practitioner contact at Princess Alexandra (for germane clinical trials) -- and who also kindly replied to my original inquiry and said PV-10 is not a standard of care, and that the matter would be addressed -- have gone unanswered. See Correction (April 19, 2016) on the blog's Archived News IV page.
Updated (7/17/16): H/t InvestorVillage poster leave_the_gun for being a reminder for me to re-visit ILI cost under this blog news item (I previously had surfaced the following article under A selection of literature (June 30, 2016) below).
Amgen's Ma et al., "Use Patterns and Costs of Isolated Limb Perfusion and Infusion in the Treatment of Regional Metastatic Melanoma: A Retrospective Database Analysis," Adv Ther. 2016; 33: 282–289 examined the cost of ILI/isolated limb perfusion (ILP):
"The mean cost of ILP/ILI performed in the inpatient setting was US$36,758 (±27,124) per procedure. The range of the cost varied considerably from US$8202 to US$154,008, with a median cost of US$26,905."Cost only comprised inpatient hospitalization expense; other costs and expenses were not included. The data appear to be left-skewed (e.g., mean cost is greater than median cost) -- see the paper's cost data here.
The authors note the following limitations of their analysis:
"While ILI is currently more common than ILP, there is no separate procedure code (CPT) differentiating ILP from ILI in the claims data. This precludes an analysis of use patterns and costs separately for ILP and ILI. Cost per procedure could be different for ILP and ILI because the ILP technique involves a more technically complex and invasive operative procedure, requiring open surgical cannulation of the vessels at the root of the extremity. In comparison to ILP, ILI is a simplified and minimally invasive operative procedure. Secondly, the study population was composed of commercially insured and Medicare-insured patients, and the patterns and costs of these procedures may not be representative of all patients with regional metastatic melanoma, especially the uninsured or those covered by Medicaid. Comparison of ILP/ILI with other alternative therapies for limb melanoma metastases including electrochemotherapy, local therapies (interleukin, Rose Bengal, electrodessication), and systemic therapies was outside the scope of the current study. This topic warrants further research.
The current study focused only on hospitalization cost associated with ILP/ILI, which can be seen as direct medical costs. Indirect costs associated with ILP/ILI were not examined due to lack of indirect cost information in the database. Considering that metastatic melanoma affects the working age population, indirect costs related to the recovery period at home, concomitant medication such as antibiotics or painkillers, and rehabilitation processes could be considerable and add to the overall costs. Furthermore, the indirect cost associated with complication or morbidity due to ILP/ILI could also be substantial. Thus, the total costs of ILP/ILI to society can be more expensive than said in the healthcare claims data."Updated (7/17/16): When PeterMac and Princess Alexandra medical institutions compare the costs of PV-10 and ILI, and note that PV-10 is more cost effective, I believe they are referring to the setting (outpatient vs. inpatient) and direct hospital cost (zero days of hospital stay vs. multiple days). From a public hospital in a single payer healthcare system's perspective, PV-10 is delivered in an outpatient setting requiring no substantial cost to the hospital, while ILI is complex procedure delivered in an inpatient setting.
ILI for melanoma is a cancer therapy (my lingo) that comprises or requires:
- a surgical procedure under general anesthesia,
- equipment such as a syringe pump system,
- a chemotherapy drug such as melphalan,
- a hospital stay of some length, and
- medical staff (physicians [surgeon, oncologist] and nurse practitioners/physician's assistants/nurses) to (a) undertake the procedure and (b) monitor the patient during his or her stay.
Consider Giles et al., "Isolated limb infusion chemotherapy for melanoma: an overview of early experience at the Adelaide Melanoma Unit," Cancer Manag Res. 2013; 5: 243–249, which compares the cost, among other things, of ILI and ILP {my underlined emphasis}:
"The advantages of ILI [over ILP] are its technical simplicity and practicality, good tolerability in patients (especially the elderly), and the relative ease of repeating the procedure(s). ILI also has a relatively low complication rate compared with isolated limb perfusion, and does not require specialized extracorporeal bypass facilities, invasive surgery, or blood transfusion. Further, the reduced operative time and cost required per procedure makes it economically more attractive for health budgets than isolated limb perfusion. Lower rates of limb toxicity and morbidity, including compartment syndrome, peripheral neuropathy, infection, and venous thrombosis, are also significant additional economic advantages...
The current analysis of our procedures indicates that ILI is a particularly useful method for controlling metastatic limb melanoma, with high efficacy and results comparable with those reported using isolated limb perfusion chemotherapy regimens, avoiding a more invasive and costly procedure."PV-10 for melanoma is a cancer therapeutic that comprises or requires:
- a syringe,
- ablative immunotherapy drug compound PV-10,
- medical staff (a physician or mid-level provider) to inject the patient's lesions, and
- no hospital stay.
If and when PV-10 is approved in Australia, there will be a treatment cost for the drug. Under the special access scheme, I believe PV-10 is provided free of charge to Australian hospitals.
Weird (July 15, 2016)
 |
| Image source |
- An existing standard of care at a medical institution (Princess Alexandra Hospital in Australia),
- A preferred treatment to an approved therapy (isolated limb infusion [ILI]) where appropriate,
- By the hospital to be better for the patients over the approved therapy, and
- More cost effective for the medical institution.
Australia: Consider Kroon et al., "Australian Multicenter Study of Isolated Limb Infusion for Melanoma," Annals of Surgical Oncology, April 2016, Volume 23, Issue 4, pp 1096-1103:
"Results
The median patient age was 74 years (range 28–100) and 59 % of patients were female. Overall response rate was 75 % (complete response [CR] 33 %; partial response 42 %). Stable disease was seen in 18 % of patients and progressive disease in 7 %. Wieberdink grade III or higher was seen in 30 % of the cases. No toxicity-related amputations occurred, and median survival was 44 months. In patients with a CR, median survival was 80 months (p = 0.014). On multivariate analysis, Breslow thickness, lower-limb ILI, and a procedure performed at the Melanoma Institute Australia remained significant predictors for response, although not for survival."The U.S.A./Sweden: Consider Dossett et al., "Clinical Response and Regional Toxicity Following Isolated Limb Infusion Compared with Isolated Limb Perfusion for In-Transit Melanoma," Ann Surg Oncol. 2016 Jul;23(7):2330-5.
"A total of 203 patients were reviewed (ILI = 94, ILP = 109). There were no differences in age, sex, or N-stage between groups; however, BOD was higher for the ILI group (high BOD 58 vs. 44 %, p = 0.04). Regional toxicity was minimal (Grade IV < 1 % in ILI and 2 % in ILP, p = 0.40). Overall response rate (ORR) was 53 % for ILI versus 80 % for ILP (p < 0.001). Median overall survival (OS) was 46 months for ILI versus 40 months for ILP (p = 0.31). A high BOD [hazard ratio (HR) 3.02, 95 % confidence interval (CI) 1.85-4.93, p < 0.001] and N3 disease (HR 1.58, 95 % CI 1.01-2.48, p = 0.04) were associated with worse OS, whereas there was no difference in OS by procedure (p = 0.20)."ILI and PV-10 will be the subject of PV-10 investigator Professor/Dr. Mark Smithers, MD's (Princess Alexandra) presentation at Locoregional Melanoma 2016 in Australia (Peter MacCallum Cancer Centre): "Injectable therapy versus limb infusion for intransit disease: Which treatment for which patient?"
Australia, again (July 14, 2016)
1. Provectus issued a press release and made an associated 8-K filing in regards to the company's Aussie subsidiary and office, Establishes Australian Subsidiary. In particular, COO and interim CEO Peter Culpepper noted:
"With a subsidiary in Australia, we are bringing our corporate structure in line with our scientific work. Our research and development program has been international from the very beginning, and now, Provectus is an international company. The new unit should make it easier to work with the Australian regulatory authorities, and having an office in the region may facilitate our work in Asian markets as Sydney is just two hours ahead of Beijing, Hong Kong and Singapore. If and when PV-10 receives approval in Australia and other nations in the region, we will have pre-positioned ourselves to develop a sales and marketing force."Is some form of early approval in the cards, or is the potential but incremental benefit only from R&D and clinical trial cost reimbursement? Per Peter in the PR:
"Provectus has already been very active in Australia for years because of our research into PV-10 as an investigational treatment for melanoma. In fact, we began our phase 1 study of PV-10 in 2005 at the Sydney Melanoma Unit in North Sydney and the Newcastle Melanoma Unit in Waratah, both in New South Wales. Since then, we have also worked with the Princess Alexandra Hospital in Brisbane, Queensland, the Royal Adelaide Hospital in Adelaide, South Australia, and the Peter MacCallum Cancer Centre in Melbourne, Victoria."2. The company also made another 8-K filing today, this time regarding the suspension of its Cantor "equity line of credit," or at-the-money (ATM) offering program/vehicle:
"On July 13, 2016, Provectus Biopharmaceuticals, Inc. (the “Company”) suspended sales of its common stock under the Controlled Equity Offering Sales Agreement, dated as of April 30, 2014, by and between the Company and Cantor Fitzgerald & Co. The Company expects the suspension to last until December 31, 2016, but no assurances can be given that the Company will not extend the suspension beyond that date. The Company also has the ability to lift the suspension prior to December 31, 2016, should it desire to make sales of its common stock under the Controlled Equity Offering Sales Agreement prior to December 31, 2016."The Cantor financing facility was not mentioned in the May 5th 1Q16 10-Q filing:
"As a result of its ability to manage variable expenses and minimal fixed costs, the Company believes its cash and cash equivalents on hand at March 31, 2016 will be sufficient to meet its current and planned operating needs until at least 12 months from the date these financial statements are issued without consideration being given to additional cash inflows that might occur from the exercise of outstanding warrants or future sales of equity securities." {Page 13 under Liquidity and Capital Resources}Cantor was last mentioned in the March 30th 2015 10-K filing:
"By managing variable cash expenses due to minimal fixed costs, we believe our cash and cash equivalents on hand at December 31, 2015 will be sufficient to meet our current and planned operating needs until into 2017 without consideration being given to additional cash inflows that might occur from the exercise of existing warrants or future sales of equity securities. In addition, on April 30, 2014, the Company entered into a Controlled Equity OfferingSM Sales Agreement with Cantor Fitzgerald & Co., as sales agent (“Cantor”), under which the Company may issue and sell shares of its common stock having an aggregate offering price of up to $50,000,000 from time to time through Cantor, acting as sales agent." {page 42 under Capital Structure}A reason or reasons for doing this suspension could comprise or include:
- New accounting firm/auditor Marcum no longer required the window dressing of access to capital (in this case via Cantor's ATM offering) that former auditor BDO did,
- Cost reduction related to keeping the facility open; see "Additionally, legal expense increased by about $500,000 primarily due to our NYSE MKT listing and the Controlled Equity OfferingSM Sales Agreement with Cantor and investor relations and related travel expenses increased approximately $1,100,000 in 2014 over 2013" on page 46 of the 2015 10-K, and
- A "cleaner slate" in regards to other, future, potential, higher tier investment banking relationships.
It would appear Provectus was not invited to Cantor Fitzgerald's 2nd Annual Healthcare Conference (July 12-13). I'm not necessarily linking the two together. While there may be some administrative, cost and/ or accounting-related benefits to the ATM suspension, going forward or in addition, Provectus' board of directors and management team must be much better on the corporate development side than they have been in the past. The excuse that there is little one can do when one is a sub-$100 million company ring very hollow (and, by this point, are objectively refutable).
3. I note a visit from Sanofi-Aventis Singapore to the blog.
"Intralesional treatment for advanced melanoma: what's on the horizon?" (July 14, 2016)
Updated below.
 |
| Click to enlarge. |
Updated (7/13/16): From the same publication, Future Medicine, Dr. Agarwala wrote in 2012: "Intralesional therapy for metastatic melanoma with a focus on PV-10."
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. Putting It All Together HOT Melanoma 2012 aka “What will you do on Monday?” |
 |
| Click to enlarge. |
 |
| Image source |
- October 2014: The III Eurasian Melanoma and Skin Cancers Forum in Suzdal, Russia; "Overview of Intralesional Therapy for Melanoma: Focus on PV-10,"
- May 2015: The American Society of Clinical Oncology's Annual Meeting in Chicago; "A Changing Topography: The Role of Intralesional Therapy in Melanoma,"
- December 2015: The Melanoma Bridge 2015 meeting in Naples, Italy: "Overview of Intralesional Therapy for Melanoma,"
- May 2016: The Melanoma Immunotherapy Regional Meeting in Dubrovnik, Croatia; "The Changing Paradigm in Immuno-oncology: The Past vs. the Future in the Treatment of Melanoma," and
- July 2016: The 6th European Post-Chicago Melanoma/Skin Cancer Meeting in Munich, Germany; "Current Clinical Trials Soft Tissue and Skin Metastases."
October 2014: PV-10 BTD denial (May)
 |
| Click to enlarge |
 |
| Click to enlarge |
May 2015: PV-10 pivotal melanoma Phase 3 trial (April)
 |
| Click to enlarge |
 |
| Click to enlarge |
December 2015: T-Vec approval (October)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
May 2016
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
July 2016
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated below.
1. H/t InvestorVillage poster Warlord who noted Provectus' Phase 1 neuroendocrine tumors (NET) liver mets trial has started recruiting at Australia's The Queen Elizabeth Hospital (Adelaide).
 |
| Click to enlarge. Image source |
2. Provectus issued a press release and made an associated 8-K filing in regards to an ESMO 2016 poster presentation the company's clinical investigators should make, Announces Acceptance of Abstract for Poster Presentation at European Society for Medical Oncology 2016 Congress. The abstract/poster are entitled "Intralesional Rose Bengal for Stage III and IV Melanoma."
Will there be substantive information on the poster, or will it a trials in progress type like that presented at ASCO 2016, "Intralesional rose bengal for treatment of melanoma?"
Updated (7/19/16): I located more information on the above poster presentation by downloading the latest (or final) ESMO 2016 program. First, as I mostly thought, the poster is a trials-in-progress one about the two melanoma trials Provectus currently is running, like the poster at ASCO 2016. Second, as I mostly thought too, Germany's Dr. Axel Hauschild, MD, PhD is/will be a clinical investigator for one or more of the company's trials; see 10-Q Notes (May 10, 2016) on the blog's Archived News V page:
"Updated (5/10/16): As of this writing, the transcript of the call is not yet available. I have two speculations. First, I speculate the lead investigator of the European portion of the pivotal melanoma Phase 3 trial is (is going to be) Dr. Axel Hauschild, MD, PhD, Professor and Head of the Interdisciplinary Skin Cancer Center at the Department of Dermatology, University Hospital Schleswig-Holstein in Kiel, Germany."ESMO 2016 (Hauschild is the third author listed):
 |
| Click to enlarge |
 |
| Click to enlarge |
I noted in July 2, 2016 blog post Shapeshifter that concentration influences Rose Bengal's shape transformation (sphering). Intralesionally (intratumorally) injected PV-10 for oncology is a 10% solution of Rose Bengal, while topically applied PH-10 for dermatology is a 0.001% to 0.01% gel of the active pharmaceutical ingredient (API) Rose Bengal.
In regards to PH-10's mechanism of action, Provectus previously speculated "[a]pparent local down-regulating action on aberrant immune expression in skin." See a slide from one of the company's 2014 corporate presentations below.
 |
| Click to enlarge. Orange oval emphasis is mine. |
"I think it'll be very interesting. When we started the mechanism work for PV-10, we discovered some things that we predicted and we discovered some things that were a pleasant surprise. And hopefully we'll have the same case for PH-10."My sense is that, at a minimum, Dr. Krueger confirmed what company principals had predicted regarding PH-10 and down-regulation.
Do Dr. Jedd Wolchok/Sloan Kettering Understand Immunotherapy's History? (July 12, 2016)
Updated below.
It would appear that Memorial Sloan Kettering Cancer Center (MSKCC) recently (i.e., that is 2015, perhaps earlier) embraced the history of the medical institution's involvement with harnessing the immune system to fight and treat cancer by describing the work of Dr. William Coley, MD, "now considered the “Father of Cancer Immunotherapy,”" on its website. See MSKCC webpage "Immunotherapy: Revolutionizing Cancer Treatment since 1891." Dr. Coley began his career as a bone surgeon at New York Cancer Hospital, which later became part of MSKCC.
When writing about immunotherapy, and usually pointing to Bristol-Myers' anti-CTLA-4 drug ipilimumab (Yervoy) and/or anti-PD-1 drugs pembrolizumab (Merck & Co., Keytruda) and nivolumab (Bristol-Myers, Opdivo), many mainstream media and medical writers and journalists often include in their introduction descriptions of Dr. Coley's work. I think it makes for a good story, as the writers endeavor to link history, and descriptions and lessons from the past, to the present day, and potentially our future.
MSKCC says Dr. Coley's work "paved the way for the modern immunotherapies that are helping patients today."
Route of delivery matters. Dr. Coley's work, and his approach to treatment, comprise two key feature, one of which nearly all who write about him (but not everyone) routinely ignore, conflating his discoveries, observations and conclusions with drugs incapable of delivering what he experimented with in order to seek better patient outcomes.
Coley's approach to the treatment of cancer was composed of (a) a "drug compound," the heated-killed bacteria known as Coley’s toxins whose actions following treatment somehow engaged the immune system (e.g., fever), and (b) the route of administration by injection of the dead bacteria into the patient's tumors.
The key feature of Coley's work that is ignored, conflated, confused or misunderstood: the manner in which the drug, drug compound, biologic or small molecule is delivered. Yervoy, Keytruda and Opdivo are immunotherapies that are intravenously administered to patients; they are not injected into patient tumors.
Google "Wolchok" and "Coley," and thousands of results are returned. MSKCC's Dr. Jeff Wolchok, a medical oncologist, apparently uses Coley's story when he (Dr. Wolchok) explains immunotherapy. Dr. Wolchok, holder of MSKCC's Lloyd J. Old Chair for Clinical Investigation, was a student of Dr. Lloyd Old, MD, who (according to Memorial Sloan Kettering):
"...did some of the first modern research on immunotherapy, with a substance called BCG, now an FDA-approved treatment for bladder cancer. BCG is made from a weakened version of the bacterium that causes tuberculosis. Experts think Coley’s toxins may have worked in a similar manner to BCG — jumpstarting an immune response to cancer by provoking one against the bacteria."This work of Dr. Old appears to be during the 1950s. For example, see "Effect of Bacillus Calmette-Guérin Infection on Transplanted Tumours in the Mouse." Who am I to say or write this, but perhaps Dr. Old was focused on one of the two key features, the use of a biologic (i.e., the "drug compound") to engage the immune system. He may have ignored Coley's work's other feature, route of delivery.
The administration of BCG (aka Bacillus Calmette-Guérin) for bladder cancer is intravesical, which means it is "put directly into the bladder through a catheter, instead of being injected into a vein or swallowed." See the illustration below.
 |
| Image source |
Ironically, the second aspect of Coley's work, route of delivery, was explored in the 1970s with BCG (or BCG immunotherapy) in metastatic melanoma when the drug was it was directly injected into metastatic melanoma lesions limited to the skin; see, for example, "BCG Immunotherapy of Malignant Melanoma: Summary of a Seven-year Experience." Unfortunately, the immunotherapy failed a Phase 3 trial; see 2004 paper "Mature results of a phase III randomized trial of bacillus Calmette–Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I–III melanoma (E1673):"
"In what to our knowledge is the largest ever trial to test the role of BCG as adjuvant therapy for melanoma, no benefit for BCG was observed for patients with AJCC Stage I–III disease. The mature results of the current trial projected to 30 years confirmed the negative results of previous smaller studies utilizing this agent."What does Dr. Coley's work tell us about how to treat cancer via immunotherapy? Is it about the drug compound? Is it about the route of administration? Or, as I believe (because of Rose Bengal), is it both?
Consider April 2016's "Germ of an Idea: William Coley's Cancer-Killing Toxins", which more appropriately places Dr. Coley's work into context:
"That was all Coley needed to proceed directly to human trials, and Zola would become his first test subject. Coley filled a syringe with living Streptococcus pyogenes, known to induce erysipelas attacks, and injected the solution directly into Zola’s tumor. It took awhile — in fact, it took repeated injections over five months — but finally, an hour after one particular injection in October, Zola broke out into sweaty chills, and his body temperature soared to 105 degrees...
“Coley injected his first patient a century ago, and what he saw was almost identical to what we saw in our first patient,” says Saurabh Saha, a partner with Atlas Venture, former BioMed Valley researcher and senior author of the study...”
C. novyi is really a two-pronged weapon against cancer: It germinates in tumors and releases cancer-killing enzymes, and it may also trigger an immune response similar to Coley’s Toxin. Since C. novyi survives only in oxygen-poor environments — tumors can be notoriously void of oxygen — the bacteria die when they reach healthy, oxygen-rich tissues, sparing collateral damage. Essentially, the injections perform highly precise biosurgery from the inside out."Updated (7/31/16): Does The New York Times understand cancer immunotherapy's history? The NYT's Denise Grady wrote "Harnessing the Immune System to Fight Cancer" on July 30th. In it she references Dr. Coley's name 18 times, and presumably uses his work as a vehicle to discuss Dr. James Allison's immune checkpoint inhibitor work. Interestingly, this author, while invoking Coley's biologic material he injected into patients, does not mention the route of delivery Coley used. She uses the verb "inject," but does not say where:
"Dr. Coley began to inject terminally ill cancer patients with Streptococcal bacteria in the 1890s. His first patient, a drug addict with an advanced sarcoma, was expected to die within weeks, but the disease went into remission and he lived eight years.
Dr. Coley treated other patients, with mixed results. Some tumors regressed, but sometimes the bacteria caused infections that went out of control. Dr. Coley developed an extract of heat-killed bacteria that came to be called Coley’s mixed toxins, and he treated hundreds of patients over several decades. Many became quite ill, with shaking chills and raging fevers. But some were cured."Ironically, she references radiation, which is making a resurgence because of the growing understanding/belief that local treatments to/on tumors may unlock the gateway to the immune system's reaction around the body:
"Early in the 20th century, radiation treatment came into use. Its results were more predictable, and the cancer establishment began turning away from Coley’s toxins. Dr. Coley’s own institution, Memorial Hospital (now Memorial Sloan Kettering Cancer Center) instituted a policy in 1915 stating that inpatients had to be given radiation, not the toxins. Some other hospitals continued using them, but interest gradually waned. Dr. Coley died in 1936."See, for example, June 2015's "June Podcast: The Abscopal Effect with Sandra Demaria."
Finally, it is interesting to note that Allison, Wolchok and others submitted a patent application (published in 2014) for the use of an oncolytic virus with immune checkpoint inhibitors via the injection of the virus into tumors.
Updated pivotal melanoma Phase 3 trial protocol (July 11, 2016)
Provectus' CTO Dr. Eric Wachter, PhD appears to have amended the trial protocol faster than expected.
 |
| Click to enlarge. |
1. Official title: "PV-10 Intralesional Injection vs Systemic Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma Without Distant Metastases" was changed to "PV-10 Intralesional Injection vs Systemic Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma"
| Click to enlarge. Image source |
| Click to enlarge. Image source above |
| Click to enlarge. Image source above |
| Click to enlarge. Image source above |
Second, the change of the official title, for me, feels like a refinement of the ultimate label (by removing the phrase "without distant metastases."
On another note, the compassionate use/expanded access program (special access scheme in Australia), as noted previously, was closed.
 |
| Click to enlarge. |
"Studies such as these, using combination therapies will continue to advance the field and head-to-head comparisons of immune coreceptor blockade in combination with regional chemotherapy or other intralesional treatments such as PV-10 (Rose bengal) should continue to be pursued. Several examples of these combination strategies already exist, including National Cancer Institute (NCI) trial 0262600 looking at T-VEC and pembrolizumab, NCI trial 01323517 combining isolated limb infusion using melphalan with ipilimumab, and NCI trial 02115243, which is being modified to look at combining nivolumab and ipilimumab neoadjuvantly prior to isolated limb infusion with melphalan." {underlined emphasis is mine}H/t @bradpalm1 for the article from where the above quote comes: "What is the OPTiMal Way to Manage In-Transit Disease?," Olino, K et al. Ann Surg Oncol (2016).
Some thoughts about the article, and some related thoughts include:
- An interesting Conflicts of Interest statement: "Despite efforts to maintain clinical equipoise during the conduct of a clinical trial, there is always room for potential conflict of interest in a high-stakes corporate-sponsored drug registration trial. The role of industry in ‘marketing’ trials to increase visibility of a therapeutic agent is well-documented. Several studies have demonstrated that not only do industry-sponsored trials have more manuscripts per trial in the literature than non-industry-sponsored trials, but there are also frequently more subset analyses published from industry-sponsored trial datasets. As such, similar to ASCO’s new policy, we feel that lead investigators and lead authors should not receive honoraria or consulting fees from companies sponsoring trials in which their compound is being evaluated as a prerequisite for publication in this journal."
- Great Summary questions:
- "While researchers are expanding the armamentarium of agents to use in a novel fashion, we must continue to work towards determining what role, if any, regional treatments play in the rapidly changing landscape of melanoma therapies." — Recall MD Anderson's Dr. Davies' comments pictured below about the next frontier in treating melanoma: regional disease; see Category vs. Agent (June 30, 2016) below.
- "This challenge needs to push surgeons leading clinical trials to objectively answer two questions: (i) Can a regional therapeutic generate a clinically effective systemic immune response? (ii) Can regional therapeutics augment the clinical effectiveness of systemically administered immunotherapy?" — To me, these questions are another way of asking whether the so-called local agent (intralesional [IL] or intratumoral [IT] drugs and drug compounds) can generate a systemic effect.
- Amgen's IL drug talimogene laherparepvec/T-Vec/Imlygic is a questionable product (e.g., difficult and awkward for physician/provider use and/or re-use, expensive and awkward to ship, store and handle) with a marginal clinical value proposition (e.g., much weaker immunologic signalling) compared to PV-10. In terms of FDA approval, however, T-Vec was "the first guy through the wall," and that approval is good for the category of IL drugs and agents, whether those agents are ablative immunotherapies like PV-10 or oncolytic virus immunotherapies like Cavatak. Nevertheless, BioVex/Amgen's pivotal Phase 3 trial design has been regarded as poor (e.g., comparator/control arm, primary endpoint). The authors of the abovementioned paper also criticize Patient Selection:
- "The issue of appropriate patient selection also leads to some practical concerns about the T-VEC trial. There is difficulty applying the treatment methodology of the investigators into clinical practice based on descriptions of what constituted ‘unresectable’ disease and the heterogeneous manner in which treatment was administered. Furthermore, treatment was administered in such a way to favor treating new lesions first and then established lesions, even if the established lesions were larger. We need to be cautious when interpreting the data since, inadvertently, such an approach could lead changing the overall reported treatment responses (i.e. easier to get a >50 % response for a 6 mm tumor than for a 2 cm tumor)."
- The University of Texas Medical Branch at Galveston authors — Dr. Olino (The Johns Hopkins School of Medicine, The Memorial Sloan Kettering Cancer Center) and Dr. Tyler (Dartmouth Medical School, Duke University Medical Center, MD Anderson Cancer Center) — are both surgical oncologists. As such, they highlight a key point in cancer treatment {underlined emphasis is mine}:
- "There are multiple approaches to managing patients with stage IIIb, IIIc, and IVa disease, ranging from systemic treatments with checkpoint blockade inhibitors to targeted treatments for individuals who have a BRAF mutation. Regional treatments include surgical excision, intralesional therapy with compounds such as TVEC, and regional chemotherapy approaches if disease is confined to the extremity. There can be significant differences of opinion, even among surgeons, as to the optimal initial approach, as questions as simple as ‘How do you define resectable in-transit disease?’ vary from practice to practice. There is also frequently a split between medical oncologists and surgical oncologists as to which approach is best. Medical oncologists tend to favor systemic approaches with immunotherapy as a way to try to achieve a long-term complete response. Surgical oncologists, who have historically managed these patients with regional approaches, see excellent long-term disease control in 30–40 % of patients and, as such, frequently desire to delay systemic therapy with its attendant morbidity for when patients actually need it. Our personal approach, is that we feel all patients with stage IIIb, IIIc, and IVa disease should be presented at a multidisciplinary tumor board where local expertise and comfort with different treatment options can be balanced with the unique characteristics of a patient’s presentation to tailor a treatment plan for that individual. This treatment plan would not only consider participation in various clinical trials but also consider sequential interventions in the event of initial treatment failure."
- Insofar as PV-10 is used as a monotherapy to [demonstrate its ability in Provectus' pivotal melanoma Phase 3 trial] prevent or forestall the spread of the disease from Stage IIIB-C and IV M1a to Stage IV M1b-c, a notion more comfortable to the surgical oncologist, the company's Phase 1b study combining PV-10 and pembrolizumab in patients exclusively with Stage IV disease is meant to provide medical oncologists with a better tool than what currently is available today (i.e., checkpoint inhibitors as monotherapies).
- Back in 2014, Forbes' Matthew Herper did not appear to know or understand the difference between how surgical and medical oncologists operate, pardon the pun. See November 1, 2014 blog post Florey, Chain & Heatley⎟Coley⎟sort of, maybe, possibly, conceivably, probably...
Speaking about isolated limb infusion, the language in the trial protocol of an Australian study at Princess Alexandra Hospital of topical imiquimod or diphenylcyclopropenone for the management of cutaneous in-transit melanoma metastases, which has begun recruiting, remains unchanged {my underlined emphasis}:
See [Australian] Standard of Care? (April 11, 2016) on the blog's Archived News V page. It would see the correction was a non-correction. See Correction (April 19, 2016), also on the same blog page."Historical control. Following completion of the TIDAL Melanoma Study a comparison to retrospective data will be made for patients treated with isolated limb infusion and PV-10 treatments. These modalities represent the existing standard of care at our institution. Data for these control treatments were derived from institutional records between 1996 - 2014.PV-10 treatment involves an intra-lesional injection of Rose Bengal solution into in-transit melanoma metastases and has been investigated as a part of another phase II trial completed at our centre. For the formal treatment results of this investigation please refer to: Thompson JF, et al. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann Surg Oncol. 2014.Isolated limb infusion involves isolating the vascular supply to a limb affected by in-transit melanoma metastases and instilling a loco regional chemotherapeutic agent, melphalan. For the formal institutional treatment results of from therapy please refer to: Barbour AP, et al. Isolated Limb Infusion for Malignant Melanoma: Predictors of Response and Outcome. Ann Surg Oncol. 2009."
Finally, returning to the paper above, the physician authors [in my view] properly reference William Coley's work: "Intratumoral treatment as a form of regional immunotherapy can trace its origin back to 1892 with the description of Coley’s toxin." See also October 15, 2015 blog post Still Standing. Google (for news of) "immunotherapy" and "Coley," and see how many articles invoke Coley and non-injectable immunotherapies (e.g., checkpoint inhibitors); see, for example, yesterday's Allentown Morning Call article "On the cusp of a cure:"
"On the one hand, immunotherapy seems to have come out of nowhere. In Siddhartha Mukherjee’s Pulitzer Prize-winning history of cancer, published just six years ago, immunotherapy doesn’t even rate an index entry. On the other hand, the first indication that the immune system could be coaxed into fighting cancer was documented more than a century ago by William Coley, a surgeon at the New York Cancer Hospital, the precursor to Memorial Sloan Kettering.
Coley noticed one of his cancer patients experienced a complete remission following an unrelated bacterial infection. Over the next several decades, Coley injected the bacteria, which became known as Coley’s toxins, into more than 900 cancer patients, most of whom had inoperable sarcomas, or tumors. About 10 percent of the patients were entirely cured, apparently because the toxins somehow jogged the immune system into action. Even so, lacking a theoretical framework to explain the underlying biological mechanisms at work, Coley’s insights were widely dismissed and nearly forgotten.
William Coley was a surgeon at the New York Cancer Hospital, the precursor to Memorial Sloan Kettering. He documented more than a century ago that the immune system could be coaxed into fighting cancer.
Not until the end of the 20th century did researchers give immunotherapy a second, serious look. In the mid-1990s, James Allison led a team of scientists at the University of California, Berkeley Cancer Research Laboratory that determined a biological molecule called CTLA-4 regulated immune system T cells. Allison theorized, correctly, that CTLA-4 was inhibiting T cells from attacking cancer cells. In a landmark paper published in 1996, Allison’s team showed that blocking CTLA-4 could slow and even stop tumor growth in mice. The findings launched a race to develop drugs for human use."Desert > Beach (July 8, 2016)
Updated below, again.
For the love of small furry animals (and the occasional large one), would someone save me from this humidity (57%? sure...)?
1. H/t a shareholder who informed me of Provectus' COO and interim CEO Peter Culpepper's video interview (with Wendy Gillette) at the Marcum MicroCap Conference (June 1-2). During the interview Peter said (paraphrasing) that he expects an interim data readout for the pivotal melanoma Phase 3 trial this year, which would be generally consistent with the company's CTO Dr. Eric Wachter, PhD's guidance on the May 10th 1Q16 business update conference call; see Potential 2H16 data catalysts (July 6, 2016) below.
I believe it is possible for the interim readout to occur this year or early next year per Eric's current guidance, or sometime in 2017 later than such guidance (see Potential 2H16 data catalysts (July 6, 2016) below). Both of my beliefs could exist within the same universe.
On the one hand, a data readout could be undertaken using a prescribed time trigger, of which I have previously written. See, for example, July 23, 2015 blog post "Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV." Such a time trigger, or safety valve, would help when there is a significant difference in the distribution curve of events between the treatment arm (PV-10) and the original control arm (chemotherapy). Simply put, PV-10 would not generate any disease progression events (i.e., a horizontal progression-free survival curve [in a perfect world, which the world is not]), and chemotherapy eventually would generate all events (i.e., a curve that would mostly fall-off after a few weeks or more). It is possible the trigger has been set within the timeframe of Eric's current guidance.
On the other hand, I am far from experienced enough to understand the impact on a prescribed time trigger from the inclusion of Amgen's intralesional (IL) drug T-Vec as a comparator. Is a T-Vec event distribution curve normal like chemotherapy's, or is it more favorably skewed and if so by how much? T-Vec-treated lesions progress prior to response (PPR); see "Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial," Ann Surg Oncol (2016) under Category vs. Agent (June 30, 2016) below. It is possible measurement of T-Vec treated tumors might generate events under RECIST 1.1. In addition, what is the impact on the enrolling the necessary number of patients for statistical separation of the treatment and control arm PFS curves if the number of trial sites has been slow to ramp up to the necessary number to achieve the necessary number of patients?
I can understand how complex and/or difficult it might be to assess a time trigger that may be subsequently influenced by (a) the addition of another comparator (T-Vec), one potentially better than the original control (chemotherapy) and (b) the impact of amending a trial protocol that delays the opening of trial sites. But I do not yet understand by how much. And given Eric's track record of missing his own deadlines, I am inclined to believe his guidance potentially underestimate matters (i.e., he has over-promised and is at risk of under-delivering).
Updated (7/9/16): Andtbacka et al.'s "Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial" observed that 16% of the T-Vec treatment arm (n = 48 out of 295) achieved a durable response of their injected lesions, which is "an objective response lasting continuously ≥ 6 months." Of the 48 patients, almost half of them experienced an enlargement of their injected lesion(s) -- disease pseudoprogression -- before the lesion(s) shrunk (partial response) or was(were) destroyed (complete response).
At a lesion level, and so not directly comparable to a patient level, the"median time to response of responding injected lesions from baseline was 9.3 weeks (IQR 5.1–17.1 weeks). Put another way, it would seem that any pseudoprogression could or would occur within 9+ weeks. This may suggest a good number of T-Vec patients in Provectus' pivotal melanoma Phase 3 trial's comparator arm (investigator's choice of chemotherapies DTIC/TMZ or oncolytic virus T-Vec) would generate a disease progression event by at least the second clinical assessment (of tumor size) and certainly by the first comprehensive assessment.
Updated (7/9/16): Andtbacka et al.'s "Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial" observed that 16% of the T-Vec treatment arm (n = 48 out of 295) achieved a durable response of their injected lesions, which is "an objective response lasting continuously ≥ 6 months." Of the 48 patients, almost half of them experienced an enlargement of their injected lesion(s) -- disease pseudoprogression -- before the lesion(s) shrunk (partial response) or was(were) destroyed (complete response).
 |
| Click to enlarge. Figure 2 of the abovementioned paper |
 |
| Click to enlarge. Image source |
2. H/t a regular hatter on the below.
 |
| Click to enlarge. |
A link to the US PTO's Trademark Electronic Search System (TESS) for the above (but that may timeout) is here. The tipper/shareholder commented to me (slightly edited by me for readability):
Management replaced the gambling dice with the medicinal mixture word tinctura; the die is cast (alea iacta est) is understood to indicate that events have passed a point of no return.
Wikipedia: "We know from [Caesar's journals] that Caesar is not taking this lightly. He knows that if he marches on Rome with his armies, then he is a public enemy, and that he will either have to win, or die. For a Roman patrician like Julius Caesar there is no life without military service; there is no life without service to the state. He cannot simply 'go native' and stay in Gaul, and he does realise that if he goes back to Rome, he would be killed. At this time the northernmost border of the Roman territory in Italy is the River Rubicon. Once someone crosses the River Rubicon, he's in Roman territory. A general must not cross that boundary with his army – he must do what the Romans call lay down his command, which means surrender his right to order troops, and certainly not be carrying weapons. Caesar and his armies hesitate quite a while at this river while Caesar decides what to do, and Caesar tells us that he informs his soldiers that it's a little tiny bridge across the river, but once they cross it they'll have to fight their way all the way to Rome, and Caesar is well aware that he's risking not just his own life, but those of his loyal soldiers, and he might not win. Pompey is a formidable enemy. It's also impossible to avoid the fact that Caesar was attacking the state, and as a patrician Roman this would have been very difficult for him, equivalent to beating up your father. He wouldn't have done any of this lightly. Finally he makes a decision, it's time to go, and he uses a gambling metaphor: he says 'Roll the dice', 'Alea jacta est'. Once the dice start rolling they cannot be controlled, even though we don't know what it is as the dice roll and tumble. Julius and his men swiftly cross the river and they march double time toward Rome, where they almost beat the messengers sent to inform the Senate of their arrival."
Updated (7/8/16): When the company trademarked the name "PV-10" or the phrase "when patients win, we all win," Provectus described its goods and services, in whole or part, respectively, as "medicinal and pharmaceutical preparations, namely, pharmaceutical medications for the treatment and prevention of cancers, melanoma, breast cancer, basal cell carcinoma, squamous cell carcinoma and cancers of the liver." In the above "tinctura datum est" trademark, goods and services are described as "Scientific research, development, consulting, design, engineering and testing in the fields of biotechnology and pharmaceuticals; Chemical, biochemical and biological research and analysis services; Medical laboratory services; Medical and scientific research, namely, conducting clinical trials for pharmaceutical preparations for the treatment of cancer." Underlined emphasis is mine.
3. When Peter refers to interim data, as he did on the May 10th 1Q16 business update conference call, see Potential 2H16 data catalysts (July 6, 2016) again below, he could be referring to both the pivotal melanoma Phase 3 trial (see #1 above) and melanoma combination Phase 1b study. The latter, as I have speculated, could be presented at SMR 2016.
3. When Peter refers to interim data, as he did on the May 10th 1Q16 business update conference call, see Potential 2H16 data catalysts (July 6, 2016) again below, he could be referring to both the pivotal melanoma Phase 3 trial (see #1 above) and melanoma combination Phase 1b study. The latter, as I have speculated, could be presented at SMR 2016.
4. H/t InvestorVillage poster canis_star for reminding me of Roche and Amgen's June 2015 collaboration combining the former's anti-PD-L1 drug compound and the latter's IL drug in patients with (a) triple-negative breast cancer and (b) colorectal cancer with liver metastases. Where are the trial protocols? As of this writing, I cannot find anything related to this work on ClinicalTrials.gov.
5. There is no mention on the table below of IL agents. All appear to be systemically delivered compounds. While chemotherapy is referenced, I am surprised radiotherapy is not.
 |
| Click to enlarge. Tweet image source |
6. Compare where immune checkpoint inhibitors are more successful, and where they are not.
 |
| Click to enlarge. Tweet image source |
 |
| Click to enlarge. Tweet image source |
Melanoma equals "hot" tumor, or [very] immunogenic. Hepatocellular carcinoma (HCC) equals less hot or "cold" tumor, or not very immunogenic or non-immunogenic.
Sorafenib is not a great drug, but it is considered in some situations as standard of care:
"Significant progress in the treatment of HCC was therefore made with the approval of the multikinase inhibitor sorafenib for this indication. The approval was based upon two placebo-controlled randomized trials which for the first time could demonstrate a survival benefit in HCC patients treated with sorafenib. The majority of patients included in these studies were in ECOG performance status (PS) 0 or 1 and had an adequate liver function classified as ChildPugh A (CP-A).
However, in clinical practice the majority of patients with advanced HCC have severe liver cirrhosis and substantial comorbidity, compromising their general medical condition and liver function.
Despite the lack of evidence of a survival benefit, many HCC patients with Child-Pugh B and even C liver cirrhosis are treated with sorafenib. Therefore a study of the efficacy and tolerability of sorafenib in an unselected patient population was warranted.
Moreover, one of the challenges in the treatment with sorafenib is the difficulties in assessing tumor response by traditional response criteria. The pivotal trial by Llovet et al. reported a very modest response rate of only 2%. The lack of a correlation between objectively observed response and clinical benefit complicates treatment evaluation and clinical decision making." (Quote link) {underlined emphasis is mine}In 2015, folks were excited about immune checkpoint inhibitors and HCC. For example:
"Extremely encouraging results from the interim analysis of the phase I/II nivolumab trial (CA209-040 trial) evaluating the efficacy of nivolumab in patients with hepatocellular carcinoma (HCC) were reported at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago from May 29 to June 2, 2015...
The overall objective response rate was 19% (n=8), including the two patients who achieved CR (5%). Disease control rates were 67% (n=28) for stable disease (SD) or better and 33% (n=14) for progressive disease (PD), indicating an extremely favorable study outcome."In 2016, not so much. For example:
"Treatment with the PD-1 inhibitor nivolumab (Opdivo) demonstrated an objective response rate (ORR) of 9% as a second-line therapy for patients with hepatocellular carcinoma (HCC), according to updated results from the phase I/II CheckMate-040 study presented at the 2016 ASCO Annual Meeting. In previous findings from the study, the ORR was 19%; however, for the latest update, the investigators cautioned that data were preliminary and so they may have underestimated the response rate. In the study, responses were frequently delayed, with a complete response occurring after 24 months of treatment. When considering those with stable disease, the disease control rate was 65%."Updated (7/8/16): 7. It dawned on me that when Eric said on the March 16th 4Q15/CY15 business update conference call that, in regards to PH-10, Provectus would have a preliminary analysis of its mechanism of action study completed in March, if not April, he may well have meant what he said. A preliminary analysis would be completed. He did not say, of course, that he would necessarily share the results immediately. He did say on the same call:
"I think it'll be very interesting. When we started the mechanism work for PV-10, we discovered some things that we predicted and we discovered some things that were a pleasant surprise. And hopefully we'll have the same case for PH-10."
Updated below, again.
1. More liver Phase 1 study results: ESMO 2016, the ESMO 2016 congress, October 7-11.
Updated 7/19/16): Based on the ESMO 2016 program only noting Provectus' melanoma trials in progress poster presentation, liver data are unlikely or will not be presented here.
2. Initial melanoma Phase 1b study results combining PV-10 and pembrolizumab in Stage IV patients: SMR 2016, the 13th International Congress of the Society for Melanoma Research, November 6-9 (SITC 2016 [November 9-13] might also be a venue, but I would lean towards SMR for clinical data).
Updated (7/6/16): Recall Provectus' COO and interim CEO's comments from the May 10th 1Q16 business update conference call: "It is conceivable the interim data will be available prior to one of our upcoming conference calls. And if so, it is possible that we will hold a special update call separate from the filing related call." The 3Q16 filing (and presumably call) would be done during the week of November 7th (Monday), which could suggest SMR rather than SITC.
3. PH-10 mechanism of action study results: Sometime in 2016? On the March 16th 4Q15/CY15 business update conference call Provectus' Dr. Eric Wachter, PhD noted in regards to a question about PH-10 mechanism of action work:
"We are working with a major research university on analyzing skin biopsies that were collected three times during the course of the study for each patient; at the beginning of the study pretreatment, after four weeks of application of vehicle, and then four weeks after application of PH-10. That work is essentially complete. We are in the process of receiving that this week. And I think that it's possible that we will have a preliminary analysis of that completed this month. Certainly if not this month, early into the next month, the month of April."We're well past the end of April.
Updated (7/6/16): I'm hard pressed to believe the pivotal melanoma Phase 3 trial interim readout is anything but a 1H17 event (and close to the end of the half than its beginning), despite Eric's comments on the May 10th 1Q16 business update conference call (when discussing the Imlygic-focused protocol changes):
"Questioner: All right. Thank you. I just want to follow up, and just as a result of you being behind by six months, did I hear Peter right by him saying that interim data from P3 will still be coming out this year even though you're six months behind schedule? How can that be?
Dr. Eric Wachter: We were projecting mid-year previously, and now that would occur near the end of the year or early next year with current projections."Based on a very limited data set, Eric tends to take several months from when he discusses protocols or protocol amendments to file them on CliniclTrials.gov, which merely is a time marker; he has said, for the Imlygic-focused protocol that changes were implemented several weeks before it was filed on CT.gov. The ASCO changes he proposed (see Trials in progress (June 9, 2016) below) might be filed on CT.gov in September/October, and perhaps implemented as early as September.
So, from late-summer or early-fall, how long might it take to complete the interim readout, given these ASCO-proposed changes? The proposed changes purportedly would speedup recruitment, but I'll guess another 3-month delay might be in the cards, strictly due to making and filing the changes, which may or may not be addressed by faster enrollment.
A bridge (July 3, 2016)
Updated below: 9/3/16.
Last year at Melanoma Bridge 2015, organized by Fondazione Melanoma Onlus under the auspices of National Cancer Institute “Fondazione G. Pascale" (Naples, Italy), Sidra Medical and Research Center (Doha, Qatar), the Italian Association of Medical Oncology (AIOM) and the Society for Immunotherapy of Cancer (SITC), PV-10/Rose Bengal key opinion leader and St. Luke's Health Network's Dr. Sanjiv Agarwala, MD presented "Overview of intralesional oncolytic therapy" during the News on Immunotherapy session.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated (9/3/16): Dr. Agarwala's presentation at Melanoma Bridge 2016 appears to have a name now: "Innovative combination strategies – oncolytic therapy and systemic immunotherapy approaches" (setting aside the digital burp that cause the '–').
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Australia (July 1, 2016)
Updated below, again, once more.
"A long time ago in a galaxy far, far away....," specifically November 2010, Provectus met with the Therapeutic Goods Administration purportedly to review a path for approval of PV-10 in Australia. See the associated press release here. At the time, Dr. Eric Wachter, PhD, head of the company's pre-clinical/clinical development program and later name Provectus' CTO presumably authored most if not all of the the words in release, which noted:
"The recent meeting focused on manufacturing, characterization and specifications for PV-10, along with a review of clinical data and anticipated Phase 3 study design and endpoints. The proposed primary endpoint of progression free survival, which Provectus proposed to the U.S. Food and Drug Administration (FDA) earlier this year in its first end-of-Phase-2 meeting with FDA, was deemed appropriate for assessment of efficacy in light of established European Medicines Agency (EMEA) standards adopted by TGA. Use of interim data from the first half of Phase 3 study subjects, in conjunction with safety data collected in earlier studies of PV-10 for melanoma, was discussed to allow early evaluation for marketing approval for metastatic melanoma, and TGA agreed that these data should be sufficient for this review if the analysis confirmed efficacy."Setting aside what may or may not have been circulated vis a vis meeting details and specifics at the time, the above release paragraph (with my underlined emphasis) appears to frame details of an agreed upon evaluative pathway: trial protocol design, appearing to focus in the release primarily on the primary endpoint; and some number of patients, comprised of the pivotal melanoma Phase 3 trial and prior clinical work. Since then (November 2010), I don't believe there has been further guidance from management regarding the pathway to approval in Australia.
About five and half years later (May/June 2016) Provectus establishes an Australian subsidiary. To what end? In An Australian office? (June 16, 2016) below I wrote about, as an example, CAR T company and U.S.-based Bluebird Bio, which established an Australian subsidiary, Bluebird Bio Australia Pty Ltd, in March 2014. Was Bluebird expecting approval in Australia when it set up the sub? I don't believe so. Rather, this company (before or around the time of creating the Australian sub) planned to treat an Australian patient, which occurred later that year ; see its December 2014 press release "bluebird bio Announces Data Demonstrating First Four Patients with β-Thalassemia Major Treated with LentiGlobin™ are Transfusion-Free." I would imagine Bluebird's approach to cancer treatment, unlike Provectus', requires proximate work to extract material (T cells) from a patient before it (the biologic material) is put back into him or her. I cannot imagine this material is shipped back to the U.S. (Cambridge, Massachussetts, or wherever Bluebird's production or lab facility is), and then returned to Australia. I would have to believe such work would be (would have been) done in Australia, requiring staff and lab space, which would generated SGA and/or R&D expense that would be run through the Australian sub, which then would roll-up to the U.S. parent's income statement, balance sheet and statement of cash flows. As such, Bluebird's near-term business goal for establishing its Australian subsidiary was to run expenses through it (and likely use it for liability reasons as well).
Provectus has run trials in Australia in the past and shipped product there. There has not appeared to be a historical clinical trial-based requirement to establish a subsidiary. What is the requirement now? Perhaps, in light of the introduction to this blog news item, a different question to ask is "What is the current requirement for Provectus to obtain approval in Australia?" That is, has the landscape, generally and specifically, changed from 2010 to 2016, such that the "terms" for an early evaluation for marketing approval may be different now?
Updated (7/2/16): H/t a regular hatter who provided the Australian Securities and Investments Commission (ASIC) details for Provectus Biopharmaceuticals Australia Pty Ltd. See three screenshots below (the original document is available as an automatic download using this link)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Source link is here. |
 |
| Click to enlarge. Source image |
 |
| Click to enlarge. Source image |
 |
| Click to enlarge. Source image |
 |
| Click to enlarge. Across the street at the time |
 |
| Click to enlarge. |
Is Provectus' Australia subsidiary for cost reimbursement related to the PV-10 Phase 1 NET liver mets, or a pathway to approval for PV-10 in melanoma?
Updated (7/2/16): In a continued dialog on the topic of Australia with the hatter of the hat tip immediately above, he or she noted Delpharm Consultants involvement with Novartis Fluad; see page 187 of "The Australian Immunisation Handbook, 9th Edition" (2008). This piqued my interest to search the TGA's website for "Delpharm Consultants," which returns 12 items.
One item of note to me was a January-June 2012 AusPAR for U.S.-based Osiris Therapeutics' Prochymal that had Delpharm as the sponsor. Prochymal is a stem cell therapy. It received marketing authorization in Canada in May 2012 and marketing consent in New Zealand in June 2012. Osiris sold Prochymal to Australia's Mesoblast in October 2013, which re-labelled the drug MSC-100-IV. The story, good and bad, continues. A November 2015 short thesis is here. The drug was licensed to a Japanese company, JCR Pharmaceuticals, which launched the drug in Japan in February 2016.
From June 28, 2016 TGA webpage "Australian Public Assessment Reports for prescription medicines (AusPARs):"
"An AusPAR provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve an application.
Before a prescription medicine can be made available in Australia, the company legally responsible for supplying the product must lodge a submission with the TGA. The TGA then evaluates the safety, quality and effectiveness of the product to determine if the benefits to people taking the medicine outweigh the risks."The Prochymal AusPAR references the acronym CER, which stands for clinical evaluation report. A CER, paraphrasing or taking from this link here (albeit for a medical device), contains a comprehensive analysis of the clinical data relevant to the drug, authored by a clinical expert competent in the appropriate field and able to give an objective assessment of the clinical data that are present. Unlike for the Prochymal/Osiris AusPAR that used Delpharm as a sponsor -- I can find no references to an Osiris Therapeutics Australia Pty Ltd, or a variation on the name -- another AusPAR uses the drug sponsor's Australian subsidiary, AstraZeneca Pty Ltd. An AusPAR appears to be a post-decision work product.
I'm very much guessing at the following. Consider the verbiage and figure below from "Transparent Regulations for Prescription Medicines: The Australian Way."
"Prescription medicines applications are classified into three categories:
Category 1: Applications for new chemical entities, new biological entities, new combination products, extension of indications, major variations, new generics, minor variations etc. fall under the category 1 application. Evaluation time: 255 working days from the date of acceptance."
 |
| Click to enlarge. |
"The TGA is introducing pilot changes to the pre-submission phase of the prescription medicines registration process. Participation in the pilot will be possible for all Type A (New Chemical and New Biological Entities and Biosimilars) and Type D (New Generic Medicine) applications accompanied by a validated version 3.0 eCTD. The pilot commenced on 1 February 2016 and will run until 30 September 2016.
The pre-submission pilot will test the effectiveness of removing the need to provide a 'Module 2 or equivalent' with your Pre-submission Planning form (PPF). It's anticipated that this will reduce the pre-submission workload for applicants and reduce overall registration timeline by at least one month."Fooling around with the estimator...
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated (7/3/16): According to the curriculum vitae (CV) of Princess Alexandra Hospital's Dr. Mark Smithers, MD, Delpharm was a co-sponsor of Provectus' "A Phase 2 Study of Intralesional PV-10 in the Treatment of Metastatic Melanoma."
 |
| Click to enlarge. Image source (third search return) |
 |
| Click to enlarge. |
June blog readership statistics.
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated below.
It seems to me that Huntsman Cancer Institute's Dr. Robert Andtbacka, MD might be an intralesional (IL) therapy key opinion leader (KOL), a "category KOL," while Rutgers Cancer Institute of New Jersey's Dr. Howard Kaufman, MD is a talimogene laherparepvec (T-Vec) KOL and St. Luke's University Health Network Dr. Sanjiv Agarwala, MD is a PV-10 (rose bengal) KOL, both agent KOLs.
Furthermore, one or both of Andtbacka and Agarwala might also be IL combination KOLs too, more so than other clinicians who were/are IL agent KOLs (e.g., Kaufman), or KOLs of immunotherapies that could be combined with IL agents (e.g., NYU Langone Medical Center's Weber, Memorial Sloan Kettering Cancer Center's Chapman, UCLA's David Geffen School of Medicine's Ribas, etc.). Agarwala has been discussing the combination of IL agents with immunotherapies (immune checkpoint inhibitors) and targeted therapies (e.g., vemurafenib) as early as 2012. Andtbacka has succinctly described the clinical value proposition of combining IL agents with immune checkpoint inhibitors (see A selection of literature (June 30, 2016) immediately below):
- IL agents do not add toxicity to the combination,
- IL agents may enhance the effect of checkpoint inhibitors, and
- IL agent-checkpoint inhibitor combination response rates are better than those of either treatment alone.
Both Agarwala and Andtbacka currently are involved in IL agent-checkpoint inhibitor clinical trials:
- Agarwala (3): PV-10 + pembrolizumab (SLUHN 2015-97), SD-101 + pembrolizumab (SLUHN 2015-93), HF-10 + ipilimumab (SLUHN 2014-66)
- Andtbacka (4): PV-10 + pembrolizumab (expected), pIL-12 electroporation + pembrolizumab (89469), SD-101 + pembrolizumab (89466), CAVATAK (Coxsackievirus A21) + ipilimumab (88893)
It struck me as a little odd that former/current Amgen (T-Vec) and Provectus (PV-10) consultant and Moffitt Cancer Center's Dr. Jonathan Zager wrote the below of IL therapies for cancer in June 9th's "An Update on Talimogene Laherparepvec:"
"Another advantage is a potential “bystander effect,” where a systemic response is induced and untreated lesions show regression. Unfortunately, this has only been seen with select intralesional therapies such as PV-10."T-Vec doesn't produce a bystander effect? Isn't that, a bystander effect (in non-treated lesions) presumably resulting from harnessing or priming or doing something to the immune system upon injection of treated lesions the raison d'être of oncolytic [virus] immunotherapies? It is of small molecule and ablative immunotherapies likes PV-10.
Provectus' metastatic melanoma Phase 2 trial prospectively explored the idea and concept behind the bystander effect because some patients had all of their lesions (disease) treated, and some did not.
 |
| Click to enlarge. ASCO 2014 image source |
 |
| Click to enlarge. Same image source as the above screenshot |
 |
| Click to enlarge. |
- Patient-level: 23% CR and 54% ORR for PV-10, vs. 0% CR and 48% ORR for T-Vec,
- Lesion-level, treated: 74% CR and 80% ORR, vs. 46% CR and 67% ORR, and
- Lesion-level, untreated (bystander): 54% CR and 59% ORR, vs. 30% CR and 41% ORR.
It is interesting that Kaufman felt the need to undertake a bystander analysis of T-Vec...now.
Updated (7/4/16): Associate Professor and Deputy Chair, Department of Melanoma Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center's Dr. Michael Davies, MD, PhD's June 2016 presentation "New Paradigms ‐ The Rapidly Evolving Landscape and Management of Advanced Melanoma" highlighted the next frontier, as he termed it, in treating melanoma: regional disease. Those regional diseased-focused slides included:
Amgen and its T-Vec key opinion leaders & clinicians (e.g., Andtbacka, Ross, Zager, Kaufman) continue to work on establishing the IL agent "bystander" bona fides, with Andtbacka et al., "Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial," Ann Surg Oncol (2016). H/t @bradpalm1.
A selection of literature (June 30, 2016)Updated (7/4/16): Associate Professor and Deputy Chair, Department of Melanoma Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center's Dr. Michael Davies, MD, PhD's June 2016 presentation "New Paradigms ‐ The Rapidly Evolving Landscape and Management of Advanced Melanoma" highlighted the next frontier, as he termed it, in treating melanoma: regional disease. Those regional diseased-focused slides included:
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
"Purpose
Talimogene laherparepvec (T-VEC) is an oncolytic immunotherapy designed to induce tumor regression of injected lesions through direct lytic effects, and of uninjected lesions through induction of systemic antitumor immunity. In this study, we describe the patterns and time course of response to T-VEC from the phase III OPTiM trial of 436 patients with unresected stages IIIB–IV melanoma...
Conclusions
T-VEC demonstrates response in injected and uninjected lesions, and represents a new first-in-class oncolytic virus immunotherapy for patients with melanoma. These data also suggest that, similar to other novel immunotherapies, more patients may have benefited from treatment with T-VEC due to the delayed nature of tumor regression. Opportunities to enhance T-VEC activity could include direct injection of visceral lesions and/or combination with immune checkpoint inhibitors. Early reports from a phase Ib clinical trial of T-VEC and ipilimumab suggest a response rate of 56% and a CR rate of 33%; a randomized phase II study is ongoing. Future studies will likely focus on defining the specific mechanisms by which T-VEC mediates tumor regression and the development of rational combination therapies to improve the potential of this agent for patients with cancer, in both melanoma and other tumors."
Updated below.
January 2016: "Use Patterns and Costs of Isolated Limb Perfusion and Infusion in the Treatment of Regional Metastatic Melanoma: A Retrospective Database Analysis" (Amgen paper)
"Comparison of ILP/ILI with other alternative therapies for limb melanoma metastases including electrochemotherapy, local therapies (interleukin, Rose Bengal, electrodessication), and systemic therapies was outside the scope of the current study. This topic warrants further research...The use of ILP and ILI was associated with long hospital stays and high costs. The results of this study may provide useful source data for the economic evaluation of treatment options for regional metastatic melanoma."April 2016: "A Role for Intralesional Monotherapy in Melanoma: Debate" (HemOnc Today Melanoma and Cutaneous Malignancies conference)
"Andtbacka’s case for the superiority of intralesional therapies combined with systemic therapies (ie, checkpoint inhibitors such as ipilimumab, nivolumab, pembrolizumab), was based on 3 points: intralesional therapies do not add toxicity; they may enhance the effect of checkpoint inhibitors; and response rates with the combinations are better than with either treatment alone."June 2016 (9th): "An Update on Talimogene Laherparepvec" (Moffitt Cancer Center)
"Another area that is changing the landscape in the treatment of metastatic melanoma is the use of intralesional therapy. Coley et al first developed intralesional therapy in 1893 when regression of a locally advanced tumor was seen after injection of a bacterial toxin. Since treatments are injected directly into the lesion, higher concentrations can be used with less risk of overall systemic toxicity. Another advantage is a potential “bystander effect,” where a systemic response is induced and untreated lesions show regression. Unfortunately, this has only been seen with select intralesional therapies such as PV-10."June 2016 (11th): "New Paradigms ‐ The Rapidly Evolving Landscape and Management of Advanced Melanoma" (MD Anderson)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
"From a time perspective, from an understanding of disease perspective, and also for understanding what may or may not work, I think the neoadjuvant approach in patients who have in-transit disease and palpable lymph node disease will be beneficial."The replacements, brah (June 28, 2016)
Updated below.
At the April 9, 2015 panel session Provectus held in New York -- see Audiophile (April 19, 2015) and Kick-off (June 22, 2015) on the blog's Archived News III page -- the company said its pivotal melanoma Phase 3 trial would comprise 25 sites in the U.S. and 10 in Australia (not including sites Provectus might or might not have in other countries, such as China, India and Brazil).
On the company's May 7, 2015 1Q15 conference call, in regards to the pivotal melanoma Phase 3 trial, Eric said: "As I've noted previously, we expect enrollment to be approximately one-third from the U.S.; one-third from Australia; and one-third from the rest of the world." See Conference Call, part 3 (May 10, 2015) on the blog's Archived News III page.
On Provectus' May 11th 1Q16 business update conference call, Eric's Eric's plan to address the Phase 3 being behind schedule was:
"First, we’ve enlisted the support of a very prominent clinical investigator in Germany to lead the European portion of our study. He joins similarly selective leads for each of the geographic regions, North America, Oceania, Brazil and China that the study stands. Second, we’ve been working with our lead site in Australia to assure that it can fulfill a nationwide regulatory role under the National Ethics Application System, or NEAS, as additional Australian sites join the study. Third, we’ve begun exploring expansion of our study to additional regions including Argentina, a country with demographics and melanoma incidents similar to the U.S. And four, we’ve begun streamlining clinical operations to show that human and capital resources are focused on our core mission."In response to a question on the same 1Q16 call about the path for and prospects of approval in Australia, Eric said:
Okay, Pete mentioned that early accelerated or expedited, we can use all sorts of terms, may be in a big fashion processes for approval in Australia, we are assessing, we’ve been assessing them since inception of our clinical work in Australia. One of the aspects of closing the expanded access protocol is that we now have an opportunity to collate all of the data from that process to be able to actually use it for at least supportive purposes if not better higher purposes in terms of regulatory approvals. So we’ll be continuing to review that opportunity in Australia on an ongoing basis. And as the case dictates, we may elect to move forward in Australia on a regulatory stance that’s faster or slower than in the U.S."Do clinical sites in the European Union (EU), Brazil, Argentina and Mexico essentially replace (rather than augment) Australian sites in an evolving path of a pivotal melanoma Phase 3 trial?
Updated (6/29/16): H/t regular finders of hats InvestorVillage poster canis_star who observed sponsors of every approved drug in Australia had a registered Australian company (i.e., with a name that included Pty Limited). Reviewing one of Australia's Therapeutic Goods Administration (TGA)' web pages the poster linked to, June 2, 2016's Prescription medicines: registration of new chemical entities in Australia, all sponsors in 2014, 2015 and 2016 were Australian subsidiaries, whether titled with a name that included Pty Ltd or, in the case of Bayer, Australia Ltd (i.e., Bayer Australia Ltd).
I previously address managed entry/access in Australia under blog news item Managed entry agreements/Managed access programmes (April 16, 2016) on the Archived News V page. In 2011, Australia introduced a managed entry scheme for listing new medicines pending the availability of more conclusive evidence of cost-effectiveness to support continued listing at a higher price (source: taken from Wonder et al., "Australian Managed Entry Scheme: A New Manageable Process for the Reimbursement of New Medicines?").
A March 2015 working document entitled "Framework for the Managed Access Programme for submissions to the PBAC" notes {underlined emphasis is mine}:
"The Managed Access Programme (MAP) is a mechanism that enables the Pharmaceutical Benefits Schedule (PBS) listing of products, under special circumstances of high unmet clinical need, on terms that allow for the resolution of otherwise unacceptable clinical or economic uncertainty for the Pharmaceutical Benefits Advisory Committee (PBAC). It seeks to enhance the quality and strength of evidence provided to decision-makers in reimbursement applications...
In most cases where there are not sufficient high quality data to clarify the efficacy and safety of a drug, randomisation in a Human Research Ethics Committee approved clinical trial is an appropriate standard of care. Such trials are not subsidised via the PBS. The application of the MAP mechanism is thus restricted to submissions where the PBAC agrees that, based on the data available, the proposed drug would provide a significant net clinical benefit, but the PBAC is uncertain about the magnitude of that benefit and therefore the price that should be paid, and when:
-- there is a high and urgent unmet clinical need for the drug;
-- the PBAC would not otherwise recommend the listing of the drug at the proposed price because the extent of the clinical effect is uncertain and/or there is a high cost to the PBS overall and/or on a per patient basis; and
-- there is evidence that can reliably be reported and evaluated within a reasonable timeframe, which the PBAC is satisfied would resolve the identified area of uncertainty."In a January 2015 article entitled "Is it possible to manage pbs managed entry schemes?," the author (Toby Gould, Market Access, Commercial Eyes) noted:
"The PBS Managed Entry Scheme provides an opportunity for new medicines to gain PBS listing earlier than previously possible. Whilst this opportunity sounds good, as suggested by the absence of references to Managed Entry Scheme proposals in PBAC meeting agendas and Public Summary Documents, it appears that there is a reluctance to pursue a Managed Entry route to market from industry.
Reluctance to pursue market entry under a Managed Entry Scheme may be due to a range of regulatory and commercial issues affecting when and how a new product is brought to market. However, a gap in the original framework for Managed Entry Schemes was the high degree of uncertainty about the mechanism(s) for sponsors to be able renegotiate a price that reflects the overall value of their product when mature evidence addressing areas of PBAC uncertainty was available."The author further noted:
"Whilst overall industry experience with the PBS Managed Entry Scheme appears to be limited, two positive recommendations under a Managed Entry Scheme were outlined in the November 2014 outcomes of the PBAC meeting (crizotinib [Xalkori®, Pfizer] and trametinib [Mekinist®, GSK].
In both cases the PBAC outlined that there would be no opportunity for sponsors to seek a higher price upon the presentation of more mature evidence, a position seemingly in conflict with the framework for Managed Entry Schemes published by the Department of Health."The concluded:
"Perhaps reflecting the reluctance of industry to pursue Managed Entry Schemes, the PBAC is set to consider a draft ‘Managed Access Programme’ framework at the March 2015 meeting. Whether or not this programme will represent a pragmatic evolution of the Managed Entry Scheme will be determined over the course of 2015."This draft was referenced above the Gould article. The July 2016 Pharmaceutical Benefit Advisory Committee (PBAC) meeting agenda included a major submission for trametinib "to seek PBAC reconsideration of the cost-effectiveness of trametinib for the treatment of metastatic melanoma and to fulfil the requirements of the Managed Entry Scheme."
Is Eric pursuing a managed entry scheme approach in Australia for PV-10 for the treatment of in transit melanoma? Both Peter MacCallum Cancer Centre (PeterMac) in Melbourne and Princess Alexandra Hospital in Brisbane presented abstracts about in transit melanoma patients they treated from 2010-14 and 2005-15, respectively. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) on the blog's Archived News V page.
2. A visitor from Paris' Université Pierre et Marie Curie (UPMC)/Pierre and Marie Curie University visited the blog earlier today for some time. He or she visited, among other pages, June 13, 2015
blog post Reproducibility, the Hallmark of Western Science.
A 2015 paper including UPMC co-authors was entitled "Trial Watch—Oncolytic viruses and cancer therapy," Pol et al., Oncoimmunology. 2015 Dec 8;5(2):e1117740. eCollection 2016. One of the authors (at UPMC). There is a relationship between UPMC and France's INSERM, both medical research institutions. One of the paper's author is INSERM and Gustave Roussy Cancer Center's Dr./Prof. Laurence Zitvogel, MD, PhD, whose current research includes tumour immunology and immunotherapy.
When you Google "rose bengal" and "Zitvogel," papers by Panzarini et al. come up regarding Rose Bengal Acetate PhotoDynamic Therapy that reference Dr. Zitvogel's work (i.e., published papers) and, for example, immunogenic and tolerogenic cell death.
In Moffitt Cancer Center's PV-10 mechanism of action paper "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1" paper, the authors reference Panzarini (one paper) and Zitvogel (three papers).
What is UPMC's interest in Rose Bengal and PV-10?
3. H/t InvestorVillage poster Area51 for the below, Provectus' lawsuit against former Chairman, CEO and a co-founder Dr. Craig Dees, PhD:
 |
| Click to enlarge. |
At June 15th, Provectus' short interest as a percent of float was 0.8%. For several different reasons, the company is not relevant to the capital markets.
 |
| Click to enlarge. Image source: WSJ Short Interest: NYSE MKT Highlights |
 |
| Click to enlarge. Tweet image source. Source data: WSJ Short Interest: Nasdaq Highlights |
Additive: 1 + 1 < 2. Synergistic: 1 + 1 > 2 (best case, >> 2) (June 25, 2016)
Updated below.
Following up on the blog news item immediately below, The bar (June 24, 2016), additive in the context of immuno-oncology combinations might be represented as one plus one is less than or equal to two; that is, efficacy (e.g., response rate) of the combination would be better than the individual efficacies of the pair's components. The sum of the parts is greater than the whole.
A synergistic combination, however, could or should generate efficacy greater than the sum of the individual efficacies, and, in a best case, much greater than the sum; that is, the whole is greater than the sum of the parts.
Taking MD Anderson's Dr. Merrick Ross, MD's combo therapy for melanoma slide -- see ASCO 2016: "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist" (June 22, 2016) -- I tried to illustratively model the difference between additivity and synergism.
 |
| Click to enlarge. |
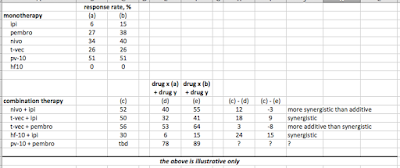 |
| Click to enlarge. |
More importantly, I return to the question I asked below. Would/will a PV-10 + pembro combination be additive in its results, or synergistic? If the pairing is additive, I'd imagine we see response rates (the Phase 1b study's secondary endpoints are progression-free survival and objective response rate) greater than ~60%.
If the pairing is synergistic, what would/could the response rate be? If the pairing is synergistic, how synergistic could it be?
Updated (6/27/16): If one was to try to compare apples-to-apples — still "sort of," and still very carefully and with upteen caveats — you'd have to consider the following before trying to assess or speculate about the outcome of the Phase 1b study combining PV-10 and pembrolizumab (n.b. I am trying to do the former, and I do have my own thoughts and beliefs about it, without treading on the latter):
- The objective response rate for PV-10 for Stage IV patients in Provectus' metastatic melanoma Phase 2 trial was 26% (no complete responses); PV-10 treatment in the Phase 2 study was limited to up to four injections per lesion over a 16-week period; patients were limited in the number of lesions they could have treated with PV-10 (up to 20); and, tumor measurement was based on modified RECIST,
- Pembrolizumab's KEYNOTE 001 study comprised Stage IV patients, and yielded an overall objective response rate of 34%,
- Provectus' Phase 1b combination study comprises only Stage IV patients; provides PV-10 treatment every three weeks, similar to the treatment schedule for pembrolizumab (but more frequent than the treatment schedule of the company's pivotal melanoma Phase 3 trial that provides PV-10 treatment every four weeks); treats all injectible lesions; and, measures tumor change based on RECIST 1.1.
The point of the "math" in regards to additive versus synergistic is this, and it's general and not meant to be actual or specific. If PV-10 and pembrolizumab are additive for patients, response rate could be 60% or less (it might be higher [or lower] given the differences between how tumors are treated and measured in PV-10's Phase 1b combination study compared to its Phase 2 monotherapy or single agent study); that is less than or equal to (very, very roughly speaking) the sum of 26% and 34%. If the pairing is synergistic, the response rate could be 60% or more; if the pairing is very synergistic, the response rate could be much higher.
The bar (June 24, 2016)
What is the bar for a combination therapy? A tweet from Dr. Vanessa Lucey, PhD/@vmlucey provides:
 |
| Click to enlarge. |
"...the effect of combination therapy with IL PV-10 and co-inhibitory blockade is mediated by CD8+ T cells, and that depletion of both CD4+ T cells and CD25+ Tregs significantly enhances anti-tumor immunity in a melanoma model."Provectus' press release on the abstract noted:
"Shari Pilon-Thomas, Ph.D., who leads the research team at Moffitt, noted, "Our results show that combining intralesional PV-10 with anti-PD-1 co-inhibitory blockade not only suppresses tumor growth vs. either agent alone but also yields marked increases in tumor-specific T cell activation against injected tumor."
Eric Wachter, Ph.D., Chief Technology Officer of Provectus, observed, "The nonclinical data reported by our collaborators at Moffitt reaffirm the crucial role T cells play in response to tumor ablation with intralesional PV-10, and further demonstrate the potential value of combining PV-10 with T cell directed checkpoint inhibition, such as the anti-PD-1 agent pembrolizumab. Intriguingly, these data also highlight possible strategies for augmenting this paradigm by harnessing additional targets in T cell signaling.""Synergistic? I think so.
Now consider a tweet from Merrilyn Datta/@merrilyn57:
 |
| Click to enlarge. |
But what could be the bar for perceived success of PV-10 and pembrolizumab? The bar might include an objective response rate of at least ~60% for melanoma (a so-called "solid tumor cancer").
 |
| Click to enlarge. Tweet image source @vmlucey |
Mostly cloudy. A high of 88, and 62% humidity. (June 23, 2016)
Updated below, again.
1. Provectus principals Dr. Timothy Scott, PhD (President), Dr. Eric Wachter, PhD (CTO) and Peter Culpepper (COO, and interim CEO) have a number of stock options that expire today; 1.05 million, 680K and 1 million, respectively. No significant amount of options are available to Dr. Scott and Peter until 2020, and Eric until 2025. If some of these options are exercised, one would imagine Form 4s would be filed by or on Monday, June 27th.
 |
| Click to enlarge. Image source |
- Dr. Scott has acquired/purchased less than 665K shares via stock option exercise (~32% of his share ownership) during his co-founder, officer/executive and board member tenure, and has forfeited almost as many options as he has exercised (~80%). One aspect of his share ownership, and that of Eric's, is that both received shares at Provectus' inception and, I believe, were issued stock in lieu of payment for services,
- Eric has acquired/purchased nearly 5.6 million shares via option exercise (~80%) during his co-founder, officer/executive and board member tenure, and forfeited no options,
- Peter acquired/purchased nearly 2 million shares via option exercise (100%) during his officer/executive tenure; this figure is nearly three times that of Dr. Scott.
Updated (6/28/16): It does not appear any of management's (and former management's) June 23rd $1.02-struck options were exercised (totaling 3.78 million shares of common stock, or >1% on a fully diluted basis). The forfeitures would be the first for Eric and Peter, and not unsurprising for Dr. Scott. At an approximately 65% discount to Thursday's closing share price, however, exercising may well have been a bridge too far for managers who previously have exercised options notably out-of-the-money (OTM).
 |
| Click to enlarge. |
3. H/t a shareholder and periodic hatter in regards to another melanoma combination of NewLink's IDO inhibitor indoximod and co-inhibitory blockade. The Phase 1b results of indoximod/ipilimumab combination in seven patients yielded an objective response rate (ORR) (by RECIST) of 29% (14% complete response [CR]). Compare this result to epacadostat/pembrolizumab's combination of 57% and 29%, respectively. NewLink's Phase 2 study (this company is the sponsor; there is no collaborator) appears to be a non-randomized clinical trial of three arms: indoximod + ipilimumab, indoximod + nivolumab, and indoximod + pembrolizumab.
Updated (6/23/16): H/t the same hatter who noted NewLink presented more recent combination data at ASCO 2016:
- Indoximod + pembrolizumab had a 53% ORR (8 of 15) and a 13% CR, and
- Indoximod + co-inhibitory blockade (i.e., ipi, nivo and pembro) had a 36% ORR (10 of 28) and a 10% CR.
The results remain in the range or ballpark of past initial combination results for melanoma; that is, ~50%'ish:
 |
| Click to enlarge. Image source |
4. Of the potential amendments to the pivotal melanoma Phase 3 trial protocol, see Trials in progress (June 9, 2016) below, the third (i.e., an aggregate diameter of target lesions of at least 1 cm [currently 1 to 5 lesions of at least 1 cm in longest diameter]) could be the most important because classic in-transit patients are similar to the example in Lippey et al.'s Figure 1, which observed that (a) the majority of patients Peter MacCallum treated with PV-10 had lesions <1 cm (12 of 19 patients) and (b) the number of lesions for most patients was <10 (13 of 19) (see Table II in Lippey et al.).
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
Broader label for the single agent Vs. A combination of agents (June 22, 2016)
A. First, let me establish the backdrop for this blog news item, which is a tweet (of an article by Endpoints' John Carroll) by John Carroll (formerly of FierceBiotech): "Gary Glick gets $27M to leap back into immuno-oncology R&D with Novartis, Atlas and Abingworth in his corner."
Does some or all of the below sound familiar, if you're a shareholder of Provectus, which has small molecule ablative immunotherapy Rose Bengal (PV-10)?
Of course, Moffitt Cancer Center authors published their work on PV-10's mechanism of action in May: "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1."
B. And second, let me further expand the backdrop of this post by further commenting on some features or aspects of Incyte’s IDO1 inhibitor in combination with Merck’s anti-PD-1 therapy in patients with advanced or metastatic melanoma; see Back to the future (June 15, 2016) below.
I think the company's clinical investigators may have a good amount of relevant clinical data to socialize with Big Pharma at the Post-Chicago meeting, and/or to present at SMR or SITC 2016.
2. Provectus CTO Dr. Eric Wachter, PhD's randomized combination therapy Phase 2 trial appears to be designed to expand the label of single agent PV-10 into Stage IV disease for its use in combination with, perhaps, explicitly pembrolizumab and implicitly at least all anti-PD-1 agents -- rather than designed to get the approval of PV-10 and pembrolizumab as a pairing or combination. See PV-10 + [insert name here] (September 23, 2015) on the blog's Archived News IV page.
Consider the following combination trials for advanced melanoma:
The trials seeking approval of a combination utilize a placebo in their design; Provectus' does not (at least not yet). Another interesting point, at least to me, is the similarity in a certain trial design of ipi + nivo, epa + pembro, and PV-10 + pembro: disease progress measurement using RECIST 1.1 (for ORR and PFS).
By running a randomized trial that compares PV-10 to standard of care (SOC) in the manner of PV-10 + SOC vs. SOC, rather than a randomized trial that includes a placebo in the manner of PV-10 + SOC vs. placebo + SOC, I believe Eric then could expand (in context) the single agent label and still pursue co-marketing with Merck and other PD-1 drug or drug compound owners. If he were to run a trial with a placebo, I would imagine he would be seeking the approval of the pairing, and reduce his leverage somewhat in discussions with the other drug in the pairing (and potentially other prospective partners with a pairable PD-1).
ASCO 2016: "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist" (June 22, 2016)
H/t a regular hatter and shareholder: Provectus updated its Melanoma Clinical Trial Update website page to include a melanoma symposium held (it would appear) at ASCO 2016 entitled "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist."
Symposium faculty were investigators/clinicians familiar with immune checkpoint inhibitors and intralesional agents, and from medical organizations that are clinical trial sites for Provectus' pivotal melanoma Phase 3 trial and the company's melanoma combination Phase 1b/2 study: Dr. Robert Andtbacka, MD (Chair)/Huntsman Cancer Institute, Dr. Sanjiv Agarwala, MD/St. Luke's Cancer Center, and Dr. Merrick Ross, MD/MD Anderson.
Table-setting slides:
Selected slides from Dr. Ross' portion, focusing on IL drugs/agents and their potential treatment roles. Note that he refers to them here, and has in the past, as "oncolytic immunotherapies," while Provectus is trying to differentiate between biologic T-Vec/Imlygic and chemical Rose Bengal/PV-10 by calling the latter an "ablative immunotherapy" in its (Provectus', PV-10's) branding.
The fors have it (June 17, 2016)
Updated below.
Provectus made an 8-K filing related to voting results on its annual meeting of stockholders' proposals. All proposals passed. My 2016 votes were described in June 14, 2016 blog post Proxy Vote.
Voting results from 2015 are below; my 2015 votes were described in May 30, 2015 blog post Proxy Vote.
Updated (6/19/16): Upon further reflection of the voting this year (and from last year, and compared to the year before), here are a few more thoughts:
"a new synthesis patent...will make it very clear that our protection of Rose Bengal for therapeutic use is in fact ours" (June 17, 2016)
Updated below, again, once more. Updated: 8/22/16.
A biotechnology company's intellectual property comprises its preclinical and clinical data, and its patents, patent applications, trade secrets (if applicable), etc. Provectus' COO and interim CEO Peter Culpepper noted at the 2016 BIO CEO & Investor Conference in February (my underlined emphasis):
The company received another patent (utilizing the same original name) in March 2016 (#9,273,022), which was a division of patent #8,530,675 (application number 12/884,833), having been allowed about three months earlier in November 2015. Provectus discussed the new synthesis patent in the company's March 1st press release, "Awarded Patent Extending Protection of the PV-10 Manufacturing Process:"
It might be approved in about three months, or September 2016.
Updated (7/18/16): Provectus issued a press release and made an associated 8-K filing regarding the notice of allowance (NOA) of the above patent application, Receives Patent Allowance for Use of Halogenated Xanthenes in Pharmaceutical Compositions and as Medicaments. The issue fee payment was received by the USPTO last week on July 15th, which likely was the necessitating process step for the company prior to press releasing the NOA.
The press release's title succinctly describes the value to Provectus' intellectual property portfolio. Rose Bengal is the company's lead investigational agent for both oncology and dermatology applications. It could be called a first-in-class halogenated xanthene*. There are, however, other members of the halogenated xanthene, all of which belong to Provectus, that a potential competitor might have used to seek approval, if they could, to challenge PV-10 (and PH-10) if and when approved.
Takeaway: The PR, and particularly the title, highlight Provectus' ownership of all halogenated xanthenes (the so-called analogs of Rose Bengal) in regards to owning and making them for therapeutic use (i.e., synthesis for pharmaceutical composition for medicaments). That is, the company owns the first-in-class, the second-in-class, the third-in-class, etc.
Provectus' CTO Dr. Eric Wachter, PhD's quotes in the PR are useful:
Takeaway: Peter's last quote should remind folks that Provectus is much farther ahead than most companies in its situation in regards to manufacturing, logistics and, in general, supply chain.
* There are other labels of relevance to a broader discussion of class, such as a first-in-class ablative immunotherapy.
** A discussion of suitable valuation methods (e.g., discounted cash flow/net present value, peak sales multiple, precedent transactions, etc.) requires context of course.
Updated (7/24/16): H/t a hatter who believes the allowed patent will issue (be awarded) by early-to-mid-August (based on the July 15th issue fee payment date and the current US PTO schedule. Perhaps there is something else contained in the about-to-be-granted daughter synthesis patent that management is waiting on. Peter’s quote that "[i]nvestigational drug product generated using this proprietary technology is being used in all ongoing clinical trials of PV-10, including the pivotal phase 3 trial in melanoma (NCT02288897)" makes the hatter wonder whether this new patent would provide an additional layer of IP protection in Eric’s compulsive mind that then would facilitate the release of some data.
Updated (8/4/16): The patent will be awarded on August 23rd.
Updated (8/22/16): The patent was awarded.
A. First, let me establish the backdrop for this blog news item, which is a tweet (of an article by Endpoints' John Carroll) by John Carroll (formerly of FierceBiotech): "Gary Glick gets $27M to leap back into immuno-oncology R&D with Novartis, Atlas and Abingworth in his corner."
Does some or all of the below sound familiar, if you're a shareholder of Provectus, which has small molecule ablative immunotherapy Rose Bengal (PV-10)?
"“It’s very clear that checkpoint inhibitors, to frame it in context, are here to stay,” explains Glick. “They’ve made tremendous strides, with treatment options for patients with no other option.” But they are also limited to the number of patients and indications that they target now, still facing hurdles in the tumor microenvironment.
Glick believes he can blaze a trail with small molecules that can take some of those hurdles down, working both as single agents and in combination with checkpoint inhibitors which can facilitate an immune system attack on tumors. There’s also a strategy for developing new drugs for autoimmune diseases.
“There are a number of targets in the innate immune system that have the ability to turn cold tumors hot,” says Glick. “What’s unique is that we have these compounds, when these targets are turned, that do not lead to a cytokine storm that can lead to an adverse event.”" {underlined emphasis is mine}As an aside, I wrote about the innate immune system in March 21, 2014 blog post PV-10 is not bigger than Mother Nature:
More interestingly, and don't take my word for it — because, in truth, who am I in the grand scheme of things — "High mobility group box 1 (HMGB1) is a ubiquitous nuclear protein that is implicated in various aspects of the innate immune system as a damage-associated molecular pattern molecule and a late mediator of inflammation, as well as in principal cellular processes, such as autophagy and apoptosis" {bolded and underlined emphasis is mine}, source: Lee et al., Yonsei Med J. 2014 Sep; 55(5):1165-76."Interestingly, dendritic cells are a component of the innate immune system. "Dendritic cells serve as a link between the bodily tissues and the innate and adaptive immune systems, as they present antigen to T cells, one of the key cell types of the adaptive immune system." My takeaway, here, is dendritic cells are a critical link or bridge between the two layers (innate and adaptive) of the immune system. Dendritic cells."
Of course, Moffitt Cancer Center authors published their work on PV-10's mechanism of action in May: "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1."
B. And second, let me further expand the backdrop of this post by further commenting on some features or aspects of Incyte’s IDO1 inhibitor in combination with Merck’s anti-PD-1 therapy in patients with advanced or metastatic melanoma; see Back to the future (June 15, 2016) below.
- February 2014: The parties (Merck, Incyte) enter into the collaboration for the abovementioned combination trial for several metastatic cancers. Incyte is to be the sponsor of the trial. Costs are to be shared by the parties. Incyte notes "...its collaboration with Merck follows preclinical studies that suggested a combination of INCB24360 and MK-3475 may enhance the antitumor immune response more than either agent alone." (MK-3475 was pembrolizumab's original name),
- June 2014: The study begins, A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of Pembrolizumab (MK-3475) in Combination With Epacadostat (INCB024360) in Subjects With Selected Cancers (INCB 24360-202 / MK-3475-037 / KEYNOTE-037/ ECHO-202),
- July-August 2015: The study does a data readout: "As of August 21, 2015, 54 patients were enrolled. This report includes safety data on 28 patients (melanoma [n=11], RCC [n=5], NSCLC [n=5], TCC [n=3], EA and SCCHN [n=2 each]) and 19 patients evaluable for efficacy as of July 13, 2015."
- October 2015: The parties expand the collaboration to begin a pivotal trial of the combination in patients with advanced melanoma. Under the terms of the agreement, the parties also agree to, "...for a period of two years, not to initiate new pivotal studies of an IDO1 inhibitor in combination with a PD-1/PD-L1 antagonist as first-line therapy in advanced or metastatic melanoma with any third party. During this time, the companies will each offer the other the opportunity to collaborate on any new pivotal study involving an IDO1 inhibitor in combination with a PD-1/PD-L1 antagonist for types of melanoma and lines of therapy outside of the current collaboration agreement,"
- November 2015: The parties release data of the Phase 1 work at SITC 2015. A table from Gangadhar et al., Journal for ImmunoTherapy of Cancer 2015 3(Suppl 2):O7 is below.
 |
| Click to enlarge. Image source |
- June 2016: The pivotal melanoma study begins, A Phase 3 Study of Pembrolizumab + Epacadostat or Placebo in Subjects With Unresectable or Metastatic Melanoma, and avoids Stage III or Stage IV patients amenable to local therapy.
The Phase 1 phase or portion of the Incyte/Merck collaboration had a primary endpoint of objective response rate (ORR) and a secondary endpoint of progression-free survival (PFS). Upon how much data (and what kind) did Incyte and Merck rely in order to advance metastatic melanoma from the Phase 1 study to a pivotal, randomized Phase 3 trial?
- Results of ORR and, probably, PFS (i.e., endpoints state above),
- ~12 months (i.e., June 2014 to July 2015 above), a timeframe more relevant to PFS than ORR but that would provide perspective on the durability of ORR, and
- A melanoma N (of the trial), perhaps, of 7.
All of Incyte's epacadostat's clinical trials for melanoma are in combination with another agent: pembrolizumab (pivotal Phase 3, recruiting), a vaccine therapy (Phase 2, recruiting), nivolumab (Phase 1/2, recruiting) and durvalumab (Phase 1/2, recruiting).
Finally, the parties (Merck, Incyte) observe the data in July/August, when they are readout, and further expand their relationship (i.e., melanoma) a couple of months later (October) before presenting the data at a medical conference (November). What should be clear is that Incyte is working with Merck to get a combination therapy approved that would be marketed as a combination, like approved ipilimumab (Yervoy) and nivolumab (Opdivo).
1. So, how much data, and what kind, does Provectus require to secure a co-development partner (to combine its drug with PV-10 for at least melanoma), setting aside for now a discussion of terms and conditions?
- November 2014: At SITC 2015, Moffitt presents preclinical work indicating that a combination of PV-10 and co-inhibitory blockade (CTLA-4, PD-1 and PD-L1) improves anti-tumor immunity and regression of melanoma.
- September 2015: Provectus initiates its own program combining PV-10 and pembrolizumab for Stage IV patients, with a primary endpoint of PFS and secondary endpoint of ORR.
- March 2016: The Phase 1b portion of Provectus' combination therapy program may be transitioning to a Phase 2; see PV-10 Phase II Combination With Pembrolizumab (March 17, 2016) on the blog's Archived News V page.
- June-July 2016: The 6th Post-Chicago Meeting on Melanoma/Skin Cancer is held in Munich, six to nine months after September to November. Six-month PFS rates for pembrolizumab ranged from 34-38%; median PFS was 5-6 months.
2. Provectus CTO Dr. Eric Wachter, PhD's randomized combination therapy Phase 2 trial appears to be designed to expand the label of single agent PV-10 into Stage IV disease for its use in combination with, perhaps, explicitly pembrolizumab and implicitly at least all anti-PD-1 agents -- rather than designed to get the approval of PV-10 and pembrolizumab as a pairing or combination. See PV-10 + [insert name here] (September 23, 2015) on the blog's Archived News IV page.
Consider the following combination trials for advanced melanoma:
 |
| Click to enlarge. |
By running a randomized trial that compares PV-10 to standard of care (SOC) in the manner of PV-10 + SOC vs. SOC, rather than a randomized trial that includes a placebo in the manner of PV-10 + SOC vs. placebo + SOC, I believe Eric then could expand (in context) the single agent label and still pursue co-marketing with Merck and other PD-1 drug or drug compound owners. If he were to run a trial with a placebo, I would imagine he would be seeking the approval of the pairing, and reduce his leverage somewhat in discussions with the other drug in the pairing (and potentially other prospective partners with a pairable PD-1).
ASCO 2016: "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist" (June 22, 2016)
H/t a regular hatter and shareholder: Provectus updated its Melanoma Clinical Trial Update website page to include a melanoma symposium held (it would appear) at ASCO 2016 entitled "The Role of Immunotherapy in the Medical Management of Melanoma: An Overview for the Oncologist."
 |
| Click to enlarge. Image source |
Table-setting slides:
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
Updated below.
Provectus made an 8-K filing related to voting results on its annual meeting of stockholders' proposals. All proposals passed. My 2016 votes were described in June 14, 2016 blog post Proxy Vote.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
- There were several perspectives on the same and different topics from all sides (e.g., management, the board of directors, shareholders in attendance, shareholders not in attendance, etc.),
- Shareholders who care, and care to voice their views, are growing in number, on an absolute basis, and as a percent of total shares outstanding*. For example, the total number of votes on directorship and compensation more than doubled from 2013 to 2016, even though the number of shares outstanding increased by ~50%.
 |
| Click to enlarge. |
I believe shareholder opinion (and the desire and motivation to make their opinion known) will be important if and when it comes times to vote on an appropriate or inappropriate buyout figure,
- Shareholder voices and opinions can matter, even they don't get what they want (for now). Shareholder disapproval of Provectus managers and its board increased dramatically over the last two years.
 |
| Click to enlarge. |
I believe both management and the board are hearing the growing chorus of valid criticism (irrespective of whether the singers voted for or against),
- In my view, whether they voted for or withhold/against (or abstained), shareholders understand the growing, potential, broadscale clinical value of Rose Bengal (and understand it well), even if worth is not yet reflected in the share price and market capitalization (and hasn't been for quite some time).
A more recent example of growing clinical value (or, at a minimum, growing clinical interest) might be pediatric oncology. There was biomedical literature from the 1960s about the use of Rose Bengal in children; see Rose Bengal in children (hepatoblastoma, radiopharmaceutical) (May 6, 2016) on the blog's Archived News V page. Amgen recently began exploring the use of its intralesional agent (IL) and oncolytic immunotherapy talimogene laherparepvec (T-Vec; Imlygic) in children with advanced non-CNS tumors; this trial protocol was first received by ClinicalTrials.gov in December 2015, and trial is not yet recruiting as of the last CT.gov change in April 2016. Small molecule, halogenated xanthene and ablative immunotherapy PV-10 should have widespread applicability for pediatric solid tumor cancers.
Longtime shareholders of all perspectives hoped the company, and thus the share price, would have been farther ahead (higher) in 2010 when Provectus management thought accelerated approval on the basis of the company's metastatic melanoma Phase 2 results was viable — before Bristol-Myers' anti-CTLA-4 immunotherapy ipilimumab/Yervoy overturned the melanoma playing field with its pivotal trial success and drug approval in 2011. Pfizer's CTLA-4 inhibitor and ipi relative tremelimumab failed its pivotal trials around the same time; the co-inhibitory blockade compound was resurrected by AstraZeneca, which recently compared the approved Yervoy/anti-PD-1 nivolumab/Opdivo melanoma combination to its anti-PD-L1 durvalumab/treme pairing.
These same shareholders thought IL agent and ablative immunotherapy PV-10 would have its day in 2014 — before breakthrough therapy designation was denied by the FDA. In an almost Gladiator-esque final scene ("I will see you again, but not yet...not yet"), clinical benefit derived from tumor destruction and shrinkage was recognized by the Agency and its advisory panels for T-Vec in April 2015 (committee meeting), and again in October (drug approval).
Here shareholders are, in 2016, with a considerable amount of attention being paid to PV-10, arguably, and finally. I believe the drug and the company are approaching both regulatory and commercial validation. I also believe there can be no intellectually honest disagreement over the attention being paid to PV-10 by clinicians and key opinion leaders, the FDA and other global regulatory bodies, and Big Pharma and Big Biotech.
Both Provectus' management and the board have a lot of work still to do, and room for improvement to do it. The company's CTO Dr. Eric Wachter, PhD needs to show more integrity regarding efficient and effective communications about process and progress; as much as (and perhaps more than) he believes clinical data should be generated with the utmost integrity. The board should try to be be balanced, active, knowledgeable and invested in its understanding of the growing, potential, broadscale clinical value of Rose Bengal, and in its discussions and interactions with management — ultimately for patients, and thus for shareholders.
Both Provectus' management and the board have a lot of work still to do, and room for improvement to do it. The company's CTO Dr. Eric Wachter, PhD needs to show more integrity regarding efficient and effective communications about process and progress; as much as (and perhaps more than) he believes clinical data should be generated with the utmost integrity. The board should try to be be balanced, active, knowledgeable and invested in its understanding of the growing, potential, broadscale clinical value of Rose Bengal, and in its discussions and interactions with management — ultimately for patients, and thus for shareholders.
* Not a fully diluted number
Updated below, again, once more. Updated: 8/22/16.
A biotechnology company's intellectual property comprises its preclinical and clinical data, and its patents, patent applications, trade secrets (if applicable), etc. Provectus' COO and interim CEO Peter Culpepper noted at the 2016 BIO CEO & Investor Conference in February (my underlined emphasis):
"Now the intellectual property is important on Page 28. The synthesis patent, the fourth one down, that’s the new one that expires in 2031. That was necessary so you cannot buy Rose Bengal off the shelf and use it in a patient. It does not meet ICH specifications. So the new synthesis is the way that we can protect the manufacturing of Rose Bengal to the specifications necessary. However, we’ve been notified by the US PTO that there is a new patent coming, so we have a Notice of Allowance of a new synthesis patent. We’ll be sure to announce that. That’s very important as well and will make it very clear that our protection of Rose Bengal for therapeutic use is in fact ours.Previously, Provectus received its original or initial (and key) synthesis patent, "Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes," in September 2013 (#8,530,675).
The company received another patent (utilizing the same original name) in March 2016 (#9,273,022), which was a division of patent #8,530,675 (application number 12/884,833), having been allowed about three months earlier in November 2015. Provectus discussed the new synthesis patent in the company's March 1st press release, "Awarded Patent Extending Protection of the PV-10 Manufacturing Process:"
"Provectus believes that this patent, wholly owned by Provectus and conferring coverage to at least 2031, will provide further protection around the proposed commercial process for manufacturing PV-10. Investigational drug product generated using this proprietary technology is being used in all ongoing clinical trials of PV-10, including the pivotal phase 3 trial in melanoma (NCT02288897).
Provectus' efforts to bring this process development to fruition were supported by Cambrex Charles City, Inc., a subsidiary of Cambrex Corporation (NYSE:CBM, www.cambrex.com), a life sciences company that provides products and services that accelerate and improve the development and commercialization of new and generic therapeutics."It would appear Provectus has further extended the protection of its manufacturing process of Rose Bengal (and presumably the entire class of halogenated xanthenes) with another division of the parent, application number 13/916,408, that was allowed by the USPTO on June 6th.
 |
| Click to enlarge. |
Updated (7/18/16): Provectus issued a press release and made an associated 8-K filing regarding the notice of allowance (NOA) of the above patent application, Receives Patent Allowance for Use of Halogenated Xanthenes in Pharmaceutical Compositions and as Medicaments. The issue fee payment was received by the USPTO last week on July 15th, which likely was the necessitating process step for the company prior to press releasing the NOA.
 |
| Click to enlarge. |
Takeaway: The PR, and particularly the title, highlight Provectus' ownership of all halogenated xanthenes (the so-called analogs of Rose Bengal) in regards to owning and making them for therapeutic use (i.e., synthesis for pharmaceutical composition for medicaments). That is, the company owns the first-in-class, the second-in-class, the third-in-class, etc.
Provectus' CTO Dr. Eric Wachter, PhD's quotes in the PR are useful:
"The allowed subject matter originates from a divisional application, or so-called 'daughter' case, derived from the original, 'parent' case that led to issuance of U.S. Patent 8,530,675 in September, 2013. That parent case covers our novel process for synthesizing rose bengal and related analogs. This daughter case confers protection to use of a wide range of those analogs in or as therapeutic products. This subject matter was described in the parent filing, but since it covers a different patent classification than that which formed the basis for the initial set of claims issued in the '675 patent, it was necessary to seek protection as a divisional application. This allowance represents complementary protection to that afforded by the '675 patent.
Since the daughter case covers pharmaceutical use of novel rose bengal analogs that can be made using our patented synthesis process, it provides a significant potential commercial lifetime for these analogs. Such patent strategy is common in our industry, building on original innovation by allowing value to be derived from next generation pharmaceutical products, thereby driving further innovation."As are the company's COO and interim CEO Peter Culpepper:
"This patent allowance speaks to the first and second pillars in our value proposition: our intellectual property and our control of the drug supply chain. We have a robust portfolio of patents related to the production of PV-10, and this allowance extends the value of that to potential future products that build on the work we've put into PV-10.
Earlier this year, we received U.S. Patent No. 9,273,022, which extends the scope of protection of the manufacturing process conferred by our September 2013 patent to include coverage of the use of alternative raw material in manufacturing halogenated xanthenes, including rose bengal, the active pharmaceutical ingredient (API) in PV-10. That patent, which is wholly owned by Provectus and conferring coverage to at least 2031, provides further protection around our proposed commercial process for manufacturing PV-10. Investigational drug product generated using this proprietary technology is being used in all ongoing clinical trials of PV-10, including the pivotal phase 3 trial in melanoma (NCT02288897)."Takeaway: With each additional relevant patent, the so-called patent cliff for Rose Bengal (if and when approved) gets extended, and an eventual buyer of the company has a potential pipeline of analogs to follow through with in terms of R&D and hope for approval. The 2031 timeframe above also becomes relevant for valuation** purposes when building the spreadsheet related to worth of the molecule and molecular family.
Takeaway: Peter's last quote should remind folks that Provectus is much farther ahead than most companies in its situation in regards to manufacturing, logistics and, in general, supply chain.
* There are other labels of relevance to a broader discussion of class, such as a first-in-class ablative immunotherapy.
** A discussion of suitable valuation methods (e.g., discounted cash flow/net present value, peak sales multiple, precedent transactions, etc.) requires context of course.
Updated (7/24/16): H/t a hatter who believes the allowed patent will issue (be awarded) by early-to-mid-August (based on the July 15th issue fee payment date and the current US PTO schedule. Perhaps there is something else contained in the about-to-be-granted daughter synthesis patent that management is waiting on. Peter’s quote that "[i]nvestigational drug product generated using this proprietary technology is being used in all ongoing clinical trials of PV-10, including the pivotal phase 3 trial in melanoma (NCT02288897)" makes the hatter wonder whether this new patent would provide an additional layer of IP protection in Eric’s compulsive mind that then would facilitate the release of some data.
Updated (8/4/16): The patent will be awarded on August 23rd.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
An Australian office? (June 16, 2016)
Updated below.
Provectus incorporated its Australian subsidiary, Provectus Biopharmaceuticals Australia Pty Ltd, on May 18th.
The company received its Australian business number (ABN) for the local subsidiary on June 6th.
The above ABN information indicates a business location in Hornsby (a postcode of NSW 2077), a suburb of Sydney; this link, the Australian Securities and Investments Commission (ASIC) register, indicates a postcode/location of NSW 2000/Sydney.
On Provectus' May 11th 1Q16 business update conference call, however, the company's CTO Dr. Eric Wachter, PhD announced PV-10's CUP would be wound down by year-end. See Objects in mirror are closer than they appear (May 26, 2016):
CAR T company and U.S.-based Bluebird Bio established an Australian subsidiary, Bluebird Bio Australia Pty Ltd, in March 2014. Was Bluebird expecting approval in Australia when it set up the sub? No. This company treated an Australian patient towards the end of 2014; see its December 2014 press release "bluebird bio Announces Data Demonstrating First Four Patients with β-Thalassemia Major Treated with LentiGlobin™ are Transfusion-Free." Bluebird, unlike Provectus, doesn't ship treatment product from Cambridge, Massachusetts to patients. Work is required to extract material (T cells) from a patient before it (the biologic material) is put back into him or her. I do not believe this material is shipped back to the U.S., and then returned to Australia. I think such work would be done in Australia, requiring staff and lab space, which would generated SGA and/or R&D expense that would be run through the Australian sub, which then would roll-up to the U.S. parent's income statement, balance sheet and statement of cash flows.
Provectus established an Australian sub this year for what reason?
Back to the future (June 15, 2016)
Updated below.
PDT? "Targeted Photoimmunotherapy Approach for Cancer Moves Forward," National Cancer Institute (NCI) staff, NCI, April 25, 2016
There is considerable biomedical literature on the topic of Rose Bengal as photodynamic therapy (PDT) for oncology and other applications (e.g. anti-bacterial). See this PubMed link here (search terms "rose bengal" and "photodynamic therapy"):
Effect size. H/t InvestorVillage poster canis_star, who noted the start of a pivotal melanoma Phase 3 trial for the combination of Incyte's IDO inhibitor epacadostat and Merck & Co.'s immune checkpoint inhibitor pembrolizumab/Keytruda. Of note to me:
CEO Search Committee (June 13, 2016)
Last month I was asked to interview for a spot (as a shareholder or "shareholder representative") on Provectus' CEO search committee.
As I believe I understood it, the committee was to have comprised three members: (i) committee leader and independent/outside director Jan Koe, (ii) management/management representative, a co-founder and inside director Dr. Tim Scott, PhD, and (iii) a non-officer/non-director shareholder/shareholder representative. I thought I understood that the committee's recommendation of a CEO candidate would have been made to the group of independent/outside directors Koe, board chairman Al Smith IV, and Dr. Kelly McMasters, MD, PhD, and ultimately voted on by the full board. I think three shareholders were interviewed for this spot or position.
From what I now understand, the search and search process will continue without the above construct of a shareholder representative included on a/the CEO search committee. I'll go into more detail about this topic after I finish blogging the rationale of my proxy vote; see Still [road] trippin' (June 13, 2016) immediately below. I did provide my vote below in the context of (fully or mostly expecting) the abovementioned outcome or lack thereof.
Still [road] trippin' (June 13, 2016)
Provectus’ 2016 annual meeting of stockholders is scheduled for Thursday, June 16th at 4 pm EDT in New York, New York. Shareholders of record as of April 25th's close of business should have until the annual meeting to vote. Practically, however, votes probably should be received at least 48 hours ahead of the meeting (i.e., no later than Tuesday, June 14th at 4 pm EDT). This year Provectus asked shareholders to vote on three proposals:
Trials in progress (June 9, 2016)
Updated below.
Provectus issued a press release today and also made an associated 8-K filing related to its trials in progress poster at ASCO 2016, Announces Poster Presentation on PV-10 at Annual Meeting of American Society of Clinical Oncology Now Available Online.
Provectus' CTO Dr. Eric Wachter, PhD noted (presumably with the goal of trying to further accelerate enrollment):
The MD Anderson of Australia. Melbourne's Peter MacCallum Cancer Centre is holding a conference called Locoregional Melanoma 2016 on August 26 and 27th, of which Provectus is a sponsor.
The conference's scientific convenor is Dr. David Gyorki, MD, a surgical oncologist at "PeterMac."
Dr. Gyorki also is the author of correspondence of the PV-10-related compassionate use/expanded access program (special access scheme in Australia) paper Lippey et al., "Intralesional PV-10 for in-transit melanoma-A single-center experience," J Surg Oncol, 2016 May 30.
The agenda includes:
Updated below.
Dr. Eric Wachter, PhD, Provectus CTO, May 10th1Q16 business update conference call:
Updated (6/7/16): Intralesional (IL) drug Imlygic. H/t InvestorVillage poster canis_star: A June 7th article about IL T-Vec (aka talimogene laherparepvec) entitled "Oncolytic virus approved to treat melanoma" by Charles Schmidt (originally published: JNCI J Natl Cancer Inst (2016) 108 (5)). Of note to me, with my underlined emphasis:
Dees lawsuit update. H/ts to InvestorVillage posters joeytakasugi and Area51. The former provides a link to the pdf file of an updated complaint. The latter notes:
Updated below.
One key piece of of Provectus' intellectual property (e.g., patents, patent applications, trade secrets, etc.) is the company's combination therapy patent for certain combinations involving PV-10 that is jointly held with Pfizer (and awarded in August 2015): "Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer." During his Marcum Microcap Conference presentation on June 2nd, Provectus' COO and interim CEO Peter Culpepper said (again) that Pfizer doesn't gain the benefit of the combination therapy patent unless it does a transaction (buys) Provectus. If someone else buys Provectus, said buyer would gain benefit of the patent.
Provectus' Phase 1b/2 trial protocol of "PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma" was amended in two places:
In the combination trial of PV-10 and Merck & Co.'s anti-PD-1 pembrolizumab (Keytruda), some might be focused on Keytruda efficacy as a comparator to PV-10. The study program has been designed to test PV-10 + standard of care (SOC) vs. SOC, which is a very common strategy for pivotal studies (i.e., investigational agent + SOC vs SOC), and not PV-10 vs. SOC. Again, while the comparator is Keytruda, the comparison is Keytruda and PV-10.
See PV-10 + [insert name here] (September 23, 2015) on the blog's Archived News IV page. It is quite common to pair an investigational therapy with a standard of care (e.g., pivotal testing of Bristol-Myers' anti-CTLA-4 ipilimumab with systemic chemotherapy DTIC vs. DTIC) that would result (if successful) in the approval of the investigational therapy without requiring its subsequent use in the combination. Since the SOC is routinely available, no support is required or needed from the SOC's owner.
Once the above is accomplished, there may be situations where the combination is marketed together.
As noted above, Provectus' has claims covering the combination of PV-10 and other cancer treatments, so the company "owns" or controls any co-marketing (if successful) of PV-10 and Keytruda. The two could be sold separately if PV-10 garners approval as a single agent but, for instance, Merck & Co. could not suggest use of Keytruda with PV-10 unless Provectus allows it.
Updated (6/5/16): The Twitter feed I curated for this project currently comprises 15 "followings:" Paul D. Rennert/@PDRennert, Brad Loncar/@bradloncar, Luke Timmerman/@ldtimmerman, Amy Brown/@AmyEPVantage, Christopher Heery/@ChrisHeery, Jacob Plieth/@JacobPlieth, John Carroll/@JohnCBiotech, Tom Silver/@TomSilver39, Philippe Aftimos, MD/@aftimosp, Jamie Singer/@JSwatercooler (Provectus), Sally Church/@MaverickNY, Matthew Herper/@matthewherper, Adam Feuerstein/@adamfeuerstein and Peter Culpepper/@peterculpepper (Provectus).
The feed seemed to coalesce around combination therapy-oriented thoughts as ASCO 2016 began starting June 3rd (in chronological order of tweeting, oldest to newest):
And (PLoS ONE 2013): "Splenocytes isolated from tumor bearing mice treated with IL PV-10 demonstrated enhanced tumor-specific IFN-gamma production compared to splenocytes from PBS-treated mice in both models."
A cracked pipeline (June 2, 2016)
H/t a shareholder who forwarded the following article to me, "Cancer doctors face next drug challenge," David Crow, Financial Times, June 1st {underlined emphasis is mine}:
b. Provectus has advanced the therapeutic use of Rose Bengal, which is the second medicinal use of the compound. The original and first medicinal use of Rose Bengal was as a diagnostic. Isn't Rose Bengal a classical small-molecule therapeutic?
c. Provectus' intellectual property (IP) covers all halogenated xanthenes (HXs), and the company's IP continues to grow around the class of HXs, of which Rose Bengal is first-in-class. Second-, third-, etc.-in-class also are owned by Provectus. As such, generic manufacturers should find it difficult to copycat Rose Bengal and its relatives.
Funny (2008): "Biological drugs — antibodies, bioactive proteins and the like — can be expensive to produce, but they're also very difficult to copy. And the world's regulatory agencies are still working out how to tell if another firm's generic biological is truly equivalent to the original branded drug. These factors mean that biological products have a lifetime that can extend well beyond their natural patent term, unlike traditional small-molecule drugs, which are under siege from generic manufacturers."
Rose Bengal is a small molecule cancer drug made from a chemical compound. Aspects of management have been and are one thing. I agree. Chemistry, however, is another. Unless you really don't understand, um, chemistry?
Red Food Dye No. 105 (June 1, 2016)
Updated below, again.
There were several points of import for me in Australia's PeterMac's PV-10 experience (Lippey et al., see immediately below or June 1st blog post Intralesional PV-10 for In-Transit Melanoma—A Single-Center Experience).
Please note: Rather than use quotations to identify the authors' work (words), I am very liberally using (copying) their own words as I write below so as not to be too disjointed.
Among these points were:
Intralesional PV-10 for in-transit melanoma—A single-center experience (May 31, 2016)
Updated below.
One of the two abstracts related to PV-10 (for the treatment of melanoma) that were presented at the Royal Australasian College of Surgeons 85th Annual Scientific Congress (May 2-6) in Australia was published (as a paper) in the Journal of Surgical Oncology: "Intralesional PV-10 for In-Transit Melanoma—A Single-Center Experience" (Peter MacCallum Cancer Centre). A shareholder said he would try to obtain the article for me; Provectus eventually should make it available.
Two notes:
Updated (5/31/16): Speaking of research, h/t Investor Village (IV) poster canis_star: Cerwenka et al., "HMGB1: the metabolic weapon in the arsenal of NK cells," Molecular & Cellular Oncology, April 2016:
IV's canis_star also links to a May 30th article about the Cerwenka et al. work, "Natural Killer cells contain powerful toxin against tumors:"
Updated below.
Provectus incorporated its Australian subsidiary, Provectus Biopharmaceuticals Australia Pty Ltd, on May 18th.
 |
| Click to enlarge. Image source above |
 |
| Click to enlarge. Image source above. |
On Provectus' May 11th 1Q16 business update conference call, however, the company's CTO Dr. Eric Wachter, PhD announced PV-10's CUP would be wound down by year-end. See Objects in mirror are closer than they appear (May 26, 2016):
"...after very careful consideration we have recently announced to our clinical investigators that we will be winding down our expanded access program for PV-10 during the remainder of 2016. Our expanded access protocol was opened in May 2009 to provide access to PV-10 for cancer patients with cutaneous or subcutaneous lesions who are not eligible for another PV-10 trial. At that time, we were completing our Phase II study and beginning design of what would become the current Phase III study. In the interval between then and now, we commenced the Phase III study, open to patients with Stage 3 melanoma and started our Phase 1b/2 combination study open to melanoma patients with Stage 4 disease. While these studies were in the formative state, the expanded access protocol was expanded from an initial target of 25 to 30 patients to eventually provide for enrollment of up to approximately 115 patients.
As of the end of 2015, the expanded access protocol had accrued 160 patients in the U.S. and Australia with more accrued to-date. We are required to closely track all patients receiving PV-10 under all protocols to meet global reporting obligations with regard to document in use and safety of our investigational product. Since we are now in Phase III, this tracking requires markedly greater diligence. We initiated the expanded access protocol and needs not addressed within the context of formal clinical trials. Now that we have two active trials underway opened to a substantial fraction of Stage 3 and 4 melanoma patients and have reached the accrual target for the expanded access protocol, it is necessary and appropriate that we wrap up enrollment under the EA-O2 protocol.
To minimize disruption, we will continue to allow enrollment of new patients until the end of June and will continue to supply PV-10 until at least the end of December for patients commencing PV-10 on or before our June update. We appreciate the enthusiasm our investigators have shown for PV-10 under this program, and I’m sure you will appreciate that it is of most importance that use is conductive within the context of clinical trials needed to support approval of PV-10 in the USA, Australia and the rest of the world. There will be trials such as our Phase 3 melanoma trial and our Phase 1b/2 combination therapy trial." {underlined emphasis is mine}Updated (6/30/16): Why does a company based in one country require or use a subsidiary in another? Such subsidiaries ideally should generate revenues and incur costs, and so are international business entities. Running trials where the domestic (to the U.S.) company supplies drug and may pay certain trials expenses (i.e., Provectus) should not (in my view) require a foreign/international (in Australia) subsidiary. In fact, the company, Provectus, has not, having running trials treating Australian patients without the need for an Australian subsidiary to do so.
CAR T company and U.S.-based Bluebird Bio established an Australian subsidiary, Bluebird Bio Australia Pty Ltd, in March 2014. Was Bluebird expecting approval in Australia when it set up the sub? No. This company treated an Australian patient towards the end of 2014; see its December 2014 press release "bluebird bio Announces Data Demonstrating First Four Patients with β-Thalassemia Major Treated with LentiGlobin™ are Transfusion-Free." Bluebird, unlike Provectus, doesn't ship treatment product from Cambridge, Massachusetts to patients. Work is required to extract material (T cells) from a patient before it (the biologic material) is put back into him or her. I do not believe this material is shipped back to the U.S., and then returned to Australia. I think such work would be done in Australia, requiring staff and lab space, which would generated SGA and/or R&D expense that would be run through the Australian sub, which then would roll-up to the U.S. parent's income statement, balance sheet and statement of cash flows.
Provectus established an Australian sub this year for what reason?
Back to the future (June 15, 2016)
Updated below.
PDT? "Targeted Photoimmunotherapy Approach for Cancer Moves Forward," National Cancer Institute (NCI) staff, NCI, April 25, 2016
"Two new studies from NCI researchers add to growing evidence of the promise of a novel type of cancer immunotherapy that uses infrared light to activate rapid and selective killing of cancer cells."
 |
| Image source |
- E.g., 2016: "Comparing the efficacy of photodynamic and sonodynamic therapy in non-melanoma and melanoma skin cancer,"
"The trio left ORNL and founded Photogen Technologies, Inc., to commercialize a multi-photon laser technology to treat cancer.
Dees recalls that the epiphany that led to the PV-10 and PH-10 drugs occurred on a Sunday night in Photogen’s Oak Ridge Highway laboratory. They were so enthused by the idea that they rushed to the Knoxville Airport and somehow convinced the security officials to let them run a lab sample through the X-ray machines to test their theory.
The results were exactly what their epiphany had predicted, and they immediately thought, “A cure for cancer was just reduced to practice at the airport.”
Has PDT be relabeled and rebranded as photoimmunotherapy?The new approach “obsoleted our laser technology that Sunday night,” Dees said, explaining that the trio had “come up with something better and cheaper.”
Effect size. H/t InvestorVillage poster canis_star, who noted the start of a pivotal melanoma Phase 3 trial for the combination of Incyte's IDO inhibitor epacadostat and Merck & Co.'s immune checkpoint inhibitor pembrolizumab/Keytruda. Of note to me:
- Protocol first received: April 22, 2016
- The following patient population, with my bolded and underlined emphasis: "Have unresectable Stage III or Stage IV melanoma, as per AJCC staging system not amenable to local therapy,"
- An estimated enrollment of 600,
- Primary endpoints of progression-free survival (PFS) and overall survival (OS), and
- A secondary endpoint of overall response rate (ORR).
Comparing to Amgen and Merck's pivotal melanoma Phase 3 trial for the combination of the former's intralesional (IL) drug/oncolytic virus talimogene laherparepvec/T-Vec/Imlygic and pembro:
- Protocol first received: September 26, 2014
- The following patient population: "...stage IIIB to IVM1c for whom surgery is not recommended,"
- An estimated enrollment of 660,
- Primary endpoints of PFS and OS, and
- Secondary endpoints such as ORR, best overall response (BOR) rate, and durable response rate (DRR).
 |
| Click to enlarge. |
The IDO1/co-inhibitory blockade (CIB) and IL agent-oncolytic immunotherapy/CIB have roughly the same response rate (57% v. 56%); however, the former has a better complete response (CR) rate (29% v. 13%). T-Vec has been found to be more synergistic with CIB anti-CTLA4 ipilimumab/Yervoy than the anti-PD1s.
Currently, Provectus' Dr. Eric Wachter, PhD has designed the Phase 2 portion of the company's melanoma combination trial of PV-10 and pembro as:
- A population only of Stage IV patients,
- An estimated enrollment of 120,
- A primary endpoint of PFS, and
- Secondary endpoints such as ORR and OS.
I'm not certain Provectus' Phase 2 would be a pivotal trial; however, I believe it would be. Provectus' N of 120 patients is approximately 80% less than Incyte/Merck and Amgen/Merck's trials. Eric previously has said he would adjust estimate enrollment based on the treatment effect he sees in the Phase 1b study.
Updated (6/16/16): H/t InvestorVillage poster canis_star for the pembro/ipi advanced melanoma combo results from ASCO 2016: "Pembrolizumab/Ipilimumab Combo is Safe in Advanced Melanoma," Darcy Lewis, Targeted Oncology, June 14, 2016. My updated summary table is below.
Updated (6/16/16): H/t InvestorVillage poster canis_star for the pembro/ipi advanced melanoma combo results from ASCO 2016: "Pembrolizumab/Ipilimumab Combo is Safe in Advanced Melanoma," Darcy Lewis, Targeted Oncology, June 14, 2016. My updated summary table is below.
 |
| Click to enlarge. |
Australia. I located some public information yesterday regarding the company's next step here.
 |
| Image source |
As I believe I understood it, the committee was to have comprised three members: (i) committee leader and independent/outside director Jan Koe, (ii) management/management representative, a co-founder and inside director Dr. Tim Scott, PhD, and (iii) a non-officer/non-director shareholder/shareholder representative. I thought I understood that the committee's recommendation of a CEO candidate would have been made to the group of independent/outside directors Koe, board chairman Al Smith IV, and Dr. Kelly McMasters, MD, PhD, and ultimately voted on by the full board. I think three shareholders were interviewed for this spot or position.
From what I now understand, the search and search process will continue without the above construct of a shareholder representative included on a/the CEO search committee. I'll go into more detail about this topic after I finish blogging the rationale of my proxy vote; see Still [road] trippin' (June 13, 2016) immediately below. I did provide my vote below in the context of (fully or mostly expecting) the abovementioned outcome or lack thereof.
Still [road] trippin' (June 13, 2016)
Provectus’ 2016 annual meeting of stockholders is scheduled for Thursday, June 16th at 4 pm EDT in New York, New York. Shareholders of record as of April 25th's close of business should have until the annual meeting to vote. Practically, however, votes probably should be received at least 48 hours ahead of the meeting (i.e., no later than Tuesday, June 14th at 4 pm EDT). This year Provectus asked shareholders to vote on three proposals:
- A slate of five directors to serve on the company's board for a one-year term,
- An advisory vote to approve Provectus' named executive officers’ compensation, and
- The selection of accounting firm Marcum LLP as the company's independent auditor for 2016.
My Ballot
 |
| Click to enlarge. |
I will post my rationale tomorrow.
Trials in progress (June 9, 2016)
Updated below.
Provectus issued a press release today and also made an associated 8-K filing related to its trials in progress poster at ASCO 2016, Announces Poster Presentation on PV-10 at Annual Meeting of American Society of Clinical Oncology Now Available Online.
Provectus' CTO Dr. Eric Wachter, PhD noted (presumably with the goal of trying to further accelerate enrollment):
"This meeting occurred at a fortuitous time, since we have had numerous discussions with key investigators over the several months since our phase 3 protocol underwent significant updating earlier this year to address changes in standard of care for patients with locally advanced cutaneous melanoma. These discussions identified several small but important changes to patient eligibility to align protocol requirements more closely with typical patient characteristics, and we intend to implement these in the near future, particularly in light of the positive feedback we received to these at the meeting."According to the ASCO poster, potential amendment (to the pivotal melanoma Phase 3 trial) topics could include:
- Allow distant cutaneous and subcutaneous metastases in Stage IVM1a patients,
- Increase or eliminate the limit on the number of lesions at baseline (currently no more than 20 at baseline), and
- An aggregate diameter of target lesions of at least 1 cm (currently 1 to 5 lesions of at least 1 cm in longest diameter).
Updated (6/9/16): Eric also notes on the poster for PV-10 as a single agent therapy that "despite major advances, clinical trial remains the preferred option for patients with locoregional disease."
 |
| Click to enlarge. Image source |
Should PV-10 win its pivotal melanoma trial for this patient population, would it become first-line treatment for patients with locoregional disease?
Say what you'll do, and then please, please, please do what you said you'd do. From the May 10th 1Q business update conference call:
"Provectus' COO and interim CEO Peter Culpepper: This year is shaping up to be a year of significant change for Provectus. Among other things, we expect to have interim data from our Phase 3 study of PV-10 as a treatment for locally advanced cutaneous melanoma. See initial data from our Phase 1b/2 trial of PV-10 in combination with Pembrolizumab for metastatic melanoma, expand our clinical program in hepatic cancers, and advance our interactions with perspective corporate partners. Our clarity on these topics will be more precise with each passing quarterly conference call.
I believe that in our third quarter call this coming November, we will be in an excellent position to discuss the status and timing of data and the effects on our business becomes with evermore advanced clinical data.
To get to this potentially important infraction point, we can expand a number of sites and increase the speed at which we recruit patients and we are working to do that. Currently, clinicaltrials.gov shows that our Phase 3 study has five sites recruiting patients and another two sites in process of so doing. We expect many more sites in the coming weeks." {underlined emphasis is mine}One of the seven sites on CT.gov at the time (Australia's Princess Alexandra Hospital) turned from "not yet recruiting" to "recruiting" since May 10th. It's been more than four weeks, which I believe exceeds "coming." So, where's the "many" Peter?
The MD Anderson of Australia. Melbourne's Peter MacCallum Cancer Centre is holding a conference called Locoregional Melanoma 2016 on August 26 and 27th, of which Provectus is a sponsor.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
Updated below.
Dr. Eric Wachter, PhD, Provectus CTO, May 10th1Q16 business update conference call:
"Nonetheless, despite approval of Imlygic and a number of new drugs for melanoma in recent years, the body that defines standard of care for the US for patients with cancer, the National Comprehensive Cancer Network, still recommends clinical trial as the preferred option for patients with locally advanced cutaneous melanoma. I believe this clearly indicates that there's still room for new therapies, such as PV-10."The table evolved from Table IV, Publications for Comparison of Lippey et al., "Intralesional PV-10 for in-transit melanoma-A single-center experience," J Surg Oncol, 2016 May 30:
 |
| Click to enlarge. |
- "Complete responses were achieved in nearly 11% of treated patients, and treatment lengthened overall survival to a median of 23.3 months, compared with 18.0 months among control subjects who took the endogenous immune stimulator granulocyte–macrophage colony-stimulating factor."
- Unlike Food and Drug Administration approval, which applies to melanoma of all stages, European approval will probably be limited to stage 3 and 4M1a—tumors of the skin and lymph nodes." Note PV-10's success with objective response and complete response in Stage IIIB-IVM1a melanoma patients.
- "Howard Kaufman, MD, chief of the surgical oncology division at Rutgers Robert Wood Johnson Medical School in New Brunswick, NJ,...T-Vec’s global principal investigator" St. Luke Cancer Center's Dr. Sanjiv Agarwala is PV-10's equivalent, I believe.
- "James Gulley, MD, PhD, head of the immunotherapy section at the National Cancer Institute, said T-Vec has the potential to become a powerful therapeutic tool. “We need new and better ways to turn T cells against cancer, and that’s what T-Vec appears to do,” he said. But Gulley said that T-Vec’s greatest benefits will probably be achieved in combined treatment with immune checkpoint inhibitors, such as ipilumumab, a CTLA-4 inhibitor, and pembrolizumab, an anti–PD-1 agent." Interestingly, Dr. Gulley tweeted today:
 |
| Click to enlarge. Tweet image source |
Provectus' pivotal trial for PV-10 and Stage III-VM1a melanoma compares the investigation ablative immunotherapy PV-10 to systemic chemotherapy and oncolytic viral therapy Imlygic.
Dees lawsuit update. H/ts to InvestorVillage posters joeytakasugi and Area51. The former provides a link to the pdf file of an updated complaint. The latter notes:
"The only difference between the original complaint and the amended one is Provectus wants the court to approve the appointment of a receiver "to oversee and manage the proper foreclosure and disposition of the pledged stock is necessary and appropriate.""Question: Who owns what? Answer: Not Pfizer. (June 3, 2016)
Updated below.
One key piece of of Provectus' intellectual property (e.g., patents, patent applications, trade secrets, etc.) is the company's combination therapy patent for certain combinations involving PV-10 that is jointly held with Pfizer (and awarded in August 2015): "Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer." During his Marcum Microcap Conference presentation on June 2nd, Provectus' COO and interim CEO Peter Culpepper said (again) that Pfizer doesn't gain the benefit of the combination therapy patent unless it does a transaction (buys) Provectus. If someone else buys Provectus, said buyer would gain benefit of the patent.
Provectus' Phase 1b/2 trial protocol of "PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma" was amended in two places:
| Click to enlarge. |
 |
| Click to enlarge. |
See PV-10 + [insert name here] (September 23, 2015) on the blog's Archived News IV page. It is quite common to pair an investigational therapy with a standard of care (e.g., pivotal testing of Bristol-Myers' anti-CTLA-4 ipilimumab with systemic chemotherapy DTIC vs. DTIC) that would result (if successful) in the approval of the investigational therapy without requiring its subsequent use in the combination. Since the SOC is routinely available, no support is required or needed from the SOC's owner.
Once the above is accomplished, there may be situations where the combination is marketed together.
As noted above, Provectus' has claims covering the combination of PV-10 and other cancer treatments, so the company "owns" or controls any co-marketing (if successful) of PV-10 and Keytruda. The two could be sold separately if PV-10 garners approval as a single agent but, for instance, Merck & Co. could not suggest use of Keytruda with PV-10 unless Provectus allows it.
Updated (6/5/16): The Twitter feed I curated for this project currently comprises 15 "followings:" Paul D. Rennert/@PDRennert, Brad Loncar/@bradloncar, Luke Timmerman/@ldtimmerman, Amy Brown/@AmyEPVantage, Christopher Heery/@ChrisHeery, Jacob Plieth/@JacobPlieth, John Carroll/@JohnCBiotech, Tom Silver/@TomSilver39, Philippe Aftimos, MD/@aftimosp, Jamie Singer/@JSwatercooler (Provectus), Sally Church/@MaverickNY, Matthew Herper/@matthewherper, Adam Feuerstein/@adamfeuerstein and Peter Culpepper/@peterculpepper (Provectus).
The feed seemed to coalesce around combination therapy-oriented thoughts as ASCO 2016 began starting June 3rd (in chronological order of tweeting, oldest to newest):
- The left-hand side of the Cancer Immunity Cycle, the agonists or stimulatory agents (e.g., 4-1BB, OX40, etc.)
 |
| Click to enlarge. Tweet image sources here and here |
- What really is a good combination partner? Another checkpoint inhibitor? Back to the future with chemotherapy?
 |
| Click to enlarge. Tweet image source |
- 4-1BB in melanoma: 0% ORR -- but note pharmacodynamic (PD) trends: CD8+ T cells, IFN gamma, etc.
 |
| Click to enlarge. Tweet image sources here and here |
- 4-1BB safety?
 |
| Click to enlarge. Tweet image source |
- OX40 (Roche version) incremental?
 |
| Click to enlarge. Tweet image source here and here |
- Immunotherapy treatment time: Too long. Dr. Eric Whitman, MD below is an investigator in Provectus' pivotal melanoma Phase 3 trial of PV-10 vs. Systemic Chemotherapy or Oncolytic Virus (Stage IIIB-IVM1a)
 |
| Click to enlarge. Tweet image source |
- Who needs a proprietary combination with a protected agent?
 |
| Click to enlarge. Tweet image source |
- Proprietary combinations...
 |
| Click to enlarge. Tweet image source |
 |
| Click to enlarge. Tweet image source |
- A peek into the future of cancer care? Priming anti-PD-L(1)s...
 |
| Click to enlarge. Tweet image source |
- Intralesional drug (T-Vec) + Co-inhibitory blockade drug (pembrolizumab) for advanced melanoma: 57% ORR, 24% CR
 |
| Click to enlarge. Tweet image source |
- Partner issues...
 |
| Click to enlarge. Tweet image source |
 |
| Click to enlarge. Tweet image source |
PV-10 + co-inhibitory blockade (AACR 2016): Mice said "They also conclude that "the effect of combination therapy with IL PV-10 and PD-1 blockade is mediated by CD8+ T cells, and depletion of either CD4+ T cells or CD25+ Tregs enhances anti-tumor immunity in the M05 melanoma model."
And (PLoS ONE 2013): "Splenocytes isolated from tumor bearing mice treated with IL PV-10 demonstrated enhanced tumor-specific IFN-gamma production compared to splenocytes from PBS-treated mice in both models."
- Leader or table stakes?
 |
| Click to enlarge. Tweet image source |
- Chen said intralesional (IL) agents occupied Step #s 1-3. IL T-Vec is highlighted in Step #2 below.
 |
| Click to enlarge. Tweet image source |
H/t a shareholder who forwarded the following article to me, "Cancer doctors face next drug challenge," David Crow, Financial Times, June 1st {underlined emphasis is mine}:
"“We have to think about fairness,” says Dr Julie Vose, president of Asco. “We’re trying wherever it’s suitable to talk about value and cost.”
A study comparing the price of 23 major cancer drugs in different countries could spark a debate when it is published at the conference, she says.
One of the problems with the latest generation of cancer drugs is that they are biologic medicines made from living cells rather than chemical compounds, making it harder for generic manufacturers to make cheaper copycat versions.
Instead, generic manufacturers must try to make biosimilar versions of the drug that are not direct copies, and some big pharma companies are warning these might not be as good as the “real thing”."a. Bristol-Myers' ipilimumab (anti-CTLA-4, Yervoy) and nivolumab (anti-PD-1, Opdivo), Merck & Co.'s pembrolizumab (anti-PD-1, Keytruda), and Amgen's talimogene laherparepvec (oncolytic virus, Imlygic) are biologic medicines. Rose Bengal (PV-10) is a chemical compound
b. Provectus has advanced the therapeutic use of Rose Bengal, which is the second medicinal use of the compound. The original and first medicinal use of Rose Bengal was as a diagnostic. Isn't Rose Bengal a classical small-molecule therapeutic?
c. Provectus' intellectual property (IP) covers all halogenated xanthenes (HXs), and the company's IP continues to grow around the class of HXs, of which Rose Bengal is first-in-class. Second-, third-, etc.-in-class also are owned by Provectus. As such, generic manufacturers should find it difficult to copycat Rose Bengal and its relatives.
Funny (2008): "Biological drugs — antibodies, bioactive proteins and the like — can be expensive to produce, but they're also very difficult to copy. And the world's regulatory agencies are still working out how to tell if another firm's generic biological is truly equivalent to the original branded drug. These factors mean that biological products have a lifetime that can extend well beyond their natural patent term, unlike traditional small-molecule drugs, which are under siege from generic manufacturers."
Rose Bengal is a small molecule cancer drug made from a chemical compound. Aspects of management have been and are one thing. I agree. Chemistry, however, is another. Unless you really don't understand, um, chemistry?
 |
| Image source |
Updated below, again.
There were several points of import for me in Australia's PeterMac's PV-10 experience (Lippey et al., see immediately below or June 1st blog post Intralesional PV-10 for In-Transit Melanoma—A Single-Center Experience).
Please note: Rather than use quotations to identify the authors' work (words), I am very liberally using (copying) their own words as I write below so as not to be too disjointed.
Among these points were:
- Patient population — PeterMac, or Peter MacCallum Cancer Centre noted the patient population as unresectable local recurrence and in-transit metastasis (ITM) of cutaneous melanoma, or American Joint Committee on Cancer (AJCC) Stage IIIB and IIIC, which can present a therapeutic challenge because these patients have (x) a longer survival than patients with AJCC Stage IV disease but (y) local symptoms, particularly ulceration, bleeding, and pain, that can be associated with significant debility and impact on quality of life.
- Takeaway: PeterMac's experience is yet another example of a clear, guideline-/patient population-based, "label ready" use for PV-10. It more than likely does not comprise the eventual, entire melanoma label for the investigational compound, but it does re-emphasize the "base label" that should enable PV-10 to make its way into commerce.
- Treatment with PV-10 — Most patients (12 of 19, 63%) received only one course of treatment. Ten patients (53%) did not have all lesions injected because of the number of lesions present. Nevertheless, the headline response data were disease control (complete or partial response or disease stability) of 68% (13 of 19) and complete response of 26% (5).
- Takeaway: Provectus' CTO Dr. Eric Wachter, PhD has always emphasized that the compassionate use/expanded access program (special access scheme in Australia), of which PeterMac has been a long-time site, has informed him and, in truth, treating physicians, how best to use PV-10. For AJCC IIIB/C disease, which was expanded to include IVM1a as part of the amended protocol for Provectus pivotal melanoma Phase 3 trial where all of a patient's lesions would be injected with PV-10, or for the non-medical person that is me, the physician and his or her patient has to hit all disease, and keep hitting all disease, until the disease goes away (achieves complete response) -- or it (the disease) doesn't. The subgroup analysis of Provectus' metastatic melanoma Phase 2 trial of patients who had all of their disease treated (all were Stage IIIB-C) generated 82% disease control (20 of 28) and 50% complete response (14). See also ASCO 2014 and ESMO 2014.
- Injection site pain with PV-10 — In PeterMac's initial experience, lesions were injected in the clinic under local anesthetic; however, it proved to be highly uncomfortable to inject multiple lesions. As a result, all but two of its 19 patients had their lesions injected under intravenous sedation in the operating room. Provectus' Phase 2 study reported that 80% of the 80 patients experienced injection site pain. PeterMac concluded, however, that factors predictive of response included the presence of ulceration, blistering, eschar, or pain following injection.
- Takeaway: "No pain, no gain," it would appear. No pain, no local immune response. In 2013 Provectus observed that loco-regional blistering was prognostic for (predictive of) responses to PV-10 in injected lesions. Phase 2 patients who experienced such blistering enjoyed a much greater response in their injected lesions than those patients whose lesions did not experience this loco-regional blistering phenomenon: 91% vs. 54% disease control (32 vs. 48 patients, p = 0.001), and 66% vs. 42% complete response (p = 0.61).
- Predictors of patient and tumor response, and thus PV-10 success — PeterMac noted predictors of complete response as age (p = 0.004) and lesion size (0.004, 0.008). The younger, the better. The smaller or lesser (the lesion, the grouping of them), the better. The number of injected lesions and the time from primary diagnosis to treatment were not predictive. The presence of ulceration, blistering, eschar, or pain following injection was predictive of response.
- Takeaway: Treating cancer earlier or when disease burden is lower (i.e., when lesions are smaller or groups of lesions are less, presumably) should seem obvious. Being younger and presumably having a stronger immune system should seem obvious, too.
- Marketing campaigns aside, what's best for the patient? — PeterMac noted that immune checkpoint as well as kinase inhibitors may be associated with significant toxicity. In the case of Keytruda and Opdivo, they require frequent hospital visits for infusions.
- Takeaway: As PeterMac wrote, the effective treatment options for patients with unresectable metastases have increased and the prognosis for these patients has significantly improved. PV-10, when approved, should further help.
- Food coloring >> Herpes — PeterMac noted that Amgen's approved intralesional (IL) therapy talimogene laherparepvec (T-Vec, Imlygic) generated a response rate for patients with all lesions injected (all disease treated) of 33% in its pivotal melanoma trial. The subgroup analysis of Provectus' metastatic melanoma Phase 2 trial of patients who had all of their disease treated (all were Stage IIIB-C) generated a response rate of 71%.
- Takeaway: PeterMac believes that for an elderly patient with ITM, a simple and effective local therapy with minimal side effects is an attractive option. Perhaps the same could apply to younger patients, or any patient. T-Vec is far from a simple therapy or an effective product. It also has weaker immunologic signalling. I could go on...
Updated (6/7/16): Provectus issued a press release today and also made an associated 8-K filing related to the company announcing access to a PV-10-peer-reviewed publication by clinicians from Australia's Peter MacCallum Cancer Centre (East Melbourne), Announces Publication of Article on PV-10 for In-Transit Melanoma in Journal of Surgical Oncology. The paper is entitled "Intralesional PV-10 for In-Transit Melanoma - A Single Centre Experience." Underlined emphasis below is mine.
Since the press release very likely was written by Provectus' CTO Dr. Eric Wachter, PhD, I tend to focus on the items he highlights in it:
- See above, Treatment with PV-10, and Predictors of patient and tumor response
- "Dr. Lippey reported, "After a median follow up of 11.7 months, disease control was achieved in 63% of patients. Five patients (26%) achieved a complete response, another five (26%) patients achieved a partial response, and two patients had stable disease (11%) at the time of last follow-up. Seventy-four percent (14/19) of patients had a clinical response at time of first follow-up (median time 21 days); range 8–91 days. Younger patients and those with smaller lesions were more likely to respond to treatment." The median age of patients in this series was 80 years."
- See above, Treatment with PV-10
- "Dr. Lippey also noted, "Ten patients did not have all lesions injected, primarily due to the number of lesions present. A bystander response was noted in un-injected lesions in 50% of patients who did not have all their lesions directly injected.""
- See Melanoma (June 7, 2016) above, or Table IV, Publications for Comparison from the publication itself
- "Eric Wachter, CTO of Provectus commented, "The results reported here are consistent with other evaluations of PV-10 in melanoma, and highlight both the rapid ablative properties and the immunologic features of PV-10 as an investigational ablative immunotherapy.""
Sometimes when I think of Provectus, the co-founders (former and current) and principals, and certain operational aspects of the company, I can't help but recall a scene from The Other Guys ("... and work your mouth like a puppet"). Casbah.
May 2016 blog readership stats.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
New SEC filing. Provectus filed a prospectus, Form 424(b)(3), related to the re-opened warrant exchange program. See New SEC filing: Reopened Warrant Exchange (May 13, 2016) on the blog's Archived News V page. I compared this "new" prospectus to the "original" one filed January 20, 2016 using Text Compare! Of note was Eric intending to exchange the balance of the warrants he held (359,719; page 22), while board of directors member Jan Koe not intending to exchange his balance (100K). Re-reading the prospectus also reminded me of the following, which I had wanted to run down when reading the relevant quarterly filing for the topic (underlined emphasis is mine; see 1Q16 cash flow statement screenshot from the filing thereafter):
"Accounting Treatment
The modification of the exercise price of the Existing Warrants and the Replacement Warrants are treated as an inducement to enter into the Exchange Offer. As such, the difference between the actual fair value of the Existing Warrants as of the date of their exchange and the actual fair value of the modified Existing Warrants and the fair value of the Replacement Warrants will be recorded as an incentive expense in connection with the early warrant exercise, with an offsetting entry to additional paid-in-capital. The fair value of the Existing Warrants exchanged will be determined using the Black-Scholes pricing model as of the date of the exchange. The fair value of the modified Existing Warrants and the Replacement Warrants issued will be determined using the Black-Scholes pricing model on the Closing Date. The Exchange Offer does not modify any terms of the warrant agreements or the current accounting treatment for the un-exchanged warrants."
 |
| Click to enlarge. Page 4 |
Updated below.
One of the two abstracts related to PV-10 (for the treatment of melanoma) that were presented at the Royal Australasian College of Surgeons 85th Annual Scientific Congress (May 2-6) in Australia was published (as a paper) in the Journal of Surgical Oncology: "Intralesional PV-10 for In-Transit Melanoma—A Single-Center Experience" (Peter MacCallum Cancer Centre). A shareholder said he would try to obtain the article for me; Provectus eventually should make it available.
Two notes:
- "Nineteen patients with in-transit melanoma were treated with intralesional PV-10 between 2010 and 2014. Disease control (complete or partial response or disease stability) was achieved in 68% of patients with 26% having a complete response," and
- "Patients were considered to have a complete response if all lesions (including un-injected lesions) were eradicated by the treatment."
 |
| Click to enlarge |
 |
| Click to enlarge. Orange rectangular emphasis is mine |
Abstract: Targeting tumor glycolysis would hit the main energy source of cancer. We show that Natural Killer (NK) cells pursue this strategy. They employ High Mobility Group Box 1 (HMGB1) protein - a well-known pro-inflammatory cytokine - to specifically target glycolysis in cancer cells. This opens up new perspectives for cancer immunotherapy.In May, Moffitt Cancer Center published its work on PV-10's mechanism of action, "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1."
IV's canis_star also links to a May 30th article about the Cerwenka et al. work, "Natural Killer cells contain powerful toxin against tumors:"
"In this toxic mix, pathologists have identified the High Mobility Group Box 1 (HMGB1) protein as a highly effective natural agent against cancer: It paralyzes a mechanism of energy production that is generally used by tumor cells, but not by healthy body cells. This type of 'cell murder' by the immune system has so far been unknown..."Immunotherapies normally aim to enhance the immune system so it can recognize cancer cells better and fight them more effectively. A therapy with HMGB1 would carry the advantage that it uses the weapons of the natural immune system without depending on its functionality, and still manages to target cancer cells in a very selective manner," explains Gdynia."
Combination (May 29-30, 2016)
Entry #1, May 29th. At a macro level, that is the intersection of the regulator (e.g., the FDA) and industry, a shift in focus on combination therapy has overtaken the initial roll-out of immunotherapies (specifically co-inhibitory blockade, with emphasis on inhibitory) that:
Entry #2, May 30th. The laying of Provectus' combination groundwork could have started in 2011, when preparation of its combination therapy patent might have started; the patent application itself was filed by Pfizer and the company in March 2012.
Provectus' "combination timeline," however, comprises or includes other relevant events and key milestones, such as:
Objects in mirror are closer than they appear (May 26, 2016)
As the American Cancer Society's Compassionate Drug Use webpage notes:
It is very interesting to note (see * below) that Amgen closed it's U.S. CUP in February 2016 (T-Vec was approved [as Imlygic] by the FDA in October 2015), a program that started May 2014 (First Received Date on ClinicalTrials.gov). The Big Biotech's CUP in select European countries, which started in October 2014, remains open (Last updated on CT.gov was January 2016). Note the following comment from T-Vec's Europe CUP protocol, with my underlined emphasis:
By several measures, Provectus' CUP has been quite a success. Originally designed for 115 patients, and having an initial target of 25-30, approximately 160 patients were treated in the U.S. and Australia through the end of 2015 and more were treated in 2016.
On Provectus' May 11th 1Q16 business update conference call, however, the company's CTO Dr. Eric Wachter, PhD announced PV-10's CUP would be wound down by year-end:
Maybe Eric could have offered a third reason for closing the CUP: one wouldn't necessarily need a program to offer access to an investigational drug if said drug were no longer investigational (i.e., if said drug were indeed approved). Said a different way, melanoma patients wouldn't need a CUP if they had access to the drug on an approved basis. At a minimum, Australia's Therapeutic Goods Administration's (TGA's) pathway to prospective approval for PV-10 probably is very fluid in regards to timing. Eric said on the 1Q16 CC that SAS enrollment of new patients would continue until the end of June, and PV-10 would be supplied to those patients (enrolling by or before the end of June) until at least the end of December.
So, one reading of that timing, for Australia (one country of Provectus' two-country CUP/SAS) at least, could be potential TGA approval of some sort in 2H16 at the earliest. Another optimistic reading could be that Eric expects FDA approval of a sort (accelerated approval?) by the time the CUP is wound down, or maybe and more likely shortly thereafter or thereafter.
Afterall, he did say Provectus was six months behind its initial schedule for reaching the interim and final analysis triggers for the company's pivotal melanoma Phase 3 trial (i.e., year-end 2016, or into early-2017).
* T-Vec CUP programs:
From time to time I have the opportunity to discuss science-related and other matters with Provectus' CTO Dr. Eric Wachter, PhD. The traditional threshold that defines a small molecule appears to be a molecular weight (MW) about 500 g/mol; so, was I wrong to assume or label Rose Bengal as a small molecule despite an MW of about 974. Rose Bengal is an exception in that its MW is high because of the presence of its four iodides, which total about 507 g/mol (or ~52% of the molecule's weight). Rose Bengal is not a macromolecule, polymer or biomolecule, so it is best classified as a small molecule, albeit a very heavy one. See also 3. Small molecules — a need to return to a "new" age of chemistry enlightenment under Challenges & Opportunities (May 22, 2016) on the blog's Archived News V page.
The work by Moffitt Cancer Center shows Rose Bengal (PV-10) kills via necrosis, although there is some evidence of apoptosis. The preclinical data from the University of Illinois at Chicago showing apoptosis presumably confirms under certain conditions that apoptosis may be observed. The pharmacology upon intralesional (IL) injection of PV-10 clearly is necrosis-based on the very rapid destruction of tumors. Unfortunately, it is very difficult to study such processes in vivo, hence the ambiguity once the process is moved in vitro.
Foote et al., "A phase 2 study of intralesional PV-10 followed by radiotherapy for localized in transit or recurrent metastatic melanoma," J Clin Oncol 34, 2016 (suppl; abstr e21072) conclude:
"The combination of IL PV-10 and radiotherapy resulted in lesion specific, normal tissue sparing, ablation of melanoma tumors with minimal local or systemic adverse effects. The study results justify expanded evaluation in a randomized trial" {Underlined emphasis is mine}. Radiation would be the choice for the control arm such a study; that is, PV-10 + standard of care (SOC) vs SOC.
Dimeski et al., “Interference from Rose Bengal with total bilirubin measurement,” Clinical Chemistry 55(5):1040-41, note that Rose Bengal "gums up" (my words) scientific measurement equipment. Dimeski's letter to the editor commented on interferences noted in laboratory samples collected during Provectus' metastatic melanoma Phase 2 study.
The company has conferred with him on this topic since the publication. Dimeski and colleagues hail from, wait for it, the Department of Chemical Pathology at Brisbane, Australia's Princess Alexandra Hospital.
Other note:
St. Luke's Cancer Center medical oncologist, intralesional (IL), melanoma key opinion leader Dr. Sanjiv Agarwala was the keynote speaker at the Melanoma Immunotherapy Regional Meeting in Dubrovnik, Croatia on May 20th. Agarwala's slide deck is here.
"Melanoma rates in Croatia are steadily and markedly rising;" see Barbarić et al., "Incidence and mortality trends of melanoma in Croatia, 1988-2008," Croat Med J. 2012 Apr; 53(2): 135–140. Of note to me, where both Amgen's T-Vec/Imylgic and Provectus's PV-10 were mentioned:
What is the treatment effect size, very roughly speaking of course, of T-Vec compared to PV-10?
- Began with the approval of ipilimumab (anti-CTLA-4) in 2011,
- Continued with the "excitement" of the anti-PD-1s at ASCO in 2013 and their approvals (for advanced melanoma) in 2014, and
- Ended in 2015 with the realization immune checkpoint inhibitors (a) have modest response rates, (b) do not work on a supermajority of cancer patients and (c) need help to work better.
The last point is particularly interesting to me. Generally speaking, it has dawned on the industry as a whole that initiating the cancer immunity cycle requires something to occur at the beginning (i.e., Steps 1-3) to allow the end to finish better (i.e., Step 7) so as to continue the cycle — which is the essence of a self-perpetuating cycle (that continues to self-perpetuate once "perpetuated" or initiated).
Recent Novartis material helped to cogently define for me the help immune checkpoint inhibitors require; see slide number 56 below.
 |
| Click to enlarge. |
Novartis is of course "talking its book" (or drug pipeline), but the Big Pharma's comments above the shortcomings of co-inhibitory blockade, which those formerly excited folks probably now better realize and understand (one would hope) is not a panacea. Strengths and shortcomings include:
- Anti-CTLA-4: Impressive memory, but low response rate and inflammatory toxicity,
- Anti-PD-1: Broader activity (than anti-CTLA-4), but an incomplete picture of durability of responses, and
- Both appear to require pre-existing anti-tumor immunity for clinical benefit, but most patients fail to manifest significant endogenous responses
Entry #2, May 30th. The laying of Provectus' combination groundwork could have started in 2011, when preparation of its combination therapy patent might have started; the patent application itself was filed by Pfizer and the company in March 2012.
 |
| Click to enlarge. |
- November 2012, when the company first presented preclinical data establishing its early combination premise of PV-10 with other cancer treatments,
- July 2013, when Moffitt Cancer Center published preclinical data establishing that the injection of PV-10, alone, could lead to a systemic tumor-specific immune response,
- November 2014, when Moffitt presented preclinical data establishing PV-10 could be combined with the entire category of co-inhibitory blockade; that is, all immune checkpoint inhibitors (e.g., anti-CTLA-4, anti-PD-1, anti-PD-L1),
- August 2015, when the U.S. Patent and Trademark Office awarded the combo patent to Pfizer and Provectus,
- September 2015, when Provectus initiated a Phase 1b trial combining PV-10 and anti-PD-1 pembrolizumab (approved in 2014 at Keytruda) for Stage IV melanoma, and
- May 2016, when Moffitt published preclinical and clinical data establishing that the injection of PV-10, alone, could lead to systemic anti-tumor immunity.
Entry #3, May 30th. I use Celldex and Bristol-Myers' May 2014 immuno-oncology combination therapy deal and clinical development program (CDP) as a reference point for several reasons (e.g., deal structure, trial design, study cost sharing, study sponsorship, etc.). The study program was/is "A Dose Escalation and Cohort Expansion Study of Anti-CD27 (Varlilumab) and Anti-PD-1 (Nivolumab) in Advanced Refractory Solid Tumors."
Among other things:
- The upfront payment of $5 million Bristol paid Celldex probably was related to the transaction's reduction in future milestone payments and royalties (from an old Celldex deal with Medarex, which Bristol bought in 2009, where potential milestone payouts were waived and royalty rates were reduced, than the actual immuno-oncology relationship,
- Study costs were/are shared by the two parties,
- Celldex was the study program's Sponsor and Responsible Party (ClinicalTrial.gov terms), while Bristol was the/a Collaborator,
- Recruitment for the Phase 1 portion began in January 2015, after nivolumab was approved for advanced melanoma in December 2014, and
- Certain rights afforded to Bristol by Celldex included (i) exclusivity for combining the latter's drug with the former's and (ii) an exclusivity period of doing a license deal after the study program is completed.
Entry #4, May 30th. In addition to wanting to understand the framework and positioning of combination therapy trials (e.g., "deal" terms, what [compound] helps what [compound] and why, trial design, who pays for what, etc.), like in the case of Bristol-Celldex, I also wanted to understand initial outcomes. For example, in the combination category of intralesional (IL) agents and checkpoint inhibitors, Amgen's talimogene laherparepvec (T-Vec) appears to have more synergy with Bristol-Myers' anti-CTLA-4 ipilimumab than it (T-Vec) has with Merck & Co.'s anti-PD-1 pembrolizumab.
But returning to Celldex's varlilumab and Bristol's nivolumab, initial Phase 1 data was presented at AACR 2016, "Phase 1 results from the combination of an immune activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events." Germane trial information included changes in tumor infiltrating lymphocytes (TILs), PD-L1 expression, immune biomarkers such as CD4 and T regulatory cells, and anti-tumor responses. Phase 1 addressed non-small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), ovarian carcinoma, colorectal cancer (CRC), and melanoma. Phase 2 will address NSCLC, SCCHN, CRC, renal cell carcinoma (RCC) and glioblastoma (GBM).
Entry #5, May 30th. From 2012-2015, Provectus protected its intellectual property (IP) of PV-10 in combination with other cancer treatments. IP, including patents and in the company's case trade secrets, probably are most important asset Provectus owns.
Around the same time the company began establishing its early combination premise of PV-10 with other cancer treatments, albeit in preclinical fashion. 2015 saw the company transition into the clinic with pembrolizumab following the key patent's issuance: "A Phase 1b/2 Study of PV-10 Intralesional Injection in Combination With Systemic Immune Checkpoint Inhibition for Treatment of Metastatic Melanoma." 2016 saw clinical confirmation of the combination premise materialize by way of combining PV-10 and radiotherapy: "A phase 2 study of intralesional PV-10 followed by radiotherapy for localized in transit or recurrent metastatic melanoma."
What makes PV-10 a good combination therapy partner is the robustness of the upstream trigger for a systemic anti-tumor response that is the drug compound's ablation (upon injection). In fact, PV-10 can lead to anti-tumor immunity ("induce"), which is the prerequisite for the immune checkpoint inhibitors to work better ("boost").
Initial Phase 1b results might be presented at SMR 2016 (November 6-9).
Entry #5, May 30th. From 2012-2015, Provectus protected its intellectual property (IP) of PV-10 in combination with other cancer treatments. IP, including patents and in the company's case trade secrets, probably are most important asset Provectus owns.
Around the same time the company began establishing its early combination premise of PV-10 with other cancer treatments, albeit in preclinical fashion. 2015 saw the company transition into the clinic with pembrolizumab following the key patent's issuance: "A Phase 1b/2 Study of PV-10 Intralesional Injection in Combination With Systemic Immune Checkpoint Inhibition for Treatment of Metastatic Melanoma." 2016 saw clinical confirmation of the combination premise materialize by way of combining PV-10 and radiotherapy: "A phase 2 study of intralesional PV-10 followed by radiotherapy for localized in transit or recurrent metastatic melanoma."
What makes PV-10 a good combination therapy partner is the robustness of the upstream trigger for a systemic anti-tumor response that is the drug compound's ablation (upon injection). In fact, PV-10 can lead to anti-tumor immunity ("induce"), which is the prerequisite for the immune checkpoint inhibitors to work better ("boost").
Initial Phase 1b results might be presented at SMR 2016 (November 6-9).
 |
| Image source |
"[Physicians/providers" use the term “compassionate use” to refer to the treatment of a seriously ill patient using a new, unapproved drug when no other treatments are available. Drugs that are being tested but have not yet been approved by the US Food and Drug Administration (FDA) are called investigational drugs. These drugs are generally available only to people who are taking part in a clinical trial (a research study that is testing the drug). Being able to use one of these drugs when you are not in a clinical trial is most commonly referred to as compassionate use."Since 2009, Provectus' has run a compassionate use (expanded access) program (CUP/EAP) in the U.S. and special access scheme (SAS) in Australia to provide patients with access to PV-10 for cutaneous or subcutaneous cancerous tumors. The CUP/EAP/SAS protocol does not make specific reference to melanoma (although many cutaneous or subcutaneous tumors are melanoma-related), like Amgen's talimogene laherparepvec's (T-Vec's Imlygic's) melanoma-focused CUPs*, and so a range of cancer tumors were treated with PV-10.
It is very interesting to note (see * below) that Amgen closed it's U.S. CUP in February 2016 (T-Vec was approved [as Imlygic] by the FDA in October 2015), a program that started May 2014 (First Received Date on ClinicalTrials.gov). The Big Biotech's CUP in select European countries, which started in October 2014, remains open (Last updated on CT.gov was January 2016). Note the following comment from T-Vec's Europe CUP protocol, with my underlined emphasis:
"Eligible subjects will be treated with talimogene laherparepvec until the subject has achieved a complete response, all injectable tumors have disappeared, clinically relevant (resulting in clinical deterioration or requiring change of therapy) disease progression beyond 6 months of therapy, intolerance of protocol treatment, or until talimogene laherparepvec receives marketing authorization approval in Europe for the treatment of melanoma, whichever occurs first."The European Commission approved Imlygic in December 2015.
By several measures, Provectus' CUP has been quite a success. Originally designed for 115 patients, and having an initial target of 25-30, approximately 160 patients were treated in the U.S. and Australia through the end of 2015 and more were treated in 2016.
 |
| Click to enlarge. |
"...after very careful consideration we have recently announced to our clinical investigators that we will be winding down our expanded access program for PV-10 during the remainder of 2016. Our expanded access protocol was opened in May 2009 to provide access to PV-10 for cancer patients with cutaneous or subcutaneous lesions who are not eligible for another PV-10 trial. At that time, we were completing our Phase II study and beginning design of what would become the current Phase III study. In the interval between then and now, we commenced the Phase III study, open to patients with Stage 3 melanoma and started our Phase 1b/2 combination study open to melanoma patients with Stage 4 disease. While these studies were in the formative state, the expanded access protocol was expanded from an initial target of 25 to 30 patients to eventually provide for enrollment of up to approximately 115 patients.
As of the end of 2015, the expanded access protocol had accrued 160 patients in the U.S. and Australia with more accrued to-date. We are required to closely track all patients receiving PV-10 under all protocols to meet global reporting obligations with regard to document in use and safety of our investigational product. Since we are now in Phase III, this tracking requires markedly greater diligence. We initiated the expanded access protocol and needs not addressed within the context of formal clinical trials. Now that we have two active trials underway opened to a substantial fraction of Stage 3 and 4 melanoma patients and have reached the accrual target for the expanded access protocol, it is necessary and appropriate that we wrap up enrollment under the EA-O2 protocol.
To minimize disruption, we will continue to allow enrollment of new patients until the end of June and will continue to supply PV-10 until at least the end of December for patients commencing PV-10 on or before our June update. We appreciate the enthusiasm our investigators have shown for PV-10 under this program, and I’m sure you will appreciate that it is of most importance that use is conductive within the context of clinical trials needed to support approval of PV-10 in the USA, Australia and the rest of the world. There will be trials such as our Phase 3 melanoma trial and our Phase 1b/2 combination therapy trial."Eric's reasons for closing the CUP then were (a) the availability of two active clinical trials for a substantial fraction of Stage III/IV melanoma patients, and (b) reaching and then exceeding the program’s original accrual design and initial targets. Nevertheless, why shut down what clearly appeared to be a successful program benefitting patients in both the U.S. and Australia?
Maybe Eric could have offered a third reason for closing the CUP: one wouldn't necessarily need a program to offer access to an investigational drug if said drug were no longer investigational (i.e., if said drug were indeed approved). Said a different way, melanoma patients wouldn't need a CUP if they had access to the drug on an approved basis. At a minimum, Australia's Therapeutic Goods Administration's (TGA's) pathway to prospective approval for PV-10 probably is very fluid in regards to timing. Eric said on the 1Q16 CC that SAS enrollment of new patients would continue until the end of June, and PV-10 would be supplied to those patients (enrolling by or before the end of June) until at least the end of December.
So, one reading of that timing, for Australia (one country of Provectus' two-country CUP/SAS) at least, could be potential TGA approval of some sort in 2H16 at the earliest. Another optimistic reading could be that Eric expects FDA approval of a sort (accelerated approval?) by the time the CUP is wound down, or maybe and more likely shortly thereafter or thereafter.
Afterall, he did say Provectus was six months behind its initial schedule for reaching the interim and final analysis triggers for the company's pivotal melanoma Phase 3 trial (i.e., year-end 2016, or into early-2017).
* T-Vec CUP programs:
- U.S.: NCT02147951, Expanded Access Protocol of Talimogene Laherparepvec for Subjects With Unresected, Stage lllB to IVM1c Melanoma (aka A Phase 3b, Multicenter, Open-label, Single-arm, Expanded Access Protocol of Talimogene Laherparepvec for the Treatment of Subjects With Unresected, Stage lllB to IVM1c Melanoma) — no longer available
- Europe: NCT02297529, Expanded Access Study of Talimogene Laherparepvec for Treatment of Subjects With Unresected Stage IIIB-IVM1c Melanoma (aka A Phase 3b, Multicenter, Open-label, Single-arm, Expanded Access Protocol of Talimogene Laherparepvec for the Treatment of Subjects in Europe With Unresected Stage IIIB to IVM1c Melanoma)
 |
| Image source |
The work by Moffitt Cancer Center shows Rose Bengal (PV-10) kills via necrosis, although there is some evidence of apoptosis. The preclinical data from the University of Illinois at Chicago showing apoptosis presumably confirms under certain conditions that apoptosis may be observed. The pharmacology upon intralesional (IL) injection of PV-10 clearly is necrosis-based on the very rapid destruction of tumors. Unfortunately, it is very difficult to study such processes in vivo, hence the ambiguity once the process is moved in vitro.
Foote et al., "A phase 2 study of intralesional PV-10 followed by radiotherapy for localized in transit or recurrent metastatic melanoma," J Clin Oncol 34, 2016 (suppl; abstr e21072) conclude:
"The combination of IL PV-10 and radiotherapy resulted in lesion specific, normal tissue sparing, ablation of melanoma tumors with minimal local or systemic adverse effects. The study results justify expanded evaluation in a randomized trial" {Underlined emphasis is mine}. Radiation would be the choice for the control arm such a study; that is, PV-10 + standard of care (SOC) vs SOC.
Dimeski et al., “Interference from Rose Bengal with total bilirubin measurement,” Clinical Chemistry 55(5):1040-41, note that Rose Bengal "gums up" (my words) scientific measurement equipment. Dimeski's letter to the editor commented on interferences noted in laboratory samples collected during Provectus' metastatic melanoma Phase 2 study.
 |
| Click to enlarge. |
Other note:
St. Luke's Cancer Center medical oncologist, intralesional (IL), melanoma key opinion leader Dr. Sanjiv Agarwala was the keynote speaker at the Melanoma Immunotherapy Regional Meeting in Dubrovnik, Croatia on May 20th. Agarwala's slide deck is here.
 |
| Click to enlarge. |
 |
| Click to enlarge |
 |
| Click to enlarge. |





No comments:
Post a Comment