Offering, Part II (June 30, 2015)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
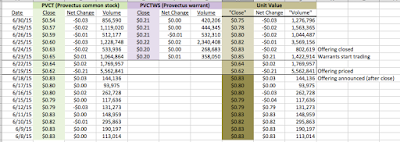
Having announced the proposed Maxim Group-organized public offering of common stock and warrants on June 18th, Provectus closed the transaction on June 24th (associated 8-K filing), whereby the company:
- Sold 17.5 million common stock shares (at $0.75 per share) -- receiving gross proceeds of $13.125 million,
- Issued 17.5 million warrants (with an $0.85 exercise price),
- Paid fees to Maxim of $1.05 million, and
- Issued an over-allotment of 2.625 million warrants to Maxim the option of which the firm subsequently exercised.
 |
| Click to enlarge. |
On June 18th (associated 8-K filing), in conjunction with the above fundraising, the company also reduced the equity line of credit it previously had with Alpha Capital Anstalt (established in July 2013, associated 8-K filing) to $10 million from an original $30 million figure. Provectus was and still is able to put (sell) shares to Anstalt as part of the arrangement; Anstalt cannot call (buy) shares.
An end-of-the-quarter-financing to meet hard and/or soft requirements of accounting firm BDO and/or the New York Stock Exchange should come as no surprise. Peter's historical approach has been to top up Provectus' cash balance a few days to a few weeks before quarter-end, typically with a Network 1 Financial retail investor-based fundraising but occasionally with Maxim retail investor participation (e.g., during the three months ended December 31, 2012, and September 30 and December 31, 2013). My expectation had been for a further $4-5 million drawdown on the existing Network 1 private placement vehicle. According to a source purportedly familiar with the situation but not authorized to publicly speak about it (I had to use that phrase at least once), gross proceeds of $5 million were available to Peter from Network 1 under the terms of the placement (i.e., $1 per unit and 50% warrant coverage [one common stock + half of one warrant] and a $1.25 exercise price) (prior to the announcement of the Maxim offering, Provectus' share price was trading around or above 80 cents). Had this path been taken again, dilution would have been 7.5 million shares and warrants, or 2.9%.
Peter's eventual approach was unexpected: gross proceeds of $13.1-15.1 million, $0.75 per unit and 100% warrant coverage [one common stock + one warrant], an $0.85 exercise price, purportedly mostly institutional investors, and Maxim as the sole bookrunner. This decision caused dilution of 37.6-40.3 million shares and warrants, or 13.7-15.7% -- nearly 5.5 times as much as the "usual" Network 1 raise (i.e., 40.3 ÷ 5.5).
Why did Peter choose Maxim over Network 1 (e.g., why this much, why them, why now)? As a [long-time] shareholder I do feel more than a little run over by the transaction, and I question his choice(s). Nevertheless, I believe there's some good process in Peter's approach, and he may achieve the good-to-great outcome he seeks (i.e., a much higher share price) because of it [that is, process can't make a bad deal good, but could make a good deal great].
Piecing together information at the time, with more than a little guessing thrown in, it seemed that in 2012 Peter (and thus Provectus management and its board of directors) sought to raise money at a substantially higher and much more respectful (to shareholders) valuation as well as upgrade the shareholder base to a higher proportion of institutional investors through a Big Pharma- (or brand name life sciences institutional investor-) led "IPO" of company preferred stock (PVCTP) on the NASDAQ, built on the foundation of Provectus (Eric) attaining a special protocol assessment ("SPA") from the FDA to commence a pivotal melanoma Phase 3 trial. His behind-the-scenes process presumably began earlier, but Peter's overt process began June 2012 when he filed a $100 million mixed securities (preferred stock, common stock, warrants) shelf offering. This was followed in September by the filing of the contemplated transaction's prospectus, for a Series A 8% convertible preferred stock together with Series D Warrants (on the preferred stock) (with Maxim as the "IPO's" sole bookrunner. The strategy seemed sound and the process appeared good, but the outcome was unfortunate and the execution was atrocious. Eric unfortunately could not reach agreement with the FDA on an acceptable [to him] Phase 3 trial design. With no SPA, no lead investor materialized (Big Pharma or institutional fund) and the round could not/did not come together. It's unclear to me when Peter found out Eric did not have an SPA for him, but with hindsight it was probably a month before he filed the "IPO's" prospectus (when he raised money via Network 1); he later raised money again through Network 1 in November after he terminated the offering in October (which I believe he should have done much sooner, especially after he realized he did not have the foundation for the fund raising).
Nearly three years later Peter may be at it again with Maxim and the "IPO" of a series of warrants (PVCTWS). This time, however, the foundations for the round may mostly fall under his role and responsibilities (business and corporate development) rather than exclusively Eric's (clinical development). A larger institutional investor-led round with Maxim, rather than the retail investor-only round with Network 1, provides him with more capital to complete and/or start clinical trial, potentially equity research coverage, and possibly leverage in collaboration discussions, all as he seeks to raise Provectus' market capitalization in anticipation of the interim analysis of the pivotal melanoma Phase 3 trial. Peter now should have the capital to pay for the full Phase 3 trial (I believe his estimate of ~$6 million or $25K per patient) and maybe the 1b portion of the liver Phase 1b/2 trial(s). He better get full throated research coverage from Maxim's Jason Kolbert; existing shareholders paid dearly for it (although, research coverage from lower tier investment banking firms like Maxim are akin to "the moon does not exist if nobody is looking at it"). The delay in arriving at deal with Sinopharm more than likely has been a lack of agreement on the upfront payment figure (Provectus wants bank, Sinopharm has offered nothing), and being able to walk away from the deal may enable a deal to get done. Thus, in July and August the company should see two liver data "stories" presented as posters at conferences, the publication of the combination therapy patent jointly owned by Provectus and Pfizer (it already has been allowed by the US PTO), and the fuller kick-off of the pivotal melanoma Phase 3 trial. Shareholders also may see long sought after pharmaceutical company collaborations.
Peter and Provectus failed the share price in 2012. They currently are failing the share price in 2015. Craig et al. have swung for the fences yet again. I hope they've finally connected this time for shareholders.
Seeking Alpha, and Me (June 27, 2015)
Seeking Alpha ("SA") published blog post Eric's Process under my name today. The SA version is here. As agreed, SA included the blog's landing page disclosure ("Disclosure" in bold below) and provided a link to the blog's Disclosure page ("See additional disclosures here" below).
 |
| Click to enlarge. |
When SA publishes my blog posts as SA articles under my name, another author also publishes an article at or around the same time.
 |
| Articles published on SA on June 27th |
 |
| Articles published on SA on January 21st and 22nd |
Provectus' primary and secondary liver cancer program (i.e., hepatocellular carcinoma or HCC, and cancers metastatic to the liver) is comprised of three threads, or treated groups:
- A Main Study Group of 6 patients,
- An Expansion Cohort 1 of up to 24 patients, and
- An Expansion Cohort 2 of 6-12 patients.
 |
| Click to enlarge. |
It might be possible to guess, based on the abstract made accessible today of Provectus' Phase 1 liver trial -- see Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver (June 24, 2015) below -- and Dr. Agarwala's presentation at the company's April 9th panel discussion in New York -- see "Oops" (April 10, 2015) -- the following:
- The abstract/poster presentation at the ESMO 17th World Congress on Gastrointestinal Cancer in July in Barcelona covers the Main Study Group,
- The abstract/poster presentation at the 6th Asia-Pacific Primary Liver Cancer Expert Meeting in July in Osaka covers the Expansion Cohort 1, and
- A possible abstract/poster presentation at the European Cancer Congress 2015 in September in Vienna might cover the Expansion Cohort 2.
Barcelona may tell the "basic story" of a Phase 1 dose-escalating safety study that also includes some perspective on efficacy. Osaka may communicate an "enhanced or more detailed story" of the higher dose group with a greater emphasis on efficacy in both HCC and a broader array of liver metastases. Vienna, if the company presents there (this of course assumes they will submit an abstract; notification is early-July), may tell the story of comparing PV-10 and sorafenib, the standard of care for HCC.
 |
| Click to enlarge. |
Provectus issued a press release today and made an associated 8-K filing (that included another item) regarding an award it received at the AAPI's 33rd Annual Convention and Scientific Assembly, AAPI Honors Provectus Biopharmaceuticals During 33rd Annual Convention and Scientific Assembly. In December 2014 the company announced it had agreed to agreed to sponsor the activities of the American Association of Physicians of Indian Origin (AAPI). In January, at the organization's 2015 Global Healthcare Summit in Mumbai, India, AAPI announced Provectus would be the inaugural member of AAPI's Global Clinical Trial Network. See AAPI – Global Clinical Trials Network (January 6, 2015) below. The trial network's website is here, and a screenshot is below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
Liver, Part I (June 24, 2015)
The abstract made accessible today of Provectus' Phase 1 liver trial -- see Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver (June 24, 2015) below -- appears to be consistent with the sneak peak of clinical data provided in Dr. Agarwala's presentation at the company's April 9th panel discussion in New York; see "Oops" (April 10, 2015) below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Three initial subjects with either HCC or cancer metastatic to the liver will receive 0.25 mL PV-10 per cc lesion volume (Lv) to a single lesion (up to a maximum dose of 7.5 mL PV-10). If none of the initial three subjects experiences a new and persistent CTCAE Grade 3 or greater non-hematological or any Grade 4 hematological toxicity over a 28-day follow-up interval, an additional three subjects will be enrolled and similarly treated with PV-10 administered at 0.50 mL per cc Lv (up to a maximum dose of 15 mL PV-10) provided no new and persistent Grade 3 or greater non-hematological or any Grade 4 hematological toxicity occurs. {Underlined emphasis is mine}The abstract notes, among other things, (i) long-term follow-up (9-15 months) of four patients, two of whom had partial response of their injected tumors (i.e., an objective response of 50%) (6-15 months on Agarwala's slide), (ii) temporary elevated enzyme levels (transient elevation of ALT [alanine transaminase] and AST [aspartate aminotransferase]), and (iii) adverse events were resolved without sequelae (no long-term sequelae).
Conforming the abstract with Agarwala's slides, two of the initial 6 study patients must have died so that no long-term follow-up was possible; thus, the abstract does not address 4 other patients, as noted on the slides. Twelve primary and secondary liver cancer lesions were treated, per the slides; however, the abstract notes the response of four patient lesions but not the others and no cancerous make-up. The poster, available next week, presumably should provide more information.
Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver (June 24, 2015)
ESMO World Congress on Gastrointestinal Cancer 2015:
Introduction: Intralesional PV-10, a 10% solution of rose bengal, has recently demonstrated high rates of complete response and durable local control in metastatic melanoma. The current Phase 1 study is assessing safety, pharmacokinetics, and preliminary efficacy of PV-10 in subjects with non-resectable hepatocellular carcinoma or cancer metastatic to the liver.
Methods: Subjects having at least one liver tumor ≥ 1 cm are administered a single percutaneous intralesional injection of PV-10 to one Target Lesion at dose of 0.25 or 0.50 mL per cm3 lesion volume. Plasma concentrations of PV-10 from 1 hour to 28 days after injection are measured. Radiologic assessments of the injected Target Lesion are performed to determine response over initial 28 day and long-term 9-15 month periods. Serum levels of potential liver injury markers are measured, and adverse events recorded.
Results: In an initial study cohort, six subjects received PV-10. Significant adverse events were limited to injection site and photosensitivity reactions, and resolved without sequelae. All injected tumors were stable in size at 28 days, and of 4 that had long-term assessment, 2 had partial response, for a long-term tumor-specific objective response rate of 50%. PV-10 plasma levels decreased rapidly in a bi-exponential pattern, with initial and terminal phase half-lives of 4.5 and 100 hours, respectively. Elevated liver enzymes levels subsided within a week of treatment.
Conclusion: Preliminary efficacy in treatment of liver tumors with PV-10 was observed. Toxicity was transient, and treatment had acceptable tolerability. The study is continuing at three study centers with two expansion cohorts to assess response in hepatocellular carcinoma and other cancers metastatic to the liver.First Melanoma, Then Liver (June 22, 2015)
This week PV-10 & melanoma-related information and data will be presented at the 5th European Post-Chicago Melanoma / Skin Cancer Meeting on Thursday/Friday, and presumably at the "Melanoma: from basic science to clinical applications" conference on Wednesday-Friday. Provectus is a sponsor of both. The accepted abstract to the former is below. While the poster should look similar to the one Eric presented at the April 9th panel discussion in New York (see Trials in progress (May 27, 2015) below), there may be new information in the bottom right hand corner of the poster (e.g., conclusions, etc.) presented later this week. I'm more interested in learning what Moffitt Cancer Center's Dr. Vernon Sondak, MD has to say and show during his oral presentation.
 |
| Click to enlarge. |
Voting Results (June 22, 2015)
Provectus made an 8-K filing today in which the company included proxy voting results. 2014's results are here. See also below, and also May 30th blog post Proxy Vote.
 |
| Click to enlarge. |
 |
| Image source |
Calendar (June 21, 2015)
Provectus updated its Calendar of Events page for July to include a Best of ASCO Meeting put on by the Chinese Society of Clinical Oncology (CSCO) in Hangzhou, China from July 9-12. A CSCO description of the 2013 event is here.
 |
| Click to enlarge. |
H/t InvestorVillage PVCT poster Starlight66, who noted Provectus' 8-K filing related to the Maxim fundraising contained the below in regards to the company's outside director Jan Koe:
| Click to enlarge. |
A bearish viewpoint or perspective on the filing paragraph above: Koe may not have agreed to a 180-day "lock-up" of his Provectus holdings because he sees a bad or less than positive outcome on the horizon and/or in the future for the company, and wants to sell sooner rather than later. As noted above, insider directors Dees and Scott, officers Wachter and Culpepper, and other outside directors McMasters and Smith agreed to the lock-up. A "bullish" viewpoint or perspective: Alternatively, Koe may not have agreed to the lock-up because he is aware of actual and potential news, and wants to capture a return on investment for his shares and stock options (before the 6-month period, which is a generic or customary time frame) if and when he departs the board. There can be other reasons or explanations too.
Offering (June 19, 2015)
Provectus issued a press release today and made several SEC filings -- 8-K, prospectus supplement, adjustment to a prospectus supplement (related to the Alpha Capital Anstalt "equity line of credit") and warrant registration statement -- regarding a fundraising it did/is doing, Provectus Biopharmaceuticals, Inc. Announces Pricing of Public Offering to Raise $13.1 Million. Existing shareholder dilution should be 14-16%; see below.
 |
| Click to enlarge. |
Provectus' Peter Culpepper is presenting at the 2015 BIO International Convention today — slides are available here. A new slide:
 |
| Click to enlarge. |
Repeatability, the other side of the Does-It-Work coin (June 15, 2015)
Key aspects of PV-10's clinical value proposition — tumor ablation (the local effect of tumors [into which PV-10 is injected] going away) and tumor-specific immune responses (the systemic effect of distant, non-injected tumors going away [following aforementioned injection]) in multiple solid tumor cancers — that Craig, Tim and Eric originally demonstrated were reproduced by Moffitt Center, whose initial work was reproduced by the University of Illinois at Chicago. See my blog post Reproducibility, the Hallmark of Western Science.
One side of the "does it work" coin is reproducibility, the degree of agreement of testing or measuring by different observers in different laboratories. The other side is repeatability, the degree of agreement of testing or measuring by the same observer in the same laboratory. [I used a variation of a portion of the Wikipedia entry on reproducibility.] For a longtime, in their investor presentation, Provectus summarized the cofounders' work (primarily Craig's) to repeat the abovementioned aspects of PV-10's clinical value proposition.
 |
| Click to enlarge. Provectus investor presentation, February 2012 |
 |
| Click to enlarge. |
An interesting Twitter thread from @jmccarter80 this past week.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Third party non-clinical work (June 5, 2015)
You may recall I asked Eric on Provectus' 4Q14 conference call held March 13th:
"Good afternoon, Dr. Wachter...can you speak to the third-parties outside of Moffitt for example that may be undertaking preclinical and/or clinical work using PV-10?"I of course was hoping learn (i) what other researchers and research organizations might be undertaking non-/pre-clinical research (murine model work) on PV-10, and (ii) what other clinicians and medical institutions might be part of the company's compassionate use program.
Provectus' Chief Technology Officer Dr. Eric Wachter, PhD replied:
"I cannot comment on other third-party work that may or may not be underway with regard to non-clinical work with PV-10. Obviously that is something that may be of interest and if it is, and we haven't disclosed this, it’s probably a sensitive nature."Today we learned (see today's blog post Intralesional Injection of Rose Bengal Induces an Anti-tumor Immune Response and Potent Tumor Regressions in a Murine Model of Colon Cancer -- (abstract P134, see page S86) a group from the University of Illinois at Chicago's ("UIC's") Department of Surgical Oncology, the Ajay Maker Laboratory, published some results from their preclinical work with PV-10 and colorectal cancer.
According to the lab's website:
The Maker laboratory focuses on studying tumor biology in order to better understand markers of malignant transformation and to create novel approaches to target cancer cell death. His team is researching the role of the immune system in cancer, specifically in gastrointestinal cancers that have metastasized and spread to the liver. The team is working towards identifying immunotherapeutic agents for these conditions and initiating clinical trials to investigate them. Dr. Maker’s research has been supported by foundations and pharmaceutical companies; and he currently is the principal investigator on a multi-year federal research grant from the National Cancer Institute/National Institutes of Health to study the immunobiology of GI cancers. {Underlined emphasis is mine. Note: I underline the reference to the NCI/NIH because of the institutions, the grant and the nature of that work, and not because of any implication with respect to PV-10}Further, the lab's research focus is:
Dr. Maker’s research focuses on cancers of the gastrointestinal tract and hepatopancreatobiliary system. During his post-doctoral training he performed translational immunotherapy research under Dr. Steven Rosenberg at the National Institutes of Health, where he and his colleagues were of the first to identify the utility of an anti-CTLA4 antibody as an anti-cancer treatment for patients with metastatic melanoma, and investigated its immunological mechanism of action in Phase I/II trials with Ipilimumab. Since that time, his lab has been interested in understanding the response of the immune system to primary gastrointestinal cancers and their metastases. They have determined that a tumor microenvironment reflective of T-cell proliferation and activation is associated with improved survival in patients with colorectal liver metastases. They have since identified candidate genes and cytokines that are potential therapeutic targets for enhancing the anti-tumor immune response in these patients and are currently developing techniques to stimulate this process.
In addition, Dr. Maker has an interest in using targeted therapy to incite cancer cell apoptosis. He and his colleagues have developed a technique to sensitize resistant gastrointestinal cancer cells to TRAIL induced apoptosis by manipulating tumor cell glucose metabolism. Investigation of the mechanisms responsible for this synergy have identified unique signaling pathways and miRNA targets that may provide tumor-specific targets for therapy.
Additionally, Dr. Maker’s laboratory is interested in identifying immunologic markers of cancer in pancreatic cystic tumors. A great challenge in the clinical management of mucinous tumors of the pancreas is determination of appropriate timing for pancreatic resection. He and his colleagues have previously determined the utility of EUS-FNA in evaluation of mucinous cysts, and have identified unique mucin and cytokine markers of pancreatic malignancy. Along with colleagues in the Branch-Duct IPMN International Expert Conference Biomarkers Working Group, they have reported upon current and future biomarkers for pancreatic cystic tumors. {Underlined emphasis is mine.}Biotechnology and pharmaceutical companies produce and publish non-clinical work exclusively of their employees:
- At AACR 2015, for example, Amgen produced a couple of internally generated posters (i.e., the author line was comprised exclusively of Amgen employees) for intraslesional agent talimogene laherparepvec ("T-Vec") -- see Talilmogene laherparepvec increases the anti-tumor efficacy of the anti-PD-1 immune checkpoint blockade and Talimogene laherparepvec activates systemic T-cell-mediated anti-tumor immunity.
- Provectus of course has generated those "internal" posters as well; see Generation of an Antitumor Response and Immunity Using a Small Molecule Drug (PV-10) (SITC 2012) and Combination of PV-10 Immuno-chemoablation and Systemic anti-CTLA-4 Antibody Therapy in Murine Models of Melanoma (AACR 2013).
- NewLink published preclinical combination study work (their drug + anti-PD1/PD-L1 antibodies) at AACR 2014 (April), co-conducted by NewLink employees and Georgia Regents University Research Institute researchers/employees.
- In another example, at an ASCO 2014 presentation of a Phase 1/2 melanoma study, principal investigators (that included Moffitt Cancer Center's Dr. Jeffrey Weber, M.D., Ph.D.) noted "[p]reclinical data support antitumor synergy for INCB024360 when administered with an antibody antagonist to checkpoint receptors," referencing a October 2013 (submitted)/February 2014 (published) SITC journal paper* (the paper, however, does not present preclinical combo work on the subject drug but another related Incyte compound), co-conducted by Celldex employees and the University of Chicago researchers/employees.
Provectus calls PV-10 an ablative immunotherapy because of the drug's two-step mechanism of action. See below.
 |
| Click to enlarge. |
Moffitt Cancer Center said (no Provectus employees in the author byline), in their July 2013 paper entitled Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer:
"Treatment of the subcutaneous lesion with a single injection of IL PV-10 led to regression of the injected lesion as well as the distant B16 melanoma lung metastases. Anti-tumor immune responses were measured in splenocytes collected from mice treated with IL PBS or PV-10. Splenocytes isolated from tumor bearing mice treated with IL PV-10 demonstrated enhanced tumor-specific IFN-gamma production compared to splenocytes from PBS-treated mice in both models." {Bold emphasis is mine}UIC said (no Provectus employees in the author byline), in their March 2015 abstract entitled Intralesional Injection of Rose Bengal Induces an Anti-tumor Immune Response and Potent Tumor Regressions in a Murine Model of Colon Cancer -- (abstract P134, see page S86):
"Treatment of subcutaneous tumors with a single injection of intralesional PV-10 led to near complete responses in all animals within days of exposure and significant regression of the injected lesions compared to controls (n=6 per group, p=0.027). PV-10 treatment was associated with occasional bystander responses in contralateral untreated tumors and trended towards a decreased rate of growth in these lesions. Splenocytes isolated from tumor bearing mice treated with PV-10 displayed enhanced tumor-specific IFN-γ production compared to splenocytes from PBS-treated mice (p = 0.025)." {Bold and underlined emphasis is mine}You may recall from my May 2015 blog post Revolutionize, Perfect, Quintessential, Moffitt's Dr. Sarnaik, MD said in an inteview with Dermatology Times of inteferon gamma (IFN-gamma, IFN-γ):
The addition of autologous tumor cells to purified CD8+ cells obtained from the post-treatment blood sample of one patient stimulated release of interferon-gamma, the “quintessential antitumor cytokine” {Underlined emphasis is mine}Moffitt's Dr. Jeffrey Weber, MD, PhD: "Cytokines are the messengers of the immune system. Cytokines are substances, either proteins or glycoproteins, secreted by immune cells. They have autocrine and paracrine functions, so that they function locally or at a distance to enhance or suppress immunity. In cancer therapy, we generally use cytokines to enhance immunity."
My takeaways include:
- PV-10 is an ablative immunotherapy,
- PV-10 ablation -- the destruction or regression of injected tumors or lesions -- has been shown in multiple cancer indications by multiple third parties,
- PV-10's tumor-specific immunotherapeutic capability -- the destruction or regression of non-injected, distant or "bystander" tumors or lesions, and by virtue of the production of tumor specific IFN-γ -- has been shown in multiple cancer indications by multiple third parties.
Through the wall, and beyond (June 2, 2015)
In April I wrote about the joint meeting of the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC") and Oncologic Drugs Advisory Committee ("ODAC"), which voted by an overwhelming 22-to-1 margin in favor (i.e., yes to the question) of Amgen's talimogene laherparepvec ("T-Vec") having an overall favorable benefit-risk profile for the treatment of injectable regionally or distantly metastatic melanoma, and thus supporting traditional approval of the drug. See my blog post entitled The first guy through the wall.
Amgen further expanded its combination therapy approach for T-Vec by announcing on May 29th it would combine the intralesional ("IL") agent with Merck's approved anti-PD-1 agent Keytruda in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN). See ASCO Day #2, sampling (May 30, 2015) below. Merck's press release is here.
The Big Biotech previously had early-stage trials combining T-Vec with Bristol-Myers' approved Yervoy (anti-CTLA-4) and Merck's Keytruda for advanced melanoma. Merck and Amgen announced the T-Vec-Keytruda combo would be moving towards a randomized Phase 3 trial.
Today Amgen announced it was combining T-Vec with Roche's investigational anti-PDL-1 drug atezolizumab in triple negative breast cancer, and colorectal cancer with liver metastases (both Phase 1b studies). Amgen's press release is here.
Takeaways/questions:
- Presumed approval, by virtue of the overwhelming advisory committee vote above (official approval only comes of course from the FDA), presumably helped further expand Amgen's clinical program for T-Vec.
- Amgen appears to be moving T-Vec firmly toward use in combination (with 3 Big Pharma and 4 indications, and the use of T-Vec in combination with radiation in patients with locally advanced soft tissue sarcomas).
- Is Big Pharma saying immune checkpoint inhibitors (first CTLA-4, now PD-1 and PD-L1s) need help?
- How does this affect or influence the combination component of Provectus' clinical development program?
 |
| Click to enlarge. Image source |
"...provides digital media, media training, crisis and issues management, and financial communications/IR services. The company’s practice areas include consumer, corporate, healthcare, public affairs, social impact, technology, beauty, and global China practice. Allison & Partners LLC was founded in 2001 and is headquartered in San Francisco, California. It has additional offices in Atlanta, Beijing, Chicago, Dallas, London, Los Angeles, Paris, Portland, New York, Phoenix, San Francisco, Paris, Singapore, Lyon, San Diego, Seattle, Shanghai, Silicon Valley, and Washington. As of May 4, 2010, Allison & Partners LLC operates as a subsidiary of MDC Partners Inc." {Underlined emphasis is mine.}Allison+Partners describes itself as "...a strategic public relations (PR) firm specializing in media relations, digital PR marketing, and communications management services." A couple of interesting screenshots from the firm's website are below.
Provectus' Chief Operating Officer and Chief Financial Officer's Peter Culpepper's reference to the Pharmaceutical Research and Manufacturers of America ("PhRMA"), also an Allison+Partners' client, is relevant or somewhat notable. PhRMA "...represents the country’s leading biopharmaceutical researchers and biotechnology companies" (ironically, I played a very small role on a team that provided consulting work to PhRMA and Pfizer [a lifetime ago]).
Off-topic, in another technology-driven industry, but related to China, it was interesting to note of the new PR firm (April 2015): "Allison+Partners will drive awareness of online lodging marketplace Airbnb among Greater China’s 100 million annual outbound international travelers through a series of high-profile features, familiarization events and media-based awareness programs."
Provectus' PR references Matthew Della Croce, President, Europe and Global Corporate for Allison+Partners. See here for a press release of his original hire at Allison+Partners, and here for his webpage at the firm. Allison+Partners' Todd Aydelotte, Managing Director in the firm's Corporate Practice, is Provectus' new media relations contact.
My initial takeaways and questions include:
- A high quality and global firm,
- A clear upgrade over the overlapping media/PR roles of prior consultant Bill Gordon and investor relations firm Porter, LeVay & Rose (where he was or was not currently hanging out). See The Doctor Is In (May 2, 2015) below,
- Without putting too excessive a weight on this takeaway, one would imagine there was material deliberation and a selection process and decision by Allison+Partners to take Provectus on as a client, as there would be for the company to retain the PR firm, and
- Management's timing seems appropriate, and is interesting, as the pivotal melanoma Phase 3 trial ramps up, primary and secondary liver cancer data is presented for the first time, and commercial validation (geographic license(s), drug co-development) possibly abounds.
 |
| Image source |
"The same misunderstanding happens in David vs. Goliath fights in business, which Gladwell substantiates with numerous case studies and research examples in his recently published book. Most fail to recognize the advantages an underdog brand has when it faces off against a competitor who has strength, size, and wealth. And that's exactly why nimble, upstart companies, with their new solutions to old problems, often can best Goliaths."Although, Provectus shareholders ultimately hope Goliath ends up adopting (buying) David's weapon to defeat cancer.
Provectus issued a press release yesterday and made an associated 8-K filing regarding the education session at ASCO 2015 chaired by Dr. Sanjiv Agarwala, MD of St. Luke's University Hospital and Health Network (and a key PV-10 clinical investigator), Provectus Biopharmaceuticals' Data on PV-10 as Intralesional Treatment of Melanoma Presented at 2015 American Society of Clinical Oncology Annual Meeting. See On the payroll (April 3, 2015) below. Slides used during the session are here. The session was entitled A Changing Topography: The Role of Intralesional Therapy in Melanoma. Slides of interest to me included are below.
May Blog Stats (June 1, 2015) (Updated 6/2/15: title updated; thank you)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
ASCO Day #2, sampling (May 30, 2015)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Note "Complete Response" |
 |
| Click to enlarge. Topalian, a John Hopkins physician previously has referred to PV-10 as an autologous vaccine. |
One of the Tweeters in my curated Twitter feed for this project (an investment in Provectus), @bradloncar (some I follow directly, others I follow indirectly and look to those I follow directly to curate by their retweets), highlighted two important items that generated rhetorical but necessary observational questions in my mind, and one very insightful table.
First, is PV-10 a cancer immunotherapy, and is Provectus a cancer immunotherapy company? I understand in relation to market capitalization this is form over substance, but like the chicken and her egg when does substance lead to form?
 |
| Click to enlarge. @bradloncar's Tweet thread for this |
Clinically, Moffitt previously also said, via a Provectus press release: "The Moffitt researchers presented clinical data on 8 melanoma patients that demonstrated significant decreases in melanoma cells in injected tumors and uninjected bystander tumors 7-14 days after PV-10 injection as evidenced by pathologic evaluation confirmed with immunohistochemical staining of biopsy specimens for melA (a marker of melanoma). The researchers showed that these changes in tumors were accompanied by increased populations of CD3+, CD4+ and CD8+ T cells along with NKT cells in peripheral blood. T cells from one patient were purified and exhibited increased interferon-gamma expression when exposed to the patient's pre-treatment melanoma cells." (quote source: Provectus press release regarding Moffitt's PV-10 AACR 2014 poster presentation)
Second, is PV-10 a "T-cell technology," and is Provectus a T-cell technology company? I consider T-cell technology and cancer immunotherapy interchangeable (and merely labels).
 |
| Click to enlarge. @bradloncar's Tweet thread for this |
Third, the needs/wants of Big Pharma of so-called immuno-oncology assets:
 |
| Click to enlarge. @bradloncar's Tweet thread for this |
Associating with my April 2015 blog post entitled The first guy through the wall, a post mostly about the joint meeting of the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC") and Oncologic Drugs Advisory Committee ("ODAC") that voted by an overwhelming 22-to-1 margin in favor (i.e., yes to the question) of T-Vec having an overall favorable benefit-risk profile for the treatment of injectable regionally or distantly metastatic melanoma, and thus supporting traditional approval of the drug, T-Vec received substantial pre-ASCO media coverage. Notable articles include from May 27th:
- AFP: Trial shows virus treatment effective against skin cancer,
- Gizmodo: Herpes-Based Drug Shown to Successfully Treat Aggressive Skin Cancer,
- The Japan Times: Modified herpes virus able to destroy skin cancer cells, trigger immune response in trial,
- Eqsuire: At Last, Great News About Herpes (pun intended, I am sure),
- U.S. News & World Report: Health Buzz: How the Herpes Virus Can Kill Cancer,
- The Independent: World first as scientists use cold sore virus to attack cancer cells (a UK newspaper), and
- European Pharmaceutical Review: World first as viral immunotherapy T-VEC shows patient benefit in Phase III trial (May 26th).
- First, starting a Phase 1 combination therapy trial in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN), and
- Second, moving towards a global, randomized, Phase 3 study evaluating the combination in regionally or distally metastatic melanoma.
Amgen terminated BioVex's Phase 3 trial in T-Vec's head and neck cancer trial (as a monotherapy) shortly after it acquired the private company. See, for example, my August 2012 blog post entitled Intralesionally-Delivered Local Agents with Potential Systemic Benefits: PV-10, Allovectin-7 and T-Vec (formerly OncoVEX). It is unsurprising a combination approach for T-Vec is being considered. Recall Moffitt's Dr. Jeffrey Weber, MD, PhD from ASCO 2014:
Potential synergistic activity of T-VEC with other checkpoint inhibitors is also anticipated. “Now there are some interesting drugs, and T-VEC is one of them,” said Jeffrey Weber, MD, PhD, director of the Donald A. Adam Comprehensive Melanoma Research Center, Moffit Cancer Center. “I see this as a niche drug that would be best used to prime the immune system and follow up with a drug such as pembrolizumab, nivolumab, ipilimumab, or a combination of those.”Merck and Amgen previously undertook a Phase 1b combination trial, which was discussed at ASCO 2014 -- Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma -- and will be discussed at ASCO 2015 -- A multicenter, open-label trial of talimogene laherparepvec (T-VEC) plus pembrolizumab vs pembrolizumab monotherapy in previously untreated, unresected, stage IIIB-IV melanoma. The former focused on objective response, and the latter on survival.
Expired (May 29, 2015)
It would appear Provectus' Chairman and CEO Dr. Craig Dees, PhD, and President Dr. Timothy Scott, PhD each let 325K stock options expire (i.e., "forfeited" in the language of the company's 10-K/Q filings). I make this observation based on the lack of Form 4 filings related to option exercises for May 19th and May 25th (i.e., the notion the executives would have had 2 days following option expiration to make the filings had they exercised the options). The screenshots below (purple emphasis is mine) come from the 2015 proxy statement.
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Image source |
The abstract/presentation bears the same name, Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver, as the abstract/presentation to be made the day before on July 2nd at the 17th World Congress on Gastrointestinal Cancer in Barcelona, Spain. The two presentations are about 14 hours apart.
According to management, (i) Dr. Sanjiv Agarwala, MD of St. Luke's University Hospital and Health Network (and a key PV-10 clinical investigator) will present the Osaka poster (Dr. Paul Goldfarb, MD, a surgical oncologist and the principal investigator of Provectus' liver Phase 1 trial, only is a co-author), while (ii) Provectus' Chief Technology Officer Dr. Eric Wachter, PhD, a European Society for Medical Oncology ("ESMO") member will present the Barcelona poster. Provectus press released on May 14th that Dr. Agarwala would present the Barcelona poster. See Mish Mash (May 19, 2015) below.
Trials in progress (May 27, 2015)
Provectus issued a press release today and made an associated 8-K filing regarding a poster presentation, and presentation in June in Munich, Germany, Provectus Biopharmaceutical's Abstract Accepted for Poster Presentation at 5th European Post-Chicago Melanoma / Skin Cancer Meeting. Moffitt Cancer Center's Dr. Vernon Sondak, MD, who will make the presentation, has done so before on the topic of Rose Bengal/PV-10 last year at the 4th European Post-Chicago meeting. See Intralesional Therapy for Melanoma (June 29, 2014) on the blog's Archived News II page. Sondak is a consultant to Provectus. The abstract/poster is entitled Trials in Progress: Intralesional Rose Bengal vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma.
 |
| Click to enlarge |
Advanced Cutaneous Melanoma. The preceding link are the slides related to the poster. A photograph of the actual poster is to the right.
"Trials in progress" appears to be industry terminology/jargon/vernacular for abstracts/posters of that cover all phases of clinical research (phases I to III, supportive care, nonpharmacologic interventions) but are ongoing and have not reached prespecified endpoints for analysis. Inclusion of results is considered improper.
I believe the company may use this venue, a medical conference, to provide a summary of the status of the pivotal melanoma Phase 3 trial.
Eric said on the May 7th conference call:
I don’t anticipate that company will provide site by site or patient by patient announcements of study milestones since this will be highly unusual for our industry but we do anticipate providing periodic summaries of study status. And also as noted by Pete, when we open new centers, these will be shown on the NCI clinical trials registry website.Interestingly, the company did provide a form of these announcements earlier in its history. The table below is of those announcements for the melanoma Phase 2 trial. Provectus may repeat some aspect of this history as/when Eric deems it appropriate, and in what form/format he deems appropriate.
 |
| Click to enlarge. |
Provectus updated its September Calendar of Events website page to include the notation of The 18th Annual Meeting of Chinese Society of Clinical Oncology (CSCO) in Xiamen, China from September 16-20, 2015.
 |
| Click to enlarge. |
 |
| Image source |
- Safety, and tissue sparing,
- Local and systemic efficaciousness (effectiveness),
- Multi-indication viability,
- Synergistic combination with other therapies and families of therapeutic agents,
- Ease of physician use as well as supportive of patient compliance,
- Ease of use, re-use, shipment, storage and handling, and
- Affordability (globally).
Together with Amgen's talimogene laherparepvec ("T-Vec"), PV-10 has established a role for intralesional agents in the treatment of melanoma. The role, however, is perhaps "nuanced:"
- Melanoma, as a cancer, is pernicious, vicious, and can spread slowly and methodically, or spread in a shotgun manner,
- Local and regional disease -- in light of historically available therapies and therapeutics (e.g., surgery, chemotherapy) -- can spread to visceral disease,
- Melanoma tumors are immunogenic ones -- ironically, the same pernicious and vicious disease holds within it the potential, if catalyzed effectively and sufficiently, to generate an immune response by the body to forestall or defeat the disease itself, and
- Combination therapy, most especially for late-stage disease might be best approached using the combination of an intralesional agent and immune checkpoint blockade.
But while it has taken sometime to establish the role of a so-called local agent to find its place in the treatment decision tree of a so-called systemic disease, local-regional (or loco-regional) therapy is commonplace in the treatment of other solid tumor cancers.
The next major prong of Provectus clinical development program is liver cancer, comprised of (i) presenting clinical results of the company's Phase 1 and expanded Phase 1 studies and (ii) establishing pathways to approval domestically in the U.S. and internationally in China among other geographies.
Primary liver cancer ("liver cancer").
According to PubMed (search term: Goldfarb PM[Author]) Goldfarb has authored or co-authored 21 papers*, the last in 2002. From the little information available from Provectus, the patients that comprised the original Phase 1 clinical trial were treated for primary liver cancer (hepatocellular carcinoma or HCC). One metastatic colorectal cancer tumor in a patient also was treated, I believe; however, the bulk of the treatment and thus observation was of liver cancer or HCC. The focus of the poster's narrative may be on the successful overall survival of these patients. While there may be some discussion about response rates, the time that has elapsed since these patients were treated would seem to make a survival discussion more relevant.
Although I am surprised the "lead author" is Goldfarb, I shouldn't be because he was/is the original, initial and lead clinical investigator, and it would appear industry tradition is for such person to headline a poster or peer-reviewed publication. Provectus' Chief Technology Officer Dr. Eric Wachter, PhD also should be an author/contributor (as he has been with the company's melanoma presentations and publications). I believe (surmise) an apparent key opinion leader relatively new to PV-10, an interventional radiologist, will be added to the author/presenter line.
Cancers metastatic to the cancer/Metastatic cancer in the liver/Secondary liver cancer.
"Next up," on July 2nd, the day before Goldfarb's poster presentation, is Provectus and clinical investigator Dr. Sanjiv Agarwala, MD's (a medical oncologist) presentation at the 17th World Congress on Gastrointestinal Cancer in Barcelona, Spain.The next major prong of Provectus clinical development program is liver cancer, comprised of (i) presenting clinical results of the company's Phase 1 and expanded Phase 1 studies and (ii) establishing pathways to approval domestically in the U.S. and internationally in China among other geographies.
Primary liver cancer ("liver cancer").
"Primary liver cancer, also known as hepatocellular carcinoma, is a cancer that begins in the liver." "When compared to the United States, liver cancer is much more common in developing countries within Africa and East Asia. In some countries, it is the most common cancer type."First up, in regards to presenting liver cancer clinical results, is Provectus and clinical investigator Dr. Paul Goldfarb, MD's (a surgical oncologist) presentation at the 6th Asia-Pacific Primary Liver Cancer Expert (APPLE 2015) Meeting on July 3rd in Osaka, Japan.
According to PubMed (search term: Goldfarb PM[Author]) Goldfarb has authored or co-authored 21 papers*, the last in 2002. From the little information available from Provectus, the patients that comprised the original Phase 1 clinical trial were treated for primary liver cancer (hepatocellular carcinoma or HCC). One metastatic colorectal cancer tumor in a patient also was treated, I believe; however, the bulk of the treatment and thus observation was of liver cancer or HCC. The focus of the poster's narrative may be on the successful overall survival of these patients. While there may be some discussion about response rates, the time that has elapsed since these patients were treated would seem to make a survival discussion more relevant.
Although I am surprised the "lead author" is Goldfarb, I shouldn't be because he was/is the original, initial and lead clinical investigator, and it would appear industry tradition is for such person to headline a poster or peer-reviewed publication. Provectus' Chief Technology Officer Dr. Eric Wachter, PhD also should be an author/contributor (as he has been with the company's melanoma presentations and publications). I believe (surmise) an apparent key opinion leader relatively new to PV-10, an interventional radiologist, will be added to the author/presenter line.
Cancers metastatic to the cancer/Metastatic cancer in the liver/Secondary liver cancer.
"The liver is also the most common site to which cancer has spread (metastasized) from other parts for the body, most commonly the colon, lungs, or breast. When this happens, it is NOT liver cancer. Instead, the cancer is named for the organ it originated from (the primary site). For example, colon cancer that spreads to the liver is called metastatic colon cancer. It is not liver cancer. In the U.S., metastatic cancer in the liver is far more common than primary liver cancer."
According to PubMed (search term: Agarwala SS[Author]) Agarwala has authored or co-authored 95 papers*, the last (as of the writing of this blog news item) in May 2015. Although the abstract/poster presentation is entitled "Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver," I would imagine (without any benefit of querying Provectus management) the focus of the poster's narrative may be on patients treated in the expanded Phase 1 study where:
- There is a comparative component to the treatment -- PV-10 + sorafenib vs. sorafenib,
- Possibly as many or more patients had cancer metastatic to the liver (secondary liver cancer) than primary liver cancer,
- Successful response rates of these patients might be the focus with a relatively smaller view into survival (since the expanded trial really began recruiting in 2012), and
- Some time, space and discussion might be given to the response of the cancer in the organ where the cancer in the liver originated -- that is, possibly an aswer to the question "Did the primary cancer go away after the secondary liver cancer was treated with PV-10?
- Table 1 details "Studies (>30 Patients) of Hepatic Resection in Patients With Liver Metastases From Ocular or Cutaneous Melanoma," and 5-year survival rates or 0-42%,
- Table 2 details "Studies (>10 Patients) of Systemic Therapy for Patients With Metastatic Ocular Melanoma," and overall response rates of 0-29% and median overall survival of 6-14 months,
- Table 3 details "Studies (>15 Patients) of Hepatic Intra-Arterial Chemotherapy for Hepatic Metastases From Ocular or Cutaneous Melanoma," and overall response rates of 2-36% and median overall survival of 8.5-21 months,
- Table 4 details "Studies (>15 Patients) of Hepatic Arterial Embolization Techniques for Hepatic Metastases From Ocular or Cutaneous Melanoma," and overall response rates of 0-39% and median overall survival of 5-14.4 months, and
- Table 5 details "Studies (>15 Patients) of Hepatic Perfusions for Hepatic Metastases From Ocular or Cutaneous Melanoma,"and overall response rates of 2-62% and median overall survival of 9.9-12.1 months.
"Rose Bengal for melanoma treatment: will it translate to the clinic?" (May 23, 2015)
H/t @bradpalm1 for the article link posted on InvestorVillage: St. Luke's University Hospital and Health Network (Bethlehem, Pennsylvania) medical oncologist and key PV-10 clinical investigator Dr. Sanjiv Argarwala, MD authored a recent article in Melanoma Management entitled Rose Bengal for melanoma treatment: will it translate to the clinic? Notable comments in the article include:
Among strong justifications for ongoing interest in intralesional therapies is the fact that about 10% of patients with locally advanced melanoma develop in-transit cutaneous metastases. Also, local/satellite/in-transit lesions represent about the same percentage of primary melanomas. Many of these lesions are accessible to injection, and treating them may be clinically important not only because of the potential to alleviate such symptoms as ulceration, bleeding, infection and pain, but also because many of these lesions remain localized for a considerable period of time and successful local regional therapy may provide durable responses and defer systemic therapy until it is inevitable.
While the new systemic immunotherapies are a groundbreaking advance, response rates are far from absolute, often not durable in the case of BRAF-mutated disease, and for most patients cure remains elusive. Serious and in some cases fatal adverse events have been reported, and the risk of significant morbidities related to uncontrolled locoregional disease is high.
The most likely application of PV-10 and other intralesional therapies, many agree, will be in combination with systemic immunotherapies. The low toxicities found with PV-10 especially, but generally with all of the intralesional injectables (usually transient injection site inflammation), make combining them with immune checkpoint inhibitors (anti-CTLA-4 or anti-PD-1) or targeted small molecules (BRAF and MEK kinase inhibitors) easy and attractive. The preliminary findings from the T-VEC/ipilimumab trial noted above are encouraging.
Beyond melanoma, evaluations of PV-10 in treatment of primary and metastatic tumors of the liver are ongoing. Testing of PV-10 with the newer systemic immunotherapies is being planned actively, as well.
Many unanswered questions remain. How well will PV-10 combine with systemic immunotherapies? How strong, in fact, are the systemic immune responses evoked by local tumor ablation, and how extensive and valuable for patients are the quality of life improvements? Of course, only future clinical trials can further resolve these and other uncertainties. The promise suggested by the research to date is compelling – and if confirmed, translating these benefits to the clinic is a high priority. {Underlined emphasis is mine}Melanoma Management has produced 4 issues thus far (2 in 2014, and 2 in 2015 to date). The editorial advisory board includes senior editor and Australian PV-10 clinical investigator Dr. John Thompson, and editors Dr. Peter Hersey, MD, PhD (an Australian PV-10 investigator) and Moffitt Cancer Center's Dr. Jonathan Zager, MD (a consultant to Provectus).
Agarwala's last article was I believe was Current Opinion in Oncology's March Intralesional therapy for advanced melanoma: promise and limitation. His medical practice includes a strong emphasis on conducting clinical trials, and he previously was an investigator for systemic immunotherapy as well as other intralesional agents (i.e., Amgen's T-Vec, Vical's Allovectin-7). In 2014's fourth quarter Agarwala traveled to the III Eurasian Melanoma and Skin Cancers Forum in Suzdal, Russia and the Beijing International Melanoma Congress in China in October, and the Society for Melanoma Research International Congress in Zurich, Switzerland in November to speak about intralesional agents and PV-10. He will be chairing an education session from 8-9:15 am on May 31st at ASCO 2015 entitled Locoregional Therapies in the Setting of Systemic Treatment Advances: What's Next? (part of the conference's Track(s): Melanoma/Skin Cancers; Developmental Therapeutics and Translational Research).
Don't pimp my data (May 22, 2015)
On January 29, 2015, Provectus' Chief Technology Officer Dr. Eric Wachter, PhD met with the FDA to discuss the company's pivotal melanoma Phase 3 trial design (in hopes of finalizing it). Eleven days later, on February 9th, Provectus issued press release Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study. Eric said:
Quote #1: "We are extremely grateful for the careful review conducted by the Agency. The outcome of the review does not affect the fundamental design of the study nor the patient population, but does affect certain details concerning some secondary end points and statistical analysis matters, such as the treatment of missing data. We are making a number of small changes to the protocol in light of this review, and will issue a final version later this month." {Underlined emphasis is mine}He also said:
Quote #2: "We have eight sites, four in the U.S. and four in Australia, in our expanded access program currently using PV-10 for melanoma and other cutaneous malignancies. We expect that they will provide a path to quickly starting enrollment upon completion of the review period. In addition, we have been qualifying additional sites that will join the study pending action by their respective Institutional Review Boards." {Underlined emphasis is mine}On March 12th, 42 days after the January FDA meeting the final protocol was posted on ClinicalTrials.gov. Eric ended up missing his self-imposed end-of-February deadline (from February 9th). Four days later, on March 16th, Provectus issued press release Amended Protocol of PV-10 for Phase 3 Study as Treatment for Melanoma Now Available Online. No management person was quoted, but for this new item a germane sentence was:
The Company does not require additional review from the U.S. Food and Drug Administration(the "FDA") to start the phase 3 study, and has begun the process of gaining approval from the Institutional Review Board (IRB) of each individual site for the amended protocol. {Underlined emphasis is mine}On April 15th, 34 days after the final protocol was posted (on March 12th) and 76 days after the January FDA meeting Provectus issued press release Opens Patient Enrollment; Begins Phase 3 International FDA Comparative Clinical Trial of PV-10 for Melanoma. No management person was quoted, but for this new item a germane sentence was:
The Company is seeking 225 patients and enrollment has begun at St. Luke's University Hospital and Health Network, Bethlehem, PA, the first study site to be opened, with additional sites to be added in the coming weeks and months. {Underlined emphasis is mine}One day later on April 16th, following the associated press release (rather than the other way around) the ClinicalTrials.gov site for the Phase 3 trial was updated to reflect the addition of the St. Luke's site.
On Provectus' May7th conference call, Eric reminded shareholders:
I don’t anticipate that company will provide site by site or patient by patient announcements of study milestones since this will be highly unusual for our industry but we do anticipate providing periodic summaries of study status. And also as noted by Pete, when we open new centers, these will be shown on the NCI clinical trials registry website.Thirty-six days after the .gov site (the NCI clinical trials registry website) was updated for the first trial site of St. Luke's (5+ weeks > "coming weeks") and 113 days after the January FDA meeting no new clinical trial sites have been added. Eric missed another self-imposed deadline of "coming weeks."
My historically-oriented sense is, like with reporting the 2014 calendar year compassionate use program enrollment figure, promptly updating the NCI website is not a priority with Eric (taken, I suppose, in the context of grander or more important [to him] items or process steps). He should have returned from China with Peter today. As a result, I wouldn't be surprised if Eric updates the site next week now that he's back in Knoxville. In reality, however, it may only be through the middle to the end of June ("i.e., "coming months" starting in mid-April) that a more substantive picture of on-line sites becomes available.
Sometimes you have to laugh (May 20, 2015)
On Twitter today:
 |
| Click to enlarge |
 |
| Click to enlarge. Tweet thread |
Last year, presumably before he "knew" Jamie, Brad briefly commented about Provectus -- you know, thoughts one way or another -- around the time of the company's submission and the subsequent denial of its breakthrough therapy designation application to the FDA.
 |
| Click to enlarge. Tweet threads are here. |
 |
| Image source |
In situ vaccination (May 20, 2015)
At SITC 2012 Provectus principals presented the poster titled below. A link to the complete poster is here.
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
"T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient's individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFβ activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFβ neutralization during radiation therapy effectively generates CD8+ T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNγ and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFβ blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti–PD-1 antibodies extended survival achieved with radiation and TGFβ blockade. Thus, TGFβ is a fundamental regulator of radiation therapy's ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFβ neutralization offers a novel individualized strategy for vaccinating patients against their tumors." {Underlined emphasis is mine}
The above combination of anti-PD-1, blockade of TGFβ and radiation should be cost prohibitive; however, the language of radiation being able to generate an in situ vaccine is interesting, and the potential subsequent synergy with immune checkpoint blockade is consistent with industry thought elsewhere.
H/t @bradpalm1: "Fascinating stuff, particularly the observation that radiation therapy upregulates the expression of PD-L1 and PD-1 which may act to ultimately suppress T-cell activation and function."
Osaka + Liver (May 20, 2015)
A second liver-related abstract/poster by Provectus could have been accepted, this time by/at the 6th Asia-Pacific Primary Liver Cancer Expert (APPLE 2015) Meeting. Dr. Paul Goldfarb, MD is a surgical oncologist and the principal investigator of Provectus' liver Phase 1 trial (as well as the expanded liver Phase 1 trial). He also is a member of the company's strategic advisory board. Sharp Memorial Hospital of San Diego is a compassionate use program ("CUP") site.
 |
| Image source, see page 3 of 6 |
Poster Hombre77 on InvestorVillage's Provectus message board wrote "I think he's got it wrong when he associates PVCT's poster presentations with the specific conference sessions referenced above" in regards to Mish Mash (May 19, 2015)'s Cáncer al hígado below. Poster viewing times already were established, and not necessarily associated with scientific agenda sessions. Thank you.
 |
| Click to enlarge. |
Note in the related January 2014 press release:
The lead author, Faraz Kazmi, a Senior Scientist at XenoTech said, “The FDA guidance for industry on drug-drug interactions requires an in vitro assessment of the potential liability for a new drug to engender drug-drug interactions. These experiments are important because they provide the basis for safe, rational clinical trial design and are important determinants toward the ultimate goal, which is patient safety.”While Moffitt Cancer Center may have established through its SITC 2014 poster and associated work that their "...murine studies support combination therapy with IL PV-10 and co-inhibitory blockade," perhaps similar or comparable in vitro drug-drug interaction work has been or is being done to provide the basis for the safe, rational clinical trial design of the combination of PV-10 and an immune checkpoint inhibitor for advanced melanoma.
 |
| Tweet thread |
Rather than taking a biochemistry approach to develop drugs that kill cancer cells, Provectus Biopharmaceuticals (NYSE MKT:PVCT) is using physical chemistry by harnessing the unique properties of Rose Bengal, a first-in-class halogenated xanthene. {Underlined emphasis is mine}I guess it depends on who says it, and how they say it (and I suppose whether they say it): Provectus Biopharmaceuticals reached an agreement with regulators on the design of a pivotal clinical trial intended to get its melanoma drug approved. The phase III study will enroll 225 patients with locally advanced cutaneous melanoma. The patients will be treated with either PV-10 or systemic chemotherapy. When half of the events (disease progression) have occurred, an interim analysis will be performed on the primary endpoint, progression-free survival.
Management's said all of the above, of course, but they haven't said this: If the interim analysis is successful, Provectus will be able to seek accelerated approval in the U.S. They (Peter) just hinted at it. If they know, say it. If they don't (or aren't comfortable or willing to say), don't hint.
 |
| Asia Biotech Invest |
See the link here, and navigate forward using the toolbar at the top of the screen.
Mish Mash (May 19, 2015)
 |
| Click to enlarge. |
Compassionate use. Provectus' compassionate use program ("CUP") was opened in 2009 (June in Australia, October in the U.S., and all patients in Provectus' metastatic melanoma Phase 2 trial were treated by September). It reached a low point of 8 new patients in 2012 before adding double-digit new patients again in 2013. See Compassionate Use/Expanded Access (January 5, 2015) and CUP (January 11, 2015) below. The 2014 figure may be around 50.
Cáncer al hígado. Provectus issued a press release last week and made an associated 8-K filing regarding liver data presentation in July in Spain, Provectus Biopharmaceuticals' Poster Presentation on PV-10 Clinical Data from Phase 1 Study for Cancers of the Liver Scheduled for Thursday, July 2, 2015 at 17th World Congress on Gastrointestinal Cancer. As I speculated in Potpourri (May 1, 2015) below, Dr. Sanjiv Agarwala, MD of St. Luke's University Hospital and Health Network (and a key PV-10 clinical investigator) is the lead author/presenter. Judging from the presentation times (10:30 to 11:00 a.m. and 4:55 to 5:25 p.m. local time) and the conference's scientific program, Agarwala and the poster appear to be in the Esophageal Cancer (which doesn't make sense to me) and Rare Tumors: Neuroendocrine Tumors, Gastrointestinal Stromal Tumors and Anal Cancer sessions, respectively.
Sinopharm. Provectus issued a press release yesterday and made an associated 8-K filing regarding the extension of the memorandum of understanding ("MOU") between the company and Sinopharm, Provectus Biopharmaceuticals Extends for Additional 60 Days Memorandum of Understanding with Sinopharm-China State Institute of Pharmaceutical Industry and Sinopharm A-Think Pharmaceutical Co., Ltd. Notably, in a quote by management in the press release:
"We remain optimistic that a definitive licensing agreement with our Chinese partners can be achieved. The extra time is needed to ensure that nothing in the agreement undermines the legal position of Provectus or Sinopharm under Chinese or American law, and to ensure that the timetable for all deliverables is realistic - and clearly defined taking into account the regulations regarding new drug applications under the Chinese regulatory system." {Underlined emphasis is mine}The underlined portion above makes me wonder what if any aspects of December 2014's 25th U.S.-China Joint Commission on Commerce and Trade influenced Sinopharm-Provectus deliverable discussions. Interestingly, Sinopharm folks apparently also spoke with Moffitt Cancer Center folks:
"Since the signing of the MOU, management of Provectus and senior personnel at Sinopharm-CSIPI and Sinopharm A-THINK have held numerous conference calls, have met face-to-face in both China and the US, and Chinese scientists on staff at Sinopharm have discussed in person PV-10 and its clinical results with the lead investigators at St. Luke's University Hospital and Health Network and Moffitt Cancer Center." {Underlined emphasis is mine}Notable funding. The "bio-Twittersphere" (@JacobPlieth) notes today the venture capital funding of an oncolytic virus company (PsiOxus Therapeutics) as well as a selected pipeline oncolytic virus drug compounds. The table pictured in the aforementioned tweet is copied below.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. c 2014. |
 |
| Click to enlarge. Original October 2014 image source. |
Interestingly, both Viralytics and PsiOxus Therapeutics also are endeavoring to deliver their respective investigational drug compounds intravenously (and not just intratumorally).
Downer (May 18, 2015)
13F filings for the period ending March 31, 2015 showed a decrease in institutional share holdings of Provectus: approximately 4.25 million share owned/held (down from 4.91 million as at 12/31/14, or -13%), and 2.28% of shares outstanding (not a fully diluted figure) (down from 2.65%).
 |
| Click to enlarge |
Life sciences investor Knoll Capital Management, which initiated a position in the quarter ending 6/30/14, tapped out after three quarters.
 |
| Click to enlarge |
Ongoing (May 14, 2015)
I recently had the opportunity to speak with an individual asked by existing Provectus investors to undertake a desk-based review of the company (i.e., read and review publicly available materials about the drug and management team). I believe I understood him to not have previously come across PV-10 or Provectus prior to the request to diligence the situation. His CV described a person with substantial, identifiable and notable business, academic, operational and research experience in both physical and biological sciences in both the private and public sectors.
His perspectives included (paraphrasing):
SAR (May 14, 2015)
When Eric said "Moving forward on the regulatory side now will allow us to move forward in Asia with or without the assistance of the corporate partner," he may have meant that in addition to exploring entry into China directly, there is another gateway into the country. Consider this July 2013 article entitled Hong Kong to become popular venue for pharmaceutical companies to conduct drug tests:
 |
| Image source |
His perspectives included (paraphrasing):
- Provectus' Chairman and CEO Dr. Craig Dees, PhD was well respected at Oak Ridge National Laboratory,
- PV-10 appeared to have significant potential as a cancer therapy,
- Management may need assistance in the form of more experienced managerial personnel to better deal with the FDA, and navigate and manage regulatory affairs, and
- Management has to parallel process at a greater volume and faster rate.
SAR (May 14, 2015)
When Eric said "Moving forward on the regulatory side now will allow us to move forward in Asia with or without the assistance of the corporate partner," he may have meant that in addition to exploring entry into China directly, there is another gateway into the country. Consider this July 2013 article entitled Hong Kong to become popular venue for pharmaceutical companies to conduct drug tests:
"In the old days, most trials were done in the West," says Henry Yau, assistant registrar of HKU's Clinical Trials Centre based at Queen Mary Hospital. "There were two reasons: because the industry was there, and drug companies like to do research in their home countries; and because a US company, for example, might not be able to do a trial overseas - if it wanted to sell a drug in Britain, for example, it would have to repeat the trials there." That all started to change in the mid-1990s, when the International Conference on Harmonisation, a body that combines the medical regulatory authorities of the US, European Union and Japan, produced Good Clinical Practice, a quality standard for the regulation of trials. It "made drug regulation a global business", Yau says, and at a stroke made international trials feasible. {Underlined emphasis is mine}
And:
"In terms of the future in Hong Kong, early drug development is important. In China, recruitment is really fast, so you can easily do a big phase three trial there. So our role in Hong Kong is in phase one and two, and then you go to the mainland or wherever for phase three." {Underlined emphasis is mine}
And:
"The biggest priority to increase the number of trials in Hong Kong is being the gateway to China." Getting drugs approved on the mainland can be complicated, she adds, with a clinical trial certificate from the State Food and Drug Administration taking about a year. In Hong Kong, trial certificates take about three months, but the Hong Kong portion of a mainland trial will only be recognised if a certificate from the FDA is forthcoming. "Efficiency is a competitive advantage of Hong Kong, and under this system, that advantage is not as big as it could be," says Chan. With drug patents only lasting 20 years, a few months can make a big difference to a pharmaceutical company; Chan says the industry would like a mainland FDA-approved certificate issued in Hong Kong. {Underlined emphasis is mine}
Small Molecules (May 13, 2015)
H/t shareholder @bradpalm1 for the article entitled Fire Up the Immune System to Fight Cancers and Boost Portfolios: John McCamant. The article did not discuss Provectus.
H/t the article & link from a shareholder: A December 2012 post from the China Law Blog entitled The China MOU (Memorandum of Understanding). Use Them At YOUR Peril. Dan Harris of Harris & Moure:
Provectus is a sponsor of a conference entitled Melanoma: from basic science to clinical applications, which will be held June 24-26, 2015 in Reykjavik, Iceland. Other sponsors include Bristol-Myers, Amgen and Viralytics (an Australian-based oncolytic virus immunotherapy company). Conference organizers include Dr. Robert Andtbacka, MD of the Huntsman Cancer Institute in Salt Lake City, Utah.
Updated: When I asked Peter what is/was the goal of sponsoring the Iceland melanoma conference, he replied "Really, the same as all the melanoma conferences; namely, enhanced visibility with various KOLs."
Conference Call, part 3 (May 10, 2015)
Some final thoughts and questions about the May 7th 1Q15 conference call are below.
Regarding the pivotal melanoma Phase 3 trial, Eric: "As I've noted previously, we expect enrollment to be approximately one-third from the U.S.; one-third from Australia; and one-third from the rest of the world."
Note Conference Call (May 9, 2015) below. On the company's March 12th conference call for CY 2014 Eric also said, this time on the topic of Provectus' Asia Phase 1b/2 liver trial:
First, Eric confirmed the company was preparing to file the Chinese version of an "investigational new drug" application ("IND") to the sFDA/cFDA (see Silk Route (April 4, 2015) below), which would permit commencing and/or participating in a clinical trial in the country.
For China the standard of care ("SOC") would be the local therapy of choice (e.g., radiofrequency ablation ["RFA"], transcatheter arterial chemoembolization ["TACE"], etc.), while the Western SOC would be sorafenib. A 2012 sample paper is Efficacy of radiofrequency ablation to treat advanced hepatocellular carcinoma (Wu et al., Peking University Cancer Hospital and Institute).
Fourth, in my view, there is "deal daylight" between Provectus and Sinopharm, which I'm not sure will be resolved by the May 16th expiration date of memorandum of understanding ("MOU") between the two companies such that a deal would be consummated.
Peter said on the May 7th call:
Provectus held its 1Q15 conference call on Thursday, May 7th. A surprisingly poor quality transcript from Seeking Alpha is here. Details for hearing a replay of the conference may be found here.
On the company's March 12th conference call for CY 2014, Eric said on the topic of combining PV-10 with an immune checkpoint inhibitor for advanced melanoma:
Swap out Bristol-Myers' ipilimumab for Merck U.S.' pembrolizumab, and most of Eric's "very concrete and interesting things" from the May 7th call already were said by Agarwala on April 9th, and by Peter on March 3rd (see Updated presentation slides (March 3, 2015) and the particular slide {purple markup is mine} below).
Eric's pertinent comments from the May 7th call were:
Takeaways & Questions: It's likely Moffitt Cancer Center is at least one of the lead clinical sites (and Dr. Jeffrey Weber is likely one of the lead investigators). Would/could the Phase 2 trial be a pivotal one? If so, wouldn't the FDA have to review and opine on this trial's protocol? Management has costed the Phase 1b trial at about $1 million to carry out. I would imagine having a Big Pharma partner only for a melanoma combination therapy program is inefficient and not strategically acceptable to management. A more comprehensive program involving the combination of PV-10 and pembrolizumab for other indications (e.g., non-small cell lung cancer, prostate, renal cell carcinoma, etc.) would be.
The letter Q (May 7, 2015)
In advance of today's quarterly conference call after the stock market closes, Provectus made its 1Q15 quarterly filing (and issued an associated press release and made an associated 8-K filing). What was/is of initial note and interest to me follow:
1. During the first quarter, the company raised gross proceeds of $776,000 via the open and existing Network 1 private placement ($15 million "face value" vehicle. I believe the money was raised (i.e., taken in/subscriptions accepted) at the end of March (i.e., last week of the month). Money was last raised at the end of the fourth quarter (i.e., taken in December 31st). The balance of available vehicle is about $6.4 million.
Updated: Takeaway: Peter did not raise a sum of money that mitigated or paid for the quarter's operating burn, as he has done in the past. He also is not carrying a cash balance as high as he has in the past: $14,170,733 as at March 31st vs. $17,391,601 as at 12/31/14, $17,773,680 as at 9/30/14, $18,126,036 as at 6/30/14, $16,648,916 as at 3/31/14 (Provectus moved the NYSE MKT in May 2014). The other side of the trade might say he could not raise the money. The other side of the other side of the trade might say he believes he does or would not have to (see 3. below).
2. Monthly cash burn for the first quarter appears to be $1.395 million, which is flat to 4Q14 ($1.392 million), which was 20% higher than 3Q14, which was a tad higher than 2Q14, which was about 20% higher than 1Q14, which was flat to 4Q13, which was 37% higher 3Q13. Takeaway: Peter is not running a $1 million-a-month cash burn.
3. In the May 7th press release management added the sentence, "The sale of common stock was reduced since we are seeking to minimize dilution to our existing stockholders where practicable by limiting the issuance of our equity securities."
4. Contrasted with the March 12th press release in which Provectus was "...a development-stage oncology and dermatology biopharmaceutical company," it now is "...a clinical-stage oncology and dermatology biopharmaceutical company." {Italicized emphasis is mine}
5. Forward-looking statements in the May 7th PR were revised from "...to license PV-10, our melanoma drug product candidate, and other solid tumors such as liver cancer..." to "...to license PV-10, our melanoma drug product candidate, and other solid tumors such as cancers of the liver..." {Italicized emphasis is mine} Management said cancers of the liver comprises primary (hepatocellular carcinoma) and secondary (metastatic) liver cancer.
6. Management changed "will" from the 2014 10-K to "should" in the 10-Q. See below:
We believe our continued development of PV-10 with existing funds should yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of solid tumor indications due to its unique immuno-chemoablation mechanism of action known as ablative immunotherapy or oncolytic immunotherapy. The primary ablative mechanism of PV-10 is followed by a secondary immunomodulatory mechanism. Likewise, we believe our development of PH-10 with existing funds should yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of inflammatory dermatoses due to its unique non-steroidal anti-inflammatory mechanism of action. {Italicized emphasis is mine} Management also included broader mechanism of action categorization or labelling: i.e., ablative immunotherapy (recent labeling of PV-10) and oncolytic immunotherapy (that some folks like MD Anderson's Dr. Merrick Ross use to describe PV-10, and Amgen's T-Vec without the virus aspect).
7. As noted in Stock Option Exercises (January 8, 2015) below, "One employee of the Company forfeited 300,000 stock options on January 7, 2015." I believe the employee is Craig.
8. In regards to the Stipulated Settlement Agreement and Mutual Release (the “Settlement”), "$266,667 was repaid by the Executives as of March 31, 2015." This reduced balance sheet item Short-term receivable — settlement from $733,333 as at 12/31/14 to $466,666 as at 3/31/15.
Updated: [@alanrobertross noted this:] 9. "During the three months ended March 31, 2015, 3,693,898 warrants were forfeited." I had read this, but didn't think it important.
Data (and details) are needed (May 5, 2015)
If [as I wrote in my March 2015 follow-up investment letter] early-stage drug development company described Provectus from 2002 to 2015, and 2015 marks the beginning of the Company’s transition into a regulatory-focused and data-driven organization, then Provectus needs to generate data as well as details about data it (and I think, reasonably, third parties) will generate (e.g., trial sites, trial protocols, Moffitt pre-clinical and clinical data, etc.).
As I wrote in Potpourri (May 1, 2015) below I hope Eric delivers on the expectations and timing he set during the March 12th conference call: concrete details of the proposed Asian (Chinese) Phase 1b/2 study of PV-10 plus standard care for hepatocellular carcinoma, and a potential Phase 1b/2 study of PV-10 and an immune checkpoint inhibitor, among other things.
Provectus issued a press release and filed an associated 8-K today regarding allowance of the company's synthesis patent application by the European Patent Office, and issuance by the Japanese Patent Office. The patent was issued by the U.S. Patent & Trademark Office in 2013, and the application was allowed by the Chinese Patent Office earlier this year.
The company appears to have selected a new PR firm (Oglivy?). Upgrading this function is nice, but data generation is much, much nicer.
Eric (and maybe Peter) may be attending the 2015 Accelerating Anticancer Agent Development and Validation Workshop, May 6-8 in Bethesda, Maryland; I believe Eric (and Jamie, and maybe Peter) attended the 2014 workshop: "The workshop is designed for both scientists and consumer advocates with clinical trial experience that have an interest in new approaches to developing or enhancing agents or combinations of agents for the diagnosis, treatment or prevention of cancer."
It's possible Provectus may be using Quintiles as its contract research organization ("CRO") for the melanoma Phase 3 trial.
"So what’s driving the increase?" (May 4, 2015)
Morrison & Foerster, April 2015:
It's an oldie, but a goodie (May 3, 2015)
H/t a shareholder, who wrote:
In 2013 Moffitt Cancer Center noted IFN-gamma production in murine model work involving PV-10, but IFN-gamma is a type II interferon. In 2014 Moffitt further noted from their mousie work that PV-10 led to HMGB1 release.
As an aside, a reminder of that 2014 work (see my March 9, 2014 blog post), which noted:
But, the TDLNs are immune-privileged sites. "Immune responses and associated inflammation in certain parts of the body, including brain, eye, testes, placenta, and fetus, carry a high risk of lethal organ dysfunction or reproductive failure. These tissues, which have evolved to be protected, to a variable degree, from immune responses, are called immune privileged sites."
Once cancer cells from upstream tumors enter downstream lymph nodes (i.e., enter the TDLNs), the disease becomes ever so more dangerous. This infiltration of the TDLNs by diseased cells, and thus into an immune-privileged area where a protective immune response to the invaders is not sufficiently or strongly initiated, allows the cancer cells (I think) to grow much more unfettered and un-fought by the body's immune system than they potentially would elsewhere in the body. "Lymph nodes that lie immediately downstream of tumors [tumor-draining lymph nodes (TDLNs)] undergo profound alterations due to the presence of the upstream tumor. The antigen-presenting cell population in TDLNs becomes modified such that tumor-derived antigens are cross-presented by host cells in a tolerizing fashion. In addition, the number and suppressor activity of regulatory T cells (Tregs) are increased in the TDLN. Emerging evidence suggests that some of these Tregs may be generated de novo against specific tumor-derived antigens, and thus they arise as a direct consequence of antigen presentation in the TDLN. Others may represent Tregs against self-antigens, which undergo preferential activation in the tolerogenic milieu of the TDLN. The TDLN thus becomes an anatomic context in which presentation of new antigens not only fails to elicit a protective immune response but also actively creates systemic tolerance. In this regard, the TDLN displays features analogous to classical immune privilege. Accumulating evidence thus suggests that the TDLNs, although small in size, may exert a profound tolerizing influence on the rest of the immune system. These mechanisms will need to be interrupted in order for clinical anti-tumor immunotherapy to be successful." {bolded emphasis is mine}
Thus, preventing the TDLNs from naturally suppressing what otherwise would be a normal immune response to tumor fluid or fragments or discharge emanating from an upstream tumor would be key, as noted above, to more successful anti-tumor immunotherapy: "Regional lymph nodes are the first site for melanoma metastases. The sentinel node (SN), on the direct lymphatic drainage pathway, which usually harbors first metastases, demonstrates significant suppression in its ability to respond to antigenic stimulation...Antigen presentation by dendritic cells (DCs) is the most potent means to initiate a T cell immunity." So, if an otherwise immune-privileged site like the TDLNs are unable to mount an immune fight or response, success likely would be achieved by encouraging the infiltration of DCs into these lymphoid organs (i.e., the TDLNs) to start and continue the fight.
But, is a systemic immunotherapy able to achieve the recruitment of DCs to the TDLNs, or is a local therapy better suited? "In recent years, it has become apparent that immunoregulatory processes influence cancer development. The key players in tumor progression are mainly present in the microenvironment of the tumor and the draining lymph nodes. Interventions aimed at shifting tumor-promoting actions toward effective tumor-eradicating immunity are thus foremost required locally. As immune-modulating therapy has been shown to cause many adverse side effects when administered systemically, we strongly advocate the further development of local treatment for cancer immunotherapy" {Bolded emphasis is mine}
As Craig has said for a while the path to defeating cancer, a systemic disease, is through local therapy that has systemic properties. Rapid ablation of injected tumors upstream from the TDLNs creates tumor fragments (antigens) that are taken up from antigen presenting DCs that infiltrate the TDLNs to create an immune response in an area that desperately needs one.
Stanford's Dr. Peter Lee, M.D. noted that: "Whether TDLNs are invaded by cancer cells is a key prognostic indicator for patients with breast and other cancers. Lymph nodes are immune organs and TDLNs are key sites of tumor-immune interactions...TDLNs may be immunologically altered and demonstrated the clinical significance of T cell and dendritic cell (DC) decreases in predicting relapse in breast cancer." Or, here too.
PV-10's rapid ablations fights cancer's foot soldiers on the front lines (i.e., the injected tumors). By getting the body to send its own solders into the TDLNs to fight cancer cells there, metastatic disease around the body has its supply lines cut. PV-10 breaks tolerance. Is Moffitt showing antigen presenting DCs migrating (infiltrating) into the "tolerizing" environment of a TDLN and overcoming tolerance (resistance)? Have they figured the mechanism to overcoming tolerance (resistance); that the migration of antigen presenting DCs is what breaks tolerance? Of course, the sentence in the Moffitt 2014 abstract is based on their murine model (pre-clinical work).
The Doctor Is In (May 2, 2015)
The American Academy of Dermatology designated the month of May as National Melanoma Skin Cancer Prevention Month. The Provectus-sponsored video below appears to be an interview of St Luke's University Health Network's Dr. Sanjiv Agarwala, MD for other media persons then to potentially interview him to further discuss:
With the start of Provectus' pivotal melanoma Phase 3 trial specifically, or perhaps more broadly the transition from an early-stage drug development company into a regulatory-focused and data-driven organization, I think it's time (long overdue, but I'm being very generous) that management improve their media relations function: i.e., swap out Bill Gordon for a more nationally recognized firm/person(s) that is/are dramatically better.
Potpourri (May 1, 2015)
Allspice. On Thursday Provectus issued a press release and filed an associated 8-K regarding presentation of liver cancer data (both primary [hepatocellular] and secondary [metastatic]) at the European Society for Medical Oncology's ("ESMO's")17th World Congress on Gastrointestinal Cancer in Barcelona, Spain from July 1-4. The poster presentation's title is Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver. The abstract should be available on June 24th. Management discussed this on March 12th when they wrote: "The Company expects to report initial data at one or more international cancer conferences this summer." Aside from Provectus' Dr. Eric Wachter, PhD, I imagine the abstract/poster's co-authors would include the investigators at the liver cancer trial sites: Sharp Memorial Hospital's Dr. Paul Goldfarb, MD (San Diego), The Southeastern Center for Digestive Disorders & Pancreatic Cancer's Dr. Alexander Rosemurgy, MD (Tampa) and St Luke's University Health Network's Dr. Sanjiv Agarwala, MD. I imagine Dr. Agarwala would be the "lead" author.
Cinnamon bark. Judging from Provectus' Calendar of Events for September, if accepted, additional liver data may well be presented at the 18th European Cancer Organization ("ECCO") – 40th ESMO European Cancer Congress in Vienna, Austria from September 25-29. The "lead" author for this abstract/poster should be a key opinion leader relatively new to the company (I believe I've confirmed the name outside of speaking to management), and who is an interventional radiologist. "Interventional radiology uses minimally invasive image-guided procedures to diagnose and treat diseases in nearly every organ system." By contrast, Goldfarb and Rosemurgy are surgical oncologists, and Agarwala is a medical oncologist.
Cloves. Provectus may have engaged the investment banking part of Maxim Group to assist the company with exploring, negotiating and/or consummating a regional license transaction for PV-10 in India. The Maxim i-banker may be Ritesh Veera. While I cringe at the mention of this M-word (call it a reflexive [almost natural] action), I hope the i-banking side of the firm treat's Provectus — as, you know, a client — better than its broker side did in 2012.
Lavender flowers. Speaking of Maxim: Even though Provectus is part of Maxim equity research analyst Jason Kolbert's coverage universe (as of April 26/27th), and even though Provectus' notes on its website analyst coverage by Maxim, I don't believe Kolbert has yet issued a research note on the company (unless he's done so only to institutional investors privately or confidentially). The last note on Provectus Maxim (and from any equity research analyst) was, I believe, in February 2014 by Dr. Echo He, MD, PhD (a private one meant for institutional investors and clients, and which was sent to me by another shareholder)(see below).
Reading Dr. He's note at the time, it appeared she was skeptical Provectus would be successful with its breakthrough therapy application because (i) the 28-patient subgroup might have been considered a limited sample size and (ii) the retrospective analysis of symptom relief might have been considered guidance for further clinical studies rather than stand-alone evidence.
Since Wall Street for the most part is a pay-to-play business, a successful India transaction via Veera (and thus potential fees to Maxim) might lead to a note from Kolbert. Cantor Fitzgerald likely won't initiate research coverage until Provectus draws down on its equity facility.
Marjoram leaves. "Investment bank" New Jersey's Network 1 Financial and Chinese investment bank Tririver Capital appear to be representing Provectus in China, and Maxim may be repping the company in India, who is Provectus using for Brazil (and perhaps more broadly South America)? I don't think they have representation yet in presumed conversations with international and domestic pharmaceutical companies. Updated 5/3/15: Management said they had investment banking representation in Brazil from a local firm.
Orange peel. Management removed slides from both Dr. Argawala and Eric's April 9th panel discussion presentations in New York that were later posted on the company's website. I am embarrassed for them by the decision made, action carried out, explanations provided and verbs used therein. I hope Eric delivers on the expectations and timing he set during the March 12th conference call: concrete details of the proposed Asian (Chinese) Phase 1b/2 study of PV-10 plus standard care for hepatocellular carcinoma, and a potential Phase 1b/2 study of PV-10 and an immune checkpoint inhibitor — "That’s why I mentioned in my opening comments that we expect to have a very concrete and interesting things to present on this particular topic when we have the next meeting, this next conference call."
Annual Meeting Proxy (May 1, 2015)
Provectus filed its Form 14A in advance of its June 19, 2015 annual meeting, which will be held in Orlando this year (rather than its historical location of Knoxville). Updated 5/1/15: H/t a shareholder...The 2015 AAPI Convention will be held in Orlando from June 17th to 21st.
The three "core" questions being put to a vote are (as they have been from year to year): (i) to elect directors to serve on the company's board for a one-year term, (ii) to conduct an advisory vote to approve the compensation of the principals (officers), and (iii) to ratify the selection of BDO as Provectus' auditor.
Upcoming May option exercise dates from page 18 include:
April Blog Stats (May 1, 2015)
Intralesional therapy for metastatic melanoma (April 30, 2015)
H/t a shareholder: Intralesional therapy for metastatic melanoma, December 2014, Vol. 15, No. 18 , Pages 2629-2639 (doi:10.1517/14656566.2014.967682), Sarah Sloot, Omar M Rashid, and Jonathan S Zager
So you're saying they're already obsolete [as monotherapies]? (April 28, 2015)
{Tongue somewhat in cheek} Dr. Sally Church, PhD: Where do we go beyond monotherapy with immune checkpoints?
Bag End (April 27, 2015)
The FDA re-posted its review of Amgen's talimogene laherparepvec in advance of Wednesday's advisory committees meeting. The original review is here. The revised review, which contains questions for discussion and one voting question, is here:
Mixed Bag (April 27, 2015)
The FDA provided materials in advance of Amgen's talimogene laherparepvec's ("T-Vec's") 2015 Cellular, Tissue and Gene Therapies Advisory Committee meeting (see here). Subsequent to its initial upload, the Agency removed its T-Vec review, which I was able to download before it was taken down. The document, entitled Cellular, Tissue, and Gene Therapies Advisory Committee and Oncologic Drugs Advisory Committee Meeting, April 29, 2015, BLA 125518, talimogene laherparepvec (Amgen), made for a useful and insightful read. The advisory committees meeting — a joint meeting of the Cellular, Tissue and Gene Therapies Advisory committee ("CTGTAC") and Oncologic Drugs Advisory Committee ("ODAC") — is on April 29th from 8 am to 6 pm.
T-Vec's pivotal Phase 3 trial focused on patients with unresectable stage IIIB, stage IIIC or stage IV melanoma; that is, advanced melanoma (and not locally advanced cutaneous melanoma, PV-10's indicated patient population). Stage IIIB/C patients comprised 30% of T-Vec's trial's patient population.
For now, I walk away with the following (in no particular order; generally and specifically related to T-Vec, and Provectus/PV-10):
1. Leading up to the FDA review, questions surrounded T-Vec's Phase 3 trial's primary endpoint (i.e., durable [objective] response rate ["DRR"]), complete response ["CR"] or partial response ["PR"] maintained for at least 6 months, and beginning at any point within 12 months of initiating therapy) and comparator (i.e., control arm patients were treated with GM-CSF).
2. The Agency highlighted several concerns with the trial's design and execution, including:
3. The FDA commented: "The definition of the primary endpoint allowed a subject to be counted as “durable responder” (DR) even if the subject developed new lesions, relapse, or progression of disease after the 6- month period when the durable response was recorded (See Section 6.1)." {Underlined emphasis is mine}
H/t shareholder @bradpalm1 for the article entitled Fire Up the Immune System to Fight Cancers and Boost Portfolios: John McCamant. The article did not discuss Provectus.
TLSR: As drug, antibody and cellular therapeutics become more specialized to specific or orphan diseases, the costs of the therapies will go up as the specific patient population goes down. Are the Centers for Medicare & Medicaid and private payers going to pay these higher rates if the patient can be treated more effectively the first time?
JM: Yes. Over the long term, we'll have a bigger question. We will be so effective at treating patients with the specific diseases that we will have to make difficult decisions when it comes to allocating resources. But I really believe that the cost of goods sold (COGS) will be very important. We discussed this topic in the April 30, 2015, issue of Medical Technology Stock Letter, particularly when it comes to immunotherapy or combinations of three, four drugs. A small molecule inherently gives the investor and, of course, the patient, a much better cost of goods than almost any other therapeutic modality. Small molecules also tend to be safer, and they offer convenience in that you may be able to keep cancer patients out of infusion centers for many years. We think small molecules are going to be one of the keys to making immunotherapy more accessible and more broadly available, because of their cost and ease of use. {Underlined emphasis is mine}And:
TLSR: Anthera has a $170M market cap right now. Is this large enough for institutions to start taking positions?
JM: It is on the edge right now. Given the Phase 3 nature of the story, we are seeing some institutions begin to nibble at Anthera, basically because they see the valuation disconnect. You have a Phase 3 name that's out of favor, and if b-mod does work, the risk/reward is outstanding. {Underlined emphasis is mine}
ASCO 2015 abstracts now are available here. Amgen's talimogene laherparepvec's abstracts are here. Of initial note to me was Survival, safety, and response patterns in a phase 1b multicenter trial of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma, and:
The data cutoff was Dec 22, 2014 with all patients at least 17 months from start of treatment. 18 patients received T-VEC+ipi; grade 3/4 treatment-emergent AEs occurred in 32% and grade 3/4 immune-related AEs occurred in 2 patients with no treatment-related deaths. Per irRC, ORR was 56% (33% CR) and DRR was 44%. Median time to response was 5.3 months (range 2.6-5.7). Median progression-free survival (PFS) was 10.6 months (2.6-19.3+). Median overall survival (OS) was not reached; 12-month and 18-month survival were 72.2% and 67%. On a lesion level, 24 and 11 of 35 injected index lesions and 8 and 5 of 16 uninjected index lesions regressed ≥ 50% and 100%, respectively. {Underlined emphasis is mine}
Complete response ("CR") (by modified WHO) for T-Vec's pivotal metastatic melanoma Phase 3 trial was 11% [f/n 1]. CR for one of ipi's pivotal metastatic melanoma Phase 3 trials was 1.5% [f/n 2]. The T-Vec+ipi poster from ASCO 2014 concluded: "In consideration with published reports, these data, although preliminary, suggest higher CR and OR rates than either agent alone and earlier responses after ipi initiation during T-VEC+ipi than with ipi alone."
T-Vec's melanoma Phase 3 trial's 12-month overall survival ("OS") rate was 73.7% and ipi's was 45.6%, compared with the combination's 72.2%. The 18-month OS rate of the combination was 67%, while ipi's was 33.2% and eyeballing T-Vec's was 68% (f/n 3, page 44); a 24-month figure was reported for T-Vec.
The China MOU (May 12, 2015)Updated 5/14/15: Since preliminary results were presented at ASCO last year there has been criticism these results should not be given too much credence because of the very small number of patients (i.e., 18). Notwithstanding, the work illustrates the paradigm that an intralesional therapy that elicits a T cell response may offer potent synergy with T cell-based systemic immunotherapy.
Updated 5/14/15: T-Vec exhibited a trend toward survival benefit in its Phase 3 trial on which further statistical doubt was cast by the FDA's analysis for/during the CTGTAC/ODAC meeting at the end of April. Nonetheless, both analyses (the original, and the adjusted) indicated a trend toward benefit, so it's likely the combination of T-Vec and ipi should as well. It is growing in understanding there is delayed response for immunotherapies, so early OS results like the above should be interpreted with caution. And, of course, comparing OS between studies is nearly impossible, particularly in this case because T-Vev's Phase 3 trial enrolled about 25% Stage III patients while the ipi study was less than 3% Stage III. The early demographics for the combination study suggest a patient population similar to that of T-Vec's Phase 3 study. Until the randomized phase data are reported the only strong conclusion one might be able to make from these early data is that it appears there's no negative impact on OS. It would seem likely improved response rate data for the combo may translate to OS benefit as well, but this remains to be proven.
H/t the article & link from a shareholder: A December 2012 post from the China Law Blog entitled The China MOU (Memorandum of Understanding). Use Them At YOUR Peril. Dan Harris of Harris & Moure:
"In the common law tradition like that in the United States, an MOU means little. Only a signed deal really counts. This is not true in the civil law tradition. In the civil law tradition, there is the concept of good faith negotiation. Under that concept, it is not acceptable to simply walk away from an MOU if that would constitute “bad faith.” Common law lawyers hate the concept, but it is deeply ingrained into the civil law tradition. In fact, it is a core concept in the Chinese contract law of 1999. Since the traditions are so different, you can see where conflicts may arise.
...
In practice, the Chinese side often will try to turn an MOU into a concrete commitment when it suits them and ignore it when it does not. This is how most people behave and it should be no surprise. The problem is that under Chinese law the Chinese side might be justified in insisting that the MOU is binding if the behavior of the foreign side constitutes bad faith."From Dan's June 5, 2013 post China MOU. Like I Really Care.:
"AK (interviewer): So you don’t feel like there’s much weight to a press release or an announcement or a photo opportunity around an MOU?
DH (Dan): I feel like there’s no weight. In fact, my first reaction is, why are you announcing something that may or may not happen down the road? If something’s really going to happen down the road — if something’s really imminent down the road — then why not just wait until you’ve gotten down the road? Why are you taking the risk of acting as though some big thing has happened, and then two or three months later what are you going to do when it really doesn’t come to fruition? Are you going to issue another press release saying, “oh we were just kidding three months ago,” or are you going to do what most companies do, which is just say nothing?
...
AK: In your blog you do warn that MOUs with Chinese organizations can sometimes actually be more than they appear.
DH: That’s exactly right. That’s sort of the flip side of all of this, and that is that MOUs, in North America but even more so in China, sometimes are not really MOUs—they’re contracts. And the American company doesn’t realize that. We’ve had companies come to us and say “hey, could you help us with this MOU and then we’re going to have to figure out what to do to by way of a contract.” And then we look at the MOU and we tell them that under Chinese law this [what they thought was an MOU] is a contract. And a lot of times in those instances, that’s how the Chinese company views the MOU, and the American company didn’t even realize it. So they’ve gone over [to China] and signed something without the authority of the higher-ups in their own company, and that something they signed is a two-or three-year contract that they nobody ever really approved. So that’s the flip side of all of this. Just calling something an MOU doesn’t mean it’s an MOU as is commonly defined by North American business people."From Dan's June 7, 2013 post China MOUs. I Really Do Care. Deeply.:
But for some reason, a number of readers missed this aspect of the post and I ended up getting hit with a flurry of emails pointing out how I was wrong to be so flippant about MOUs with China. To the extent my interview was not clear, I accept the criticism and I want to clarify. The point of my interview was to note how outsiders to an MOU have no idea regarding the meaning of the MOU as such documents can range from “hey let’s cooperate” all the way to a binding deal. Generally though, if a company is going to announce an MOU, it means that they likely do not have a binding deal (or at least do not realize that they have a binding deal). Put simply, if the company has a binding deal or thinks it has a binding deal, why bother announcing an MOU?
The post was not intended to minimize the importance of the MOU that you may go off and sign. Far from it as these documents can be critical to anyone doing business in China.
...
China MOUs can matter. China MOUs can be important. China MOUs can be binding. Believe it."From Dan's April 2014 post Negotiating With Chinese Companies. The Pros And Cons of MOUs.:
"MOUs are common with China business for the simple reason that Chinese companies love them. But why do Chinese companies love them? In our experience, we see them used typically to achieve the following two things:
1. To memorialize in writing the existing state of the agreement before the underlings at the Chinese company pass it on to their boss or bosses for approval. We frequently see this at large Chinese companies, particularly SOEs
2. To memorialize in writing the existing state of the agreement and then to use that written document as a starting point for additional negotiations intended to favor the Chinese company only.
If you are negotiating with a Chinese company that insists on an MOU, you should try to discern the reason the MOU is so important and if it is for reason number two above, you should make clear that once the MOU is signed, you will not be in a position to re-negotiate critical terms and you should stick by that statement."Where? (May 11, 2015)
Provectus is a sponsor of a conference entitled Melanoma: from basic science to clinical applications, which will be held June 24-26, 2015 in Reykjavik, Iceland. Other sponsors include Bristol-Myers, Amgen and Viralytics (an Australian-based oncolytic virus immunotherapy company). Conference organizers include Dr. Robert Andtbacka, MD of the Huntsman Cancer Institute in Salt Lake City, Utah.
Updated: When I asked Peter what is/was the goal of sponsoring the Iceland melanoma conference, he replied "Really, the same as all the melanoma conferences; namely, enhanced visibility with various KOLs."
Conference Call, part 3 (May 10, 2015)
Some final thoughts and questions about the May 7th 1Q15 conference call are below.
Regarding the pivotal melanoma Phase 3 trial, Eric: "As I've noted previously, we expect enrollment to be approximately one-third from the U.S.; one-third from Australia; and one-third from the rest of the world."
I do not believe Eric noted this previously.Peter: "Given our current rate of expenditures and our ability to curtail or defer certain controllable expenditures, I reiterate what I said during our last call, we do not anticipate needing to raise additional capital to further develop PV-10 on our own to treat locally advanced cutaneous melanoma, cancers of the liver, recurrent breast cancer, bladder cancer, lung cancer, pancreatic cancer, and other indications because we plan to strategically monetize PV-10 through appropriate regional license transactions, license PH-10 for psoriasis and other related indications described as inflammatory dermatoses and also complete the spin-out of Pure-ific Corporation and the other non-core subsidiaries."
It would seem to me Peter raised money via Provectus' Network 1 Financial private placement vehicle after his last call, which was March 12th. If I am correct, he raised money at the end of March when I believe he accepted subscriptions, albeit notably a quarter or less of what he raised the two previous times when he sought to roughly offset the company's quarterly cash burn rate. See The letter Q (May 7, 2015) below.Peter: "I am honored to now ask Eric to continue with the conference call and his prepared comments before we take questions from those on the call."
Why is Peter honored?Eric: "Enrollment is also continuing under our extended access protocol for PV-10 with well over melanoma patients having received PV-10 in the U.S. and Australia."
How many new patients were enrolled/treated in the compassionate use program for calendar year 2014?Peter: "It's very gratifying with all the people that we recognize and particularly recognizing Eric and his cofounders Craig Dees and Tim Scott."
Why is he recognizing Provectus' co-founders now? (Don't they have to score the touchdown or win the game first?)Peter: "The innovation that has been landmark and the industry point frankly with this type of compound was resolved and to be able do this work now in the final phase, there are so many opportunities before us and coming to get us is quite profound."
Landmark, and profound? I've written myself PV-10 (presumably the innovation Peter is referring to) "...exemplifies innovation over incrementalism, meaningful over marginal, productized technology over hypothetical, and changing the world over accepting the status quo, with not an insignificant amount of serendipity over contrivance. In sum, these form the quintessential essence of a paradigm shift in the treatment of cancer," but I'm just some guy on the Internet writing a blog. It's a little odd and perhaps a little interesting for Peter to publicly say this.Peter: "These by far the most exciting times of the company and we're very much looking forward to literally these coming weeks and months this quarter, next quarter, literally this year."
Literally these coming weeks? Alrighty then.Eric: "I would speculate that we can see that what was clearly very strong headwinds a year ago are maybe abating here in Washington and that presumably bodes well for future success with PV-10."
KeyConference Call, part 2 (May 10, 2015)
Note Conference Call (May 9, 2015) below. On the company's March 12th conference call for CY 2014 Eric also said, this time on the topic of Provectus' Asia Phase 1b/2 liver trial:
Prepared remarks: In addition, our Phase 1 study of PV-10 for liver RECIST continuing to recruit patients, in particular those with tumorous metastatic liver. This work is continuing to provide valuable insight into potential additional areas of development since a range of cancers are metastasized to liver. It is also continuing to be supportive of our Pivot Asia for hepatocellular carcinoma or HCC and is a key driving force behind our partnering activities in China and elsewhere.
We expect to report initial data from this study at one or more international cancer conferences in the summer. We are also in continued dialogue with the key opinion leaders in Asia to guide – of our proposed phase 1b/2 study of PV-10 plus standard care for HCC. As with our melanoma combination study, I expect to have very concrete details to discuss at our next quarterly conference call. {Underlined emphasis is mine}Eric's pertinent comments from the May 7th 1Q15 conference call were:
Additionally, our Phase 1 study of PV-10 for liver tumor has continued to recruit patients at our clinical sites in the U.S. especially those with tumors metastatic to liver and is providing valuable insight into potential additional areas for development, since a range of cancers metastasized to liver.
We also expect this study to be instrumental in achieving our Pivot Asia for hepatocellular carcinoma or HCC and it is playing an important role in our partnering activities in China and elsewhere. As Pete noted, we will report initial data from the study at the ESMO Congress on Gastrointestinal Cancer in early July with additional data presentations possible during the second half of the year.
We've also begun the preparatory process for regulatory filing necessary to begin clinical work for this indication in China, in support of an anticipated corporate partnership as well as to begin enrollment for the Phase 3 melanoma study.
With regard to HCC planning and design of our post Phase 1b/2 study of PV-10 plus standard of care for HCC remains unchanged. Moving forward on the regulatory side now will allow us to move forward in Asia with or without the assistance of the corporate partner.{Underlined emphasis is mine}In my view, I don't think Eric met the expectation he set of sharing very concrete details. He did, however, provide more process details (along with Peter) that help frame for me the company's potential entry into China.
First, Eric confirmed the company was preparing to file the Chinese version of an "investigational new drug" application ("IND") to the sFDA/cFDA (see Silk Route (April 4, 2015) below), which would permit commencing and/or participating in a clinical trial in the country.
Prepared remarks: "We've also begun the preparatory process for regulatory filing necessary to begin clinical work for this indication in China, in support of an anticipated corporate partnership as well as to begin enrollment for the Phase 3 melanoma study."
In response to a question: "As I mentioned in my introductory remarks, we are working to prepare the regulatory filings to allow that process to commence in China and that’s very -- keen to filing an IND in U.S but with the Chinese format for that particular document. We are working with a couple of CROs to get our investigational drug documentation from U.S. into the corporate format so that IND can be conveyed to the Chinese FDA in appropriate structure." {Underlined emphasis is mine}Second, at least as I read it in the context of the growing familiarity and potential relationship between Beijing Cancer Hospital's Dr. Jun Guo (Beijing Cancer Hospital is also known as Peking Cancer Hospital), Provectus and Eric have their local medical institution [site] that would conduct/participate in a PV-10 trial and whose institutional review board ("IRB") would accept or not disapprove of the IND for it to be effective (along with the acceptance or non-disapproval of the cFDA).
Eric, in response to a question: "I would like to make an additional comment on the topic of China. Of course as I mentioned it's a valuable corporate endeavor from the partnering and financial side, but from the clinical development side, it's a compelling story, both for our melanoma indication and for hepatocellular carcinoma. So we're doing an appropriate thing right now which is moving forward in a position that will allows us to get into China with or without a partner in China."
In response to a question: "So, the liver protocol Asia, the concept for that study has been further developed. The consummation of that since it is in Asia specific study, hinging principally on China is somewhat longer horizon than we might have anticipated a year ago or even six months ago where we developed a full appreciation of the regulatory climate in China which just as the U.S. is a moving target. That being said, again I will reiterate my comments earlier that from the clinical development as to appropriate patients to allow clinical development to move swiftly and potentially to make important difference in China. That being said, we think that that will help to provide a compelling argument in each side of the process." {Underlined emphasis is mine}Third, the framework or outline of a Chinese Phase 1b/2 trial for hepatocellular carcinoma already had been presented, by Agarwala at the April 9th panel discussion in New York, and thus Eric said on the May 7th call "With regard to HCC planning and design of our post Phase 1b/2 study of PV-10 plus standard of care for HCC remains unchanged" (see "Oops" (April 10, 2015) below).
 |
| Click to enlarge. |
Fourth, in my view, there is "deal daylight" between Provectus and Sinopharm, which I'm not sure will be resolved by the May 16th expiration date of memorandum of understanding ("MOU") between the two companies such that a deal would be consummated.
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
Prepared remarks: "We met with Sinopharm executives last month in Shanghai and expect further discussions this month which we expect to announce prior to the current MoU expiration, May 16. We continue to work with potential partners in China and elsewhere to enter the optimal transaction possible for stockholder, patients and PV-10 development."It does seem the Sinopharm-Provectus "relationship" and efforts to achieve a mutually acceptable deal are productive (i.e., both parties continue to talk) and may not result in a deal, and that there also may be deal/commercial interest from another party in China (if you believe Peter). The question, it seems, is price.
Peter, in response to a question: "...there is no doubt we'll enter into a partnership in China in my mind. It's very clear with our active meetings in China, it's crystal clear. So, there is significant interest in the partnership. There is no question about that at all. We meet with numerous entities. We have the Sinopharm MoU and I want this to be very clear. I guess there is seems to be a little confusion phone call, there is no doubt that partners want to access and have relationship, a commercial relationship with PV-10, there is no doubt at all. The only question like I said in my prepared remark is how to optimize the transaction for the benefit of stockholders, patients and PV-10 developments."Conference Call (May 9, 2015)
Provectus held its 1Q15 conference call on Thursday, May 7th. A surprisingly poor quality transcript from Seeking Alpha is here. Details for hearing a replay of the conference may be found here.
On the company's March 12th conference call for CY 2014, Eric said on the topic of combining PV-10 with an immune checkpoint inhibitor for advanced melanoma:
Prepared remarks: "Throughout our consultations with key clinical opinion leaders, we’ve also been attuned to the need to address the needs of patients with more advanced disease. With enrollment now complete in the mechanism of action study of PV-10 of Moffitt Cancer Center, and initial results reported on that study and on the companion nonclinical study to assess combination of PV-10 with immune checkpoint inhibition, we are making progress on design of our proposed study to assess PV-10 in combination with immune checkpoint inhibition in patients with advanced melanoma.
We continue to expect this to be structured as a Phase 1b/2 study with a modest sized single arm Phase 1b component and expedited safety and efficacy endpoints supporting expansion to a larger randomized Phase 2 component. Endpoints for Phase 1b are likely to comprise acute safety as a combination regimen and objective response rate at three to four months. For Phase 2, we anticipate endpoints of progression free survival at overall survival. With three agents now approved in the US and also available in other key locations abroad, I expect to have very concrete details on this work to discuss at our next quarterly conference call. Importantly now that we have options for this systemic agent, this study can commence with or without the assistance of a partner." {Underlined emphasis is mine}
In response to a question: "...yes, absolutely, so that is certainly a very important area of development in melanoma and a number of other oncologic indications. We just went to melanoma research alliance annual meeting in Washington a week or so ago. And one of the core discussion topics at the meeting was how to facilitate studies to move those types of combination efforts forward in a more efficient fashion. One of the reasons we’ve been on the sidelines although we’ve been public in talking about our ideas in this regard is that we’ve been looking for the correct fit for an initial effort in that area. As I mentioned in my introductory comments, now that we have three approved immune checkpoint inhibitor agents available to us through standard of care, we have wealth of opportunities. And we are in the process of making final decisions on what initial work with one or more of those candidate drugs would look like. We’ve described that study a number of times, and I outlined it today Phase 1b/2 study is still exactly what we expect to commence. We’ve talked with prospective investigators for the study, they agree that that is the appropriate study design. It is a robust study design, quick and efficient study design that has proven to be of value in other context, combination of other agents with immune checkpoint inhibitors in melanoma. And so I think we have a solid basis for moving forward.
That’s why I mentioned in my opening comments that we expect to have a very concrete and interesting things to present on this particular topic when we have the next meeting, this next conference call. It is indeed a very exciting area and it is an opportunity that we see as important for expanding the value of PV-10 from patients with locally advanced disease, patients that have truly advanced – candidates that may not be perfect candidates for single agent PV-10 therapy, it appeared to be well matched for combination regimens based on our modeling and non-clinical data that we’ve collected through our collaboration with Moffitt." {Underlined emphasis is mine}On April 9th, during the panel session Provectus held in New York, however, the slide below (n.b. purple markups on the slide are mine) -- from October 2014 I believe (See "Oops" (April 10, 2015) below) -- was presented.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Eric's pertinent comments from the May 7th call were:
"And in addition to the progress on the combination therapy patent front that Pete mentioned, we've also made substantial progress towards commencement of our proposed clinical study of PV-10 in combination with immune checkpoint inhibition. We have identified the investigators who will lead this work, the agent to be used in conjunction with PV-10, the patient population and the dosing schedule for both agents along with the study end-point. To assess potential benefit of PV-10 for patients with advance melanoma, this phase 1b/2 study will incorporate a modest sized single arm Phase 1b component with expedited safety and efficacy end point supporting expansion to a larger randomized Phase 2 component.
End points for phase 1b are expected to comprise assessment of acute safety of the combination regimen and objective response rate at three to four months. For the Phase 2 portion, end points will be progression-free survival and overall survival.
Once the protocol addressing each of these areas is complete, we believe the pieces are in place to commence clinical work on this important second development path for PV-10 and melanoma. Since the checkpoint inhibitor we expect to use is licensed in the U.S., we can commence this study with or without the systems or a partner." {Underlined emphasis is mine}In the end, concrete and interesting likely were Eric's May 7th "revelations" that (i) investigators for the melanoma combination therapy have been identified (or chosen), (ii) the Phase 2 trial protocol is being finalized, and (ii) Provectus is ready to proceed with the Phase 1b trial with or without a Big Pharma partner.
Takeaways & Questions: It's likely Moffitt Cancer Center is at least one of the lead clinical sites (and Dr. Jeffrey Weber is likely one of the lead investigators). Would/could the Phase 2 trial be a pivotal one? If so, wouldn't the FDA have to review and opine on this trial's protocol? Management has costed the Phase 1b trial at about $1 million to carry out. I would imagine having a Big Pharma partner only for a melanoma combination therapy program is inefficient and not strategically acceptable to management. A more comprehensive program involving the combination of PV-10 and pembrolizumab for other indications (e.g., non-small cell lung cancer, prostate, renal cell carcinoma, etc.) would be.
The letter Q (May 7, 2015)
 |
| Image source |
1. During the first quarter, the company raised gross proceeds of $776,000 via the open and existing Network 1 private placement ($15 million "face value" vehicle. I believe the money was raised (i.e., taken in/subscriptions accepted) at the end of March (i.e., last week of the month). Money was last raised at the end of the fourth quarter (i.e., taken in December 31st). The balance of available vehicle is about $6.4 million.
 |
| Click to enlarge. |
2. Monthly cash burn for the first quarter appears to be $1.395 million, which is flat to 4Q14 ($1.392 million), which was 20% higher than 3Q14, which was a tad higher than 2Q14, which was about 20% higher than 1Q14, which was flat to 4Q13, which was 37% higher 3Q13. Takeaway: Peter is not running a $1 million-a-month cash burn.
3. In the May 7th press release management added the sentence, "The sale of common stock was reduced since we are seeking to minimize dilution to our existing stockholders where practicable by limiting the issuance of our equity securities."
4. Contrasted with the March 12th press release in which Provectus was "...a development-stage oncology and dermatology biopharmaceutical company," it now is "...a clinical-stage oncology and dermatology biopharmaceutical company." {Italicized emphasis is mine}
5. Forward-looking statements in the May 7th PR were revised from "...to license PV-10, our melanoma drug product candidate, and other solid tumors such as liver cancer..." to "...to license PV-10, our melanoma drug product candidate, and other solid tumors such as cancers of the liver..." {Italicized emphasis is mine} Management said cancers of the liver comprises primary (hepatocellular carcinoma) and secondary (metastatic) liver cancer.
6. Management changed "will" from the 2014 10-K to "should" in the 10-Q. See below:
We believe our continued development of PV-10 with existing funds should yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of solid tumor indications due to its unique immuno-chemoablation mechanism of action known as ablative immunotherapy or oncolytic immunotherapy. The primary ablative mechanism of PV-10 is followed by a secondary immunomodulatory mechanism. Likewise, we believe our development of PH-10 with existing funds should yield proof-of-concept evidence to support expected best-in-class clinical benefit to treat a wide range of inflammatory dermatoses due to its unique non-steroidal anti-inflammatory mechanism of action. {Italicized emphasis is mine} Management also included broader mechanism of action categorization or labelling: i.e., ablative immunotherapy (recent labeling of PV-10) and oncolytic immunotherapy (that some folks like MD Anderson's Dr. Merrick Ross use to describe PV-10, and Amgen's T-Vec without the virus aspect).
7. As noted in Stock Option Exercises (January 8, 2015) below, "One employee of the Company forfeited 300,000 stock options on January 7, 2015." I believe the employee is Craig.
8. In regards to the Stipulated Settlement Agreement and Mutual Release (the “Settlement”), "$266,667 was repaid by the Executives as of March 31, 2015." This reduced balance sheet item Short-term receivable — settlement from $733,333 as at 12/31/14 to $466,666 as at 3/31/15.
Updated: [@alanrobertross noted this:] 9. "During the three months ended March 31, 2015, 3,693,898 warrants were forfeited." I had read this, but didn't think it important.
 |
| Click to enlarge. |
 |
| Image source |
As I wrote in Potpourri (May 1, 2015) below I hope Eric delivers on the expectations and timing he set during the March 12th conference call: concrete details of the proposed Asian (Chinese) Phase 1b/2 study of PV-10 plus standard care for hepatocellular carcinoma, and a potential Phase 1b/2 study of PV-10 and an immune checkpoint inhibitor, among other things.
Provectus issued a press release and filed an associated 8-K today regarding allowance of the company's synthesis patent application by the European Patent Office, and issuance by the Japanese Patent Office. The patent was issued by the U.S. Patent & Trademark Office in 2013, and the application was allowed by the Chinese Patent Office earlier this year.
The company appears to have selected a new PR firm (Oglivy?). Upgrading this function is nice, but data generation is much, much nicer.
Eric (and maybe Peter) may be attending the 2015 Accelerating Anticancer Agent Development and Validation Workshop, May 6-8 in Bethesda, Maryland; I believe Eric (and Jamie, and maybe Peter) attended the 2014 workshop: "The workshop is designed for both scientists and consumer advocates with clinical trial experience that have an interest in new approaches to developing or enhancing agents or combinations of agents for the diagnosis, treatment or prevention of cancer."
It's possible Provectus may be using Quintiles as its contract research organization ("CRO") for the melanoma Phase 3 trial.
"So what’s driving the increase?" (May 4, 2015)
Morrison & Foerster, April 2015:
"The data shows that buyers are paying more up front for products at all stages of development than in the past. This likely reflects two market forces. First, the IPO market and access to capital are allowing biotechnology companies to drive harder bargains. Companies are less willing to accept modest up-front payments in licensing transactions when they have a viable alternative to raise capital and continue development on their own, while retaining 100 percent of the rights and future economic potential of their products. For the biotechnology company, it becomes a question of dilution: the company can suffer a certain amount of equity dilution by selling stock, or it can have its economic rights to its assets diluted by licensing them to a pharmaceutical company (or large biotechnology company) for a share of the future economics. The availability of a viable capital-raising path has no doubt altered this calculus for many sellers in the market."
 |
| Click to enlarge. |
H/t a shareholder, who wrote:
"Now that the mechanism of action of checkpoint inhibition is now understood and recognized (a relatively recent phenomenon), we know that patient survival is more strongly correlated with strengthening of adaptive immunity (through the expression of stimulatory tumor antigens and amplification of the T-cell response) rather than attempts to kill the tumor any way possible and at any cost. The great revelation is certainly to debulk the tumor and attempt to prevent mechanical/structural issues (such as lumen obstructions, pathological bone fractures, perforations, etc.), but that the focus should be on first providing intact tumor antigens for the adaptive immune system through immunogenic cell death (via radiation, low-dose chemotherapy, oncolytic viral therapy, PV-10, HDAC inhibitors, etc.) such that tumors become in vivo vaccine factories and then augmenting this vaccine response with amplifying checkpoint inhibition."He also pointed me to an article by Bezu et al. (Front. Immunol., 24 April 2015 | doi: 10.3389/fimmu.2015.00187) entitled Combinatorial strategies for the induction of immunogenic cell death. The article begins:
"The expression “immunogenic cell death” (ICD) generally refers to a functionally peculiar case of regulated cell death (RCD) that – in immunocompetent hosts – is capable of activating an adaptive immune response against dead cell-associated antigens. Of note, ICD generally (but not obligatorily) manifests with apoptotic morphological features, and at least some of its manifestations depend on components of the apoptotic apparatus. Irrespective of these morphological and biochemical considerations, immunocompetent mice injected s.c. with cancer cells succumbing to bona fide ICD (in the absence of any adjuvant) develop a cellular immune response associated with the establishment of immunological memory that protects them from a subsequent challenge with living cells of the same type. {Underlined emphasis is mine}The article concludes:
"In spite of old beliefs, cancer cells continuously interact with the immune system: first, as they are generated by healthy cells upon malignant transformation; second, as they evolve and acquire additional neoplastic features; and third, when they are challenged with therapeutic interventions. During the last decade, such a conceptual revolution, i.e., considering tumors as entities that can be detected and destroyed by the immune system, has paved the way toward the development of novel therapeutic agents conceived to re(instate) anticancer immunity, and some of these interventions have already been licensed for use in humans by international regulatory agencies. In addition, it has become clear that many therapeutics that had been used for decades in the clinic are efficient (for the most part) because they engage the host immune system against malignant cells. ICD is one of the several mechanisms through which cytotoxic chemotherapeutics, targeted anticancer agents as well as some forms of radiotherapy can elicit tumor-targeting immune responses. Identifying novel ICD inducers as well as measures that convert non-immunogenic RCD into bona fide ICD is of primordial importance. Promising preclinical results and preliminary clinical findings suggest, indeed, that agents that promote CALR exposure, ATP secretion, type I IFN production, HMGB1 release or stimulate the downstream signal transduction pathway may considerably improve the clinical profile of conventional therapeutic regimens (Figure 1). A systematic investigation of the ability of currently available anticancer agents to elicit the abovementioned ICD-associated processes in human cancer cells of distinct histological origin is urgently awaited. These data may pave the way to the clinical implementation of combinatorial immuno(chemo)regimens that efficiently promote ICD and hence mediate complete tumor regression in a high proportion of patients." {Underlined emphasis is mine}Figure 1 from the article (noted above) is below.
 |
| Image source |
As an aside, a reminder of that 2014 work (see my March 9, 2014 blog post), which noted:
"...there is a significant increase in the number of DCs infiltrating the tumor-draining lymph nodes after IL injection of PV-10. These findings suggest that PV-10 treatment leads to the release of DC activating factors and DC recruitment."Tumor-draining lymph nodes, or "TDLNs," are "[l]ymph nodes that lie immediately downstream of tumors." Lymph nodes act as filters for the body’s lymphatic drainage system, catching cancer cells in the fluid draining away from a tumor. It's easy to understand the importance of the lymph nodes to metastatic disease, and thus the relationship the TDLNs (lymph nodes where cancer cells themselves have infiltrated, grow in number, and journey to near and distant parts of the body) have with cancer. Lymph nodes are an immune system organ. What organs make up the immune system? Bone marrow, thymus, spleen and lymph nodes. "Lymph nodes are a bit like the spleen, but instead of filtering blood, they filter lymph. Lymph is composed of fluids which drain from tissues. It is collected at various locations throughout the body and circulates through a series of lymph nodes, eventually returning to the blood for circulation" (previously linked source).
But, the TDLNs are immune-privileged sites. "Immune responses and associated inflammation in certain parts of the body, including brain, eye, testes, placenta, and fetus, carry a high risk of lethal organ dysfunction or reproductive failure. These tissues, which have evolved to be protected, to a variable degree, from immune responses, are called immune privileged sites."
Once cancer cells from upstream tumors enter downstream lymph nodes (i.e., enter the TDLNs), the disease becomes ever so more dangerous. This infiltration of the TDLNs by diseased cells, and thus into an immune-privileged area where a protective immune response to the invaders is not sufficiently or strongly initiated, allows the cancer cells (I think) to grow much more unfettered and un-fought by the body's immune system than they potentially would elsewhere in the body. "Lymph nodes that lie immediately downstream of tumors [tumor-draining lymph nodes (TDLNs)] undergo profound alterations due to the presence of the upstream tumor. The antigen-presenting cell population in TDLNs becomes modified such that tumor-derived antigens are cross-presented by host cells in a tolerizing fashion. In addition, the number and suppressor activity of regulatory T cells (Tregs) are increased in the TDLN. Emerging evidence suggests that some of these Tregs may be generated de novo against specific tumor-derived antigens, and thus they arise as a direct consequence of antigen presentation in the TDLN. Others may represent Tregs against self-antigens, which undergo preferential activation in the tolerogenic milieu of the TDLN. The TDLN thus becomes an anatomic context in which presentation of new antigens not only fails to elicit a protective immune response but also actively creates systemic tolerance. In this regard, the TDLN displays features analogous to classical immune privilege. Accumulating evidence thus suggests that the TDLNs, although small in size, may exert a profound tolerizing influence on the rest of the immune system. These mechanisms will need to be interrupted in order for clinical anti-tumor immunotherapy to be successful." {bolded emphasis is mine}
Thus, preventing the TDLNs from naturally suppressing what otherwise would be a normal immune response to tumor fluid or fragments or discharge emanating from an upstream tumor would be key, as noted above, to more successful anti-tumor immunotherapy: "Regional lymph nodes are the first site for melanoma metastases. The sentinel node (SN), on the direct lymphatic drainage pathway, which usually harbors first metastases, demonstrates significant suppression in its ability to respond to antigenic stimulation...Antigen presentation by dendritic cells (DCs) is the most potent means to initiate a T cell immunity." So, if an otherwise immune-privileged site like the TDLNs are unable to mount an immune fight or response, success likely would be achieved by encouraging the infiltration of DCs into these lymphoid organs (i.e., the TDLNs) to start and continue the fight.
But, is a systemic immunotherapy able to achieve the recruitment of DCs to the TDLNs, or is a local therapy better suited? "In recent years, it has become apparent that immunoregulatory processes influence cancer development. The key players in tumor progression are mainly present in the microenvironment of the tumor and the draining lymph nodes. Interventions aimed at shifting tumor-promoting actions toward effective tumor-eradicating immunity are thus foremost required locally. As immune-modulating therapy has been shown to cause many adverse side effects when administered systemically, we strongly advocate the further development of local treatment for cancer immunotherapy" {Bolded emphasis is mine}
As Craig has said for a while the path to defeating cancer, a systemic disease, is through local therapy that has systemic properties. Rapid ablation of injected tumors upstream from the TDLNs creates tumor fragments (antigens) that are taken up from antigen presenting DCs that infiltrate the TDLNs to create an immune response in an area that desperately needs one.
Stanford's Dr. Peter Lee, M.D. noted that: "Whether TDLNs are invaded by cancer cells is a key prognostic indicator for patients with breast and other cancers. Lymph nodes are immune organs and TDLNs are key sites of tumor-immune interactions...TDLNs may be immunologically altered and demonstrated the clinical significance of T cell and dendritic cell (DC) decreases in predicting relapse in breast cancer." Or, here too.
PV-10's rapid ablations fights cancer's foot soldiers on the front lines (i.e., the injected tumors). By getting the body to send its own solders into the TDLNs to fight cancer cells there, metastatic disease around the body has its supply lines cut. PV-10 breaks tolerance. Is Moffitt showing antigen presenting DCs migrating (infiltrating) into the "tolerizing" environment of a TDLN and overcoming tolerance (resistance)? Have they figured the mechanism to overcoming tolerance (resistance); that the migration of antigen presenting DCs is what breaks tolerance? Of course, the sentence in the Moffitt 2014 abstract is based on their murine model (pre-clinical work).
The Doctor Is In (May 2, 2015)
The American Academy of Dermatology designated the month of May as National Melanoma Skin Cancer Prevention Month. The Provectus-sponsored video below appears to be an interview of St Luke's University Health Network's Dr. Sanjiv Agarwala, MD for other media persons then to potentially interview him to further discuss:
- What is the importance of early melanoma detection when it comes to treatment?
- Would you briefly compare melanoma versus squamous cell carcinoma versus basal cell carcinoma? What sets melanoma apart?
- How are late-stage melanoma lesions treated today? Is there room for improvement?
- What roles does the immune system play against melanoma?
- What are intralesional therapies and how do they work?
- Why may late-stage melanoma patients need to try multiple approaches?
- What are some newer therapies that are promising and pushing ahead in clinical trials?
- Where can patients get information on new trials?
With the start of Provectus' pivotal melanoma Phase 3 trial specifically, or perhaps more broadly the transition from an early-stage drug development company into a regulatory-focused and data-driven organization, I think it's time (long overdue, but I'm being very generous) that management improve their media relations function: i.e., swap out Bill Gordon for a more nationally recognized firm/person(s) that is/are dramatically better.
Potpourri (May 1, 2015)
 |
| Image source |
Cinnamon bark. Judging from Provectus' Calendar of Events for September, if accepted, additional liver data may well be presented at the 18th European Cancer Organization ("ECCO") – 40th ESMO European Cancer Congress in Vienna, Austria from September 25-29. The "lead" author for this abstract/poster should be a key opinion leader relatively new to the company (I believe I've confirmed the name outside of speaking to management), and who is an interventional radiologist. "Interventional radiology uses minimally invasive image-guided procedures to diagnose and treat diseases in nearly every organ system." By contrast, Goldfarb and Rosemurgy are surgical oncologists, and Agarwala is a medical oncologist.
Cloves. Provectus may have engaged the investment banking part of Maxim Group to assist the company with exploring, negotiating and/or consummating a regional license transaction for PV-10 in India. The Maxim i-banker may be Ritesh Veera. While I cringe at the mention of this M-word (call it a reflexive [almost natural] action), I hope the i-banking side of the firm treat's Provectus — as, you know, a client — better than its broker side did in 2012.
 |
| Screenshot from May 1st |
 |
| Click to enlarge |
Since Wall Street for the most part is a pay-to-play business, a successful India transaction via Veera (and thus potential fees to Maxim) might lead to a note from Kolbert. Cantor Fitzgerald likely won't initiate research coverage until Provectus draws down on its equity facility.
Marjoram leaves. "Investment bank" New Jersey's Network 1 Financial and Chinese investment bank Tririver Capital appear to be representing Provectus in China, and Maxim may be repping the company in India, who is Provectus using for Brazil (and perhaps more broadly South America)? I don't think they have representation yet in presumed conversations with international and domestic pharmaceutical companies. Updated 5/3/15: Management said they had investment banking representation in Brazil from a local firm.
Orange peel. Management removed slides from both Dr. Argawala and Eric's April 9th panel discussion presentations in New York that were later posted on the company's website. I am embarrassed for them by the decision made, action carried out, explanations provided and verbs used therein. I hope Eric delivers on the expectations and timing he set during the March 12th conference call: concrete details of the proposed Asian (Chinese) Phase 1b/2 study of PV-10 plus standard care for hepatocellular carcinoma, and a potential Phase 1b/2 study of PV-10 and an immune checkpoint inhibitor — "That’s why I mentioned in my opening comments that we expect to have a very concrete and interesting things to present on this particular topic when we have the next meeting, this next conference call."
Annual Meeting Proxy (May 1, 2015)
 |
| Image source |
The three "core" questions being put to a vote are (as they have been from year to year): (i) to elect directors to serve on the company's board for a one-year term, (ii) to conduct an advisory vote to approve the compensation of the principals (officers), and (iii) to ratify the selection of BDO as Provectus' auditor.
Upcoming May option exercise dates from page 18 include:
- Each of Craig Dees and Tim Scott: 325,000 on May 19th and 25th (next, December),
- Peter Culpepper: 33,334 on May 25th (next, December), and
- Eric Wachter: None.
Scott and Wachter had about $33K withheld from their respective salaries in 2014 in connection with the settlement of the shareholder lawsuit (see page 16). Wachter was added as a defendant in the class action lawsuit (see page 30), and the Hurtado lawsuit was stayed pending a motion by Provectus to dismiss the class action lawsuit.
April Blog Stats (May 1, 2015)
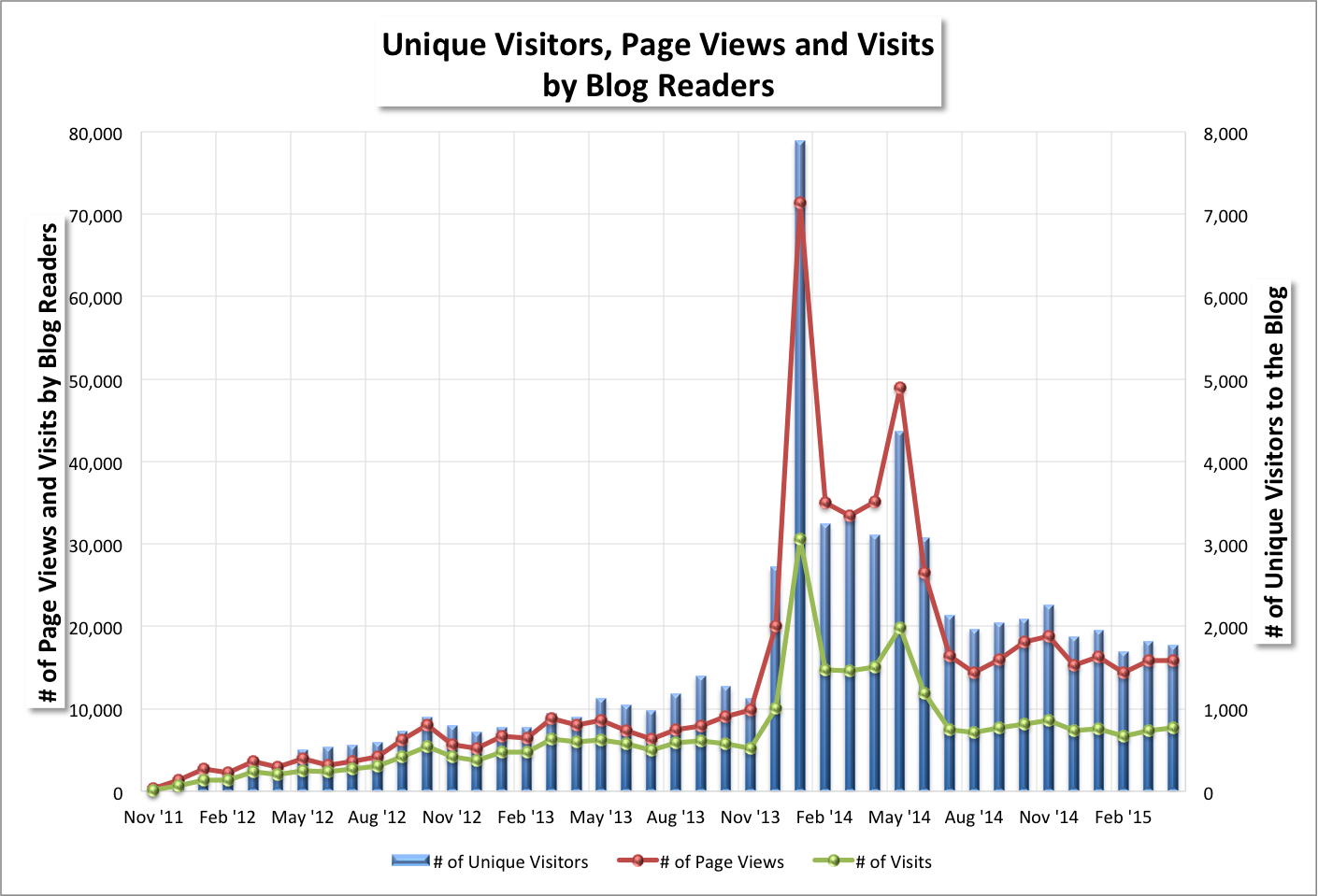 |
| Click to enlarge. |
 |
| Click to enlarge. |
H/t a shareholder: Intralesional therapy for metastatic melanoma, December 2014, Vol. 15, No. 18 , Pages 2629-2639 (doi:10.1517/14656566.2014.967682), Sarah Sloot, Omar M Rashid, and Jonathan S Zager
Introduction: Intralesional therapy for metastatic melanoma has some advantages over systemic therapy. Local drug administration allows for delivery of an increased concentration of the agent and reduced systemic exposure, thereby increasing local efficacy and limiting toxicity. Moreover, since in vivo tumor nodules contain the tumor antigens, this tumor tissue may serve as an autologous vaccine to induce systemic immunity. This so-called ‘bystander effect’, where uninjected distant lesions exhibit a response, has been reported in select intralesional therapy trials.
Areas covered: This review will give an overview of the working mechanisms, clinical evidence and side effects for available intralesional and topical therapies and summarize the most recent developments in this field.
Expert opinion: The ideal treatment approach for locoregionally advanced melanoma should be multidisciplinary and tailored to the patient, taking into consideration patient-related, tumor-related factors (such as location, tumor burden, mutation status) and previous treatments received. It will likely not be a single therapy, but rather a combination of injectable treatments, regional perfusions and systemic therapies.
 |
| Click to enlarge. |
{Tongue somewhat in cheek} Dr. Sally Church, PhD: Where do we go beyond monotherapy with immune checkpoints?
 |
| Click to enlarge. Image source, purple oval emphasis is mine |
 |
| Image source |
- Discussion: Please discuss the benefit of talimogene laherparepvec for the proposed indication,
- Discussion: Please discuss the safety of talimogene laherparepvec for the proposed indication,
- Discussion: Considering the evidence of effectiveness and safety of talimogene laherparepvec, and the current landscape of available therapies for melanoma, please discuss whether talimogene laherparepvec has an overall favorable benefit-risk profile for some population other than the proposed indicated population. If for some other population, please describe that population.
- Discussion: Please discuss whether the dosing instructions (including both dose and regimen, for both individual lesions and for the subject) provided for Study 005/05 would be sufficient to inform the use of talimogene laherparepvec by healthcare providers in clinical practice. If not, please discuss any additional dosing instructions that would be helpful.
- Discussion: Regarding the shedding and potential transmission of talimogene laherparepvec from patients treated with the product: a) Please discuss the available data from the ongoing shedding study and the potential risk for transmission to close contacts (e.g., immunocompromised, infants, pregnant women) and health care providers (HCP), and b) Please discuss Amgen’s proposed postmarketing protocol 20130193 and identify any recommendations for modification of the protocol. Please consider whether the protocol design is adequate to capture (with qPCR confirmation) cases of talimogene laherparepvec transmission to close contacts, should they occur. Are there additional measures that should be included in the postmarketing study to ensure that samples can be collected and testing can be performed in a timely manner for suspected herpetic lesions in close contacts or HCPs
- Advisory Committee Discussion: Study 005/05 met its primary objective by demonstrating a higher durable response rate (DRR) in the talimogene laherparepvec group than in the control (GM-CSF) group. Concerns regarding the study results include uncertainty regarding the clinical meaningfulness of the durable responses (e.g., considering the limited evidence of a systemic effect), and uncertainty regarding an effect on overall survival. Discussion: Please discuss the benefit of talimogene laherparepvec for the proposed indication, particularly considering the results of Study 005/05 and the concerns outlined above.
- Advisory Committee Discussion: In Study 005/05, the most common treatment-emergent adverse events that occurred more commonly with talimogene laherparepvec included fatigue, chills, pyrexia, nausea, influenza-like illness, and injection site pain. Serious adverse events attributed to the study treatment included cellulitis at the injection site and injection site reactions. Discussion: Please discuss the safety of talimogene laherparepvec for the treatment of injectable regionally or distantly metastatic melanoma.
- Advisory Committee Discussion: There may be subgroups of the proposed indicated population for whom talimogene laherparepvec would have a more favorable benefit-risk profile. For example, some patients (e.g., patients with Stage IIIB or IIIC melanoma; patients whose tumors do not have a BRAF mutation) may have few treatment options and want a treatment that avoids the potential toxicities associated with the currently approved therapies. Discussion: Considering the evidence of effectiveness and safety of talimogene laherparepvec, and the current landscape of available therapies for melanoma, please discuss whether talimogene laherparepvec has an overall favorable benefit-risk profile for some population other than the proposed indicated population. If for some other population, please describe that population. {Underlined emphasis is mine}
- Question to the Advisory Committee: The Study 005/05 protocol specified that talimogene laherparepvec (up to 4 mL total) was to be injected into one or more cutaneous or subcutaneous (SC) or nodal melanoma lesions every 2 weeks until clinically relevant disease progression occurred or there was no residual tumor to inject (Section 4.4). However, the actual dose administered, and the dosing regimen, were subject to investigator discretion, and varied considerably among the study subjects. Discussion: Please discuss whether the dosing instructions (including both dose and regimen, for both individual lesions and for the subject) provided for Study 005/05 would be sufficient to inform the use of talimogene laherparepvec by healthcare providers in clinical practice. If not, please discuss any additional dosing instructions that would be helpful. {Underlined emphasis is mine}
- Advisory Committee Discussion: Talimogene laherparepvec is a replication-competent virus derived from an attenuated HSV-1 isolate. As such, talimogene laherparepvec is expected to have biological properties that are similar to wild type HSV-1 with regard to viral shedding and potential transmission and latency/symptomatic reactivation. However, to date, there are limited data on talimogene laherparepvec shedding, which serves as a proxy for transmission. Thus, there are concerns that viral shedding may expose healthcare providers (HCP) and close patient contacts to talimogene laherparepvec. Regarding the shedding and potential transmission of talimogene laherparepvec from patients treated with the product: a. Please discuss the available data from the ongoing shedding study and the potential risk for transmission to close contacts (e.g., immunocompromised, infants, pregnant women) and health care providers (HCP), and b. Please discuss Amgen’s proposed postmarketing protocol 20130193 and identify any recommendations for modification of the protocol. Please consider whether the protocol design is adequate to capture (with qPCR confirmation) cases of talimogene laherparepvec transmission to close contacts, should they occur. Are there additional measures that should be included in the postmarketing study to ensure that samples can be collected and testing can be performed in a timely manner for suspected herpetic lesions in close contacts or HCPs?
- Question to the Advisory Committee: The proposed indication for talimogene laherparepvec is for the “treatment of injectable regionally or distantly metastatic melanoma.” Please consider the background information and evidence of benefit and safety provided in the briefing document, as well as the presentations and discussions during this meeting. Voting Question: Does talimogene laherparepvec have an overall favorable benefit-risk profile for the treatment of injectable regionally or distantly metastatic melanoma? In voting, please consider only whether the available evidence would support traditional approval, not Accelerated Approval. {Bold emphasis is mine}
As the FDA review document noted, "Study 005/05 enrollment was not limited to any subgroup, and subgroup analyses are generally not reliable with regard to an intervention’s safety or efficacy in the subgroup. In addition, Study 005/05 does not provide any direct comparison of talimogene laherparepvec to available therapies, for the study as a whole or for any subgroups." Further: "FDA has the regulatory flexibility to consider this BLA for either traditional approval or Accelerated
Approval. FDA could approve the product under the Accelerated Approval pathway for either the
proposed indicated population, or for a subgroup of the proposed population. However, the BLA
submission does not contain any statements from the Applicant regarding how the available data might support Accelerated Approval. In the absence of a submission that presents the Applicant’s position regarding Accelerated Approval, and the absence of FDA review of such a submission, a full and fair consideration of the Accelerated Approval pathway for use of talimogene laherparepvec is not feasible at the time of this Advisory Committee meeting. For this reason, although the Committee discussion may include consideration of Accelerated Approval, FDA asks the Committee to vote only on the question of traditional approval for talimogene laherparepvec.
Despite response and safety benefits acknowledged by the FDA, the lack of overall survival ("OS") benefit for an indicated population where is germane may sink T-Vec's candidacy for approval, and probably would garner a majority "no" response to the voting question above.
Additionally, despite Amgen's combination therapy trials of T-Vec and Yervoy/ipilimumab (Phase 1b complete) (Stage IIIB, IIIC, IVM1a, IVM1b, or IVM1c disease) and T-Vec and Keytruda/pembrolizumab (recruiting) (Stage IIIB to IVM1c Melanoma), and should the consensus committee vote be no, Amgen may have to run a further trial comprised exclusively of Stage III melanoma patients, like Provectus's PV-10's pivotal Phase 3 trial, to garner approval consideration as a monotherapy.
Provectus/PV-10 implications of a "no" vote: E.g., More, faster melanoma Phase 3 trial enrollment?, Pending a successful trial outcome, out the door as the first intralesional ("IL") (local) agent approved for melanoma (cancer)?
A "yes" vote: E.g., Recognition of IL agents' role in treating metastatic melanoma?, Halo effect for PV-10 when it is next up in the regulatory approval queue (pending a successful trial outcome)?
Provectus/PV-10 implications of a "no" vote: E.g., More, faster melanoma Phase 3 trial enrollment?, Pending a successful trial outcome, out the door as the first intralesional ("IL") (local) agent approved for melanoma (cancer)?
A "yes" vote: E.g., Recognition of IL agents' role in treating metastatic melanoma?, Halo effect for PV-10 when it is next up in the regulatory approval queue (pending a successful trial outcome)?
Mixed Bag (April 27, 2015)
 |
| Image source |
T-Vec's pivotal Phase 3 trial focused on patients with unresectable stage IIIB, stage IIIC or stage IV melanoma; that is, advanced melanoma (and not locally advanced cutaneous melanoma, PV-10's indicated patient population). Stage IIIB/C patients comprised 30% of T-Vec's trial's patient population.
 |
| FDA review document, page 32, 5.2 Subject Characteristics (highlight is mine) |
1. Leading up to the FDA review, questions surrounded T-Vec's Phase 3 trial's primary endpoint (i.e., durable [objective] response rate ["DRR"]), complete response ["CR"] or partial response ["PR"] maintained for at least 6 months, and beginning at any point within 12 months of initiating therapy) and comparator (i.e., control arm patients were treated with GM-CSF).
2. The Agency highlighted several concerns with the trial's design and execution, including:
- Subject or investigator bias: "based on knowledge of the treatment assignment" and "regarding the relative benefit of talimogene laherparepvec and the control may have influenced the determination that it was in the best interest of the subject to stop treatment or to be given other therapy for melanoma",
- Asymmetric dropouts: "There were more control group subjects than talimogene laherparepvec group subjects who discontinued study treatment at or before 3 months, 56.0% versus 29.2%,"
- Early study discontinuations in subjects in the control arm:
 |
| FDA review document, page 35, 5.4 Study Conduct, 5.4.1 Duration of Response Assessment |
- Missing study assessments: "Eligibility violations were reported for 26 subjects in the talimogene laherparepvec arm and seven subjects in the control arm. Protocol deviations, including missing more than one clinical assessment, were reported in 36 (12.2%) subjects in the talimogene laherparepvec arm and five (3.5%) subjects in the control arm. Most protocol deviations were about eligibility violations and missing scans. FDA analysis showed that nine subjects in the talimogene laherparepvec arm and four subjects in the control arm were missing more than 1 sequential response assessment. FDA analysis of protocol deviations suggested that these had no more than a minor effect on the study results." (i.e., p still <0.0001) {Underlined emphasis is mine}
 |
| FDA review document, page 37, 6.1 Primary Endpoint, 6.1.1. Primary Endpoint Results |
4. The FDA commented: "Inclusion criterion “multiple superficial melanoma lesions which in aggregate have a total diameter of ≥ 10 mm” allowed enrollment of subjects who had only small or very small lesions. Inclusion of such subjects raises concerns regarding the reliability of injection, and particularly reliability of measurement, both at the baseline and during assessments of response." {bold and underlined emphasis is mine}. BioVex's OncoVEX inclusion criteria from ClinicalTrials.gov:
 |
| Image source |
PV-10 inclusion criteria for its pivotal melanoma Phase 3 trial is below:
 |
| Image source |
5. T-Vec safety: "Serious adverse events associated with talimogene laherparepvec included cellulitis, impaired wound healing, and immune-mediated disease (e.g., glomerulonephritis). Shedding data were limited. The applicant has proposed a pharmacovigilance plan to collect postmarketing safety data." {Underlined emphasis is mine}
And: "With respect to the transmission risk of talimogene laherparepvec, in premarket studies there was an absence of rigorous follow-up of suspected herpetic infections, in study subjects or contacts, to detect possible talimogene laherparepvec infection. The potential risk of talimogene laherparepvec transmission to contacts in the post-licensure period needs to be evaluated."
On the brighter side for intralesional agents at large, including PV-10:
This was a helpful reminder of what Eric described as the hypothesis of PV-10's pivotal melanoma Phase 3 trial (see Audiophile (April 19, 2015) below): "The study is predicated on two hypotheses. One is if we make lesions go away we will forestall progression of the patient's [Stage IIIB/C] disease. We'll show that with our progression free survival primary endpoint."
 |
| FDA review document, page 14, 2.1 Melanoma Overview |
6. From the FDA's review of T-Vec (page 64, 10 Issues and Discussions, 10.1 Evidence of Effectiveness):
"However, the study results for the primary endpoint are statistically robust. Therefore, FDA believes that any bias that might have occurred in the study conduct would not change the study results sufficiently to alter the overall interpretation that talimogene laherparepvec had an effect on durable response rate. " {Underlined emphasis is mine}
7. From the FDA's review of T-Vec (pages 66-7, 10 Issues and Discussions, 10.2 Patient Population):
"The available therapies for Stage IIIB, IIIC, and Stage IV melanoma include products with clinically important toxicities (see Section 12). Due to concern regarding these potential toxicities, some patients with melanoma may not be willing to take any of the currently available therapies. For such patients, talimogene laherparepvec may offer an important safety advantage over the currently approved therapies."
Considering that melanoma patients now have multiple treatment options, it is unclear whether talimogene laherparepvec offers an acceptable benefit-risk profile for the proposed indicated population. However, there may be melanoma patients for whom talimogene laherparepvec would be an appropriate alternative to the currently approved therapies. For example, 16.3% of subjects in the talimogene laherparepvec group had a durable response, but subgroup analyses showed a durable response in 33.0% of subjects with Stage IIIB or IIIC melanoma who received talimogene laherparepvec, and a durable response in 23.9% of subjects who received talimogene laherparepvec as first-line therapy. Talimogene laherparepvec’s overall benefit-risk profile might be more favorable in such patients, or patients with fewer treatment options, than in the proposed indicated population.
Study 005/05 enrollment was not limited to any subgroup, and subgroup analyses are generally not reliable with regard to an intervention’s safety or efficacy in the subgroup. In addition, Study 005/05 does not provide any direct comparison of talimogene laherparepvec to available therapies, for the study as a whole or for any subgroups. Nevertheless, there may be patients with melanoma who do not have good treatment options, and for whom talimogene laherparepvec would be safe and effective." {Underlined emphasis is mine}
My takeaways include (i) Eric's work to ensure as complete and fine-grained Phase 3 protocol for PV-10, (ii) the FDA's highlighting Stage III melanoma patients need better and safer options than the approved immune checkpoint inhibitors, and (iii) while T-Vec may or may not be approved for its proposed indicated population of patients with metastatic or advanced melanoma (Stave IV patients), it may well have to run a trial of Stage III patients to be approved if it isn't for this biologics license application ("BLA").
Provectus' next quarterly conference call is scheduled for May 7th.
Provectus also appears to have changed its forward looking statements in press releases after March 16th, from...:
...to:
Say What? (April 26, 2015)
Provectus' CFO/COO Peter Culpepper was interviewed by BioPharm Insight on April 22nd. The interview, entitled Provectus prioritizes pharma partners with clinical-stage PD1/PDL1s, provided some useful nuggets.
First, management's position (from the article and discussions with them) is that if they don't get "paid" (i.e., "The company hopes to ink a deal similar to the clinical trial codevelopment deal between Bristol-Myers Squibb and Celldex Therapeutics (NASDAQ:CLDX), announced 14 May 2014.") to combine PV-10 with an immune checkpoint inhibitor (approved or late-stage investigational), they'll run their own trial of PV-10 and Merck's Keytruda (pembrolizumab). Paid means either getting an upfront payment and sharing trial costs, or having trial costs picked up by the partner.
The author writes:
Second, Provectus has interest in combining PV-10 with other categories of drugs, such as targeted therapies. For example, liver cancer drug Nexavar (sorafenib) is a targeted therapy.
Third, one of the company's pivotal melanoma Phase 3 clinical trial sites may be Memorial Sloan Kettering:
Third, he reaffirms management's true intent for PV-10 (and the potential basis for its possible true worth), combination therapy-driving valuation notwithstanding:
Get with The Program (April 22, 2015)
With the start of Provectus' Phase 3 clinical trial of PV-10 [as a monotherapy] versus systemic chemotherapy (dacarbazine) for unresectable locally advanced cutaneous melanoma [exclusively] in Stage III patients, attention turns to two other aspects of the company's clinical development program ("CDP"). Provectus' next quarterly conference call should be held in early- to mid-May to coincide with the filing of the company's first quarter 10-Q. On the last call, March 12th, Eric said of the melanoma and liver CDPs:
For example, Merck KGgaA's approach to its anti-PD-L1 agent avelumab has been to run "indication specific" and "indication general" trials:
Hepatocellular carcinoma ("HCC") ("primary liver cancer"), and Cancer metastatic to the liver ("metastatic liver cancer") ("secondary liver cancer"). As Eric noted during Provectus' April 9th panel discussion, the so-called Asia pivot reflected the company's better understanding of the differences of market applicability and interest regarding HCC and metastatic liver cancer. That is, generally speaking, the "market" for the former is primarily in Asia, while the "market" for the latter is primarily in the U.S. (and I then would presume the so-called developed world).
Provectus' next quarterly conference call is scheduled for May 7th.
 |
| Image source |
 |
| March 16, 2015 PR: Provectus Biopharmaceuticals' Amended Protocol of PV-10 for Phase 3 Study as Treatment for Melanoma Now Available Online |
 |
| April 27th PR (when I noticed the change): Provectus Biopharmaceuticals To Present at Asia Biotech Invest 2015 Conference Wednesday, May 20, 2015, Hong Kong |
 |
| Image source |
First, management's position (from the article and discussions with them) is that if they don't get "paid" (i.e., "The company hopes to ink a deal similar to the clinical trial codevelopment deal between Bristol-Myers Squibb and Celldex Therapeutics (NASDAQ:CLDX), announced 14 May 2014.") to combine PV-10 with an immune checkpoint inhibitor (approved or late-stage investigational), they'll run their own trial of PV-10 and Merck's Keytruda (pembrolizumab). Paid means either getting an upfront payment and sharing trial costs, or having trial costs picked up by the partner.
The author writes:
"When asked about timing of a partnership deal, Culpepper said discussions would take place in parallel process with the melanoma Phase Ib/II trial. There is no set launch date for the Phase Ib/II trial, but the company is meeting with key opinion leaders next week and will know more by 1 May, Culpepper said."Underlined emphasis is mine.Peter and Provectus' CTO Dr. Eric Wachter, Ph.D. are said to be in Brazil next week (as discussed on the company's March 12th conference call) to [my speculation] meet with either or both of the potential lead investigator (and his or her medical institution) and the prospective pharmaceutical company licensee of PV-10 for the country. That licensee may have interest in local use of the drug, as a monotherapy and in combination with one of their agents. Recall I wrote under Silk Route (April 4, 2015) below that arriving at the point a drug may be used in a country for a clinical trial requires the submission of an IND (an "investigational new drug" application), which should be comparable to the FDA process of (a) submitting an IND application to (i) the local biopharmaceutical regulatory agency and (ii) the institutional review board ("IRB") of a local medical institution [site] that would conduct a PV-10 trial that (b) both have to accept or not disapprove of the IND for it to be effective. The local IND, like in the U.S., permits a Phase 1 study to commence in the respective country. What's relevant about May 1st?
Second, Provectus has interest in combining PV-10 with other categories of drugs, such as targeted therapies. For example, liver cancer drug Nexavar (sorafenib) is a targeted therapy.
Third, one of the company's pivotal melanoma Phase 3 clinical trial sites may be Memorial Sloan Kettering:
"Enrollment on the Phase III has already begun at St. Luke's University Hospital, Bethlehem, Pennsylvania, according to a 15 April press release. In addition, the company hopes to bring on MD Anderson Cancer Center in Houston, Texas, some Australian sites, and another high-profile group like Memorial Sloan Kettering in New York, he said."In my opinion, Peter would not have dropped such knowledge unless Sloan Kettering currently was being added. MD Anderson is a PV-10 compassionate use program ("CUP") site. The question in my mind is "who is the Sloan Kettering investigator:" Dr. Paul Chapman, MD (preferred, in my view) or Dr. Jedd Wolchok, MD, PhD (see #Trending (April 9, 2015) below).
Third, he reaffirms management's true intent for PV-10 (and the potential basis for its possible true worth), combination therapy-driving valuation notwithstanding:
"The ultimate goal for PV-10, an intralesional therapy injected directly into solid tumors, is to use it as a neoadjuvant therapy, or as an adjunct to surgery, Culpepper added."Amgen currently is recruiting for its neoadjuvant trial of intralesional agent talimogene laherparepvec ("T-vec") in Florida (Florida Hospital Memorial Medical Center), Kentucky, Utah and Switzerland: Efficacy and Safety of Talimogene Laherparepvec Neoadjuvant Treatment Plus Surgery Versus Surgery Alone for Melanoma. Utah means Dr. Robert Andtbacka, MD and Huntsman Cancer Institute. Kentucky may mean the University of Louisville, which also is a PV-10 CUP site.
Get with The Program (April 22, 2015)
 |
| Image source |
"Throughout our consultations with key clinical opinion leaders, we’ve also been attuned to the need to address the needs of patients with more advanced disease. With enrollment now complete in the mechanism of action study of PV-10 of Moffitt Cancer Center, and initial results reported on that study and on the companion nonclinical study to assess combination of PV-10 with immune checkpoint inhibition, we are making progress on design of our proposed study to assess PV-10 in combination with immune checkpoint inhibitions in patients with advanced melanoma.
We continue to expect this to be structured as a Phase 1b/2 study with a modest sized single arm Phase 1b component and expedited safety and efficacy endpoints supporting expansion to a larger randomized Phase 2 component. Endpoints for Phase 1b are likely to comprise acute safety as a combination regimen and objective response rate at three to four months. For Phase 2, we anticipate endpoints of progression free survival at overall survival. With three agents now approved in the US and also available in other key locations abroad, I expect to have very concrete details on this work to discuss at our next quarterly conference call. Importantly now that we have options for this systemic agent, this study can commence with or without the assistance of a partner." {Underlined emphasis is mine}
And: "In addition, our Phase 1 study of PV-10 for liver RECIST continuing to recruit patients, in particular those with tumorous metastatic liver. This work is continuing to provide valuable insight into potential additional areas of development since a range of cancers are metastasized to liver. It is also continuing to be supportive of our Pivot Asia for hepatocellular carcinoma or HCC and is a key driving force behind our partnering activities in China and elsewhere.
We expect to report initial data from this study at one or more international cancer conferences in the summer. We are also in continued dialogue with the key opinion leaders in Asia to guide – of our proposed phase 1b/2 study of PV-10 plus standard care for HCC. As with our melanoma combination study, I expect to have very concrete details to discuss at our next quarterly conference call."Combination therapy for melanoma. More than just running a combination therapy trial for melanoma (see "Oops" (April 10, 2015) below) with or without a Big Pharma partner, I am curious about the strategy of why, what, where and how to run combinations of PV-10 and {insert your PD-1 or PD-L1 drug here}. With the Pfizer patent for combining PV-10 and systemic immunotherapies now allowed, what is Provectus management's approach to this part of its CDP; a trial specific to melanoma, or a broader relationship and trial for solid tumors?
For example, Merck KGgaA's approach to its anti-PD-L1 agent avelumab has been to run "indication specific" and "indication general" trials:
- Indication specific includes (based on protocols/trials filed on www.clinicaltrials.gov) (i) merkel cell carcinoma, sponsored by subsidiary EMD Serono in the U.S., Australia, France, Germany, Italy, Japan, Spain and Switzerland, and (ii) non-small cell lung cancer, EMD Serono, in the U.S., Argentina, Australia, and a host of other countries.
- Indication general includes (iii) metastatic or locally advanced solid tumors (with expansion to gastric cancer), Merck KGaA, in Japan, and (iv) selected tumor indications, EMD Serono, in the U.S. and several other countries.
Takeaway: As such, I'm curious about how, if at all, Provectus' conversations about a geographic license deal in India evolves into a potential relationship. See Just visiting (January 30, 2015) below. For example, could India be a "test bed" for the treatment of multiple and certain solid tumor indications with PV-10 and a checkpoint inhibitor (rather than merely such a combination only for melanoma)?
Key components of the CDP, for both HCC and metastatic cancer very likely would have to be the presentation of clinical data, and better definition of the regulatory pathway(s) to approval. I speculated Eric may present initial data about the Phase 1 and expanded Phase 1 trials (e.g., hepatic objective response rate, overall survival to date) at the 17th World Congress of Gastrointestinal Cancer, July 1-4, in Barcelona, Spain, and then "cancer-free" data at The European Cancer Congress, September 25-29, in Vienna, Austria. See They Live (April 11, 2015) below.
A further nuance or distinction of how the data are presented could be indication; that is, HCC vs. metastatic cancer. Metastatic cancer may be presented at the 17th World Congress of Gastrointestinal Cancer (if applied to, and if accepted) (with some data on HCC), while the presentation at The European Cancer Congress (if applied to, and if accepted) might have an HCC focus.
Takeaway: Is the China liver CDP manifested as a Phase 1b for HCC in the country, presumably after an IND is filed there, or as a third cohort of the expanded Phase 1 trial? Is a U.S. liver CDP manifested as a Phase 1b for metastatic cancer? And, what and where are the data?
Combo (April 20, 2015)
Provectus issued a press release and made an associated 8-K filing today regarding the allowance of its patent with Pfizer for the use of PV-10 in combination with systemic immunotherapy agents in treatment of cancer. The press release reminded me of at least two things.
First, the PR notes "The allowed claims cover use of PV-10 in combination with systemic inhibitors of immune system down-regulation, such as anti-CTLA-4, PD-1 and PD-L1 antibodies, along with enhancers of immune system up-regulation, such as IL-2 and interferon-gamma." Underlined emphasis is mine. This reminds me of Wolchok, Allison and Mount Sinai's Newcastle disease virus combination patent application filed last year (see #Trending (April 9, 2015) below): "In addition, described herein are methods for treating cancer comprising administering Newcastle disease viruses in combination with an agonist of a co-stimulatory signal of an immune and/or an antagonist of an inhibitory signal of an immune cell." Again, underlined emphasis is mine.
Second, the PR references Pfizer's Dr. Eagle. I've previously written about Bristol-Myer's ipilimumab's Pfizer relative tremelimumab ("treme"). See here (September 2012) and here (April 2013). It was found, "...at the 2008 ASCO annual meeting, that the phase III trial of tremelimumab for the treatment of advanced melanoma did not have a positive result with regard to its primary endpoint." In 2011 AstraZeneca's MedImmune in-licensed treme from Pfizer and assumed global development rights. Pfizer retained the rights to use treme with specified types of combination therapies. {Bold emphasis is mine} Some believe there still is a role for treme in the treatment of cancer. At the annual meeting of the American Association of Cancer Research currently going on in Philadelphia, information from the University of Pennsylvania was presented about the combination of Pfizer's agonist (co-stimulatory agent) and anti-CD40 antibody — CP-870,893 ("pedaling faster") — and Pfizer's antagonist (co-inhibitor agent) and anti-CTLA4 antibody ("releasing the brakes") —treme:
Abstract: Combination of agonistic CD40 monoclonal antibody CP-870,893 and anti-CTLA-4 antibody tremelimumab in patients with metastatic melanoma
The audio of Provectus' April 9th panel discussion is available, and [in my view] makes for a good listen. Panel members comprised Provectus' CTO Dr. Eric Wachter, PhD and CFO/COO Peter Culpepper, medical oncologist Dr. Sanjiv Agarwala, MD (St. Luke's) and surgical oncologist Dr. Merrick Ross (MD Anderson). There are several comments that struck me, such as (transcribing precisely or roughly):
Sometimes (often?), it might be smart to keep things simple. If data drive the share price, market capitalization, and the deal, then its straightforward to observe Provectus has not generated enough "data:" e.g., presentation (or publishing) of pre-clinical or clinical data, filing of trial protocols, starting of studies and trials, establishing regulatory clarity, etc. — data that are visible to and can be digested and evaluated by investors and the market. Arguably, management hasn't generated data for several years. So it's no wonder the share price has stagnated.
Take, for example, the melanoma [monotherapy] clinical development program ("CDP") (see the table below), which has been the company's lead indication. One could argue, 2014's volatility notwithstanding — January 2014's reporting of December 2013's Type C meeting, March 2014's submission of the breakthrough therapy designation application and its May denial — no "data" has been generated since 2012. Moffitt Cancer Center has been more active, and visible.
With less overall data generated, the same can be said of Provectus' melanoma [combination therapy] CDP.
Liver CDP substance has been sparse, too. Of course, a possible look into the data may have been revealed during Provectus' April 9th panel discussion; see, for example, They Live (April 11, 2015) below.
Ditto once again for the CDPs of other cancer indications, and PH-10.
The takeaway for me with this blog news item is, as I wrote in a revisiting of my investment letter, March 2015's Why I Remain Long Provectus Biopharmaceuticals: "If early-stage drug development company described Provectus from 2002 to 2015, 2015 marks the beginning of the Company’s transition into a regulatory-focused and data-driven organization" {Underlined emphasis is mine}.
Among other "data" in 2015:
Provectus issued a press release and made an associated 8-K filing today announcing it opened patient enrollment for the company's pivotal Phase 3 trial of PV-10 (versus systemic chemotherapy dacarbazine) for unresectable locally advanced cutaneous melanoma. As expected (see Reactions (March 12, 2015) below), the first clinical site open is St. Luke's University Hospital and Health Network in Bethlehem, Pennsylvania. St. Luke's investigator is Dr. Sanjiv Argawala, MD, a medical oncologist.
Last week, on April 10th, an article entitled Phase 3 trial to compare PV-10 with chemotherapy in stage III melanoma discussed the trial and quoted Agarwala, who also participated in Provectus' panel discussion last Thursday. Notable quotes included:
Provectus' CTO Dr. Eric Wachter, PhD addressed the melanoma Phase 3 trial during last Thursday's panel session. Selected slides follow:
PV-10 becomes the latest intralesional ("IL") agent to enter a pivotal melanoma Phase 3 trial. Prior IL agents included Vical's Allovectin-7 (velimogene aliplasmid), which failed its Phase 3 trial in 2013, and Amgen's talimogene laherparepvec ("T-Vec'"), which barely passed its trial (when results were reported in 2013). Both agents were indicated for advanced melanoma, where Stage 3 patients comprised 52% and 30% of the trial populations, respectively. PV-10's Phase 3 trial is for locally advanced cutaneous melanoma, and Stage 3 patients make-up the entire N.
Elaborating on Eric's last slide above, Demographics and Response Data for Recent Melanoma Immunotherapy Studies:
Previous/recent immunotherapy studies involving IL agents (Allovectin-7, T-Vec) and checkpoint inhibitors (ipi, pembro, nivo) have been carried out for advanced melanoma, where Stage 3 patients are in the minority or nearly non-existent. PV-10's trial is exclusively comprised of Stage 3 patients who will have all of their melanoma disease treated as needed without limitation (unlike in the Phase 2 trial, which limited treatment).
Although it may be comprehensive and inclusive to question whether PV-10 may succeed in its Phase 3 trial, more germane, honest and relevant is to query the trial's enrollment rate in order to ascertain how quickly the trial might reach, at a minimum or at least, the interim analysis checkpoint.
Preview (April 15, 2015)
Provectus' CFO/COO Peter Culpepper's slides for his presentation at the ChinaBio Partnering Forum 2015 later tonight (U.S. time zones) may be read starting here. There do not appear to be any changes to the deck from used by Peter at the Growth Capital Expo in Las Vegas on Monday.
The World Congress of Lymphology (April 15, 2015)
Provectus is a sponsor of the 25th World Congress of Lymphology, September 7-11, in San Francisco. It's likely Moffitt Cancer Center's Dr. Jonathan Zager, MD, a surgical oncologist, will speak about PV-10 in the session he co-chairs on September 9th (see Therapy against In-Transit Metastatic Melanoma on the right). Zager may have been or still is a member (see page 15 of the May 2014 link immediately prior)(and this probably compensated as such) of Eric's oncology advisory board.
The conference itself seems to me [as a non-medical person] very specific: i.e., "lymphology, the study of the integrated lymphatic system (lymphatics, lymph nodes and lymphoctyes." From an October 2012 article entitled Progression of cutaneous melanoma: implications for treatment that included Zager as a co-author: "The survival rates of melanoma, like any type of cancer, become worse with advancing stage. Spectrum theory is most consistent with the progression of melanoma from the primary site to the in-transit locations, regional or sentinel lymph nodes and beyond to the distant sites." And from 2008 (another author): "When melanoma spreads, it does so invariably by the lymphatic system which drains to the regional lymph nodes. Uncommonly, melanoma can become trapped in the lymphatic vessels and grow to cause tumour nodules in the skin and subcutaneous tissues between the primary site and the regional lymph node basin. These nodules are termed in-transit metastases and carry an ominous prognosis." Of course, Moffitt's murine model work (presented in April 2014 at AACR) demonstrated injection of tumors with PV-10 "...led to an infiltration of dendritic cells to the lymph nodes draining the treated tumors."
Note the patient population for Provectus' pivotal melanoma Phase 3 trial below.
My takeaway for Provectus' sponsorship of this event: Increase awareness of the Phase 3 trial for better enrollment.
_f_z_r (April 14, 2015)
Provectus' strategic advisory board member Dr. Craig Eagle, MD [now] is General Manager of Pfizer Oncology Canada.
They Live (April 11, 2015)
St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala, MD's presentation at the company's panel discussion in New York on Thursday, before management removed his last eight slides from the deck put up onto the website, potentially provided insight into the framing of clinical liver data to be presented this year at a medical conference or two.
As background, Agarwala's original presentation provided data on 10 patients from Provectus' Phase 1 and expanded Phase 1 liver trials (see the two slides below; purple writing is mine). Takeaways could include:
Provectus initiated the Phase 1 trial in October 2009 with the goal of enrolling and treating up to six patients. Initial enrollment, the treatment of the first cohort of three patients, was completed by September 2010. The second cohort (of three patients) was treated by January 2011 (n.b. the linked press release is dated January 27, 2011 on the page listing PRs in that timeframe; however, the PR itself is dated January 27, 2010). Based on the company's unchanging liver slide from its previous corporate website presentation, it would appear at least 5 HCC lesions and 1 CRLM lesion initiall were treated. Agarwala noted 7 HCC and 5 metastatic lesions were treated across 10 patients.
Provectus expanded the liver Phase 1 trial's protocol (the "expanded Phase 1 liver trial"), but had not reported any further information on the trial's status until Thursday (i.e., no press releases on accrual/enrollment and/or treatment). It would appear 4 patients from the expanded Phase 1 were included in Agarwala's slides (i.e., 6 from the Phase 1 + 4 from the expanded Phase 1 = 10) (it has been rumored upward of 20 patients were treated in the expanded trial).
The cancer-free dates for patients in the Phase 1 trial, where the relevant time frame is at least five years of survival, should be August and December 2015, respectively. See below.
H/t a shareholder for sparking this thought: Noting the 50-month survival reference as well as a reference to a planned combination study with ipilimumab for melanoma in H2-2014 means part or all of Agarwala's Thursday presentation very likely also was given [by him] at the 2014 Beijing International Melanoma Congress on October 18 in Beijing, China (because of the calculated timing above, and because pembrolizumab was approved in September [and management pivoted to it as the preferred partner in a combination study towards year-end or into 2015]). See below, and also 2014 Beijing International Melanoma Congress (October 7, 2014) on the blog's Archived News II page.
Takeaways of this news item post could include: Eric may present initial data about the Phase 1 and expanded Phase 1 trials (e.g., hepatic objective response rate, overall survival to date) at the 17th World Congress of Gastrointestinal Cancer, July 1-4, in Barcelona, Spain, and then "cancer-free" data at The European Cancer Congress, September 25-29, in Vienna, Austria.
"Oops" (April 10, 2015)
Provectus removed eight slides from Dr. Agarwala's panel discussion presentation (see link immediately below) when his presentation was put up on the company's website. As one shareholder put it, "Interesting how they snuck in the liver data into yesterday’s slide presentation like it was some casual update. Why wouldn’t this be framed into more of a public disclosure unless much more was coming?" Two of the slides are below in Initial Clinical Testing: HCC and Liver Mets (April 10, 2015). All missing slides follow, with commentary on (in purple) the slide.
Initial Clinical Testing: HCC and Liver Mets (April 10, 2015)
Provectus released slide presentations from yesterday's panel discussion by Provectus' CTO Dr. Eric Wachter, PhD and St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala, MD. Of note was Agarwala's comment regarding clinical liver overall survival data (second slide below):
Share (April 9, 2015)
Provectus-related material provided to attendees of the panel discussion. The folder on the left contains various items, while the Word document on the right is a list of said items in the folder. Clicking on the folder icon will (should) open it in a new browser tab and display its contents. Clicking on the document icon will (should) open it in a new tab too for reading.
Panelling (April 9, 2015)
A shareholder in attendance at today's panel discussion should provide feedback to the blog later today. Watch this space.
Updated 4/9/15: A shareholder provided a picture of Provectus' poster that would be included in the upcoming weekend's HemOnc today Melanoma and Cutaneous Malignancies conference in New York.
Of note [at least for me] is the right hand side of the poster.
Management also said they expect 25 clinical sites in the U.S. and 10 (primary and satellite locations) in Australia for the upcoming pivotal melanoma Phase 3 trial. MD Anderson's Dr. Merrick Ross, MD commented the clinical site numbers speak to both the interest in this trial and the difficulty with finding patients for it.
Calendars (April 9, 2015)
Provectus has a new webpage, Calendar of Events (h/t an investor), that details company events. For example, Provectus is
#Trending (April 9, 2015)
It would appear PV-10 — a so-called front-end, starter of the engine or stepper on the gas pedal, but all immune system priming or activating — and the associated intellectual property surrounding this use (i.e., the combination therapy patent application jointly filed with Pfizer) is growing in importance to Provectus' business strategy and intrinsic valuation. With regard to the former, what opportunities exist for the company to strike a uni- or multi-indication co-development deal to combine PV-10 with a immune checkpoint inhibitor, or should/does Provectus do its own combination therapy trial with a reimbursable inhibitor? With regard to the latter, how much value could accrue to the company from this aspect of cancer treatment (i.e., late-stage disease, combination therapy/therapeutics)?
Hat tip to @bradpalm1 for leading me to the following 2014 paper and patent application. The paper is entitled Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Authors were Dmitriy Zamarin, Rikke B. Holmgaard, Sumit K. Subudhi, Joon Seok Park, Mena Mansour, Peter Palese, Taha Merghoub, Jedd D. Wolchok and James P. Allison. {Bold emphasis is mine}
Clinical trial sites. Provectus previously said all compassionate use program ("CUP") locations would be clinical trial sites for the company's upcoming pivotal melanoma Phase 3 trial; specifically, 8 sites of which one was not yet named. My take on actual and potential sites is below.
I don't think all 7 named CUP sites will be active in the trial (i.e., Sharpe Memorial, for example, primarily was/is a liver trial site). Four additional sites might come on-line sooner (i.e., Huntsman, Roswell Park, Moffitt, and Peter MacCallum). Interestingly, Provectus might have sites in four of Australia's six states. Additionally of note, Melanoma Institute Australia has a total of 18 campuses and Peter MacCallum has five.
Cancer immunotherapy stock index. Forbes contributor Luke Timmerman recently wrote about Brad Loncar's Loncar Cancer Immunotherapy Index in an article entitled Cancer Immunotherapy Is All Over The Science News -- And Now It Has Its Own Stock Index (April 7th). Timmerman wrote:
The "small" biotechnology category of Loncar's index is comprised primarily of companies with checkpoint inhibitors and ex vivo immunotherapies that sport an average market capitalization (as of April 8th) of $2.2 billion.
Mentor's index was comprised primarily of cancer vaccine companies (or in vivo approaches to immunotherapy) that had much small market capitalizations in 2009 (than Loncar's comparable category) (note: Provectus and Advaxis were added in 2010 and 2011, respectively).
In 2013 Mentor's index had grown to add a few more stocks but still was dominated by cancer vaccine companies with small market capitalizations.
Mentor's index valued today (setting aside bankrupt companies) has an average company market capitalization one-quarter of Loncar's comparable category, perhaps representing the in vivo-ex vivo divide.
He Got [Immune] Game (April 8, 2015)
As I wrote in Talimogene laherparepvec (March 20, 2015) below, in February Amgen announced two FDA advisory committees would review its intralesional agent (oncolytic immunotherapy) talimogene laherparepvec ("T-Vec") for the treatment of patients with injectable regionally or distantly metastatic melanoma. The committees comprised the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC"), and the Oncologic Drugs Advisory Committee ("ODAC"). The meeting date is April 29, 2015, at which time Amgen's Biologics License Application ("BLA") for T-Vec would be discussed. Amgen submitted its BLA for T-Vec to the Agency in August 2014. T-Vec's PDUFA date is October 27, 2015.
According to Tarius SAC Tracker, "[r]eportedly, the FDA had initially set the date for July 28, 2015, but pushed it back for Amgen to submit additional information." {Underlined emphasis is mine}
Amgen (i.e., Amgen researchers) submitted two T-Vec-related abstracts to the 2015 annual meeting of the American Association of Cancer Research ("AACR"). The first is Talimogene laherparepvec activates systemic T-cell-mediated anti-tumor immunity:
Familiarity (April 7, 2015)
Today Provectus issued a press release and filed an associated 8-K related to PV-10 data and use being presented at St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala's HemOnc Today Melanoma and Cutaneous Malignancies Annual Meeting in New York at the end of this week. PV-10 data and use has been presented at Agarwala's conference since 2011 (i.e., this is the fifth year). 2011's PR is here, 2012's is here, 2013's is here, and 2014's is here. The conference has had a portion of it dedicated to "Local Regional Therapy for Melanoma," such as the 2012 portion of the agenda below.
As Provectus presumably waits for clinical site institutional review boards ("IRBs") to approve the final pivotal melanoma Phase 3 protocol, it is interesting to note (a) how little has changed in terms of the broad brush strokes of a Phase 3 trial management contemplated...
...and (b) combination with other therapies.
Huntsman Cancer Institute's and likely Provectus melanoma Phase 3 principal investigator Dr. Robert Andtbacka presented (c) the 2012 overview below of intralesional agents in development...
...and (d) comparison of intralesional agent response rate following injection of tumors.
Remember, Andtbacka also was/is a paid consultant for several intralesional agent compound companies: Amgen (OncoVEX/T-Vec), Vical (Allovectin-7) and Viralytics (Coxsackievirus A21). Recently Andtbacka said of T-Vec:
Silk Route (April 4, 2015)
Provectus' March 12th conference call provides some insight into the process of achieving a deal in China with Sinopharm for a regional license of PV-10. If or when a deal gets inked should depend on the process, and achieving certain milestones or checking certain boxes, as opposed to whether the parties can arrive (or have arrived) at a mutually agreeable set of deal terms and conditions.
A precedent transaction would be Aeterna Zentaris and Sinopharm A-Think, where the former licensed a drug to the latter, and also transferred its relevant technology to the other. Aeterna received a non-refundable $1 million fee, presumably at signing, for the transfer of its technology. A-Think agreed to make additional payments upon achieving certain pre-established regulatory and commercial milestones, and to pay royalties on future net sales. A-Think is responsible for all of the development, production, registration and commercialization of the drug. Because of confidentiality, the license agreement (see Article 6, Milestone Payments; Royalties) between the parties did not disclose either milestone event or payment details for (i) approval (section 6.1) [there are several events & payments], (ii) commercial (6.2) [there are several events & payments], (iii) royalties (6.3.1), and (iv) combination products (6.3.2) [there are several events & payments]. Without this information (i.e., milestone and subsequent payment), it's difficult to evaluate and assess Aeterna's deal, and more importantly use it to potentially project Provectus'.
Provectus' process with Sinopharm A-Think thus far may be more about the former endeavoring to complete (together with the latter) certain approval- or regulatory-related milestones (acceptance by or approval of the Chinese FDA ["sFDA/cFDA"]) before consummating the transaction. The March 12th conference call does not tell us anything about what the specific regulatory milestones that Provectus might be trying to knock-out are, but they might well include some of those required by the comparable FDA process, such as (a) submitting an investigational new drug ("IND") application to (i) the sFDA/cFDA and (ii) the institutional review board ("IRB") of the Chinese medical institution [site] conducting a PV-10 trial that (b) both have to accept or not disapprove of the IND for it to be effective. The Chinese IND, like in the U.S. and around the world, permits a Phase 1 study to commence in the country.
Here is what the conference call told us and/or may be telling us:
Huntsman Cancer Institute and University of Utah surgical oncologist, and talimogene laherparepvec ("T-Vec") (Amgen) and Allovectin-7 (Vical) pivotal Phase 3 trial investigator Dr. Robert Andtbacka, MD recently disclosed he is a consultant to Provectus. He remains a consultant to Amgen (formerly BioVex, the innovator of T-Vec), and was a consultant to Vical. PV-10, T-Vec and Allovectin-7 are intralesional agents. The disclosure was made at Moffitt Cancer Center's Dr. Jeffrey Weber's, MD, PhD's 11th Annual International Symposium on Melanoma and Other Cutaneous Malignancies, which was held in Miami on March 7th. Andtbacka [as far as I can tell or research on the Web] did not disclose an affiliation with Provectus in 2014, but did cut a video about PV-10's upcoming Phase 3 trial while discussing T-Vec in Zurich at the 2014 annual meeting of the Society of Melanoma Research Congress (see Mind the gap (January 24, 2015) below).
This could (should) mean Hunstman and Andtbacka would be a clinical trial site and investigator, respectively, for Provectus' pivotal melanoma Phase 3 study. Also part of Weber's conference's faculty was Dr. Ahmad Tarhini, MD, a medical oncologist from the University of Pittsburgh.
Coinciding with the 2015 annual meeting of the Society of Surgical Oncology ("SSO") in Houston from March 25th to 28th, an educational activity sponsored by Amgen (donation) and Provectus (grant) was held on March 27th proximate to the SSO annual meeting, and entitled Advances in Immunotherapy for Advanced Melanoma: Integrating Intralesional and Anti-PD-1 Immunotherapies into Current Practice. Faculty included Andtbacka, St Luke’s University Hospital & Health Network and PV-10 investigator Dr. Sanjiv Agarwala, MD (medical oncologist) and MD Anderson Cancer Center and PV-10 investigator Dr. Merrick Ross, MD (surgical oncologist).
Dr. Argawala is chairing an education session from 8-9:15 am on May 31st at ASCO 2015 entitled Locoregional Therapies in the Setting of Systemic Treatment Advances: What's Next? (part of the conference's Track(s): Melanoma/Skin Cancers; Developmental Therapeutics and Translational Research). The panel also includes Dr. Tarhini. I imagine both T-Vec and PV-10 will be discussed.
March Blog Stats (April 2, 2015)
Primary and Metastatic Liver Cancer Work (March 29, 2015)
Management said they would present clinical liver cancer trial data at one or more international conferences this summer. The expanded liver Phase 1 trial started in September 2012. Per the trial’s protocol patients were divided into two cohorts; one received PV-10, and the other both PV-10 and sorafenib. Prior to treating patients in the PV-10-plus-sorafenib cohort, however, Provectus conducted an in vitro study that demonstrated a low risk of clinically relevant drug-drug interaction between Rose Bengal and sorafenib derivatives. The original liver cancer trial clinical site was Sharp Memorial Hospital of San Diego, California. Second site The Southeastern Center for Digestive Disorders & Pancreatic Cancer of Tampa, Florida began recruiting in April 2013 (investigator: Dr. Alexander Rosemurgy, MD). Third site St Luke's University Health Network of Bethlehem, Pennsylvania began recruiting in February 2014 (investigator: Sanjiv Agarwala, MD). Trial changes can be found here.
After reviewing the Provectus' Insurance expense categorized under Research and development in the company's 10-Ks, a considerable number and the bulk of trial's liver patients may have been treated in 2013, with some treated in 2014. Provectus' insurance expense includes insurance for clinical trials and the company's compassionate use program ("CUP").
Interestingly and notably, the company has not had any clinical trial insurance claims to date, according to Provectus' CFO/COO Peter Culpepper (if he accurately answered my question, which was whether Provectus had incurred any claims through the end of 1Q15).
I tabulated Insurance expense over time. See the table below.
Insurance can go up or down for several reasons: A higher or lower number of on-going clinical trials (e.g., more/different sites, more patients). Different indications would warrant difference insurance costs. Claims should increase insurance expense. More or less patients treated in the CUP may increase or decrease costs. Additionally, Peter may have prepaid insurance expenses in one year for another.
With no trials, save the CUP, running from 2010 to about 2012, it would appear a "baseline insurance expense" could be about $90-100K. To what would one attribute the more than $60K and $15K increases [above baseline] in 2013 and 2014, respectively? An increase in CUP patients is one answer (the number of new CUP patients in 2014 is forthcoming). Another answer also could be more treated liver patients. The increase in insurance expense would seem to substantiate the rumor of 20ish patients treated in Provectus' expanded liver Phase 1 trial.
Underlining (March 26, 2015)
Provectus' CFO/COO Peter Culpepper's BIO Asia International Conference presentation slides of March 25th are available here. Whoever advised Peter on this version of his presentation sure must like to underline words.
The third of three clinical trial sites for Provectus' PH-10 Phase 2 mechanism of action study (A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10), Miami, Florida's International Dermatology Research, has begun recruiting.
Underlining notwithstanding, there were several interesting and notable changes to Peter's Tokyo presentation (right, below), compared to the one he gave in Zurich at the European Life Science CEO Forum & Exhibition on March 3rd (left, below).
"In vivo adaptive immunity" is new language from management, with in vivo referring to performed or taking place in a living organism.
I thought it was a good add and reminder that median progression-free survival ("PFS") was not reached for Stage III patients in Provectus metastatic melanoma Phase 2 trial.
It's good to read PV-10's liver program will have a higher profile in 2015. And...?
Peter's language regarding the Phase 1b/2 trial of PV-10 in combination with immune checkpoint inhibition, first pembrolizumab and then the others seems to diminish the value of the initial message of introducing pembro in the first place. Moffitt mechanism of action data will be published. Liver data arrives in 2015.
I do not believe Provectus' combination therapy claims that cover anti-PD-1 and anti-PD-L-1 therapeutics are included in the above mentioned 58 claims that are public, but covered in those that are yet to be made public on the U.S. Patent and Trademark Office website.
DMF stands for Drug Master File. I'm curious why Peter inserted this acronym here, and now. A DMF is a submission to the FDA of information, usually concerning the confidential detailed information about Chemistry, Manufacturing and Controls ("CMC") of a drug product or a component of a drug Product. No company must file a DMF, unless information contained in DMF is used to support a new drug application ("NDA")(or an IND or ANDA) and the NDA references the DMF (in which case the Agency would review the DMF). Recent DMFs are a better indicator of intent to manufacture than older DMFs.
Although management said they are assessing potential clinical study of other indications like breast and pancreatic cancer in Provectus' recent 10-K filing (see page 10), it is highly unlikely such work would commence this year given management’s already-taxed mental bandwidth.
Investigational Immunotherapy Agents for Melanoma (March 23, 2015)
H/t @bradpalm1: From a March 24th article by Dr. Lisa Raedler, Ph.D. in Targeted Oncology entitled Tumor Antigens and Their Role in Immune Response in Melanoma:
Work in progress (March 23, 2015)
I am writing an updated investment letter (working title: Why I Remain Long Provectus Biopharmaceuticals), which would be a follow-up to my September 2013 letter entitled Why I'm Long Provectus Biopharmaceuticals (Pharmaceuticals prior to the company's 2013 name change). My current draft summary points include:
In February Amgen announced two FDA advisory committees would review its intralesional agent (oncolytic immunotherapy) talimogene laherparepvec ("T-Vec") for the treatment of patients with injectable regionally or distantly metastatic melanoma. The committees comprised the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC"), and the Oncologic Drugs Advisory Committee ("ODAC"). The meeting date is April 29, 2015, at which time Amgen's Biologics License Application ("BLA") for T-Vec would be discussed. Amgen submitted its BLA for T-Vec to the Agency in August 2014. T-Vec's PDUFA date is October 27, 2015. According to Tarius SAC Tracker, "[r]eportedly, the FDA had initially set the date for July 28, 2015, but pushed it back for Amgen to submit additional information."
Is it a given that the Agency approves T-Vec for the above indication? Might the PDUFA date be pushed out again? The ODAC meeting should be revealing and likely pivotal in nature, in terms of gleaning the FDA's perspectives on (i) T-Vec's pivotal trial's comparator, which was GM-CSF, (ii) viral shedding, which references the risk of transmission of the virus upon which the immunotherapy is constructed (T-Vec was engineered from herpes simplex 1 [HSV-1]), and (iii) the relevance of the trial's primary endpoint, which was durable response rate ("DRR") (defined as the rate of CR or PR lasting continuously for 6 or more months). The pivotal trial met its primary endpoint: 16% DRR in the T-Vec arm v. 2% in the GM-CSF arm (p<0.001).
Conference Presentations (March 18, 2015)
Provectus will be presenting at the 12th Annual BIO Asia International Conference, which runs from March 24-25 in Tokyo (investor/financial-oriented).
Move the chains (March 17, 2015)
On Provectus' March 12th conference call, CFO & COO Peter Culpepper said in regards to the company's discussions with Chinese pharmaceutical company/drug distributor Sinopharm:
To score the touchdown of larger hoped for at signing/upfront monies, it appeared [based on Peter's response to Dr. Gullem above] that Provectus required both melanoma and liver trial protocols (Phase 3 and Phase 1b/2, respectively) appropriate to China to be completed. Peter may have thought such a deal was doable given the then circumstances, and was thwarted (again) when Eric surprised (or was surprised) in late-December 2014 by having to conduct a further late-January 2015 meeting with the FDA related to the melanoma Phase 3 trial protocol (see December 22nd press release Provectus Biopharmaceuticals to Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment).
What was to have been ready-to-go in 3Q14 (i.e., melanoma) only now (i.e., as we near the end of 1Q15) is good-to-go, and I believe it is unlikely Eric will have the liver protocol for China in place before the memorandum of understanding ("MOU") expires in about 2 months.
Assuming (i) a mutually acceptable deal indeed may be had and (ii) there are enough at-signing (upfront) dollars acceptable to Provectus management, it would seem prudent for them to (a) consummate a transaction with Sinopharm on the basis of the recently filed, final, melanoma Phase 3 protocol and thus collect some at-signing money, (b) shunt previously hoped for/contemplated additional at-signing dollars related to an in-place liver protocol to a near-term date when Eric can deliver this protocol (e.g., by the end of 2Q15), and (c) not have extend the MOU for a second time.
Presumably without having to settle for a lesser overall deal but rather consummate whatever originally anticipated deal with the same dollars but different dates that presumably encouraged them to enter into an MOU with Sinopharm in the first place, Provectus management would have moved the chains with an eye still on reaching the end zone.
e.g., pain, infection, significant bleeding (March 16, 2015)
On May 23rd, Provectus released FDA correspondence dated May 16, 2014 revealing the Agency turned down the company's request for Breakthrough Therapy Designation ("BTD") for PV-10, and locally advanced cutaneous melanoma. In this denial letter, the FDA noted:
In the May 23rd conference call that followed release of the BTD denial letter, Eric said:
Today the company issued press release Provectus Biopharmaceuticals' Amended Protocol of PV-10 for Phase 3 Study as Treatment for Melanoma Now Available Online, and filed an associated 8-K. Among other things in it, Provectus confirmed "The previous secondary endpoint of "change in total symptom score from baseline using the patient reported Skindex-16 instrument (to be assessed 12 weeks after Day 1)" has been re-assigned exploratory endpoint status..." but also noted "...this may change once the Company completes an ongoing assessment of the suitability of the Skindex-16 instrument for this patient population."
Key endpoint measures ostensibly remain progression-free survival ("PFS"), complete response rate ("CRR") and overall survival ("OS").
If PRO remains shunted to exploratory status or ultimately remains tangential in nature and importance to the trial's outcome — merely a measurable outcome that does not rise to the level of needing to be passed— then I would wonder whether and if that is tacit acknowledgement by the FDA that making the tumor go away is tantamount to making a patient's disease symptom(s) go away, and another illustration of Agency support for the applicability of the drug to a defined patient population in need of better treatment solutions (and thus a clear pathway to approval).
Intralesional Therapy for In-transit and Satellite Metastases in Melanoma (March 15, 2015)
April 2015, Surgical Oncology Clinics of North America. Drs. Kendra J. Feeney, MD and Michael J. Mastrangelo, MD, Department of Medical Oncology, Sidney Kimmel School of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania
Note: Rose bengal = PV-10 (and PH-10)
Speed, Brah (March 14, 2015)
On Thursday's conference call, Provectus' Chief Technology Officer Dr. Eric Wachter, Ph.D. established [for me] a baseline from which to examine certain other features of the company's pivotal melanoma Phase 3 trial:
A decision tree might look like this:
Changes to Eric's initial protocol were made and went live on ClinicalTrials.gov today. See More/Official Differences (March 13, 2015) and Differences (March 13, 2015). Taken in total, I believe these changes may (i) make it easier to enroll and thus (ii) increase trial enrollment more than initially anticipated or estimated, and potentially (iii) improve results:
Side-by-side changes from ClinicalTrials.gov for Provectus' upcoming pivotal Phase 3 trial for locally advanced cutaneous melanoma may be found here at the .gov site. Additional changes include patients who are are BRAF V600 wild-type (formally BRAF V600E), and:
The key is below.
Differences (March 13, 2015)
Differences between what should be Provectus' final protocol (March 11, 2015) for its pivotal melanoma Phase 3 trial and its initial protocol (November 7, 2014) are below:
1. Purpose
2. Endpoints (Outcome measures)
4. Arms
5. Detailed description
6. Inclusion criteria
Final Protocol (March 13, 2015)
H/t a shareholder: Provectus' protocol for its upcoming pivotal melanoma Phase 3 trial has been updated on ClinicalTrials.gov: PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma
Reactions (March 12, 2015)
Initial reactions to Provectus' conference call ("CC") include:
New Patent (March 10, 2015)
A new patent issued today on the US PTO's website, Topical medicaments and methods for photodynamic treatment of disease {bold emphasis is mine}. The abstract reads:
Fresh (March 10, 2015)
Buttressing management's belief in PV-10's multi-indication viability beyond the clinical trial data produced to date in melanoma, liver cancer, and breast cancer are data generated in the Provectus' compassionate use program and by third parties such as Moffitt Cancer Center and other investigators/researchers.
In Peter's March 3rd European Life Science CEO Forum & Exhibition presentation he highlighted the company's plans for 2015 that also included data generation and presentation by Provectus and Moffitt.
On Thursday's conference call I'd like to hear Eric provide a comprehensive outline of pre-clinical and clinical work having been and being conducted with PV-10, such as:
Provectus last updated the market on the company's progress with the Therapeutic Goods Administration ("TGA"), Australia's version of the FDA in November 2010 in a press release entitled Provectus Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia, noting:
I understand Eric met with the TGA during his visit to Australia in December, which also coincided with the Annual Scientific Meeting ("ASM") of the Clinical Oncology Society of Australia ("COSA"). If one may assume (a) he updated the TGA on PV-10's status, so to speak, and (b) there remains the above mentioned path to approval, the questions then may be (i) whether and when Eric would submit an application to the TGA to move forward, and (ii) whether the TGA would move independent of the FDA.
Updated 3/6/15: Patients counting toward the 300 figure must be those treated only for melanoma. As a result, 328 above would be lessened by 12 (breast), 6 + 20 (liver), and some number of non-melanoma CUP patients treated from 2009-2014. It's more than likely a rough number of at least 50 patients from the melanoma Phase 3 trial would push the count above 300.
Network 1 (March 5, 2015)
Network 1 (Testaverde, Hemming) are on the west coast of Florida fundraising for Provectus, which likely will draw down ~$4 million (i.e., a quarter's operating burn) of the remaining $7.2 million of the $15 million private placement. See Sources and Uses (February 19, 2015) below. Fundraising is a process that includes introducing the company to prospective investors and updating existing ones as you contemplate forming capital, with a decision to accept/take money potentially coming later.
Oddities (March 4, 2015)
From Peter's recent European conference presentation, when comparing it to the baseline one so as to understand what additional comments and statements he added (a sampling is below):
[Above] Thank you?
[Above] What does their objective mean?
[Above] See blog post They ARE out to get me..., and Amgen's upcoming clinical trial entitled Phase 2, Open-Label, Single-arm Trial to Evaluate the Correlation Between ORR and Baseline Intratumoral CD8+ Cell Density in Subjects With Unresected Stage IIIB to IVM1c Melanoma With Talimogene Laherparepvec (underlined emphasis in mine). One might say, [very] loosely, T cell density is a proxy of a sort for adaptive immunity.
[Above] My hunch is the addition of Fast Track and AA (accelerated approval) reflect their hope and goal of the steps of the regulatory process following the generation of sufficient interim data from the upcoming pivotal melanoma Phase 3 trial. The addition of Brazil as a potential geographic license deal that would not interfere with eventually hoped global licensure is notable. Who is the large cap biotech that they're speaking to?
Updated presentation slides (March 3, 2015)
One of this blog's most popular posts is January 2012's The Wisdom Of Crowds. I'm appreciative of the many eyes (irrespective of their "side of the trade") that are focused on Provectus. Peter updated the slides following what I linked to yesterday; see Presentation slides (March 2, 2015) below. Among the changes of note:
Provectus' Peter Culpepper is presenting at the 8th Annual European Life Science CEO Forum & Exhibition tomorrow — slides are available here. There does not appear to be additional information beyond what was included in February's BIO CEO slide deck.
Cost-benefit (March 2, 2015)
Bristol-Myers announced the FDA accepted their biologics license application ("BLA") for ipilimumab as an adjuvant treatment for patients with stage 3 melanoma who are at high risk of recurrence today, noting a projected FDA action date (a PDUFA date) of October 28, 2015. The trial protocol on ClinicalTrials.gov is here. Adjuvant therapy is treatment given in addition to the primary one, which in this case was surgery. The trial compared surgery (used first) + ipi (used second) versus surgery (used first) + a placebo (used second). Biologics generally are approved by FDA via a BLA rather than an new drug application ("NDA"). I would imagine Bristol-Myers will be successful in this outcome, as it seems to me there is no "standard" for adjuvant treatment in the NCCN guidelines.
While important to be aware of, I don't believe the above is meaningfully relevant to
From an June 2014 article authored by Joe Barber about the Bristol-Myers trial entitled Bristol-Myers Squibb's Yervoy improves recurrence-free survival in Phase III melanoma trial:
Prior Art (February 28, 2015)
There was considerable discussion by Provectus management about a so-called co-development deal that would see PV-10 combined with a checkpoint inhibitor mid- to late-last year. Consider Peter's comments at a Chicago investor gathering held during ASCO (early-June), his comments on the company's June 19, 2014 and November 6, 2014 conference calls, and management's discussion in Provectus' 3Q 2014 filing. This talk was followed up by Moffitt's pre-clinical combination work involving PV-10 and co-inhibitory blockade at SITC 2014 (November). In November 2014 Pfizer announced a global strategic alliance with Merck Germany and licensed the latter's anti-PD-L1 therapeutic, while revealing the existence of its own anti-PD-1 therapeutic.
If Peter had said in June, for example, that one could expect (among other things) a co-development deal within 90 days, you'd imagine there may have been discussions along those lines with a prospective partner or two. Part of the no-outcome time that has since elapsed assuredly results from Eric as a rate limiting step (from the perspective of producing a suitable Phase 1b/2 trial protocol for a combination therapy pairing). Another contributory factor may have been the waiting for Moffitt's independent pre-clinical combination therapy work to be presented (and, possibly, a peer-reviewed publication, which of course has not yet materialized). Additionally, prospective partners may want to firmly establish the value proposition for combining their drugs with PV-10; intralesional agent T-Vec's preliminary results with ipilimumab (response rate and immunologic signalling) should help this discussion.
But I wonder how much of the dampening on Provectus' ability and/or speed to arrive at a co-development deal with a Big Pharma holding a checkpoint inhibitor (if one assumes there is sufficient medical and scientific rationale to do so) is Pfizer's doing. I think it's a very safe assumption Provectus has regularly engaged Pfizer's legal department (at a minimum) starting before both companies filed the combination therapy patent application in March 2012 and since. Both companies may be equally conservative when it comes to addressing how they protect intellectual property, or one may be more conservative than the other and thus lead the process (over the other) as a result. In reading an October 2012 article by Gene Quinn (h/t a shareholder) entitled The Impact of the America Invents Act on the Definition of Prior Art, I wonder whether Pfizer, out of an abundance of caution (and conservatism), insisted the combination patent be formally and fully allowed before permitting Provectus to enter into a co-development deal and/or commence a combination therapy trial.
N = 7 (February 27, 2015)
Moffitt embarked on a Phase 1 feasibility study in humans, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study, to "...to find out more about how PV-10 works in melanoma tumors." The trial protocol was submitted to ClinicalTrials.gov in December 2012. At AACR 2014 (April) some data was presented on the first eight patients (N = 8). Total enrollment was to have been 15.
More than a year earlier, in a February 2013 Cancer Watch article entitled Back to Phase 1: Understanding Systemic Effects of PV-10, it was written:
Moffitt changed the status of the Phase 1 study from Recruiting to Active, not recruiting this week. All fifteen patients were enrolled and treated.
Combinatorics (February 26, 2015)
In the category of too much time on my hands..., re-visiting #3 of Some things I think or guess (February 15, 2015) below, I erred in my speculative math of when Provectus’ combination therapy patent with Pfizer could issue. Using the company's synthesis patent (far from a determinative or comprehensive approach), I compared the times between filing and publishing the patent application, and between filing and allowing of the patent. My original "math" suggested an approval timeframe for the combination therapy patent as late as May (Q2), because I adjusted for the extra month or so it took between filing and publishing the combination patent application compared to the synthesis one. If I simply assume it'll take about the same time for both patents to be or have been allowed, it is conceivable the combination patent could be approved by the USPTO in early-March.
Far more important than the mathematical gymnastics above, I am curious about the following:
Having an approved patent such as this should have value, particularly as the focus grows on the stimulatory aspect of a stimulatory-inhibitory drug-drug combination. But how much value, and how is this value "unlocked" or monetized? And how much value does Pfizer ascribe to it?
Market Making (February 22, 2015)
Provectus' designated market marker ("DMM," formerly known as a specialist) at the NYSE MKT (formerly the American Stock Exchange) is J. Streicher & Co. It is possible Provectus may change DMMs to KCG Americas, speculation based solely on the increase of shares held by the firm's parent KCG Holdings ("KCG") as of its 12/31/14 13F SEC filing (I would presume Peter proactively had conversations with the firm starting at least in 4Q14). Market makers that "...exercise investment discretion over $100 million or more in Section 13(f) securities are required to file Form 13F." According to Provectus, they do not intend to switch DMMs.
Moving from Streicher to KCG should be a good thing because the latter is much larger than the former, and would bring more heft to market making activities for Provectus' stock. KCG has a net worth (i.e., total assets minus total liabilities) potentially more than 40 times that of Streicher. KCG also reported a market value of $6.1 billion of stocks held in the above mentioned 13F filing (I don't believe Streicher has ever made such filings, and none in recent memory).
According to the NYSE, DMMs "...manage a physical auction to combine with an automated auction that includes algorithmic quotes from other DMMs and market participants, are required to meet stringent NYSE depth and continuity standards and, to encourage participation and bolster market quality, are on parity with Display Book and floor broker quotes." Other NYSE MKT DMMs include Brendan E. Cryan and Company, KCG Americas and Virtu Financial Capital Markets (it's not clear from the link when this list was last updated, possibly 2014).
DMMs vary by the size of their balance sheets, among other characteristics and parameters. The smaller DMMs are J. Streicher ($17.5 million net worth as at 9/30/04, the last statement of financial condition is here; no 13F filings) and Brendan E. Cryan (no statements appear to be available). The much larger ones are KCG Americas (KCG has a $745 million net worth as at 6/30/14) and Virtu (a net worth of $203 million as at 12/31/13, unaudited; $813 million market value of stock holding as of the firm's 12/13/14 13F filing). KCG was formed in 2013 by the merger of Knight Capital Group and Getco, when the latter acquired the former in the wake of Knight's 2012 electronic trading blow-up.
My takeaway for this news item is: Knight has much, much greater size heft than Streicher, possibly 40-60 times more depending on the parameter e.g., balance sheet, stock holdings, etc.). Should (when) market interest increase in Provectus' stock, KCG would be far better placed to handle it than Streicher [ever could].
Sources and Uses (February 19, 2015)
Re-visiting #2 of Some things I think or guess (February 15, 2015) below, I think the company will raise money to offset another quarterly burn.
Provectus' CFO & COO Peter Culpepper's definition of fund raising is different from mine. He may consider fundraising the act of accepting subscriptions from a private placement, or when the money hits the company's bank account. Fundraising is a process, which may or may not culminate with the deposit. As he prepares for the end of Q1, Peter probably already has begun the fundraising process, with the likelihood but not certainty he will raise about $4 million (i.e., a quarter's worth of cash burn) at the end of March or the beginning of April.
Ending calendar/fiscal year 2014, Provectus may have had about $21 million of cash on hand. To arrive at this figure, I assumed an increase in 4Q14 monthly burn ($1.2 million) over the 3Q14 figure ($1.157 million).
The threshold to keep in the good graces of the NYSE seems to be the combination of a minimum cash balance ($18 million), fund raising ability (confirmed sources, and at or above $1 per common share [warrant coverage appears not to count]), and regulatory [and maybe commercial] progress (maybe going so far as to show FDA correspondence to the stock exchange).
Assuming the same 4Q14 monthly cash burn for 1Q15, it's unlikely Peter has to raise money until the end of the quarter, when Provectus' cash balance should fall below $18 million.
Provectus' $1 million or so monthly operating expense likely also covers the company's pivotal Phase 3 trial expense where the amount is converted into a monthly expense, lumpy as it may be from time to time.
Peter should be able to readily access the balance of the Network 1 Financial private placement already in place and from which Provectus previously drew down $7.8 million in gross proceeds, leaving him a balance of $7.2 million. The terms of the placement are units priced at $1.00 per common share, including 50% warrant coverage with a $1.25 exercise price (5-year term).
For planning purposes Peter may see the $7.2 million balance as a confirmed and easily accessible source of funds for the company to clear 2Q15 and 3Q15's operating expenses, or very nearly so. If PVCT's share price is below a dollar when the time comes to raise money, Peter would turn to Network 1. If, however, the share price is significantly above $1, he then could turn to Cantor and draw money from the at-the-money ("ATM") financing vehicle already in place there.
One way (Network 1) or the other (Cantor), Peter very likely has to raise money going into Q2, which means I don't believe he concludes a regional geographic license transaction in Q1. I also don't believe potential catalysts in this quarter, largest among them the commencement of the pivotal melanoma Phase 3 trial, would push the share price meaningfully above $1, indicating fundraising dollars this would come from Network 1 again.
Eric may file the U.S. liver Phase 1b/2 protocol (sorafenib vs. PV-10) with the FDA in Q2, and finalize the China liver one (country-specific local ablation technology [possibly radiofrequency ablation or "RFA"] vs. PV-10) in the same quarter. If this plays out — that is, Eric meets his timeline — it is possible a transaction, which surely requires a finished liver protocol as a necessary and potentially sufficient condition, is consummated.
Should a deal be done, the money (i.e., whatever payment amount is available at signing) may arrive 30 to 60 days thereafter depending on how quickly Provectus and Peter move to definitive agreements with the partner (e.g., Sinopharm). If there truly has been material progress towards a deal and Peter has been smart, the term sheet has been finalized and the definitive agreements have been framed if not prepped.
More than likely, the money arrives in Q3, which would mean raising (either at the end of Q2 or at the beginning of Q3) up to one more quarter of operating expenses. If a China deal is consummated on good to great terms, Peter should be able to draw from the Cantor ATM (hopefully given a much higher share price than $1).
It's not clear to me whether a U.S. liver Phase 1b/2 trial can start without regional transaction license money (or a materially higher share price resulting from a transaction announcement that makes raising money for this trial expense worth it from a dilution calculation perspective) because I do not believe the additional liver trial cash expense is accounted for in Provectus' monthly operating expense (as I believe the melanoma Phase 3 trial is). Interestingly, a U.S. liver Phase 1b trial may be sized at 20-25 patients (allowing certain data to be collected in hopes/with the thought of "pivotalizing" the Phase 2 portion), which then might be affordable within current monthly burn boundaries (at $40-50,000 per U.S. liver patient).
My takeaways from this news item include: (i) Provectus likely will raise a quarterly burn offset amount, (ii) Peter should do so towards the end of the first quarter, or the beginning of the second, (iii) the company likely would obtain those funds via Network 1 unless potential catalysts materialize and push the share price above the threshold for securing capital via Cantor, and (iv) further fundraising, as well as the timing of such, would be strongly influenced by the status of the advancement of the liver clinical development program in Q2.
Institutional Ownership (February 18, 2015)
As reported through February 18th thus far, Provectus shares held by institutions who made SEC form 13F filings (~4.9 million) and shares held as a percent of those outstanding (2.7%) increased during the quarter ending December 31, 2014 (from ~4.1 million and ~2.3%, respectively, at 9/31/14). Data was collected from Whalewisdom.com.
Numbers (February 17, 2015)
Re-visiting #1 of Some things I think or guess immediately below, I don't believe the outcome of the trial necessarily or exclusively is predicated by the comparison (i.e., "horizontal separation") of the median progression-free survival ("PFS") of the PV-10 treatment arm compared to the median PFS of the chemotherapy (DTIC or TMZ) control arm. The latter appears to be well established in the literature and by clinicians (and probably well understood by the FDA). The former should continue to extend so long as lesions and tumors are treated with PV-10, and patients remain in the trial (and are evaluable).
I wonder if (think) the outcome of Provectus' upcoming pivotal Phase 3 trial for locally advanced melanoma, and the possibility of early success, is focused on the "vertical separation" of the two PFS curves at 12 weeks, the trial's first assessment period. A timeframe of 12 weeks, or 3 months, should exceed the established or understood efficacy of chemotherapy.
So, rather than count the number of patients enrolled and treated in the trial (2:1 randomization, 2 of three patients enter the PV-10 treatment arm and 1 patient enters the chemotherapy control arm) to reach horizontal clinical significance (N_h), I wonder if (think) the number of patients is that required to reach vertical clinical significance (N_v, at 12 weeks).
The trial's projected hazard ratio (I use a baseline assumption of the last publicly noted figure of 0.545, see blog post Hazard ratio, and other stat stuff), together with its' alpha and power (and assumptions and projections of clinical performance of its arms) may help a layperson grossly estimate N_v, which my very rough and probably too simplistic math suggests could be the enrollment and treatment of ~125 patients (the trial has been designed to enroll a total of 225 patients). On the other hand, the trials' actual hazard ratio may suggest only about ~50 patients may be necessary for the vertical separation of the PFS curves at 12 weeks to reach clinical significance.
Some things I think or guess (February 15, 2015)
#1. I think speculating when the trial could reach a successful outcome requires estimating when half of trial’s N (number of patients) will be enrolled and treated, and thus estimating the enrollment rate.
#2. I think the company will raise money to offset another quarterly burn.
#3. I think Provectus’ combination therapy patent with Pfizer will issue in April or May, paving the way (given this additional level or stage of intellectual property protection) for a co-development deal to be struck to combine PV-10 with another company’s checkpoint inhibitor.
#4. Sometime in Q2, I think Eric will file the Phase 1b/2 liver protocol (for the U.S.: PV-10 v. sorafenib), paving the way for the trial to start.
#5. I guess the company will present Phase 1 liver data with an overall survival orientation (and possibly preliminary expanded Phase 1 data with a tumor regression orientation) at ASCO (May 29-June 2).
Coming Soon (February 13, 2015)
Eric's guidance is informative but not instructive; his actions, however, more often are both. I'm of the view or opinion Provectus will start its pivotal Phase 3 trial (registration study) for locally advanced cutaneous melanoma in March. I lean toward this more not solely because of his quote in Provectus' Provectus Biopharmaceuticals Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study press release of "We are making a number of small changes to the protocol in light of this review, and will issue a final version later this month," but because he recently made a change (February 11th) to the company's compassionate use (expanded access) program's study details on ClinicalTrials.gov. The specifics of the change are not fully described, however; only a change having been made is noted. The CUP was established in June (Australia) and October (U.S.) 2009 after the metastatic melanoma Phase 2 trial was being wound down (patient accrual ended in May, patients were observed for 52 weeks). Certain patients from the Phase 2 trial eventually were transitioned to the CUP. Such programs have the exclusion criteria of cancer patients who are eligible for an existing clinical trial. With the Phase 3 trial about to start, it would make sense for Eric also to make some changes to the CUP.
Two down, one to go (February 10, 2015)
H/t a shareholder: The second of three clinical trial sites for Provectus' PH-10 Phase 2 mechanism of action study (A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10), University of North Carolina School of Medicine (Department of Dermatology), has begun recruiting. Texas' DermResearch began recruiting last month.
Who's on first, What's on second, Review's on third (February 10, 2015)
In the company's press release Provectus Biopharmaceuticals Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study, Eric said:
Ablative Immunotherapy (February 6, 2015)
PV-10 may be as much a singularly unique drug as Rose Bengal may be a singularly unique molecule. A good read about Rose Bengal ("RB"), PV-10 and Provectus' principals remains Walter Alexander's August 2010 article entitled Rose Bengal: From a Wool Dye to a Cancer Therapy. A water-soluble dye created by Gnehm in 1882, RB weighs less than 1,000 Daltons. I'm still struck by RB's near century of prior clinical use:
As of January 2015, there are 3,720 medical literature citations of RB listed on PubMed, more than 235 of them related to cancer. The key to the story of PV-10, and Provectus, is that the therapeutic benefits of RB remained hidden until the 1980s, when sufficient quantities were first administered in preclinical studies.
At that time the FDA and Japanese Ministry of Health and Welfare were scrutinizing artificial food colorings. Japanese researchers Ito et al. evaluated red food dye No. 105 (RB) tumorigenicity, observing "dose-dependent survival increases in the mice receiving Rose Bengal" in their 1986 paper.
I believe as early as 1998, although it may have been a few years later, Provectus’ multi-disciplinary team of founders, a molecular virologist (Craig Dees), a chemical engineer (Tim Scott) and a chemist (Eric Wachter), were looking for drug candidates having antineoplastic activity separately came across Rose Bengal. According to Alexander's recounting their story of discovery, a commercial data search by them identified several hundred candidates. Dees et al.'s own subsequent screening promptly zeroed in on RB. Subsequent preclinical tests with bacterial and cancer cell lines quickly demonstrated RB’s impressive cytotoxic activity. Follow-on animal and human studies confirmed RB, delivered directly into tumors, was a selective and potent agent for ablating cancers and harnessing the immune system.
The Medical Review(s) component of the drug approval package for Bristol-Myer's anti-PD-1 therapeutic Opdivo (nivolumab) included an interesting blurb about a reviewer's concern regarding possible bias. Investigators at 8 clinical trial sites disclosed payments by Bristol-Myers totaling ~$12 million for 26 research grants, contracts, and speaking honoraria and bureau fees.
The reviewer compared the objective response rate ("ORR") of the dataset (N = 167, Opdivo = 120, investigator's choice of chemotherapy = 47) upon which application for approval was made against the ORR of the subgroup of patients from non-conflicted sites (N_noConflict = 127, Opdivo = 90, chemotherapy = 37). He or she concluded the exclusion of conflicted sites did not impact efficacy evaluation.
Same as it ever was (February 3, 2015)
Provectus updated it's website presentation, which historically has served as one of Peter's corporate communication mediums, on January 14th to reflect his view (as understood from Eric) of the start of the company's pivotal Phase 3 trial for locally advanced cutaneous melanoma.
The previous version (at least from what I track/tracked) from December 15, 2014, prior to Provectus' December 22nd press release Provectus Biopharmaceuticals to Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment, guided the below:
Intralesional therapy for advanced melanoma: promise and limitation (February 2, 2015)
Medical oncologist, St. Luke’s Cancer Center staffer, Professor of Medicine at Temple University School of Medicine and Provectus principal investigator Dr. Sanjiv Agarwala, MD is out with a Current Opinion in Oncology article entitled Intralesional therapy for advanced melanoma: promise and limitation, which focuses on the patient population who would benefit from intralesional therapy (PV-10); see Keep talking (and keep doing) (January 29, 2015) below.
H/t a shareholder:
January Blog Stats (February 1, 2015)
Just visiting (January 30, 2015)
Merck KGaA visited the blog from Darmstadt, Hessen, Germany. Merck entered into a $2.5 billion deal and collaboration with Pfizer in November to develop and commercialize Merck's PD-L1 agent and Pfizer's PD-1 agent.
Keep talking (and keep doing) (January 29, 2015)
Surgical oncologist, MD Anderson Cancer Center staffer, and Provectus principal investigator Dr. Merrick Ross, MD recently described oncolytic immunotherapies ("OIs") as rupturing tumors in a manner similar to a multivalent vaccine. See Knick-knacks (January 28, 2015) below. It's an interesting label. PV-10 is, of course, not a vaccine; however, management has described the drug's immune response (mechanism of action #2) deriving from its chemoablation (mechanism of action #1) "...as tantamount to “in-situ vaccination” (SITC 2012)— in situ to mean "on site," "locally," where it takes place, etc., and vaccination to mean stimulate the immune system to develop adaptive immunity.
Additionally, "[v]accines may be monovalent (also called univalent) or multivalent (also called polyvalent). A monovalent vaccine is designed to immunize against a single antigen or single microorganism. A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms."
While PV-10 does generate cancer immunity ("...induces tumor-specific T cell-mediated immunity in multiple histologic subtypes..."), it is not a vaccine because it is not constructed to immunize against particular antigens. Whatever antigens exist in tumors injected with PV-10 subsequently become, if successful, the antigens against which PV-10 immunizes.
H/t a shareholder: Dr. Suzanne Topalian, MD, Professor of Surgery and Oncology, and Director, Melanoma Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University speaks about Immunotherapy for Melanoma Treatment: Approved and Investigational Drugs (October 2014), and notes local injection (i.e., intralesional therapy, and PV-10) under tumor vaccines (presumably sponsored by the Melanoma Research Alliance). The video of her presentation follows the picture below. Her discussion of melanoma vaccine platforms begins at 8:09.
H/t a shareholder: Medical oncologist, St. Luke’s Cancer Center staffer, Professor of Medicine at Temple University School of Medicine and Provectus principal investigator Dr. Sanjiv Agarwala, MD appears to be out with a Current Opinion in Oncology article entitled Intralesional therapy for advanced melanoma: promise and limitation, which focuses on the patient population who would benefit from intralesional therapy (PV-10):
In his article entitled Provectus seeking Indian PV-10 API patent and says it is "more than a match" for skin cancer competitors, in-PharmaTechnologist.com writer Gareth MacDonald quotes Provectus' CFO & COO Peter Culpepper as saying "“we are more than able to match the competition and beat it in the US." Provectus' Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isobenzofuran-1,9-xanthen]-3-one (Rose Bengal) and Related Xanthenes, which [according to the company] was filed as an application with the State Intellectual Property Office of the People's Republic of China ("SIPO") in September 2010, the same time as it was filed with the U.S. Patent and Trademark Office ("PTO"). The PTO published the synthesis patent application in March 2011. SIPO published it in July 2012, and allowed it in January 2015. In the case of India, the application was filed in March 2012 and published in May 2013. It's nice to hear Peter speak like this; however, keep doing [more].
Knick-knacks (January 28, 2015)
Comments by Dr. Robert Andtbacka, MD., Associate Professor, University of Utah School of Medicine, and Merrick Ross, MD, Professor, University of Texas MD Anderson Cancer Center at The Society for Melanoma Research annual congress in Zurich, Switzerland (November 13 to 16) about oncolytic immunotherapies were interesting:
Pfizer's recent Q4 2014 conference call provided an interesting answer from its Chairman and CEO, Ian Read.
Provectus principals have only bought shares through stock option exercises (i.e., insider buys or purchases). There have been no insider sales since the company's inception. Insider buying is considered a bullish signal, although such buying often can be poorly timed:
Below is a table of Eric's exercises from 2011 to date (preliminary metastatic melanoma Phase 2 clinical trial data was presented in November 2010). Eric's exercise of 1 million stock options on September 6, 2011 (transaction date; the filing date was September 7th) was excluded because all principals exercised that grant that day (as it was bonus compensation).
Below is a graphic of the above exercises combined with several key (but not exhaustive) regulatory affairs outcome dates.
In 2011 Eric regularly exercised a roughly regular amount of stock options: ~94K every 28 days.
He did not undertake another exercise for nearly a year (347 days, from December 5, 2011 to November 16, 2012) despite Provectus's January 18, 2012 press release Provectus Receives Guidance From FDA On Pathway to Approval for Phase 3 Trial of PV-10 For Metastatic Melanoma. He later expanded in October 2, 2012 press release Provectus Pharmaceuticals Presents Final Phase 2 Melanoma Data at ESMO 2012, commenting on the Phase 3 study design:
The company's May 13, 2013's Provectus Updates Shareholders in Its Annual CEO Letter cryptically then but understandably now contained more Eric-speak of lack of regulatory affairs progress:
Almost 10 months (310 days) have passed since Provectus submitted via overnight courier its BTD application, and Eric exercised options again, this time at a ~25% premium to the share price. He may be more confident again about PV-10's regulatory pathway, and the process of reaching this critically important destination.
Stock Option Exercise (January 26, 2015)
Provectus' Dr. Eric Wachter, Ph.D., exercised options to buy underlying common stock, and filed an associated Form 4 today. Specifics about this exercise may be found in the table below. See Stock Option Exercises (January 8, 2015) below for complete tables for other management principals.
Provectus updated the commercial strategy slide of the corporate website presentation. See Commercial Strategy (January 14, 2015) below.
Dermatology & PH-10 (January 26, 2015)
H/t a shareholder: Provectus' mechanism of action study for PH-10—A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10—now is recruiting.
Mind the gap (January 24, 2015)
The current National Comprehensive Cancer Network ("NCCN") guidelines for melanoma still appear to list "clinical trial" as the top recommendation for patients with disease where resection is not curative.
Provectus' upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma, which the FDA determined met the criteria for a serious or life-threatening disease or condition, addresses a patient population for which, again, clinical trial is the preferred primary treatment.
One of the topics of discussion between Provectus and the FDA (as noted in the company's December 2014 press release Provectus Biopharmaceuticals to Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment) may be the gap that exists between the standard of care ("SOC") for this patient population (which is NCCN guidelines) and labeling of systemic immunotherapies. Bristol-Myers' ipilimumab, for example, is indicated for unresectable or metastatic melanoma (underlined emphasis is mine). While discussions with the Agency may yield changes in eligibility requirements that could increase the speed of enrollment of patients in Provectus' study, these changes could influence a prospective label for PV-10 in the event the trial is successful and the drug ultimately is approved.
The germane inclusion criterion (for the above) is "Failed, did not tolerate, or not a candidate for (due to co-morbidities, pre-existing autoimmune disease or drug unavailability) treatment with ipilimumab or another immune checkpoint inhibitor." Addressing this successfully (for Provectus) may allow PV-10 to bridge this gap, become SOC, and take its place in NCCN guidelines.
T-Vec and Allovectin-7 principal investigator Dr. Robert Andtbacka (of the Huntsman Cancer Institute) cut a video about PV-10's upcoming Phase 3 trial while discussing T-Vec in Zurich at the 2014 Society of Melanoma Research Congress during a satellite symposium sponsored by Amgen and entitled Oncolytic Immunotherapy – Engaging the Immune System to Target Melanoma. See below.
Andtbacka was an NCCN Guidelines panel member for version 1.2015. Three Provectus principal investigators and consultants (Thompson, Khushalani and Ross) also were members.
Missing Something (January 22, 2015)
Modified (T-Vec and PV-10 added to original Credit Suisse illustration/table) from source tweet.
Returning to Seeking Alpha, sort of (January 21, 2015)
Seeking Alpha ("SA") reached out to me on January 13th, presumably as part of a routine campaign to gather content from bloggers for SA, to ask if they could publish some of my blog posts as SA articles. SA previously asked me this last August—see Seeking Alpha, and this blog (August 26, 2014) on the blog's Archived News II page—but I turned down the SA Director/Editor who contacted me then in large part because of the looseness and incompleteness of his communication on the topic.
According to SA Director/Editor Abby Estikangi-Carmel who contacted me last week, SA offered to monitor Connecting the dots...Provectus Biopharmaceuticals for articles they thought were suitable for their website, and post them under my SA account, which has been dormant since October 2013 (see the blog's Disclosures page). She also said at the time that SA would back-post a couple of my blog posts using their original publication dates to update my SA presence.
Before agreeing to their request, I asked Ms. Carmel several questions including:
They also initially placed only my blog's disclaimer under the articles as a disclosure, rather than [what Ms. Carmel originally agreed to do, which was to] link to the blog's Disclosures page. SA tweeted the articles:
SA also commented on StockTalk on my behalf:
Takeaway: I am not returning to SA in any real sense—I will not be publishing articles or or post Instablog posts there myself, nor will I be replying to comments under articles SA publishes. The website essentially served the purpose I originally had intended, which was to publish an investment letter (and thesis) about Provectus (see September 2013's Why I'm Long Provectus Pharmaceuticals). SA may or will choose to publish as articles from this blog whatever posts they select, and whenever they select them. "Returning" under the above mentioned situation and conditions seems fine to me, as SA's website and community is a much larger forum than this blog to "talk my book"/"pump."
A Twisted Interview (January 21, 2015)
Provectus' CFO/COO Peter Culpepper was interviewed by The Wall Street Transcript ("TWST"). The interview was first published Friday via TWST subscription. The company made it available today. It appears the interview was conducted in late-2014, probably December. Three items struck me.
1. Pfizer's tremelimumab. Peter said "Pfizer first approached us because they wanted us to combine PV-10 with their initial anti-CTLA-4 immune checkpoint blockade, which is an analog of Bristol’s."
It's interesting to read Peter short comment above this situation. See, for example, November 2011's blog post The Usual Suspect[s], and other posts by searching for tremelimumab on the blog using the search tool.
Takeaway: Beginning around the time Pfizer's Dr. Craig Eagle, M.D. commenced his relationship with the company, he wanted to do a transaction with Provectus—in the above case, an out-license from Pfizer to Provectus. What does this history, now confirmed (sort of), do for me today? Nothing; however, it does frame certain other Pfizer fiction.
2. Protocol ordering. Peter said "Yes, there is an order. The order we’ve established is the Phase III melanoma study is filed; we’re waiting for FDA interaction to make sure all the particulars are fine. Then, they will submit the Phase Ib/II combination study design, which we said is underway, and we are finalizing it. As a matter of fact, we’re meeting with key opinion leaders on this topic next week. Then, we do the Ib/II liver study. We’ve been meeting with individuals also for our liver program on a regular basis. That probably will come very shortly after the combo, and we’ve met with individuals globally...So the Ib/II for liver would follow very closely after the combo, and then, breast unfortunately would be after that, but it would be the same study design instead of, say, using sorafenib or the checkpoint blockade as the combo piece. We would use chemotherapy or radiation, but breast would definitely come after Phase III is underway and enrolling, the Phase Ib/II combo is underway and enrolling, and Phase Ib/II liver is underway and enrolling."
So: i. melanoma Phase 3, ii. melanoma combination Phase 1b/2, iii. liver Phase 1b/2, and iv. breast Phase 1b/2. Previously, I had understood management's guidance was melanoma, liver, and melanoma combo. See, for example, Provectus' Q3 2014 conference call.
Takeaway: The impact of the re-ordering is negligible to nothing. I merely am struck, however, by the fact Peter changed the order, because [for me] it convey's Eric order, as said order may have been influenced or guided by Peter's business and corporate development activities.
3. San Antonio Breast Cancer Symposium ("SABCS"). Peter said "So I would say, with the next three PV-10 studies, certainly we’ll see those studies generating meaningful data in 2015. I hope the answer will be yes in breast. We’re pushing on that. We have already established a relationship with a breast cancer advocacy group. We plan to be at San Antonio Breast Cancer Symposium in San Antonio in December. We’re very focused, and of course, we’ve done clinical work in breast, so we know PV-10 is appropriate for breast, and we’ve been encouraged by potential partners to be treating breast cancer, particularly in China and India where breast cancer is a top concern, but liver cancer is of course also a cancer of very significant concern."
Cancer clinical trial work has evolved from Phase 1, 2 and 3 trials to, somewhat recently, Phase 1b/2 trials that may lead to pivotal trials suitable for drug approval consideration. See terminology provided by Eli Lilly and Company:
Takeaway: For Provectus, among other things, Eric's throughput has been a rate-determining step for the progress of the clinical development program. The company may be able to participate in 2015 SABCS if he improves his work-rate.
Combinations & Category Building (January 20, 2015)
Combinations of therapies, where one of the the two combined therapies is a checkpoint inhibitor (e.g., anti-CTLA-4, anti-PD-1), and thus combination therapy clinical trials, appear to be predominantly if not exclusively focused on advanced melanoma.
There are lots of different ideas about how and what to combine. Combinations can include therapeutics (i.e., one drug with another), and therapies like surgery and radiotherapy (i.e., a drug with radiotherapy). For this blog news item, I'm going to focus on the combination of two drugs from two different categories—specifically, co-stimulatory and co-inhibitory. Checkpoint inhibitors fall into the co-inhibitory ("co-in") category, and intralesional agents like T-Vec and PV-10 would fall into the co-stimulatory ("co-stim") category. Current approaches appear to focus on exploring, very generally speaking, (i) two co-ins and (ii) a co-stim and a co-in. It strikes me (i) represents doubly releasing the brakes of the immune system, while (ii) may suggest starting its engine and then releasing its brakes.
Success of combination therapies (and more broadly speaking, success of cancer treatments) ultimately may derive from the immunogenicity of cancer tumors to which the combination therapies are applied. More available immunogenic material in tumors (i.e., melanoma) may make it easier and more amenable for checkpoint inhibitors to work (as monotherapies) than cancers where tumors are less immunogenic or not immunogenic at all (i.e., pancreatic cancer). As a result, (i) above may see two co-ins trying to make what they can of available immunogenicity, while (ii) above may see the co-stim improve and increase tumor immunogenicity for the co-in to then do its thing.
As an aside, "tumour immunogenicity varies greatly between cancers of the same type in different individuals and between different types of cancer." Melanoma tumors are considered more immunogenic than lung cancer tumors; however, renal cell carcinoma, melanoma, lung cancer and prostate cancer "...tumors may be more immunogenic than once believed." Interestingly, Bristol-Myer's melanoma trial of its PD-1 Opdivo (nivolumab) generated a 40% objective response rate ("ORR"). The Big Pharma's non-small cell lung cancer trial for Opdivo generated a 15% ORR.
When Moffitt Cancer Center's Dr. Jeffrey Weber called T-Vec a "a niche drug that would be best used to prime the immune system," he may have meant priming the immune system when treating melanoma (or, at best, a limited number of cancer indications). As a therapy engineered from herpes simplex 1 (HSV-1), while T-Vec could be administered into cutaneous, subcutaneous or nodal lesions in its clinical trials, injections of visceral lesions were not permitted. See PV-10 & Amgen's Talimogene Laherparepvec (June 9, 2014) on the blog's Archived News II page. All of T-Vec's clinical trials to date are melanoma-related. A trial for squamous cell carcinoma of the head and neck was terminated was terminated, presumably by Amgen, after the Big Biotech acquired Biovex (the privately held Boston-based that developed T-Vec when it was called OncoVEX).
Priming the immune system for melanoma and other cancer indications likely means, from the perspective of those who believe generating as many antigens as possible for the antigen-presenting cells ("APCs") to take-up/present to the immune system (i.e., T-cells, natural killer cells and other immune system components), engaging as many tumors as possible and harnessing/increasing those tumors' immunogenicity.
Moffitt Cancer Center fully understands PV-10 is efficacious in multiple cancer indications. For example, see Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer.
PV-10 has been injected in melanoma, breast cancer and liver cancer via clinical trials, and other indications treated by Provectus' compassionate use program. If PV-10 has the potential to prime the immune system for treating melanoma, I imagine it has the potential to prime the immune system for other cancer indications.
Category building (January 19, 2015)
It bears watching T-Vec's progress as part of its category building of "[intralesional] oncolytic immunotherapies," and how Amgen's IL agent establishes the category's bona fides in terms of priming the immune system. The notion of a stimulatory agent (e.g., PV-10, T-Vec, vaccines, etc.) making non-immunogenic tumors immunogenic, and immunogenic ones more so recently has gained traction as a novel and additional approach to treating cancers, and to making checkpoint blockade agents more relevant.
As much as PV-10 is exploring new ground (middle stage patients with cutaneous and subcutaneous melanoma), T-Vec, via its acquisition by Amgen, is exploring more new ground (in combination with checkpoint blockade agents for late-stage patients here and here, and surgery in middle-to-latish stage patients having cutaneous, subcutaneous and nodal melanoma) beyond its initial pathway to licensure (metastatic melanoma/late stage patients).
Conference Presentations (January 16, 2015)
Provectus appears to be presenting at the 17th annual BIO CEO & Investor Conference—February 9-10 in New York (investor/financial-oriented)—and the 8th Annual European Life Sciences CEO Forum (the company is a sponsor)—March 4-5 in Zurich (partnering/networking-oriented).
Commercial Strategy (January 14, 2015)
Provectus' updated their corporate website presentation to include a slide entitled PV-10: Commercial Strategy (see below).
It is a good idea to communicate such information and thoughts in this way, and better layout management's strategy and sentiment with respect to monetizing PV-10. I'd also build on the slide's bullet points:
Provectus' compassionate use program ("CUP"), or expanded access program ("EAP"), which the company initiated in 2009 (June in Australia, October in the U.S., and all patients in Provectus' metastatic melanoma Phase 2 trial were treated by September) reached a low point of 8 new patients in 2012 before adding double-digit new patients in 2013, very likely in 2014 and potentially in 2015 (based on CUP site physician requests prior to year-end). See Compassionate Use/Expanded Access (January 5, 2015) below. Despite the approvals of ipilimumab (2011), peginterferon alfa-2b (2011), vemurafenib (2011), dabrafenib (2013), trametinib (2013), pembrolizumab (2014) and nivolumab (2014), there still appears to be room and demand for PV-10.
It is odd Provectus' facilitation of PV-10 expanded access has been used by physicians so much with so few issues or fanfare, especially considering CUP patients are there because they have run out of options. I have not been able to find information on EAP results of other oncology drugs from U.S.-based sites; however, there have been papers written about the results of international patients from using these therapeutics. I imagine this dearth of data could be chalked up to U.S. federal and state patient privacy regulations.
Provectus' CUP has existed in various forms since inception, including multiple physician-sponsored ones, Phase 2 continuation expanded access, a "pure" EAP (i.e., a treatment size IND), and other oddities. There does not appear to be a lot of pure EAPs (i.e., treatment size or treatment INDs).
There also does not appear to be a lot of oncology EAPs in general, perhaps more more than 30 such programs a year.
All of Provectus' FDA filings and correspondence letters should be stored in the company's electronic data room. CUP data, which may find its way into various filings but mostly in annual reports and special filings (i.e., investigator brochures to accompany PV-10 and PH-10 developments), presumably could provide a visitor with a more comprehensive view of treated patients and their histories.
Let me get this straight... (January 10, 2015)
She's on the strategic advisory board to help Provectus prepare for when other pharmaceutical companies come calling to license PV-10 and/or acquire the company?
Nothing to see here, move along.
"Time value of money" (January 9, 2015)
In recent years, when discussing Provectus's potential acquisition (as a company designed from the start to be sold, rather than one focused on out-licensing multiple drug candidates), Craig consistently said (paraphrasing) in Phase 3; that is, sometime during the company's pivotal Phase 3 melanoma trial. I don't believe, however, that he was being too specific or general. All along I think he's been saying that in order for management to the get the lofty price they seek for Provectus and its rather unique drug with unique mechanisms of action and immune response, they'd have to generate enough mature data for Big Pharma to move.
According to Silicon Valley Bank ("SVB"), "[i]n the last few years, there has been a noticeable shift in the direction of big exit M&A activity from early-stage to the later-stage." SVB defines the "big exit" as "...private venture-backed M&A with at least $75 million upfront in biotech..." I am using private venture-backed as a proxy for Provectus Biopharmaceuticals. Provectus is neither venture-backed nor private, but has operated as de facto one: "closely held" by a core network of retail investors, and governed pretty much only by management (and then only by a subset until recently).
Interestingly, using data through 2012, "[t]he time from founding of a company to trade sale has significantly increased since 2005. Whereas the average “time to exit” was a bit more than 5 years in 2005 it now has increased to almost 9 years, indicating that VC-backed biopharma companies have to develop their products in later-stage clinical trials before they can orchestrate a trade sale." A trade sale is European lingo for M&A or an acquisition. Provectus was founded in 2002.
Discussing the same with Peter, he has not said in Phase 3. Rather, Peter said (paraphrasing) Provectus would be acquired when Big Pharma could finally ascertain its time value of money for PV-10, which has been fluid because the FDA and Eric were not able to converge on the initial regulatory approval pathway to licensure until very recently. Before finalizing certain operational aspects of a fully agreed upon pivotal Phase 3 trial for locally advanced cutaneous melanoma—2015—there was an appeal for Breakthrough Therapy Designation ("BTD") for locally advanced cutaneous melanoma but of course a question of what would come with BTD in terms of additional clinical steps prior to submission of a new drug application ("NDA"). Before BTD—2013-2014—there was the pursuit of an agreement on a special protocol assessment ("SPA") for a pivotal Phase 3 trial for metastatic melanoma. Before the SPA—2012-2013—there was the pursuit of an accelerated path to approval: 2009/10-2011/12.
Let's say that one way Big Pharma thinks about acquisition, licensing and partnering is to evaluate companies with Phase 3 assets that could be launched in a year, fit their profiles, and fill gaps in their pharmaceutical portfolios (see, for example, this 2009 paper). I take "could be launched in a year" to mean (i) a pivotal trial underway for (ii) a specific indication in (iii) a distinct patient population, all of which Provectus now established.
Thus, if the time value of money now can be estimated—launch time frames may be projected when Provectus' Phase 3 trial begins (the assumption of course is when the trial succeeds, not if)—it then would not be surprising for Big Pharma to begin calculating.
Provectus's relationship with Pfizer is odd, to say the least. It is odd in terms of how Pfizer appears to be acting, and odd in terms of how Provectus management has acted. Pfizer has added more and more members to Provectus's strategic advisory board ("SAB") member, since it's first addition of Dr. Craig Eagle, M.D., when these folks easily could have been working quietly with the company with no need for fanfare. The counterpoint to this argument could be that such work might have been deemed material and thus reportable by Provectus' legal counsel. Provectus acquiesced to burying the second and third SAB additions in press releases without effectively leveraging Pfizer's clear interest and growing relationship into a much higher market capitalization. Pfizer seemingly has established the longest and largest look under Provectus' tent at what appears to be no tangible or material cost to Pfizer, and ostensibly no visibly tangible or material benefit to Provectus. I think it's reasonable to observe management has given the appearance of putting all of Provectus' eggs in Pfizer's basket without commensurate benefit.
Angelo's addition, at this moment in time, does speak more to regulatory validation than it does commercial validation. In reconsidering Craig and Peter's comments, an pharmaceutical industry professional like Angelo who formulates and substantiates drug in-licensing/acquisition business cases would seem to suggest Big Pharma (Pfizer, at least) agrees PV-10's regulatory pathway finally is set, and thus the time value of money now can be calculated. Provectus presumably just has to begin this necessary leg of the journey.
Stock Option Exercises (January 8, 2015)
Provectus' Dr. Tim Scott, Ph.D., exercised options to buy underlying common stock, and filed an associated Form 4 on January 7th. CFO/COO Peter Culpepper exercised options too on January 7th; his Form 4 is here. Specifics about these two exercises may be found in the table below.
It is likely Chairman and CEO Dr. Craig Dees, Ph.D. forfeited the 300,000 stock options that would have expired today, based on his forfeiture of 325,000 options in 2014. Peter and Chief Technology Officer Dr. Eric Wachter, Ph.D. have no options that expire in 2015; however, both Craig and Scott each have 525,000 that are due.
Notes (January 7, 2015)
The Immune System. The immune system has many components, such as white blood cells, antibodies, bone marrow, a complement system, interferon, hormones, the spleen, and the thymus. According to the preceding linked website, white blood are a large collection of different cells that work together to destroy stuff that include leukocytes, lymphocytes, monocytes, granulocytes, B-cells, plasma cells, T-cells, helper T-cells, killer T-cells, suppressor T-cells, natural killer cells, neutrophils, eosinophils, basophils, phagocytes, and macrophages.
"Immunotherapy is treatment that uses your body's own immune system to help fight cancer." According to the American Cancer Society, "Immunotherapy includes treatments that work in different ways. Some boost the body’s immune system in a very general way. Others help train the immune system to attack cancer cells specifically." So, what components of the immune system should an immunotherapeutic boost and/or train?
T-cell based therapies abound, like adoptive cell therapy (take out, put back in): Cellectis, Juno Therapeutics, Bluebird Bio, Kite Pharma, etc. There's a potential CAR-T killer, backed by Dr. Patrick Soon-Shiong, M.D., (see Christmas Cheer: Sorrento, NantWorks, and Conkwest Create Keiretsu) because it's focus is on natural killer cells, and not T-cells. There's a focus on regulatory T cells, or Treg cells, by an Amgen veteran backed by Celgene and Kleiner Perkins. Don't forget Big Pharma's PD-1s and PD-L1s that, um, release the brakes of the immune system.
And then there's PV-10, which seems to involve several immune system components, that we know of for now...
Unencumbered oncology asset. "Unencumbered" means one does not have to share revenue with anyone else. An unencumbered oncology assets like PV-10 should provide management with more options to maximize Provectus' asset value.
A universal biomarker for cancer. Is an important biomarker for the disease the acidic microenvironment of a tumor? See, for example, the work of The combined effort of the three laboratories of Dr. Oleg Andreev (URI), Dr. Yana Reshetnyak (URI) and Dr. Donald Engelman (Yale University) directed toward the development of pH (Low) Insertion Peptide (pHLIP) nanotechnology platform for cancer imaging and treatment:
H/t Investor Village/Wile_E_Coyote: AAPI – Global Clinical Trials Network launched during Global Healthcare Summit 2015 in Mumbai
"According to Dr. Ravi Jahagirdar, President of AAPI, the new AAPI initiative is about “educating physicians regarding clinical trials – an extensive network provided by AAPI Membership – across the United States including in small towns, and ranging from large groups that can run the trials themselves, to solo or small groups that will serve as a referral source to a designated center for research and/or clinical trials.” AAPI could be instrumental for any pharmaceutical Research companies in USA or globally in potentially recruiting a Phase 3 or 4 trial in the USA as part of its introduction of new products to a worldwide market.
Dr. Ravi announced that Provectus Biopharmaceuticals, a development stage oncology research company has committed to be the inaugural member of the Global Clinical Trial network. AAPI is looking forward to creating a large network of Pharmaceutical companies to make this initiative a great success. The networking opportunities from a group that is known for such, leading to a close knit execution of projects in research and/or clinical trials, provides an incredible opportunity for any participating organization.
“I am very excited about potential for Indian investigators through this network to be part of clinical trials with PV 10 in melanoma other malignancies,” said Dr. Agarwala, Chief of Medical Oncology & Hematology at St. Luke’s Cancer Center and Professor of Medicine at Temple University School of Medicine and has been Principal Investigator of Provectus clinical trials involving immunotherapy and targeted therapy for melanoma.
“Connecting to the brand of AAPI and its extensive US Physician network will lead to an increased visibility of pharmaceutical clinical trials here in the USA,” commented Dr. Joseph Chalil, Strategic Advisor to Provectus Biopharmaceuticals, who attended the event."
Compassionate Use/Expanded Access (January 5, 2015)
Provectus' compassionate use program ("CUP") has grown since its inception in 2009, June in Australia and October in the U.S. The program uses PV-10 to treat cutaneous (on the skin) or subcutaneous (under the skin) tumors irrespective of cancer. Some safety and efficacy information has been published about CUP patients, such as 2010's A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series and 2013's Novel use of Rose Bengal (PV-10) in two cases of refractory scalp sarcoma. In both cases, patients were not from the U.S. (I believe they were all Australian). For the most part, however, there are no published data about the program's results (e.g., indications treated, safety issues [if any], efficacy, survival, etc.). Through 2013 year-end, according to the company's website, "more than 100" participated (a cumulative figure, I believe). Numbers for past year-ends may be found in Provectus' annual CEO shareholder letters or updates. See the table and graph below.
The cumulative number of patients treated through 2014 year-end should be made available by the company on or around its early- to mid-March conference call that should coincide with Provectus' 10-K filing. I speculate, based on anecdotal comments attributed (but unconfirmed) to principal investigators whose hospitals also are PV-10 CUP sites (Dr. Sanjiv Agarwala's St. Luke's and Dr. Merrick Ross' MD Anderson), 25-50 new patients may have participated in 2014. The table and graph below reflect the upper end of this range.
For comparison, the company has treated through presumably 2013 year-end, in terms of published numbers on its website, "...12 in a Phase 1 recurrent breast cancer study, 6 in a Phase 1 metastatic liver cancer study, 20 in a Phase 1 melanoma study, 80 in a Phase 2 melanoma study, 10 in an investigator-initiated study of PV-10 chemoablation followed by radiotherapy, and over 100 patients enrolled in an active Compassionate Use Program for PV-10." Presented another way, through 2014 year-end, 118 patients in Provectus' sponsored clinical trials, and potentially 125 or more patients in the CUP—more treated CUP patients than clinical trial ones.
Network 1 Financial Revisited (January 3, 2015)
Updated from the news item immediately below: Peter corrected me by (paraphrasing) noting the first two line items of the table in my January 2nd news item were the same (thus, I appear to have misread the November 6th 10-Q). So, two tranches (not three) have been raised; the December 31st tranche was the second (not the third). Gross proceeds of $7.8 million have been raised (not $11.4 million); $7.2 million remains unused (not $3.6 million). A revised table is below:
He commented (paraphrasing) that he used Network 1 at this point in time because they were the only firm that could sell Provectus stock at $1.00 per share, and was substantially above market. Using Cantor's "ATM" facility would have seen him raise money under $1.00. Peter continued that Provectus is maintaining the company's NYSE MKT listing by what they (Provectus) is doing on the capital formation front. Network 1 may not give them equity research coverage, but he believes they provide something far more valuable at this point in time; namely, substantially above market financing. The share price ranged from a $0.93 intraday high to a $0.76 intraday low in December. The closing high and low were $0.92 and $0.77, respectively, which occurred on consecutive days (December 5th and 4th, respectively). A December stock price chart is below:
If you carry out a quick 'n dirty analysis of (i) the number of shares issued at-the-market (say, $0.80 per share) via Cantor and (ii) the number of total shares, assuming warrant exercises, via Network 1 (at $1.00 per share for the common stock, and a $1.25 exercise price), the dilutive difference [in the grand scheme of things] probably is de minimis. See below, which is a very basic analysis, and does seek to quantify the full range of opportunities and challenges of funding through each firm.
The larger point Peter was trying to make, I believe, was the paramount importance of financing at $1-plus from an NYSE relationship perspective. Something also to consider, which Peter did not address with me, is Provectus' demonstrated ability and flexibility -- via/using Network 1 -- to raise money as and when he needs it. Shareholders may not like these quarter-by-quarter or period-by-period financings (I certainly have grown tired of them), but at this point in time (the operative perspective) they permit the company to not have to take or accept just any license transaction until such time as they can generate more meaningful data and/or get the deal they want.
Peter confirmed (paraphrasing) the fundraising decision-making process and outcome incorporated Provectus' accounting firm BDO's year-end activity (i.e., process, analysis and review, and decision).
Most importantly (for me), Peter did what he said he would do, again. In this case, whether one agrees with it/him or not, it was a quarterly fundraising to offset quarterly burn, and he disclosed it when it was appropriate to do so (i.e., very proximate to when he did it). It's an important trait and approach that did not change, which is notable, relevant and important, and that is potentially much more revealing, insightful and helpful when applied to other Provectus topics and situations within Peter's purview.
Network 1 Financial, again (January 2, 2015)
Provectus raised a third tranche of money using its $15 million private placement with Network 1 Financial -- ~$4.1 million this time, leaving $3.7 million unused -- and issued an 8-K related to it. Information on the first and second tranches may be found at the bottom of page 12 in the company's November 6th 10-Q filing.
I previously thought the company had utilized the placement's remaining placement (see Capital Formation (November 21, 2014) below). In retrospect, however, it turned out some prospective participants (i.e., some Network 1 clients) were preparing their subscriptions (by selling existing Provectus stock they held in order to participate), which Provectus later accepted on December 31st.
Peter continues to frustrate through his continued, exclusive use of Network 1. Frustration derives from the continued use of a firm, Network 1, that has not, does not and will never provide equity research coverage of Provectus. Peter's seeming comfort to keep paying Network 1 healthy fees to raise money for him in exchange for nothing (other than the net proceeds of those financings) continues to be annoying and frustrating. That Network 1 facilitated Sinopharm's introduction to the company should have no bearing, since I presume the broker-dealer will be handsomely paid for it (i.e., a mid- to high single digit percent of the upfront payment due Provectus if and when a deal is consummated).
At best, Network 1's continued use is of no value because the company gets nothing in return (i.e., no research coverage). At worst, it is of negative value because Provectus effectively pays Network 1 not to cover it. To be fair, research coverage for mid-to large cap companies, let alone nano- to small cap ones, ranges from scarce to non-existent. There has been periodic coverage of Provectus by Rodman & Renshaw, Maxim Group, Roth Capital, etc., which isn't to say the Janneys, Aegiss, etc. of the world are any better or worse (this kind of firm are not the white shoe institutions whose "credibility" one would have hoped could have been lent to Provectus) but they are better than nothing, and most certainly better than choosing to do nothing.
Given the kind of superlative drug candidate PV-10 is (and the paradigm shift in the treatment of cancer I contend it also is), were Provectus more successful at capital formation, I wouldn't have objected to the company giving Wall Street and its denizens the middle finger. Management's long-standing lack of success on the capital formation front obfuscates their business strategy, which I think is intelligent and sound, and diminishes their clinical trial capital efficiency. By using the same firm over and over again, and effectively eschewing Wall Street equity research quid pro quo, Provectus' capital formation has been much more expensive (i.e., more dilutive) than it should have been.
It's very likely Peter found it expeditious to use Network 1. In the end, my comments above probably are much ado about little more than nothing. While repeated behavior, objectively of poor quality, is not a good thing, this Network 1 raise (i.e., each tranche, and all of the tranches) speaks to choosing expeditiousness over value at this point in time (i.e., essentially 2014's third and fourth quarters). It would have been better, and also a change for the better, to use Cantor and a portion of Provectus' $50 million Controlled Equity Offering Sales Agreement with the firm to potentially bring in institutional investors who could be a higher quality of shareholder than Network 1 clients. Peter may have deemed a "road show" to meet Cantor clients (as prospective investors), which likely would have to have required Eric's presence and participation, not worth their time.
Peter's historical challenge on the capital formation front arguably has been Eric repeatedly missing timing expectations (and some issues related to Craig and Tim that appear to be firmly in the past). Missing deadlines doesn't necessarily equate to doing a bad job, making bad or incorrect choices, or having bad or ineffective process(es). It can simply mean the guy doing clinical development mis-setting expectations for (and not fully comprehending the role, responsibility and implications of) the guy doing capital formation results in costlier dilution, a lower market capitalization than otherwise, and company brand tarnish. Furthermore, lather, rinse, repeat is of course not good.
Peter may have believed Eric could deliver a ready-to-enroll pivotal melanoma Phase 3 trial protocol sooner than he eventually will (see Like Watching Sausage Being Made (December 29, 2014) below), which could have permitted a China deal to be consummated and thus its potential upfront payment to be triggered, which then would have mitigated some or all three of the Network 1 tranches.
Cash on hand at year-end probably could or should be about $20 million, give or take. I estimated cash on hand as at November 5th, or roughly at the end of the third quarter, of about $20 million. See Notepad (November 6, 2014) below. I speculate the ~$3.6 million in net proceeds from the December 31st tranche may cover Provectus' fourth quarter operating expense. Peter seemed to say as much on the company's November 6th conference call:
A potential trigger for a China regional transaction could be the completion of Provectus's discussion with the FDA about operational aspects of the melanoma Phase 3 trial, which according to the company should be in January or early-February, rather than the filing of a liver Phase 1b/2 trial protocol.
No change in behavior: I appreciate Peter's timely disclosure of the fundraising event and amount. That there was no change in Peter's approach to disclosure is a good thing. He could have shunted information about the fundraising to Provectus' 10-K, which should be filed in early- to mid-March. Having timely information about the tranche should allow shareholders to promptly calculate dilution, and establish observations and draw conclusions on topics incorporating cash balances, cash burn needs and rates.
Combo (April 20, 2015)
 |
| Image source |
First, the PR notes "The allowed claims cover use of PV-10 in combination with systemic inhibitors of immune system down-regulation, such as anti-CTLA-4, PD-1 and PD-L1 antibodies, along with enhancers of immune system up-regulation, such as IL-2 and interferon-gamma." Underlined emphasis is mine. This reminds me of Wolchok, Allison and Mount Sinai's Newcastle disease virus combination patent application filed last year (see #Trending (April 9, 2015) below): "In addition, described herein are methods for treating cancer comprising administering Newcastle disease viruses in combination with an agonist of a co-stimulatory signal of an immune and/or an antagonist of an inhibitory signal of an immune cell." Again, underlined emphasis is mine.
Second, the PR references Pfizer's Dr. Eagle. I've previously written about Bristol-Myer's ipilimumab's Pfizer relative tremelimumab ("treme"). See here (September 2012) and here (April 2013). It was found, "...at the 2008 ASCO annual meeting, that the phase III trial of tremelimumab for the treatment of advanced melanoma did not have a positive result with regard to its primary endpoint." In 2011 AstraZeneca's MedImmune in-licensed treme from Pfizer and assumed global development rights. Pfizer retained the rights to use treme with specified types of combination therapies. {Bold emphasis is mine} Some believe there still is a role for treme in the treatment of cancer. At the annual meeting of the American Association of Cancer Research currently going on in Philadelphia, information from the University of Pennsylvania was presented about the combination of Pfizer's agonist (co-stimulatory agent) and anti-CD40 antibody — CP-870,893 ("pedaling faster") — and Pfizer's antagonist (co-inhibitor agent) and anti-CTLA4 antibody ("releasing the brakes") —treme:
Abstract: Combination of agonistic CD40 monoclonal antibody CP-870,893 and anti-CTLA-4 antibody tremelimumab in patients with metastatic melanoma
Combination therapy with αCD40 and treme was well-tolerated in patients with metastatic melanoma, with rates of CRS and other toxicity similar to previously reported rates for αCD40 or treme treatments alone. Overall ORR was 27.3%, with median OS of 26.1 months. Given the tolerability, antitumor activity, and biomarker evidence of immune activation, expanded study of this combination should be pursued.Trial protocol: Tremelimumab and CP-870,893 in Patients With Metastatic Melanoma
RATIONALE: Monoclonal antibodies, such as tremelimumab and CD40 agonist monoclonal antibody CP-870,893, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry cancer-killing substances to them. Giving tremelimumab together with CD 40 agonist monoclonal antibody CP-870, 893 may kill more tumor cells. PURPOSE: This phase I trial is studying the side effects and best dose of giving tremelimumab together with CD40 agonist monoclonal antibody CP-870,893 in treating patients with metastatic melanoma.Article: Combining Two Investigational Immunotherapy Drugs Safe, With Early Signs of Effectiveness
“This novel combination of an immunostimulatory molecule and a checkpoint inhibitor was found to be safe. There was real concern that stimulating the immune system while ‘cutting the brakes’ with checkpoint inhibition could lead to increased incidence or severity of immune side effects. We did not see this,” said Bajor. “Secondarily, the clear clinical evidence of response to this combination, even in some patients with highly morbid visceral disease, was striking.”
After a median follow-up of 22 months, the overall response rate (ORR) was 27 percent, which included complete responses in two patients and partial responses in four patients. The median progression-free survival (PFS) was 2.5 months and the median overall survival (OS) was 26.1 months. The side effects were nonoverlapping and similar to those which had been seen when each of these agents was tested independently, according to Bajor.Audiophile (April 19, 2015)
 |
| Image source |
- Agarwala: Injectable agents are pretty hot right now in melanoma and other cancers
- Agarwala: We all know that the lymph node is where all the action is
- Agarwala: So the Phase 1b trial of a combination with ipilimumab is in the very late planning stages
- Agarwala: Or liver metastases, and then under imaging guidance have an interventional radiologist ("IR") inject the tumor. This not done by me or my nurse. This will be done by an IR person that should be in a suite so you have the control and these patients are injected and then kept in the hospital overnight for observation and it really hasn't been any toxicity except pink urine. This drug does get into the circulation when you inject it in the liver and you turn pink, which is no big deal. So there is some interesting data with liver as well and it's well tolerated. I was a little surprised by this when Eric first mentioned this to me. Injecting the liver, that sounds a little dangerous. I was impressed. I took a little while to actually join the trial, and I did and have done several patients at my center now and really have had no issues. You just need to make sure the liver is fit to be injected by certain tests that we do pre-treatment and then monitor them carefully. So again a safe treatment and you can see an example of a nice response in the liver. {Underlined emphasis is mine}
- Agarwala: This is a relatively straightforward agent discovered to have this chemo-ablative effect almost by accident and then with more study getting to know better the mechanism of action which is both local and distant.
- Agarwala: I think the potential to do liver-based trials in countries like China and India is infinite.
- Wachter: So the question is why would one want to address local regional disease with focal treatments and intralestional therapy for example and this is a point of disagreement that would exist. Typically if we were to draw a line between Sanjiv [Argawala] the medical oncologist and Merrick [Ross] the surgical oncologist because they see patients typically with a very different extent of their disease. The domain of the medical oncologist typically has patients with a systemic disease where the disease has migrated from the original site and developed in other parts of the body. A surgical oncologist typically see patients with disease that still in the affected area that was originally presented with melanoma is the primary tumor can then present. What a colleague of mine refer to a stepwise progression and I thought that was a great characterization. Where over a period of months or years will recur in the vicinity of the primary. Some patients immediately have what he also referred to a shotgun presentation a shotgun progression where the disease will present distant sites. We still don't understand the difference between these two patient populations but there are certainly two very distinct populations of patients that present with these patterns of disease the ones that have local regional disease typically will have survival in many years from original onset of that disease. Those that show distant metastasis typically have much shorter survival.
- Wachter: The study is predicated on two hypotheses. One is if we make lesions go away we will forestall progression of the patient's disease. We'll show that with our progression free survival primary endpoint. We also believe if we make lesions go away the symptoms of the disease go away, and we will assess that with these assessments of symptoms based on patient-reported outcomes and investigator assessments.
- Ross: He was talking about the cascade path where the hypothesis of injectable agents actually work and can start to cascade how the tumor gets destroyed is an important first step. There is something called program cell death. When cancer cells die, that's called apoptosis. That is not an immune stimulating process, as opposed to necrosis which actually is so. So it's important to know PV-10 injection causes necrosis, which is a stimulating process. So that's an important distinction and that's why it's important that you use agents that causes necrosis because most of these patients even though they tend to have disease it seems to be localized to a certain part of the body.
- Agarwala: Maybe you know that number of sites is not atypical for a study of this size. In fact I think it's an underestimate in my opinion. I think we're going to need more sites potentially However it's a good start.
- Ross: I think your question is a good one and I think the answer to that is actually both statements that you said are probably correct. There that it is a little bit difficult for this particular trial in the US because of the landscape. But that but also the fact that multiple sites will be involved means that will probably accrue relatively fast.
- Wachter: The current Phase 1 study has two patient populations, patients with hepatocellular carcinoma and patients with other cancers that have metastasized to the liver. These are very different diseases obviously. They're all cancers but one is originated in the liver or one is originate elsewhere in the body and metastasized to the liver. We knew going into the phase one study that we were going to get a range of patients in that not a static population. Sanjeev showed a slide. It's a little little bit dated but gave you a flavor for the types of patients that have landed in that study cohort. What that study cohort allows us to do is develop and test hypotheses about whether PV-10 might have merit either in treating other patients with that particular disease matter started to liver let's say colorectal cancer for example. Or maybe even it may give us an indication if it's working very well in the metastatic liver then it could be advanced to treatment of primary recurrent disease elsewhere in the body and so that study is providing us with a tremendous amount of hypothesis value.
- Wachter: I also commented during the discussion there may be some strategic reasons to keep that [Phase 1 liver] study open...Just in developing a program that will involve China and one of the unique aspects of working in China is that oftentimes it's to the advantage of the Western study sponsored to conduct Chinese studies as part of an international study. And I won't go into the details of why that would be. However we would anticipate that that phase one study may lead to opening of another study cohort that would involve patients that are particularly important in China but would also involve those similar patients elsewhere in the world.
- Culpepper: Prior to a strategic global partnership say with one of the big pharma entities that we're close to in my personal opinion the Pfizer patent is joint patent application for a combination patent is probably the most interesting when you think about how we can monetize this because there's been a lot of activity between development companies like us with big pharma along these lines of the co-development.
Sometimes (often?), it might be smart to keep things simple. If data drive the share price, market capitalization, and the deal, then its straightforward to observe Provectus has not generated enough "data:" e.g., presentation (or publishing) of pre-clinical or clinical data, filing of trial protocols, starting of studies and trials, establishing regulatory clarity, etc. — data that are visible to and can be digested and evaluated by investors and the market. Arguably, management hasn't generated data for several years. So it's no wonder the share price has stagnated.
Take, for example, the melanoma [monotherapy] clinical development program ("CDP") (see the table below), which has been the company's lead indication. One could argue, 2014's volatility notwithstanding — January 2014's reporting of December 2013's Type C meeting, March 2014's submission of the breakthrough therapy designation application and its May denial — no "data" has been generated since 2012. Moffitt Cancer Center has been more active, and visible.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Among other "data" in 2015:
- New PH-10 "data:" a Phase 2 mechanism of action study underway,
- New melanoma monotherapy "data:" a pivotal Phase 3 trial [registration study] underway,
- New melanoma combination therapy "data:" issuance of the Pfizer patent, potentially a Phase 1b study protocol filed and/or a trial started, and
- New liver "data:" actual data, and a Phase 1b study protocol filed and/or a trial started.
 |
| Click to enlarge. Unaltered image source |
Last week, on April 10th, an article entitled Phase 3 trial to compare PV-10 with chemotherapy in stage III melanoma discussed the trial and quoted Agarwala, who also participated in Provectus' panel discussion last Thursday. Notable quotes included:
- “This is a trial design that is difficult to do. We're going to need a lot of centers, and there will be smaller groups per center because these are not easy patients to find. But we're not going to deprive patients of the ability to get checkpoint inhibitors if that is the right treatment for them.”
- “In clinical trial development, we have to make a trial that is fair to the drug and also fair to the patients. I think this trial fits this design.”
- “We observed the highest response rate in patients in whom we injected all lesions, so that is the reason why in the phase 3 trial we will select patients for whom we can inject every lesion.”
- “Even though we are going to test PV-10 as a single-agent treatment, I firmly believe the future of these intralesional agents is in combination.”
"I believe investors, collectively, are remarkably accurate when it comes to identifying cancer drugs destined to fail phase III studies. In this way, the Feuerstein-Ratain Rule is a very reliable negative prognostic indicator...Externally, investors reward small companies when drugs advance into later stages of clinical trials. If you've ever sat through an investor presentation, you've seen companies make a big deal about having a cancer drug in a phase III trial. I call it the "phase III badge..." The message here is not small companies are incapable of developing successful cancer drugs. Rather, if a small company does have a successful cancer drug in its early-stage pipeline, investors and/or larger companies tend to notice rather quickly...The market for good cancer drugs is very competitive, when a small-cap company advances a drug on its own into phase III studies, investors should see a red flag. Assume the market and larger companies have vetted that drug and found it lacking."The Feuerstein-Ratain rule predicts failure of oncology Phase 3 studies of companies that, four months before final study results are announced, have market capitalizations under $300 million.
Provectus' CTO Dr. Eric Wachter, PhD addressed the melanoma Phase 3 trial during last Thursday's panel session. Selected slides follow:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Preview (April 15, 2015)
Provectus' CFO/COO Peter Culpepper's slides for his presentation at the ChinaBio Partnering Forum 2015 later tonight (U.S. time zones) may be read starting here. There do not appear to be any changes to the deck from used by Peter at the Growth Capital Expo in Las Vegas on Monday.
The World Congress of Lymphology (April 15, 2015)
 |
| Click to enlarge. |
The conference itself seems to me [as a non-medical person] very specific: i.e., "lymphology, the study of the integrated lymphatic system (lymphatics, lymph nodes and lymphoctyes." From an October 2012 article entitled Progression of cutaneous melanoma: implications for treatment that included Zager as a co-author: "The survival rates of melanoma, like any type of cancer, become worse with advancing stage. Spectrum theory is most consistent with the progression of melanoma from the primary site to the in-transit locations, regional or sentinel lymph nodes and beyond to the distant sites." And from 2008 (another author): "When melanoma spreads, it does so invariably by the lymphatic system which drains to the regional lymph nodes. Uncommonly, melanoma can become trapped in the lymphatic vessels and grow to cause tumour nodules in the skin and subcutaneous tissues between the primary site and the regional lymph node basin. These nodules are termed in-transit metastases and carry an ominous prognosis." Of course, Moffitt's murine model work (presented in April 2014 at AACR) demonstrated injection of tumors with PV-10 "...led to an infiltration of dendritic cells to the lymph nodes draining the treated tumors."
Note the patient population for Provectus' pivotal melanoma Phase 3 trial below.
 |
| Click to enlarge. |
_f_z_r (April 14, 2015)
Provectus' strategic advisory board member Dr. Craig Eagle, MD [now] is General Manager of Pfizer Oncology Canada.
They Live (April 11, 2015)
 |
| Image source |
As background, Agarwala's original presentation provided data on 10 patients from Provectus' Phase 1 and expanded Phase 1 liver trials (see the two slides below; purple writing is mine). Takeaways could include:
- Safety ("generally well tolerated"),
- Notable survival in terms of both duration ("up to 50 months") and rate ("7 of 10 patients alive"), and
- Multi-indication viability (i.e., hepatocellular carcinoma ("HCC"), or primary liver cancer; and cancers metastatic to the liver [i.e., colorectal cancer or CRLM, non-small cell lung cancer or NSCLC, melanoma or Mel, and ovarian cancer or Ova], or secondary cancer).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. 2013 version of the slide. |
The cancer-free dates for patients in the Phase 1 trial, where the relevant time frame is at least five years of survival, should be August and December 2015, respectively. See below.
H/t a shareholder for sparking this thought: Noting the 50-month survival reference as well as a reference to a planned combination study with ipilimumab for melanoma in H2-2014 means part or all of Agarwala's Thursday presentation very likely also was given [by him] at the 2014 Beijing International Melanoma Congress on October 18 in Beijing, China (because of the calculated timing above, and because pembrolizumab was approved in September [and management pivoted to it as the preferred partner in a combination study towards year-end or into 2015]). See below, and also 2014 Beijing International Melanoma Congress (October 7, 2014) on the blog's Archived News II page.
 |
| Click to enlarge. |
"Oops" (April 10, 2015)
Provectus removed eight slides from Dr. Agarwala's panel discussion presentation (see link immediately below) when his presentation was put up on the company's website. As one shareholder put it, "Interesting how they snuck in the liver data into yesterday’s slide presentation like it was some casual update. Why wouldn’t this be framed into more of a public disclosure unless much more was coming?" Two of the slides are below in Initial Clinical Testing: HCC and Liver Mets (April 10, 2015). All missing slides follow, with commentary on (in purple) the slide.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Provectus released slide presentations from yesterday's panel discussion by Provectus' CTO Dr. Eric Wachter, PhD and St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala, MD. Of note was Agarwala's comment regarding clinical liver overall survival data (second slide below):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Provectus-related material provided to attendees of the panel discussion. The folder on the left contains various items, while the Word document on the right is a list of said items in the folder. Clicking on the folder icon will (should) open it in a new browser tab and display its contents. Clicking on the document icon will (should) open it in a new tab too for reading.
Panelling (April 9, 2015)
A shareholder in attendance at today's panel discussion should provide feedback to the blog later today. Watch this space.
Updated 4/9/15: A shareholder provided a picture of Provectus' poster that would be included in the upcoming weekend's HemOnc today Melanoma and Cutaneous Malignancies conference in New York.
 |
| Click to enlarge. |
 |
| Click to enlarge. Poster quadrant 1. |
 |
| Click to enlarge. Table, Poster quadrant 1. |
 |
| Click to enlarge. Poster quadrant 4. |
Calendars (April 9, 2015)
Provectus has a new webpage, Calendar of Events (h/t an investor), that details company events. For example, Provectus is
- Presenting at Asia Biotech Invest 2015, May 19-21, in Hong Kong (conference website confirmation is here),
- A sponsor of (and presumably presenting at) the 15th Annual Biotech in Europe Forum, September 29-30, in Basel, Switzerland (conference website confirmation is here),
- Presenting at Australia Biotech Invest 2015, October 5-6, in Melbourne, Australia (conference website confirmation is here).
Of course, the company and/or its data will participate in and/or be presented at:
- The April 9th Provectus panel,
- HemOnc today Melanoma and Cutaneous Malignancies, April 10-11, in New York, and
- Growth Capital Expo 2015, April 12-14, in Las Vegas.
So, it begs the question, why were other events posted, such as
- AACR 2015, April 18-22, in Philadelphia,
- ASCO 2015, May 29-June 2, in Chicago,
- 23rd World Congress of Dermatology, June 8-13, in Vancouver, Canada,
- 5th European Post-Chicago Melanoma/Skin Cancer Meeting, June 25-26, 2015, in Munich, Germany (conference website confirmation is here),
- 17th World Congress of Gastrointestinal Cancer, July 1-4, 2015, in Barcelona, Spain (liver data here?),
- 25th World Congress of Lymphology September 7-11, in San Francisco,
- ECCO 2015, September 25-28, in Vienna, Austria
- 11th EADO (European Association of Dermato Oncology) Congress and the 8th World Meeting of Interdisciplinary Melanoma/Skin Cancer Centers, October 28-31, in Marseille, France,
- SITC 2015, November 4-8, 2015, in National Harbor, Maryland, and
- 12th International Congress of the Society for Melanoma Research, November 18-21, in San Francisco?
It would appear PV-10 — a so-called front-end, starter of the engine or stepper on the gas pedal, but all immune system priming or activating — and the associated intellectual property surrounding this use (i.e., the combination therapy patent application jointly filed with Pfizer) is growing in importance to Provectus' business strategy and intrinsic valuation. With regard to the former, what opportunities exist for the company to strike a uni- or multi-indication co-development deal to combine PV-10 with a immune checkpoint inhibitor, or should/does Provectus do its own combination therapy trial with a reimbursable inhibitor? With regard to the latter, how much value could accrue to the company from this aspect of cancer treatment (i.e., late-stage disease, combination therapy/therapeutics)?
Hat tip to @bradpalm1 for leading me to the following 2014 paper and patent application. The paper is entitled Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Authors were Dmitriy Zamarin, Rikke B. Holmgaard, Sumit K. Subudhi, Joon Seok Park, Mena Mansour, Peter Palese, Taha Merghoub, Jedd D. Wolchok and James P. Allison. {Bold emphasis is mine}
Abstract: "Preexisting lymphocytic infiltration of tumors is associated with superior prognostic outcomes in a variety of cancers. Recent studies also suggest that lymphocytic responses may identify patients more likely to benefit from therapies targeting immune checkpoints, suggesting that therapeutic efficacy of immune checkpoint blockade can be enhanced through strategies that induce tumor inflammation. To achieve this effect, we explored the immunotherapeutic potential of oncolytic Newcastle disease virus (NDV). We find that localized intratumoral therapy of B16 melanoma with NDV induces inflammatory responses, leading to lymphocytic infiltrates and antitumor effect in distant (nonvirally injected) tumors without distant virus spread. The inflammatory effect coincided with distant tumor infiltration with tumor-specific CD4+ and CD8+ T cells, which was dependent on the identity of the virus-injected tumor. Combination therapy with localized NDV and systemic CTLA-4 blockade led to rejection of preestablished distant tumors and protection from tumor rechallenge in poorly immunogenic tumor models, irrespective of tumor cell line sensitivity to NDV-mediated lysis. Therapeutic effect was associated with marked distant tumor infiltration with activated CD8+ and CD4+ effector but not regulatory T cells, and was dependent on CD8+ cells, natural killer cells, and type I interferon. Our findings demonstrate that localized therapy with oncolytic NDV induces inflammatory immune infiltrates in distant tumors, making them susceptible to systemic therapy with immunomodulatory antibodies, which provides a strong rationale for investigation of such combination therapies in the clinic." {Bold emphasis is mine}The patent application is entitled Newcastle Disease Viruses and Uses Thereof. Inventors were Peter Palese, Adolfo Garcia-Sastre, Dmitriy Zamarin, James Allison and Jedd Wolchok. Applicants and assignees were Memorial Sloan Kettering Cancer Center and Icahn School of Medicine at Mount Sinai. Dr. Peter Palese, PhD is Professor & Chair of Microbiology and Professor of Medicine, Infectious Diseases at Mount Sinai. Dr. Jedd Wolchok, MD, PhD is Chief, Melanoma and Immunotherapeutics Service and Lloyd J. Old Chair for Clinical Investigation at Memorial Sloan Kettering. Dr.. Adolfo García-Sastre, PhD is Professor of Microbiology and of Medicine (Infectious Diseases) and Co-Director, Global Health and Emerging Pathogens Institute at Mount Sinai.
"Described herein are chimeric Newcastle disease viruses engineered to express an agonist of a co-stimulatory signal of an immune cell and compositions comprising such viruses. Also described herein are chimeric Newcastle disease viruses engineered to express an antagonist of an inhibitory signal of an immune cell and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer. In addition, described herein are methods for treating cancer comprising administering Newcastle disease viruses in combination with an agonist of a co-stimulatory signal of an immune and/or an antagonist of an inhibitory signal of an immune cell." {Bold emphasis is mine}@bradpalm1 commented (paraphrasing):
Dr. Jim Allison, PhD, who conducted preclinical development that initially demonstrated antibodies directed at CTLA-4 could result in tumor regressions in mice, is trying to commercialize the combination use of engineered NDV with checkpoint inhibitors ("CIs") to exploit the unique local/systemic T-cell effects and associated CI synergies he and Zamarin had noted in earlier papers. He obviously envisions the added need for an efficient front-end to potentiate the effects and broaden the indications for these new backend immunotherapeutic agents, but apparently hitched his wagon to the wrong horse in this race. It’s very telling that Allison, of all people, predicted the future need to provoke specific immunogenic cell death of the targeted cancers before the full potential of these checkpoint antibodies could be maximized. Ironically, the initial modified NDV tested in Phase 1 studies against advanced solid tumors (given intravenously rather than intralesionally) was designated PV701.I am struck by:
- The clear admission by key opinion leaders ("KOLs") (particularly Allison) that the back-end/releasers of the brakes — immune checkpoint inhibition/inhibitors — need help from the front-end/starters of the engine/steppers on the gas pedals to be/remain relevant,
- The clear admission by KOLs (especially Allison) that local agents (intralesional agents)— and thus the route of delivery (i.e., via tumors) — is critically important to the treatment solution, and to the continued relevance of systemic therapeutics like inhibitors, and
- KOLs and their medical institutions patenting potential solutions because they [obviously] see both potential in and profit from it.
Clinical trial sites. Provectus previously said all compassionate use program ("CUP") locations would be clinical trial sites for the company's upcoming pivotal melanoma Phase 3 trial; specifically, 8 sites of which one was not yet named. My take on actual and potential sites is below.
 |
| Click to enlarge. |
Cancer immunotherapy stock index. Forbes contributor Luke Timmerman recently wrote about Brad Loncar's Loncar Cancer Immunotherapy Index in an article entitled Cancer Immunotherapy Is All Over The Science News -- And Now It Has Its Own Stock Index (April 7th). Timmerman wrote:
"Brad Loncar, an independent investor in Lenexa, Kansas with a history of investing in cancer immunotherapy, is rolling out a new index that is the first to round up all the players in this emerging sector within biotech." {Underlined emphasis is mine}It's not the first. An earlier version of a cancer immunotherapy stock index is the Mentor Capital Cancer Immunotherapy Index, which started on July 10, 2009.
The "small" biotechnology category of Loncar's index is comprised primarily of companies with checkpoint inhibitors and ex vivo immunotherapies that sport an average market capitalization (as of April 8th) of $2.2 billion.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
As I wrote in Talimogene laherparepvec (March 20, 2015) below, in February Amgen announced two FDA advisory committees would review its intralesional agent (oncolytic immunotherapy) talimogene laherparepvec ("T-Vec") for the treatment of patients with injectable regionally or distantly metastatic melanoma. The committees comprised the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC"), and the Oncologic Drugs Advisory Committee ("ODAC"). The meeting date is April 29, 2015, at which time Amgen's Biologics License Application ("BLA") for T-Vec would be discussed. Amgen submitted its BLA for T-Vec to the Agency in August 2014. T-Vec's PDUFA date is October 27, 2015.
According to Tarius SAC Tracker, "[r]eportedly, the FDA had initially set the date for July 28, 2015, but pushed it back for Amgen to submit additional information." {Underlined emphasis is mine}
Amgen (i.e., Amgen researchers) submitted two T-Vec-related abstracts to the 2015 annual meeting of the American Association of Cancer Research ("AACR"). The first is Talimogene laherparepvec activates systemic T-cell-mediated anti-tumor immunity:
"Taken together these data supports that talimogene laheparepvec activates a systemic T cell mediated anti-tumor immune response."The second is Talilmogene laherparepvec increases the anti-tumor efficacy of the anti-PD-1 immune checkpoint blockade:
"Our findings demonstrate that localized therapy with talimogene laherparepvec augments the systemic anti-tumor immune response seen with anti-PD-1 therapy, providing a strong rationale for continued investigation of such combinations in the clinic."BMO Capital equity research analyst Dr. Jim Birchenough, MD wrote in March:
In our view these data back fill some of the observations that have been reported in the clinic from combination trials of [T-Vec] with a checkpoint inhibitor. The AMGN models appear to provide a sound basis for evaluating strategies to boost the effectiveness of [T-Vec] in both injected and non-injected tumors. Ongoing trials of [T-Vec] with [Yervoy] or [Keytruda] are expected to complete in 2016 and while the 200 patient [Yervoy] trial is open label the [Keytruda] trial contains a randomized phase 2 portion. The [T-Vec] melanoma filing is scheduled to be vetted by FDA’s ODAC April 29th, with a PDUFA data of October 2015. T-VEC has the potential to define how oncolytic viruses can be used to help vaccinate patients with their own tumors, but AMGN’s investment level in the project is currently minimal given AMGN’s investment in the larger Repatha opportunity.Two years ago at AACR 2013, Moffitt Cancer Center (Provectus only provided PV-10 for the cancer center's independent experiments) submitted its abstract Intralesional injection with PV-10 induces a systemic anti-tumor immune response in murine models of breast cancer and melanoma:
"In total, these studies support the induction of tumor-specific T cell-mediated immunity after single treatment with IL-PV-10 in multiple histologic subtypes."Five months ago at the 2014 annual meeting of the Society for Immunotherapy of Cancer ("SITC"), Moffitt submitted its abstract Efficacy of intralesional injection with PV-10 in combination with co-inhibitory blockade in a murine model of melanoma:
"Together, these studies support the induction of increased tumor-specific immunity after co-inhibitory blockade in combination with IL PV-10 therapy."What will come of T-Vec's April FDA advisory committee review meetings? What additional information was provided to the FDA to support the oncolytic virus' approval; the above? Could T-Vec's approval evaluation be pushed out further? How much attention and support to this investigational compound is Amgen giving? What if any implications might any of this have on other investigational oncolytic viruses?
Familiarity (April 7, 2015)
Today Provectus issued a press release and filed an associated 8-K related to PV-10 data and use being presented at St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala's HemOnc Today Melanoma and Cutaneous Malignancies Annual Meeting in New York at the end of this week. PV-10 data and use has been presented at Agarwala's conference since 2011 (i.e., this is the fifth year). 2011's PR is here, 2012's is here, 2013's is here, and 2014's is here. The conference has had a portion of it dedicated to "Local Regional Therapy for Melanoma," such as the 2012 portion of the agenda below.
 |
| Click to enlarge. |
 |
| 2012: Update on Intralesional Ablative Therapies, Dr. Merrick Ross, MD Anderson, Slide 36 |
 |
| 2012: Update on Intralesional Ablative Therapies, Dr. Merrick Ross, MD Anderson, Slide 39 |
 |
| 2012: Intralesional Therapy for Systemic Disease: Where Will It Fit In?, Andtbacka, Slide 6 |
 |
| 2012: Intralesional Therapy for Systemic Disease: Where Will It Fit In?, Andtbacka, Slide 21 |
 |
| 2012: Intralesional Therapy for Systemic Disease: Where Will It Fit In?, Andtbacka, Slide 2 |
“Our patients are living longer, and we’re able for the first time in melanoma to potentially talk about a cure, which is something we have not been able to talk about before,” said Dr. Robert Andtbacka, who is a surgical oncologist. Andtbacka said the new treatment teaches the body to heal itself.Agarwala presented an updated version of Andtbacka's pipeline slide at the III Eurasian Melanoma and Skin Cancers Forum, in Suzdal, Russia in October 2014.
 |
| 2014: Slide 8 |
Provectus' March 12th conference call provides some insight into the process of achieving a deal in China with Sinopharm for a regional license of PV-10. If or when a deal gets inked should depend on the process, and achieving certain milestones or checking certain boxes, as opposed to whether the parties can arrive (or have arrived) at a mutually agreeable set of deal terms and conditions.
A precedent transaction would be Aeterna Zentaris and Sinopharm A-Think, where the former licensed a drug to the latter, and also transferred its relevant technology to the other. Aeterna received a non-refundable $1 million fee, presumably at signing, for the transfer of its technology. A-Think agreed to make additional payments upon achieving certain pre-established regulatory and commercial milestones, and to pay royalties on future net sales. A-Think is responsible for all of the development, production, registration and commercialization of the drug. Because of confidentiality, the license agreement (see Article 6, Milestone Payments; Royalties) between the parties did not disclose either milestone event or payment details for (i) approval (section 6.1) [there are several events & payments], (ii) commercial (6.2) [there are several events & payments], (iii) royalties (6.3.1), and (iv) combination products (6.3.2) [there are several events & payments]. Without this information (i.e., milestone and subsequent payment), it's difficult to evaluate and assess Aeterna's deal, and more importantly use it to potentially project Provectus'.
Provectus' process with Sinopharm A-Think thus far may be more about the former endeavoring to complete (together with the latter) certain approval- or regulatory-related milestones (acceptance by or approval of the Chinese FDA ["sFDA/cFDA"]) before consummating the transaction. The March 12th conference call does not tell us anything about what the specific regulatory milestones that Provectus might be trying to knock-out are, but they might well include some of those required by the comparable FDA process, such as (a) submitting an investigational new drug ("IND") application to (i) the sFDA/cFDA and (ii) the institutional review board ("IRB") of the Chinese medical institution [site] conducting a PV-10 trial that (b) both have to accept or not disapprove of the IND for it to be effective. The Chinese IND, like in the U.S. and around the world, permits a Phase 1 study to commence in the country.
Here is what the conference call told us and/or may be telling us:
- Peter and Eric are traveling to China in April to advance the process that may or may not include signing a deal with Sinopharm (if the process has not sufficiently advanced). Peter: "We anticipate visits with Sinopharm over the next month and expect to be successful in entering into a partner transaction in China although there can be no assurance that any such transaction will occur," [Aside: Why say you expect to enter into a transaction and then follow up with a canned "no assurance" risk statement? Say it better, or say nothing at all.]
- The regulatory process in China probably began last summer. Eric: "And so what we have been learning through our work in Asia over last nine months is how to tailor a program that we had originally contemplated for western program to a nascent area where the disease is endemic and that really is important as evidenced by the slow rate of accrual of patients in our US-based liver study." {Underlined emphasis is mine}
- The process might take 12-18 months. Eric: "So in the part of the world like China which is an interesting case, we might be able to access a large number of patients that would be eligible for the study but for instance the approval process to get the study going, not technical review of the protocol and modifications, I have gone to the agency but just the fundamental review process for conducting a clinical trial where an investigation drug can be very prolonged, often times 12 to 18 months." {Underlined emphasis is mine}
- sFDA/cFDA acceptance of PV-10 (and its IND in China?) may be achieved by the Company's May conference call. Eric: "We are also in continued dialogue with the key opinion leaders in Asia to guide – of our proposed phase 1b/2 study of PV-10 plus standard care for HCC. As with our melanoma combination study, I expect to have very concrete details to discuss at our next quarterly conference call." I suppose one might ask whether Eric is sandbagging and might be "early," or if he accurately conveyed the likely timeframe.
Huntsman Cancer Institute and University of Utah surgical oncologist, and talimogene laherparepvec ("T-Vec") (Amgen) and Allovectin-7 (Vical) pivotal Phase 3 trial investigator Dr. Robert Andtbacka, MD recently disclosed he is a consultant to Provectus. He remains a consultant to Amgen (formerly BioVex, the innovator of T-Vec), and was a consultant to Vical. PV-10, T-Vec and Allovectin-7 are intralesional agents. The disclosure was made at Moffitt Cancer Center's Dr. Jeffrey Weber's, MD, PhD's 11th Annual International Symposium on Melanoma and Other Cutaneous Malignancies, which was held in Miami on March 7th. Andtbacka [as far as I can tell or research on the Web] did not disclose an affiliation with Provectus in 2014, but did cut a video about PV-10's upcoming Phase 3 trial while discussing T-Vec in Zurich at the 2014 annual meeting of the Society of Melanoma Research Congress (see Mind the gap (January 24, 2015) below).
 |
| Click to enlarge. |
Coinciding with the 2015 annual meeting of the Society of Surgical Oncology ("SSO") in Houston from March 25th to 28th, an educational activity sponsored by Amgen (donation) and Provectus (grant) was held on March 27th proximate to the SSO annual meeting, and entitled Advances in Immunotherapy for Advanced Melanoma: Integrating Intralesional and Anti-PD-1 Immunotherapies into Current Practice. Faculty included Andtbacka, St Luke’s University Hospital & Health Network and PV-10 investigator Dr. Sanjiv Agarwala, MD (medical oncologist) and MD Anderson Cancer Center and PV-10 investigator Dr. Merrick Ross, MD (surgical oncologist).
Dr. Argawala is chairing an education session from 8-9:15 am on May 31st at ASCO 2015 entitled Locoregional Therapies in the Setting of Systemic Treatment Advances: What's Next? (part of the conference's Track(s): Melanoma/Skin Cancers; Developmental Therapeutics and Translational Research). The panel also includes Dr. Tarhini. I imagine both T-Vec and PV-10 will be discussed.
 |
| Click to enlarge. |
 |
| Click to enlarge |
 |
| Click to enlarge |
Management said they would present clinical liver cancer trial data at one or more international conferences this summer. The expanded liver Phase 1 trial started in September 2012. Per the trial’s protocol patients were divided into two cohorts; one received PV-10, and the other both PV-10 and sorafenib. Prior to treating patients in the PV-10-plus-sorafenib cohort, however, Provectus conducted an in vitro study that demonstrated a low risk of clinically relevant drug-drug interaction between Rose Bengal and sorafenib derivatives. The original liver cancer trial clinical site was Sharp Memorial Hospital of San Diego, California. Second site The Southeastern Center for Digestive Disorders & Pancreatic Cancer of Tampa, Florida began recruiting in April 2013 (investigator: Dr. Alexander Rosemurgy, MD). Third site St Luke's University Health Network of Bethlehem, Pennsylvania began recruiting in February 2014 (investigator: Sanjiv Agarwala, MD). Trial changes can be found here.
After reviewing the Provectus' Insurance expense categorized under Research and development in the company's 10-Ks, a considerable number and the bulk of trial's liver patients may have been treated in 2013, with some treated in 2014. Provectus' insurance expense includes insurance for clinical trials and the company's compassionate use program ("CUP").
Interestingly and notably, the company has not had any clinical trial insurance claims to date, according to Provectus' CFO/COO Peter Culpepper (if he accurately answered my question, which was whether Provectus had incurred any claims through the end of 1Q15).
I tabulated Insurance expense over time. See the table below.
 |
| Click to enlarge. |
With no trials, save the CUP, running from 2010 to about 2012, it would appear a "baseline insurance expense" could be about $90-100K. To what would one attribute the more than $60K and $15K increases [above baseline] in 2013 and 2014, respectively? An increase in CUP patients is one answer (the number of new CUP patients in 2014 is forthcoming). Another answer also could be more treated liver patients. The increase in insurance expense would seem to substantiate the rumor of 20ish patients treated in Provectus' expanded liver Phase 1 trial.
Underlining (March 26, 2015)
Provectus' CFO/COO Peter Culpepper's BIO Asia International Conference presentation slides of March 25th are available here. Whoever advised Peter on this version of his presentation sure must like to underline words.
The third of three clinical trial sites for Provectus' PH-10 Phase 2 mechanism of action study (A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10), Miami, Florida's International Dermatology Research, has begun recruiting.
Underlining notwithstanding, there were several interesting and notable changes to Peter's Tokyo presentation (right, below), compared to the one he gave in Zurich at the European Life Science CEO Forum & Exhibition on March 3rd (left, below).
 |
| Click to enlarge. |
 |
| Add caption |
 |
| Add caption |
 |
| Add caption |
 |
| Add caption |
 |
| Add caption |
 |
| Add caption |
Investigational Immunotherapy Agents for Melanoma (March 23, 2015)
H/t @bradpalm1: From a March 24th article by Dr. Lisa Raedler, Ph.D. in Targeted Oncology entitled Tumor Antigens and Their Role in Immune Response in Melanoma:
"According to Sasha E. Stanton, MD, PhD, of the University of Washington School of Medicine and Fred Hutchinson Cancer Research Center, “Our body’s response to foreign antigens is inflammation. But, to protect from autoimmunity, its response to self-antigens is dampened. Tumors are very smart. They figure out ways to subvert antitumor responses by taking advantage of this dampening effect.”
Although melanoma and other cancer cells have a wide variety of ways to avoid immune detection, an equally large array of therapeutic strategies exist to boost T-cell, DC, and immune mediator functioning. Table 2 lists novel immunotherapy agents in development for melanoma. The ultimate goal of these therapies is to ensure that fully functional tumor-specific T cells eliminate melanoma cells and establish active, ongoing, and long-term immune surveillance against new cancer cells.
Stanton explained, “Melanoma is leading the way in immunotherapy. It is an immunogenic tumor. While targeting programmed death 1 (PD-1) is a great start, it is not a home run. There are a lot of ways to modify tumors and make PD-1 more effective. T-VEC [talimogene laherparepvec] and other vaccines that use multiple tumor-associated antigens are particularly exciting.”"
 |
| Clicl to enlarge. |
 |
| Image source |
- My investment thesis is intact. Up until this point FDA regulatory clarity was lacking (and therefore some measure of commercial validation has been absent) despite compelling clinical and business value propositions for Provectus’ investigational oncology and dermatology compounds. Clarity now appears explicit, and thus I remain long the stock.
- The initial pathway to oncology approval for ablative immunotherapy PV-10 is set, beginning with a pivotal Phase 3 trial (registration study) for Stage IIIB and Stage IIIC patients with unresectable locally advanced cutaneous melanoma (versus systemic chemotherapy), where there should be the opportunity for PV-10 to become the standard of care for this patient population if successful.
- The oncology clinical development program should expand this year to include Phase 1b/2 trials of PV-10 in combination with immune checkpoint inhibition for patients with advanced melanoma, and as a monotherapy for patients with hepatocellular carcinoma or cancer metastatic to the liver. This expansion would affirm PV-10’s multi-indication viability, utility across disease stage spectrum, and functionality either as a monotherapy or an immune system primer.
- Undervalued inflammatory dermatoses therapeutic PH-10 should garner more attention, beginning with a Phase 2 mechanism of action study that started earlier this year. Together with a number of completed preclinical toxicity studies, clinical mechanism data due by year-end should facilitate Agency discussions about a consensus pivotal Phase 3 trial design (and therefore commercial validation in the way of a global or regional license deal).
- More preclinical and clinical data should be presented or published this year and next about liver cancer (clinical), PV-10 mechanism (clinical), PV-10 in combination with immune checkpoint inhibition (preclinical) and PV-10 in combination with external beam radiation therapy (clinical). Overall survival-focused clinical data, together with the Phase 3 trial, would strengthen PV-10’s proposition to cancer patients that complete response of cancerous tumors after PV-10 injection results in disease staying away.
- Regulatory clarity should facilitate initial commercial validation, such as one or more regional geographic PV-10 licenses in China, India and/or other countries (outside of the U.S., Japan and Western Europe).
- Favorable pharmaceutical industry trends should further improve M&A valuation prospects as Big Pharma separates into three categories for oncology franchises: Those with immune checkpoint inhibition that admit the need for a primer, those who do not fully admit such need, and those who have neither stimulatory nor inhibitory compounds but know they desperately need something.
In February Amgen announced two FDA advisory committees would review its intralesional agent (oncolytic immunotherapy) talimogene laherparepvec ("T-Vec") for the treatment of patients with injectable regionally or distantly metastatic melanoma. The committees comprised the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC"), and the Oncologic Drugs Advisory Committee ("ODAC"). The meeting date is April 29, 2015, at which time Amgen's Biologics License Application ("BLA") for T-Vec would be discussed. Amgen submitted its BLA for T-Vec to the Agency in August 2014. T-Vec's PDUFA date is October 27, 2015. According to Tarius SAC Tracker, "[r]eportedly, the FDA had initially set the date for July 28, 2015, but pushed it back for Amgen to submit additional information."
Is it a given that the Agency approves T-Vec for the above indication? Might the PDUFA date be pushed out again? The ODAC meeting should be revealing and likely pivotal in nature, in terms of gleaning the FDA's perspectives on (i) T-Vec's pivotal trial's comparator, which was GM-CSF, (ii) viral shedding, which references the risk of transmission of the virus upon which the immunotherapy is constructed (T-Vec was engineered from herpes simplex 1 [HSV-1]), and (iii) the relevance of the trial's primary endpoint, which was durable response rate ("DRR") (defined as the rate of CR or PR lasting continuously for 6 or more months). The pivotal trial met its primary endpoint: 16% DRR in the T-Vec arm v. 2% in the GM-CSF arm (p<0.001).
Conference Presentations (March 18, 2015)
Provectus will be presenting at the 12th Annual BIO Asia International Conference, which runs from March 24-25 in Tokyo (investor/financial-oriented).
Move the chains (March 17, 2015)
On Provectus' March 12th conference call, CFO & COO Peter Culpepper said in regards to the company's discussions with Chinese pharmaceutical company/drug distributor Sinopharm:
"Negotiations are ongoing, with our mutual objective clear to get the deal right both for us and for our Chinese counterparts. Dr. Zhidan Jia, Chief Executive Officer of Sinopharm A-THINK, has said, "I am happy with the progress we made so far as our relationship with PVCT moves forward. Sinopharm will do more research on the recent filed PV-10 Phase 3 trial protocol. It will allow us to understand the drug better, and will help both parties to move forward in the Chinese market." We anticipate visits with Sinopharm over the next month and expect to be successful in entering into a partner transaction in China, although there can be no assurance that any such transaction will occur."Peter quoted a quote from Provectus' November 14, 2014 press release entitled Provectus Biopharmaceuticals Extends Memorandum of Understanding with Sinopharm-China State Institute of Pharmaceutical Industry and Sinopharm A-THINK Pharmaceutical Co., Ltd. He of course neglected to include in his March 12th remarks a certain sentence from the November 14th press release:
"The Company stated that it is hopeful that a contract will be finalized in the coming weeks, and this extension illustrates that there is sufficient interest on both sides to continue to work out the details."Peter's comments from Provectus's August 7, 2014 conference call were instructive on the probability and timing of a regional China license deal with Sinopharm or another geographic party:
 |
| Click to enlarge. |
What was to have been ready-to-go in 3Q14 (i.e., melanoma) only now (i.e., as we near the end of 1Q15) is good-to-go, and I believe it is unlikely Eric will have the liver protocol for China in place before the memorandum of understanding ("MOU") expires in about 2 months.
Assuming (i) a mutually acceptable deal indeed may be had and (ii) there are enough at-signing (upfront) dollars acceptable to Provectus management, it would seem prudent for them to (a) consummate a transaction with Sinopharm on the basis of the recently filed, final, melanoma Phase 3 protocol and thus collect some at-signing money, (b) shunt previously hoped for/contemplated additional at-signing dollars related to an in-place liver protocol to a near-term date when Eric can deliver this protocol (e.g., by the end of 2Q15), and (c) not have extend the MOU for a second time.
Presumably without having to settle for a lesser overall deal but rather consummate whatever originally anticipated deal with the same dollars but different dates that presumably encouraged them to enter into an MOU with Sinopharm in the first place, Provectus management would have moved the chains with an eye still on reaching the end zone.
e.g., pain, infection, significant bleeding (March 16, 2015)
On May 23rd, Provectus released FDA correspondence dated May 16, 2014 revealing the Agency turned down the company's request for Breakthrough Therapy Designation ("BTD") for PV-10, and locally advanced cutaneous melanoma. In this denial letter, the FDA noted:
We have reviewed your request and while we have determined that treatment of “locally advanced cutaneous melanoma” meets the criteria for a serious or life-threatening disease or condition, the preliminary clinical evidence you submitted does not indicate that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Therefore, designation as a Breakthrough Therapy cannot be granted at this time.
The preliminary clinical data provided in your request for Breakthrough Therapy designation are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma; however, the preliminary clinical data do not demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. This determination is based on the paucity of data on endpoints indicative of clinical benefit (e.g., pain, infection, significant bleeding) and our inability to determine the clinical significance of the reduction in the size in one to 10 target lesions in patients with locally advanced melanoma, who may have additional untreated cutaneous, subcutaneous, or visceral sites of disease. The information provided on durability of response is also of unclear clinical significance given the modifications to RECIST. Finally, there was also insufficient information provided in the package to ascertain improvement in or relief of tumor-related symptoms of pain, bleeding, or tumor ulceration. We acknowledge that a subset of patients with pre-treatment pain was identified, with summary data presented for two post-treatment time-points (at 8 and 13 weeks). However, from the information provided using visual analogue scale (VAS) for assessment of pain is incomplete as it did not capture pain throughout the course of treatment, no information on concomitant pain medications were provided, and it is not clear that the results observed were clinically significant, as no information was provided on validation of the VAS instrument used. {Bold and underlined emphasis is mine}Collaborating at the time with other shareholders who had reached out to folks who routinely dealt with the FDA (and not affiliated with Provectus) to gain their opinion of the denial letter's contents, this consensus view of the letter was (paraphrasing): The Agency (a part of it, it seemed) was asking for more information (i.e., data on patient reported outcomes ["PROs"]), rather than commenting on the efficacy, applicability and/or the "approvability" of the drug. Let's assume that read (pardon the pun) was/is accurate. Then, Eric presumably have had to return with prospective endpoints indicative of determining clinical benefit for the patient population with locally advanced cutaneous melanoma (i.e., measures of pain, infection, significant bleeding). In other words, he had to better address symptomatic assessment in an eventual Phase 3 trial to gather the additional information the FDA sought in order to facilitate the approval of the drug.
In the May 23rd conference call that followed release of the BTD denial letter, Eric said:
"...Because of the lack of requirements for patients to have pain symptoms upon enrollment [in the melanoma Phase 2 trial], only a small fraction of patients had clinically significant pain at baseline. So, we analyzed those patients and presented them that analysis of those data in context of the objective response data. We found that there was a strong relationship between the two types of data, that there was simply not enough of the symptomatic, or symptomology data to show a statistical function. I have to conclude that that’s the principal basis for the rejection of the [BTD] application. I'd say that it was our assumption going into the application that improvement in symptoms, if we made the patient’s symptoms go away was tantamount to -- I’m sorry -- if we make the patient’s lesions go away that’s tantamount to making the patient’s symptoms of that disease go away. We don’t seem to have been successful in convincing the Agency of that."On Provectus' June 2014 ASCO and September 2014 ESMO posters that followed BTD denial, Eric discussed PROs as a secondary endpoint for the pivotal melanoma Phase 3 trial, and symptomatic assessment. The initial draft protocol submitted to the FDA included "Change in total symptom score from baseline using the patient reported Skindex-16 instrument" as a secondary endpoint.
Today the company issued press release Provectus Biopharmaceuticals' Amended Protocol of PV-10 for Phase 3 Study as Treatment for Melanoma Now Available Online, and filed an associated 8-K. Among other things in it, Provectus confirmed "The previous secondary endpoint of "change in total symptom score from baseline using the patient reported Skindex-16 instrument (to be assessed 12 weeks after Day 1)" has been re-assigned exploratory endpoint status..." but also noted "...this may change once the Company completes an ongoing assessment of the suitability of the Skindex-16 instrument for this patient population."
Key endpoint measures ostensibly remain progression-free survival ("PFS"), complete response rate ("CRR") and overall survival ("OS").
If PRO remains shunted to exploratory status or ultimately remains tangential in nature and importance to the trial's outcome — merely a measurable outcome that does not rise to the level of needing to be passed— then I would wonder whether and if that is tacit acknowledgement by the FDA that making the tumor go away is tantamount to making a patient's disease symptom(s) go away, and another illustration of Agency support for the applicability of the drug to a defined patient population in need of better treatment solutions (and thus a clear pathway to approval).
Intralesional Therapy for In-transit and Satellite Metastases in Melanoma (March 15, 2015)
April 2015, Surgical Oncology Clinics of North America. Drs. Kendra J. Feeney, MD and Michael J. Mastrangelo, MD, Department of Medical Oncology, Sidney Kimmel School of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania
Note: Rose bengal = PV-10 (and PH-10)
 |
| Click to enlarge. |
On Thursday's conference call, Provectus' Chief Technology Officer Dr. Eric Wachter, Ph.D. established [for me] a baseline from which to examine certain other features of the company's pivotal melanoma Phase 3 trial:
"Note that for pivotal studies such as this one, the protocol has to be prospective - that means that everything you need to measure must be set in stone prior to enrolling the first patient. Thus, assuring agreement on design of the study is crucial if a study is to play a pivotal role in support of approval...
So, after thorough consultation with key clinical opinion leaders in the U.S. and abroad to assure feasibility, and repeated consultation with the FDA, I can state that this randomized study is built on a firm foundation, using conventional definitions and endpoints in a patient population in need of new treatment options."From here, I am focused on the speed of enrollment, and thus the time it may take for the trial to recruit, enroll and treat 113 patients, or potentially a lesser number.
A decision tree might look like this:
 |
| Click to enlarge. |
- Having failed [or not a candidate for] at least one systemic immunotherapy (final protocol) is a "crisper" delineation and lower threshold (less of a constraint) than having failed [or not a candidate for] systemic immunotherapy (initial protocol) (i.e., failed at least one vs. failed the category {that is, failed all}),
- BRAF V600E (initial) is a subset of BRAF V600 (final),
- Treating all visible disease in the PV-10 arm (final) is better than treating no more than 25 study lesions (initial), and should positively influence overall survival as well as better help substantiate the trial's thesis of demonstrating that tackling disease at Stage III [by hitting all of it that is visible] may forestall or prevent its expansion to Stage IV,
- One tumor visible for treatment (final) rather than two (initial), albeit a larger one, is a lower threshold (less of a constraint),
- Events progression to also include increase in size and/or number of lesions (final) is a "crisper" and more expansive definition of progression, with a potential greater effect or impact on the control arm,
- Removal of PRO as a secondary endpoint and its shunting to merely a measurable outcome means it does not rise to the level of needing to be passed, and also and more importantly might reflect acknowledgement by the FDA that making the tumor go away is tantamount to making a patient's disease symptom(s) go away, and
Eric, from Provectus' May 23, 2014 conference call: "...Because of the lack of requirements for patients to have pain symptoms upon enrollment [in the melanoma Phase 2 trial], only a small fraction of patients had clinically significant pain at baseline. So, we analyzed those patients and presented them that analysis of those data in context of the objective response data. We found that there was a strong relationship between the two types of data, that there was simply not enough of the symptomatic, or symptomology data to show a statistical function. I have to conclude that that’s the principal basis for the rejection of the [BTD] application. I'd say that it was our assumption going into the application that improvement in symptoms, if we made the patient’s symptoms go away was tantamount to -- I’m sorry -- if we make the patient’s lesions go away that’s tantamount to making the patient’s symptoms of that disease go away. We don’t seem to have been successful in convincing the Agency of that."
- Removal of language following "When the last subject randomized to the comparator arm has completed the Week 13 visit..." would seem to facilitate a more rapid complete crossover of control arm patients should the trial be called early.
Side-by-side changes from ClinicalTrials.gov for Provectus' upcoming pivotal Phase 3 trial for locally advanced cutaneous melanoma may be found here at the .gov site. Additional changes include patients who are are BRAF V600 wild-type (formally BRAF V600E), and:
 |
| Click to enlarge. |
Differences (March 13, 2015)
Differences between what should be Provectus' final protocol (March 11, 2015) for its pivotal melanoma Phase 3 trial and its initial protocol (November 7, 2014) are below:
1. Purpose
 |
| Initial Protocol, November 7, 2014 (click to enlarge) |
 |
| Final Protocol, March 11, 2015 (click to enlarge) |
 |
| Initial Protocol, November 7, 2014 (click to enlarge) |
 |
| Final Protocol, March 11, 2015 (click to enlarge) |
 |
| Initial Protocol, November 7, 2014 (click to enlarge) |
 |
| Final Protocol, March 11, 2015 (click to enlarge) |
 |
| Initial Protocol, November 7, 2014 (click to enlarge) |
 |
| Final Protocol, March 11, 2015 (click to enlarge) |
 |
| Initial Protocol, November 7, 2014 (click to enlarge) |
 |
| Final Protocol, March 11, 2015 (click to enlarge) |
H/t a shareholder: Provectus' protocol for its upcoming pivotal melanoma Phase 3 trial has been updated on ClinicalTrials.gov: PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma
Reactions (March 12, 2015)
 |
| Image source |
- Overall, a positive call with good information provided, some questions not answered, and other questions still needing to be asked,
- Melanoma Phase 3 trial: The CC (Eric's comments, Network 1's Damon Testaverde comments) and some additional context (see Network 1 (March 5, 2015) below) might suggest at least one clinical site could be recruiting, enrolling and potentially treating patients before the end of March — St. Luke's Hospital and Health Network, Bethlehem, PA,
- Data: I'm looking forward to presentation of liver data later this year (see Some things I think or guess (February 15, 2015) below), as I am presentation of PV-10 plus radiation data in patients with advanced melanoma (interim, according to Eric),
- Liver Phase 1b/2 trial protocol/trial: It's unclear how much progress Eric will be able to achieve on this item by the end of Q2, although he seemed to relish turning his attention to liver now that the melanoma Phase 3 trial was underway. Will the company commence a trial (unlikely), or will the protocol at least be filed with the FDA (potentially)?,
- China, India, Brazil: It seems like [for China at least] a filed liver Phase 1b/2 protocol is necessary for a transaction, or transactions, to be consummated.
New Patent (March 10, 2015)
A new patent issued today on the US PTO's website, Topical medicaments and methods for photodynamic treatment of disease {bold emphasis is mine}. The abstract reads:
New photodynamic, topically-applicable medicaments and certain medical uses of such photodynamic medicaments for treatment of human or animal tissue are described, wherein a primary active component of such medicaments is a halogenated xanthene. The halogenated xanthenes constitute a family of potent photosensitizers that become photoactivated upon illumination of the treatment site with visible wavelengths of light. In preferred embodiments, such medicaments are used for treatment of a variety of conditions affecting the skin and related organs; the mouth and digestive tract and related organs; the urinary and reproductive tracts and related organs; the respiratory tract and related organs; various other internal or external tissue surfaces, such as tissue surfaces exposed during surgery; and for treatment of a variety of conditions related to microbial or parasitic infection. In another preferred embodiment, such medicaments are produced in various formulations including liquid, semisolid or aerosol delivery vehicles.Previously language was "intracorporeal, " situated or occurring within the body. Today's patent's language is "topical," designed for or involving application to or action on the surface of a part of the body. The topical focus (or delivery) thus should be related to Provectus' dermatological agent PH-10, and its inflammatory dermatoses applications. I imagine the company would press release this tomorrow, and further explain its import (in the PR and/or on Thursday's conference call). PV-10, and PV-10 synthesis is protected into 2031. This new patent may afford some additional runway (out to 2035?).
Fresh (March 10, 2015)
 |
| Image source |
In Peter's March 3rd European Life Science CEO Forum & Exhibition presentation he highlighted the company's plans for 2015 that also included data generation and presentation by Provectus and Moffitt.
 |
| Click to enlarge. |
- Provectus: Phase 1 and expanded Phase 1 liver cancer data, Compassionate use program data, as well as goals and plans for other indications (clinical or pre-clinical),
- Moffitt: Clinical mechanism of action data, pre-clinical combination therapy data, and also a summary overview of the breadth and depth of PV-10 work undertaken by the cancer center,
- Foote et al.: Dr. Matthew Foote's follow-up study combining a single injection of PV-10 followed by radiotherapy (approximately 25 patients). Foote's initial exploration comprised three patients who achieved ostensibly complete responses. Medical Xpress' March 9th article entitled Radiation plus immunotherapy combo revs up immune system to better attack melanoma, study suggests details a University of Pennsylvania study combining ipilimumab (applied second) and radiotherapy (applied first) that achieved 18% partial response, 18% stable disease and 64% progressive disease), and
- Other places where formal and/or contractual relationships exist involving clinical and/or pre-clinical work with PV-10.
Provectus last updated the market on the company's progress with the Therapeutic Goods Administration ("TGA"), Australia's version of the FDA in November 2010 in a press release entitled Provectus Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia, noting:
"Use of interim data from the first half of Phase 3 study subjects, in conjunction with safety data collected in earlier studies of PV-10 for melanoma, was discussed to allow early evaluation for marketing approval for metastatic melanoma, and TGA agreed that these data should be sufficient for this review if the analysis confirmed efficacy."The use of interim data from a Phase 3 trial was necessary to reach a total of at least 300 patients treated with PV-10. I believe more than 300 patients have been treated with PV-10 through at least the end of 2014.
 |
| Provectus' website |
 |
| Click to enlarge. |
Updated 3/6/15: Patients counting toward the 300 figure must be those treated only for melanoma. As a result, 328 above would be lessened by 12 (breast), 6 + 20 (liver), and some number of non-melanoma CUP patients treated from 2009-2014. It's more than likely a rough number of at least 50 patients from the melanoma Phase 3 trial would push the count above 300.
Network 1 (March 5, 2015)
Network 1 (Testaverde, Hemming) are on the west coast of Florida fundraising for Provectus, which likely will draw down ~$4 million (i.e., a quarter's operating burn) of the remaining $7.2 million of the $15 million private placement. See Sources and Uses (February 19, 2015) below. Fundraising is a process that includes introducing the company to prospective investors and updating existing ones as you contemplate forming capital, with a decision to accept/take money potentially coming later.
Oddities (March 4, 2015)
From Peter's recent European conference presentation, when comparing it to the baseline one so as to understand what additional comments and statements he added (a sampling is below):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated presentation slides (March 3, 2015)
One of this blog's most popular posts is January 2012's The Wisdom Of Crowds. I'm appreciative of the many eyes (irrespective of their "side of the trade") that are focused on Provectus. Peter updated the slides following what I linked to yesterday; see Presentation slides (March 2, 2015) below. Among the changes of note:
- H/t a shareholder, and regular hatter: The introduction of Brazil as a prospective geographic region for potential licensure (see slides 21 and 25), and
- H/t canis_star on the PVCT Investor Village stock chat board: The comment Merck US's approved anti-PD-1 agent pembrolizumab (Keytruda) could (will?) be the pairing in a combination therapy P1b/2 trial for late-stage melanoma (see slide no. 17).
Provectus' Peter Culpepper is presenting at the 8th Annual European Life Science CEO Forum & Exhibition tomorrow — slides are available here. There does not appear to be additional information beyond what was included in February's BIO CEO slide deck.
Cost-benefit (March 2, 2015)
Bristol-Myers announced the FDA accepted their biologics license application ("BLA") for ipilimumab as an adjuvant treatment for patients with stage 3 melanoma who are at high risk of recurrence today, noting a projected FDA action date (a PDUFA date) of October 28, 2015. The trial protocol on ClinicalTrials.gov is here. Adjuvant therapy is treatment given in addition to the primary one, which in this case was surgery. The trial compared surgery (used first) + ipi (used second) versus surgery (used first) + a placebo (used second). Biologics generally are approved by FDA via a BLA rather than an new drug application ("NDA"). I would imagine Bristol-Myers will be successful in this outcome, as it seems to me there is no "standard" for adjuvant treatment in the NCCN guidelines.
 |
| Click to enlarge. |
- PV-10 (used alone) as a montherapy for stage 3 patients having unresectable locally advanced cutaneous melanoma, because [in my view] Provectus and the FDA have defined a suitable patient population for whom treatment with the company's drug is relevant and should have clinical benefit, and
- PV-10 (used first) in a combination therapy with co-inhibitory blockade (used second) to treat stage 4 patients, because patients receiving this pairing also would have unresectable disease that has expanded to visceral spread.
From an June 2014 article authored by Joe Barber about the Bristol-Myers trial entitled Bristol-Myers Squibb's Yervoy improves recurrence-free survival in Phase III melanoma trial:
Bristol-Myers Squibb noted that 48.8 percent of patients in the Yervoy arm discontinued treatment due to adverse effects, with most managed using standard protocols, although five patients in the Yervoy arm died due to the treatment. Conversely, 1.7 percent of patients in the placebo arm experienced treatment-related adverse effects, and no patients died as a result of treatment. Ron Peck, the company's global development leader for Yervoy, said a 10 mg dose of Yervoy was used in the trial and may have contributed to the side effect rate, while a 3 mg dose is being used in later studies.
However oncologist Jeffery Weber of H. Lee Moffitt Cancer Center suggested that the result "raises as many questions as it answers," particularly with regards to cost. Weber estimated that because the trial used a higher dose than that currently approved for Yervoy and treated patients for a longer period, the full regimen would cost as much as $1.5 million per patient. In response, Bristol-Myers Squibb spokeswoman Sarah Koenig said the company is "sensitive to concerns about the rising costs of healthcare, including pharmaceuticals" and is "actively evaluating ways to address other Yervoy dosing regimens being studied" to enable access for patients in need. She added that the drugmaker is "in [the] process of evaluating options based on these results and it is premature to speculate on our regulatory plans." {Bold and underlined emphasis is mine}February Blog Stats (March 1, 2015)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
If Peter had said in June, for example, that one could expect (among other things) a co-development deal within 90 days, you'd imagine there may have been discussions along those lines with a prospective partner or two. Part of the no-outcome time that has since elapsed assuredly results from Eric as a rate limiting step (from the perspective of producing a suitable Phase 1b/2 trial protocol for a combination therapy pairing). Another contributory factor may have been the waiting for Moffitt's independent pre-clinical combination therapy work to be presented (and, possibly, a peer-reviewed publication, which of course has not yet materialized). Additionally, prospective partners may want to firmly establish the value proposition for combining their drugs with PV-10; intralesional agent T-Vec's preliminary results with ipilimumab (response rate and immunologic signalling) should help this discussion.
But I wonder how much of the dampening on Provectus' ability and/or speed to arrive at a co-development deal with a Big Pharma holding a checkpoint inhibitor (if one assumes there is sufficient medical and scientific rationale to do so) is Pfizer's doing. I think it's a very safe assumption Provectus has regularly engaged Pfizer's legal department (at a minimum) starting before both companies filed the combination therapy patent application in March 2012 and since. Both companies may be equally conservative when it comes to addressing how they protect intellectual property, or one may be more conservative than the other and thus lead the process (over the other) as a result. In reading an October 2012 article by Gene Quinn (h/t a shareholder) entitled The Impact of the America Invents Act on the Definition of Prior Art, I wonder whether Pfizer, out of an abundance of caution (and conservatism), insisted the combination patent be formally and fully allowed before permitting Provectus to enter into a co-development deal and/or commence a combination therapy trial.
N = 7 (February 27, 2015)
Moffitt embarked on a Phase 1 feasibility study in humans, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study, to "...to find out more about how PV-10 works in melanoma tumors." The trial protocol was submitted to ClinicalTrials.gov in December 2012. At AACR 2014 (April) some data was presented on the first eight patients (N = 8). Total enrollment was to have been 15.
 |
| Click to enlarge. |
While adoptive cell transfer offers the advantage that enough T cells can be obtained for infusion in all patients, the T-cell receptors transfected into the T cells have a limited antigen-specificity. The strategy works, Dr. Sarnaik said, only about half the time. “We generate large numbers of T-lymphocytes, but we don’t have control over their quality. We think one of the limitations is that the T cells you get out of the tumor just aren’t good enough.” PV-10, however, does cause an immune response, suggesting that a combination treatment may improve the quality of the T-lymphocytes and have a greater impact on the disease.
When Shari Pilon-Thomas, PhD, also a Moffitt researcher, demonstrated that T-lymphocytes recovered from mice treated with PV-10 do appear to be of a higher quality, as evidenced by stronger tumor reactivity, the stage was set for Dr. Sarnaik’s current 15-patient pilot study. In it, one of two resectable melanoma tumors is injected with PV-10. Both are removed several weeks later. Serum is assessed before and after treatment to look for changes in the infiltration of immune cells. In patients with an immune response, PV-10 therapy can be continued.
“This is a straightforward study that will give a yes or no answer,” Dr. Sarnaik said.
Investigators will monitor carefully for known PV-10 adverse events, such as strong sun sensitivity, and interactions with diuretics, older psychiatric medications and some topical agents. Because drug concentrations are high only locally, intralesional therapy produces limited toxicities. The study will be completed in a year, with data analysis requiring another 6 months. First results may be ready in a year, however.In an April 2014 article entitled PV-10 decreases melanoma cells in tumours by ecancer reporter Janet Fricker that followed-up on this work, Moffitt's Dr. Shari Pilon-Thomas, Ph.D. was quoted saying:
“Ironically, the original aim of the trial to assess tumour-infiltrating lymphocytes was thwarted when biopsies of patient tumours collected just seven to 10 days after PV-10 injection no longer contained viable tumour tissue.”Fricker then wrote "Studies are now underway in an additional seven patients to take biopsies and blood samples at more frequent time intervals after PV-10 injection to elucidate the pathways more clearly." In the same article Fricker quoted Eric as saying:
“The study shows that there are plausible immunological processes that explain the bystander response observed in patients. This isn’t a spurious event, it’s a bona fide immunological response.”By June at ASCO 2014 13 patients were enrolled but more data only on the first 8 were presented.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
In the category of too much time on my hands..., re-visiting #3 of Some things I think or guess (February 15, 2015) below, I erred in my speculative math of when Provectus’ combination therapy patent with Pfizer could issue. Using the company's synthesis patent (far from a determinative or comprehensive approach), I compared the times between filing and publishing the patent application, and between filing and allowing of the patent. My original "math" suggested an approval timeframe for the combination therapy patent as late as May (Q2), because I adjusted for the extra month or so it took between filing and publishing the combination patent application compared to the synthesis one. If I simply assume it'll take about the same time for both patents to be or have been allowed, it is conceivable the combination patent could be approved by the USPTO in early-March.
 |
| Click to enlarge. |
- Do additional claims include anti-PD-1 and PD-L1 therapeutic agents (i.e., checkpoint blockade), and also other immune-inhibitory molecules (e.g., IDO, TIM-3, LAG-3)? The current published application only covers anti-CTLA-4 agents.
- How are economics of the patent shared with Pfizer? I presume Pfizer only shares in revenues derived from anti-CTLA-4 combinations, since the Big Pharma had owned one of these agents (i.e., tremelimumab) prior to the inception of the patent application. Does Pfizer have an economic claim on all checkpoint blockade agents? Do these economics extend beyond Provectus' acquisition by another company, or are they limited to the scenario of Pfizer buying Provectus?
 |
| Click to enlarge. |
Market Making (February 22, 2015)
 |
| Image source |
Moving from Streicher to KCG should be a good thing because the latter is much larger than the former, and would bring more heft to market making activities for Provectus' stock. KCG has a net worth (i.e., total assets minus total liabilities) potentially more than 40 times that of Streicher. KCG also reported a market value of $6.1 billion of stocks held in the above mentioned 13F filing (I don't believe Streicher has ever made such filings, and none in recent memory).
 |
| Click to enlarge |
DMMs vary by the size of their balance sheets, among other characteristics and parameters. The smaller DMMs are J. Streicher ($17.5 million net worth as at 9/30/04, the last statement of financial condition is here; no 13F filings) and Brendan E. Cryan (no statements appear to be available). The much larger ones are KCG Americas (KCG has a $745 million net worth as at 6/30/14) and Virtu (a net worth of $203 million as at 12/31/13, unaudited; $813 million market value of stock holding as of the firm's 12/13/14 13F filing). KCG was formed in 2013 by the merger of Knight Capital Group and Getco, when the latter acquired the former in the wake of Knight's 2012 electronic trading blow-up.
My takeaway for this news item is: Knight has much, much greater size heft than Streicher, possibly 40-60 times more depending on the parameter e.g., balance sheet, stock holdings, etc.). Should (when) market interest increase in Provectus' stock, KCG would be far better placed to handle it than Streicher [ever could].
Sources and Uses (February 19, 2015)
 |
| Image source |
Provectus' CFO & COO Peter Culpepper's definition of fund raising is different from mine. He may consider fundraising the act of accepting subscriptions from a private placement, or when the money hits the company's bank account. Fundraising is a process, which may or may not culminate with the deposit. As he prepares for the end of Q1, Peter probably already has begun the fundraising process, with the likelihood but not certainty he will raise about $4 million (i.e., a quarter's worth of cash burn) at the end of March or the beginning of April.
Ending calendar/fiscal year 2014, Provectus may have had about $21 million of cash on hand. To arrive at this figure, I assumed an increase in 4Q14 monthly burn ($1.2 million) over the 3Q14 figure ($1.157 million).
 |
| Click to enlarge |
Assuming the same 4Q14 monthly cash burn for 1Q15, it's unlikely Peter has to raise money until the end of the quarter, when Provectus' cash balance should fall below $18 million.
 |
| Click to enlarge. |
Peter should be able to readily access the balance of the Network 1 Financial private placement already in place and from which Provectus previously drew down $7.8 million in gross proceeds, leaving him a balance of $7.2 million. The terms of the placement are units priced at $1.00 per common share, including 50% warrant coverage with a $1.25 exercise price (5-year term).
 |
| Click to enlarge. |
One way (Network 1) or the other (Cantor), Peter very likely has to raise money going into Q2, which means I don't believe he concludes a regional geographic license transaction in Q1. I also don't believe potential catalysts in this quarter, largest among them the commencement of the pivotal melanoma Phase 3 trial, would push the share price meaningfully above $1, indicating fundraising dollars this would come from Network 1 again.
Eric may file the U.S. liver Phase 1b/2 protocol (sorafenib vs. PV-10) with the FDA in Q2, and finalize the China liver one (country-specific local ablation technology [possibly radiofrequency ablation or "RFA"] vs. PV-10) in the same quarter. If this plays out — that is, Eric meets his timeline — it is possible a transaction, which surely requires a finished liver protocol as a necessary and potentially sufficient condition, is consummated.
Should a deal be done, the money (i.e., whatever payment amount is available at signing) may arrive 30 to 60 days thereafter depending on how quickly Provectus and Peter move to definitive agreements with the partner (e.g., Sinopharm). If there truly has been material progress towards a deal and Peter has been smart, the term sheet has been finalized and the definitive agreements have been framed if not prepped.
More than likely, the money arrives in Q3, which would mean raising (either at the end of Q2 or at the beginning of Q3) up to one more quarter of operating expenses. If a China deal is consummated on good to great terms, Peter should be able to draw from the Cantor ATM (hopefully given a much higher share price than $1).
It's not clear to me whether a U.S. liver Phase 1b/2 trial can start without regional transaction license money (or a materially higher share price resulting from a transaction announcement that makes raising money for this trial expense worth it from a dilution calculation perspective) because I do not believe the additional liver trial cash expense is accounted for in Provectus' monthly operating expense (as I believe the melanoma Phase 3 trial is). Interestingly, a U.S. liver Phase 1b trial may be sized at 20-25 patients (allowing certain data to be collected in hopes/with the thought of "pivotalizing" the Phase 2 portion), which then might be affordable within current monthly burn boundaries (at $40-50,000 per U.S. liver patient).
My takeaways from this news item include: (i) Provectus likely will raise a quarterly burn offset amount, (ii) Peter should do so towards the end of the first quarter, or the beginning of the second, (iii) the company likely would obtain those funds via Network 1 unless potential catalysts materialize and push the share price above the threshold for securing capital via Cantor, and (iv) further fundraising, as well as the timing of such, would be strongly influenced by the status of the advancement of the liver clinical development program in Q2.
Institutional Ownership (February 18, 2015)
As reported through February 18th thus far, Provectus shares held by institutions who made SEC form 13F filings (~4.9 million) and shares held as a percent of those outstanding (2.7%) increased during the quarter ending December 31, 2014 (from ~4.1 million and ~2.3%, respectively, at 9/31/14). Data was collected from Whalewisdom.com.
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Image source |
I wonder if (think) the outcome of Provectus' upcoming pivotal Phase 3 trial for locally advanced melanoma, and the possibility of early success, is focused on the "vertical separation" of the two PFS curves at 12 weeks, the trial's first assessment period. A timeframe of 12 weeks, or 3 months, should exceed the established or understood efficacy of chemotherapy.
So, rather than count the number of patients enrolled and treated in the trial (2:1 randomization, 2 of three patients enter the PV-10 treatment arm and 1 patient enters the chemotherapy control arm) to reach horizontal clinical significance (N_h), I wonder if (think) the number of patients is that required to reach vertical clinical significance (N_v, at 12 weeks).
 |
| Click to enlarge. Illustrative PFS curves |
Some things I think or guess (February 15, 2015)
#2. I think the company will raise money to offset another quarterly burn.
#3. I think Provectus’ combination therapy patent with Pfizer will issue in April or May, paving the way (given this additional level or stage of intellectual property protection) for a co-development deal to be struck to combine PV-10 with another company’s checkpoint inhibitor.
#4. Sometime in Q2, I think Eric will file the Phase 1b/2 liver protocol (for the U.S.: PV-10 v. sorafenib), paving the way for the trial to start.
#5. I guess the company will present Phase 1 liver data with an overall survival orientation (and possibly preliminary expanded Phase 1 data with a tumor regression orientation) at ASCO (May 29-June 2).
Coming Soon (February 13, 2015)
Eric's guidance is informative but not instructive; his actions, however, more often are both. I'm of the view or opinion Provectus will start its pivotal Phase 3 trial (registration study) for locally advanced cutaneous melanoma in March. I lean toward this more not solely because of his quote in Provectus' Provectus Biopharmaceuticals Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study press release of "We are making a number of small changes to the protocol in light of this review, and will issue a final version later this month," but because he recently made a change (February 11th) to the company's compassionate use (expanded access) program's study details on ClinicalTrials.gov. The specifics of the change are not fully described, however; only a change having been made is noted. The CUP was established in June (Australia) and October (U.S.) 2009 after the metastatic melanoma Phase 2 trial was being wound down (patient accrual ended in May, patients were observed for 52 weeks). Certain patients from the Phase 2 trial eventually were transitioned to the CUP. Such programs have the exclusion criteria of cancer patients who are eligible for an existing clinical trial. With the Phase 3 trial about to start, it would make sense for Eric also to make some changes to the CUP.
Two down, one to go (February 10, 2015)
H/t a shareholder: The second of three clinical trial sites for Provectus' PH-10 Phase 2 mechanism of action study (A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10), University of North Carolina School of Medicine (Department of Dermatology), has begun recruiting. Texas' DermResearch began recruiting last month.
Who's on first, What's on second, Review's on third (February 10, 2015)
"As noted in our press release of December 22, 2014, when we submitted the protocol to the Agency in November 2014, we included a brief list of questions about certain operational aspects of the protocol. The FDA subsequently indicated that a formal meeting was appropriate to assure that these questions were addressed in a timely and comprehensive manner. As is typical for such meetings, we provided a more extensive list of questions in the formal meeting package. This led to a very thorough and helpful review of the protocol as a result of the meeting."
"We are extremely grateful for the careful review conducted by the Agency. The outcome of the review does not affect the fundamental design of the study nor the patient population, but does affect certain details concerning some secondary end points and statistical analysis matters, such as the treatment of missing data. We are making a number of small changes to the protocol in light of this review, and will issue a final version later this month."
"We have eight sites, four in the U.S. and four in Australia, in our expanded access program currently using PV-10 for melanoma and other cutaneous malignancies. We expect that they will provide a path to quickly starting enrollment upon completion of the review period. In addition, we have been qualifying additional sites that will join the study pending action by their respective Institutional Review Boards." {Colored, bolded, underlined emphasis is mine}Review refers to the review of the protocol conducted by the FDA during the January 29th Type C meeting. Review refers to the review of the small changes to the protocol [emanating from the aforementioned FDA review] by the CUP/Phase 3 trial site IRBs, likely indicating patient enrollment could start in late-February. Given Eric's track record of missing his own timelines and deadlines, it's more likely the trial will start sometime in March. I think he needs to demonstrate a better quality of Provectus leadership (and thus public company stewardship) by, among other things, writing cogent press releases.
Ablative Immunotherapy (February 6, 2015)
PV-10 may be as much a singularly unique drug as Rose Bengal may be a singularly unique molecule. A good read about Rose Bengal ("RB"), PV-10 and Provectus' principals remains Walter Alexander's August 2010 article entitled Rose Bengal: From a Wool Dye to a Cancer Therapy. A water-soluble dye created by Gnehm in 1882, RB weighs less than 1,000 Daltons. I'm still struck by RB's near century of prior clinical use:
- Added to safranin victoria yellow for ocular pneumococcal infection (Römer, 1914),
- A stain for visualizing corneal ulcers (Kleefeld, 1919), and
- A marker for impaired liver function (Delprat, 1925).
As of January 2015, there are 3,720 medical literature citations of RB listed on PubMed, more than 235 of them related to cancer. The key to the story of PV-10, and Provectus, is that the therapeutic benefits of RB remained hidden until the 1980s, when sufficient quantities were first administered in preclinical studies.
At that time the FDA and Japanese Ministry of Health and Welfare were scrutinizing artificial food colorings. Japanese researchers Ito et al. evaluated red food dye No. 105 (RB) tumorigenicity, observing "dose-dependent survival increases in the mice receiving Rose Bengal" in their 1986 paper.
I believe as early as 1998, although it may have been a few years later, Provectus’ multi-disciplinary team of founders, a molecular virologist (Craig Dees), a chemical engineer (Tim Scott) and a chemist (Eric Wachter), were looking for drug candidates having antineoplastic activity separately came across Rose Bengal. According to Alexander's recounting their story of discovery, a commercial data search by them identified several hundred candidates. Dees et al.'s own subsequent screening promptly zeroed in on RB. Subsequent preclinical tests with bacterial and cancer cell lines quickly demonstrated RB’s impressive cytotoxic activity. Follow-on animal and human studies confirmed RB, delivered directly into tumors, was a selective and potent agent for ablating cancers and harnessing the immune system.
Labeling (marketing) PV-10 still is a work in progress. Some time ago Eric had coined the phrase immuno-chemooablative. See for example my blog post $PVCT: Florey, Chain & Heatley. St. Luke Cancer Hospital's Dr. Sanjiv Agarwala, MD and MD Anderson's Dr. Merrick Ross, MD have described PV-10 as an oncolytic immunotherapy. See for example my post "PV-10 delivers greatest effects when all lesions are injected", or Knick-knacks (January 28, 2015) and Keep talking (and keep doing) (January 29, 2015) below. Johns Hopkins University's Dr. Suzanne Topalian, MD for example referred to the drug as an autologous tumor vaccine (along with T-Vec and Allovectin-7).
Ablative immunotherapy probably is the most apt descriptor of PV-10 thus far. As an ablative immunotherapy PV-10 presents a two-pronged approach to fighting cancer, the local effect of tumor ablation, and the systemic effect of a tumor-specific immune response:
- Local: The patient’s tumor burden is rapidly reduced after injection of PV-10 into cancerous lesions. Selective targeting by RB minimizes side effects. Unlike many other cancer drugs, PV-10 does not rely on a single pathway to work, and has no known resistance.
- Systemic: PV-10 causes regression of untreated tumors. The drug potentially prolongs Progression-free survival. PV-10 possibly may be combined with immunomodulatory drugs and other systemic therapies for use in lesions that are inaccessible to a direct injection of PV-10.
It probably will take more time to both substantiate and further elucidate the 'no resistance' claim. PV-10 has been shown to be effective on refractory tumors with many prior interventions that investigators have used it on. In the compassionate use program PV-10 presumably has been used to treat patients for years with continued efficacy. Fundamental research by Moffitt supports the notion of pathologic complete response ("pCR") notion coupled with attendant immunologic signaling evidence ("[t]he core of any definition of pCR is achieving no residual histological evidence of tumor...").
I also think the import of RB's physical chemistry may be obscured by most folks' focus on the biological chemistry of other drug candidates.
The pillars of Provectus' intellectual property protection approach thus far comprise (i) second medicinal use, (ii) method of use, (iii) formulation, (iv) synthesis, and (v) combination. Second medicinal use refers to the utilization of RB as a therapeutic, where before it had been used as a diagnostic. Provectus' synthesis patent is a near (if not actual) composition of matter patent because the amount of the removal of impurities in the new synthesis approach exceeds 2%, a threshold that apparently constitutes a new molecule (and thus, it would appear, composition of matter).
Opdivo (February 4, 2015) |
| Click to enlarge. |
 |
| Click to enlarge. |
Provectus updated it's website presentation, which historically has served as one of Peter's corporate communication mediums, on January 14th to reflect his view (as understood from Eric) of the start of the company's pivotal Phase 3 trial for locally advanced cutaneous melanoma.
 |
| January 14, 2015 version |
 |
| December 15, 2014 |
Medical oncologist, St. Luke’s Cancer Center staffer, Professor of Medicine at Temple University School of Medicine and Provectus principal investigator Dr. Sanjiv Agarwala, MD is out with a Current Opinion in Oncology article entitled Intralesional therapy for advanced melanoma: promise and limitation, which focuses on the patient population who would benefit from intralesional therapy (PV-10); see Keep talking (and keep doing) (January 29, 2015) below.
H/t a shareholder:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Merck KGaA visited the blog from Darmstadt, Hessen, Germany. Merck entered into a $2.5 billion deal and collaboration with Pfizer in November to develop and commercialize Merck's PD-L1 agent and Pfizer's PD-1 agent.
Keep talking (and keep doing) (January 29, 2015)
Surgical oncologist, MD Anderson Cancer Center staffer, and Provectus principal investigator Dr. Merrick Ross, MD recently described oncolytic immunotherapies ("OIs") as rupturing tumors in a manner similar to a multivalent vaccine. See Knick-knacks (January 28, 2015) below. It's an interesting label. PV-10 is, of course, not a vaccine; however, management has described the drug's immune response (mechanism of action #2) deriving from its chemoablation (mechanism of action #1) "...as tantamount to “in-situ vaccination” (SITC 2012)— in situ to mean "on site," "locally," where it takes place, etc., and vaccination to mean stimulate the immune system to develop adaptive immunity.
Additionally, "[v]accines may be monovalent (also called univalent) or multivalent (also called polyvalent). A monovalent vaccine is designed to immunize against a single antigen or single microorganism. A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms."
While PV-10 does generate cancer immunity ("...induces tumor-specific T cell-mediated immunity in multiple histologic subtypes..."), it is not a vaccine because it is not constructed to immunize against particular antigens. Whatever antigens exist in tumors injected with PV-10 subsequently become, if successful, the antigens against which PV-10 immunizes.
H/t a shareholder: Dr. Suzanne Topalian, MD, Professor of Surgery and Oncology, and Director, Melanoma Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University speaks about Immunotherapy for Melanoma Treatment: Approved and Investigational Drugs (October 2014), and notes local injection (i.e., intralesional therapy, and PV-10) under tumor vaccines (presumably sponsored by the Melanoma Research Alliance). The video of her presentation follows the picture below. Her discussion of melanoma vaccine platforms begins at 8:09.
 |
| Click to enlarge. |
H/t a shareholder: Medical oncologist, St. Luke’s Cancer Center staffer, Professor of Medicine at Temple University School of Medicine and Provectus principal investigator Dr. Sanjiv Agarwala, MD appears to be out with a Current Opinion in Oncology article entitled Intralesional therapy for advanced melanoma: promise and limitation, which focuses on the patient population who would benefit from intralesional therapy (PV-10):
 |
| Click to enlarge. |
Knick-knacks (January 28, 2015)
Comments by Dr. Robert Andtbacka, MD., Associate Professor, University of Utah School of Medicine, and Merrick Ross, MD, Professor, University of Texas MD Anderson Cancer Center at The Society for Melanoma Research annual congress in Zurich, Switzerland (November 13 to 16) about oncolytic immunotherapies were interesting:
Dr. Ross elaborated on the rationale for combining systemic immunotherapies with ablative intralesional treatments in an interview. “I am actually very enthusiastic about the combination of these oncolytic immunotherapies with the other targeted immunotherapies like the anti-CTLA-4 or anti-PD-1 agents,” he said. The anti-PD-1 and anti-CTLA-4 agents affect precise targets: for example, blocking a specific receptor to activate T cells and make the immune system function, he said. They differ from “classic” immunotherapies that elicit very specific T-cell responses through antigen presentation.
The oncolytic immunotherapies, including an oncolytic virus such as T-VEC or the chemical ablator PV-10, rupture the tumor in a manner similar to a multivalent vaccine, releasing antigens potentially specific to the tumor. This may include antigens that were “hidden” to the immune system when the tumor was intact, Dr. Ross said. Chemotherapies and BRAF inhibitors, he continued, probably cause oncolysis by inducing apoptosis. This programmed cell death may not cause the tumor to express antigens in a way that is useful to the immune system. “So the type of oncolysis may be very relevant. The tumor ablative agents could be synergistic with ipilimumab. It would make sense because they act at different places in the immune system.”
In Zurich, Dr. Andtbacka reported that analysis of biomarkers in the phase 1b/2 trial showed increases from baseline after treatment with T-VEC and further increases after ipilimumab treatment in total and activated CD8 T cells in peripheral blood. Increases in CD4 T cells expressing inducible T-cell costimulator also followed ipilimumab treatment, indicating upregulated CTLA-4 blockade. Importantly, patients with disease control after T-VEC had more than 1.4 times as many activated CD8 T cells, while four out of five patients with tumor growth did not have such increases. The phase 2 study comparing T-VEC plus ipilimumab to T-VEC is going forward and adding patients based on these findings. {Underlined emphasis is mine.}Compared to T-Vec, PV-10 generates more robust immunological signaling, and should improve ipilimumab's, among other checkpoint inhibitors', benefit in combination with an oncolytic immunotherapy for late-stage patients with high disease burden and bulk.
 |
| Click to enlarge. |
Your next question comes from Chris Schott from JPMorgan.
Chris Schott - JPMorgan: Great, thanks very much, just a couple of questions here. It’s first for Ian. I believe you mentioned that your business development is now focused on creating value in the near-term given the way of stage pipeline. I guess if it’s a shift from the prior focus of what we are thinking about in 2014? And then the second question on business development, when you look at valuations resetting kind of across the biopharma space, is that reducing the opportunity set as you look at these targets given the valuation discipline you piloted for Pfizer?
Ian Read - Chairman & CEO: Well I think you know if we would have seen -- we would have been concerned about the state of our research and if we hadn’t strengthened our research as we did with the deal with Merck on the immuno-oncology asset, then we may have felt we needed to do more business development in acquiring assets in our research. But I feel our research pipeline middle-stage, late-stage is strong and I would rather take our capital right now and direct it to opportunities to accelerate the EPS growth as I think we’ve got the right balance of capital allocation in the medium to long-term and on the innovative side.
So it’s just with your balance of where you are deploying your capital and where you think you’ve areas you want to strengthen. That been said, if there was a piece of intellectual property that added huge value we thought, we could develop it and we would not be shy in acquiring that intellectual property. I do think that the values are high at the moment in many sectors. We're disciplined, but you know when we find an appropriate deal that will meet our strategies of strengthening our businesses and accelerating shareholder value, we feel very comfortable that we’ve the ability to do those deals. {Underlined emphasis is mine.}Indicative (January 27, 2015)
 |
| Image source |
"The key criticism of following insider buying is that while insiders may know their businesses well, they are not sophisticated investors, as a result, their purchases can be poorly timed. This is compounded by the fact that executives usually view their company through rose colored glasses."More than any other executive—given his role and responsibilities, and personality—Eric's stock option exercise behavior is likely to be indicative of his view, at the point in time of the exercise, of the company's regulatory affairs progress.
Below is a table of Eric's exercises from 2011 to date (preliminary metastatic melanoma Phase 2 clinical trial data was presented in November 2010). Eric's exercise of 1 million stock options on September 6, 2011 (transaction date; the filing date was September 7th) was excluded because all principals exercised that grant that day (as it was bonus compensation).
 |
| Click to enlarge |
 |
| Click to enlarge. |
He did not undertake another exercise for nearly a year (347 days, from December 5, 2011 to November 16, 2012) despite Provectus's January 18, 2012 press release Provectus Receives Guidance From FDA On Pathway to Approval for Phase 3 Trial of PV-10 For Metastatic Melanoma. He later expanded in October 2, 2012 press release Provectus Pharmaceuticals Presents Final Phase 2 Melanoma Data at ESMO 2012, commenting on the Phase 3 study design:
"This study is designed to demonstrate delay or avoidance of progression of melanoma from a locoregional disease to a life-threatening systemic stage. The proposed study population experienced excellent response to PV-10 in the Phase 2 study, and this bodes well for the planned Phase 3 study, where subjects will receive treatment on an even more aggressive schedule than that allowed in Phase 2. Since our last meeting with FDA in late 2011, we have carefully studied the outcome of different sub-populations of subjects in the Phase 2 study, reviewed the criteria to be used for unambiguous assessment of response, and fostered relationships with key parties that will provide support in execution of the Phase 3 study. We look forward to formal review of the resultant protocol and supportive programmatic elements with FDA under the SPA process."The lack of achieving a special protocol assessment ("SPA") with the Agency at that time (i.e., no agreement on endpoints or patient population) likely led to the unsuccessful but poorly handled PVTCP "IPO," which caused the share price to sink in 2012's fourth quarter. Eric's exercise of ~667K options ~90% above the then share price may have been his way of showing commitment to working through the regulatory pathway challenges at the time.
The company's May 13, 2013's Provectus Updates Shareholders in Its Annual CEO Letter cryptically then but understandably now contained more Eric-speak of lack of regulatory affairs progress:
"Provectus is finalizing details for submission of a pivotal Phase 3 randomized controlled trial ("RCT") of PV-10 for metastatic melanoma, suitable for Special Protocol Assessment ("SPA"), to the Food and Drug Administration ("FDA"). While preparation for submission of our SPA has taken longer than expected, it is crucial to remember that oncology presents a moving playing field. Fine tuning of the study design is expected to mitigate clinical efficacy risk, optimize patient accrual, and increase FDA's confidence that the study design and protocol will ensure the best possible outcome for our pivotal trial. We have every reason to believe this key milestone will be achieved in 2013."His next exercise, of almost 640K options at ~60% discount to the share price, would come ~16 months (489 days) later on March 20, 2014, a few days before Provectus' March 24, 2014 press release Provectus Biopharmaceuticals Inc. Submits Application to FDA to Receive Breakthrough Therapy Designation for PV-10 for Treatment of Melanoma. Eric likely fully believed the company would achieve breakthrough therapy designation ("BTD").
Almost 10 months (310 days) have passed since Provectus submitted via overnight courier its BTD application, and Eric exercised options again, this time at a ~25% premium to the share price. He may be more confident again about PV-10's regulatory pathway, and the process of reaching this critically important destination.
Stock Option Exercise (January 26, 2015)
 |
| Click to enlarge. |
Dermatology & PH-10 (January 26, 2015)
H/t a shareholder: Provectus' mechanism of action study for PH-10—A Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10—now is recruiting.
Mind the gap (January 24, 2015)
The current National Comprehensive Cancer Network ("NCCN") guidelines for melanoma still appear to list "clinical trial" as the top recommendation for patients with disease where resection is not curative.
Provectus' upcoming pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma, which the FDA determined met the criteria for a serious or life-threatening disease or condition, addresses a patient population for which, again, clinical trial is the preferred primary treatment.
 |
| Click to enlarge. Source link |
The germane inclusion criterion (for the above) is "Failed, did not tolerate, or not a candidate for (due to co-morbidities, pre-existing autoimmune disease or drug unavailability) treatment with ipilimumab or another immune checkpoint inhibitor." Addressing this successfully (for Provectus) may allow PV-10 to bridge this gap, become SOC, and take its place in NCCN guidelines.
T-Vec and Allovectin-7 principal investigator Dr. Robert Andtbacka (of the Huntsman Cancer Institute) cut a video about PV-10's upcoming Phase 3 trial while discussing T-Vec in Zurich at the 2014 Society of Melanoma Research Congress during a satellite symposium sponsored by Amgen and entitled Oncolytic Immunotherapy – Engaging the Immune System to Target Melanoma. See below.
Andtbacka was an NCCN Guidelines panel member for version 1.2015. Three Provectus principal investigators and consultants (Thompson, Khushalani and Ross) also were members.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Returning to Seeking Alpha, sort of (January 21, 2015)
 |
| Image source |
According to SA Director/Editor Abby Estikangi-Carmel who contacted me last week, SA offered to monitor Connecting the dots...Provectus Biopharmaceuticals for articles they thought were suitable for their website, and post them under my SA account, which has been dormant since October 2013 (see the blog's Disclosures page). She also said at the time that SA would back-post a couple of my blog posts using their original publication dates to update my SA presence.
Before agreeing to their request, I asked Ms. Carmel several questions including:
- How would SA post my posts; that is, what action would I have to take? Or would SA simply do it themselves? (Paraphrasing SA) SA would do it all for me.
- I note my own disclosures on the blog's Disclosures page. How would they address that in SA's disclosure on my posts? (Paraphrasing SA) SA would link to my disclosure page at the bottom of each article.
- Would it be safe to assume my posts would not be edited by their editorial team, but merely copied verbatim? (Paraphrasing SA) Posts would be copied verbatim, and only edits for grammatical errors and the like would be made.
They also initially placed only my blog's disclaimer under the articles as a disclosure, rather than [what Ms. Carmel originally agreed to do, which was to] link to the blog's Disclosures page. SA tweeted the articles:
SA also commented on StockTalk on my behalf:
Takeaway: I am not returning to SA in any real sense—I will not be publishing articles or or post Instablog posts there myself, nor will I be replying to comments under articles SA publishes. The website essentially served the purpose I originally had intended, which was to publish an investment letter (and thesis) about Provectus (see September 2013's Why I'm Long Provectus Pharmaceuticals). SA may or will choose to publish as articles from this blog whatever posts they select, and whenever they select them. "Returning" under the above mentioned situation and conditions seems fine to me, as SA's website and community is a much larger forum than this blog to "talk my book"/"pump."
A Twisted Interview (January 21, 2015)
Provectus' CFO/COO Peter Culpepper was interviewed by The Wall Street Transcript ("TWST"). The interview was first published Friday via TWST subscription. The company made it available today. It appears the interview was conducted in late-2014, probably December. Three items struck me.
1. Pfizer's tremelimumab. Peter said "Pfizer first approached us because they wanted us to combine PV-10 with their initial anti-CTLA-4 immune checkpoint blockade, which is an analog of Bristol’s."
It's interesting to read Peter short comment above this situation. See, for example, November 2011's blog post The Usual Suspect[s], and other posts by searching for tremelimumab on the blog using the search tool.
Takeaway: Beginning around the time Pfizer's Dr. Craig Eagle, M.D. commenced his relationship with the company, he wanted to do a transaction with Provectus—in the above case, an out-license from Pfizer to Provectus. What does this history, now confirmed (sort of), do for me today? Nothing; however, it does frame certain other Pfizer fiction.
2. Protocol ordering. Peter said "Yes, there is an order. The order we’ve established is the Phase III melanoma study is filed; we’re waiting for FDA interaction to make sure all the particulars are fine. Then, they will submit the Phase Ib/II combination study design, which we said is underway, and we are finalizing it. As a matter of fact, we’re meeting with key opinion leaders on this topic next week. Then, we do the Ib/II liver study. We’ve been meeting with individuals also for our liver program on a regular basis. That probably will come very shortly after the combo, and we’ve met with individuals globally...So the Ib/II for liver would follow very closely after the combo, and then, breast unfortunately would be after that, but it would be the same study design instead of, say, using sorafenib or the checkpoint blockade as the combo piece. We would use chemotherapy or radiation, but breast would definitely come after Phase III is underway and enrolling, the Phase Ib/II combo is underway and enrolling, and Phase Ib/II liver is underway and enrolling."
So: i. melanoma Phase 3, ii. melanoma combination Phase 1b/2, iii. liver Phase 1b/2, and iv. breast Phase 1b/2. Previously, I had understood management's guidance was melanoma, liver, and melanoma combo. See, for example, Provectus' Q3 2014 conference call.
Takeaway: The impact of the re-ordering is negligible to nothing. I merely am struck, however, by the fact Peter changed the order, because [for me] it convey's Eric order, as said order may have been influenced or guided by Peter's business and corporate development activities.
3. San Antonio Breast Cancer Symposium ("SABCS"). Peter said "So I would say, with the next three PV-10 studies, certainly we’ll see those studies generating meaningful data in 2015. I hope the answer will be yes in breast. We’re pushing on that. We have already established a relationship with a breast cancer advocacy group. We plan to be at San Antonio Breast Cancer Symposium in San Antonio in December. We’re very focused, and of course, we’ve done clinical work in breast, so we know PV-10 is appropriate for breast, and we’ve been encouraged by potential partners to be treating breast cancer, particularly in China and India where breast cancer is a top concern, but liver cancer is of course also a cancer of very significant concern."
Cancer clinical trial work has evolved from Phase 1, 2 and 3 trials to, somewhat recently, Phase 1b/2 trials that may lead to pivotal trials suitable for drug approval consideration. See terminology provided by Eli Lilly and Company:
- Phase 1 studies are mainly concerned with evaluating a drug’s safety profile, including the safe dosage range.
- Phase 1b studies are usually conducted in patients diagnosed with the disease, or condition for which the study drug is intended, who demonstrate some biomarker, surrogate, or possibly clinical outcome that could be considered for "proof of concept."
2015 SABCS will be held December 8-12, 2015. Abstracts, if following the 2014 conference's form, should have a submission deadline around June 10th. Provectus' Phase 1 trial for recurrent breast cancer had a primary and secondary outcome time frame measures varying from 7-21 days to 5-7 weeks. I don't doubt Peter believed at the time he did the TWST interview that it may be possible for Provectus to recruit, treat and observe breast cancer patients, measure their progress, and complete and submit an abstract to SABCS before June.
Takeaway: For Provectus, among other things, Eric's throughput has been a rate-determining step for the progress of the clinical development program. The company may be able to participate in 2015 SABCS if he improves his work-rate.
Combinations & Category Building (January 20, 2015)
Combinations of therapies, where one of the the two combined therapies is a checkpoint inhibitor (e.g., anti-CTLA-4, anti-PD-1), and thus combination therapy clinical trials, appear to be predominantly if not exclusively focused on advanced melanoma.
There are lots of different ideas about how and what to combine. Combinations can include therapeutics (i.e., one drug with another), and therapies like surgery and radiotherapy (i.e., a drug with radiotherapy). For this blog news item, I'm going to focus on the combination of two drugs from two different categories—specifically, co-stimulatory and co-inhibitory. Checkpoint inhibitors fall into the co-inhibitory ("co-in") category, and intralesional agents like T-Vec and PV-10 would fall into the co-stimulatory ("co-stim") category. Current approaches appear to focus on exploring, very generally speaking, (i) two co-ins and (ii) a co-stim and a co-in. It strikes me (i) represents doubly releasing the brakes of the immune system, while (ii) may suggest starting its engine and then releasing its brakes.
Success of combination therapies (and more broadly speaking, success of cancer treatments) ultimately may derive from the immunogenicity of cancer tumors to which the combination therapies are applied. More available immunogenic material in tumors (i.e., melanoma) may make it easier and more amenable for checkpoint inhibitors to work (as monotherapies) than cancers where tumors are less immunogenic or not immunogenic at all (i.e., pancreatic cancer). As a result, (i) above may see two co-ins trying to make what they can of available immunogenicity, while (ii) above may see the co-stim improve and increase tumor immunogenicity for the co-in to then do its thing.
As an aside, "tumour immunogenicity varies greatly between cancers of the same type in different individuals and between different types of cancer." Melanoma tumors are considered more immunogenic than lung cancer tumors; however, renal cell carcinoma, melanoma, lung cancer and prostate cancer "...tumors may be more immunogenic than once believed." Interestingly, Bristol-Myer's melanoma trial of its PD-1 Opdivo (nivolumab) generated a 40% objective response rate ("ORR"). The Big Pharma's non-small cell lung cancer trial for Opdivo generated a 15% ORR.
When Moffitt Cancer Center's Dr. Jeffrey Weber called T-Vec a "a niche drug that would be best used to prime the immune system," he may have meant priming the immune system when treating melanoma (or, at best, a limited number of cancer indications). As a therapy engineered from herpes simplex 1 (HSV-1), while T-Vec could be administered into cutaneous, subcutaneous or nodal lesions in its clinical trials, injections of visceral lesions were not permitted. See PV-10 & Amgen's Talimogene Laherparepvec (June 9, 2014) on the blog's Archived News II page. All of T-Vec's clinical trials to date are melanoma-related. A trial for squamous cell carcinoma of the head and neck was terminated was terminated, presumably by Amgen, after the Big Biotech acquired Biovex (the privately held Boston-based that developed T-Vec when it was called OncoVEX).
Priming the immune system for melanoma and other cancer indications likely means, from the perspective of those who believe generating as many antigens as possible for the antigen-presenting cells ("APCs") to take-up/present to the immune system (i.e., T-cells, natural killer cells and other immune system components), engaging as many tumors as possible and harnessing/increasing those tumors' immunogenicity.
Moffitt Cancer Center fully understands PV-10 is efficacious in multiple cancer indications. For example, see Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer.
PV-10 has been injected in melanoma, breast cancer and liver cancer via clinical trials, and other indications treated by Provectus' compassionate use program. If PV-10 has the potential to prime the immune system for treating melanoma, I imagine it has the potential to prime the immune system for other cancer indications.
Category building (January 19, 2015)
It bears watching T-Vec's progress as part of its category building of "[intralesional] oncolytic immunotherapies," and how Amgen's IL agent establishes the category's bona fides in terms of priming the immune system. The notion of a stimulatory agent (e.g., PV-10, T-Vec, vaccines, etc.) making non-immunogenic tumors immunogenic, and immunogenic ones more so recently has gained traction as a novel and additional approach to treating cancers, and to making checkpoint blockade agents more relevant.
 |
| Click to enlarge. |
Conference Presentations (January 16, 2015)
Provectus appears to be presenting at the 17th annual BIO CEO & Investor Conference—February 9-10 in New York (investor/financial-oriented)—and the 8th Annual European Life Sciences CEO Forum (the company is a sponsor)—March 4-5 in Zurich (partnering/networking-oriented).
Commercial Strategy (January 14, 2015)
Provectus' updated their corporate website presentation to include a slide entitled PV-10: Commercial Strategy (see below).
 |
| Click to enlarge. |
- PV-10 is an advanced investigational clinical oncology drug with great global potential
- Provectus will continue to communicate with and evaluate potential partners in selected global geographies with the clear intention of controlling the drug's supply chain and actively participating in the process of commercializing PV-10
- The Company will continue to actively pursue, investigate and diligence business development opportunities of both internal and external origin prior to obtaining interim randomized data from PV-10's pivotal melanoma Phase 3 trial
- Our main assumption: Obtaining interim randomized Phase 3 clinical data for PV-10 (an unencumbered asset) before signing a global agreement should provide the greatest ROA for the Company and ROI for Provectus' shareholders
 |
| Click to enlarge. |
Provectus' compassionate use program ("CUP"), or expanded access program ("EAP"), which the company initiated in 2009 (June in Australia, October in the U.S., and all patients in Provectus' metastatic melanoma Phase 2 trial were treated by September) reached a low point of 8 new patients in 2012 before adding double-digit new patients in 2013, very likely in 2014 and potentially in 2015 (based on CUP site physician requests prior to year-end). See Compassionate Use/Expanded Access (January 5, 2015) below. Despite the approvals of ipilimumab (2011), peginterferon alfa-2b (2011), vemurafenib (2011), dabrafenib (2013), trametinib (2013), pembrolizumab (2014) and nivolumab (2014), there still appears to be room and demand for PV-10.
It is odd Provectus' facilitation of PV-10 expanded access has been used by physicians so much with so few issues or fanfare, especially considering CUP patients are there because they have run out of options. I have not been able to find information on EAP results of other oncology drugs from U.S.-based sites; however, there have been papers written about the results of international patients from using these therapeutics. I imagine this dearth of data could be chalked up to U.S. federal and state patient privacy regulations.
Provectus' CUP has existed in various forms since inception, including multiple physician-sponsored ones, Phase 2 continuation expanded access, a "pure" EAP (i.e., a treatment size IND), and other oddities. There does not appear to be a lot of pure EAPs (i.e., treatment size or treatment INDs).
 |
| Click to enlarge. Source link. |
 |
| Click to enlarge. Source link. |
Let me get this straight... (January 10, 2015)
She's on the strategic advisory board to help Provectus prepare for when other pharmaceutical companies come calling to license PV-10 and/or acquire the company?
Jay: Run over your plan one more time, 'cause I'm struggling with it. You neuralyzed memory of the Light but left yourself clues. A photo pointing to a key in a pizzeria which opens a locker at Grand Central. In the locker, we'll find another clue.In-Pharma Technologist People On The Move:
Kay: I like to keep my enemies confused.
Jay: We're all confused, Kay.
— Men In Black II
 |
| Click to enlarge. |
"Time value of money" (January 9, 2015)
 |
| Image source |
According to Silicon Valley Bank ("SVB"), "[i]n the last few years, there has been a noticeable shift in the direction of big exit M&A activity from early-stage to the later-stage." SVB defines the "big exit" as "...private venture-backed M&A with at least $75 million upfront in biotech..." I am using private venture-backed as a proxy for Provectus Biopharmaceuticals. Provectus is neither venture-backed nor private, but has operated as de facto one: "closely held" by a core network of retail investors, and governed pretty much only by management (and then only by a subset until recently).
 |
| Click to enlarge. Healthcare M&A Report 2013 |
 |
| Click to enlarge. Healthcare M&A Report 2014 |
 |
| Click to enlarge. Healthcare M&A Report 2014 |
 |
| Click to enlarge. Healthcare M&A Report 2014 |
 |
| Click to enlarge. |
Let's say that one way Big Pharma thinks about acquisition, licensing and partnering is to evaluate companies with Phase 3 assets that could be launched in a year, fit their profiles, and fill gaps in their pharmaceutical portfolios (see, for example, this 2009 paper). I take "could be launched in a year" to mean (i) a pivotal trial underway for (ii) a specific indication in (iii) a distinct patient population, all of which Provectus now established.
Thus, if the time value of money now can be estimated—launch time frames may be projected when Provectus' Phase 3 trial begins (the assumption of course is when the trial succeeds, not if)—it then would not be surprising for Big Pharma to begin calculating.
From Provectus' December 17, 2014 press release Provectus Biopharmaceuticals To Sponsor American Association of Physicians of Indian Origin: "Provectus has also expanded the membership of its Strategic Advisory Board with the addition of Deanna Angello, Director, Commercial Strategy and New Business Planning for the Global Established Pharma business at Pfizer Inc. In this role, she is focused on new business opportunities for the US region, notably in areas such as licensing of new assets, mergers and acquisitions, and strategic partnerships...Dr. Craig Dees, Ph.D., CEO of Provectus, said, "We are delighted to have Deanna join our Strategic Advisory Board. She is a highly experienced, forward-thinking healthcare and pharmaceutical strategist, with commercial experience in marketing and business development and we look forward to working with her in the coming months." {Bold emphasis is mine.}
From Ms. Angelo's LinkedIn page (it would appear) as posted on Investor Village: "Leading growth strategies and partnerships with potential revenues > $500M by managing cross-functional partners, formulating robust business cases, and performing due diligence to thoroughly assess the commercial value and fit of in-licensing and acquisition opportunities." {Bold emphasis is mine.} Another biographical link, which I referenced in my December 21, 2014 post Let Me Tell You A Story About Pfizer, has since been taken down.I doubt Ms. Angelo is helping Provectus with developing a drug launch plan and team as much as she may be helping to assess the commercial value and fit and thus construct the business case for the in-license/acquisition of PV-10. She also may be lending her experience in drug pricing, a relevant topic of late.
Provectus's relationship with Pfizer is odd, to say the least. It is odd in terms of how Pfizer appears to be acting, and odd in terms of how Provectus management has acted. Pfizer has added more and more members to Provectus's strategic advisory board ("SAB") member, since it's first addition of Dr. Craig Eagle, M.D., when these folks easily could have been working quietly with the company with no need for fanfare. The counterpoint to this argument could be that such work might have been deemed material and thus reportable by Provectus' legal counsel. Provectus acquiesced to burying the second and third SAB additions in press releases without effectively leveraging Pfizer's clear interest and growing relationship into a much higher market capitalization. Pfizer seemingly has established the longest and largest look under Provectus' tent at what appears to be no tangible or material cost to Pfizer, and ostensibly no visibly tangible or material benefit to Provectus. I think it's reasonable to observe management has given the appearance of putting all of Provectus' eggs in Pfizer's basket without commensurate benefit.
Angelo's addition, at this moment in time, does speak more to regulatory validation than it does commercial validation. In reconsidering Craig and Peter's comments, an pharmaceutical industry professional like Angelo who formulates and substantiates drug in-licensing/acquisition business cases would seem to suggest Big Pharma (Pfizer, at least) agrees PV-10's regulatory pathway finally is set, and thus the time value of money now can be calculated. Provectus presumably just has to begin this necessary leg of the journey.
Stock Option Exercises (January 8, 2015)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Notes (January 7, 2015)
 |
| Image source |
"Immunotherapy is treatment that uses your body's own immune system to help fight cancer." According to the American Cancer Society, "Immunotherapy includes treatments that work in different ways. Some boost the body’s immune system in a very general way. Others help train the immune system to attack cancer cells specifically." So, what components of the immune system should an immunotherapeutic boost and/or train?
T-cell based therapies abound, like adoptive cell therapy (take out, put back in): Cellectis, Juno Therapeutics, Bluebird Bio, Kite Pharma, etc. There's a potential CAR-T killer, backed by Dr. Patrick Soon-Shiong, M.D., (see Christmas Cheer: Sorrento, NantWorks, and Conkwest Create Keiretsu) because it's focus is on natural killer cells, and not T-cells. There's a focus on regulatory T cells, or Treg cells, by an Amgen veteran backed by Celgene and Kleiner Perkins. Don't forget Big Pharma's PD-1s and PD-L1s that, um, release the brakes of the immune system.
And then there's PV-10, which seems to involve several immune system components, that we know of for now...
Unencumbered oncology asset. "Unencumbered" means one does not have to share revenue with anyone else. An unencumbered oncology assets like PV-10 should provide management with more options to maximize Provectus' asset value.
 |
| Click to enlarge. Image source. |
"The metabolism of cells in injured or diseased tissues often results in the acidification of the extracellular environment, so acidosis might be useful as a general marker for the imaging and treatment of diseased states if an effective targeting method can be developed" (Targeting diseased tissues by pHLIP insertion at low cell surface pH, Andreev et al., Front. Physiol., 13 March 2014 | doi: 10.3389/fphys.2014.00097) {Bold and underlined emphasis is mine.}AAPI – Global Clinical Trials Network (January 6, 2015)
H/t Investor Village/Wile_E_Coyote: AAPI – Global Clinical Trials Network launched during Global Healthcare Summit 2015 in Mumbai
 |
| Provectus strategic advisory board member Dr. Joseph Chalil, M.D. of Boehringer Ingelheim (far left) and principal investigator Dr. Sanjiv Agarwala, M.D. of St. Luke's Cancer Center (far right) |
Dr. Ravi announced that Provectus Biopharmaceuticals, a development stage oncology research company has committed to be the inaugural member of the Global Clinical Trial network. AAPI is looking forward to creating a large network of Pharmaceutical companies to make this initiative a great success. The networking opportunities from a group that is known for such, leading to a close knit execution of projects in research and/or clinical trials, provides an incredible opportunity for any participating organization.
“I am very excited about potential for Indian investigators through this network to be part of clinical trials with PV 10 in melanoma other malignancies,” said Dr. Agarwala, Chief of Medical Oncology & Hematology at St. Luke’s Cancer Center and Professor of Medicine at Temple University School of Medicine and has been Principal Investigator of Provectus clinical trials involving immunotherapy and targeted therapy for melanoma.
“Connecting to the brand of AAPI and its extensive US Physician network will lead to an increased visibility of pharmaceutical clinical trials here in the USA,” commented Dr. Joseph Chalil, Strategic Advisor to Provectus Biopharmaceuticals, who attended the event."
Compassionate Use/Expanded Access (January 5, 2015)
Provectus' compassionate use program ("CUP") has grown since its inception in 2009, June in Australia and October in the U.S. The program uses PV-10 to treat cutaneous (on the skin) or subcutaneous (under the skin) tumors irrespective of cancer. Some safety and efficacy information has been published about CUP patients, such as 2010's A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series and 2013's Novel use of Rose Bengal (PV-10) in two cases of refractory scalp sarcoma. In both cases, patients were not from the U.S. (I believe they were all Australian). For the most part, however, there are no published data about the program's results (e.g., indications treated, safety issues [if any], efficacy, survival, etc.). Through 2013 year-end, according to the company's website, "more than 100" participated (a cumulative figure, I believe). Numbers for past year-ends may be found in Provectus' annual CEO shareholder letters or updates. See the table and graph below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Network 1 Financial Revisited (January 3, 2015)
Updated from the news item immediately below: Peter corrected me by (paraphrasing) noting the first two line items of the table in my January 2nd news item were the same (thus, I appear to have misread the November 6th 10-Q). So, two tranches (not three) have been raised; the December 31st tranche was the second (not the third). Gross proceeds of $7.8 million have been raised (not $11.4 million); $7.2 million remains unused (not $3.6 million). A revised table is below:
He commented (paraphrasing) that he used Network 1 at this point in time because they were the only firm that could sell Provectus stock at $1.00 per share, and was substantially above market. Using Cantor's "ATM" facility would have seen him raise money under $1.00. Peter continued that Provectus is maintaining the company's NYSE MKT listing by what they (Provectus) is doing on the capital formation front. Network 1 may not give them equity research coverage, but he believes they provide something far more valuable at this point in time; namely, substantially above market financing. The share price ranged from a $0.93 intraday high to a $0.76 intraday low in December. The closing high and low were $0.92 and $0.77, respectively, which occurred on consecutive days (December 5th and 4th, respectively). A December stock price chart is below:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Peter confirmed (paraphrasing) the fundraising decision-making process and outcome incorporated Provectus' accounting firm BDO's year-end activity (i.e., process, analysis and review, and decision).
Most importantly (for me), Peter did what he said he would do, again. In this case, whether one agrees with it/him or not, it was a quarterly fundraising to offset quarterly burn, and he disclosed it when it was appropriate to do so (i.e., very proximate to when he did it). It's an important trait and approach that did not change, which is notable, relevant and important, and that is potentially much more revealing, insightful and helpful when applied to other Provectus topics and situations within Peter's purview.
Network 1 Financial, again (January 2, 2015)
Provectus raised a third tranche of money using its $15 million private placement with Network 1 Financial -- ~$4.1 million this time, leaving $3.7 million unused -- and issued an 8-K related to it. Information on the first and second tranches may be found at the bottom of page 12 in the company's November 6th 10-Q filing.
I previously thought the company had utilized the placement's remaining placement (see Capital Formation (November 21, 2014) below). In retrospect, however, it turned out some prospective participants (i.e., some Network 1 clients) were preparing their subscriptions (by selling existing Provectus stock they held in order to participate), which Provectus later accepted on December 31st.
Peter continues to frustrate through his continued, exclusive use of Network 1. Frustration derives from the continued use of a firm, Network 1, that has not, does not and will never provide equity research coverage of Provectus. Peter's seeming comfort to keep paying Network 1 healthy fees to raise money for him in exchange for nothing (other than the net proceeds of those financings) continues to be annoying and frustrating. That Network 1 facilitated Sinopharm's introduction to the company should have no bearing, since I presume the broker-dealer will be handsomely paid for it (i.e., a mid- to high single digit percent of the upfront payment due Provectus if and when a deal is consummated).
At best, Network 1's continued use is of no value because the company gets nothing in return (i.e., no research coverage). At worst, it is of negative value because Provectus effectively pays Network 1 not to cover it. To be fair, research coverage for mid-to large cap companies, let alone nano- to small cap ones, ranges from scarce to non-existent. There has been periodic coverage of Provectus by Rodman & Renshaw, Maxim Group, Roth Capital, etc., which isn't to say the Janneys, Aegiss, etc. of the world are any better or worse (this kind of firm are not the white shoe institutions whose "credibility" one would have hoped could have been lent to Provectus) but they are better than nothing, and most certainly better than choosing to do nothing.
Given the kind of superlative drug candidate PV-10 is (and the paradigm shift in the treatment of cancer I contend it also is), were Provectus more successful at capital formation, I wouldn't have objected to the company giving Wall Street and its denizens the middle finger. Management's long-standing lack of success on the capital formation front obfuscates their business strategy, which I think is intelligent and sound, and diminishes their clinical trial capital efficiency. By using the same firm over and over again, and effectively eschewing Wall Street equity research quid pro quo, Provectus' capital formation has been much more expensive (i.e., more dilutive) than it should have been.
It's very likely Peter found it expeditious to use Network 1. In the end, my comments above probably are much ado about little more than nothing. While repeated behavior, objectively of poor quality, is not a good thing, this Network 1 raise (i.e., each tranche, and all of the tranches) speaks to choosing expeditiousness over value at this point in time (i.e., essentially 2014's third and fourth quarters). It would have been better, and also a change for the better, to use Cantor and a portion of Provectus' $50 million Controlled Equity Offering Sales Agreement with the firm to potentially bring in institutional investors who could be a higher quality of shareholder than Network 1 clients. Peter may have deemed a "road show" to meet Cantor clients (as prospective investors), which likely would have to have required Eric's presence and participation, not worth their time.
Peter's historical challenge on the capital formation front arguably has been Eric repeatedly missing timing expectations (and some issues related to Craig and Tim that appear to be firmly in the past). Missing deadlines doesn't necessarily equate to doing a bad job, making bad or incorrect choices, or having bad or ineffective process(es). It can simply mean the guy doing clinical development mis-setting expectations for (and not fully comprehending the role, responsibility and implications of) the guy doing capital formation results in costlier dilution, a lower market capitalization than otherwise, and company brand tarnish. Furthermore, lather, rinse, repeat is of course not good.
Peter may have believed Eric could deliver a ready-to-enroll pivotal melanoma Phase 3 trial protocol sooner than he eventually will (see Like Watching Sausage Being Made (December 29, 2014) below), which could have permitted a China deal to be consummated and thus its potential upfront payment to be triggered, which then would have mitigated some or all three of the Network 1 tranches.
Cash on hand at year-end probably could or should be about $20 million, give or take. I estimated cash on hand as at November 5th, or roughly at the end of the third quarter, of about $20 million. See Notepad (November 6, 2014) below. I speculate the ~$3.6 million in net proceeds from the December 31st tranche may cover Provectus' fourth quarter operating expense. Peter seemed to say as much on the company's November 6th conference call:
"Yes, I think that’s a fair way to be very specific about it. If we’re not able to raise or to secure non-dilutive cash, then we will need to go that the capital market to offset burn. That’s right. And that’s the importance of generating meaningful data and the importance of monetizing the assets to minimize dilution."This financing buys at least one more quarter for Eric. The timing of the acceptance of the subscriptions of the third tranche could be related to the quarter/year-end process of and review by Provectus' accounting firm BDO. If so, Peter should not have to revisit the topic of sufficient capital levels (because the third tranche should have met BDO's requirement for the time being), and thus should not have to raise money again until the end of the first quarter of 2015 (at which time, in the absence of upfront payments from regional transactions, he would draw down on the balance, or thereabouts, of the $15 million placement, assuming the share price has not risen above the offering's price point).
A potential trigger for a China regional transaction could be the completion of Provectus's discussion with the FDA about operational aspects of the melanoma Phase 3 trial, which according to the company should be in January or early-February, rather than the filing of a liver Phase 1b/2 trial protocol.
No change in behavior: I appreciate Peter's timely disclosure of the fundraising event and amount. That there was no change in Peter's approach to disclosure is a good thing. He could have shunted information about the fundraising to Provectus' 10-K, which should be filed in early- to mid-March. Having timely information about the tranche should allow shareholders to promptly calculate dilution, and establish observations and draw conclusions on topics incorporating cash balances, cash burn needs and rates.


































No comments:
Post a Comment