News items from July to December 2015.
Mix & Match (December 29, 2015)
Updated below.
Calendar of Events. Provectus added the 12th International Conference of the Asian Clinical Oncology Society (theme -- Cancer in Asia: Bridging the Gaps) from April 8-10 in New Delhi, India to its calendar of events for the month. Lead melanoma Phase 3 trial investigator and St. Luke's medical oncologist Dr. Sanjiv Agarwala is a member of the conference's faculty.
Updated (12/29/15): H/t InvestorVillage poster Juggernaut for informing me about Provectus' updates to its November 2016 Calendar of Events webpage, specifically the 9th International Symposium of Cancer Immunotherapy from November 16-19 in Nanjing, China. Of course, the company may or may not attend and/or participate.
One of the conference pages is the graphic of the milestones in the development of active immunotherapy, taken from the paper Therapeutic vaccines for cancer: an overview of clinical trials by Melero et al. (Nat Rev Clin Oncol. 2014 Sep;11(9):509-24). See below, with my purple emphasis.
 |
| Click to enlarge. Image source |
A., the reference to William Coley (1893) (also see "Training The Immune System To Fight Cancer Has 19th-Century Roots" (December 28, 2015) below).
B., the reference to the discovery of tumor-specific antigens (1957), followed by the reference to the discovery of molecularly de defined tumor antigens recognized by human T cells (1991). During an ASCO 2010 investor presentation, Eric presented about the bystander effect, which is a tumor-specific response brought on by PV-10 treatment: "Response in untreated proximal and visceral lesions consistent with immunologic process. PV-10 chemoablation yields immediate reduction in tumor burden. Ablation appears to recruit immune cells to exposed tumor antigens" [f/n 1].
The key aspect of PV-10's clinical value proposition is the local effect of tumor ablation upon injection (the first of its dual mechanisms of action) that produces the systemic effect of a tumor-specific immune response and eventually anti-tumor immunity (the second mechanism).
Moffitt Cancer Center and the University of Illinois independently, and independently from each other, reproduced Craig’s original work, which first demonstrated PV-10’s two-prong approach to successfully fighting cancer in multiple indications: Tumor ablation, the local effect of destroying injected tumors; a tumor-specific immune response, the systemic effect of destroying non-injected tumors; tumor-specific IFN-gamma/γ production; and multi-indication viability in at least melanoma, breast cancer and colorectal cancer.
C., the reference to Burnet's proposal of the theory of immunosurveillance (1967). The theory of immune surveillance suggests, according to Peggs et al., "...that the immune system plays a key role in suppressing tumor growth and that the incidence of cancer would be much greater were it not for the ability of the immune system to identify and eliminate nascent tumor cells...While the immune system appears capable of eliminating or containing early tumor growth, some tumor cells escape detection and eventually cause cancer." Said another way, when thinking about the growing potential role and promise of cancer immunotherapy, "...we continually develop malignant cells every day that are consumed by the immune system to prevent tumor development, and the immunotherapy drugs seem to target the failure of immune recognition and immune response" (Dr. Peter Salgo, M.D.). See September 21, 2014 blog post Co-stimulatory & Co-inhibitory.
In September 2014, one of the SITC presentation's co-authors and Moffitt Cancer Center assistant professor and researcher, Dr. Shari Pilon-Thomas, Ph.D., co-authored an online OncLive article entitled Immunotherapy Combined With Chemotherapy for Pancreatic Cancer: A Game Changer? (see September 18, 2014 blog post Treating Cancer). In it Dr. Pilon-Thomas and her fellow authors write:
Of note, the immune system’s involvement in cancer development and progression has sparked much interest in recent years. The model of the cancer-immunity cycle suggests an interplay of immune-suppression and immune-stimulation. In normal individuals, a state of immunosurveillance is in place. However, within the tumor microenvironment, inhibitory signals and immunosuppressive cells are present and tip the scale in favor of immune suppression.
Continued: The idea of the cancer-immunity cycle proposes that, for a cancer immune response to be generated, the net balance between immune stimulation versus immune suppression must be tipped in favor of the former. Studies in various cancers have suggested that tumors evade the immunogenic process mostly by factors that promote immunosuppression. {Underlined emphasis is mine}
Achieving T cell immunity almost if not actually by definition should mean overcoming resistance to cancer, thus overcoming checkpoint blockade and mitigating the need to artificially release the brakes.
Should stimulation via stimulatory therapeutics and therapies start the engine and enables the gas pedal to be stepped on sufficiently and appropriately (i.e., with minimal or manageable side effects or adverse events) so as to achieve T cell immunity, brakes may not be necessary once the car is moving (in context, and given the car [the immune system] can drive itself and not careen off the road because it then should know what it is doing).
Over time, however, road friction may start slowing the car down to the point where waning immunosurveillance (the immune system recognizing and eliminating continuously arising cancerous cells) no longer can protect the patient from relapse (analogous to how waning varicella zoster antibody titers may result in a bout of shingles). Keeping the brakes disengaged, especially with non-immunogenic tumors, should have some role going forward, making Merck, Bristol-Myers, Roche, AstraZeneca, Pfizer and other companies’ checkpoint inhibitors not necessarily obsolete as much as persnickety. See February 18, 2015 blog post The Early Obsolescence of Checkpoint Inhibitors.
[F/n 1] ASCO 2010 Investor Briefing Presentation, Clinical Program Overview, Provectus, Dr. Eric Wachter, PhD, slide 42 of 55
Imylgic. From from an ideal intralesional (IL) agent, Amgen's T-Vec (Imylgic) is shipped to physicians in a -90˚C to -70˚C storage container, and requires ultra-low temperature refrigeration onsite. PV-10 is use, re-used, shipped and handled at room temperature.
 |
| Click to enlarge. Purple emphasis is mine. Image source |
 |
| Click to enlarge. Image via image source |
13 weeks. Provectus' Phase 1b study of PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma has an "investigational Treatment Phase of the study" of 13 weeks. The company's completed Phase 2 Study of Intralesional PV-10 for Metastatic Melanoma saw complete responses mostly require 1-2 injections of Rose Bengal, but in some cases up to 4 treatments. Patients in the Phase 1b study of PV-10 and Merck's Keytruda (pembrolizumab) will have up to 5 treatments of both therapies. Five is greater than four, presumably for good measure.
"Training The Immune System To Fight Cancer Has 19th-Century Roots" (December 28, 2015) |
| Click to enlarge. Purple emphasis is mine. Image source |
Previous related entry:
- November 1, 2014 blog post Florey, Chain & Heatley⎟Coley⎟sort of, maybe, possibly, conceivably, probably...
"A novel immunotherapy drug is credited for successfully treating former President Jimmy Carter's advanced melanoma. Instead of killing cancer cells, these drugs boost the patient's immune system, which does the job instead. Immunotherapy is cutting-edge cancer treatment, but the idea dates back more than 100 years, to a young surgeon who was willing to think outside the box."Her story is about William Coley.
William Coley is a pioneer of cancer immunotherapy (late 1800s), and considered by many to be the "Father of Immunotherapy." According to Wikipedia (see immediately prior link):
"Coley developed the theory that post-surgical infections had helped patients to recover better from their cancer by provoking an immune response. He began to experiment by deliberately causing this phenomenon, injecting bacteria directly into people being treated – but because this had the adverse effect of causing infection he then switched to using dead bacteria."Hoption Cann et al. (link at the top of the post) write about Coley's work:
"Coley considered several points crucial to a patient’s survival. First and foremost was to imitate a naturally occurring acute infection, and thus, inducing a fever was essential. Injections were optimally administered daily (or every other day) for the first month or two. To avoid immune tolerance to the vaccine, the dosage was gradually increased over time (depending on patient response). The vaccine was injected directly into the primary tumour and metastases, when accessible. Finally, a minimum six month course of weekly injections was followed to prevent disease recurrence." {Underlined emphasis is mine.}Coley injected the bacteria/vaccine/toxins into cancerous tumors and cancer metastases.
In her article, all Davis mentions in regards to injection is the paragraph below:
"So why did Stein's cancer go away and stay away after he got a bacterial infection? Coley speculated that the strep infection had reversed the cancer. and wondered what would happen if he tried to reproduce the effect by deliberately injecting cancer patients with bacteria."Why then, when writing about cancer immunotherapy and associated approved and investigational checkpoint blockade drugs like Yervoy (anti-CTLA-4, Bristol-Myers), Keytruda (anti-PD-1, Merck), Opdivo (anti-PD-1, Bristol-Myers), MPDL3280A (anti-PD-L1, Roche/Genentech), MEDI4736 (anti-PD-L1, AstraZeneca/MedImmune), etc., do some folks include what seems like obligatory yet obtuse nods to Coley? Google, for example, coley immunotherapy pd-1.
Coley's vaccine and these inhibitors both engage the immune system. But although the mechanism of action of Coley's vaccine (or Coley's toxins as it also was known) was and remains unknown, it is believed the approach led to specific and non-specific immune responses (paragraph source/sentence taken from: Rational approaches to human cancer immunotherapy, Davis et al.). Interestingly, according to Davis et al.'s article, Coley's injected-bacteria-into-accessible-tumors-and-metastases was, "[a]s late as 1934, “Coley’s toxins” was the only known systemic treatment for cancer" (article footnote). {Underlined emphasis is mine.} A treatment injected into tumors that generates an immune response is called a systemic treatment for cancer. Interesting...
Checkpoint blockade therapeutic agents are non-specific immunotherapies. "Non-specific immunotherapies don’t target cancer cells specifically. They stimulate the immune system in a more general way..."
What does a non-specific immunotherapy specifically train the immune system about?
CTLA-4s (e.g., Yervoy), PD-1s (e.g., Keytruda, Opdivo), PD-L1s, etc. are non-specific immunotherapies.
"According to a 1965 article that was published in A Cancer Journal for Clinicians (1):
“In 1952, a bibliography of the literature, and, in 1953, a report on 30 inoperable cases which had been treated by Coley's mixed toxins and had survived thereafter for periods of from 1 to 47 years (20 cases had a survival of over 20 years) were published. The report is said to be based on a comparative analysis of over 1,200 cases treated with Coley's toxins, and 300 cases in which intercurrent infections played a part. Over 270 cases were said to have shown complete regression of the tumor, but the 30 inoperable cases were selected for the report because the diagnoses had been confirmed by microscopic examination, and some information on their subsequent history was available. Of the 30 tumors, 7 were classified as carcinoma, 19 as various types of sarcoma, 2 as malignant melanoma, and 2 as giant cell tumors.”
A complete response rate of 22% (270 out of 1200) was impressive by the standards of the 1960's and today. But, despite these results, the article states that the, “American Cancer Society has found no evidence that treatment with Coley's mixed toxins results in any objective benefit in the treatment of cancer in human beings.” It is difficult to reconcile this conclusion with the results cited in the same article. Perhaps the results were simply not believed as they were authored by Mrs. Helen Coley Nauts, Executive Director of the New York Cancer Research Institute. Mrs. Nauts was the daughter of William Coley." (paragraph source link) {Underlined emphasis is mine.}
Coley's approach generated notable complete responses.
“In 1952, a bibliography of the literature, and, in 1953, a report on 30 inoperable cases which had been treated by Coley's mixed toxins and had survived thereafter for periods of from 1 to 47 years (20 cases had a survival of over 20 years) were published. The report is said to be based on a comparative analysis of over 1,200 cases treated with Coley's toxins, and 300 cases in which intercurrent infections played a part. Over 270 cases were said to have shown complete regression of the tumor, but the 30 inoperable cases were selected for the report because the diagnoses had been confirmed by microscopic examination, and some information on their subsequent history was available. Of the 30 tumors, 7 were classified as carcinoma, 19 as various types of sarcoma, 2 as malignant melanoma, and 2 as giant cell tumors.”
A complete response rate of 22% (270 out of 1200) was impressive by the standards of the 1960's and today. But, despite these results, the article states that the, “American Cancer Society has found no evidence that treatment with Coley's mixed toxins results in any objective benefit in the treatment of cancer in human beings.” It is difficult to reconcile this conclusion with the results cited in the same article. Perhaps the results were simply not believed as they were authored by Mrs. Helen Coley Nauts, Executive Director of the New York Cancer Research Institute. Mrs. Nauts was the daughter of William Coley." (paragraph source link) {Underlined emphasis is mine.}
Coley's approach generated notable complete responses.
NPR's Davis writes in regards to Coley's daughter, Helen Coley Nauts:
"And quite possibly, that's where Coley's legacy would have ended except for this: After Coley's death in 1936, his daughter, Helen Coley Nauts, started looking through her father's papers while doing research for his biography. She found about 1,000 files of patients her father had treated with Coley's toxins. She spent years carefully analyzing these cases and could see that he had extraordinary rates of success in regressing some cancerous tumors. She couldn't get anyone interested in studying her father's work, so she decided to do it herself. With a small grant, in 1953 Helen Coley Nauts started the Cancer Research Institute, dedicated to understanding the immune system and its relationship to cancer."
Which brings us to a chicken-and-egg question. Which comes first, the complete response, or the immune response? For PV-10, successfully generating a complete response leads to a good to great immune response.
Per medical writer Walter Alexander's recent article PV-10 in Metastatic Melanoma: Rapid Responses Led Phase 3 (he's written about PV-10 before, including PV-10 Moves Forward):
* The article came to my attention because of @bradloncar's tweet in my Twitter feed for this project/investment.
The high-percent response rates in bystander lesions underscored the importance of elucidating the mechanism underlying PV-10's activity. That meant going back to bench investigations. The operant question for researchers, according to Shari A. Pilon-Thomas, PhD, Moffitt Cancer Center Immunology Program, was: "Is it just because you inject the drug and it goes everywhere and then kills tumor cells at other sites? Or is injecting PV-10 inducing a T-cell response, such that T-cells travel throughout the body and kill tumors in their various locations?"
In a poster presentation at the 2013 meeting of the American Association of Cancer Research, she pointed to evidence suggesting that an immune-mediated process underlies PV-10 responses in untreated lesions. First, responses in untreated lesions occurred only when responses had occurred in injected lesions, and second, responses in bystander lesions typically were delayed in comparison with responses in injected lesions, Dr Pilon-Thomas noted.
Dr Pilon-Thomas has previously shown in murine models that induced flank tumors treated with PV-10, as compared with placebo, were about a third of the size, and bystander lesions were about 30% smaller. At the same time, concentrations of interferon-gamma, a cytokine critical for innate and adaptive immunity (including tumor control) and for activating macrophages, were increased more than fivefold.
These findings, along with those from other studies, led Dr Pilon-Thomas to conclude, "We think that when you inject PV-10 into a tumor, it destroys the tumor, releasing tumor fragments that are then taken up by immune cells. The immune cells travel to the lymph nodes where they 'educate' or activate T-cells, which can in turn travel anywhere in the body."
Her research also showed that PV-10-induced immunity is tumor specific.
Further evidence of immune responses induced by PV-10 come from another study conducted at the Moffitt Cancer Center, this time involving eight patients with dermal and/or subcutaneous metastatic melanoma. The findings, presented at this year's ASCO annual meeting in a highlighted poster session by Amod Sarnaik, MD, a surgical oncologist at the Moffitt Cancer Center, showed that intralesional PV-10 was associated with a significant increase (P = .03) in circulating cytotoxic CD8+ T-cells, a potential mechanism for a tumor-specific immunologic effect secondary to tumor ablation.
In this study of eight patients, each patient had two study lesions that were sampled by biopsy before treatment; one of the two lesions was injected with intralesional PV-10, and then both residual sites were completely excised 1 to 2 weeks after PV-10 injection. Tumors were compared before and after treatment to determine pathologic complete response (pCR).
PV-10 resulted in pCR in the posttreatment biopsy specimens of both PV-10-injected and uninjected study lesions in four of the eight patients, and all eight exhibited at least partial regression of the injected lesion.
Six of these eight patients had metastatic disease that was refractory to previous treatment with immunologics (ipilimumab [Yervoy, Bristol-Meyers Squibb Company] and anti-PD-1 therapy) and BRAF-mutation inhibitor (vemurafenib [Zelboraf, Hoffman-La Roche]). After PV-10, four of these six patients had pCRs in both the injected and uninjected lesions. {Underlined emphasis is mine.}PV-10 generates a complete response in order to generate/which is followed by an immune response.
* The article came to my attention because of @bradloncar's tweet in my Twitter feed for this project/investment.
Form 4 (December 23, 2015)
Updated below.
Provectus CTO Dr. Eric Wachter, PhD exercised 120K of his 985K June 23, 2016 options ($1.02 exercise price) on Monday. His Form 4 filing is here. The closing share price on December 21st was $0.40. Eric's stock activity list is here.
| Click to enlarge. Image source (as at 12/31/14) |
The company's CFO/COO Peter Culpepper exercised the balance of 175K options ($0.94 strike price) that were to have expired on December 9th. See Stock option grants (December 10, 2015) below. The closing share price that day was $0.46.
Eric has exercised the most number of stock options of Provectus' four principals. His previous exercises included ~640,000 shares with exercise prices of $0.75, $0.93 and $0.95 on March 20, 2014. The closing share price that day was $2.40. The company submitted its breakthrough therapy designation on March 24th.
Updated (12/23/15): From the viewpoint of two sides of the trade (negative and neutral), Eric's stock option exercise noted above may or may not merit consideration. From the other side of the trade (positive), his exercise is in keeping with his personality, his beliefs, his knowledge, his data, etc. Eric's option exercise rationale may well have included year-end tax planning consideration. He (and I believe very likely or with certainty) alone is fully aware of the breadth and depth of Provectus' preclinical and clinical development program, and global regulatory affairs interactions.
 |
| Click to enlarge. |
 |
| Image source |
I thought the article/interview provided a continuing reminder of the unique history (development, diagnostic) of Rose Bengal, and of the serendipity of the innovation of Provectus founders -- Chairman and CEO Dr. Craig Dees, PhD, President Dr. Timothy Scott, PhD, and Eric.
In the century and then some of the existence of the molecule, its therapeutic application only was first explored and investigated by Craig, Dr. Scott and Eric, and then by third parties around the world who undertook studies following Craig et al.'s initial work:
- The "Japanese" (1986): Induction of thyroid tumors in (C57BL/6N x C3H/N)F1 mice by oral administration of 9-3',4',5',6'-tetrachloro-o-carboxy phenyl-6-hydroxy-2,4,5,7-tetraiodo-3-isoxanthone sodium (Food Red 105, rose bengal B) -- How ironic that these researchers' study of potentially carcinogenicity/tumorigenicity agents observed dose-dependent survival increased in the mice receiving Rose Bengal, and then did nothing...?
- Another "American" (2012): Selective toxicity of rose bengal to ovarian cancer cells in vitro,
- The "Iranians" (2014): Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages -- Mousavi previously published initial work with Australian Hersey, and Eric. See Interference (November 27, 2015) below, and
- The "Italians" (2014): Rose Bengal Acetate PhotoDynamic Therapy (RBAc-PDT) Induces Exposure and Release of Damage-Associated Molecular Patterns (DAMPs) in Human HeLa Cells.
"“I was determined to find some investigators and developers who were trying to do something different. I told a headhunter to find me some crazy people who were doing cancer research—and she did. The biggest problem in medical innovation is finding the true trailblazers, and I found that with the founders of Provectus.”" {Underlined emphasis is mine}When Peter endeavors to communicate about Provectus management's expectations about the type and size of exit the company would entertain, he needs to stop describing it in short form or ensure the writer clearly understands what Peter means. Levy concludes his article with:
"What is clear from our discussion is the enthusiasm the COO has for PV-10 and his belief in its abilities. As for Provectus’ future, Culpepper pointed to Celgene’s deal with Abraxis for its lead candidate Abraxane as the ideal transaction, when the company’s goals are achieved. “We do want to have an exit,” he comments. But for now, the highly promising work and the PV-10 trials continue." {Underlined emphasis is mine}Peter means management wants (i) a multi-billion dollar upfront payment and (ii) an earn-out structure (a tradable contingent value right [CVR]; see, for example, here) that would capture royalties from the sales of PV-10 (and PH-10 and other Rose Bengal-based therapeutic applications) through current patent expiration of 2031 and potentially beyond. The Celgene-Abraxis transaction description is here. He fully understands Provectus' market capitalization must rise dramatically from current levels for management to attain their desired upfront payment, let alone their hoped for, presumably well crafted CVR.
Levy's last paragraph opens with a sentence that I think nicely ties this part of today's news item, Peter's interview with yesterday's, Eric stock option exercise:
"What is clear from our discussion is the enthusiasm the COO has for PV-10 and his belief in its abilities."I'd say Eric has clear enthusiasm for PV-10, and data and knowledge (as yet publicly undisclosed) in its abilities.
Maybe it was just about single agent IP (December 22, 2015)
Following up on Oncolytic virus(es) + Anti-angiogenic agent(s) (December 21, 2015) below, searching the US PTO patent and patent application websites for the search term "Catherex" yielded two patents and one patent application (all under the title Viral vectors and their use in therapeutic methods). I incorrectly noted the "patent" below, Use of oncolytic viruses and antiangiogenic agents in the treatment of cancer, as awarded; according to the US PTO PAIR website, it was abandoned by MediGene -- which assigned the above patent/patent applications to Catherex -- in 2012.
 |
| Click to enlarge. |
Investor/industry conferences. H/t InvestorVillage poster who noted the March 2016 Bio Pharma ASIA Convention 2016 item was added to Provectus' Calendar of Events webpage(s) for that month. Along with that event, other investor and industry (non-medical) events include:
- January 11-13, 2016: Biotech Showcase 2016 (San Francisco), where the company is presenting at 3:30 pm PT on January 13th,
- February 8-9: The 18th Annual BIO CEO & Investor Conference (New York) -- no mention yet of the company presenting here,
- March 22-24: BioPharma Asia Convention 2016 (Singapore), which it would seem management might attend, and
- May 10-11: Bio€quity Europe 2016 (Copenhagen, Denmark) -- no mention yet of the company presenting here.
On my curated Twitter feed @bradloncar noted this morning Amgen's acquisition of Catherex. @JacobPlieth, also on the same feed, suggested the M&A rationale might have been [for Amgen and its oncolytic virus T-Vec Imlygic] to sidestep Catherex's oncolytic virus intellectual property (IP) infringement.
 |
| Click to enlarge. Image source |
One such Catherex patent, Use of oncolytic viruses and antiangiogenic agents in the treatment of cancer, was awarded in 2008.
I believe the Catherex acquisition is more about building Amgen/Imlygic's IP portfolio, particularly as it relates to combination oncology therapies (i.e., an oncolytic virus + [fill in the blank]).
"The process of formation of new blood vessels from pre-existing blood vessels is termed as angiogenesis." See Samant et al., Recent Advances in Anti-Angiogenic Therapy of Cancer, Oncotarget. 2011 Mar; 2(3): 122–134. The authors note:
"Since angiogenesis is critical for tumor growth and metastasis, anti-angiogenic treatment is a highly promising therapeutic approach...
There are currently seven approved anti-cancer therapies in two primary categories:
Monoclonal antibodies directed against specific pro-angiogenic growth factors and/or their receptors
Small molecule tyrosine kinase inhibitors (TKIs) of multiple pro-angiogenic growth factor receptors."As of the writing of the above papers, the four approved mabs were/are bevacizumab (Avastin), cetuximab (Erbitux), panitumumab (Vectibix), and trastuzumab (Herceptin). The three approved TKIs (nibs) were/are erlotinib (Tarceva), sorafenib (Nexavar), and sunitinib (Sutent).
The above noted Catherex patent notes, among others, the following claims:
- "1. A combination of at least one oncolytic virus and at least one antiangiogenic agent,"
- "10. The combination of anyone of claims 1 to 9, wherein said antiangiogenic agent is selected from the group consisting of agents that target the vascular endothelial growth factor (VEGF) pathway, an integrin, a matrix metalloproteinase (MMP) and/or protein kinase C beta (PKCβ), or a combination thereof,"
- "13. The combination of claim 12, wherein said monoclonal antibody is Bevacizumab (Avastin®), 2C3, or HuMV833 or a combination thereof," {Bold emphasis is mine}
- "33. The combination of claim 32, wherein said monoclonal antibody is cetuximab (Erbitux®), panitumumab (Vectibix®), nimotuzumab, matuzumab, zalutuzumab, mAb 806, or IMC-1 1 F8," {Bold emphasis is mine}, and
- "34. The combination of anyone of claims 1 to 9, wherein said antiangiogenic agent is a tyrosine kinase inhibitor,"
- "36. The combination of any of claims 34 or 35, wherein said tyrosine kinase inhibitor is selected from the group consisting of sunitinib (SUl 1248; Sutent®), SU5416, SU6668, vatalanib (PTK787/ZK222584), AEE788, ZD6474, ZD4190, AZD2171 , GW786034, sorafenib (BAY 43-9006), CP-547,632, AG013736, YM-359445, gefitinib (Iressa®), erlotinib (Tarceva®), EKB-569, HKI-272, and Cl-1033, preferably wherein the tyrosine kinase inhibitor is ZD6474." {Bold emphasis is mine}
Rock the vote. I also imagine Provectus management would like folks (shareholders) to vote for the company's nomination [presumed self-nominated or media relations-nominated] for Most Buzzworthy Public Company at the 2016 CEO & Investor Conference.
Companies on the Most Buzzworthy Public Company ballot are below:
Official contest rules may be found here.
Status update (December 20, 2015)
Previous related entries below:
A. As noted in the previous entries below on this topic, one comp might be Aeterna Zentaris:
Friday Grab bag (December 18, 2015)
New Zealand. In regards to a poster presented at the 2015 annual conference of the New Zealand Hospital Pharmacists’ Association in August in Napier on the hospital pharmacy compounding of an injection of 10% Rose Bengal for a patient with unresectable melanoma*, Provectus' CFO/COO Peter Culpepper responded:
BMS v. Merck. In regards to Bristol-Myers PD-1 lawsuit against Merck -- see, for example, September 2014 FiercePharma article Bristol-Myers socks Merck's brand-new Keytruda with PD-1 patent suit -- note A New Merck: Reviewed blog's December 12, 2015 entry BMS/Ono Immuno-Oncology Patent In United Kingdom Held Valid, Over Merck Objections:
The company's continued approach to protecting its intellectual property presumably should further narrow or tighten the access to the active pharmaceutical ingredient.
** See Drug substance/product synthesis (December 8, 2015) below.
Intellectual property II. The New Zealand hospital pharmacy compounding situation highlights the important the company placed on having key IP in place (awarded patents, etc.) before commencing its pivotal melanoma Phase 3 trial, which in this regard would be the initial synthesis patent -- something Provectus' CTO Dr. Eric Wachter, PhD only admitted in the aftermath of the denial of the FDA's breakthrough therapy designation in May 2014:
Sanlorenzo et al. note in their December 2015 article JAMA Dermatology article Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression* that:
Lo et al. provide a December 2015 opinion piece on the above in JAMA Oncology titled Prognostic Significance of Cutaneous Adverse Events Associated With Pembrolizumab Therapy**.
In 2013 Provectus and its metastatic melanoma Phase 2 clinical trial investigators presented Locoregional Disease Control in Metastatic Melanoma: Exploratory Analyses From Phase 2 Testing of Intralesional Rose Bengal at the European Cancer Congress. On this poster the company and its investigators noted loco-regional blistering experienced by 40% of Phase 2 trial patients, who they observed "exhibited markedly greater response vs. those not experiencing this phenomenon. Further, the poster co-authors observed "[b]listering may be indicative of nascent local immunologic response."
Screenshots related to this blistering/immunologic response phenomenon from Provectus' ECC 2013 poster are below; fuzzy purple emphasis is mine.
* JAMA Dermatol. 2015 Nov 1;151(11):1206-12.
** JAMA Oncol. 2015 Dec 1;1(9):1340-1.
Trial Math: Meeting the Primary Endpoint (December 16, 2015)
Array BioPharma (Nasdaq: ARRY) reported top-line results from its ongoing Phase 3 clinical trial of binimetinib in patients with advanced NRAS-mutant melanoma today, which included noting median progression-free survival (PFS) of the control arm (systemic chemotherapy dacarbazine [DTIC]) as 1.5 months. Array's trial included patients with advanced (Stage IIIC) unresectable or metastatic (Stage IV) NRAS Q61 mutation-positive cutaneous or unknown primary melanoma.
Provectus' pivotal melanoma Phase 3 trial currently is comparing PV-10 (treatment arm) and systemic chemotherapy (DTIC or temozolomide [TMZ]) (control arm) in patients with unresectable locally advanced cutaneous melanoma (Stage III disease). See PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma.
DTIC and TMZ are well known systemic chemotherapies. The former is administered intravenously, while the latter is a pill. Their performance is well documented. PFS for chemotherapy (DTIC or TMZ) is approximately 2 to 3 months: e.g., Middleton et al. (2000), Patel et al. (2011) [see footnote 13], Hauschild et al. (2012) [see reference 1], etc. According to Eric, "[t]he performance of DTIC and TMZ are well documented and generally yield a normal distribution of events (for example, refer to Middleton et al.)." Middleton et al., for example, note median PFS of 1.9 months for TMZ and 1.5 months for DTIC.
In Provectus' metastatic melanoma Phase 2 trial PFS of patients who had all their lesions injected was 9.8 months [1]. I believe Eric designed the trial for a 90% power to detect a 70% improvement in median PV-10 PFS (over DTIC/TMZ PFS) — if the trial's DTIC/TMZ median PFS is 1.5 months then PV-10's must be >2.55 months, or if 2 then >3.4, or if 2.5 then >4.25.
The 2015 annual conference of the New Zealand Hospital Pharmacists’ Association (NZHPA) was held in Napier, New Zealand in at the end of August.
See pages 79 (title) and 92 (full abstract).
Moffitt Cancer Center's immune mechanism of action work, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study, as noted on the center's SITC 2015 poster (bottom right-hand corner), was "supported by the Cancer Center Support Grant P30 CA076292 from the National Cancer Institute and NCI 5K23CA178083-02 (AAS)."
Grant 5K23CA178083-02 is mentored patient-oriented research where the grantee/mentee/principal investigator/Moffitt's Dr. Amod Sarnaik, MD.
Two snapshots from the Notice of Award are below -- a screenshot of a portion of the first page, and a screenshot of a portion of a subsequent page.
The above of course confirms management's comments regarding an upcoming Moffitt paper focused at least on PV-10's immune mechanism of action. I would imagine a 1H16 publication date.
Research (December 14, 2015)
Photosensitized rose bengal induced phototoxicity on human melanoma cell line under natural sunlight exposure:
Prom (December 11, 2015)
Patient reported outcome (PRO) is an "Other Outcome Measures" of Provectus' pivotal Phase 3 trial, PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma. The key words here are "locally advanced cutaneous melanoma." The relevance of PROs, PRO measures (or PROMs), etc. may positively impact a drug's label and claims therein, and may help in the education of both physicians and patients.
See below:
Gabrail Cancer Center in Canton, Ohio, in addition to being a place where work is being done on a sponsor-not-yet-identified study entitled Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (this study does not turn up on ClinicalTrials.gov, also is conducting the following studies:
I am grateful to Provectus' Chairman and CEO Dr. Craig Dees, PhD and CTO Dr. Eric Wachter, PhD for regularly and routinely discussing with me over time facets and features of small molecule Rose Bengal, the class of halogenated xanthenes, PV-10, PH-10, etc. A biotechnology or pharmaceutical company protects their drug compounds through, among other things, branding, patents, regulatory approvals, and keeping secrets.
While historically falling short in their efforts to manage Wall Street (investor) expectations (it is not a bad thing to buck Wall Street, but as public company managers there are objective thresholds that arguably should or must be routinely met in paying attention to/serving investors and shareholders), discussions with Provectus management about the whys and whats of Rose Bengal or PV-10 help strengthen investor education and resolve in regards to, among other things, the company's drug development program.
I also try to keep up-to-date with current, historical, domestic and global uses of Rose Bengal. As a reminder, Rose Bengal is a German industrial dye created in Germany in 1882. Remember the MIT guy who said "Most drugs are really German dyes?" See blog news item “Most drugs are really German dyes” (August 3, 2015) below. Used as a dye/makeup for Asian (Indian, Bangladeshi) women for marriages and religious festivals, other uses of Rose Bengal over the last century have included
Stock option grants (December 10, 2015)
Updated below again, again.
Provectus' Board of Directors' (BOD's) Compensation Committee, BOD, and management granted themselves a total of 1.75 million common stock options (12/9/2025 expiry; i.e., 10 years from yesterday) with an exercise price of $0.75. Company principals received 400K options each, and outside BOD members received 50K each.
In addition, Provectus' CFO/COO Peter Culpepper exercised the balance of 175K options ($0.94 strike price) that were to have expired on December 9th (he exercised an initial amount of 29,786 on January 7, 2015; so, 145,214 + 29,786 = 175,000) [e/n 1]. See the table below.
E/n 1: Below.
Updated (12/10/15): Prior option awards to Provectus management have had 10-year expirations.
I imagine Provectus management would argue yesterday's grants to the co-founders, principals and officers -- Chairman and CEO Dr. Craig, Dees, PhD, President Dr. Timothy Scott, PhD, CTO Dr. Eric Wachter, PhD, and CFO/COO Peter Culpepper) -- were (i) good, because the options had an above market exercise price of $0.75 (December 9th closing share price $0.46), and (ii) warranted, given the so-called milestones they achieved this year (see below, for example, from October Australia Biotech Invest 2015 presentation).
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
Previous related entries below:
- Status update (October 5, 2015),
- "Comp" (November 24, 2015), and
- Extension(s) (December 9, 2015).
A. As noted in the previous entries below on this topic, one comp might be Aeterna Zentaris:
- Transaction date: December 1, 2014
- Sinopharm subsidiary: A-THINK Pharmaceutical
- Therapeutic area: Oncology
- Drug compound: Zoptarelin doxorubicin
- Initial indication: Endometrial cancer (cancer of the lining of the womb)
- Initial annual market size: Perhaps comparable to the U.S. market, whether or not adjusted for potential differences in treatment pricing (e.g., $300-400 million) -- [e/n 1, 2]
- Agreements: Master Collaboration; License; Technology Transfer and Technical Assistance
- Other indications: per Aeterna: ovarian, prostate, breast and bladder -- [e/n 1]
- Initial annual U.S. market size: per Aeterna: > $500 million -- [e/n 1]
Terms of the license agreement were not disclosed at the time of Aeterna's filings related to the license and other agreements, and have not yet been disclosed. Confidential treatment (i.e., redaction) was requested for certain portions of the license agreement, but by whom and why? Aeterna itself or on behalf of Sinopharm? Wouldn't the licensor (Aeterna) want to "brag" about its financial terms with the licensee (Sinopharm) if they were sufficiently notable?
B. Another comp could be Oramed Pharmaceuticals:
- Transaction date: November 30, 2015
- Sinopharm subsidiary/affiliate: Sinopharm Capital, Hefei Tianhui Incubator of Technologies
- Therapeutic area: Diabetes
- Drug compound: Oral insulin capsule ORMD-0801
- Indication: Type I and II
- Annual market size: $3.5 billion, annually growing at a double-digit percentage rate (Type II diabetics account for 95% of the market) -- [e/n 3]
- Agreements: License; Investment
Terms of the license agreement were disclosed at the time of Oramed's press release on the signing of a non-binding letter of intent with Sinopharm in July 2015. Finalized terms were:
- License
- $3 million upon execution,
- $8 million in near-term payments subject to Oramed entering into certain agreements,
- Up to $27 million* after the achievement of certain milestones, and
- 10% royalty on net sales
- Investment
- $12 million at $10.39 (closing share price on 11/30/15: $9.10, range $8.80-10.74)
C. I would imagine if a good deal can be had with Sinopharm (A-Think, CSIPI), Provectus management would very much want to disclose financial terms.
A Seeking Alpha article on the topic of Oramed's deal with Sinopharm referenced an August 17th Bloomberg article entitled China to Speed Up New Drug Approvals in Boost For Multinationals. A few things were notable (and of which I needed reminding):
a. "Foreign companies will now be able to conduct clinical trials in China simultaneously with overseas trials.
b. "Drugmakers will also be able to use data from international clinical trials to apply to import their products into China," and
c. "Innovative drugs including those for AIDS, cancer, major infectious diseases and rare illnesses are eligible for expedited review and approval."
It would appear injectable cancer medications are nothing new to China, and the focus of PV-10's use in the country and its SARs presumably is its cost effective clinical value proposition as a single agent or monotherapy.
[E/n 1] Aeterna Zentaris, Improving Life… Transforming Value, August 12, 2015
[E/n 2] Nasdaq: ICAD, Investor Presentation, April 2013
[E/n 3] MLV & Co. on Oramed Pharmaceuticals, Sinopharm LOI For China Rights To ORMD-0801, July 7, 2015. See also FBR & Co. on Oramed Pharmaceuticals, Potential Blockbuster Insulin Drug; Awaiting Data from Phase IIb Study; Initiating at Outperform, November 18, 2015
* "Up to $27 million" = "Up to $50 million" - $3 million - $8 million - $12 million
Friday Grab bag (December 18, 2015)
 |
| Image source |
"We are well aware of situations like this. We either get them to stop or include them as clinical sites. Unfortunately much confusion has been generated on this topic. People need to understand that PV-10 has to be sold and manufactured with our synthesis of Rose Bengal, because only that synthesis meets ICH specifications for therapeutic drug products. Anybody trying to prepare Rose Bengal and/or PV-10 for purposes of therapeutic use are not only violating our patents, but are also operating illegally within their own healthcare environment."* See Hospital Pharmacy Compounded Rose Bengal 10% for Unresectable Melanoma (December 15, 2015) below, and December 15, 2015 blog post Compounding.
BMS v. Merck. In regards to Bristol-Myers PD-1 lawsuit against Merck -- see, for example, September 2014 FiercePharma article Bristol-Myers socks Merck's brand-new Keytruda with PD-1 patent suit -- note A New Merck: Reviewed blog's December 12, 2015 entry BMS/Ono Immuno-Oncology Patent In United Kingdom Held Valid, Over Merck Objections:
"In at least one theater (London) — in this truly global patent battle — over immuno-oncology breakthrough products — Merck has come up a little short. In the United Kingdom, at the trial court level, a sitting patent judge has held that BMS, claiming through an Ono Pharmaceutical Co. Ltd. (Japan) patent license, holds a valid and enforceable U.K. patent. MSD had contended that the subject patent was invalid."Intellectual property I. I wonder whether Provectus' allowed continuation patent application** of its awarded synthesis patent further addresses resorcinol, from which Rose Bengal (RB) is derived.
 |
| Click to enlarge. From the USPTO PAIR website |
 |
| Click to enlarge. Image source |
Intellectual property II. The New Zealand hospital pharmacy compounding situation highlights the important the company placed on having key IP in place (awarded patents, etc.) before commencing its pivotal melanoma Phase 3 trial, which in this regard would be the initial synthesis patent -- something Provectus' CTO Dr. Eric Wachter, PhD only admitted in the aftermath of the denial of the FDA's breakthrough therapy designation in May 2014:
"Over the same period, we worked on modernization of our PV-10 supply chain as evidenced by a recently issued U.S. patent September of 2013 covering methods for manufacturing Rose Bengal to modern quality standards. I think absent this work, it is unlikely that early investigation drug product was used up to that time could have been qualified for phase three use, and certainly not for support of an NDA."Prognostic Significance of Cutaneous Adverse Events Associated With Immunotherapies (December 17, 2015)
Sanlorenzo et al. note in their December 2015 article JAMA Dermatology article Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression* that:
"Pembrolizumab therapy was associated with cutaneous AEs in 42% of patients. The development of cutaneous AEs, especially of hypopigmentation in patients with melanoma, could point toward better treatment response."
 |
| Click to enlarge. Purple emphasis is mine. |
 |
| Click to enlarge. |
Screenshots related to this blistering/immunologic response phenomenon from Provectus' ECC 2013 poster are below; fuzzy purple emphasis is mine.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
** JAMA Oncol. 2015 Dec 1;1(9):1340-1.
Trial Math: Meeting the Primary Endpoint (December 16, 2015)
"We firmly believe that Phase 3 testing should not be started unless you can adequately predict the outcome. It's critical to understand what the drug is doing, which patients are most likely to benefit, what other options those patients have, and which endpoints would be most convincing for government agencies to approved the labelled indication for the drug."
-- Provectus' CTO Dr. Eric Wachter, PhD, June 19, 2014 conference call
Array BioPharma (Nasdaq: ARRY) reported top-line results from its ongoing Phase 3 clinical trial of binimetinib in patients with advanced NRAS-mutant melanoma today, which included noting median progression-free survival (PFS) of the control arm (systemic chemotherapy dacarbazine [DTIC]) as 1.5 months. Array's trial included patients with advanced (Stage IIIC) unresectable or metastatic (Stage IV) NRAS Q61 mutation-positive cutaneous or unknown primary melanoma.
Provectus' pivotal melanoma Phase 3 trial currently is comparing PV-10 (treatment arm) and systemic chemotherapy (DTIC or temozolomide [TMZ]) (control arm) in patients with unresectable locally advanced cutaneous melanoma (Stage III disease). See PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma.
DTIC and TMZ are well known systemic chemotherapies. The former is administered intravenously, while the latter is a pill. Their performance is well documented. PFS for chemotherapy (DTIC or TMZ) is approximately 2 to 3 months: e.g., Middleton et al. (2000), Patel et al. (2011) [see footnote 13], Hauschild et al. (2012) [see reference 1], etc. According to Eric, "[t]he performance of DTIC and TMZ are well documented and generally yield a normal distribution of events (for example, refer to Middleton et al.)." Middleton et al., for example, note median PFS of 1.9 months for TMZ and 1.5 months for DTIC.
In Provectus' metastatic melanoma Phase 2 trial PFS of patients who had all their lesions injected was 9.8 months [1]. I believe Eric designed the trial for a 90% power to detect a 70% improvement in median PV-10 PFS (over DTIC/TMZ PFS) — if the trial's DTIC/TMZ median PFS is 1.5 months then PV-10's must be >2.55 months, or if 2 then >3.4, or if 2.5 then >4.25.
[1] Subgroup Efficacy in Patients Receiving Intralesional Rose Bengal to All Existing Melanoma in Phase II Study PV-10-MM-02, European Society For Medical Oncology, Abstract #1120P, September 2014.As the pivotal trial's principal (lead) investigator, St. Luke's Cancer Center's Dr. Sanjiv Argawala, MD, said last year following ESMO 2014, “The progression free survival of 9.8 months compares favourably with historical progression-free survivals of less than 2.5 months for DTIC/TMZ.” [2]
[2] Janet Fricker, PV-10 delivers greatest effects when all lesions are injected, Pharmiweb.com, October 15, 2014For more on this topic see on the blog:
- June 23, 2014: Trial Math: Meeting the Primary Endpoint, Pt. 1,
- June 26, 2014: Trial Math: Meeting the Primary Endpoint, Pt. 2,
- February 12, 2015: Hazard ratio, and other stat stuff, and
- August 4, 2015: Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part VI.
The 2015 annual conference of the New Zealand Hospital Pharmacists’ Association (NZHPA) was held in Napier, New Zealand in at the end of August.
 |
| Image source |
Hospital Pharmacy Compounded Rose Bengal 10% for Unresectable MelanomaRajan Ragupathy, Barnard J, Goddard J, Van Staden M, Vickers J, Waikato District Health Board, Hamilton
Introduction
Intralesional injection with agents that stimulate an immunogenic anti-tumour response, such as Rose Bengal, may be a treatment option for unresectable melanoma [1-3]. A proprietary Rose Bengal 10% formulation(PV-10) is undergoing clinical trials, but the raw material is readily available.
Case description
The patient was a 70 year old female with unresectable melanoma, who had exhausted all chemotherapy, radiotherapy and surgical options. Palliative intralesional injection with Rose Bengal 10% was proposed to prevent further ulceration and alleviate pain. Efforts to gain clinical trial or compassionate supply access to PV-10 proved unsuccessful. Pharmacy was requested to compound the injection. Staff performed a literature search, and considered legal, ethical, DHB clinical governance and HML compliance implications. Pharmacy then developed a method of compounding the injection, sourced Rose Bengal and prepared the treatment. (Solubility was a particular challenge, as a 10% solution was 100 times the listed solubility of Rose Bengal). The patient was treated with 0.5ml to 1 ml injected into each target lesion and discharged.
Discussion
This case illustrates principles that apply to all novel treatments. Legal requirements regarding new medicines, and the Health and Disability Commissioner’s guidance on such treatments must be adhered to. It is important to consider the difference between novel treatments based on recent evidence, and treatments intended in any way to generate further evidence. (The latter being experimental treatments that would require research approval). Compounding such products is a complex operation, and this case required input from medicines information, clinical trials, supply chain and aseptic compounding personnel. Compounding is best performed in an aseptic environment to ensure product quality and operator safety.
Conclusion
Preparing novel treatments in hospital pharmacies is possible, but complex. Careful consideration of legal and ethical issues is needed, especially for vulnerable patients who have no other treatment options.
Justification for presentation
We are aware of a further case where a hospital pharmacy has been requested to compound Rose Bengal 10%. The promise of this treatment may lead to other requests. This case also illustrates principles that apply to other novel treatments.
References
1. Damian DL. Topical Immunotherapy with Diphencyprone for in Transit and Cutaneously Metastatic Melanoma. Journal of Surgical Oncology 2014; 109:308–313Publication (December 15, 2015)
2. Thompson JF. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Research 2008; 18:405–411
3. Ross MI. Intralesional Therapy With PV-10 (Rose Bengal) for in-Transit Melanoma. Journal of Surgical Oncology 2014;109:314–319
 |
| Click to enlarge. Image source |
Grant 5K23CA178083-02 is mentored patient-oriented research where the grantee/mentee/principal investigator/Moffitt's Dr. Amod Sarnaik, MD.
Two snapshots from the Notice of Award are below -- a screenshot of a portion of the first page, and a screenshot of a portion of a subsequent page.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Research (December 14, 2015)
 |
| Image source |
- Abstract: Rose Bengal (RB) is an anionic water soluble xanthene dye, which used for many years to assess eye cornea and conjunctiva damage. RB showed strong absorption maxima (λmax) under visible light followed by UV-B and UV-A. RB under sunlight exposure showed a time dependent photodegradation. Our results show that photosensitized RB generates 1O2 via Type-II photodynamic pathway and induced DNA damage under sunlight/UV-R exposure. 2’dGuO degradation, micronuclei formation, single and double strand breakage were the outcome of photogenotoxicity caused by RB. Quenching studies with NaN3 advocate the involvement of 1O2 in RB photogenotoxicity. RB induced linoleic acid photoperoxidation which was parallel to 1O2 mediated DNA damage. Oxidative stress in A375 cell line ( human melanoma cell line) was detected through DCF-DA assay. Photosensitized RB decreased maximum cellular viability under sunlight followed by UV-B and UV-A exposures. Apoptosis was detected as a pattern of cell death through the increased of caspase-3 activity, decreased mitochondrial membrane potential as well as PS translocation through inner to outer plasma membrane. Increased cytosolic levels of Bax also advocate the apoptotic cell death. We propose a p53 mediated apoptosis via increased expression of Bax gene and protein. Thus, the exact mechanism behind RB phototoxicity was the involvement of 1O2 which induced oxidative stress mediated DNA and membrane damage, finally apoptotic cell death under natural sunlight exposure. The study suggests that after the use of RB, sunlight exposure may avoid to prevent from its harmful effects.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
See below:
- New Moffitt Cancer Center study related to PV-10? (July 21, 2015),
- Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part V (July 24, 2015),
- Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015), and
- Recruiting (September 2, 2015).
Gabrail Cancer Center in Canton, Ohio, in addition to being a place where work is being done on a sponsor-not-yet-identified study entitled Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (this study does not turn up on ClinicalTrials.gov, also is conducting the following studies:
- Qualitative interviews with with people diagnosed with ovarian cancer and their caregivers,
- Qualitative interviews with Acute Lymphocytic (Lymphoblastic) Leukemia (ALL) Patients for the development of a new patient reported outcome measure, and
- A qualitative interview to evaluate symptoms of NSCLC and content validity of associated measures.
I am grateful to Provectus' Chairman and CEO Dr. Craig Dees, PhD and CTO Dr. Eric Wachter, PhD for regularly and routinely discussing with me over time facets and features of small molecule Rose Bengal, the class of halogenated xanthenes, PV-10, PH-10, etc. A biotechnology or pharmaceutical company protects their drug compounds through, among other things, branding, patents, regulatory approvals, and keeping secrets.
While historically falling short in their efforts to manage Wall Street (investor) expectations (it is not a bad thing to buck Wall Street, but as public company managers there are objective thresholds that arguably should or must be routinely met in paying attention to/serving investors and shareholders), discussions with Provectus management about the whys and whats of Rose Bengal or PV-10 help strengthen investor education and resolve in regards to, among other things, the company's drug development program.
 |
| Image source |
- An additive to safranin victoria yellow for ocular pneumococcal infection,
- A stain for visualizing corneal ulcers,
- A marker for impaired liver function,
- A food dye, an insecticide,
- A cancer therapeutic, and
- A dermatology therapeutic.
"Molecules that are virtually insoluble in certain solvents may be uploaded to "hostile" phases by dendrimers. Prime examples of this phenomenon are Eosin Y, EY, and Rose Bengal, RB, that are not soluble in CH2Cl2 where they can, however, be solvated through the interaction with a fourth generation dendrimer of polypropylene amine, POPAM-4D. The two dyes share the same carbon framework and differ for the pattern of halogenation, and yet their cosolvation varies over a factor of 4: six Eosin Y and ∼25 Rose Bengals are solvated by the macromolecule. Leveraging on a previous report where molecular dynamics simulations of 12 EY@POPAM-4D in CH2Cl2 showed a reduction to the experimental limit of 6, we now perform similar calculations with an excess, i.e., 40, of RB@POPAM-4D. The simulations quantitatively reproduce the cosolvation effect. They also provide a microscopic understanding of its origin and of motions-interactions of the macromolecule and both of its guests."You never know where an exploration to better understand Rose Bengal and Provectus' platform drug substance and oncology/dermatology compounds will lead you.
Stock option grants (December 10, 2015)
Updated below again, again.
Provectus' Board of Directors' (BOD's) Compensation Committee, BOD, and management granted themselves a total of 1.75 million common stock options (12/9/2025 expiry; i.e., 10 years from yesterday) with an exercise price of $0.75. Company principals received 400K options each, and outside BOD members received 50K each.
In addition, Provectus' CFO/COO Peter Culpepper exercised the balance of 175K options ($0.94 strike price) that were to have expired on December 9th (he exercised an initial amount of 29,786 on January 7, 2015; so, 145,214 + 29,786 = 175,000) [e/n 1]. See the table below.
 |
| Click to enlarge. |
| Click to enlarge. Image source: 2015 Form 14A |
I imagine Provectus management would argue yesterday's grants to the co-founders, principals and officers -- Chairman and CEO Dr. Craig, Dees, PhD, President Dr. Timothy Scott, PhD, CTO Dr. Eric Wachter, PhD, and CFO/COO Peter Culpepper) -- were (i) good, because the options had an above market exercise price of $0.75 (December 9th closing share price $0.46), and (ii) warranted, given the so-called milestones they achieved this year (see below, for example, from October Australia Biotech Invest 2015 presentation).
 |
| Click to enlarge. |
According to management, the rationale for the stock option grants would be contained in 2016's Form 14A (the definitive proxy statement), as would the list of comparable companies utilized by Provectus' BOD's compensation committee.
Stock option grants to the outside board members (of 50K each) seems in keeping with board member cash and stock option compensation paid/issued each year. In the past, such grants were issued some period of time after the annual meeting of the company (e.g., in June or July). In 2014, outside board member stock option grants were made at the end of July. See, for example, Provectus option grants to board member Al Smith.
Yesterday's compensation-related option grants (awards) to Provectus management were the first since 2011 and 2010, when the managers issued themselves 1MM options each. For example, see Tim Scott's 2011 issuance, 2010 issuance, and full list of option activity. As a result of a prior legal settlement, management forfeited almost half of the 2011 and 2010 issuances; see Scott's forfeitures here and here.
I'm curious why they, management, thought it would be okay, via the BOD's compensation committee, to award sizeable option grants/awards now? Year-end? Settlement of the lawsuit(s)? News in the not so distant future (since they'd then have to issue options with exercise prices presumably commensurate with the then share price so as not to issue so-called cheap stock)? Etc.?
Updated (12/11/15): Chairman and CEO Dr. Craig Dees, PhD and President Dr. Timothy Scott, PhD let their December 9th options expire (200K for each of them). During the calendar years 2014 and 2015 Craig (2014 and 2015) and Dr. Scott (2015) forfeited totals of 1.15 million and 525K stock options, respectively. See below.
Updated (12/12/15): Their forfeitures presumably relate mostly, if not exclusively, to their lack of cash on hand, rather than any lack of commitment to or belief in Provectus and/or its/their cause. Management, through historically early and/or out-of-the-money stock option exercises (particularly Eric and Peter, and on occasion Dr. Scott) and a complete absence of insider sales (Craig et al.), have tried to demonstrate this commitment to shareholders and Wall Street.
I'm curious why they, management, thought it would be okay, via the BOD's compensation committee, to award sizeable option grants/awards now? Year-end? Settlement of the lawsuit(s)? News in the not so distant future (since they'd then have to issue options with exercise prices presumably commensurate with the then share price so as not to issue so-called cheap stock)? Etc.?
Updated (12/11/15): Chairman and CEO Dr. Craig Dees, PhD and President Dr. Timothy Scott, PhD let their December 9th options expire (200K for each of them). During the calendar years 2014 and 2015 Craig (2014 and 2015) and Dr. Scott (2015) forfeited totals of 1.15 million and 525K stock options, respectively. See below.
 |
| Click to enlarge. Image source. Underlined emphasis is mine |
 |
| Click to enlarge. Image source. Dotted lined emphasis is mine. |
 |
| Click to enlarge. Image source. Dotted line emphasis is mine. |
According to management, the forfeited options are non-qualified options (NQOs) that are exercised for cash (as opposed to cashless exercise). Cash outlays for Craig and Dr. Scott's forfeited options would appear to have to have been approximately $975K (for 1.15 million options) and $430K (525K), respectively. See the table below.
 |
| Click to enlarge. |
Extension(s) (December 9, 2015)
H/t InvestorVillage poster gymmee regarding Moffitt Cancer Center revising, again, its estimated primary completion date for the center's PV-10 immune mechanism of action study, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study (n = 15) -- this time to October 2016 (from February 2016).
The study itself began in January 2013 (the protocol was filed in December 2012). Patient data were first presented at AACR 2014 (April) -- n_initial = 8. More data on n_initial was presented at ASCO 2014 (May/June). ecancer reporter Janet Fricker's PV-10 decreases melanoma cells in tumours article from April 2014 noted "“[i]ronically, the original aim of the trial to assess tumour-infiltrating lymphocytes was thwarted when biopsies of patient tumours collected just seven to 10 days after PV-10 injection no longer contained viable tumour tissue,” said Pilon-Thomas" (Dr. Shari Pilon-Thomas, PhD, a Moffitt researcher) and "[s]tudies are now underway in an additional seven patients to take biopsies and blood samples at more frequent time intervals after PV-10 injection to elucidate the pathways more clearly" (i.e., n_subsequent = 7, for a total of n = 15). Patient data on n = 14 were presented at SITC 2015.
According to Provectus, Moffitt increased the completion date to extend patient tracking (in the center's non-therapeutic study). Prior completion dates appear to be October 2015, June 2015 and December 2014.
On other topics:
Squeezing. Roche allies with cell engineering startup SQZ on $500M+ immuno-oncology pact, December 7th by John Carroll (FierceBiotech):
Drug substance/product synthesis (December 8, 2015)
I've long been impressed by the strategy, quality of writing, and breadth and depth of Provectus' intellectual property (IP) portfolio. IP, and its instantiation, were a key and critical focus of our corporate venture capital team (and our due diligence, and assessment of value drivers and valuation) back in the day (e.g., telecom deregulation, the Internet boom & bust, some life sciences investing, etc.).
One of the company's key IP/patents was the drug product/substance synthesis to International Conference on Harmonisation (ICH) guidelines patent, Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes, awarded in September 2013.
Management continued/continues to broaden and deepen the IP portfolio, submitting to the US Patent and Trademark Office (US PTO) for consideration a continuation of the synthesis patent, Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes, which was made public on the US PTO patent application full text image database website in November 2014.
It looks like this patent application was allowed at the end of November 2015; see below:
Sorafenib (December 7, 2015)
Provectus' Phase 1/expanded Phase 1 liver trial comprised/comprises a main study group and two expansion cohorts, indications that include both HCC and cancers metastatic to the liver, and sorafenib as standard of care (for the 2nd expansion cohort). See below.
The company released initial liver cancer data mid-year:
Bridges (December 3, 2015)
Updated below again, again.
Provectus updated its Calendar of Events page for December to include Melanoma Bridge 2015.
According to the organizing entity's website, "..."Melanoma Bridge 2015" and, for the first time, “ImmunoTherapy Bridge...”are being organized on behalf of Fondazione Melanoma Onlus, under the auspicies of Istituto Nazionale Tumori Fondazione "G. Pascale", Sidra Medical and Research Centre, the Italian Association of Medical Oncology, and the Society for Immunotherapy of Cancer (SITC)."
PV-10 clinical investigator (lead investigator for Provectus' pivotal melanoma Phase 3 trial) Dr. Sanjiv Argawala is speaking on December 3rd. The conference/meeting runs from December 1st to 4th in Naples, Italy:
Updated (12/3/15): Whoops! Today is December 3rd. H/t a shareholder:
Updated (12/3/15): H/t InvestorVillage poster Juggernaut/Tweeter @DukeDiligence for informing me about Provectus' updates to its August and September 2016 Calendar of Events webpages.
Updated (12/3/15): Dr. Agarwala's Melanoma Bridge presentation is available on Provectus' Melanoma Clinical Trial Update webpage. A couple of slides from the presentation are below:
In addition to the Melanoma Clinical Trial Update webpage, Provectus also added a Cancers of the Liver Clinical Trial Update webpage.
 |
| Click to enlarge. |
The study itself began in January 2013 (the protocol was filed in December 2012). Patient data were first presented at AACR 2014 (April) -- n_initial = 8. More data on n_initial was presented at ASCO 2014 (May/June). ecancer reporter Janet Fricker's PV-10 decreases melanoma cells in tumours article from April 2014 noted "“[i]ronically, the original aim of the trial to assess tumour-infiltrating lymphocytes was thwarted when biopsies of patient tumours collected just seven to 10 days after PV-10 injection no longer contained viable tumour tissue,” said Pilon-Thomas" (Dr. Shari Pilon-Thomas, PhD, a Moffitt researcher) and "[s]tudies are now underway in an additional seven patients to take biopsies and blood samples at more frequent time intervals after PV-10 injection to elucidate the pathways more clearly" (i.e., n_subsequent = 7, for a total of n = 15). Patient data on n = 14 were presented at SITC 2015.
According to Provectus, Moffitt increased the completion date to extend patient tracking (in the center's non-therapeutic study). Prior completion dates appear to be October 2015, June 2015 and December 2014.
On other topics:
Squeezing. Roche allies with cell engineering startup SQZ on $500M+ immuno-oncology pact, December 7th by John Carroll (FierceBiotech):
"The big idea here is that SQZ can insert tumor related antigens into cells extracted from patients and then put them back in the patient. The company discovered, almost accidentally, that when you squeeze a cell the right way it will disrupt the surface, opening doors to introduce molecules inside the cell. In addition to cancer and immuno-oncology, where cell engineering has inspired a tsunami of new development programs, the company sees some big potential for autoimmune diseases.
"Your immune system gets activated against fragments," says Sharei, noting that a cell will throw out these fragments to the surface, flagging an immune system attack as T cells attack the cancer. And for Roche, it's a chance to take the lead in a new, potentially groundbreaking tech niche for cell therapies, a field that has been dominated by rivals like Novartis." {Underlined emphasis is mine}
December 8th by John Carroll (FierceBiotech):
"Aside from gaining a marquee industry name for its list of collaborators at a time Pieris has been working on making its mark in the popular field of immuno-oncology, the biotech also banks a $6.4 million upfront with a commitment of up to $409 million in milestones--a classic back-ended deal that leaves the real payoff further down the road when the potential of new drug candidates can be better evaluated." {Underlined emphasis is mine}
Already proven. Cash-rich Gilead hits acquisition trail, December 7th by David Crow (The Financial Times):
Mr Bischofberger said that Gilead was looking particularly for target companies that have drugs that are already proven to work in early-stage trials. Such groups tend to be more expensive but they carry a much lower risk of failing later on.
“We have typically preferred to wait for answers before we jump in,” he said. “Philosophically, we prefer to wait for more certainty and pay more money, which is what we did with Pharmasset, rather than getting something cheap with uncertainty.”
Aeterna Zentaris. The company, which (i) entered into a collaboration agreement with Sinopharm (Sinopharm A-Think) in December 2014 but did not disclose deal terms save for a non-refundable $1 million fee to it*, (ii) did a 100-to-1 reverse split in October/November 2015 of ~633 million shares outstanding (building on a 6-to-1 reverse split in October 2012 of ~112 million shares outstanding) (iii) had a Maxim Group/Jason Kolbert price target of $2 in October 2015 (pre-reverse split) when the share price was less than 10 cents, (iv) issued a PR about its first patient enrolled in a confirmatory Phase 3 in November 2015, and (v) received a new price target from Maxim/Kolbert of $11 ($200 split adjusted) immediately after the reverse split was effective and the share price opened at $4.58 and closed at $8.52, raised money today through Maxim (as sole book runner).
 |
| Click to enlarge. Image source |
After Provectus' COO/CFO Peter Culpepper's Maxim fundraising in June, I wrote under Offering, Part I (June 29, 2015) on the blog's Archived News III page:
"He better get full throated research coverage from Maxim's Jason Kolbert; existing shareholders paid dearly for it (although, research coverage from lower tier investment banking firms like Maxim are akin to "the moon does not exist if nobody is looking at it")."I'm going to guess by this point research coverage initiation of/a new price target on Provectus from Maxim/Kolbert (or McCarthy or [fill in the blank] analyst) will not happen in a timely or meaningful way, and doesn't really matter at this point (I don't believe it matters or mattered to Peter, frankly). I had figured and still figure as much but was disappointed no quo materialized for the quid he paid (on behalf of existing shareholders at the time) to Maxim.
* See Status update (October 5, 2015) below.
Drug substance/product synthesis (December 8, 2015)
I've long been impressed by the strategy, quality of writing, and breadth and depth of Provectus' intellectual property (IP) portfolio. IP, and its instantiation, were a key and critical focus of our corporate venture capital team (and our due diligence, and assessment of value drivers and valuation) back in the day (e.g., telecom deregulation, the Internet boom & bust, some life sciences investing, etc.).
One of the company's key IP/patents was the drug product/substance synthesis to International Conference on Harmonisation (ICH) guidelines patent, Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes, awarded in September 2013.
Management continued/continues to broaden and deepen the IP portfolio, submitting to the US Patent and Trademark Office (US PTO) for consideration a continuation of the synthesis patent, Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes, which was made public on the US PTO patent application full text image database website in November 2014.
It looks like this patent application was allowed at the end of November 2015; see below:
 |
| Click to enlarge. |
Provectus' Phase 1/expanded Phase 1 liver trial comprised/comprises a main study group and two expansion cohorts, indications that include both HCC and cancers metastatic to the liver, and sorafenib as standard of care (for the 2nd expansion cohort). See below.
 |
| Click to enlarge. |
- Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver (July 1, 2015),
- ESMO World GI Poster (July 1, 2015),
- Thoughts about Barcelona (July 2, 2015),
- Comparable, Consistent PV-10 Pharmacokinetics (July 3, 2015), and
- Clinical Trial (July 4, 2015) -- all below.
The table below reflects the initial information on the trial, and is added to the table above.
 |
| Click to enlarge. |
Data on 13 patients were presented at Barcelona and Osaka. There has been hearsay (rumor) that 30-40 patients were treated. Have patients in Expansion Cohort 2 (EC2 = EC 2.1 and EC 2.2), those patients originally on sorafenib then receiving PV-10, been treated? If a >30-40 number is veracious, it might/would mean patients in all groups/cohorts were treated (i.e., 3 + 3 + <24 + 3-to-6 + 3-to-6 = <36-to-42).
In order to gain approval of a single agent where is a standard of care (SOC), one compares the agent and SOC by running studies and trials of agent + SOC and agent + SOC v. SOC.
The Barcelona/Osaka data noted survival up to 54 months, but the time period for tracking patients in order to present such data required time to elapse.
I believe EC 2, the "sorafenib" cohort, began recruiting patients in April 2013.
At the time of the Barcelona and Osaka conferences (July 2015), medical writer Janet Fricker wrote articles about PV-10 (and Provectus), in particular ESMO GI: PV-10 shows potential in hepatocellular carcinoma and metastatic liver disease, which mentioned the sorafenib GIDEON study (first interim analysis is here, second interim analysis is here):
"The recent GIDEON study showed sorafenib, currently the only approved first line treatment in HCC, delivers a median overall survival of 10 months in patients under 70 years, and 20 months in patients older than 70 years."
GIDEON Study: Hepatocellular carcinoma in elderly patients: Final results of the Italian cohort of GIDEON study, ESMO GI 2015
In order to distinguish the agent's efficacy over the SOC, the incremental improvement of agent + SOC, if any, is attributed to the agent. That is, value_agent+SOC minus value_SOC equals value_agent.
In order to distinguish the agent's efficacy over the SOC, the incremental improvement of agent + SOC, if any, is attributed to the agent. That is, value_agent+SOC minus value_SOC equals value_agent.
Depending on, first, whether patients in EC2 indeed were treated, and then second, how many were treated, Provectus' CTO Dr. Eric Wachter, PhD might want/have to allow time to elapse following EC2 patient treatment in order to attempt to achieve overall survival (OS) longer than 10 to 20 months, and presumably substantially so. What could substantially mean? 12 months, say? Thus, Eric would have to show survival of 22 to 32 months or more. Let's say recruitment for EC2 took 12 months (who knows), then you might get something like the below:
 |
| Click to enlarge. |
On the left we assume GIDEON median OS of patients older than 70 years (20 months), and on the right we assume GIDEON median OS of patients younger than 70 years (10 months). We also assume waiting 12 more months to show substantial improvement contributed by PV-10 (i.e., 32 and 22 months, respectively).
Takeaway: Maybe Provectus has EC2 data. Maybe Eric has/wants to wait until he can show suitable OS "curve separation" (between PV-10+sorafenib, and literature results of sorafenib alone).
Bridges (December 3, 2015)
Updated below again, again.
Provectus updated its Calendar of Events page for December to include Melanoma Bridge 2015.
 |
| Click to enlarge. |
PV-10 clinical investigator (lead investigator for Provectus' pivotal melanoma Phase 3 trial) Dr. Sanjiv Argawala is speaking on December 3rd. The conference/meeting runs from December 1st to 4th in Naples, Italy:
 |
| Click to enlarge. Colored boxy emphasis is mine. Image source |
 |
| Click to enlarge. Image source |
The bottom left-hand corner picture/intralesional therapy table has been updated to include PV-10 under the Phase 3 trial column:
 |
| Click to enlarge. Image source from above. |
 |
| Click to enlarge. Image source |
The company's webpages note the joint meeting of the 16th World Congress on Cancers of the Skin and the 12th European Association of Dermato-Oncology conference (2016), which will run from August 31st to September 3rd in Vienna, Austria. The abstract submission deadline currently appears to be Spring 2016.
Rose Bengal/PV-10-related pre-clinical and clinical data has been presented before at these meetings (there is a third, the World Congress of Melanoma):
- 2009: Interim data from Provectus' metastatic melanoma Phase 2 trial at the joint meeting of 7th World Congress on Melanoma and 5th Congress of the European Association of Dermato-Oncology, and
- 2013: Mechanism of action data* at the joint meeting of the 8th World Congress of Melanoma and the 9th Congress of the European Association of Dermato-Oncology.
I wonder whether Provectus would use the 2016 conference/meeting to present interim data from the pivotal melanoma Phase 3 trial.
* With the benefit of hindsight, I wonder whether Nascimento et al. were/are one of Eric's pre-clinical development work stringers. The MOA work was entitled Rose Bengal - Phototoxicity Versus Intrinsic Cytotoxicity (Nascimento, P.; Baesler, K.; Knuever, J.; Douglas, G.; Anfosso, A.; Weninger, W.; Haass, N.). See page 8 of this link for the below, which is a portion of the abstract.
"Results: RB indeed had a dose-dependent cytotoxic effect on melanoma cells but not fibroblasts in the absence of light or upon exposure to red light (633 nm). In contrast, exposure to UV- or green light (561 nm) caused profound phototoxicity within minutes. In our 3D melanoma spheroid model, RB had a time- and dose-dependent effect on melanoma cell death of both proliferating and invading cells. In addition, RB exerted its toxicity through necrosis without perturbation of the cell cycle and the effects observed in the dark were independent of the phototoxic generation of ROS. Finally, we showed that RB induced autophagy in melanoma cells indicating a possible mechanism of action. Conclusion: In additions to its phototoxicity RB also exerts intrinsic cytotoxicity. In contrast, to the phototoxicity the intrinsic cytotoxicity has a wider therapeutic window. Here we showed that an interplay of cell necrosis and autophagy is one possible mechanism of action for RB."Like with the work from SSO 2015, Intralesional Injection of Rose Bengal Induces an Anti-tumor Immune Response and Potent Tumor Regressions in a Murine Model of Colon Cancer by Maker et al., no poster (only an abstract) has surfaced with regards to Nascimento/Haas' work.
Updated (12/3/15): Dr. Agarwala's Melanoma Bridge presentation is available on Provectus' Melanoma Clinical Trial Update webpage. A couple of slides from the presentation are below:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
November Blog Stats (December 1, 2015)
Blog readership statistics were mixed for November. I wrote 28 blog posts (2) and news items (26) in November compared to 20 in October (2 and 18, respectively). Month-over-month changes were:
Anti-microbials (November 30, 2015)
H/t @jeanie93 here and here:
Sunday (November 29, 2015)
This news item may be updated throughout the day.
SABs. H/t a shareholder: Provectus revised its Advisory Boards webpage, and removed the names of its Strategic and Science Advisor Board (S/SAB) members. See the first screenshot below. The company notes S/SAB members are utilized on an ad hoc basis.
A October 2015-dated screenshot (using Internet Archive: Wayback Machine) provides recent historical lists of the two SABs.
The Strategic Adivsory Board comprises/comprised, among other people, individuals from/formerly from Pfizer and Boehringer Ingelheim. The Scientific Advisory Board comprises/comprised a physcian who currently is a member of Provectus' board of directors.
XYZ/ABC. Last November, Moffitt presented their melanoma combination poster at SITC 2014 entitled Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma; i.e., PV-10 + one of {anti-CTLA4, anti-PD1, anti-PDL1}. I wrote about this in November 18, 2014's blog post Provectus notebook, where the preclinical Moffitt study comprised a single injection of PV-10. Of course, contrast that study approach, which had focused goals, with Provectus' Phase 1b/2 combination therapy clinical development program that sees Stage IV melanoma patients receive injections of PV-10 into all accessible disease (I believe) every 3 weeks for the duration of the study, together with immune checkpoint inhibitor pembrolizumab.
Blog readership statistics were mixed for November. I wrote 28 blog posts (2) and news items (26) in November compared to 20 in October (2 and 18, respectively). Month-over-month changes were:
- -11% for # of unique visitors (1,945 v. 2,187),
- +4% for # of page views (26,326 v. 25,332),
- +6% for # of visits (8,377 v. 7,884),
- -1% for # of U.S. cities from where visitors came (584 v. 590),
- +44% for # of world cities (164 v. 114), and
- No change for # of countries (50 v. 50).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
H/t @jeanie93 here and here:
- Promising new antimicrobials could fight drug-resistant MRSA infection, and
- New Antimicrobials Could Fight Drug-Resistant MRSA Infection, Georgia State Study Finds.
Selected quote from the second article above:
"A novel class of antimicrobials that inhibits the function of a key disease-causing component of bacteria could be effective in fighting methicillin-resistant Staphylococcus aureus (MRSA), one of the major drug-resistant bacterial pathogens, according to researchers at Georgia State University...
In previous work, the researchers developed novel small molecule SecA inhibitors active against the bacteria strains Escherichia coli and Bacillus subtilis by dissecting a SecA inhibitor called Rose Bengal (RB).
In this study, they evaluated two potent RB analogs for their activity against MRSA strains. The RB analogs inhibited the ATPase activities of two SecA isoforms identified in S. aureus, SaSecA1 and SaSecA2, as well as the SaSecA1-dependent protein-conducting channel. The inhibitors also reduced the secretion of three toxins from S. aureus and stopped three MRSA strains of bacteria from reproducing.
The best inhibitor in this group, SCA-50, showed strong activity against MRSA Mu50 strain and an inhibitory effect on MRSA Mu50 that was two-to-60 times more potent than all commonly used antibiotics, including vancomycin, the last resort option for treating MRSA-related infections."See March 28, 2012 blog post Xanthene Dyes Induce Membrane Permeabilization of Bacteria and Erythrocytes by Photoinactivation, and U.S. patent 8,974,363 (awarded March 10, 2015) and also the screenshot below.
 |
| Click to enlarge. Image source |
 |
| Image source |
This news item may be updated throughout the day.
SABs. H/t a shareholder: Provectus revised its Advisory Boards webpage, and removed the names of its Strategic and Science Advisor Board (S/SAB) members. See the first screenshot below. The company notes S/SAB members are utilized on an ad hoc basis.
A October 2015-dated screenshot (using Internet Archive: Wayback Machine) provides recent historical lists of the two SABs.
The Strategic Adivsory Board comprises/comprised, among other people, individuals from/formerly from Pfizer and Boehringer Ingelheim. The Scientific Advisory Board comprises/comprised a physcian who currently is a member of Provectus' board of directors.
 |
| Image source |
 |
| Click to enlarge. Image source |
XYZ/ABC. Last November, Moffitt presented their melanoma combination poster at SITC 2014 entitled Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma; i.e., PV-10 + one of {anti-CTLA4, anti-PD1, anti-PDL1}. I wrote about this in November 18, 2014's blog post Provectus notebook, where the preclinical Moffitt study comprised a single injection of PV-10. Of course, contrast that study approach, which had focused goals, with Provectus' Phase 1b/2 combination therapy clinical development program that sees Stage IV melanoma patients receive injections of PV-10 into all accessible disease (I believe) every 3 weeks for the duration of the study, together with immune checkpoint inhibitor pembrolizumab.
At the time frequent medical writer of PV-10 Janet Fricker wrote article in Medical News Today about Moffitt's SITC 2014 poster presentation entitled Melanoma shows improved regression with combination of PV-10 and checkpoint inhibitor. The article has an interesting quote from the cancer center's Dr. Shari Pilon-Thomas, Ph.D. about their work:
Interference (November 27, 2015)
Updated below again, again.
H/t a shareholder in regards to a 2009 letter to the editor (Clinical Chemistry) entitled Interference from Rose Bengal with Total Bilirubin Measurement. The authors introduce the following:
Updated (11/27/15): "Bilirubin (formerly referred to as haematoidin) is the yellow breakdown product of normal heme catabolism, caused by the body's clearance of aged red blood cells which contain hemoglobin[citation needed]. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases. It is responsible for the yellow color of bruises and the yellow discoloration in jaundice. It is also responsible for the brown color of feces, via its conversion to stercobilin, and the background straw-yellow color of urine via its breakdown product, urobilin." (Source: Wikipedia).
Total bilirubin "measures both BU and BC. Total and direct bilirubin levels can be measured from the blood, but indirect bilirubin is calculated from the total and direct bilirubin." BU = "Unconjugated" or "indirect bilirubin." BC = "Conjugated" or "direct bilirubin."
Takeaways include:
Updated (11/28/15): The 2009 Dimeski et al. letter to the editor also noted "PV-10 causes tumor necrosis, possibly owing to the release of cathepsins," which references Mousavi H, Zhang X, Gillespie S, Wachter E, Hersey P. Rose bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. [Abstract]Melanoma Res 2006;16 Suppl 1:PS8:
The September 2006 poster notes "RB induced cell death in melanoma cells but not fibroblasts. Death was predominantly due to necrosis but 2 lines also underwent apoptosis that was dependent on activation of caspases. Cell death was not due to release of Reactive oxygen species. RB is taken up into lysosomes and it is probable (but not proven) that cell death results from release of Cathepsins." Underlined emphasis in the preceding quote is mine.
Iran's Professor Seyed Hadi Mousavi, PhD (of the poster above) was Assistant Professor of Pharmacology, Department of Pharmacology and Pharmacological Research Centre of Medicinal Plants, School of Medicine, MUMS, Mashhad, Iran. PubMed notes two Rose Bengal-related articles by him:
"The spirit of our study was to determine whether combining PV-10 with a checkpoint inhibitor would enhance the systematic immune responses of the initial injection of PV-10."
Phrased via slightly different editing: The spirit of the study was to determine whether combining ABC (PV-10) with XYZ (e.g., pembro) would enhance the systematic immune responses of ABC (PV-10). Not, whether combining XYZ (pembro) with ABC (PV-10) would help XYZ (pembro).
pCR > CR. Moffitt's 2014 ASCO poster of the cancer center's feasibility (immune mechanism of action) study note of the first 8 patients treated (the study enrolled 14-15 patients in total):
pCR > CR. Moffitt's 2014 ASCO poster of the cancer center's feasibility (immune mechanism of action) study note of the first 8 patients treated (the study enrolled 14-15 patients in total):
"Methods:...Two study lesions in each patient were sampled by biopsy pre-treatment; one of the two lesions was injected with IL PV-10, then both residual sites were completely excised...Results: Treatment with IL PV-10 led to pCR in the post-treatment biopsies of both PV10-injected and uninjected study lesions in 4 of the 8 patients, and all 8 exhibited at least partial regression of the injected lesion."Takeaways:
- For this Moffitt study, patients received one injection into the target lesion. It should be noted that patients in Provectus' metastatic melanoma Phase 2 trial more often than not required 1-2 injections to achieve complete response (CR) of their injected (target) lesions. No biggie?
- In the Moffitt study, patients' injected lesions achieved pathologic complete response (pCR). I have not yet been able to find a definition for pCR as it relates to melanoma, but my sense from reading (mostly as it relates to breast cancer) that pCR (more than CR) means the disease is pretty much gone, or just gone. Pathologic relates to pathology, which relates to a pathologist who examines tissues and cells (more so than the medical or surgical oncologist). Histology (the study of the microscopic anatomy of cells and tissues) often times (much more often than not, because it's hard to achieve) does not play a role in CR measurement.
 |
| Click to enlarge. Image source |
The FDA recognizes either of two definitions of pCR for the purposes of designing trials for U.S. marketing approval: (i) "...the absence of residual invasive cancer on hematoxylin and eosin evaluation of the complete resected breast specimen and all sampled regional lymph nodes following completion of neoadjuvant systemic therapy (i.e., ypT0/Tis ypN0 in the current AJCC staging system)" or (ii) "...the absence of residual invasive and in situ cancer on hematoxylin and eosin evaluation of the complete resected breast specimen and all sampled regional lymph nodes following completion of neoadjuvant systemic therapy (i.e., ypT0 ypN0 in the current AJCC staging system)."
- In the Moffitt study, patients' injected lesions achieved 50% pCR, and 100% OR (objective response)(assuming "partial regression" quantitatively and appropriately equates to "partial response").
- In the Moffitt study, patients' non-injected lesions achieved 50% pCR.
- So, the question for me about Provectus' Phase 1b/2 study of combining PV-10 and pembro in patients with Stage IV melanoma obviously centers around patient response. If patients receive multiple treatments of PV-10 until disease has the opportunity to go away (if possible of course) at the injection site and elsewhere (and "goes away" means lesion, tissue and cell), and if pembro can extend PV-10's systematic immune response, just how much upside in terms of efficacy can a patient receving the combination regimen actually achieve?
CU/UCLA/UCSF. The melanoma Phase 3 trial and/or the melanoma combination Phase 1b study should include the following clinical sites or sites directly related to these entities: University of Colorado Denver School of Medicine, UCLA Medical Center, and University of California, San Francisco.
Updated below again, again.
 |
| Image source |
"We report an unusual cause for a false-positive interference in the total bilirubin method on the Beckman Coulter DxC 800 analyzer and for the hemolytic index (HI) measured on the Roche Modular D. A total bilirubin result of 53 μmol/L was obtained for a patient who had an earlier result of 7 μmol/L on the DxC 800 analyzer. The direct bilirubin concentration remained at <4 μmol/L, and no other results changed in subsequent multibiochemical profiles. The sample had a red/pink tinge. There was no clinical explanation for the increased bilirubin value. A sample obtained the next day had a total bilirubin result of 7 μmol/L. The patient was a participant in a trial for the treatment of severe melanoma lesions with PV-10 [100 mmol/L rose bengal (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodo-fluorescein disodium; MW, 1017.65 Da) in 9 g/L NaCl (i.e., 10% rose bengal disodium in 9 g/L NaCl); Provectus Pharmaceuticals]. PV-10 causes tumor necrosis, possibly owing to the release of cathepsins. The trial protocol is to inject PV-10 directly into the lesion and to collect a blood sample within an hour."The authors conclude:
"Some details of the success of the PV-10 trial have been published, and full details undoubtedly will be published in the near future. In light of the present findings, the trial protocol for the timing of postinjection sample collection should be reviewed. Plans are under way for additional trials of PV-10 for treating other cancers. Therefore, these interferences may become more common with the assays outlined above and potentially with others on different analytical systems."
 |
| Click to enlarge. Image source, Table 1 |
Total bilirubin "measures both BU and BC. Total and direct bilirubin levels can be measured from the blood, but indirect bilirubin is calculated from the total and direct bilirubin." BU = "Unconjugated" or "indirect bilirubin." BC = "Conjugated" or "direct bilirubin."
Takeaways include:
- The goal of continuing to diligence informational aspects of PV-10/Rose Bengal -- pre-clinical, clinical, chemical, etc. -- remains to explore and continue to validate the benefical aspects of the small molecule, including the consistency of some of these aspects over time (even long periods of time).
- Primary takeaway: The interference noted by Dimeski et al. (Clinical Chemistry, May 2009 vol. 55 no. 5 1040-1041) is similar to that noted by Kazmi et al. in In vitro inhibition of human liver cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) enzymes by rose bengal: system-dependent effects on inhibitory potential (Xenobiotica. 2014 Jul;44(7):606-14.):
"Rose bengal’s reported activity in in vitro tests is often difficult to interpret due to the compound’s unique physical and photochemical properties. Rose bengal absorbs visible light strongly with maxima of 550 nm interfering with assays detected close to this wavelength (Neckers, 1989). Rose bengal is also highly fluorescent, potentially giving false positives in assays relying on fluorescent detection. Furthermore, rose bengal is a well-known photosensitizer and is known to catalyze the release of reactive oxygen species including singlet oxygen in the presence of minimal ambient light (Kochevar & Redmond, 2000; Neckers, 1989). All of these issues can lead to the misinterpretation of results from in vitro assays with rose bengal.
In this study, we examined rose bengal as an inhibitor of CYP and UGT enzymes in multiple in vitro test systems, namely pooled HLM and cryopreserved hepatocytes (CHH) to formally ascertain its potential to cause DDIs via inhibition of drug metabolizing enzymes. Interference in these assays was minimized by manipulating assay lighting conditions, using mass spectrometry rather than fluorescence for metabolite detection and, in the case of cell viability assays, avoiding detection wavelengths where absorbance or fluorescence due to rose bengal would confound results." {Underlined emphasis is mine}
- Remember, Rose Bengal's history goes back a long while (Delprat GD. Arch Int Med 1923; 32(3):401–410) in regards to, for example, liver diagnostic tests.
 |
| Click to enlarge. Image source |
The above comes from the Proceedings of the sixth Pacific Science Congress of the Pacific Science Association, held at the University of California, Berkeley, Stanford University, and San Francisco, July 24th to August 12th, 1939.
- Rose Bengal is a unique molecule that seems inexcorably linked to the liver.
 |
| Click to enlarge. Image source |
The above and below comes from Compartmental Modeling and Tracer Kinetics by David H. Anderson (1983).
 |
| Click to enlarge. Image source. |
Updated (11/28/15): The 2009 Dimeski et al. letter to the editor also noted "PV-10 causes tumor necrosis, possibly owing to the release of cathepsins," which references Mousavi H, Zhang X, Gillespie S, Wachter E, Hersey P. Rose bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. [Abstract]Melanoma Res 2006;16 Suppl 1:PS8:
 |
| Click to enlarge. Image source |
Iran's Professor Seyed Hadi Mousavi, PhD (of the poster above) was Assistant Professor of Pharmacology, Department of Pharmacology and Pharmacological Research Centre of Medicinal Plants, School of Medicine, MUMS, Mashhad, Iran. PubMed notes two Rose Bengal-related articles by him:
- 2014: Rose Bengal suppresses gastric cancer cell proliferation via apoptosis and inhibits nitric oxide formation in macrophages, and
- 2009: Direct toxicity of Rose Bengal in MCF-7 cell line: role of apoptosis.
Further diligence discovered additional Rose Bengal-related articles by Professor Mousavi, some of which included co-author, PV-10 clinical investigator and Australia's Professor Peter Hersey, PhD (co-author of the poster above, too):
- Autumn 2006 (+Hersey): Study of rose bengal-induced cell death in melanoma cells in the absence of light
- Result: The result showed RB could induce pronounced cell death in different melanoma cell lines but not in fibroblast cells. This toxicity was predominantly induced by non-apoptotic cell death but in some cell lines, RB could also induce apoptotic cell death.
- December 2006 (+Hersey): Role of Caspases and Reactive Oxygen Species in Rose Bengal-Induced Toxicity in Melanoma Cells
- Conclusion: Both caspase-dependent and -independent pathways were induced by RB in melanoma cells. RB-induced generation of ROS does not play a significant role in RB-induced toxicity and it is independent of ROS production in melanoma cells.
- January 2008: Lysosome: as a proposed target for rose bengal in inducing cell death in melanoma cells
- Abstract: We have previously shown that rose bengal (RB) itself and not as a photosensitiser could induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. But the mechanisms of RB-induced cell death are unclear. Recently lysosome was reported to initiate different kinds of cell death including necrosis, caspase-dependent and -independent apoptosis. The hypothesis of this study is to investigate the role of lysosome in mediating RB-induced cell death in melanoma cells focusing on lysosomal protease cathepsin B (CB). We present two lines of evidence indicating a central role for the CB in mediating cell death. First, inhibition of CB would result in a strong protection against drug-induced cell death and apoptosis in melanoma cells. Simultaneous inhibition of caspases would determine that CB acts upstream or downstream the caspase cascade. Second, we show RB triggers disruption of lysosomes leading to release and activation of CB using an engineered yellow fluorescent protein-tagged CB. It is hypothesized that RB-induced cell death in melanoma cells is mediated through lysosomal CB in this novel cell death pathway. This idea points to a new tumor-suppressive role for lysosomes which may be less affected by chemotherapy-induced resistance mechanisms.
- October 2008 (+Hersey): Apoptosis: from Signalling Pathways to Therapeutic Tools
- Abstract: Apoptosis or programmed cell death is a gene regulated phenomenon which is important in both physiological and pathological conditions. It is characterized by distinct morphological features including chromatin condensation, cell and nuclear shrinkage, membrane blebbing and oligonucleosomal DNA fragmentation. Although, two major apoptotic pathways including 1) the death receptor (extrinsic) and 2) mitochondrial (intrinsic) pathway have been identified, recently endoplasmic reticulum and lysosomal pathways have been also recognized. Depending on both the cell type and the initiating factor, distinct pathways are activated. The pathways share a common final phase of apoptosis, consisting of activation of the executioner caspases and dismantling of substrates critical for cell survival. The important regulatory mechanisms include death receptors, caspases, mitochondria and Bcl-2 family proteins. Modulating of apoptosis is a novel therapeutic strategy in treatment of different diseases. These include situations with unwanted cell accumulation (cancer) and failure to diminish aberrant cells (autoimmune diseases) or diseases with an inappropriate cell loss (heart failure, stroke, AIDS and neurodegenerative diseases). Modulation of apoptosis is a novel therapeutic strategy in treatment of different diseases. Many approaches including gene therapy, antisense strategies and numerous apoptotic drugs to target specific apoptotic regulators, are currently being developed. The goal of this review is to provide a general overview of current knowledge on the process of apoptosis including morphology, biochemistry, signaling as well as a discussion of apoptosis in diseases and effective therapy.
A Google search of "cathepsin B" and HMGB1.
Updated (12/11/15): According to Provectus (likely Eric):
Updated (12/11/15): According to Provectus (likely Eric):
"We understand Mousavi was an Iranian researcher in Peter Hersey’s lab in the mid-2000s who performed many of the experiments described in the 2006 Perspective in Melanoma X poster. He subsequently returned to Iran and has been investigating RB on his own since."
A conversation (November 25, 2015)
Pivotal melanoma Phase 3 trial. At Provectus' April 9, 2015 panel discussion in New York, company CTO Dr. Eric Wachter, PhD said the trial would require or utilize 35 clinical sites, 25 in the U.S. and 10 in Australia. By saying on recent business update conference calls that the trial's patient make-up would be one-third from the U.S., one-third from Australia, and one-third from the rest of the world, Eric appears to be saying that also is roughly the geographic make-up of sites. So, the site breakdown may look something like 10ish sites in the U.S., 10 in Australia, and 10ish in Western Europe, Mexico, Brazil and China.
Because of how payments to the CRO are structured for the trial (and typically for other mid- to late-stage clinical trials), Provectus of course knows which sites eventually will come on-line. The upfront payment, for example, is based on the sites one is using (unless more sites are added later if initial or original numbers are not being met, and then more payments are made for those additional sites).
Guidance of a July 2016 interim readout was reaffirmed. Although I am not completely certain, it would appear this is when a data dump would be generated, and presumably deposited into the company's electronic data room, and not necessarily when an announcement would be made.
Combination therapy melanoma Phase 1b study. Initial data should be available before the pivotal Phase 3 trial's interim data is available. Again, available in this case more than likely means available in the electronic dataroom for prospective partners to review, rather than presented at a medical conference. I think Provectus would be hard pressed to have its initial data ready for ASCO 2016, unless management (Eric) deems it appropriate to present a subset of the enrolled patients. The T-Vec/ipi and T-Vec/pembro posters at ASCO 2014 and SMR 2015, respectively, presented initial response data on all enrolled patients (even if unconfirmed in the latter's case).
Liver Phase 1b study. Surprisingly to me, initial data should be available before the pivotal Phase 3 trial's interim data is available in this case too. This would mean the necessary protocol(s) would be filed soon, and I think but am not certain most if not all of this data would come from Asian participants. Protocol refinement appears to have taken time because of the work necessary to establish the proper dose escalation aspect of the study. In order to get a single agent approved, the study (any study for that matter) would test compound plus standard of care (SOC) versus SOC. See Clinical Trial (July 4, 2015) below, for example, or Conference Call, part 2 (May 10, 2015) on the blog's Archived News III page. The different kinds of SOCs and secondary treatments in the region would require some individual addressing as it related to the relationship between PV-10's impact and the SOC's on patient treatment and treatment outcome. Should may yield to could if more time is taken, although the time frame of liver tumor injection and assessment is around 28 days. One other physcian (not St. Luke's Dr. Sanjiv Argawala) will be involved in all three PV-10 studies and trials.
"Comp" (November 24, 2015)
Following up on Status update (October 5, 2015) below, Provectus continues to negotiate with Sinopharm, per Provectus' management comments on the 3Q15 business update call.
A "comp" may be Oramed Pharmaceuticals' negotiations with Sinopharm Capital. A timeline of this dance follows:
Anatomy of a data presentation (November 23, 2015)
There are several items related to Provectus' Phase 1b trial of the combination of PV-10 and pembrolizumab for patients with advanced melanoma (Stage IV) that bear follow-up/tracking.
First, there is the amount of PV-10 dosing. Per the trial protocol, pembrolizumab is administered per prescribing information. PV-10 also is administered along that timeline (i.e., every 3 weeks); however, no information is provided about the total amount administered, like it is in Provectus' pivotal Stage III melanoma study -- i.e., "Calculated required PV-10 dose ≤ 15 mL (based on total tumor burden)."
The goal of the Phase 3 trial for Stage III melanoma patients is of course to treat all disease (the presumption being that all disease is accessible for injection). The very nature of the Phase 1b study for Stage IV patients is that some proportion of disease is unaccessible with a needle of PV-10 (i.e., visceral disease). This visceral (unaccessible) disease cannot be injected with Rose Bengal. But how much of the accessible disease in a Stage IV patient will be treated with PV-10?
Second, there is the effectiveness (i.e., the objective response) of PV-10 in Stage IV patients. Public Stage IV data for Rose Bengal comes from the company's metastatic melanoma Phase 2 trial. Patients received initial injections of the compound at "t=0" (Day 1, Week 0) for up to 10 target lesions and upto 10 non-target ones, and then upto 3 more sets of inections (Weeks 8, 12 and 16).
The company's paper on its Phase 2 trial noted:
Phase 2 trial data is helpful when it comes to the effectiveness of PV-10 to complete ablate -- completely destroy (Prong #1 of PV-10's two prong approach to fighting cancer) -- injected tumors, which then would commence the cascade of antigen generation and presentation to dendritic cells and an eventual hoped for (expected) anti-tumor immune response. Most injected melanoma tumors require 1-2 injections for them to be destroyed.
Which brings us back to the question of how much PV-10 is being used to treat Stage IV patients in the Phase 1b trial -- the amount in the Phase 3 trial (and the potential label amount), or more (or less, I suppose).
Third, there is the speed of a PV-10/pembro response. At AACR and ASCO 2014, Moffitt noted PV-10's immunologic signalling and impact (Prong #2) within 7-14 days of injection. The Phase 2 trial noted a median time to response (TTR) of 1.9 months (or about 8 weeks). Pembrolizumab's median TTR is 12-13 weeks. Could the pairing generate responses within as little as 3 weeks?
Fourth, there is the durability of the response.
Fifth, there is the presentation of the data. It is the data, Step #3 of Peter's three deal steps (see November 22, 2015's Differences of Opinion), that [presumably] Big Pharma need and prospective investors wants to see. It seems to me there are several key or notable venues for such data to be presented during a calendar year: April: AACR, May/June: ASCO, September: ESMO, November: SITC, and November: SMR. Maybe there are other medical conferences at which a company would present important clinical data.
Timelines from the ipi+T-Vec and pembro+T-Vec combinations:
Better? (November 21, 2015)
Merck & Co. (Merck USA) issued a press release today about preliminary combination results ("early findings") of anti-PD-1 drug pembrolizumab and intralesional drug T-Vec, presented at the Society for Melanoma Research International Congress.
Updated (11/21/15): A potential baseline for evaluating the T-Vec/pembro combination comes from the results of the T-Vec/ipi combination.
Laundry list (November 20, 2015)
1. I'm still not sure why Provectus' COO/CFO Peter Culpepper and CTO Dr. Eric Wachter, PhD were in Las Vegas a couple of weeks ago. Maybe for this? -- City of Hope Multidisciplinary Approaches to Cancer Symposium (November 5th to 8th). Peter left Thursday night (the 5th) for the 2015 SITC annual meeting (6th to 8th), while Eric left Sunday evening (the 8th).
2. Did Eric ultimately present at the Third Melanoma MIB Conference in Rome, Italy on November 9th, “PV-10: a new opportunity in Europe from Provectus?” The company said boo about it.
3. Dr. Sanjiv Agarwala, Professor of Medicine, Chief, Oncology & Hematology, St Luke's Cancer Center and Temple University, will present something titled Melanoma: Integrating Molecular and Immunotherapy Into Clinical Practice on January 10th at the inaugural PRIMO Meeting in Hawaii from January 9th to 12th. Provectus vendor (for Brazil) and Cancer Action Now CEO Jeff Meehan is the lead sponsor of the event. Agarwala is connected to Meehan via Cancer Action Now, and Provectus also is a PRIMO sponsor.
Does the event and Agarwala's presentation provide a medical conference venue for melanoma combination therapy data (of PV-10 + pembrolizumab) to be shown and discussed? Or will this data be presented at either AACR 2016 or ASCO 2016?
4. Maybe Agarwala will discuss PV-10 at his 2016 HemOnc Today Melanoma and Cutaneous Malignancies conference/meeting on March 18th and 19th.
5. Will more liver data be presented at ESMO Asia 2015 in December?
6. Provectus' metastatic melanoma combination Phase 1b trial, PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma, may need to generate objective response (OR) and complete response (CR) rates north of at least 60% and 20%, respectively, (in my opinion) to garner real attention from prospective Big Pharma partners and investors at large.
7. If metastatic melanoma Phase 1b patients were treated by Agarwala at St. Luke's during the week of October 21st (a Wednesday) when the site began recruiting, the first clinical assessment (and the second round of treatment) presumably would've occurred during the week of November 11th (Week 4) (the Alfred E. Smith Memorial Foundation dinner was held on November 10th). The treatment regimen is Week 1 (Day 1), Week 4 (Day 1), Week 7 (Day 1), Week 10 (Day 1) and Week 13 (Day 1). The next clinical assessment likely would be the week of December 2nd (Week 7).
More themes (November 19, 2015)
Search The Journal for ImmunoTherapy of Cancer (the official journal of the Society for Immunotherapy of Cancer, or SITC) for HMGB1, and recent items include:
Themes (November 17, 2015)
Amgen's talimogene laherparepvec (trade name IMLYGIC) had four abstracts for the 2015 Society for Melanoma Research International Congress.
The themes the abstracts in total connote appear [to me] to convey are consistent with PV-10's clinical value proposition.
The abstracts all derive from T-Vec's pivotal melanoma Phase 3 trial (aka OPTiM), which Amgen likely will use as a key resource for physicians seeking information about the safety, efficacy and application of the approved intralesional ("IL") agent (aka approved oncolytic virus or approved oncolystic immunotherapy):
Takeaways from (a): The IL agent helps to forestall the spread (i.e., visceral metastasis) of the disease, from an earlier form (i.e., Stage IIIB-C/Stage IVM1a) to later stages (i.e., Stage IVM1b-c). Less tumor burden is predictive of success/survival.
Abstract (b) is below.
Takeaways from (b): Complete response appeared to be more likely in patients with earlier stages of disease and having lower tumor burden. Durable complete response appeared to prolong survival, and prevented recurrence.
Abstract (c) is below.
Takeaway from (c): T-Vec is not as safe as PV-10.
Abstract (d) is below.
Takeaway from (d): Durable response (continuous complete response + partial response over at least 6 months) has clinical benefit; however, this abstract has the flavor of justifying T-Vec's Phase 3 trial's endpoint, which was non-standard or sub-optimal or poorly conceived.
Institutional (November 16, 2015)
13F filings (through today) for the period ending September 30, 2015 showed a decrease in institutional share holdings of Provectus: 10.68 million share owned/held (down from 12.65 million as at 6/30/15, or about -16%), and 5.22% of shares outstanding (not a fully diluted figure) (down from 6.18%). See Institutional (August 14, 2015) below for the last institutional holding blog news item.
On November 4th Investor Village (IV) poster Tejman321 noted a purchase by Heights Capital Management (but did not provide a linked information source). The firm is an affiliate of Susquehanna International Group, which sold out of its entire position in 3Q15, and previously invested early-ish in Dendreon in 2008. Heights' 13F filing for the period notes all holdings are reported by other reporting manager(s). The amount Heights acquired, according to the IV post, was the amount Susquehanna held as at the 6/30/15 reporting period and sold during 3Q15. It is possible Susquehanna transferred ownership of the position to Heights, or something along those lines, or Susquehanna/Heights exited the position.
In the event of a securities transfer, there would've been an increase in institutional share holdings of Provectus: 13.13 million share owned/held (+4%), and 6.42% of shares outstanding (not a fully diluted figure). The graphs then would look like:
Sunday coffee (November 15, 2015)
If the rumor of an upcoming [news organization's] piece on Rose Bengal, PV-10 and/or Provectus has veracity, I hope the author(s) are thoughtful about how they frame the article's narrative.
For example, I hope he, she or they:
Some Hearsay (November 14, 2015)
Don't delay (November 13, 2015)
Following up on Provectus' CTO Dr. Eric Wachter, PhD's NDA comments -- see Data package (November 12, 2015) below, specifically "...any supportive safety or pharmacology studies that may be needed..." -- he likely means pre-clinical studies like carcinogenicity, mutagenicity, teratogenicity, effects on fertility, juvenile studies, acute studies, chronic studies, maximum tolerated dosage determination, dermal irritancy, ocular irritancy, photocarcinogenicity, animal pharmacokinetic studies, etc. See, for example, Application Number 22483Orig1s000 Pharmacology Review(s), or Application 200-890 Pharmacology Review(s).
I imagine the idea would be to conduct these studies and this work concurrent with the pivotal Phase 3 melanoma trial, as preparation for the eventual submission of the NDA.
As an example of delay (in this case something like a 6-9 month one), in a December 2014 press release Puma Biotechnology noted:
The base case should be Eric planning to undertaking the necessary NDA-related preclinical safety/pharmacology work on PV-10. Upside may well be the FDA does not require this work to be carried out given the existing literature available on Rose Bengal, thus saving Provectus money and time.
Data package (November 12, 2015)
Provectus' CTO Dr. Eric Wachter, PhD noted on the 3Q business update call:
T-Vec, or Imlygic, is consistently limited in its effect.
T-Vec achieved similar responses in its Phase 2 (single-arm) and 3 trials (randomized control). Patients in the Phase 2 trial received a median of six injection sets of T-Vec. Patients in the Phase 3 trial on T-Vec had a median treatment duration of 23 weeks, which is akin to 11 injection sets (my calculation) -- the prescribing label is an initial injection (Day 1, Week 1), followed by an injection three weeks later, followed by injections every two weeks). 54% of them (i.e., patients who received T-Vec in the Phase 3) completed 6 cycles or injection sets; 18% completed 12 cycles/sets.
Patients in Provectus' melanoma Phase 2 trial received upto only 4 treatments of PV-10. In the company's Phase 3 trial, patients will receive PV-10 every 4 weeks until disease resolution or progression. PV-10 Phase 2 results were, for Stage III patients with all disease treated, 71% objective response and 50% CR.
Interestingly, time to response of PV-10 in its Phase 2 trial of patients with all disease treated (the Phase 3 protocol) was 1.8 months (median, I am guessing), while for T-Vec in its Phase 3 trial it 4.1 months (low of 1.2, high of 16.7).
While T-Vec should display some amount of improvement over chemotherapy, PV-10's proper treatment regimen should demonstrate clear superiority over T-Vec (as well as chemotherapy).
Sources:
Non-dilutive Financing Source? (November 11, 2015)
CFDA (November 10, 2015)
Follow-up (November 10, 2015)
5. Princess Alexandra Hospital in Australia may be recruiting for the melanoma Phase 3 trial (source link here).
Listing and Going Concern Thresholds (November 8, 2015)
On Provectus' 3Q15 business update call on November 6th, company COO/CFO Peter Culpepper described the respective thresholds of Provectus' NYSE MKT listing requirement (New York Stock Exchange) and going concern status (accounting firm and auditor BDO). In regards to the listing requirement, Provectus must maintain a mininum stockholders' equity of $6 million. In regards to the going concern status, the company must main 12 months of cash on hand at all times.
Stockholders' equity is equal to total assets minus total liabilities, and is directly but not wholly influenced by cash and cash equivalents (i.e., cash on hand), a line item on the balance sheeet (under total assets). Generally speaking, as cash increases or decreases, all else being equal (in context of course), stockholders' equity would increase or decrease.
Twelve months of cash on hand at all times would be influenced by Peter's ability to constrain or restrain variable spending (given that fixed spending is, well, fixed). According to management, this 12-month cash figure is not 12 times the past quarter's cash spend (as as estimate for future spending) or 12 times an estimated million dollars per month spend, but rather a figure less than $12 million (like something in the range of $8-9 million).
In the absence of non-dilutive influxes of capital, Provectus may be forced to raise money as early as late-April/early-May (2Q16), or as late as early-August (3Q16).
Meh < Call < Squee (November 7, 2015)
Provectus held its 3Q15 business update conference call yesterday. Underlined emphases (and in certain cases bolded too) below in management quotes from the call's tranacript are mine.
CFO/COO Peter Culpepper: "Our estimated primary completion date is September 2017, and an estimated study completion date of October 2017. When 50 percent of the events required for the primary endpoint have occurred, the Independent Data Monitoring Committee will report an interim assessment of efficacy and safety. So, meaningful clinical data could come as early as the middle of next year, which is halfway through the study, as documented on clinicaltrials.gov. I stretch the word could, and we will continue to make every effort possible to keep our stockholders and the market updated."
combination therapy for melanoma for Stage 4 patients. Scientifically, combination therapy in
cancer treatment is a rapidly maturing area, where a rational [sp] combination of agents is
replacing the empirical approaches of the past. In this specific instance, we have completed
development of the protocol for Phase 1b-2 testing of PV-10 in combination with Merck’s
Keytruda in patients with Stage 4 melanoma. And we have begun recruitment of patients."
A Correction and One More Note (November 5, 2015)
Correction: Moffitt Cancer Center is not using PV-10 in a clinical trial or study. The shareholder who believed or thought Moffitt was corrected their initial assertion. See Breast Cancer (August 8, 2015) below.
Note: Through today's 3Q15 financial statement issuance, expense item Lab supplies and pharmaceutical preparations experienced its 5th consecutive quarter-over-quarter increase. If de minimis input inflation, consistency of make-up, and if assumed to be entirely comprised of vial production (all rather quick 'n dirty or lazy assumptions on my part), one could believe the production of vials of PV-10 (and "gel packs" of PH-10) continues to increase.
Notes (November 5, 2015)
1. Provectus issued a press release today and filed an associated 8-K regarding its 3Q15 financial results, Reports Third Quarter 2015 Financial Results, in conjunction with filing its 10-Q.
2. The end-of-3Q15 cash balance (i.e., B/S item Cash and cash equivalents) was $18.9 million, compared to the prior end-of-Q's $23.1 million figure.
3. The company's monthly cash burn for the quarter appears to have been $1.41 million, +5% quarter-over-quarter (the prior quarter-over-quarter change was -4%).
4. Working in the gray — e.g., using a cash balance threshold of $12 million, estimating a monthly cash burn range of $1 million (a presumed "rough" projected figure for accounting firm BDO's purposes) to $1.4 million (on the current high end of recent actual), assuming the absence of non-dilutive deal-related cash inflows, making an assumption regarding an acceptable low watermark for the then pre-raise cash balance figure, assuming an increase in the authorized share number, etc. — Provectus would have to raise money in 2 to 3 quarters (i.e., 5-7 months).
5. Language regarding cash burn runway changed to "into 2017" in the current Q (pp. 14-15) versus "well into 2017" in the prior Q (p. 14).
6. Regarding the class action lawsuits (see the current Q's No. 7 under Notes on page 11): "On October 1, 2015, the Court entered an order staying a ruling on the Motion to Dismiss pending a mediation to resolve the Securities Litigation in its entirety. A mediation occurred on October 28, 2015, and discussions are continuing." See Lawsuit Update (November 4, 2015) below.
7. There was no update to the derivative lawsuit naming the company as a nominal defendant added in 2Q15: the Donato Shareholder Derivative Lawsuit. See the current Q's No. 7 under Notes on page 12.
8. Recent quarter-over-quarter changes to R&D and G&A line items. Note: Some items (and/or portions thereof) are non-cash.
Lawsuit Update (November 4, 2015)
H/t InvestorVillage Area51 poster:
From ERT's PROFICIENCY™ 2015 West Coast Regional Conference Series: Demonstrating a Product’s Value with Optimized Endpoints in May (San Francisco and San Diego).
October 2014: "ERT’s COA consultants are working with Provectus to select a patient-reported outcome (PRO) measure of symptoms in this population that can be implemented in the Phase III clinical trial of PV-10 and ultimately support treatment benefit claims in the product label."
See also Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015) below.
SITC 2015, HMGB1, Anti-tumor immunity, Immunogenic cell death-(?) (November 3, 2015)
Updated below.
For this blog news item, set aside for the moment whether one thinks the biopharmaceutical ecosystem works, or not, in regards to drug development, broadly and inclusively speaking, and the ecosystem comprises the regulator (e.g., the FDA), medical practitioners, [pre-clinical] researchers and clinical investigators, medical and medical research institutions, Big Pharma company licencees/acquirors, peer groups (e.g., peer-reviewed publications, medical conferences), investors, various forms of media, etc.
Provectus has endeavored to work with Moffitt Cancer Center, and work with them (and other so-called stringers) at as much arm's length as is practical (sensible) and practicable (possible), to establish the veracity of the different facets and features of Rose Bengal and PV-10.
Stringers refers to medical and medical research organizations and entities working on/using PV-10 in some form or fashion (as facilitated by Provectus' CTO Dr. Eric Wachter, PhD) at some early or earlier stage of work (or until more broadly known and/or presented/published). Moffitt originally would've been a stringer (but perhaps wasn't strictly considered such because of its initial pivotal role). The Ajay Maker Laboratory of the University of Illinois at Chicago would appear to be a more contemporary or better example of a stringer.
2012: "Bystander"/Abscopal Effect, in Melanoma: At the Society of Surgical Oncology Annual Meeting, Moffitt reproduced, confirmed and, thus, validated, the "claim" PV-10 injection of melanoma tumors or lesions could lead to the shrinkage or destruction of untreated tumors or lesions: Intralesional Injection of Melanoma with Rose Bengal Induces Regression of Untreated Synchronous Melanoma In a Murine Model. At the time, this arguably confirmed, albeit pre-clinically, what was being seen clinically in the company's metatatic melanoma Phase 1 and 2 trials. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
2013: Immune Response, in Multiple Cancer Indications: At the American Association for Cancer Research Annual Meeting, Moffitt delved deeper into the workings of Rose Bengal and PV-10 to suggest a potential immune response was responsible for the "bystander effect," and did so in multiple cancers, which reproduced, confirmed and, thus, validated, more PV-10 "claims" (by Provectus): Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
2014: Anti-tumor immunity, in Melanoma: At the American Association for Cancer Research Annual Meeting, Moffitt further delved into the workings of Rose Bengal and PV-10, suggesting the potential for anti-tumor immunity to result from the compound's injection: Induction of anti-melanoma immunity after intralesional ablative therapy. HMGB1 was first mentioned here. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
2014: "Bystander"/Abscopal Effect, Clinical/Immune Responses, in Melanoma: At the American Society of Clinical Oncology Annual Meeting, Moffitt extended their preclinical work into the clinic, demonstrating the efficacy and immune response of treated and untreated lesions to PV-10: Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
October Blog Stats (November 1, 2015)
Blog readership mostly fell from September 2015 depending on the statistic. I wrote the same total number of blog posts (2) and news items (18) in October compared to the previous month (3 and 17, respectively). Month-over-month changes were:
Technology verification (October 30, 2015)
Phase 3 sites (October 30, 2015)
Updated below again.
Three more clinical sites were activated (but are not yet recruiting) for Provectus' pivotal Phase 3 melanoma trial:
Updated (10/30/15): Tabulation of clinical sites to date.
Updated (11/1/15): On Provectus' May 7th 1Q15 business update call, the company's CTO Dr. Eric Wachter, PhD said:
He said again the same thing on the company's 2Q15 call:
Route of delivery matters: The FDA approves Imlygic (talimogene laherparepvec) (October 27, 2015)
Updated below again.
The Agency approved intralesional therapeutic or agent/oncolytic immunotherapy/oncolytic virus T-Vec today. In the FDA press release, the agency refers to T-Vec as an oncolytic virus therapy, noting or referencing route of delivery six times. Underlined and bolded emphasis below is mine.
A Provectus shareholder like myself believes Amgen's T-Vec's approval is groundbreaking as an intralesionally-delivered therapy that happens to be an oncolytic virus, rather than strictly as an oncolytic virus therapy.
This differentiation in approach is because Provectus' founders believed intratumoral or intralesional injection of the right compound into cancer tumors or lesions (local delivery), rather than oral or intravenous administration (systemic delivery), would deliver the right information in the right format and the right way to the immune system. See October 15, 2015 blog post Still Standing.
Recruiting (October 22, 2015)
Updated below.
According to Provectus' ClinicalTrial.gov webpage, the company's combination therapy melanoma trial PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma now is recruiting at St Luke's University Hospital and Health Network. The study, as of this writing, does not appear on St. Luke's listing of melanoma trials in which the hospital is participating.
In addition, the third listed clinical site of the three, Atlantic Health System, is now is recruting patients for the company's signle agent melanoma trial PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma.
Updated (10/22/15): It should be clear to existing/prospective Provectus shareholders that it is not clear to company management how much more data, broadly speaking, is required for some sort of combination therapy deal with a Big Pharma having an immune checkpoint inhibitor (and/or a targeted therapy) under [mostly-to-exclusively] terms acceptable to Provectus to materalize.
Such terms might include an upfront payment, no-to-equal sharing of study costs, and Provectus' trial protocol, among other things. I don't believe who and where such a study (i.e., formally with a Big Pharma partner) is undertaken is a big deal, since it would be more likely carried out at prominent clinical sites by prominent clinical investors well versed in the use of pembrolizumab (as a single agent and in combination with another therapeutic).
In Provectus' September 23rd Announces Initiation of Phase 1b/2 Clinical Trial to Study PV-10 in Combination with Immune Check Point Inhibitor Pembrolizumab (with the byline: Study could be a "Significant Step to Co-Development Transaction"), the company's COO/CFO Peter Culpepper said:
Step #2, initiation of their own Phase 1b/2 clinical development program for combining PV-10 and an immune checkpoint inhibitor, was made (in management's view) when the study protocol was filed on ClinicalTrials.gov on September 23rd.
Step #3 is more information on PV-10's immune mechanism of action ("MOA") being made available. Moffitt Cancer Center will present a poster at SITC 2015 on November 6th that should expound further on PV-10's immune MOA: Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via HMGB1.
There probably are a couple of additional steps, or data generation items, that might be necessary to get a deal done (if one indeed can be had). Step #2.5 or Step #3.5 could be the publication of Moffit's work in a peer-reviewed publication (paying homage to the so-called peer review process), which company management has previously guided may occur before the end of the year.
Step #4, more than likely a/the requisite step, is the generation of data from the Phase 1b trial, which was noted as recruiting at St. Luke's on ClinicalTrials.gov today.
St. Luke's and Dr. Sanjiv Agarwala, MD, a medical oncologist who was a clinical investigator in Provectus' melanoma Phase 2 trial and is the lead investigator of the company's pivotal melanoma Phase 3 trial, are logical first movers because of Dr. Agarwala's influence with his hospital's institutional review board, his access to appropriate patients, etc. Other prominent sites and investigators, perhaps like Memorial Sloan Kettering Cancer Center and Dr. Paul Chapman, MD (a medical oncologist who is a consultant to Provectus), may materialize later.
In the September 23rd press release, Provectus' CTO Dr. Eric Wachter, PhD noted:
Since patient treatment would be assessed over (rather than just after) 15 weeks, data for Big Pharma to evaluate (in Provectus' electronic data room under confidentiality) should be generated shortly after patients are enrolled and treated, such as every three weeks or so given pembrolizumab's dosing schedule. Using a hypothetical first day of treatment as November 2nd, two data drops might occur before the end of the year. But how many patients and patient data drops would be necessary and sufficient for Big Pharma?
There is no small amount of risk to Provectus and its shareholders by management undertaking this study. There is the potential for the generation of autoimmune disease because of combining two immunomodulatory agents, like immune checkpoint inhibitors themselves already have shown. PV-10's remarkable specificity and presumably no-to-little clinically relevant drug-drug interaction with pembrolizumab should help mitigate this risk. I imagine Provectus has sufficient safety and efficacy experience in pre-clinical trials (mice studies) by Moffitt and the company's compassionate use/expanded access program about the combination of PV-10 and immune checkpoint inhibitors (whether PV-10 was given after or before the checkpoint inhibitor).
Another concern about the Phase 1b study might be whether it might take patients away from Provectus' Phase 3 trial. This is unlikely as the patient subsets are different. The Phase 3 trial is strictly comprised Stage III patients, while the Phase 1b study is strictly made up of Stage IV patients.
A Measure of Validation (October 21, 2015)
Provectus' CTO Dr. Eric Wachter, PhD said on the company's August 6th 2Q15 business upadate call:
One of the doctors at Princess Alexaxandra is running a trial: Topical Imiquimod or Diphenylcyclopropenone for the Management of Cutaneous In Transit Melanoma Metastases – A Phase II Single Centre Prospective Randomised Pilot Study. You can search for the trial using this link.
The aim of the study is: "To compare two different non-invasive topical immunotherapies in patients with multiple, in transit, cutaneous melanoma metastases. The hypothesis is that these treatments are at least as clinically effective as current therapies for patients with selective in transit melanoma metastases and can be used to enhance patients’ quality of life and decrease health-related costs."
Intransit disease presents in a number of different ways. For patients with small dermal deposits, physcians there are unable to treat them with PV-10 because the lesions are too small to be injected. These patients then are treated with diphenylcyclopropenone ("DPCP"). The trial being proposed would compare topical imiquimod (i.e., a cream or gel) and DPCP. The trial is not yet open to recruitment.
Patient use of PV-10 has been incorporated into this trial's proposed protocol. See below (fuzzy purple emphasis is mine): "comparative treatments."
ILI refers to isolated limb infusion, and is a regional technique for in-transit melanoma that "involves temporarily isolating the blood supply to an extremity to concentrate chemotherapy treatment there." ILI is in NCCN guidelines for the treatment of cancer (see Version 3.2015 below).
In situ vaccination (October 20, 2015)
Updated below.
From December 2014:
The phrase in situ vaccination conveys the concept of using a cancer patient's tumor (upon suitable or appropriate treatment of the tumor) to generate a lasting immune response in him or her. "In situ:" in its original place. "Vaccination:" having developed adaptive immunity.
From SITC 2012 (November 2012), three years before:
Updated (10/21/15): Further to this news item and the 10/20/15 update of Combination continuations (October 18, 2015) below, will 2016 be the year of in situ vaccines (or other kinds of agents that provoke anti-tumor immunity)?
Pharmaceutical industry consultant (and former Big Pharma clinical researcher) Dr. Sally Church, PhD wrote another article (on LinkedIn) titled Quit whining, get thinking!, which is associated with her subscription-based article entitled Why combine raditiation with immunotherapy? While acknolwedging the expense and ineffectiveness of immune checkpoint inhibitors, she seems to be evolving her views towards Provectus Chairman and CEO Dr. Craig Dees, PhD by writing:
Recall that many immune checkpoint inhibitor fans believe cancer tumor shrinkage is the successful byproduct of doing something right to the immune system. That is, release the immune system’s so-called brakes that block CTLA-4, PD-1, PD-L1, etc., thereby enabling T cells to fight the tumor. Craig, on the other hand, thought that harnessing the immune system in the right way to fight cancer was the successful byproduct of properly destroying the tumor.
The subject of Dr. Church's article on her subscription-based blog is NYU School of Medicine's Dr. Sandra Demaria's presentation at ECC 2015 entitled Molecular basis for radiotherapy in synergy with immunotherapy.
Radiotherapy (radiation), like targeted therapies and chemotherapy, according to Step #1 of Chen and Mellman's Cancer Immunity Cycle, release cancer antigens. Medscape France's Potentiate radiotherapy immunotherapy: a seductive strategy (translated) by Dr Isabelle Catala discusses Dr. Demaria's presentation:
Combination continuations (October 18, 2015)
Updated below again.
Three continuation [patent] applications, continuations of Provectus' already approved joint patent with Pfizer entitled Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (U.S. patent #9,107,887), appeared on the U.S PTO website on October 15th:
Updated (10/20/15): Some additional thoughts on the continuation applications (related to the combination therapy patent initially co-achieved with Pfizer) filed by Provectus.
1. A systemic inhibitor of immune system down-regulation. Neither the patent itself (patent no. 9,107,887) nor the relevant continuations (patent application nos. 20150290165 and 20150290318) are sufficiently detailed about what comprises a systemic inhibitor of immune system down-regulation, other than to protect [in the continuations] "a systemic inhibitor of immune system down-regulation comprises anti-CTLA-4 antibodies," which are described in more detail as "including ipilimumab and tremelimumab" [in the patent].
There was no mention in the filed continuations (as there was no mention in the granted patent itself) of PD-1 or PD-L1 or other categories of immune checkpoint inhibitors. Further, there was no any of the members in these categories: e.g., PD-1: nivolumab and pembrolizumab, PD-L1: avelumab and durvalumab). Where is the specific mention of these categories and their members of systemic inhibitors of immune system down-regulation?
Patent applications and continuations, prior to approval or granting, appear on the US PTO's Patent Application Database webstite and its Patent Application Information Retrieval website ("PAIR" website) after some amount of time following submission of the application/continuation.
Takeaways (and more questions):
Question:
H/t InvestorVillage poster canis_star: The Society for Immunotherapy of Cancer's ("SITC's") Understanding Cancer Immunotherapy patient guide.
N.B. The guide does not mention Provectus' intralesional agent PV-10; however, it does discuss Amgen's intralesional agent/oncolytic virus immunotherapy talimogene laherparepvec ("T-Vec").
SITC's patient guide includes an immunotherapy timeline.
The bottom of the timeline references William Coley, considered by many to be the "Father of Immunotherapy."
The following originally was published as November 1, 2014 blog post Florey, Chain & Heatley⎟Coley⎟sort of, maybe, possibly, conceivably, probably..., with additions made by me in this news item.
▸ William Coley was a pioneer of cancer immunotherapy (late-1800s). According to Wikipedia (see immediately prior link):
Takeaway: Coley injected the bacteria/vaccine/toxins into cancerous tumors and cancer metastases.
▸ Why then, when writing about cancer immunotherapy and associated approved and investigational checkpoint blockade drugs like Yervoy (anti-CTLA-4, Bristol-Myers), Keytruda (anti-PD-1, Merck), Opdivo (anti-PD-1, Bristol-Myers), MPDL3280A (anti-PD-L1, Roche/Genentech), MEDI4736 (anti-PD-L1, AstraZeneca/MedImmune), etc., do some folks include what seems like obligatory yet obtuse nods to Coley? Google, for example, coley immunotherapy pd-1.
Coley's vaccine and these inhibitors both engage the immune system. But although the mechanism of action of Coley's vaccine (or Coley's toxins as it also was known) was and remains unknown, it is believed the approach led to specific and non-specific immune responses (paragraph source/sentence taken from: Rational approaches to human cancer immunotherapy, Davis et al.). Interestingly, according to Davis et al.'s article, Coley's injected-bacteria-into-accessible-tumors-and-metastases was, "[a]s late as 1934, “Coley’s toxins” was the only known systemic treatment for cancer" (article footnote). {Underlined emphasis is mine.} A treatment injected into tumors that generates an immune response is called a systemic treatment for cancer. Interesting...
Checkpoint blockade therapeutic agents are non-specific immunotherapies. "Non-specific immunotherapies don’t target cancer cells specifically. They stimulate the immune system in a more general way..."
2015 Lasker Award winner Dr. James Allison, PhD[11], whose research eventually resulted in the development of immune checkpoint inhibitor and systemic immunotherapy ipilimumab, believed cancer tumor shrinkage was the successful byproduct of doing something right to the immune system. That is, releasing the immune system’s so-called brakes that block CTLA-4, thereby enabling T cells to fight the tumor.
Takeaway: CTLA-4s, PD-1s, PD-L1s, etc. are non-specific immunotherapies.
▸ "According to a 1965 article that was published in A Cancer Journal for Clinicians (1):
“In 1952, a bibliography of the literature, and, in 1953, a report on 30 inoperable cases which had been treated by Coley's mixed toxins and had survived thereafter for periods of from 1 to 47 years (20 cases had a survival of over 20 years) were published. The report is said to be based on a comparative analysis of over 1,200 cases treated with Coley's toxins, and 300 cases in which intercurrent infections played a part. Over 270 cases were said to have shown complete regression of the tumor, but the 30 inoperable cases were selected for the report because the diagnoses had been confirmed by microscopic examination, and some information on their subsequent history was available. Of the 30 tumors, 7 were classified as carcinoma, 19 as various types of sarcoma, 2 as malignant melanoma, and 2 as giant cell tumors.”
A complete response rate of 22% (270 out of 1200) was impressive by the standards of the 1960's and today. But, despite these results, the article states that the, “American Cancer Society has found no evidence that treatment with Coley's mixed toxins results in any objective benefit in the treatment of cancer in human beings.” It is difficult to reconcile this conclusion with the results cited in the same article. Perhaps the results were simply not believed as they were authored by Mrs. Helen Coley Nauts, Executive Director of the New York Cancer Research Institute. Mrs. Nauts was the daughter of William Coley." (paragraph source link) {Underlined emphasis is mine.}
Takeaway: Coley's approach generated notable complete responses.
▸ Which brings us to a chicken-and-egg question. Which comes first, the complete response, or the immune response? For PV-10, successfully generating a complete response leads to a good to great immune response.
Per medical writer Walter Alexander's recent article PV-10 in Metastatic Melanoma: Rapid Responses Led Phase 3 (he's written about PV-10 before, including PV-10 Moves Forward):
Dees thought that harnessing the immune system in the right way to fight cancer was the successful byproduct of properly destroying the tumor.
Dees et al. believed intratumoral or intralesional injection of the right compound into cancer tumors or lesions (local delivery), rather than oral or intravenous administration (systemic delivery), would deliver the right information in the right format and the right way to the immune system.
Takeaway: PV-10 generates a complete response in order to generate/which is followed by an immune response.
▸ Forbes contributor Jon Fortenbury wrote about PV-10 and Provectus in a post entitled A New Cancer Drug Worked In Over 50% Of Patients In A Phase II Trial. He subsequently updated the post to "...to include comments from an outside expert and the company's response to his criticism." Forbes staff member and editor of the section Matthew Herper later weighed in under the Comments section with:
Bristol-Myers issued a press release about PD-1 Opdivo's results for heavily pre-treated advanced squamous cell non-small cell lung cancer, generating a a Bloomberg headline: Bristol-Myers Immune Drug Improves Lung Cancer Survival:
Certainly what was notable was the improved overall survival (the quote's "the advanced lung cancer patients taking Opdivo were alive after a year on the drug") compared to historical survival figures.
As for Herper, other journalists, commentators and opiners, aside from better survival figures that are of course very good things (longer survival is better than no or shorter survival), why do they not mention or say in the same breath that generations of immunco-oncology therapeutics agents that have come (Yervoy), are here (Keytruda, Opdivo), and are to come (MPDL3280A, MEDI4736) have not had/do not have notable or memorable complete responses?
Takeaway: Partial response, good. Complete response, best.
▸ To be fair, Herper, who probably has covered biotechnology companies and the subject of oncology longer than I've held Provectus shares, and who also believes this is biology's century, may not understand the conflation Fortenbury's article, upon further editing and insertion of the "outside expert's" quote, managed to achieve.
The article described Provectus' melanoma Phase 2 trial results and, in particular, the sub-group of patients who had all of their disease treated (i.e., injected with PV-10). Why was/is this relevant? As Fortenbury writes, "If PV-10, when injected in all the cancerous lesions of a melanoma patient for longer than 16 weeks, goes on to produce positive results in the next phase, it just may be a viable treatment option for many patients with aggressive, late-stage, locally advanced melanoma." The target population of the company's upcoming pivotal Phase 3 trial are patients with un-resectable locally advanced cutaneous melanoma, which means the melanoma (i) cannot be removed by surgery (non- or un-resectable), (ii) is located in or just under the skin (cutaneous and subcutaneous, respectively), and (iii) has not metastasized to distant sites like the lungs, liver or brain.
These patients need better options, like Eric, "...patients clearly need more options: single agent options and more options for finding successful combinations that can truly change the course of this vicious disease," and Dr. Agarwala, "I think it will be an option for many patients who have a cancer disease that’s localized or regional," were quoted in the Forbes article.
Takeaway: Locally advanced cutaneous (or subcutaneous) melanoma = Melanoma that has not spread or metastasized.
This is worth repeating: The upcoming pivotal Phase 3 trial will comprise Stage III patients. The disease in Stage III has not spread. Patients in which the disease has spread are Stage IV patients.
Takeaway: Stage III ≠ Stage IV.
▸ Conflation in the article occurs when Herper asks Fortenbury to have an "outside expert" opine: PD-1 Keytruda clinical investigator and UCLA Jonsson Comprehensive Cancer Center medical oncologist with multiple clinical interests Dr. John Glaspy, M.D., MPH is quoted. Medical oncologists (like Glaspy and PV-10 principal investigator Agarwala) see mainly stage IV patients, while surgical oncologists (like PV-10 principal investigator Dr. Merrick Ross, M.D. from MD Anderson Cancer Center ) see mostly stage III patients.
The data Fortenbury's article highlights show PV-10 as a monotherapy could be an important option for patients with locally advanced cutaneous melanoma. The FDA also appears to be considering this topic too. It announced on October 8th that "melanoma, specifically unresectable loco-regional disease" was a disease candidate for public comment. See Prescription Drug User Fee Act Patient-Focused Drug Development; Request for Comments (October 8, 2014) on the blog's News page.
Takeaway: Locally advanced cutaneous melanoma = Stage III = Melanoma disease has not spread ≠ Stage IV = Metastatic melanoma with visceral disease and heavy tumor burden.
▸ Glaspy's words in the article:
Yervoy, Keytruda, Opdivo, MPDL3280A and MEDI4736 are far from curing melanoma patients because they are not achieving notable or memorable complete responses.
The melanoma component of Provectus’ clinical development program has two fundamentally different pathways to approval. In April 2015 the company commenced its pivotal Phase 3 trial of PV-10 versus systemic chemotherapy in patients with unresectable locally advanced melanoma (Stage III patients). The trial’s hypotheses are two-fold: whether complete response of injected tumors is tantamount to elimination of disease symptoms and whether PV-10 can forestall or prevent the spread of the disease from Stage III to Stage IV. If you make the tumor go away, don’t the symptoms go away too? Success with Stage III patients, and consequently with earlier stages of melanoma, Dees et al.’s vision was to replace a surgical oncologist’s scalpel with a Rose Bengal needle.
In September 2015 Provectus initiated a Phase 1b/2 trial program combining PV-10 with an immune checkpoint inhibitor (Keytruda) in patients with advanced melanoma (Stage IV) by filing the associated study protocol. Late-stage disease indicates with tumors typically inaccessible to injection. A PV-10-checkpoint inhibitor combination presents the medical oncologist (like Glaspy) with a more effective approach to reducing tumor burden (PV-10 where accessible by injection, a checkpoint inhibitor where inaccessible to injections) until the immune system can re-establish itself to finish the job Mother Nature intended it to do.
Takeaway: Partial response, not a big deal. Complete response, cured?
▸ One simple but I don't believe an over-simplistic way of looking at the situation could be surgical oncologists look at melanoma (and cancer at large) when it is first diagnosed or has not spread beyond control or has not metastasized. Medical oncologists look at melanoma (and cancer at large) when it has spread or looks like it is beyond control.
Takeaway: Surgical ocologists: PV-10. Medical oncologists: PV-10 + something else.
▸ Eric, in regards to, I believe, late-stage (Stage IV) patients (patients with distant metastases), in the Forbes article, "...more options for finding successful combinations that can truly change the course of this vicious disease," undoubtedly would have told Fortenbury about Moffitt Cancer Center presenting pre-clinical data on these combinations (i.e., PV-10 + [insert checkpoint blockade agent]) at the annual meeting of the Society for Immunotherapy of Cancer ("SITC") next week.
The idea behind combining PV-10 and a checkpoint blockader may be to generate both the specific and non-specific kinds or types of immune responses Coley's vaccine/toxins were believed to have generated. The goal of employing these kinds of combinations, to battle melanoma (cancer) once it has spread to distant sites, may be to generate both specific and non-specific immune responses.
Takeaways: Stage IV melanoma: PV-10 + one of (Yervoy, Keytruda, Opdivo, MPDL3280A, MEDI4736), Yervoy, Keytruda, Opdivo, MPDL3280A, and MEDI4736 need help.
▸ Roche's Genentech's Dr. Daniel Chen, M.D., Ph.D. quoted Roche's Genentech's Dr. Ira Mellman, M.D., both co-authors of Oncology Meets Immunology: The Cancer-Immunity Cycle, at ESMO 2014:
Takeaway: The ultimate goal of cancer treatments is to completely eliminate a tumor. Complete response.
▸ Which brings me back to where I started this post, with Florey, Chain & Heatley, and Coley.
Dees, Scott and Wachter did not discover Rose Bengal. It can be traced back to Basel, Switzerland in 1882 when a German patent was granted to Ghnem for a new family of wool dyes. Dees et al. were not the first to use Rose Bengal in an oncology setting. Japanese researchers Ito and Watanabe did so in 1986. The compound’s therapeutic benefits remained hidden until 1986 when it was given to mice by Ito and other Japanese researchers while investigating whether red food dye No. 105 (also made from Rose Bengal) caused cancer. Instead, they observed dose-dependent survival increases in the mice. The researchers, however, did not advance their observations into formal cancer studies and trials of the compound.
Craig postulated intratumoral injection, and thus the delivery of the drug via the tumor or lesion, was key. He thought it was critically important to the eventual immune response that PV-10 (Rose Bengal) be injected into a cancerous tumor or cancer metastases.
What kind of therapy is PV-10? From a 2013 Cancer Watch article entitled Back to Phase 1: Understanding Systemic Effects of PV-10:
CTLA-4, PD-1 and PD-L1 therapeutic agents are not specific enough. They do not facilitate the expression of enough antigens.
PV-10 ablation of tumors presumably leads to the generation of antigens that are presented to the immune system in (i) as large a number as possible, (ii) pristine and not un- or non-denatured shape, (iii) whole pieces and not fragments, (iv) correct conformation/the right shape, and (iv) the right context.
Rose Bengal (PV-10) of course is not a biologic, like Coley's dead bacteria. As a non-biologic, its two-prong approach to fighting cancer (dual mechanisms of action) derives from its physical chemistry.
Dees et al. have spent the better part of two decades educating much of the biopharmaceutical industry and its ecosystem about the merits of their approach to fighting cancer. Dees frames therapeutic outcomes of Rose Bengal use in the context of reproducibility, whether preclinical or clinical, study or trial, generated by the company, a clinical investigator or a third-party researcher, affiliated or unaffiliated with Provectus. He reminds that reproducibility the hallmark of Western science. If a scientist or researcher cannot repeat the outcome of an experiment, and another researcher or scientist cannot replicate the result, one should be skeptical about the veracity of the original work and claims made from it.
Rose Bengal’s track record of reproducible therapeutic features as well as preclinical and clinical results by multiple parties in multiple cancer indications in multiple species is noteworthy. Different research entities separately and independently reproduced Dees et al.’s two-prong cancer killing approach of, initially or first, tumor ablation and, subsequently or second, tumor-specific immune responses in multiple solid tumor cancers (in preclinical mouse models).
If they are ultimately successful in demonstrating Rose Bengal’s therapeutic benefits to the satisfaction of the FDA (and a Big Pharma acquirer at a commensurate valuation), Dees, Scott and Wachter’s legacy must include their steadfastness in pursuing their own philosophy for defeating the disease in the face of the regulator and industry that did not readily understand, embrace or believe the idea that a local agent could deliver meaningful, systemic, clinical benefit to cancer patients.
Takeaway: I imagine Rose Bengal/Dees et al. are more Coley's heirs than anything/anyone on SITC's immunotherapy timeline.
CD4+ > CD8+ (October 16, 2015)
H/t InvestorVillage poster FortStCowboy and another blog reader/shareholder: Roswell Park Cancer Institute (RPCI) issued a press release on October 15th entitled Special Class of T Cells Shown to Both Attack Cancer Cells and Enlist Other Immune Cells.
Ciao (October 13, 2015)
Provectus' CTO Dr. Eric Wachter, PhD will be speaking at the Third Melanoma MIB Conference in Rome, Italy in November. His presentation is entitled PV-10: a new opportunity in Europe from Provectus.
Reports from the first and second MIB conferences are here and here, respectively.
As pervasive as aspirin (October 11, 2015)
Several years ago, Provectus' CTO Dr. Eric Wachter, PhD extemporaneously said (paraphrasing) he'd liked to see PV-10 eventually be used as pervasively as aspirin. As such, it was with no small amount of irony to initially read the title of Moffitt Cancer Center's PV-10-related SITC 2015 abstract title, Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1. See SITC 2015 Preview: Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1 (October 5, 2015) and HMGB1 (October 6, 2015).
A Google News search of HMGB1 (as of this writing) yields several articles about a recent study of salicylic acid targeting HMGB1. Salicylic acid is the active ingredient of aspirin. The study, entitled Aspirin′s Active Metabolite Salicylic Acid Targets High Mobility Group Box 1 to Modulate Inflammatory Responses, noted in its abstract:
Thus, for now, we have:
Following up on Provectus' CTO Dr. Eric Wachter, PhD comments regarding a prospective, potentially pivotal, psoriasis Phase 3 trial, see the items below. Also see PH-10 (October 7, 2015) below.
Note PH-10 in the list below.
Comparing some of the late-stage development compounds to a potential Phase 3 PH-10 study:
PH-10 (October 7, 2015)
Provectus' CTO Dr. Eric Wachter, PhD and COO/CFO Peter Culpepper provided a status update on PH-10 via an interview with/article by BioPharm Insight (dated October 2nd). Several items from the interview/article struck me, including:
Takeaways:
SITC 2015 Preview: Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1 (October 5, 2015)
Updated below again.
"High-mobility group protein B1, also known as high-mobility group protein 1 (HMG-1), is a protein that in humans is encoded by the HMGB1 gene."
High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal, Lotze et al., Nature Reviews Immunology 5, 331-342 (April 2005)
> New study provides key insights into aspirin's disease-fighting abilities (September 2015)
At AACR 2014 Moffit, which has conducted murine model and clinical work on PV-10, wrote:
HMGB1 is important for activation of dendritic cells (DCs). DCs are antigen-presenting cells that process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and the adaptive immune systems.
Status update (October 5, 2015)
In July Provectus issued press release Provectus Biopharmaceuticals, Sinopharm-China State Institute of Pharmaceutical Industry and Sinopharm A-Think Pharmaceutical Co., Ltd Continue Search for Agreement on PV-10 Use in China to describe the then status of the talks between the parties related a regional geographic license of PV-10. From the PR:
Aeterna's regulatory filings do not disclose any transaction information. See below.
Network 1 Financial is acting as Provectus' "investment bank" in discussions and negotiations with Sinopharm. According to two individuals with knowledge of the situation (from two different perspectives) but who weren't authorized to speak publicly about the negotiations, payments (upfront, milestones) and royalities appear in the range of the Oramed/Sinopharm transaction (excluding the purchase of stock) for the same indications Provectus agreed to with Boehringer Ingelheim (China) (i.e., melanoma and cancers of the liver).
The Usual Suspects (October 4, 2015)
Updates in Melanoma 2015: Entering a New Therapeutic Era (registration required)
Friday (October 2, 2015)
Updated below again.
1. Special proxy vote. Provectus filed an 8-K in regards to the special proxy meeting held yesterday for the proposed increase in the company's authorized number of shares. The proposal passed with an 82% "for" vote (63% of votes were cast).
2. September Blog Stats. Blog readership fell from August 2015 depending on the statistic. I wrote 20 blog posts (3) and news items (17) in September versus 23 the previous month (6 and 17, respectively). Month-over-month changes were:
Bounce rate and pages per session metrics that changed on or around July 8th generally maintained their "trend" through September. Quarter-over-quarter changes are below:
3. Data availability. Clinical data from the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma would be available on a periodic basis (i.e., as and when regularly collected) to prospective Big Pharma partners under CDA (confidential disclosure agreement) with Provectus. Data would comprise an interim data readout, and also regular data looks for safety and efficacy. As an open label trial, it is presumably known which patients received the control drug (systemic chemotherapy) and which patients received the treatment drug (PV-10). This aspect of the clinical trial process (i.e., clinical data management, and data sharing) spurred me to think about the survivorship bias of the Feuerstein-Ratain Rule.
Are investors (collectively) that accurate when it comes to identifying cancer drugs destined to fail Phase 3 studies [copting a statement from Feuerstein's article sourced above]? Or, are companies with good drug compounds in Phase 3 acquired in advance of reporting interim data or [more than four months] prior to releasing final data (and thus not included in Feuerstein-Ratain Rule dataset), leaving low market capitalization companies with uninteresting data (which are left because Big Pharma, having potentially seen their data, are uninterested, and are thus have low market caps for a reason)?
Updated (10/3/15): 4. Lawsuit. H/t InvestorVillage poster Area51: Attorneys for both the defendants and plaintiffs filed a joint motion to stay proceedings pending mediation.
Updated (10/3/15): 5. Heard, paraphrasing.
i. Boehringer Ingelheim (China, presumably) introduced Provectus to Chinese biotechnology company Beigene, which recently entered into a Phase I study of BGB-A317, Beigene's anti-PD-1 compound. In September 2014 Boehringer Ingelheim and BeiGene announced a supply/manufacturing agreement whereby Boehringer Ingelheim BioXcellence would assist Beigene to advance its product candidates through the clinic. See Commercially Reasonable Efforts (July 18, 2015) and BioXcellence™ (July 9, 2015) below. Key opinion leader and Memorial Sloan Kettering Cancer Center's Dr. Jedd Wolchok, MD, PhD was added to Beigene's scientific advisory board in September 2015.
Item takeaways:
The balance between co-stimulation and co-inhibition is described by Inman et al.: "If sufficient co-stimulation is provided in the presence of adequate tumor-associated antigenic stimulation, the immune system will act against tumor antigen and, thus, destroy early tumors before they become fully established. Contrarily, if co-inhibitory signaling dominates, the immune system will be tolerized to tumor antigens, and the tumor will be permitted to grow unfettered and unmolested by the immune system. If neither co-stimulatory nor co-inhibitory signals dominate, the adaptive immune system may remain in a tenuous state of equilibrium, militating against tumor outgrowth with varying degrees of success."
So, it would seem to me, generalizing (or simplifying, perhaps too much):
iii.a. Certain Provectus management team members do not appear inclined to update the ClinicalTrials.gov page of Provectus' pivotal melanoma Phase 3 trial, or update the webpage in a timely fashion. For example, as of this writing, the page indicates Atlantic Health System's (AHS') site (and investigator Dr. Eric Whitman, MD) for the the company's trial is not yet recruting patients.
AHS' website, however, indicates it is open to enrollment (h/t InvestorVillage poster NickSanta):
Comparing Provectus' situation (i.e., ClinicalTrials.gov v. AHS) to another AHS/Dr. Whitman trial, open to enrollment appears to be equivalent to recrutiing.
iii.b. According to management, an unstated number of "high profile" medical institutions and clinical investigators who have treated/are treating patients with PV-10 have not wanted/do not want the names of their institutions and themselves to be identified on PV-10-related ClinicalTrials.gov webpages.
iv. Management continues to affirm the timing of an interim data readout of Provectus' pivotal melanoma Phase 3 trial as July 2016.
v. In addition to Australia and elsewhere in the U.S., melanoma Phase 3 trial sites would include locations in Brazil, Mexico, Germany and Poland. Sites are expected in India and China, but not until after the aforementioned sites in Australia, South and North America, and Europe are added.
vi. Provectus' melanoma Phase 3 trial design (and its undertaking) is as good as one with a special protocol assessment (SPA) but without the SPA's restrictions.
Is something wrong? (September 29, 2015)
Amgen's intralesional agent (IL) talimogene laherparepvec ("T-Vec") and Provectus' PV-10 are two late-stage oncology assets that, when partnered with an immune checkpoint inhibitor, may prime or activate the immune system.
In April a joint meeting of the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC") and Oncologic Drugs Advisory Committee ("ODAC") voted by an overwhelming 22-to-1 margin in favor (i.e., yes to the question) of T-Vec having an overall favorable benefit-risk profile for the treatment of injectable regionally or distantly metastatic melanoma, and thus supporting traditional approval of the drug. See the blog's April 29, 2015 post The first guy through the wall. A key moment for T-Vec, and for the category of IL agents, was when the panel concluded the reduction of injected tumors equated to/was clinical benefit.
Amgen currently is engaged in two combination therapy trials of IL agent T-Vec and an immune checkpoint inhibitor in advanced (Stage IV) melanoma. See IL-CIB Combinations (September 26, 2015) below.
Given the overwhelming margin of the CTGTAC/ODAC vote above, one would imagine the FDA would approve T-Vec, despite potential concerns over T-Vec's pivotal Phase 3 trial's questionable primary endpoint of durable response rate and questionable trial comparator of GM-CSF, and the issue of viral shedding (T-Vec is a herpes simplex virus type 1).
But, is there something wrong with T-Vec/Keytruda combination therapy trial? Amgen did not report efficacy data from the trial at the 2015 European Cancer Congress. Only safety data was reported.
The Phase 1b T-Vec/Keytruda trial itself already was odd in regards to the set-up of the lead-in period for T-Vec. The IL agent regimen began on Day 1, Week -5, and the systemic immunotherapy regimen started on Day 1, Week 0. Week -5?
The data cutoff for the trial was 6 weeks after the last patient had his or her first pembrolizumab dose. Patient enrollment was completed by March 2015. The abstract submission deadline for this late-breaking abstract was August 5th. Why was no efficacy data presented? There should have been time the data cutoff to include some efficacy data and comments onto the poster.
There are mouse analogs for an anti-CTLA agent. Provectus, for example, utilized one when they examined the combination of PV-10 and anti-CTLA-4 in melanoma at AACR 2013 (i.e., hamster anti-murine CTLA-4 anti-body 9H10). There may be no good "mouse" analog for T-Vec. Amgen utilized a surrogate called OncoVEXmuGM-CSF when they presented pre-clinical work on the combination at AACR 2015. In this case (i.e., the AACR presentation), it isn't the complete regressions of injected tumors that is important as much as the complete regressions (80%) of the uninjected ones (20%), and the level of immunologic signaling achieved.
Maybe T-Vec doesn't work in combination with Keytruda as well as it did in combination with Yervoy.
As an aside, Dr. Long, the presenter of the above abstract, is/was a consultant to Provectus.
"Intent to use" (September 26, 2015)
H/t a shareholder (a regular tipper of hats for this blog): It appears Provectus made a Trademark/Service Mark Application for PV-10 in June. See a screenshot of the webpage below.
The application appears to have a 1B filing status. See a screenshot of the webpage below.
Information regarding "1B (Intent to Use):"
The two late-stage intralesional compounds, Amgen's T-Vec and Provectus' PV-10, have been and are being combined with two of the three approved immune checkpoint inhibitors (Bristol-Myers' ipi, Merck's pembro and Bristol's nivo) in early-stage trials for patients with advanced melanoma:
A table comparing selected trial parameters of the above studies is below (I selectively noted certain parameters for comparison in yellowish-orange):
Table takeaways:
PV-10 + [insert name here] (September 23, 2015)
Updated below again.
Provectus filed its combination trial protocol on ClinicalTrials.gov, PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma.
Updated (9/23/15): Some initial thoughts (with more to follow):
Radiation (September 22, 2015)
Local (localized) or systemic radiation therapy (as a monotherapy) is a tool for oncologists to treat cancer. Radiation in one form or another can kills cancer cells, but it also kills healthy cells. See, for example, the Radiation Therapy for Cancer webpage on the National Cancer Institute's website.
"However, only recently this property of radiation has attracted the attention of immunologists seeking to induce or improve antitumor immunity. As immune checkpoint inhibitors are becoming mainstream cancer treatments, radiation oncologists have begun to observe unexpected out-of-the-field (abscopal) responses in patients receiving radiation therapy during immunotherapy. These unexpected responses were predicted by experimental work in preclinical tumor models and have clear biological bases. Accumulating experimental evidence that radiation induces an immunogenic cell death and promotes recruitment and function of T cells within the tumor microenvironment supports the hypothesis that radiation can convert the tumor into an in situ individualized vaccine." (Source: Combination of radiotherapy and immune checkpoint inhibitors, Pilones et al., Semin Radiat Oncol. 2015 Jan;25(1):28-33.) {Italicized emphasis is mine} The "abscopal effect" (above) refers "...distant tumor regression after localized irradiation."
What is the goal behind combining co-inhibitory blockage agents (i.e., immune checkpoint inhibitors) and radiation therapy? "[T]he tumor-antigen release achieved by localized radiation will promote specific tumor targeting by the adaptive immune system, which can be augmented further by systemic immune-stimulating agents. In this manner, clinicians hope to induce a phenomenon known as the abscopal effect, whereby localized radiation results in immune-mediated tumor regression in disease sites well outside of the radiation field." (Source: Combining Radiation and Immunotherapy: A New Systemic Therapy for Solid Tumors?, Tang et al., Cancer Immunol Res September 2014 2; 831) {Italicized emphasis is mine}
In order for immune checkpoint inhibitors like Keytruda and Opdivo to remain relevant, they will be combined with other therapies and therapeutics, with the resultant toxicity and cost having to both be managed in some way. See also Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer, Twyman-Saint Victor et al., Nature 520, 373–377 (16 April 2015). Note: Radiation was administered first, followed by co-inhibitory blockade. Is the idea, as noted above, that [localized] radiation reduces or destroys tumors, thus generating antigens/antigen fragements upon which the immune system may act, as the immune checkpoint inhibitor releases the breaks on the immune system?
PV-10 & Radiation Therapy. Radiation therapy has been combined with PV-10 in two situations. First, in 2010, Foote et al. reported on their combination work on 3 patients in A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series, Melanoma Research, 20:48, Jan 2010. All of the patients originally were enrolled in Provectus' metastatic melanoma Phase 2 trial (I believe). All patients achieved complete response (CR) (note detailed descriptions in the article's text); however, no discussion was provided regarding survival (that is, keep in the mind the difference or differentiation between [objective] response rate and overall survival). Note: PV-10 was administered first, followed by radiation.
Later, in 2013, Tan and Neuhaus reported on their combination work on 2 patients in Novel use of Rose Bengal (PV-10) in two cases of refractory scalp sarcoma, ANZ Journal of Surgery, 83:1-2:93, Jan 2013. Both patients were treated in the company's compassionate use/expanded access program (I believe). One patient achieved a complete response, while the other enjoyed "good" clinical effect before progressing again. Also, no discussion was provided regarding survival of the patient achieving CR. Note: Here, radiation was administered first, followed by PV-10.
Note that in the contemplated combination therapy trial of PV-10 and co-inhibitory blockade (immune checkpoint inhibition), PV-10 would be administered first, several weeks before the checkpoint inihibitor would be given to the patient.
Provectus' founders Drs. Craig Dees, PhD, Tim Scott, PhD and Eric Wachter, PhD have long posited that all patients really need is PV-10, under the key assumptions that (i) most if not all of their disease is accessible to injection and (ii) tumor burden is manageable (i.e., not too heavy).
Injecting PV-10 into most or all of the patient's tumors harnesses the immune system via/because of the tumor cell, not inspite of it; see Allison vs/& Dees (September 11, 2015) below. From Provectus' 2013 white paper PV-10 Moves Forward by medical writer Walter Alexander:
In 2010, Foote and others (Smithers et al.) began a clinical study entitled A Phase II, open-label, non-comparative study on intralesional PV-10 followed by Radiotherapy in the treatment of metastatic melanoma. The primary objective of the study was/is "to investigate the effectiveness of intralesional PV-10 followed by hypofractionated radiotherapy for the locoregional treatment of metastatic melanoma."
Eric reported on the progress of this Australian investigator-initiated study at ESMO 2012, noting 7 of up to 25 anticipated subjects were enrolled as of July of that year.
The Smither et al. trial continues, but another Foote trial/study appeared to commence in 2011: A phase I/II study of intralesional PV-10 followed by radiotherapy in the treatment of metastatic melanoma? See page 113 of the 2011 annual research report of Princess Alexandra Hospital in Brisbane, Australia. In 2012 Foote changed the objective of another trial/study of his entitled Radiotherapy followed by selective nodal dissection for bulky and/or inoperable nodal melanoma (REFORM) to read: A phase I/II study of intralesional PV-10 followed by radiotherapy in the treatment of metastatic melanoma. See page 103 of the 2012 annual report. The 2013 report reads to the right/above.
I asked Eric about the status update on the PV-10 + radiation therapy study by Foote/Smithers et al. on Provectus' March 12th 4Q14 business update conference call:
There is a theme or narrative about PV-10 that might be accurate and appropriate. PV-10 may be agnostic to tumor type (histologic subtypes) or disease presentation. PV-10 may harness the immune system through/because of the tumor/tumor cell, not inspite of it/them. In a combination therapy setting, you might give PV-10 first (even when that setting includes surgery). Radiation therapy, kinase inhibitors, immune checkpoint inhibitors, etc. may merely help reduce tumor burden in cases where there is disease inaccessible to PV-10 or heavy tumor burden, so that PV-10 can help the immune system finish off the job.
Trying to Meet Expectations One Sets (September 21, 2015)
Provectus issued a press release today and filed an associated 8-K regarding enrollment in its PH-10 mechanism of action study, Completes Patient Accrual for PH-10 Phase 2 Clinical Study of Cellular and Immunologic Changes in the Skin. See PH-10 (September 17, 2015) below. Of note to me was the company's CTO Dr. Eric Wachter, PhD's quote in the PR:
More hilarity, related to the recent (and revisted) narrative of high drug prices as a result of actions by Turing Pharmeucticals:
Sample pricing comparisons, on an absolute dollar basis:
Seeking Alpha (September 21, 2015)
SA published blog post Special Proxy Vote as Provectus Special Proxy Vote today, and also tweeted links to the article on their website.
As agreed, SA included the blog's landing page disclosure and provided a link to the blog's Disclosure page. As background, SA re-approached me in January 2015, again asking to publish blog posts as SA articles under my name. I said yes to them, but would not publish articles or post Instablog posts there myself, nor reply to comments under articles of mine SA published. When SA publishes my blog posts as SA articles under my name, another author typically publishes an article at or around the same time.
Other items on this topic:
According to the study's ClinicalTrials.gov webpage, Provectus' Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10 no longer is recruiting (i.e., the study completed recruiting its patients).
KOL update (September 16, 2015)
Dr. Paul Chapman, M.D., a medical oncologist at Memorial Sloan Kettering Cancer Center and professor of medicine at Weill Cornell Medical College now appears to be a consultant/advisor to Provectus. See a screenshot of disclosures from an August 2015 article below.
This could mean Sloan Kettering might be a trial site for a Provectus Phase 1b/2 trial of the combination of PV-10 and an immune checkpoint inhibitor for patients with advanced melanoma.
H/t a shareholder (from September 10th): Moffitt Cancer Center's Dr. Jeffrey Weber, M.D., Ph.D. — he of the "PV-10 might offer the perfect way to prime the immune system" comment — will become Deputy Director of the Laura and Isaac Perlmutter Cancer Center at NYU Langone, Co-Director of its Melanoma Program and Head of Experimental Therapeutics in November.
Special Meeting/Proxy Vote, part 4 (Saturday version) (September 12, 2015)
Provectus filed a preliminary Schedule 14A on August 21st and a definitive 14A on September 1st seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. Fully diluted shares outstanding at July 31st stood at ~294 million. The special meeting of stockholders the proxy requires takes place on October 1st. I previously wrote I would revisit this topic each Friday on the blog's Current News page until I cast our votes, which will be at least 48 hours before the meeting date and time.
I plan to vote our shares in favor of the proposal, and thus in favor of management. I'll blog our ballot and rationale in due course.
American entrepreneurship, the version where the rule proves the exceptions, is gritty, tough, dirty, full of ups and downs and highs and lows, persistent, consistent, gutwrenching, incoherent, resilient, inconsistent, replete with brilliant, befuddling, smart, dumb, arguably right and wrong decisions, and periodically presents multiple opportunities to quit. This version is especially circuitous when David knows (not thinks, not believes) he can beat Goliath, but has to learn some of how to do it correctly along the way.
Other news items on this topic:
Updated below.
Anti-CTLA-4 agent/mechanism discoverer and immunologist Dr. James Allison, PhD — he "...was the first to show that antibody blockade of a T-cell inhibitory molecule (known as CTLA-4) could lead to enhanced anti-tumor immune responses and tumor rejection" — provided a keynote address at Immune Profiling in Health and Disease conference today in Seattle, Washington. Luke Timmerman (Timmerman Report, @ldtimmerman) live tweeted several comments from Allison. Alison recently won the 2015 Lasker Award for clinical medical research for his anti-CTLA-4 work.
Generally speaking, Provectus' Chairman, CEO and co-founder Dr. Craig Dees, PhD, posits (as I see and understand it) (among other things) that one has to deal with the immune system upfront by confronting (attacking and completely killing) the tumor, doing it in vivo, and understanding one doesn't know what one doesn't know with respect to immune system mechanisms (including antigen hunting). In some and certain respects, Alison (and ipilimumab [as well as other earlier immunotherapy precursors]) and Amgen's intralesional immunotherapeutic agent T-Vec have paved the way for Craig and PV-10.
Route of delivery, how one kills the tumor, and how well one kills it all contribute to the level of a cancer agent's efficacy, and thus the level of generation of system-wide immunity. Tumors matter. In fact, a tumor could be considered a patient's "friend" (assuming the patient's phyician has the right therapeutic in hand to leverage/benefit from such "friendship").
Objective response rate (ORR) does not matter nearly as much as complete response rate (CRR), where ORR = CRR + partial response rate (PRR). What exactly are ipi, pembro, nivo, etc.'s CRRs? See slide no. 9 from May 13, 2014 blog post Provectus Biopharmaceuticals.
Yes, specificity matters. How specific can systemically delivered therapeutics ultimately be? Indeed, how specific are ipi, pembro, nivo, etc.? See Tumor [immune] response vs. Tumor-specific [immune] response (July 31, 2015) on the blog's Current News page.
Exactly.
Updated (9/11/15): See graph below.
Enough (September 9, 2015)
In my view, one of the fulcrums for voting to approve Provectus' board of directors' proposal/recommendation to increase the company's authorized number of shares is thinking [from here on out] Provectus does not need to raise cash until after an interim analysis readout of its pivotal melanoma Phase 3 trial of PV-10 vs. systemic chemotherapy for patients with unresectable locally advanced cutaneous melanoma (Stage III cancer).
This would lend credence to the belief the additional shares merely are share tools should the company's COO/CFO Peter Culpepper require them for his business and corporate development efforts.
The assumption regarding raising cash would be that it was/is mandated by (i) Provectus' accounting firm BDO to maintain the firm's going concern opinion of the company and/or (ii) the New York Stock Exchange to maintain the company's listing on the NYSE MKT. I am sure there are gray and not-so gray criteria each of the BDO and NYSE utilize in their decision-making. I'm also sure there are black-and-white criteria.
Does Provectus have sufficient cash on hand — assuming no infusion(s) of non-dilutive monies from collaborations, co-developments or licenses — so as not to have to raise cash until after an interim analysis readout? I think so.
Consider the company's balance sheet as at June 30, 2015:
After backing out the amount of net proceeds from the Maxim Group-led June offering (the PVCT.WS "IPO" of warrants on common stock) and [intangible] asset value of Provectus' patent portfolio, the company's tangible net worth (TNW) as at June 30th was north of $6 million.
I posit the key parameter in determining Provectus' TNW in any given quarter going forward is the balance sheet's line item Cash and cash equivalents. I don't believe B/S line item Total Liabilities will be particularly volatile.
I also don't believe Total Assets, adjusted for B/S line items (i) Cash and cash equivalents and (ii) Patents, net of amortization, will be particularly volatile.
As a result, and assuming an average monthly cash burn of $1.4 million, Provectus' TNW does not fall below $6 million until sometime in 3Q16, which should be after the interim analysis readout of the company's pivotal trial. Net Assets (adj.) in each of the tables below is Total Assets (as adjusted, and I assume starting at almost $5 million) minus Total Liabilities (I assume starting at nearly $1 million).
As Peter notes in the Q and K filings, by "managing variable cash expenses due to minimal fixed costs" Provectus should have flexibility to potentially comfortably extend its cash and cash equivalents such that TNW is well above a $6 million floor. For example, assume a monthly cash burn figure of, say, $1.2 million.
All of which isn't to say BDO and/ the NYSE don't invoke some gray or grayish criteria that forces Provectus and Peter to raise money prior to an interim analysis readout. Barring an earlier positive pivotal trial data readout and/or some form of non-dilutive cash infusion (related to a collaboration co-development or license transaction), however, I think it is possible-to-perhaps even probable the company would have sufficient cash on hand until after an interim analysis readout.
Numbers (September 8, 2015)
Provectus management is preparing their annual CEO letter for 2015, in which I expect the company's CTO Dr. Eric Wachter, PhD to disclose the number of patients treated in the company's compassionate use program (CUP) or expanded access protocol (EAP) for PV-10 for cutaneous or subcutaneous tumors in calendar year 2014. A historical snapshot of the CUP/EAP is included in the company's investor and online corporate presentations (a screenshot of the one from the September 1, 2015 online version is below).
Another way of viewing the CUP through the years:
Depending on the CY2014 CUP figure, what could the narrative(s) be for PV-10?
No (0) or a small number (8) of new CUP patients. With only one PV-10 trial recruiting in 2014 (the since expanded Phase 1 trial for both HCC and liver metastases) and in the face of systemic immunotherapy approvals (Keytruda, Opdivo), a possible or potential takeaway could be PV-10 is not particularly relevant.
A trend-like number (22). With only one PV-10 trial recruiting and in the face of systemic immunotherapy approvals, a possible or potential takeaway could be PV-10 has a place in an oncologist's treatment algorithm for cancer (melanoma) patients.
A significant increase (>50). A possible or potential takeaway of a more than year-over-year doubling of CUP patients might be that PV-10 is more than relevant to the cancer patient and oncology asset landscapes.
Special Meeting/Proxy Vote, part 3 (September 4, 2015)
Provectus filed a preliminary Schedule 14A on August 21st and a definitive 14A on September 1st seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. Fully diluted shares outstanding at July 31st stands at ~294 million. The special meeting of stockholders this proxy requires takes place on October 1st.
I previously wrote I would revisit this topic each Friday on the blog's Current News page until I cast our votes, which will be at least 48 hours before the meeting date and time.
I haven't determined how I will vote our shares yet. If I had to vote today, I would reject the proposal because I don't believe management has done enough to explain why they deserve shareholder approval. They appear to be trying, including but not limited to amending the reasons for their proposal (the proxy statement's Proposed Amendment) to include some process description related to compensation. See Tuesday (September 1, 2015) below, item 3.
The reasons management has given for their proposal are possibilities. They may even be potentialities. Provectus requires more authorized shares (whether they issue them or not) for the company to remain viable and relevant to BDO and the New York Stock Exchange. Becoming unviable or irrelevant under this scenario, however, does not mean the end of the world for shareholders or the end of Provectus, or the end of the potential for a good investment return.
Voting no means communicating I'd settle for a few dollars per share (give or take) in the near- to short-term by hoping that stopping the increase in authorized shares would somehow force management into a sub-optimal deal or monetization (i.e., one that they don't want but have to do).
On the other hand voting yes means I believe there's the potential for garnering more-to-many-more dollars per share at some reasonable time into the future, whether management issued more shares to remain viable and relevant (and subsequently account for that dilution) or chose to do so for whatever intelligent and strategic reason until an interim transaction (or transactions) and/or the end-game could be achieved.
I don't believe my in-the-moment opposition is particularly principled. Given management's dismal record of shareholder value mismanagement, I'm just trying determine (as best I can) if voting yes might result in a higher share price than voting no.
It's been two weeks, and a few more weeks remain before the meeting.
Other blog posts or news items on this topic:
Updated below.
Compassionate use site and Phase 3 clinical trial site University of Louisville now is recruiting patients, about a month after appearing on the trial's ClinicalTrials.gov webpage. See Pivotal Melanoma Phase 3 Trial Site (August 3, 2015) below.
Updated (9/2/15): On another but related topic, as of this writing, the Participating Trials tab of Moffitt Cancer Center's bios of Drs. Vernon Sondak, MD (a paid consultant to Provectus) and Amod Sarnaik, MD (who received research funding, and co-authored poster/paper-related work on PV-10) note Moffitt clinical trial 18158, Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma. Moffitt's Dr. Jonathan Zager, MD is listed as the investigator. See Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part V (July 24, 2015) below.
One or more of our securities (September 2, 2015)
It doesn't make sense to me that Provectus would list a "direct" equity security of its on a Asia-Pacific stock exchange (even at an above market valuation). It's not simple to do. It's seems less than smart to do so, when a more straightforward and cost effective approach might be to communicate better, meet timelines, be more thoughtful about what information to release when, underpromise and overdeliver, etc. — in successfully doing so, it should make it more compelling for domestic and international institutional and retail investors to buy the company stock, on the New York Stock Exchange. It's probably not possible as well if the equity security is tied directly to Provectus stock (i.e., a Provectus common share listed on a foreign exchange denominated in that country's currency).
H/t InvestorVillage poster canis_star: A 2012 JP Morgan pamphlet of sorts entitled Strategic Thinking For Hong Kong IPOs is informative about the pros and cons, and requirements and issues to be addressed.
Thinking back at least a year ago, maybe even longer, I recall (perhaps incorrectly) management contemplating different models for a potential China transaction to maximize geography region value capture, and thus company valuation. One was the traditional license approach of upfront, milestone (regulatory and commercial) and royalty payments (e.g., teens to low-twenties percentages of gross revenues). Another was a joint venture (JV) with the prospective marketing/distribution partner (Provectus, while an independent company, was never going to turn over manufacturing to the Chinese), whereby the goal would be to garner half of [say] EBITDA (the local partner would receive the other half). Depending on JV expenses (COGS, SG&A), it probably the case the latter (the JV) has a higher net present value than the former (the license transaction).
If management is exploring an Asia-Pacific IPO, the equity security they could have in mind could be one of a Provectus subsidiary, which then could qualify as "one...of our securities" (the language from the Schedule 14A filing). Very, very loosely speaking, one might think about Yahoo! and Yahoo! Japan, which trades on the Nikkei and is a joint venture between Yahoo! and Japanese internet company SoftBank. There are other examples of publicly traded JVs. A successful outcome for Provectus — a successful IPO however it is formed or structured — probably has several key requirements, such as a complete and presumably filed Phase 1b/2 liver cancer (hepatocellular carcinoma) trial protocol for China, a complete and potentially filed Chinese investigational new drug (IND) application with the CFDA, and an agreeable partner and completed partnership in Sinopharm and/or Boehringer Ingelheim (China). More?
A successfully-IPO'ed JV means a high valuation for the entity, which then should drag up Provectus' NYSE-based market capitalization by virtue of the company's ownership of it. Potentially a lot of work, a less than simple deal structure, a different and non-trivial amount of incremental risk, etc. for what probability of and hoped for amount of success? It has the potential to be a home run, or the possibility of being a strikeout.
Tuesday (September 1, 2015)
Updated below.
1. August Blog Stats. Blog readership mostly rose from July 2015 depending on the statistic. I wrote 23 blog posts (6) and news items (17) in August versus 31 the previous month (4 and 21, respectively). Month-over-month changes were:
Bounce rate and pages per session metrics that changed on or around July 8th generally maintained their "trend" through August. See One month does not a trend make, but.../July Blog Stats (August 1, 2015) below.
2. Lawsuits. Regarding the class action lawsuits (see the 2Q15 10-Q's No. 7 under Notes on page 10): "On June 5, 2015, Provectus filed its Motion to Dismiss the Consolidated Complaint (the “Motion to Dismiss”). On July 20, 2015, the Lead Plaintiff filed his response in opposition to the Motion to Dismiss (the “Response”). Pursuant to order of the Court, Provectus must reply to the Response no later than August 19, 2015." See Notes (August 6, 2015).
H/t InvestorVillage poster Area51: "Defendants respectfully request that this Court extend the deadline for Defendants to file the Reply from August 19, 2015 to September 18, 2015. Undersigned counsel has discussed this motion with lead counsel for Plaintiffs and is authorized to represent that counsel for Lead Plaintiff agrees to the requested relief. A proposed agreed order is filed herewith."
3. Special meeting/proxy vote. I'd like to see management discuss, revise and/or specifically address future non-cash compensation, such as some variation on the below:
Updated (9/1/15): Provectus filed its final (definitive) Schedule 14A today seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. The prior 14A filing was a preliminary one; see Special Meeting/Proxy Vote, part 2 (August 28, 2015) below.
In recent days I asked management to consider variations of addressing the topic of non-cash compensation, including different compensation approaches and (almost as if not more importantly) their communication of such. I suggested they consider (i) not including themselves (as employees as noted in the above filing paragraph where the word is struck out) as eligible for equity security (i.e., stock options) issuances made possible and available by an increase in the authorized number of shares, (ii) putting their compensation consultant-based [x] compensation committee-approved [y] non-cash compensation packages up for shareholder approval (as and if/when appropriate) and/or (iii) better describing the process by which Provectus would determine the founders and officers' future compensation, particularly the non-cash component upon hoped for interim (from "now" until "whenever") and end-game success.
Management response thus far might be considered the changes in the definitive (final) proxy filing [right-hand side of the screenshot below], compared to the initial (preliminary) one [left-hand side]: "...as determined by our compensation committee in accordance with guidance from Pearl Meyer and Partners, our independent compensation consultants..."
[x] The company employs a compensation consultant, Pearl Meyer and Partners, to determine founders and officers' compensation. Pearl Meyer would have a process for doing so.
[y] The compensation committee of Provectus' board of directors then would (more than likely) approve Pearl Meyer's recommendation (or "guidance"). I cannot find the explicit composition of the committee, save for its charter on the company's website. I imagine if the company calls it "independent," the committee would be comprised of the three directors who meet the independence requirements of the New York Stock Exchange (Smith, Koe, McMasters).
4. Branding. I communicated to management that if they're going to use vendor partners like PharmaHEALTHLabs (and Jeff Meehan) [1] combined with press releasing such use and also communicating it as a milestone [2], (i) don't in the first place unless you (ii) provide a timely update of the results of the event if you qualify the event as a milestone (e.g., essentially, why it is milestone worthy) and (iii) ensure the vendor's named website [3] is relevant, germane and serious as it relates to your own efforts, irrespective of his/its industry reputation (i.e., his/its brand as it relates to the service you utilized and your brand).
[1] The 2015 PM360 Elite, May 2015 (Entrepreneurs, Jeff Meehan, Cancer Action Now); ELITE Entrepreneur Jeff Meehan of Cancer Action Now, May 2015
[2] See below.
[3] See below.
Special Meeting/Proxy Vote, part 2 (August 28, 2015)
Last Friday Provectus filed a Schedule 14A seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. Fully diluted shares outstanding at July 31st stands at ~294 million. The special meeting of stockholders this proxy requires takes place on October 1st. See Pfizer's Just Not That Into You (August 21, 2015) below.
I haven't determined how I will vote our shares yet. If I had to vote today, I would reject the proposal because I don't believe management has done enough to more broadly explain and rationalize the approval they seek and, if approved, the potential/possible future outcome(s) for the company and shareholders.
To formulate my decision I asked (in my August 21st news item) and continue to ask three questions:
The circumstance of no shareholder-approved increase but yet the potential need for shares at some point more than likely causes Provectus' accounting firm BDO and/or the New York Stock Exchange to question the company's going concern and/or listing status, respectively. If no ability exists to issue shares BDO and/or the NYSE could question the company's future viability.
For management to try to monetize Provectus at a respectable valuation, let alone one that is commensurate with the company's worth (at least as I see it), CTO Dr. Eric Wachter, PhD and CFO/COO Peter Culpepper require the tools funded and/or made available by a positive shareholder vote. Eric and his team need to clinically develop the drug further, which may require money derived from the issuance of the new shares at some point in the future (especially if no money arrives by way of a transaction before then). Peter needs to translate this clinical development into license, collaboration, equity investment and/or sale-of-the-company transactions. In order to consummate a transaction, he may require new shares to issue to a prospective partner.
Management could (a) IPO some form of Provectus equity security on an Asian stock exchange (presumably at an above market valuation), (b) sell an equity security to a prospective partner with or without special rights or rights of first somethings (also presumably at an above market valuation), or (c) IPO and sell equity. Reason (a) alone does not make sense; IPOing on an Asian stock exchange for shits and giggles is obtuse, even by management's past standards. Reason (c) makes sense if it helps Provectus come to terms with Sinopharm, although it seems an overly complex solution to the problem of bridging differences between the parties. Reason (b) makes sense if a potential partner has expressed interest in a relationship with the company; however, the valuation of such a deal likely would have to be balanced against providing special rights or rights of first somethings to said partner.
Q2. Now could be as good a time as any for Provectus to bring this proposal to shareholders in light of the company essentially hitting its authorized share ceiling. Funding Eric is not a near-term issue given what should be a August-end cash balance of ~$19.5 million. Providing "share tools" for Peter may well be a near-term need if his business/corporate development efforts assume discussion(s) of the sale of Provectus equity to a prospective partner. Could the board and management have sought the proposal earlier? Sure. Could they seek it later? Maybe, but if current partner discussions require it in the here and now, then probably no.
It certainly would have been much more respectful to shareholders for Peter (and, in context, Eric) to discuss the proposal on the August 6th business update conference call. He of course would have to have filed the proxy statement prior to conducting the call. Putting voices (on the call) to words (in the filing) may have helped both shareholders and management.
Q3. Management hasn't done enough to this point (in terms of broad, substantive and effective communication) to deserve shareholder approval. They should be capable of rationalizing the approval they seek, and describing the possible/potential future outcome(s) for the company and shareholders if the proposal is approved. A few hundred words in an SEC filing don't cut it. Management continues to poorly communicate broadly to the very [retail] shareholders who have funded their circuitous path. The manner in which the proposal has been handled to date underscores each and every Provectus manager's continued ineffective handling of their responsibilities regarding shareholder value. That said, it's only been a week, and I continue to look forward to hearing Chairman and CEO Dr. Craig Dees, PhD et al.'s rationale/description.
I plan to revisit this topic each Friday morning on the blog's Current News page until I cast our votes, which will be at least 48 hours before the meeting date and time.
Not that good (August 27, 2015)
1. Some dialogue today on Twitter regarding side effects of anti-PD-1 therapeutics:
The above should be taken in context, and in the context of physicians being able to manage side effects. Nevertheless, fulminant type 1 diabetes:
Interestingly, two exclusion criteria are (i) "For non-hepatocellular carcinoma, there must not be acute or chronic hepatitis B virus or hepatitis C virus infection" and (ii) "For hepatocellular carcinoma, hepatitis B virus and hepatitis C virus viral load must be undetectable, and they must not have had recent treatment with certain antiviral medications." In addition, "...life expectancy should be approximately 5 months or more."
In Provectus' Phase 1 liver trial of both HCC and liver mets, note the presentation of disease PV-10 treated below from the ESMO-GI 2015 poster, and that life expectancy was greater than or equal to 12 weeks (3 months).
The available data supports PV-10's superiority over T-Vec: (i) more agnostic to tumor presentations, (ii) higher complete responses, and (iii) greater immunologic signalling.
3. An earlier article I had meant to note: Science Isn’t Broken. It’s just a hell of a lot harder than we give it credit for.
Think about the article and Heery's comment in the context of PV-10's [very] large effect size, such that trials with large Ns are not necessary.
The Doctor Is In (August 25, 2015)
A third clinical site for Provectus' pivotal melanoma Phase 3 trial was added to the trial's ClinicalTrials.gov webpage, Morristown Medical Center of Atlantic Health System. The investigator is Dr. Eric Whitman, MD, whose Twitter handle is @Melanoma_doctor. As of this writing, his site is not yet recruiting. Whitman has been aware of PV-10 for quite some time. He also appears to be the medical director of Atlantic Health's Carol G. Simon Cancer Center.
Like Huntsman Cancer Institute's Dr. Robert Andtbacka, who was an investigator on Amgen's T-Vec's melanoma Phase 3 trial and is expected to be an investigator on PV-10's melanoma Phase 3 trial, Whitman was an investigator on a melanoma Phase 2 trial of Cavatak (Coxsackievirus A21), an intralesional oncolytic virus agent.
Also, the Phase 3 trial's lead investigator, St Luke's University Hospital and Health Network may present PV-10-related information and/or data at the Nordic Melanoma Meeting in Gothenburg, Sweden in early-September.
Up (August 23, 2015)
First, let's say I assume the occurrence of:
 |
| Image source |
Because of how payments to the CRO are structured for the trial (and typically for other mid- to late-stage clinical trials), Provectus of course knows which sites eventually will come on-line. The upfront payment, for example, is based on the sites one is using (unless more sites are added later if initial or original numbers are not being met, and then more payments are made for those additional sites).
Guidance of a July 2016 interim readout was reaffirmed. Although I am not completely certain, it would appear this is when a data dump would be generated, and presumably deposited into the company's electronic data room, and not necessarily when an announcement would be made.
Combination therapy melanoma Phase 1b study. Initial data should be available before the pivotal Phase 3 trial's interim data is available. Again, available in this case more than likely means available in the electronic dataroom for prospective partners to review, rather than presented at a medical conference. I think Provectus would be hard pressed to have its initial data ready for ASCO 2016, unless management (Eric) deems it appropriate to present a subset of the enrolled patients. The T-Vec/ipi and T-Vec/pembro posters at ASCO 2014 and SMR 2015, respectively, presented initial response data on all enrolled patients (even if unconfirmed in the latter's case).
Liver Phase 1b study. Surprisingly to me, initial data should be available before the pivotal Phase 3 trial's interim data is available in this case too. This would mean the necessary protocol(s) would be filed soon, and I think but am not certain most if not all of this data would come from Asian participants. Protocol refinement appears to have taken time because of the work necessary to establish the proper dose escalation aspect of the study. In order to get a single agent approved, the study (any study for that matter) would test compound plus standard of care (SOC) versus SOC. See Clinical Trial (July 4, 2015) below, for example, or Conference Call, part 2 (May 10, 2015) on the blog's Archived News III page. The different kinds of SOCs and secondary treatments in the region would require some individual addressing as it related to the relationship between PV-10's impact and the SOC's on patient treatment and treatment outcome. Should may yield to could if more time is taken, although the time frame of liver tumor injection and assessment is around 28 days. One other physcian (not St. Luke's Dr. Sanjiv Argawala) will be involved in all three PV-10 studies and trials.
"Comp" (November 24, 2015)
Following up on Status update (October 5, 2015) below, Provectus continues to negotiate with Sinopharm, per Provectus' management comments on the 3Q15 business update call.
Discussions have continued on the basis of a memorandum of understanding signed last year with Sinopharm-China State Institute of Pharmaceutical Industry, CSIPI, the leader among all pharmaceutical research institutes in China and Sinopharm A-Think Pharmaceutical Company Limited, Sinopharm A-Think, the only injectable anti-tumor drug research and development manufacturer and distribution integrated platform within Sinopharm Group. While our working arrangement is more developed with Boehringer, management of Provectus and senior personnel and Sinopharm, CSIPI, and Sinopharm A-Think has held numerous conference calls, have met face-to-face in both China and the U.S., and Chinese scientists on staff at Sinopharm have discussed in person PV-10 and its clinical results with various lead investigators we work with globally. Some more formal relationship with them remains an option for us in China, and will endeavor to include Boehringer as well in any future developments and potential contentions as well as Network 1 Financial comments. The latter was conveyed to me by multiple third parties, but of course is hearsay.These continued discussions might be veracious, based on similar comments made by Network 1 principals to several folks who passed them on to me (but are of course heresay). A timeline follows:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
There are several items related to Provectus' Phase 1b trial of the combination of PV-10 and pembrolizumab for patients with advanced melanoma (Stage IV) that bear follow-up/tracking.
First, there is the amount of PV-10 dosing. Per the trial protocol, pembrolizumab is administered per prescribing information. PV-10 also is administered along that timeline (i.e., every 3 weeks); however, no information is provided about the total amount administered, like it is in Provectus' pivotal Stage III melanoma study -- i.e., "Calculated required PV-10 dose ≤ 15 mL (based on total tumor burden)."
The goal of the Phase 3 trial for Stage III melanoma patients is of course to treat all disease (the presumption being that all disease is accessible for injection). The very nature of the Phase 1b study for Stage IV patients is that some proportion of disease is unaccessible with a needle of PV-10 (i.e., visceral disease). This visceral (unaccessible) disease cannot be injected with Rose Bengal. But how much of the accessible disease in a Stage IV patient will be treated with PV-10?
Second, there is the effectiveness (i.e., the objective response) of PV-10 in Stage IV patients. Public Stage IV data for Rose Bengal comes from the company's metastatic melanoma Phase 2 trial. Patients received initial injections of the compound at "t=0" (Day 1, Week 0) for up to 10 target lesions and upto 10 non-target ones, and then upto 3 more sets of inections (Weeks 8, 12 and 16).
 |
| Click to enlarge. Image source. |
"Visceral Disease
Although the study was not designed to quantitatively follow lesions in visceral organs, comprehensive radiologic imaging provided some insight into whether PV-10 could have an impact on visceral disease. While a substantial fraction patients with stage IV disease were classified as not evaluable due to early progression (Table 2), four patients experienced SD or PR of their visceral disease (including patients with multiple pleural and hepatic metastases); three of these patients also exhibited SD or better outcome in their target lesions, similar to the correlation of bystander response to that of target lesions. Seven stage IV patients survived through the end of the 52-week follow-up interval, including 4 of 10 patients with M1c disease."To substantiate potential efficacy, there also is public preclinical combination data, Moffitt Cancer Center's SITC 2014 work entitled Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma, Moffitt's thus far private clinical data from its feasibility study where some patients were refractory to immune checkpoint inhibitors before receiving PV-10, and Provectus' thus far private compassionate use/expanded access program data where some patients also were refractory to immune checkpoint inhibitors before receiving PV-10.
Phase 2 trial data is helpful when it comes to the effectiveness of PV-10 to complete ablate -- completely destroy (Prong #1 of PV-10's two prong approach to fighting cancer) -- injected tumors, which then would commence the cascade of antigen generation and presentation to dendritic cells and an eventual hoped for (expected) anti-tumor immune response. Most injected melanoma tumors require 1-2 injections for them to be destroyed.
 |
| Click to enlarge. Image source |
Third, there is the speed of a PV-10/pembro response. At AACR and ASCO 2014, Moffitt noted PV-10's immunologic signalling and impact (Prong #2) within 7-14 days of injection. The Phase 2 trial noted a median time to response (TTR) of 1.9 months (or about 8 weeks). Pembrolizumab's median TTR is 12-13 weeks. Could the pairing generate responses within as little as 3 weeks?
Fourth, there is the durability of the response.
Fifth, there is the presentation of the data. It is the data, Step #3 of Peter's three deal steps (see November 22, 2015's Differences of Opinion), that [presumably] Big Pharma need and prospective investors wants to see. It seems to me there are several key or notable venues for such data to be presented during a calendar year: April: AACR, May/June: ASCO, September: ESMO, November: SITC, and November: SMR. Maybe there are other medical conferences at which a company would present important clinical data.
Timelines from the ipi+T-Vec and pembro+T-Vec combinations:
 |
| Click to enlarge. |
Merck & Co. (Merck USA) issued a press release today about preliminary combination results ("early findings") of anti-PD-1 drug pembrolizumab and intralesional drug T-Vec, presented at the Society for Melanoma Research International Congress.
 |
| Click to enlarge. |
- Step #1: Initial response data. Efficacy data of T-Vec & ipi was presented first at ASCO 2014, and comprised (i) objective response (OR) (complete response [CR] + partial response [PR]) and (ii) disease control (DC) (CR + PR + stable disease [SD]).
- Importantly, the ASCO abstract observed or concluded the following: "In consideration with published reports, these data, although preliminary, suggest higher CR and OR rates than either agent alone and earlier responses after ipi initiation during T-VEC+ipi than with ipi alone." Underlined emphasis is mine. Repeating: Higher (better) responses, earlier responses.
- Step #2: Initial durability of response data. Durability data of T-Vec & ipi was presented second at ASCO 2015, and comprised (i) progression-free survival and (ii) overall survival.
Being careful about comparing clinical trial results, I nevertheless observe the following comparisons:
- T-Vec/ipi had notably greater CR (24%) than T-Vec/pembro (13%) [again, keep in mind, the danger of comparing trials, as well as the trials' Ns), and
- T-Vec/pembro had notably greater OR (56%) than T-Vec/ipi (41%),
In addition:
- CR: pembro/IDO (29%) > ipi/T-Vec (24%) > ipi/nivo (17%) > pembro/T-Vec (13%) > pembro/ipi (4%), and
- OR: ipi/nivo (61%) > pembro/IDO and pembro/T-Vec and pembro/ipi (56%) > ipi/T-Vec (41%).
A key question: Just how much synergy exists between T-Vec and pembrolizumab (Keytruda)?
Laundry list (November 20, 2015)
 |
| Image source |
2. Did Eric ultimately present at the Third Melanoma MIB Conference in Rome, Italy on November 9th, “PV-10: a new opportunity in Europe from Provectus?” The company said boo about it.
3. Dr. Sanjiv Agarwala, Professor of Medicine, Chief, Oncology & Hematology, St Luke's Cancer Center and Temple University, will present something titled Melanoma: Integrating Molecular and Immunotherapy Into Clinical Practice on January 10th at the inaugural PRIMO Meeting in Hawaii from January 9th to 12th. Provectus vendor (for Brazil) and Cancer Action Now CEO Jeff Meehan is the lead sponsor of the event. Agarwala is connected to Meehan via Cancer Action Now, and Provectus also is a PRIMO sponsor.
 |
| Click to enlarge |
4. Maybe Agarwala will discuss PV-10 at his 2016 HemOnc Today Melanoma and Cutaneous Malignancies conference/meeting on March 18th and 19th.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
More themes (November 19, 2015)
 |
| Theme image source |
- Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1 (SITC 2015),
- Cryoablation therapy stimulates the antigen-specific T cell immune responses generated by therapeutic HPV DNA vaccine (SITC 2015, November),
- Oncolytic immunotherapy for the treatment of non-muscle invasive bladder cancer using intravesical coxsackievirus A21 (SITC 2015), and
- Combination immunotherapy: Where do we go from here? (August).
Number 1 is Moffitt Cancer Center's immune mechanism of action work on Rose Bengal/PV-10, and noted "Incubation of melanoma cells with RB led to necrosis and the release of High Mobility Group Box 1 (HMGB1), which activated DCs via up-regulation of CD40 expression. The blockade of HMGB1 significantly reduced the antigen-presenting ability of DCs."
Number 2 noted "We found that treatment with deep freezing and thawing led to up-regulation of HMGB1 in TC-1 tumor cells, rendering the tumor cells more susceptible to APCs and E7-specific CD8+ T cell-mediated killing." The authors of this work describe cryoablation therapy as "the therapeutic application of extreme cold for localized destruction of living tissue," which of course destroys both diseased and healthy tissue alike.
Tumor antigens have to be viewed in context. Physical tumor destruction techniques such as heating or freezing tissue destroy fragile antigens and disrupt their relevant contextual structures. Disruption of cell membranes and removal of lipids, proteins, and complex carbohydrates destroy the antigens’ context, which is the very thing to what immune system cells respond. Thermal destruction denatures potential antigens, changing their chemical structure so that they are no longer representative of the tumor cell. In order to work rapid destruction of tumors must preserve both antigenic structure and biological context.
Number 3 noted "[f]urthermore, CVA21 induced the expression of multiple ICD determinants (calreticulin, ATP and HMGB1 release) and the immunogenic cell marker, Fas, associated with susceptibility to immune attack." CVA21 is an ocolytic virus, like T-Vec. "With intravesical therapy, the doctor puts a liquid drug directly into the bladder (through a catheter) rather than giving it by mouth or injecting it into a vein."
I included Number 4 mainly because of author Dr. Mario Sznol, MD (in part because of Dr. Sally Church, PhD's interview with him). The article noted: "The tumor microenvironment is highly hypoxic and this can lead to altered expression of autophagy molecules, such as HMGB1. Therefore, modulation of HMGB1-driven autophagy and DAMP release may be a strategy for improving responses to chemo-resistant tumors."
PV-10/Rose Bengal's immune mechanism of action, via HMGB1, is important [in context] for the use of the compound as a single agent, and in combination with another immunomodulatory agent (such as immune checkpoint inhibitor pembrolizumab).
How important is discerning this immune MOA in regards to the joint immune MOA of a combination therapy? Management has said the preponderance of non-clinical data support the concept of combining PV-10 with a drug like pembro (Keytruda). The former elicits a functional anti-tumor T cell response in patients, while the latter increases the anti-tumor function of T cells.
How important is discerning this immune MOA in regards to the joint immune MOA of a combination therapy? Management has said the preponderance of non-clinical data support the concept of combining PV-10 with a drug like pembro (Keytruda). The former elicits a functional anti-tumor T cell response in patients, while the latter increases the anti-tumor function of T cells.
Themes (November 17, 2015)
 |
| Theme image source |
The themes the abstracts in total connote appear [to me] to convey are consistent with PV-10's clinical value proposition.
The abstracts all derive from T-Vec's pivotal melanoma Phase 3 trial (aka OPTiM), which Amgen likely will use as a key resource for physicians seeking information about the safety, efficacy and application of the approved intralesional ("IL") agent (aka approved oncolytic virus or approved oncolystic immunotherapy):
- Abstract (a): Reduced risk of developing visceral/bone metastasis (VM) in patients (pts) with stage IIIB/C/IVM1amelanoma treated with talimogene laherparepvec (T-VEC) vers us GM-CSF,
- (b): Durable complete responses (CR) in patients (pts) withstage IIIB-IV melanoma treated with talimogenelaherparepvec (T-VEC) in OPTiM,
- (c): Safety profile of talimogene laherparepvec (T-VEC) in OPTiM, a phase 3 trial for melanoma, and
- (d): Did patients in OPTiM have truly unresectable disease? Results of an independent review.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Abstract (b) is below.
 |
| Click to enlarge. |
| Click to enlarge. |
Abstract (c) is below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Abstract (d) is below.
 |
| Click to enlarge. |
Institutional (November 16, 2015)
13F filings (through today) for the period ending September 30, 2015 showed a decrease in institutional share holdings of Provectus: 10.68 million share owned/held (down from 12.65 million as at 6/30/15, or about -16%), and 5.22% of shares outstanding (not a fully diluted figure) (down from 6.18%). See Institutional (August 14, 2015) below for the last institutional holding blog news item.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
In the event of a securities transfer, there would've been an increase in institutional share holdings of Provectus: 13.13 million share owned/held (+4%), and 6.42% of shares outstanding (not a fully diluted figure). The graphs then would look like:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
For example, I hope he, she or they:
- Observe that Rose Bengal had been lying around in the plain sight of Big Pharma for decades (at least) before Provectus' co-founders came across it during their search for the ideal cancer killer. See, among other blog posts, October 15, 2015's Still Standing. There message here is something like serendipity happens when it comes to innovation.
- Maybe even observe that Rose Bengal is an industrial dye created in Germany in 1882, and quote the MIT guy who said "Most drugs are really German dyes." See blog news item “Most drugs are really German dyes” (August 3, 2015) below. The message here is something like, well, most drugs are really Germany dyes.
- Observe the difference between Rose Bengal and many other cancer drugs and investigational compounds, particularly in the immunotherapy category. Rose Bengal is a small molecule that relies on physical chemistry to effectuate its two-prong approach to fighting cancer. Other drugs and compounds are either large molecules or biologics. The message here, essentially for folks like chemist Dr. Derek Lowe, PhD, is something like, hey, small molecule, targeted therapy... The stretch or reach some folks certainly will have to make is, however, that of a small molecule having such a potentially profound impact on the immune system.
- Observe the differences in the clinical rationales of Provectus' two melanoma trials
- (a) PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma, a trial for Stage III melanoma patients, or a trial for patients with an earlier stage of disease, or a trial to show PV-10 -- when disease is accessible to injection by PV-10 -- can forestall or prevent the spread of the disease to Stage IV, and
- (b) PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma, a trial for Stage IV melanoma or a trial for patients with a later stage of disease -- Stage IV -- where disease is not wholly accessible to injection by PV-10
- Maybe even observe the difference in the type or kind of oncologist who patients may see when they have melanoma and why. For example, certain Stage III patients may see surgical oncologists, while Stage IV patients often see medical oncologists. Forbes' Matthew Herper did not appear to know or understand the difference. See November 1, 2014's blog post Florey, Chain & Heatley⎟Coley⎟sort of, maybe, possibly, conceivably, probably...
But mostly or primarily, I hope a [news organization's] article (one that most folks would believe is objective, objectively good, and that has passed through the news organization's editorial process) provides the cover for other people (general and pharmaceutical industry media alike) to write articles on and about Rose Bengal (PV-10).
Some call it "needle in a haystack investing" (maybe) but for the most part these other media entities, and frankly investors at large, are afraid of one thing or another (they may have done the diligence, they may have not done it or done enough, they still may not believe what their work has shown them) and need the assurance of others and/or other things before they'll write about or invest in Rose Bengal, PV-10 and/or Provectus (because the share price tells them otherwise). I get that. Think of it as "tide comes in and tide goes out investing" (of a sort).
Some Hearsay (November 14, 2015)
1. A [news organization's] article on PV-10 could materialize before the end of the year, which may explain why Provectus' COO/CFO Peter Culpepper said on the 3Q15 business update call:
"To build public awareness for Provectus and PV-10, we have been conducting meetings with national, regional, and local media journalists, including Reuters, CBS and more. The introductory meeting served to provide the media with necessary background on Provectus, detailed PV-10, as well as build relationships to support future company announcements."2. A pediatric oncologist may want to use PV-10 in children in a compassionate use/expanded access program setting in large part because of the compound's lack of or negligible side effects.
Don't delay (November 13, 2015)
 |
| Image source |
I imagine the idea would be to conduct these studies and this work concurrent with the pivotal Phase 3 melanoma trial, as preparation for the eventual submission of the NDA.
As an example of delay (in this case something like a 6-9 month one), in a December 2014 press release Puma Biotechnology noted:
"Puma Biotechnology, Inc. (NYSE: PBYI), a development stage biopharmaceutical company, today provided an update on the timeline for filing its New Drug Application (NDA) for the approval of PB272 (neratinib) in the extended adjuvant treatment of HER2-positive early stage breast cancer.
Puma had previously communicated that it anticipated filing the NDA for PB272 in the first half of 2015. This was based on the feedback it had previously received from regulatory agencies, which had been focused on the proposed clinical indication of HER2-positive metastatic breast cancer. Since the Company’s initial NDA filing will now be for the extended adjuvant HER2-positive early stage breast cancer indication, based on the company’s recent meetings with the U.S. Food and Drug Administration (FDA), Puma will need to submit data from preclinical carcinogenicity studies with its NDA filing in accordance with International Conference on Harmonization (ICH) guidelines. In order to accommodate this requirement, Puma intends to delay its proposed timeline for filing the NDA until the first quarter of 2016." {Underlined emphasis is mine}In Rose Bengal's case, however, the FDA should know a lot about the compound's overall safety and pharmacology profile -- given an established Agency safety profile as an intravenous hepatic diagnostic (Robengatope®) as well as a topical ophthalmic diagnostic (Rosettes® and Minims®).
The base case should be Eric planning to undertaking the necessary NDA-related preclinical safety/pharmacology work on PV-10. Upside may well be the FDA does not require this work to be carried out given the existing literature available on Rose Bengal, thus saving Provectus money and time.
Data package (November 12, 2015)
 |
| Image source |
"To this end, we've scheduled two meetings with FDA to cover manufacturing and nonclinical portions of a possible new drug application, or NDA, for PV-10 and any supportive safety or pharmacology studies that may be needed so that, if the Phase 3 study is positive, these details won't delay review of the NDA. One of these meetings has been completed, with the second scheduled for early next year. We expect both to provide crucial guidance for completion of the data package supportive of an NDA."If Eric can submit an NDA (for PV-10 in patients with unresectable locally advanced cutaneous melanoma -- Stage III melanoma) to the FDA next year, it will be because:
- He has enough data, and
- That data demonstrated PV-10's superiority over controls.
In my view, the risk to the Phase 3 is the timing of the sufficiency of data generated, rather than a question of its outcome. That is, when (i.e., how many patients) will statistically significant progression-free survival ("PFS") curve separation occur. Not if. As such, enrollment remains a key risk -- it's much slower than Eric originally expected and previously guided. The trial also is more complex with the addition of Amgen's T-Vec, another intralesional agent, as a comparator or control arm. T-Vec's addition, however, also is strategically important. Eric further noted:
"These points are very significant since the approved indication encompasses the patient population we have delineated for our Phase 3 study of PV-10 in melanoma while the additional limitation of use statement sets regulatory precedent for approval of a drug having effect limited to skin or lymph nodes. Thus, as I noted in our August conference call, when Imlygic becomes readily available as standard of care, we expect to allow its use as a comparator in those areas where it is available. Any such modifications to our study protocol should not negatively impact study timelines nor integrity study results. Quite to the contrary, approval of Imlygic not only validates our approach for seeking approval of PV-10 in patients with locally advanced cutaneous melanoma but also allows us to offer a comparator that is more attractive to patients and investigators alike. We expect to submit the necessary protocol amendment to support use of Imlygic to FDA before year’s end." {Underlined emphasis is mine}Provectus' Phase 3 trial provides an opportunity to compare PV-10 to T-Vec and also T-Vec to systemic chemotherapy along the lines of both proper endpoints (e.g., PFS, complete response rate, etc. versus durable response rate) and controls (i.e., systemic chemotherapy versus GM-CSF).
T-Vec, or Imlygic, is consistently limited in its effect.
 |
| Click to enlarge. |
Patients in Provectus' melanoma Phase 2 trial received upto only 4 treatments of PV-10. In the company's Phase 3 trial, patients will receive PV-10 every 4 weeks until disease resolution or progression. PV-10 Phase 2 results were, for Stage III patients with all disease treated, 71% objective response and 50% CR.
Interestingly, time to response of PV-10 in its Phase 2 trial of patients with all disease treated (the Phase 3 protocol) was 1.8 months (median, I am guessing), while for T-Vec in its Phase 3 trial it 4.1 months (low of 1.2, high of 16.7).
While T-Vec should display some amount of improvement over chemotherapy, PV-10's proper treatment regimen should demonstrate clear superiority over T-Vec (as well as chemotherapy).
Sources:
- Talimogene Laherparepvec, Cellular, Tissue and Gene Therapies & Oncologic Drug Advisory Committee Meeting, Sponsor material
- A Team Approach, How independent endpoint assessment committees can overcome imaging limitations.
- A Study of OncoVEXGM-CSF in Stage IIIc and Stage IV Malignant Melanoma
- Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma.
- T-VEC: The OPTiM trial, Kevin Harrington, MD PhD, UK
- IMLYGIC (talimogene laherparepvec) label/prescribing information, Amgen
- Efficacy and Safety Study of OncoVEXGM-CSF Compared to GM-CSF in Melanoma
Provectus appears to be exploring a potential non-dilutive financing source via the European Union ("EU")(specifically, via Poland/EU) with the set-up in early-September of a Polish legal entity called Provectus Biopharmaceuticals Europe.
That, or Provectus is contemplating a reverse merger into a publicly traded shell on the Warsaw Stock Exchange. Bwahaha...
It is not clear whether the source of the financing is an EU-based governmental agency or program, or a pharmaceutical company (international or U.S. offshore). According to management, Polish clinical sites in Provectus' pivotal Phase 3 trial will happen regardless of whether the company does anything with this Polish legal entity or not; however, the potential for the so-called non-dilutive financing opportunity exists because of the prospective Polish clinical sites.
The [presumed] Polish resident (Edward Stanisław Misek) named on the Polish legal entity (PROVECTUS Biopharmaceuticals EUROPE SP ZOO) presumably is required to instantiate the domestic nature of the legal entity.
 |
| Click to enlarge. Image A |
 |
| Click to enlarge. Image B |
 |
| Click to enlarge. Google translation of Image A above |
 |
| Click to enlarge. Google transaction of Image B above |
It is not clear whether the source of the financing is an EU-based governmental agency or program, or a pharmaceutical company (international or U.S. offshore). According to management, Polish clinical sites in Provectus' pivotal Phase 3 trial will happen regardless of whether the company does anything with this Polish legal entity or not; however, the potential for the so-called non-dilutive financing opportunity exists because of the prospective Polish clinical sites.
The [presumed] Polish resident (Edward Stanisław Misek) named on the Polish legal entity (PROVECTUS Biopharmaceuticals EUROPE SP ZOO) presumably is required to instantiate the domestic nature of the legal entity.
CFDA (November 10, 2015)
 |
| Click to enlarge. Image & article source |
1. Moffitt Cancer Center wrote in their SITC 2015 abstract/poster: "Intralesional (IL) therapy is under investigation to treat dermal and subcutaneous metastatic cancer. Rose Bengal (RB) is a staining agent that was originally used by ophthalmologists and in liver function studies. Previously, IL injection of RB induced regression of injected and uninjected tumors in murine models. However, the relevant mechanism is yet unknown."
The underlined sentence (my emphasis) refers to the history leading to the work reported on the poster.
The underlined sentence (my emphasis) refers to the history leading to the work reported on the poster.
2. During Provectus' 3Q15 business update call, company CTO Dr. Eric Wachter, PhD said: "The way this works with Moffitt, they are experts at something called translational medicine. And what translational medicine means is understanding therapeutic processes based on investigations in both model systems, typically either in-vitro, so in test tube, or in animals and in humans. And the translational part means that you frequently go back and forth between the two types of systems as you learn. So, in this case, it was a very interesting process where some things that were elucidated in mice were then discovered to be occurring in humans. And the opposite actually happened in the clinical studies they've completed looking at human patients. And they went back to the bench and confirmed those in mice. So, this is normal operating procedure for translational medicine groups. It allows you to benchmark what you're seeing in model systems and humans and efficiently understand what's going on in humans in a high throughput system; that is, the models." {Underlined emphasis is mine.}
The effect of PV-10 on T cells was first observed in clinical trial participants then confirmed in mice.
3. Eric also said on the update call: "To this end, we've scheduled two meetings with FDA to cover manufacturing and nonclinical portions of a possible new drug application, or NDA, for PV-10 and any supportive safety or pharmacology studies that may be needed so that, if the Phase 3 study is positive, these details won't delay review of the NDA. One of these meetings has been completed, with the second scheduled for early next year. We expect both to provide crucial guidance for completion of the data package supportive of an NDA."
These kinds of meetings are standard in the later stages of drug development as the sponsor works to assure that all topics relevant to an NDA have been covered with the Agency prior to filing the NDA.
A useful reference may be the administrative and correspondence documents related to the accelerated approval of Bristol-Myers' Opdivo (nivolumab) for metastatic melanoma on December 22, 2014. Opdivo's BLA (the biologic's version of the NDA) was submitted on and received by the FDA on July 30th. The 60-day statutory review period would have implied a date of September 28th. In particular, see Regulatory History in the July 9th meeting minutes.
- E.g., "On February 7, 2012, a CMC only meeting was held to discuss plans to support clinical trials supporting licensure and marketing approval under the cross-referenced IND 100052."
- E.g., "On October 3, 2013, a Type C meeting was held under the cross-referenced IND 104225 to provide the Agency with an update on the nivolumab global registration strategy, and potential initiation of an expanded access program (EAP), for nivolumab as monotherapy in NSCLC, melanoma, and RCC and in combination with ipilimumab for melanoma. In this meeting, FDA agreed to review a proposal for an alternate timing of the final objective response rate (ORR) analysis in Study CA209037."
- E.g., "On April 18, 2014, a pre-BLA CMC only meeting was held to obtain feedback and agreement on the contents of the BLA application and acceptability of any late components to the application."
5. Princess Alexandra Hospital in Australia may be recruiting for the melanoma Phase 3 trial (source link here).
 |
| Click to enlarge. |
 |
| Image source |
On Provectus' 3Q15 business update call on November 6th, company COO/CFO Peter Culpepper described the respective thresholds of Provectus' NYSE MKT listing requirement (New York Stock Exchange) and going concern status (accounting firm and auditor BDO). In regards to the listing requirement, Provectus must maintain a mininum stockholders' equity of $6 million. In regards to the going concern status, the company must main 12 months of cash on hand at all times.
Stockholders' equity is equal to total assets minus total liabilities, and is directly but not wholly influenced by cash and cash equivalents (i.e., cash on hand), a line item on the balance sheeet (under total assets). Generally speaking, as cash increases or decreases, all else being equal (in context of course), stockholders' equity would increase or decrease.
Twelve months of cash on hand at all times would be influenced by Peter's ability to constrain or restrain variable spending (given that fixed spending is, well, fixed). According to management, this 12-month cash figure is not 12 times the past quarter's cash spend (as as estimate for future spending) or 12 times an estimated million dollars per month spend, but rather a figure less than $12 million (like something in the range of $8-9 million).
 |
| Click to enlarge. |
Meh < Call < Squee (November 7, 2015)
 |
| Image source |
CFO/COO Peter Culpepper: "Our estimated primary completion date is September 2017, and an estimated study completion date of October 2017. When 50 percent of the events required for the primary endpoint have occurred, the Independent Data Monitoring Committee will report an interim assessment of efficacy and safety. So, meaningful clinical data could come as early as the middle of next year, which is halfway through the study, as documented on clinicaltrials.gov. I stretch the word could, and we will continue to make every effort possible to keep our stockholders and the market updated."
- Management still is guiding an interim data readout, however constructed or defined, for June/July 2016 (i.e., the middle of next year).
combination therapy for melanoma for Stage 4 patients. Scientifically, combination therapy in
cancer treatment is a rapidly maturing area, where a rational [sp] combination of agents is
replacing the empirical approaches of the past. In this specific instance, we have completed
development of the protocol for Phase 1b-2 testing of PV-10 in combination with Merck’s
Keytruda in patients with Stage 4 melanoma. And we have begun recruitment of patients."
- If I take recruitment to be one or more patients having already been injected, response data certainly could be available in January 2016 ("objective response rate assessed at four months").
Peter: Keytruda is an immune checkpoint inhibitor approved for patient--treatment of patients with
advanced or unresectable melanoma. The PV-10 mechanism of action study’s preliminary
clinical findings reported last year and further updated tomorrow by Moffitt Cancer Center,
which we just announced, showed that the immunologic effect of tumor ablation with PV-10
may be complementary to immune checkpoint inhibition. Companion preclinical testing of PV-
10 in murine models of melanoma also reported last year shows that the therapeutic effects of
PV-10 in immune checkpoint inhibition are increased when the two are used in combination.
Put simply, they may work better together, especially for late stage patients. And this Phase
1b-2 study will help us prepare for potential marketing of PV-10 as part of a combination
therapy with Keytruda.
Eric: "This class of drug [PD-1] has been shown to work favorably with PV-10 in mouse models of melanoma, as presented by our colleagues at Moffitt last November at the Annual Meeting of the Society for Immunotherapy of Cancer."
- Thus, the PD-1 agent presented in Moffitt Cancer Center's SITC 2014 poster may have been Keytruda (and not Opdivo). "Systemic administration of anti-CTLA-4 or anti-PD1 antibodies in combination with IL PV-10 resulted in increased tumor regression and improved survival in this model."
- Provectus' intellectual property ("the joint patent co-owned with Pfizer") covers PV-10 as part of a combination regimen and in combination.
Peter: "It is our understanding that as long as our stockholder equity remains above six million dollars, our common stock and tradable warrant listings on the New York Stock Exchange, NMKT [sp], continue as has been since we were listed May 16th, 2014.
- Floor #1: Helpful to know. Stockholders' equity = Total assets - Total liabilities.
CTO Dr. Eric Wachter, PhD: "PV-10 represents both unique opportunity and an incredible responsibility, because the type of agents this company is working on has the potential to change the way cancer is treated around the world. PV-10 is a small molecule, designed to be injected directly into tumors, thereby focusing its effect on disease tissue while limiting exposure to healthy tissue. We believe this focused effect in tumors have the potential to educate the immune system to find other cancer cells with the same characteristics, thereby potentially having the effect on the capacities elsewhere in the body." {Seeking Alpha call transcript}
Eric: "We have much work ahead as we continue to build out the study, and we will continue to add
sites to the website as they’re brought online, thereby, providing full transparency to patients
and other stakeholders alike. Although, the first months of site initiation have gone slower
than hoped, we’re continuing to work on accelerating site start-up, and this is expected to
continue in the present quarter and beyond."
- Still, no apparent change in the timing of the interim analysis (i.e., the middle of next year).
Eric: "Focus on strategically significant investigators and their sites in the early stage of the study helps assure that the study’s built on a firm clinical foundation necessary for successful execution. These global leaders provide the lead geographically for other investigators in their respective regions, provide key study coordination and oversight necessary to make sure the study’s conducted to the highest standards and provide crucial advice for finely tuning the study necessary to facilitate global execution. As an example, we’re about to modify the study protocol slightly to address specific needs in Brazil, voiced earlier this year by key investigators."
Eric: "The label indication for Imlygic is “local treatment of unresectable cutaneous, subcutaneous and notable lesions in patients with melanoma recurrent after initial surgery.” The agency also noted in the label that “Imlygic has not been shown to improve overall survival or have an effect on visceral metastases.” These points are very significant since the approved indication encompasses the patient population we have delineated for our Phase 3 study of PV-10 in melanoma while the additional limitation of use statement sets regulatory precedent for approval of a drug having effect limited to skin or lymph nodes. Thus, as I noted in our August conference call, when Imlygic becomes readily available as standard of care, we expect to allow its use as a comparator in those areas where it is available. Any such modifications to our study protocol should not negatively impact study timelines nor integrity study results. Quite to the contrary, approval of Imlygic not only validates our approach for seeking approval of PV-10 in patients with locally advanced cutaneous melanoma but also allows us to offer a comparator that is more attractive to patients and investigators alike. We expect to submit the necessary protocol amendment to support use of Imlygic to FDA before year’s end."
- The current construction of the pivotal melanoma Phase 3 trial is a 2:1 treatment-to-comparator randomization. As an "investigator's choice" for the comparator, I would imagine the comparator now would comprise {systemic chemthoerapy [dacarbazine or temozolomide], intralesional agent [T-Vec]}.
- Progression-free survival (PFS), the Phase 3 trial's primary endpoint, for systemic chemotherapy has been discussed on this blog (i.e., less than 12 weeks). PFS was not chosen as an endpoint in T-Vec's pivotal metastatic melanoma Phase 3 trial because an increase in lesion size and/or development of new lesion(s) prior to response was expected based on results from the compound's metastatic melanoma Phase 2 study. Instead, time to treatment failure was used. Median time to treatment failure was 8.2 months.
- Reiterating: "...the additional limitation of use statement sets regulatory precedent for approval of a drug having effect limited to skin or lymph nodes."
Eric: Since pembrolizumab is licensed in the US and Australia, we were able to commence the study without assistance of a partner. This was designed to facilitate--this study was designed to facilitate use with other drugs to enable similar testing in a straightforward manner. Thus, if ongoing negotiations with prospective corporate partners leads to interest in testing PV-10 with a different checkpoint inhibitor, or even another class of systemic drug, the design can readily accommodate such interest.
- Keytruda is administered every three weeks: Administer 2 mg/kg as an intravenous infusion over 30 minutes every 3 weeks. The metastatic melanoma combination Phase 1b study administers both PV-10 and Keytruda every three weeks (Day 1 (Week 1), Week 4, Week 7, Week 10 and Week 13). Opdivo is administered every two weeks: Administer 3 mg/kg as an intravenous infusion over 60 minutes every 2 weeks. A Phase 1b study of PV-10 and Opdivo thus would see both drugs administered every 2 weeks (Day 1 (Week 1), Week 3, Week 5, Week 7, Week 9).
Eric: "With regard to other activity in melanoma, we expect to provide an update on the status of our expanded access protocol by the end of the calendar year. And I'll note for now that approximately 140 patients have received PV-10 under this program."
- Approximately 100 patients were treated through 2013. I imagine approximately 40 patients were treated in 2014.
 |
| Click to enlarge. |
Eric: "Following up on a successful summer, we recently conducted an advisory board meeting in China that has helped to clarify plans for advancing work on HCC in Asia, along with possible companion studies in the West. While our current study has played and continues to play a crucial role in identifying promising indications for PV-10 in tumors of the liver, we will build on this study over the next several months to begin one or more Phase 1b-2 studies directed to addressing HCC in Asia.
- Separating HCC and cancers metastatic to the liver.
Eric: "To this end, we've scheduled two meetings with FDA to cover manufacturing and nonclinical portions of a possible new drug application, or NDA, for PV-10 and any supportive safety or pharmacology studies that may be needed so that, if the Phase 3 study is positive, these details won't delay review of the NDA. One of these meetings has been completed, with the second scheduled for early next year. We expect both to provide crucial guidance for completion of the data package supportive of an NDA. "
- What type of meetings are these? Type C?
- Is this an example of a pseudo-rolling submission or pseudo-rolling review?
 |
| Click to enlarge. Image source. |
Correction: Moffitt Cancer Center is not using PV-10 in a clinical trial or study. The shareholder who believed or thought Moffitt was corrected their initial assertion. See Breast Cancer (August 8, 2015) below.
Note: Through today's 3Q15 financial statement issuance, expense item Lab supplies and pharmaceutical preparations experienced its 5th consecutive quarter-over-quarter increase. If de minimis input inflation, consistency of make-up, and if assumed to be entirely comprised of vial production (all rather quick 'n dirty or lazy assumptions on my part), one could believe the production of vials of PV-10 (and "gel packs" of PH-10) continues to increase.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
1. Provectus issued a press release today and filed an associated 8-K regarding its 3Q15 financial results, Reports Third Quarter 2015 Financial Results, in conjunction with filing its 10-Q.
2. The end-of-3Q15 cash balance (i.e., B/S item Cash and cash equivalents) was $18.9 million, compared to the prior end-of-Q's $23.1 million figure.
3. The company's monthly cash burn for the quarter appears to have been $1.41 million, +5% quarter-over-quarter (the prior quarter-over-quarter change was -4%).
4. Working in the gray — e.g., using a cash balance threshold of $12 million, estimating a monthly cash burn range of $1 million (a presumed "rough" projected figure for accounting firm BDO's purposes) to $1.4 million (on the current high end of recent actual), assuming the absence of non-dilutive deal-related cash inflows, making an assumption regarding an acceptable low watermark for the then pre-raise cash balance figure, assuming an increase in the authorized share number, etc. — Provectus would have to raise money in 2 to 3 quarters (i.e., 5-7 months).
5. Language regarding cash burn runway changed to "into 2017" in the current Q (pp. 14-15) versus "well into 2017" in the prior Q (p. 14).
6. Regarding the class action lawsuits (see the current Q's No. 7 under Notes on page 11): "On October 1, 2015, the Court entered an order staying a ruling on the Motion to Dismiss pending a mediation to resolve the Securities Litigation in its entirety. A mediation occurred on October 28, 2015, and discussions are continuing." See Lawsuit Update (November 4, 2015) below.
7. There was no update to the derivative lawsuit naming the company as a nominal defendant added in 2Q15: the Donato Shareholder Derivative Lawsuit. See the current Q's No. 7 under Notes on page 12.
8. Recent quarter-over-quarter changes to R&D and G&A line items. Note: Some items (and/or portions thereof) are non-cash.
 |
| Click to enlarge. |
H/t InvestorVillage Area51 poster:
"The Parties participated in a full-day mediation session with third-party neutral Jill Sperber in New York, NY, on October 28, 2015. While the mediation session did not result in a mutually-agreeable settlement, negotiations among the Parties, with the aid of Ms. Sperber, are continuing. However, as of the date of this filing, a resolution has not yet been reached."Patient-reported outcomes (PROs) (November 4, 2015)
From ERT's PROFICIENCY™ 2015 West Coast Regional Conference Series: Demonstrating a Product’s Value with Optimized Endpoints in May (San Francisco and San Diego).
 |
| Click to enlarge. Image source (San Diego) |
See also Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015) below.
SITC 2015, HMGB1, Anti-tumor immunity, Immunogenic cell death-(?) (November 3, 2015)
Updated below.
For this blog news item, set aside for the moment whether one thinks the biopharmaceutical ecosystem works, or not, in regards to drug development, broadly and inclusively speaking, and the ecosystem comprises the regulator (e.g., the FDA), medical practitioners, [pre-clinical] researchers and clinical investigators, medical and medical research institutions, Big Pharma company licencees/acquirors, peer groups (e.g., peer-reviewed publications, medical conferences), investors, various forms of media, etc.
Provectus has endeavored to work with Moffitt Cancer Center, and work with them (and other so-called stringers) at as much arm's length as is practical (sensible) and practicable (possible), to establish the veracity of the different facets and features of Rose Bengal and PV-10.
Stringers refers to medical and medical research organizations and entities working on/using PV-10 in some form or fashion (as facilitated by Provectus' CTO Dr. Eric Wachter, PhD) at some early or earlier stage of work (or until more broadly known and/or presented/published). Moffitt originally would've been a stringer (but perhaps wasn't strictly considered such because of its initial pivotal role). The Ajay Maker Laboratory of the University of Illinois at Chicago would appear to be a more contemporary or better example of a stringer.
2012: "Bystander"/Abscopal Effect, in Melanoma: At the Society of Surgical Oncology Annual Meeting, Moffitt reproduced, confirmed and, thus, validated, the "claim" PV-10 injection of melanoma tumors or lesions could lead to the shrinkage or destruction of untreated tumors or lesions: Intralesional Injection of Melanoma with Rose Bengal Induces Regression of Untreated Synchronous Melanoma In a Murine Model. At the time, this arguably confirmed, albeit pre-clinically, what was being seen clinically in the company's metatatic melanoma Phase 1 and 2 trials. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
2013: Immune Response, in Multiple Cancer Indications: At the American Association for Cancer Research Annual Meeting, Moffitt delved deeper into the workings of Rose Bengal and PV-10 to suggest a potential immune response was responsible for the "bystander effect," and did so in multiple cancers, which reproduced, confirmed and, thus, validated, more PV-10 "claims" (by Provectus): Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
2014: Anti-tumor immunity, in Melanoma: At the American Association for Cancer Research Annual Meeting, Moffitt further delved into the workings of Rose Bengal and PV-10, suggesting the potential for anti-tumor immunity to result from the compound's injection: Induction of anti-melanoma immunity after intralesional ablative therapy. HMGB1 was first mentioned here. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
 |
| Click to enlarge, |
2015: Synergy with anti-CTLA-4, -PD-1, PD-L1, in Melanoma: At the Society for Immunotherapy of Cancer Annual Meeting, Moffitt demonstrated the potential synergy between PV-10 and immune checkpoint inhibitors: Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma. PV-10 was supplied to Moffitt, and the cancer center presumably benefited from Provectus research funding. No author of this work was from Provectus (per the poster author line).
In 2015, again, at the the Society for Immunotherapy of Cancer Annual Meeting, Moffitt presumably will better explain, or explain more, how PV-10 works: Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1.
Cells die for different reasons (because they've reached their "maturity date," because they've been injured, or because they've been infected or are diseased). How a cell dies (immunogenic, tolerogenic or 'silent') should dictate how the immune system responds to its death. Dying cells, and the manner in which they die, produce signals for the immune system to interpret, and act upon (for better or for worse).
It would appear HMGB1 plays a key role in immuogenic cell death. See, for example, Gebremeskel et al. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: Impact on clinical studies and considerations for combined therapies., Oncotarget. 2015 Oct 14.
 |
| Click to enlarge. Fuzzy purple edits to the orignal figure are mine. Image source above. |
The authors of this last paper note for chemotherapy:
"Chemotherapy has historically been thought to induce cancer cell death in an immunogenically silent manner. However, recent studies have demonstrated that therapeutic outcomes with specific chemotherapeutic agents (e.g. anthracyclines) correlate strongly with their ability to induce a process of immunogenic cell death (ICD) in cancer cells. This process generates a series of signals that stimulate the immune system to recognize and clear tumor cells. Extensive studies have revealed that chemotherapy-induced ICD occurs via the exposure/release of calreticulin (CALR), ATP, chemokine (C-X-C motif) ligand 10 (CXCL10) and high mobility group box 1 (HMGB1). This review provides an in-depth look into the concepts and mechanisms underlying CALR exposure, activation of the Toll-like receptor 3/IFN/CXCL10 axis, and the release of ATP and HMGB1 from dying cancer cells. Factors that influence the impact of ICD in clinical studies and the design of therapies combining chemotherapy with immunotherapy are also discussed."
How does PV-10 achieve anti-tumor immunity via HMGB1?
E/n 1: Green DR, Ferguson T, Zitvogel L, Kroemer G. IMMUNOGENIC AND TOLEROGENIC CELL DEATH. Nature reviews Immunology. 2009;9(5):353.
E/n 2: Bianchi, ME, Killing cancer cells, twice with one shot, Cell Death and Differentiation (2014) 21, 1–2
Updated (11/3/15): A running comparison... Comparisons can be helpful, and they also can be incorrect.
Updated (11/3/15): A running comparison... Comparisons can be helpful, and they also can be incorrect.
 |
| Click to enlarge. |
Blog readership mostly fell from September 2015 depending on the statistic. I wrote the same total number of blog posts (2) and news items (18) in October compared to the previous month (3 and 17, respectively). Month-over-month changes were:
- -3% for # of unique visitors (2,187 v. 2,257),
- -11% for # of page views (25,332 v. 28,357),
- -8% for # of visits (7,884 v. 8,590),
- -6% for # of U.S. cities from where visitors came (590 v. 631),
- -4% for # of world cities (114 v. 119), and
- +16% for # of countries (50 v. 43).
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below again.
Three more clinical sites were activated (but are not yet recruiting) for Provectus' pivotal Phase 3 melanoma trial:
- Sharp Memorial Hospital in San Diego,
- M.D. Anderson Cancer Center in Houston, and
- Princess Alexandra Hospital in Brisbane, Queensland.
Updated (10/30/15): Tabulation of clinical sites to date.
 |
| Click to enlarge. |
"Turning to status of the Phase 3 study of PV-10, we've opened our first site for enrollment of patients and are in final steps of opening a number of others. As is noted previously, these initial sites will be both in the U.S. and Australia. And as Pete noted, we're actively engaged in brining additional sites, both in these countries and in a number of other strategically selected countries such as Brazil and China into the program...
Our international efforts have indentified important prospective regional investigator and we’re optimistic about the role these investigators can play in their respective regions to lead further investigator engagement and patient approval. As I've noted previously, we expect enrollment to be approximately one-third from the U.S.; one-third from Australia; and one-third from the rest of the world. If this balance ends up shifted more to non-U.S. and non-Australian patients, our efforts to key investigators and their patients from different parts of the world should help assure that we have a diverse patient population similar to patients in the U.S." {Underlined emphasis is mine}
He said again the same thing on the company's 2Q15 call:
"As we continue to add study centers, we will monitor progress on enrollment, particularly as it relates to further optimizing implementation of the study. We continue to expect overall enrollment to be approximately one-third from the US, one-third from Australia, and one-third from the rest of the world. If this balance ends up shifted more to non-US and non-Australian patients, our efforts to access key investigators and patients from different parts of the world should help assure that we have a diverse patient population that is similar to patients in the US.
We believe the second half of this year will be crucial in determining whether the initial study timelines can be met. As we make progress toward adding sites, we will continue to monitor the impact that this is likely to have on patient accrual and assess options for adding additional sites in the regions I've noted as well as potentially adding additional regions as necessary" {Underlined emphasis is mine}Takeaways, at this point in this news item:
- How many patients does the trial require, and for what?
- Can the trial meet its timeline(s), and for what?
Let's now assume I assume, among other things, that:
- I agree with Eric's comment that "[w]e firmly believe that Phase 3 testing should not be started unless you can adequately predict the outcome." See June 26, 2014 blog post Trial Math: Meeting the Primary Endpoint, Pt. 2,
- The outcome in question is statistically significant progression-free survival (PFS) curve separation of the PV-10 treatment and systemic chemotherapy control arms,
- Statistically significant PFS curve separation requires somewhere in the roundish number range of 45 to 60 patients (2:1 randomization would imply 30 to 40 PV-10 patients, and 15 to 20 chemo ones),
- Acknowledge FDA approval based on a much smaller number of patients that also demonstrates statistically signigicant endpoint superiority is no given,
- Provectus would carry out a time- (rather than event-) based interim analysis (for safety and efficacy) somewhere in the timeframe of June to August 2016, and
- The company can enroll a sufficient number of patients by June to August 2016 (it could happen before, but that earlier timing is not central to the argument that follows).
N_hazardRatioActual (of 45-60 patients) is 20-~25% of N_HazardRatioTrial (of 225 total patients). Why is the trial's stratification comprised of one-third U.S., one-third Australian and one-third Rest of World patients?
And what did Eric mean when he repeated "If this balance ends up shifted more to non-US and non-Australian patients, our efforts to access key investigators and patients from different parts of the world should help assure that we have a diverse patient population that is similar to patients in the US?"
Returning to takeaway question #1a/b, how many patients does the trial require, and for what? Some number of patients (i.e., 45-60) would be required for the trial to be deemed successful (i.e., to achieve statistical significance), and thus for PV-10 to be approvable. Some more patients might be required to begin the approval process in a particular non-U.S. region or regions (e.g., Australia, China, Western Europe, Brazil, Mexico, etc.). One imagines that the stratification of the pivotal melanoma Phase 3 trial may also be related to PV-10 investigation for other indications (besides locally advanced cutaneous melanoms), and that countries like China may have more specific requirements than other countries (in regards to the Phase 3 trial).
 |
| Click to enlarge. |
Returning to takeaway question #2a/b, can the trial meet its timeline(s), and for what? Provectus continues to guide publicly an interim analysis would be undertaken by or around June to August, 2016 (specifically, I believe/recall, July). The most critical timeline should be the interim analysis, and thus having enough patients to demonstrate curve separation. Making certain assumptions of average enrollment per month per site (depending on region) based on some public comments, the company might be lagging.
| Click to enlarge. |
On the other hand, some different and simplistic (and potentially incorrect) assumptions yields a vastly different outcome.
 |
| Click to enlarge. |
Updated below again.
The Agency approved intralesional therapeutic or agent/oncolytic immunotherapy/oncolytic virus T-Vec today. In the FDA press release, the agency refers to T-Vec as an oncolytic virus therapy, noting or referencing route of delivery six times. Underlined and bolded emphasis below is mine.
Imlygic, a genetically modified live oncolytic herpes virus therapy, is used to treat melanoma lesions that cannot be removed completely by surgery. Imlygic is injected directly into the melanoma lesions, where it replicates inside cancer cells and causes the cells to rupture and die.
A treatment course with Imlygic consists of a series of injections into the melanoma lesions. After the initial injection, a second dose is administered three weeks later, followed by additional doses every two weeks for at least six months, unless other treatment is required or until there are no remaining injectable lesions to treat.
The safety and efficacy of Imlygic were evaluated in a multicenter study of 436 participants with metastatic melanoma that could not be surgically removed. The participants’ melanoma lesions in the skin and lymph nodes were treated with Imlygic or a comparator therapy for at least six months, or until there were no remaining injectable lesions. The study showed that 16.3 percent of the study participants who received Imlygic experienced a decrease in size of their skin and lymph node lesions, lasting for a minimum of six months, compared to 2.1 percent of the study participants receiving the comparator therapy. However, Imlygic has not been shown to improve overall survival or to have an effect on melanoma that has spread to the brain, bone, liver, lungs, or other internal organs.
The most common side effects observed in clinical study participants were fatigue, chills, fever, nausea, flu-like symptoms and pain at the injection site. Because Imlygic is a modified live oncolytic herpes virus therapy, herpes virus infection can also occur. Given this, Imlygic should not be given to individuals with suppressed immune systems or who are pregnant.Amgen, in its press release, refers to T-Vec as an oncolytic viral therapy, noting among other things that:
- "...the exact mechanism of action is unknown,"
- "Amgen intends to make IMLYGIC available to patients in the U.S. within a week. Amgen anticipates the average cost of IMLYGIC therapy to be approximately $65,000,"
- Viral shedding: "Accidental exposure to IMLYGIC™ may lead to transmission of IMLYGIC™ and herpetic infection, including during preparation and administration. Health care providers, close contacts, pregnant women, and newborns should avoid direct contact with injected lesions, dressings, or body fluids of treated patients. The affected area in exposed individuals should be cleaned thoroughly with soap and water and/or a disinfectant. Caregivers should wear protective gloves when assisting patients in applying or changing occlusive dressings and observe safety precautions for disposal of used dressings, gloves, and cleaning materials. Exposed individuals should clean the affected area thoroughly with soap and water and/or a disinfectant. To prevent possible inadvertent transfer of IMLYGIC™ to other areas of the body, patients should be advised to avoid touching or scratching injection sites or occlusive dressings,
- Lack of healing: "Impaired healing at the injection site has been reported. IMLYGIC™ may increase the risk of impaired healing in patients with underlying risk factors (e.g., previous radiation at the injection site or lesions in poorly vascularized areas). If there is persistent infection or delayed healing of the injection site, consider the risks and benefits of continuing treatment."
"We will also continue to monitor the changing treatment options available to patients in these regions and if necessary adjust certain study elements to address these changes. For instance, our last conference call was made immediately on the heels of a key FDA review committee meeting regarding possible approval of another intralesional agent for melanoma, that is T-VEC.
A decision on approval with T-VEC is scheduled for late October, and if this agent is approved and becomes readily available as standard care, we anticipate adding it as a comparator in those areas where it is available. Any such modification should not negatively impact the study timeline or--nor the integrity of study results."Takeaway: What will he say in regards to T-Vec's potential impact on Provectus' pivotal melanoma Phase 3 trial on the November 5th 3Q15 business update call? For example:
- Now that T-Vec is approved (according to Amgen, it may be available within a week), how does this affect Provectus' Phase 3 trial?
- Would the company have to replace systemic chemotherapy dacarbazine (DTIC) with T-Vec, per the FDA, and if so, would such replacement delay the Phase 3 trial?
Updated (10/28/15): Amgen still does not have a grip on how T-Vec works, which probably means BioVex founders David Latchman and Robert Coffin didn't at the time they started the company Amgen acquired in 2011. While not knowing how T-Vec (OncoVEX) did what it did, "what" was a two-prong approach to fighting cancer. Step #1, direct injection of the now approved drug into cancerous tumors and lesions would lead, locally, to their shrinking or destruction, triggering cell death and thus the release of antigens, which then would provoke the immune system to generate a systemic or bodywide response. Thus far, it does not appear Amgen has published pre-clinical work on the intravenous (systemic) delivery of T-Vec.
In September 2015, Dr. Coffin and former BioVex President and CEO Philip Astley-Sparke raised $30 million in a venture capital Series A financing to move their next attempt at oncolytic virus therapy, Replimune, forward. There is no discussion on Replimune's website on how its compound would be delivered (i.e., intratumorally or intralesionally vs. systemically or intravenously).
How does the virus get to the cancer cell? Via the tumor via injection, or via the bloodstream via intraveous administration? This would be prong no. 1 of a two-program approach, which is the provocation of the immune system prong. Prong no. 2 would be the subsequent immune system reaction and response (and its hoped for durability). Since T-Vec is an oncolytic virus, why does it require injection (multiple times) into tumors? Did Coffin believe in route of delivery, yet accepted he did not have a great compound to provoke the immune system. Or does he believe it's all about the virus (and, thus, route is not important)?
Prior to T-Vec's approval, San Diego-based Genelux's CEO and Amgen veteran Thomas Zindrick noted in Forbes (as Genelux would deliver their oncolytic virus therapy intravenously):
“What’s exciting about delivering the drug directly into the bloodstream is that we will not only get direct infection of the primary tumor, we’ll be able to infect circulating tumor cells and metastatic disease.”
In his October 27th article entitled Following pioneering approval, Versant bankrolls next-gen oncolytics player Turnstone, FierceBiotech's John Carroll notes:
"In specific, their drug is systemically administered, which should provide a leg up over T-Vec, which uses a herpes simplex virus and is delivered with an intratumoral injection...Like T-Vec, they can destroy cells and release antigens that trigger an immune response. But they are also encoding an antigen - initially MAGE A3 - that is designed to gain a specific kind of T cell attack. And the coded antigen can be switched out as the company goes after various types of cancer."Australian oncolytic virus therapy company Viralytics started with intratumoral delivery of its compound (a proprietary formulation of the common cold Coxsackievirus Type A21) before experimenting with its intravenous administration.
A Provectus shareholder like myself believes Amgen's T-Vec's approval is groundbreaking as an intralesionally-delivered therapy that happens to be an oncolytic virus, rather than strictly as an oncolytic virus therapy.
This differentiation in approach is because Provectus' founders believed intratumoral or intralesional injection of the right compound into cancer tumors or lesions (local delivery), rather than oral or intravenous administration (systemic delivery), would deliver the right information in the right format and the right way to the immune system. See October 15, 2015 blog post Still Standing.
Updated below.
According to Provectus' ClinicalTrial.gov webpage, the company's combination therapy melanoma trial PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma now is recruiting at St Luke's University Hospital and Health Network. The study, as of this writing, does not appear on St. Luke's listing of melanoma trials in which the hospital is participating.
In addition, the third listed clinical site of the three, Atlantic Health System, is now is recruting patients for the company's signle agent melanoma trial PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma.
Updated (10/22/15): It should be clear to existing/prospective Provectus shareholders that it is not clear to company management how much more data, broadly speaking, is required for some sort of combination therapy deal with a Big Pharma having an immune checkpoint inhibitor (and/or a targeted therapy) under [mostly-to-exclusively] terms acceptable to Provectus to materalize.
Such terms might include an upfront payment, no-to-equal sharing of study costs, and Provectus' trial protocol, among other things. I don't believe who and where such a study (i.e., formally with a Big Pharma partner) is undertaken is a big deal, since it would be more likely carried out at prominent clinical sites by prominent clinical investors well versed in the use of pembrolizumab (as a single agent and in combination with another therapeutic).
In Provectus' September 23rd Announces Initiation of Phase 1b/2 Clinical Trial to Study PV-10 in Combination with Immune Check Point Inhibitor Pembrolizumab (with the byline: Study could be a "Significant Step to Co-Development Transaction"), the company's COO/CFO Peter Culpepper said:
"Commercially, this is the second of three steps that we hope will significantly strengthen our hand in negotiating a co-development transaction with an immunotherapy-focused partner. Our joint patent with Pfizer was the first; this study is the second; and the third is our immune mechanism of action clinical study, which is underway at the Moffitt Cancer Center and which has completed recruitment."As noted in Peter's PR quote, Step #1, the approval of the joint patent with Pfizer, was made when the patent was awarded on August 18th (despite being allowed on April 20th).
Step #2, initiation of their own Phase 1b/2 clinical development program for combining PV-10 and an immune checkpoint inhibitor, was made (in management's view) when the study protocol was filed on ClinicalTrials.gov on September 23rd.
Step #3 is more information on PV-10's immune mechanism of action ("MOA") being made available. Moffitt Cancer Center will present a poster at SITC 2015 on November 6th that should expound further on PV-10's immune MOA: Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via HMGB1.
There probably are a couple of additional steps, or data generation items, that might be necessary to get a deal done (if one indeed can be had). Step #2.5 or Step #3.5 could be the publication of Moffit's work in a peer-reviewed publication (paying homage to the so-called peer review process), which company management has previously guided may occur before the end of the year.
Step #4, more than likely a/the requisite step, is the generation of data from the Phase 1b trial, which was noted as recruiting at St. Luke's on ClinicalTrials.gov today.
St. Luke's and Dr. Sanjiv Agarwala, MD, a medical oncologist who was a clinical investigator in Provectus' melanoma Phase 2 trial and is the lead investigator of the company's pivotal melanoma Phase 3 trial, are logical first movers because of Dr. Agarwala's influence with his hospital's institutional review board, his access to appropriate patients, etc. Other prominent sites and investigators, perhaps like Memorial Sloan Kettering Cancer Center and Dr. Paul Chapman, MD (a medical oncologist who is a consultant to Provectus), may materialize later.
In the September 23rd press release, Provectus' CTO Dr. Eric Wachter, PhD noted:
"The primary end point of tolerability in the Phase 1b portion of the study, combined with assessment of progression free survival (PFS) and objective response rate (ORR) by RECIST criteria as key secondary endpoints, assessed over a 15 week treatment interval, establish a basis for determining whether to proceed to the larger, randomized Phase 2 portion of the study." {Underlined and bolded emphasis is mine}"The Phase 1b study is a relatively short one, with "complete" data on each patient collected and tabulated after the completion of a 15-week period. If the first patient is treated in this study on, say, November 2nd (the 1st is a Sunday), endpoint data could be determined beginning mid-March 2016 (to account for some data collected 4 weeks after the 15-week period). Such data would include safety and tolerability, response, and peripheral blood mononuclear cell ("PBMC") levels.
Since patient treatment would be assessed over (rather than just after) 15 weeks, data for Big Pharma to evaluate (in Provectus' electronic data room under confidentiality) should be generated shortly after patients are enrolled and treated, such as every three weeks or so given pembrolizumab's dosing schedule. Using a hypothetical first day of treatment as November 2nd, two data drops might occur before the end of the year. But how many patients and patient data drops would be necessary and sufficient for Big Pharma?
There is no small amount of risk to Provectus and its shareholders by management undertaking this study. There is the potential for the generation of autoimmune disease because of combining two immunomodulatory agents, like immune checkpoint inhibitors themselves already have shown. PV-10's remarkable specificity and presumably no-to-little clinically relevant drug-drug interaction with pembrolizumab should help mitigate this risk. I imagine Provectus has sufficient safety and efficacy experience in pre-clinical trials (mice studies) by Moffitt and the company's compassionate use/expanded access program about the combination of PV-10 and immune checkpoint inhibitors (whether PV-10 was given after or before the checkpoint inhibitor).
Another concern about the Phase 1b study might be whether it might take patients away from Provectus' Phase 3 trial. This is unlikely as the patient subsets are different. The Phase 3 trial is strictly comprised Stage III patients, while the Phase 1b study is strictly made up of Stage IV patients.
A Measure of Validation (October 21, 2015)
Provectus' CTO Dr. Eric Wachter, PhD said on the company's August 6th 2Q15 business upadate call:
"Starting with the PV-10 Phase III study in locally advanced cutaneous melanoma, we've listed our second study site on the clinicaltrials.gov Web site and are in the final steps of opening a number of others in the US and Australia. We will continue to add sites to the Web site as they're brought online to provide full transparency to patients and stakeholders alike. However, this has gone slower than hoped. We're working to speed up site startup and anticipate that this will accelerate in the present quarter." {Underlined emphasis is mine}Of course, the quarter (the third quarter, that is) came and went. He further said:
"In Australia, we're using the new National Ethics Application Process, or NEAP, along with standard contract and indemnification agreements for the first time nationwide. These features are representative of a constantly evolving process for conduct of clinical trials, and the recent delay was necessary to properly address the changing needs of our sites. We can't change the a la carte nature of site startup in the US, but the nationwide approach in Australia is expected to expedite startup there once our initial site is active."So, there has been "slippage" in getting Australian Phase 3 sites up and running. Nevertheless, Princess Alexandra Hospital in Brisbane, Australia will be a melanoma Phase 3 trial site, and currently is one of Provectus' compassionate use/expanded access program sites. It is also where an investigator initiated study of the combination of PV-10 and radiotherapy is being conducted (h/t a shareholder and regular hatter). See Radiation (September 22, 2015) below.
One of the doctors at Princess Alexaxandra is running a trial: Topical Imiquimod or Diphenylcyclopropenone for the Management of Cutaneous In Transit Melanoma Metastases – A Phase II Single Centre Prospective Randomised Pilot Study. You can search for the trial using this link.
The aim of the study is: "To compare two different non-invasive topical immunotherapies in patients with multiple, in transit, cutaneous melanoma metastases. The hypothesis is that these treatments are at least as clinically effective as current therapies for patients with selective in transit melanoma metastases and can be used to enhance patients’ quality of life and decrease health-related costs."
Intransit disease presents in a number of different ways. For patients with small dermal deposits, physcians there are unable to treat them with PV-10 because the lesions are too small to be injected. These patients then are treated with diphenylcyclopropenone ("DPCP"). The trial being proposed would compare topical imiquimod (i.e., a cream or gel) and DPCP. The trial is not yet open to recruitment.
Patient use of PV-10 has been incorporated into this trial's proposed protocol. See below (fuzzy purple emphasis is mine): "comparative treatments."
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below.
From December 2014:
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
From SITC 2012 (November 2012), three years before:
| Click to enlarge. Image source |
 |
| Click to enlarge. Fuzzy purple emphasis is mine |
 |
| Click to enlarge. Fuzzy purple emphasis is mine |
Updated (10/21/15): Further to this news item and the 10/20/15 update of Combination continuations (October 18, 2015) below, will 2016 be the year of in situ vaccines (or other kinds of agents that provoke anti-tumor immunity)?
Pharmaceutical industry consultant (and former Big Pharma clinical researcher) Dr. Sally Church, PhD wrote another article (on LinkedIn) titled Quit whining, get thinking!, which is associated with her subscription-based article entitled Why combine raditiation with immunotherapy? While acknolwedging the expense and ineffectiveness of immune checkpoint inhibitors, she seems to be evolving her views towards Provectus Chairman and CEO Dr. Craig Dees, PhD by writing:
"...many may well have forgotten about as a valid approach for boosting the immune system, thus making it a more effective situation for subsequent therapies to do their business."The "forgotten" is radiotherapy, which is undergoing a change in emphasis from being a definitive treatment to being a component in a treatment plan. It can effect in situ vaccination, by killing cancer cells and causing antigens to be released; however, radiation therapy is not safe of course and, among other things, damages DNA, and kills both diseased and healthy tissue.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
The subject of Dr. Church's article on her subscription-based blog is NYU School of Medicine's Dr. Sandra Demaria's presentation at ECC 2015 entitled Molecular basis for radiotherapy in synergy with immunotherapy.
 |
| Click to enlarge. Image source |
"Because of its multiple actions, irradiation may therefore interfere at various levels with immunotherapy: release of antigens of tumor cells, presentation of these antigens, T cell passages of circulation in the tumor environment, tumor infiltration by T cells, finally, death of tumor cells. Furthermore, the irradiation of the primary tumor site may induce changes in distance of the immunogenicity of metastases. "In situ vaccination -> anti-tumor immunity?
Combination continuations (October 18, 2015)
Updated below again.
Three continuation [patent] applications, continuations of Provectus' already approved joint patent with Pfizer entitled Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (U.S. patent #9,107,887), appeared on the U.S PTO website on October 15th:
- 20150290165,
- 20150290309, and
- 20150290318.
Thoughts and commentary to follow.
Updated (10/19/15): See a summary table of the combination therapy patent and continuation [patent] applications below.
Updated (10/19/15): See a summary table of the combination therapy patent and continuation [patent] applications below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated (10/20/15): Some additional thoughts on the continuation applications (related to the combination therapy patent initially co-achieved with Pfizer) filed by Provectus.
1. A systemic inhibitor of immune system down-regulation. Neither the patent itself (patent no. 9,107,887) nor the relevant continuations (patent application nos. 20150290165 and 20150290318) are sufficiently detailed about what comprises a systemic inhibitor of immune system down-regulation, other than to protect [in the continuations] "a systemic inhibitor of immune system down-regulation comprises anti-CTLA-4 antibodies," which are described in more detail as "including ipilimumab and tremelimumab" [in the patent].
There was no mention in the filed continuations (as there was no mention in the granted patent itself) of PD-1 or PD-L1 or other categories of immune checkpoint inhibitors. Further, there was no any of the members in these categories: e.g., PD-1: nivolumab and pembrolizumab, PD-L1: avelumab and durvalumab). Where is the specific mention of these categories and their members of systemic inhibitors of immune system down-regulation?
Patent applications and continuations, prior to approval or granting, appear on the US PTO's Patent Application Database webstite and its Patent Application Information Retrieval website ("PAIR" website) after some amount of time following submission of the application/continuation.
Takeaways (and more questions):
- There probably is another continuation that has been filed (but is not yet evident on PAIR) that describes "a systemic inhibitor of immune system down-regulation" in more detail, and names anti-PD-1 and -PD-L1 antibody categories and also names members of these categories like avelumab, durvalumab, nivolumab and pembrolizumab.
- It's not clear to me Pfizer would be/is a co-assignee of this further continuation appliction.
- In order for Pfizer to be a meaningfully economic co-assignee on the PD-1/PD-L1 continuation application, I'd imagine they'd have to pay up for it. Provectus management has repeatedly voiced that Pfizer derives no meaningful economic benefit from the combination patents (on which it is a co-assignee) unless it acquires Provectus.
2. A systemic targeted anticancer agent. Continuation patent application no. 20150290309, which is a Provectus-only application (as sole assignee) and discussed the combination of PV-10 with targeted therapies, makes me wonder whether Provectus has examined the notion of a better front-end payload or better front-end package as the better way to treat harness the immune system for patients with late-stage or end-stage cancer (i.e., heavy disease burdened patients), rather than in combination with an immune checkpoint inhibitor.
Note the location in the cancer immunity cycle of targeted therapies.
 |
| Click to enlarge. Fuzzy purple arrow is mine. Image source |
Targeted therapies need help to create taller long-term survival curve tails. Perhaps PV-10 might help them do that, allowing for the benefit of wider short-term survival curves.
 |
| Click to enlarge. Image source |
In a 2013 Provectus white paper, company CTO Dr. Eric Wachter, PhD said:
Speaking further in an interview, he said, “I think the case has been made successfully for PV-10’s role as a potent stimulator of specific anti-tumor activity. This is evident in clinical data from Phase 1 and 2 testing, where regression of untreated bystander tumors correlated with ablation of tumors, and in these nonclinical mechanism studies. And, our recent murine studies show that this stimulation works robustly in combination with CTLA-4 blockade.”
Further studies designed to confirm the apparent synergy are underway, including one with only the low 9H10 dose/ PV-10 combination. A phase 1/2 anti-CTLA-4 dose escalation trial with PV-10 is warranted, Dr. Wachter said. Similarly, models for kinase inhibitors and an analogue for vemurafenib are being sought. Vemurafenib, like PV-10, rapidly reduces tumor burden.
PV-10 murine research demonstrated unambiguously, Dr. Wachter noted, that tumor burden is a critical variable in predicting response to a combination therapy. It has been suggested that earlier research into therapeutic melanoma vaccines faltered because tumor burden grew beyond the immune system’s capacity for control before the vaccine could develop its full effect. “We think that the combination of PV-10 with something like a kinase-inhibitor has the potential to dial back or reduce tumor burden even better than an anti-CTLA-4 agent while the systemic PV-10 immunologic effect is developing. The kinase inhibitor would do the early work against visceral disease until PV-10 can catch up and take the baton across the finish line.” {Underlined emphasis is mine}
Question:
- Is, from a meaningul clinical perspective, PV-10 in combination with a targeted therapy the so-called steak to the sizzle of PV-10 in combination with an immune checkpoint inhibitor?
H/t InvestorVillage poster canis_star: The Society for Immunotherapy of Cancer's ("SITC's") Understanding Cancer Immunotherapy patient guide.
 |
| Click to enlarge. Image source |
SITC's patient guide includes an immunotherapy timeline.
 |
| Click to enlarge. Image source, guide page no. 6 |
 |
| Click to enlarge, Image source & link above. Purple emphasis is mine |
▸ William Coley was a pioneer of cancer immunotherapy (late-1800s). According to Wikipedia (see immediately prior link):
"Coley developed the theory that post-surgical infections had helped patients to recover better from their cancer by provoking an immune response. He began to experiment by deliberately causing this phenomenon, injecting bacteria directly into people being treated – but because this had the adverse effect of causing infection he then switched to using dead bacteria." {Underlined emphasis is mine.}Hoption Cann et al. (link at the top of the post) write about Coley's work:
"Coley considered several points crucial to a patient’s survival. First and foremost was to imitate a naturally occurring acute infection, and thus, inducing a fever was essential. Injections were optimally administered daily (or every other day) for the first month or two. To avoid immune tolerance to the vaccine, the dosage was gradually increased over time (depending on patient response). The vaccine was injected directly into the primary tumour and metastases, when accessible. Finally, a minimum six month course of weekly injections was followed to prevent disease recurrence." {Underlined emphasis is mine.}Thus, Coley thought that harnessing the immune system in the right way to fight cancer was the successful byproduct of doming something correct to their tumor(s).
▸ Why then, when writing about cancer immunotherapy and associated approved and investigational checkpoint blockade drugs like Yervoy (anti-CTLA-4, Bristol-Myers), Keytruda (anti-PD-1, Merck), Opdivo (anti-PD-1, Bristol-Myers), MPDL3280A (anti-PD-L1, Roche/Genentech), MEDI4736 (anti-PD-L1, AstraZeneca/MedImmune), etc., do some folks include what seems like obligatory yet obtuse nods to Coley? Google, for example, coley immunotherapy pd-1.
Coley's vaccine and these inhibitors both engage the immune system. But although the mechanism of action of Coley's vaccine (or Coley's toxins as it also was known) was and remains unknown, it is believed the approach led to specific and non-specific immune responses (paragraph source/sentence taken from: Rational approaches to human cancer immunotherapy, Davis et al.). Interestingly, according to Davis et al.'s article, Coley's injected-bacteria-into-accessible-tumors-and-metastases was, "[a]s late as 1934, “Coley’s toxins” was the only known systemic treatment for cancer" (article footnote). {Underlined emphasis is mine.} A treatment injected into tumors that generates an immune response is called a systemic treatment for cancer. Interesting...
Checkpoint blockade therapeutic agents are non-specific immunotherapies. "Non-specific immunotherapies don’t target cancer cells specifically. They stimulate the immune system in a more general way..."
2015 Lasker Award winner Dr. James Allison, PhD[11], whose research eventually resulted in the development of immune checkpoint inhibitor and systemic immunotherapy ipilimumab, believed cancer tumor shrinkage was the successful byproduct of doing something right to the immune system. That is, releasing the immune system’s so-called brakes that block CTLA-4, thereby enabling T cells to fight the tumor.
Takeaway: CTLA-4s, PD-1s, PD-L1s, etc. are non-specific immunotherapies.
▸ "According to a 1965 article that was published in A Cancer Journal for Clinicians (1):
“In 1952, a bibliography of the literature, and, in 1953, a report on 30 inoperable cases which had been treated by Coley's mixed toxins and had survived thereafter for periods of from 1 to 47 years (20 cases had a survival of over 20 years) were published. The report is said to be based on a comparative analysis of over 1,200 cases treated with Coley's toxins, and 300 cases in which intercurrent infections played a part. Over 270 cases were said to have shown complete regression of the tumor, but the 30 inoperable cases were selected for the report because the diagnoses had been confirmed by microscopic examination, and some information on their subsequent history was available. Of the 30 tumors, 7 were classified as carcinoma, 19 as various types of sarcoma, 2 as malignant melanoma, and 2 as giant cell tumors.”
A complete response rate of 22% (270 out of 1200) was impressive by the standards of the 1960's and today. But, despite these results, the article states that the, “American Cancer Society has found no evidence that treatment with Coley's mixed toxins results in any objective benefit in the treatment of cancer in human beings.” It is difficult to reconcile this conclusion with the results cited in the same article. Perhaps the results were simply not believed as they were authored by Mrs. Helen Coley Nauts, Executive Director of the New York Cancer Research Institute. Mrs. Nauts was the daughter of William Coley." (paragraph source link) {Underlined emphasis is mine.}
Takeaway: Coley's approach generated notable complete responses.
▸ Which brings us to a chicken-and-egg question. Which comes first, the complete response, or the immune response? For PV-10, successfully generating a complete response leads to a good to great immune response.
Per medical writer Walter Alexander's recent article PV-10 in Metastatic Melanoma: Rapid Responses Led Phase 3 (he's written about PV-10 before, including PV-10 Moves Forward):
The high-percent response rates in bystander lesions underscored the importance of elucidating the mechanism underlying PV-10's activity. That meant going back to bench investigations. The operant question for researchers, according to Shari A. Pilon-Thomas, PhD, Moffitt Cancer Center Immunology Program, was: "Is it just because you inject the drug and it goes everywhere and then kills tumor cells at other sites? Or is injecting PV-10 inducing a T-cell response, such that T-cells travel throughout the body and kill tumors in their various locations?"
In a poster presentation at the 2013 meeting of the American Association of Cancer Research, she pointed to evidence suggesting that an immune-mediated process underlies PV-10 responses in untreated lesions. First, responses in untreated lesions occurred only when responses had occurred in injected lesions, and second, responses in bystander lesions typically were delayed in comparison with responses in injected lesions, Dr Pilon-Thomas noted.
Dr Pilon-Thomas has previously shown in murine models that induced flank tumors treated with PV-10, as compared with placebo, were about a third of the size, and bystander lesions were about 30% smaller. At the same time, concentrations of interferon-gamma, a cytokine critical for innate and adaptive immunity (including tumor control) and for activating macrophages, were increased more than fivefold.
These findings, along with those from other studies, led Dr Pilon-Thomas to conclude, "We think that when you inject PV-10 into a tumor, it destroys the tumor, releasing tumor fragments that are then taken up by immune cells. The immune cells travel to the lymph nodes where they 'educate' or activate T-cells, which can in turn travel anywhere in the body."
Her research also showed that PV-10-induced immunity is tumor specific.
Further evidence of immune responses induced by PV-10 come from another study conducted at the Moffitt Cancer Center, this time involving eight patients with dermal and/or subcutaneous metastatic melanoma. The findings, presented at this year's ASCO annual meeting in a highlighted poster session by Amod Sarnaik, MD, a surgical oncologist at the Moffitt Cancer Center, showed that intralesional PV-10 was associated with a significant increase (P = .03) in circulating cytotoxic CD8+ T-cells, a potential mechanism for a tumor-specific immunologic effect secondary to tumor ablation.
In this study of eight patients, each patient had two study lesions that were sampled by biopsy before treatment; one of the two lesions was injected with intralesional PV-10, and then both residual sites were completely excised 1 to 2 weeks after PV-10 injection. Tumors were compared before and after treatment to determine pathologic complete response (pCR).
PV-10 resulted in pCR in the posttreatment biopsy specimens of both PV-10-injected and uninjected study lesions in four of the eight patients, and all eight exhibited at least partial regression of the injected lesion.
Six of these eight patients had metastatic disease that was refractory to previous treatment with immunologics (ipilimumab [Yervoy, Bristol-Meyers Squibb Company] and anti-PD-1 therapy) and BRAF-mutation inhibitor (vemurafenib [Zelboraf, Hoffman-La Roche]). After PV-10, four of these six patients had pCRs in both the injected and uninjected lesions. {Underlined emphasis is mine.}The cancer tumor is not so much a patient’s enemy as it could be his or her “frenemy” (both friend and enemy). Provectus' co-founders (Chairman and CEO Dr. Craig Dees, PhD, President Dr. Timothy Scott, PhD and CTO Dr. Eric Wachter, PhD) viewed the tumor as essential to making good on the promise of anti-tumor immunity, believing tumors were repositories of a cancer patient’s known knowns, known unknowns and unknown unknowns. The immune system needed to gain access to this information (in antigenic structure and biological context) in order to effectively fight back. There arguably is more we don’t know about the immune system than we know about it. Dees’ philosophy in regards to Mother Nature’s creation (the immune system), as a result, was to help rather than change or tinker with it.
Dees thought that harnessing the immune system in the right way to fight cancer was the successful byproduct of properly destroying the tumor.
Dees et al. believed intratumoral or intralesional injection of the right compound into cancer tumors or lesions (local delivery), rather than oral or intravenous administration (systemic delivery), would deliver the right information in the right format and the right way to the immune system.
Takeaway: PV-10 generates a complete response in order to generate/which is followed by an immune response.
▸ Forbes contributor Jon Fortenbury wrote about PV-10 and Provectus in a post entitled A New Cancer Drug Worked In Over 50% Of Patients In A Phase II Trial. He subsequently updated the post to "...to include comments from an outside expert and the company's response to his criticism." Forbes staff member and editor of the section Matthew Herper later weighed in under the Comments section with:
 |
| Click to enlarge. |
An estimated 41 percent of the advanced lung cancer patients taking Opdivo were alive after a year on the drug, compared with 5.5 percent to 18 percent of these patients who historically have survived over that time-frame, Bristol-Myers said today in a statement. About 15 percent of the 117 patients in the mid-stage study responded to the treatment, one of a new class of cancer therapies that harnesses the body’s immune system to attack the disease."[P]atients in the mid-stage study responded to the treatment" above refers to objective response. If Opdivo were where we need to be in terms of better helping or utilizing the immune system, wouldn't Bristol-Myers have heralded complete response (objective response = complete response + partial response) more. The pharmaceutical company surely would have if the trial's CR were notable.
Certainly what was notable was the improved overall survival (the quote's "the advanced lung cancer patients taking Opdivo were alive after a year on the drug") compared to historical survival figures.
As for Herper, other journalists, commentators and opiners, aside from better survival figures that are of course very good things (longer survival is better than no or shorter survival), why do they not mention or say in the same breath that generations of immunco-oncology therapeutics agents that have come (Yervoy), are here (Keytruda, Opdivo), and are to come (MPDL3280A, MEDI4736) have not had/do not have notable or memorable complete responses?
Takeaway: Partial response, good. Complete response, best.
▸ To be fair, Herper, who probably has covered biotechnology companies and the subject of oncology longer than I've held Provectus shares, and who also believes this is biology's century, may not understand the conflation Fortenbury's article, upon further editing and insertion of the "outside expert's" quote, managed to achieve.
The article described Provectus' melanoma Phase 2 trial results and, in particular, the sub-group of patients who had all of their disease treated (i.e., injected with PV-10). Why was/is this relevant? As Fortenbury writes, "If PV-10, when injected in all the cancerous lesions of a melanoma patient for longer than 16 weeks, goes on to produce positive results in the next phase, it just may be a viable treatment option for many patients with aggressive, late-stage, locally advanced melanoma." The target population of the company's upcoming pivotal Phase 3 trial are patients with un-resectable locally advanced cutaneous melanoma, which means the melanoma (i) cannot be removed by surgery (non- or un-resectable), (ii) is located in or just under the skin (cutaneous and subcutaneous, respectively), and (iii) has not metastasized to distant sites like the lungs, liver or brain.
These patients need better options, like Eric, "...patients clearly need more options: single agent options and more options for finding successful combinations that can truly change the course of this vicious disease," and Dr. Agarwala, "I think it will be an option for many patients who have a cancer disease that’s localized or regional," were quoted in the Forbes article.
Takeaway: Locally advanced cutaneous (or subcutaneous) melanoma = Melanoma that has not spread or metastasized.
This is worth repeating: The upcoming pivotal Phase 3 trial will comprise Stage III patients. The disease in Stage III has not spread. Patients in which the disease has spread are Stage IV patients.
Takeaway: Stage III ≠ Stage IV.
▸ Conflation in the article occurs when Herper asks Fortenbury to have an "outside expert" opine: PD-1 Keytruda clinical investigator and UCLA Jonsson Comprehensive Cancer Center medical oncologist with multiple clinical interests Dr. John Glaspy, M.D., MPH is quoted. Medical oncologists (like Glaspy and PV-10 principal investigator Agarwala) see mainly stage IV patients, while surgical oncologists (like PV-10 principal investigator Dr. Merrick Ross, M.D. from MD Anderson Cancer Center ) see mostly stage III patients.
The data Fortenbury's article highlights show PV-10 as a monotherapy could be an important option for patients with locally advanced cutaneous melanoma. The FDA also appears to be considering this topic too. It announced on October 8th that "melanoma, specifically unresectable loco-regional disease" was a disease candidate for public comment. See Prescription Drug User Fee Act Patient-Focused Drug Development; Request for Comments (October 8, 2014) on the blog's News page.
Takeaway: Locally advanced cutaneous melanoma = Stage III = Melanoma disease has not spread ≠ Stage IV = Metastatic melanoma with visceral disease and heavy tumor burden.
▸ Glaspy's words in the article:
John Glaspy, an oncology professor at UCLA, says that “it’s not clear” whether the result are important. If they are talking about lesions that were not directly injected with the drug, the results would be meaningful. “If they are talking about the injected lesion, not so much,” Glaspy says.
When asked about it, he repeated: “Like I said, these SQ melanomas are an indolent disease, and it is not a big deal if you inject them and they regress. I don’t think you have any evidence that anybody is cured.” {Underlined emphasis is mine}Tumors need to be destroyed. That's the big deal. Fifty percent of patients in the sub-group of Provectus' Phase 2 trial who had all of their disease treated achieved a complete response (total cancer disappearance) during a treatment period that was just 16 weeks.
Yervoy, Keytruda, Opdivo, MPDL3280A and MEDI4736 are far from curing melanoma patients because they are not achieving notable or memorable complete responses.
The melanoma component of Provectus’ clinical development program has two fundamentally different pathways to approval. In April 2015 the company commenced its pivotal Phase 3 trial of PV-10 versus systemic chemotherapy in patients with unresectable locally advanced melanoma (Stage III patients). The trial’s hypotheses are two-fold: whether complete response of injected tumors is tantamount to elimination of disease symptoms and whether PV-10 can forestall or prevent the spread of the disease from Stage III to Stage IV. If you make the tumor go away, don’t the symptoms go away too? Success with Stage III patients, and consequently with earlier stages of melanoma, Dees et al.’s vision was to replace a surgical oncologist’s scalpel with a Rose Bengal needle.
In September 2015 Provectus initiated a Phase 1b/2 trial program combining PV-10 with an immune checkpoint inhibitor (Keytruda) in patients with advanced melanoma (Stage IV) by filing the associated study protocol. Late-stage disease indicates with tumors typically inaccessible to injection. A PV-10-checkpoint inhibitor combination presents the medical oncologist (like Glaspy) with a more effective approach to reducing tumor burden (PV-10 where accessible by injection, a checkpoint inhibitor where inaccessible to injections) until the immune system can re-establish itself to finish the job Mother Nature intended it to do.
Takeaway: Partial response, not a big deal. Complete response, cured?
▸ One simple but I don't believe an over-simplistic way of looking at the situation could be surgical oncologists look at melanoma (and cancer at large) when it is first diagnosed or has not spread beyond control or has not metastasized. Medical oncologists look at melanoma (and cancer at large) when it has spread or looks like it is beyond control.
Takeaway: Surgical ocologists: PV-10. Medical oncologists: PV-10 + something else.
▸ Eric, in regards to, I believe, late-stage (Stage IV) patients (patients with distant metastases), in the Forbes article, "...more options for finding successful combinations that can truly change the course of this vicious disease," undoubtedly would have told Fortenbury about Moffitt Cancer Center presenting pre-clinical data on these combinations (i.e., PV-10 + [insert checkpoint blockade agent]) at the annual meeting of the Society for Immunotherapy of Cancer ("SITC") next week.
The idea behind combining PV-10 and a checkpoint blockader may be to generate both the specific and non-specific kinds or types of immune responses Coley's vaccine/toxins were believed to have generated. The goal of employing these kinds of combinations, to battle melanoma (cancer) once it has spread to distant sites, may be to generate both specific and non-specific immune responses.
Takeaways: Stage IV melanoma: PV-10 + one of (Yervoy, Keytruda, Opdivo, MPDL3280A, MEDI4736), Yervoy, Keytruda, Opdivo, MPDL3280A, and MEDI4736 need help.
▸ Roche's Genentech's Dr. Daniel Chen, M.D., Ph.D. quoted Roche's Genentech's Dr. Ira Mellman, M.D., both co-authors of Oncology Meets Immunology: The Cancer-Immunity Cycle, at ESMO 2014:
 |
| Click to enlarge. Screen shot source: Biotech Strategy Blog. |
▸ Which brings me back to where I started this post, with Florey, Chain & Heatley, and Coley.
Dees, Scott and Wachter did not discover Rose Bengal. It can be traced back to Basel, Switzerland in 1882 when a German patent was granted to Ghnem for a new family of wool dyes. Dees et al. were not the first to use Rose Bengal in an oncology setting. Japanese researchers Ito and Watanabe did so in 1986. The compound’s therapeutic benefits remained hidden until 1986 when it was given to mice by Ito and other Japanese researchers while investigating whether red food dye No. 105 (also made from Rose Bengal) caused cancer. Instead, they observed dose-dependent survival increases in the mice. The researchers, however, did not advance their observations into formal cancer studies and trials of the compound.
Craig postulated intratumoral injection, and thus the delivery of the drug via the tumor or lesion, was key. He thought it was critically important to the eventual immune response that PV-10 (Rose Bengal) be injected into a cancerous tumor or cancer metastases.
What kind of therapy is PV-10? From a 2013 Cancer Watch article entitled Back to Phase 1: Understanding Systemic Effects of PV-10:
Echoing [Moffitt Cancer Center's] Dr. Sarnaik, Eric Wachter, PhD, Provectus chief technology officer, said that he hopes that the findings of Dr. Sarnaik’s study will point toward rational judgments about combining PV-10 with other documented therapies. “We then might want to try two or more orthogonal therapies to stress tumor cells from several different angles simultaneously, for example an immune therapy plus a metabolic therapy (e.g., a kinase inhibitor), or in a rationally designed sequence.” In a hepatocellular carcinoma model, he added, PV-10 showed significant potential for synergy with 5-fluorouracil. Provectus recently initiated clinical testing of PV-10 with the multikinase inhibitor sorafenib, again bringing in two therapies with divergent mechanisms of action.
Which category does PV-10 fall into? “I think we are getting a clearer picture of how it might be classified, but it has features of several previously unrelated categories, such as of adoptive cell transfer and vaccination,” Dr. Wachter said. “PV-10 initially reduces tumor burden through chemoablation—but then activates the immune system bringing in capacities completely orthogonal to the ablative tumor destruction,” he added.
“Amod Sarnaik’s work may give us the molecular basis for closing the loop on one of the founding concepts for going into the clinic in the first place,” Dr. Wachter commented. “Back in the preclinical days at Provectus, Craig Dees, PhD, theorized that ablation of tumors with PV-10 might lead to unmasking of tumor antigenic material. I don’t think he anticipated that it would work as well as it does.” {Underlined emphasis is mine.}Vaccines are too specific. They facilitate the expression of only one antigen. Vaccines are antigen specific.
CTLA-4, PD-1 and PD-L1 therapeutic agents are not specific enough. They do not facilitate the expression of enough antigens.
PV-10 ablation of tumors presumably leads to the generation of antigens that are presented to the immune system in (i) as large a number as possible, (ii) pristine and not un- or non-denatured shape, (iii) whole pieces and not fragments, (iv) correct conformation/the right shape, and (iv) the right context.
Rose Bengal (PV-10) of course is not a biologic, like Coley's dead bacteria. As a non-biologic, its two-prong approach to fighting cancer (dual mechanisms of action) derives from its physical chemistry.
Dees et al. have spent the better part of two decades educating much of the biopharmaceutical industry and its ecosystem about the merits of their approach to fighting cancer. Dees frames therapeutic outcomes of Rose Bengal use in the context of reproducibility, whether preclinical or clinical, study or trial, generated by the company, a clinical investigator or a third-party researcher, affiliated or unaffiliated with Provectus. He reminds that reproducibility the hallmark of Western science. If a scientist or researcher cannot repeat the outcome of an experiment, and another researcher or scientist cannot replicate the result, one should be skeptical about the veracity of the original work and claims made from it.
Rose Bengal’s track record of reproducible therapeutic features as well as preclinical and clinical results by multiple parties in multiple cancer indications in multiple species is noteworthy. Different research entities separately and independently reproduced Dees et al.’s two-prong cancer killing approach of, initially or first, tumor ablation and, subsequently or second, tumor-specific immune responses in multiple solid tumor cancers (in preclinical mouse models).
If they are ultimately successful in demonstrating Rose Bengal’s therapeutic benefits to the satisfaction of the FDA (and a Big Pharma acquirer at a commensurate valuation), Dees, Scott and Wachter’s legacy must include their steadfastness in pursuing their own philosophy for defeating the disease in the face of the regulator and industry that did not readily understand, embrace or believe the idea that a local agent could deliver meaningful, systemic, clinical benefit to cancer patients.
Takeaway: I imagine Rose Bengal/Dees et al. are more Coley's heirs than anything/anyone on SITC's immunotherapy timeline.
CD4+ > CD8+ (October 16, 2015)
H/t InvestorVillage poster FortStCowboy and another blog reader/shareholder: Roswell Park Cancer Institute (RPCI) issued a press release on October 15th entitled Special Class of T Cells Shown to Both Attack Cancer Cells and Enlist Other Immune Cells.
"The researchers identified a subset of CD4+ “helper” T cells that they call “tumor-recognizing CD4+ T cells” or TR-CD4 because of their unique ability to recognize and respond to cancer cells. These special CD4+ T cells are antigen-specific: they recognize the tumor antigen NY-ESO-1, which is expressed by many solid tumors, including ovarian, melanoma, prostate, lung, breast and synovial sarcoma cancers.
“While most studies today involving adoptive T-cell immunotherapy focus on CD8+ ‘killer’ T-cells, our team’s findings highlight a significant role for CD4+ ‘helper’ T cells, which can also play a central role in promoting antitumor immune responses,” says Kunle Odunsi, MD, PhD, Cancer Center Deputy Director, Executive Director of Roswell Park’s Center for Immunotherapy and senior author on the new study."The paper the press release references is Direct tumor recognition by a human CD4+ T-cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses.
Abstract: Tumor antigen-specific CD4+ T cells generally orchestrate and regulate immune cells to provide immune surveillance against malignancy. However, activation of antigen-specific CD4+ T cells is restricted at local tumor sites where antigen-presenting cells (APCs) are frequently dysfunctional, which can cause rapid exhaustion of anti-tumor immune responses. Herein, we characterize anti-tumor effects of a unique human CD4+ helper T-cell subset that directly recognizes the cytoplasmic tumor antigen, NY-ESO-1, presented by MHC class II on cancer cells. Upon direct recognition of cancer cells, tumor-recognizing CD4+ T cells (TR-CD4) potently induced IFN-γ-dependent growth arrest in cancer cells. In addition, direct recognition of cancer cells triggers TR-CD4 to provide help to NY-ESO-1-specific CD8+ T cells by enhancing cytotoxic activity, and improving viability and proliferation in the absence of APCs. Notably, the TR-CD4 either alone or in collaboration with CD8+ T cells significantly inhibited tumor growth in vivo in a xenograft model. Finally, retroviral gene-engineering with T cell receptor (TCR) derived from TR-CD4 produced large numbers of functional TR-CD4. These observations provide mechanistic insights into the role of TR-CD4 in tumor immunity, and suggest that approaches to utilize TR-CD4 will augment anti-tumor immune responses for durable therapeutic efficacy in cancer patients.Compare human study work on the immunologic signaling of intralesional agents PV-10 and talimogene laherparepvec below:
 |
| Click to enlarge. Purple emphasis is mine. |
Provectus' CTO Dr. Eric Wachter, PhD will be speaking at the Third Melanoma MIB Conference in Rome, Italy in November. His presentation is entitled PV-10: a new opportunity in Europe from Provectus.
 |
| Click to enlarge. |
As pervasive as aspirin (October 11, 2015)
 |
| Image source |
A Google News search of HMGB1 (as of this writing) yields several articles about a recent study of salicylic acid targeting HMGB1. Salicylic acid is the active ingredient of aspirin. The study, entitled Aspirin′s Active Metabolite Salicylic Acid Targets High Mobility Group Box 1 to Modulate Inflammatory Responses, noted in its abstract:
"Salicylic acid (SA) and its derivatives have been used for millennia to reduce pain, fever and inflammation. In addition, prophylactic use of acetylsalicylic acid, commonly known as aspirin, reduces the risk of heart attack, stroke and certain cancers. Because aspirin is rapidly de-acetylated by esterases in human plasma, much of aspirin’s bioactivity can be attributed to its primary metabolite, SA. Here we demonstrate that human high mobility group box 1 (HMGB1) is a novel SA-binding protein. SA-binding sites on HMGB1 were identified in the HMG-box domains by nuclear magnetic resonance (NMR) spectroscopic studies and confirmed by mutational analysis. Extracellular HMGB1 is a damage-associated molecular pattern molecule (DAMP), with multiple redox states. SA suppresses both the chemoattractant activity of fully reduced HMGB1 and the increased expression of proinflammatory cytokine genes and cyclooxygenase 2 (COX-2) induced by disulfide HMGB1. Natural and synthetic SA derivatives with greater potency for inhibition of HMGB1 were identified, providing proof-of-concept that new molecules with high efficacy against sterile inflammation are attainable. An HMGB1 protein mutated in one of the SA-binding sites identified by NMR chemical shift perturbation studies retained chemoattractant activity, but lost binding of and inhibition by SA and its derivatives, thereby firmly establishing that SA binding to HMGB1 directly suppresses its proinflammatory activities. Identification of HMGB1 as a pharmacological target of SA/aspirin provides new insights into the mechanisms of action of one of the world’s longest and most used natural and synthetic drugs. It may also provide an explanation for the protective effects of low-dose aspirin usage." {Underlined emphasis is mine}From a historical perspective, according to Wikipedia:
"...Hippocrates (circa 460 – 377 BC) first left historical records describing the use of powder made from the bark and leaves of the willow tree to help these symptoms...In 1763, Edward Stone, at Oxford, isolated the active ingredient of aspirin in his discovery of salicylic acid. A French chemist, Charles Frederic Gerhardt, was the first to prepare acetylsalicylic acid in 1853. In the course of his work on the synthesis and properties of various acid anhydrides, he mixed acetyl chloride with a sodium salt of salicylic acid (sodium salicylate). A vigorous reaction ensued, and the resulting melt soon solidified. Since no structural theory existed at that time, Gerhardt called the compound he obtained "salicylic-acetic anhydride" (wasserfreie Salicylsäure-Essigsäure). This preparation of aspirin ("salicylic-acetic anhydride") was one of the many reactions Gerhardt conducted for his paper on anhydrides and he did not pursue it further. Six years later, in 1859, von Gilm obtained analytically pure acetylsalicylic acid (which he called acetylierte Salicylsäure, acetylated salicylic acid) by a reaction of salicylic acid and acetyl chloride. In 1869, Schröder, Prinzhorn, and Kraut repeated both Gerhardt's (from sodium salicylate) and von Gilm's (from salicylic acid) syntheses and concluded both reactions gave the same compound—acetylsalicylic acid. They were first to assign to it the correct structure with the acetyl group connected to the phenolic oxygen. In 1897, chemists working at Bayer AG produced a synthetically altered version of salicin, derived from the species Filipendula ulmaria (meadowsweet), which caused less digestive upset than pure salicylic acid."For the moment, all we have is Moffitt's SITC 2015 presentation title that appears to implicate HMGB1 in the anti-tumor immunity Rose Bengal/PV-10 generates ("Rose Bengal" appears to be the more academically pure reference of the compound).
Thus, for now, we have:
Rose Bengal is a small molecule that laid around in plain sight of the pharmaceutical industry for about 85 years before three former Oak Ridge National Laboratory scientists re-discovered it in their search for the ideal cancer killer. The first recorded medical use of the compound was in 1914 when it was added to Safranin Victoria Yellow for the treatment of ocular pneumococcal infection. In 1998 Provectus Biopharmaceuticals’ founders, Drs. Craig Dees, PhD, Timothy Scott, PhD and Eric Wachter, PhD, identified Rose Bengal as a potentially attractive candidate for inhibiting or preventing the growth and spread of cancer tumor. Rose Bengal, or Rose Bengal disodium as it is also known, is a water-soluble German industrial dye created in 1882. It is a non-biologic whose successful two-prong approach to fighting cancer derives from physical chemistry.
Rose Bengal’s medical properties have long been established: in the clinic (as a stain for visualizing corneal ulcers, as a marker for impaired liver function), in both adults and children, in the literature (more than 3,800 references since early-October 2015 in the US National Institutes of Health's National Library of Medicine’s PubMed Central), and with the FDA (established safety profiles as an intravenous hepatic diagnostic called Robengatope®, and a topical ophthalmic diagnostic called Rosettes® or Minims®).PH-10, part 2 (October 9, 2015)
Following up on Provectus' CTO Dr. Eric Wachter, PhD comments regarding a prospective, potentially pivotal, psoriasis Phase 3 trial, see the items below. Also see PH-10 (October 7, 2015) below.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image spource. Fuzzy purple emphasis is mine. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Provectus' CTO Dr. Eric Wachter, PhD and COO/CFO Peter Culpepper provided a status update on PH-10 via an interview with/article by BioPharm Insight (dated October 2nd). Several items from the interview/article struck me, including:
- Provectus is scheduled to meet with the FDA in 1H16 to finalize details of prospective Phase 3 trials for psoriasis and atopic dermatitis (according to Eric).
- Both Phase 3s would likely have as trial Ns 200 patients, although the psoriasis trial ultimately could have a larger number of patients (according to Eric). I am curious whether Eric's trial N thus far is a reflection of PH-10's impact factor or effect size (whereby compounds with greater benefit require less patients in order to demonstrate a statistically significant performacne improvement over a comparator), or is merely efficient, or some of both.
As context, for example, Amgen and AstraZeneca's pivotal Phase 3 trial of brodalumab in patients with moderate-to-severe plaque psoriasis, reported in 2014, comprised approximately 1,800 participants. Two Pfizer pivotal Phase 3 trials of tofacitinib in patients with moderate-to-severe plaque psoriasis, reported in 2015, comprised more than 1,800 participants.
- Whatever happened to underpromising and overdelivering?
Peter: "Provectus is open for global licencing deals with big pharma for PH10 for future development and commercialisation, Chief Financial Officer Peter Culpepper said. A deal will likely be finalised after the data is available from the company's PH10 mechanismofaction (MOA) Phase II trial (NCT02322086) or potentially after its meeting with FDA in 1H16, he said."
- Another chuckle/shake of one's head.
Peter: "Asked if they are in active licensing talks, Culpepper said details are confidential but added the company's ideal licensee would be global players like Novartis (VTX:NOVN), Lausanne, Switzerland-based Galderma, Parsippany, New Jersey-based Leo Pharma or Pfizer (NYSE:PFE) that have a focus on dermatological therapeutics."
- The trials require one CRO to focus on clinical imaging, whereby "[d]etailed clinical photography will be used to allow an independent agency to do their own objective analysis" (according to Eric). As the old saying goes, a picture tells a thousand words. In an era of readily manipulated images, having a robust, reliable and independent system for image capture, assessment, presentation and archiving is more important than ever.
Takeaways:
- Moffitt Cancer Center's PV-10-related abstract was accepted for presentation at the 2015 annual meeting of the Society for Immunotherapy of Cancer in early-November. See SITC 2015 Preview: Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1 (October 5, 2015) below.
- Moffitt appears to implicate HMGB1 in PV-10's generation of anti-tumor immunity.
- All we currently have is an abstract title. There may or may not be more or much more when the abstract and poster become available.
- Implicating HMGB1 may help "sell" the PV-10 story because Moffitt may have identified a singular and primary raison d'etre for PV-10. There undoubtedly are a lot of known knowns, unknowns and unknown unknowns going on in and with the human immune system; however, it may be more palatable [for the moment] for the industry to hear PV-10's success is caused by one thing (as the industry still may not be ready to learn that the compound's outcomes may be multi-factorial or faceted).
- "Inside the cell, HMGB1 is a highly conserved chromosomal protein acting as a DNA chaperone. Outside of the cell, HMGB1 is a prototypical damage-associated molecular pattern, acting with cytokines, chemokines, and growth factors" (Source: HMGB1 in Cancer: Good, Bad, or Both?, Kang et al., Clin Cancer Res August 1, 2013 19; 4046).
- Could HMGB1 be an immune biomarker for PV-10 treatment, and thus a predictive and prognostic biomarker for this ablative immunotherapy? Read here, here and here, for example.
- It does not appear many biotechnology or pharmaceutical companies are exploring HMGB1. Genentech and Roche's focus areas, for example are below. Note HMGB1 (lower left hand side) as a stimulatory factor (purple) under Step 2, Cancer Antigen Presentation.
 |
| Click to enlarge. Image source |
- HMGB1 is a stimulatory factor in Chen and Mellman's Cancer Immunity Cycle.
 |
| Click to enlarge. Image source |
Finally, some additional background on HMGB1 from The Role of HMGB1 Signaling Pathway in the Development and Progression of Hepatocellular Carcinoma: A Review, Wang et al., Int. J. Mol. Sci. 2015, 16(9), 22527-22540:
HMGB1 is one of the HMGB family members (HMGB 1, 2, 3 and 4) with a molecular weight of 25 to 30 kDa [5], which consists 215 amino acids and three domains. The three domains include HMGB A box (9–79 aa), HMG B box (95–163 aa) and the C-terminal acidic tail (186–215 aa) [4]. In normal organs, HMGB1 acts as a positive factor to protect cells from injury. For instance, the model mice were most likely to be sensitive to liver ischemia/reperfusion [6], pancreatitis [7] and sepsis [8] if HMGB1 was knocked out in the liver, pancreas or macrophages, respectively. In contrast, HMGB1 functions as one of the damage-associated molecular patterns (DAMPs) in the sterile inflammation model by amplifying hepatic ischemia/reperfusion and acetaminophen-induced liver necrotic injury [9]. Moreover, HMGB1 has been demonstrated as a critical role in a number of cancers, including colorectal [10], breast [11,12], lung [13–16], prostate [17], cervical [18], skin [19], kidney [20,21], gastric [22–26], pancreatic [27–29], osteosarcoma [30] and leukemia [31]. As to the signal pathways of HMGB1, its receptors include receptor for advanced glycation end product (RAGE) [32,33], the toll-like receptors (TLRs, such as TLR-2, 4 and 9) [34–37], intergrin [38], α-synuclein filaments [39], proteoglycans (e.g., heparin sulfate [40]), CD24 [41], the T-cell immunoglobulin domain and mucin domain-3 (TIM-3) [42], the member of the G protein-coupled receptors CXCR4 [43], N-methyl-D-aspartate receptor (NMDAR) [44] and the triggering receptor expressed on myeloid cells-1 (TREM1) [45]. {Underlined emphasis is mine}
And:
However, the functions of HMGB1 in cancer are complicated and paradoxical because of its difference of intracellular and extracellular locations. In recent research, it has been documented as a regulator for a number of DNA events, cell differentiation, inflammatory response, cell migration, cell proliferation, cell deaths, cellular senescence, microRNA biogenesis, immune response, tissue regeneration, antibacterial, and so on [4], whereas its actions and the underlying mechanisms on the oncogenesis and advance in HCC are still unclear. {Underlined emphasis is mine}
Updated below again.
 |
| Click to enlarge. |
High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal, Lotze et al., Nature Reviews Immunology 5, 331-342 (April 2005)
High-mobility group box 1 protein (HMGB1), which previously was thought to function only as a nuclear factor that enhances transcription, was recently discovered to be a crucial cytokine that mediates the response to infection, injury and inflammation. These observations have led to the emergence of a new field in immunology that is focused on understanding the mechanisms of HMGB1 release, its biological activities and its pathological effects in sepsis, arthritis, cancer and other diseases.HMGB1 in Cancer: Good, Bad, or Both?, Kang et al., Clin Cancer Res August 1, 2013 19; 4046
"Forty years ago, high mobility group box 1 (HMGB1) was discovered in calf thymus and named according to its electrophoretic mobility in polyacrylamide gels. Now, we know that HMGB1 performs dual functions. Inside the cell, HMGB1 is a highly conserved chromosomal protein acting as a DNA chaperone. Outside of the cell, HMGB1 is a prototypical damage-associated molecular pattern, acting with cytokines, chemokines, and growth factors. During tumor development and in cancer therapy, HMGB1 has been reported to play paradoxical roles in promoting both cell survival and death by regulating multiple signaling pathways, including inflammation, immunity, genome stability, proliferation, metastasis, metabolism, apoptosis, and autophagy. Here, we review the current knowledge of both HMGB1′s oncogenic and tumor-suppressive roles and the potential strategies that target HMGB1 for the prevention and treatment of cancer."Updated (10/5/15): A sampling of HMGB1-related articles.
> New study provides key insights into aspirin's disease-fighting abilities (September 2015)
"We've identified what we believe is a key target of aspirin's active form in the body, salicylic acid, which is responsible for some of the many therapeutic effects that aspirin has," said senior author Daniel Klessig, a professor at BTI and Cornell University. "The protein, HMGB1, is associated with many prevalent, devastating diseases, including rheumatoid arthritis, heart disease, sepsis and inflammation-associated cancers, such as colorectal cancer and mesothelioma," he said.> HMGB1: A Promising Therapeutic Target for Prostate Cancer (April 2013)
High mobility group box 1 (HMGB1) was originally discovered as a chromatin-binding protein several decades ago. It is now increasingly evident that HMGB1 plays a major role in several disease conditions such as atherosclerosis, diabetes, arthritis, sepsis, and cancer. It is intriguing how deregulation of HMGB1 can result in a myriad of disease conditions. Interestingly, HMGB1 is involved in cell proliferation, angiogenesis, and metastasis during cancer progression. Furthermore, HMGB1 has been demonstrated to exert intracellular and extracellular functions, activating key oncogenic signaling pathways. This paper focuses on the role of HMGB1 in prostate cancer development and highlights the potential of HMGB1 to serve as a key target for prostate cancer treatment.> High mobility group box-1 and its clinical value in breast cancer (December 2014)
High mobility group box-1 (HMGB1) is a factor regulating malignant tumorigenesis, proliferation, and metastasis, and is associated with poor clinical pathology in various human cancers. We investigated the differential concentrations of HMGB1 in tissues and sera, and their clinical value for diagnosis in patients with breast cancer, benign breast disease, and healthy individuals.> HMGB1 in Cancer
Cancer results from multiple abnormal cell reactions and acquires ten hallmarks (Hanahan & Weinberg, 2011). When a cellular mechanism malfunctions, the resulting dam- age, if not repaired, may contribute to a cell’s development into malignancy. High mobility group box 1 (HMGB1), a chromatin associated nuclear protein and extracellular damage associated molecular pattern molecule (DAMP), has been shown to be involved in the development and growth of many cancers (Sims et al., 2010; Tang et al., 2010c). Accordingly, research in experimental animal models reveals that rea- gents that block the effects of HMGB1 are helpful in the treatment and risk reduction of several cancers, including solid tumors and leukemia.Updated (10/5/15): The title of the work above, Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1, will be presented on a poster by Moffitt Cancer Center at the 2015 annual meeting of the Society for Immunotherapy of Cancer in early-November.
At AACR 2014 Moffit, which has conducted murine model and clinical work on PV-10, wrote:
 |
| Click to enlarge. |
 |
| Click to enlarge |
Status update (October 5, 2015)
In July Provectus issued press release Provectus Biopharmaceuticals, Sinopharm-China State Institute of Pharmaceutical Industry and Sinopharm A-Think Pharmaceutical Co., Ltd Continue Search for Agreement on PV-10 Use in China to describe the then status of the talks between the parties related a regional geographic license of PV-10. From the PR:
"Dr. Zhidan Jia , Chief Executive Officer of Sinopharm A-THINK, stated, "We continue to work closely with Provectus to arrive at an agreement which defines the terms of our collaboration in bringing PV-10 to the Chinese Market. We hope to come to terms in the near future."On Provectus' August 6th 2Q15 business update call the company's COO/CFO Peter Culpepper said:
"We have continued discussions with the frame of reference established in the original memorandum of understanding, or MOU, signed last year and extended since the passing of the original termination date."There are few so-called comps (or comparable transactions) to guage what might be a good deal, if indeed a deal between Provectus and Sinopharm eventually is struck. See the table below.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
The Usual Suspects (October 4, 2015)
Updates in Melanoma 2015: Entering a New Therapeutic Era (registration required)
 |
| Click to enlarge. Fuzzy purple emphasis is mine. |
 |
| Click to enlarge. Fuzzy purple emphasis is mine. |
 |
| Click to enlarge. Fuzzy purple emphasis is mine. |
 |
| Click to enlarge. Fuzzy purple emphasis is mine. |
Updated below again.
1. Special proxy vote. Provectus filed an 8-K in regards to the special proxy meeting held yesterday for the proposed increase in the company's authorized number of shares. The proposal passed with an 82% "for" vote (63% of votes were cast).
 |
| Click to enlarge. |
- -10% for # of unique visitors (2,257 v. 2,373),
- -4% for # of page views (28,357 v. 30,557),
- -10% for # of visits (8,590 v. 9,020),
- -3% for # of U.S. cities from where visitors came (631 v. 638),
- -16% for # of world cities (119 v. 123), and
- -20% for # of countries (43 v. 54).
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. Image source |
Updated (10/3/15): 4. Lawsuit. H/t InvestorVillage poster Area51: Attorneys for both the defendants and plaintiffs filed a joint motion to stay proceedings pending mediation.
Updated (10/3/15): 5. Heard, paraphrasing.
i. Boehringer Ingelheim (China, presumably) introduced Provectus to Chinese biotechnology company Beigene, which recently entered into a Phase I study of BGB-A317, Beigene's anti-PD-1 compound. In September 2014 Boehringer Ingelheim and BeiGene announced a supply/manufacturing agreement whereby Boehringer Ingelheim BioXcellence would assist Beigene to advance its product candidates through the clinic. See Commercially Reasonable Efforts (July 18, 2015) and BioXcellence™ (July 9, 2015) below. Key opinion leader and Memorial Sloan Kettering Cancer Center's Dr. Jedd Wolchok, MD, PhD was added to Beigene's scientific advisory board in September 2015.
Item takeaways:
- If Bristol-Myers loses its patent-infringement lawsuit against Merck & Co. over the latter's PD-1 drug, doesn't that open the door to any biotechnology or pharmaceutical company developing its own PD-1 compound? At that point, how "special" do these PD-1 compounds remain?
- If an oncologist is able to achieve sufficient upfront stimulation of the immune system (with an immune system primer or activation agent, or with an immune agonist -- both immunomodulatory categories), how much need ultimately is there for back-end immune checkpoint inhibitors (also an immunomodulatory category)?
Of note, the immune system’s involvement in cancer development and progression has sparked much interest in recent years. The model of the cancer-immunity cycle suggests an interplay of immune-suppression and immune-stimulation. In normal individuals, a state of immunosurveillance is in place. However, within the tumor microenvironment, inhibitory signals and immunosuppressive cells are present and tip the scale in favor of immune suppression. {Underlined emphasis is mine}
Continued: The idea of the cancer-immunity cycle proposes that, for a cancer immune response to be generated, the net balance between immune stimulation versus immune suppression must be tipped in favor of the former. Studies in various cancers have suggested that tumors evade the immunogenic process mostly by factors that promote immunosuppression. {Underlined emphasis is mine}The theory of immune surveillance suggests, according to Peggs et al., "...that the immune system plays a key role in suppressing tumor growth and that the incidence of cancer would be much greater were it not for the ability of the immune system to identify and eliminate nascent tumor cells...While the immune system appears capable of eliminating or containing early tumor growth, some tumor cells escape detection and eventually cause cancer." Said another way, when thinking about the growing potential role and promise of cancer immunotherapy, "...we continually develop malignant cells every day that are consumed by the immune system to prevent tumor development, and the immunotherapy drugs seem to target the failure of immune recognition and immune response" (Dr. Peter Salgo, M.D.).
The balance between co-stimulation and co-inhibition is described by Inman et al.: "If sufficient co-stimulation is provided in the presence of adequate tumor-associated antigenic stimulation, the immune system will act against tumor antigen and, thus, destroy early tumors before they become fully established. Contrarily, if co-inhibitory signaling dominates, the immune system will be tolerized to tumor antigens, and the tumor will be permitted to grow unfettered and unmolested by the immune system. If neither co-stimulatory nor co-inhibitory signals dominate, the adaptive immune system may remain in a tenuous state of equilibrium, militating against tumor outgrowth with varying degrees of success."
So, it would seem to me, generalizing (or simplifying, perhaps too much):
- If co-stimulation > co-inhibition, the immune system can act decisively against cancer,
- If co-inhibition > co-stimulation, cancer overwhelms the immune system and renders it ineffective or useless, and
- If co-stimulation = co-inhibition (that is, some sort of equilibrium state), the immune system wages battles against cancer to varying degrees of success with potentially no ultimate resolution to the war itself.
iii.a. Certain Provectus management team members do not appear inclined to update the ClinicalTrials.gov page of Provectus' pivotal melanoma Phase 3 trial, or update the webpage in a timely fashion. For example, as of this writing, the page indicates Atlantic Health System's (AHS') site (and investigator Dr. Eric Whitman, MD) for the the company's trial is not yet recruting patients.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
iv. Management continues to affirm the timing of an interim data readout of Provectus' pivotal melanoma Phase 3 trial as July 2016.
v. In addition to Australia and elsewhere in the U.S., melanoma Phase 3 trial sites would include locations in Brazil, Mexico, Germany and Poland. Sites are expected in India and China, but not until after the aforementioned sites in Australia, South and North America, and Europe are added.
vi. Provectus' melanoma Phase 3 trial design (and its undertaking) is as good as one with a special protocol assessment (SPA) but without the SPA's restrictions.
Is something wrong? (September 29, 2015)
Amgen's intralesional agent (IL) talimogene laherparepvec ("T-Vec") and Provectus' PV-10 are two late-stage oncology assets that, when partnered with an immune checkpoint inhibitor, may prime or activate the immune system.
In April a joint meeting of the Cellular, Tissue and Gene Therapies Advisory Committee ("CTGTAC") and Oncologic Drugs Advisory Committee ("ODAC") voted by an overwhelming 22-to-1 margin in favor (i.e., yes to the question) of T-Vec having an overall favorable benefit-risk profile for the treatment of injectable regionally or distantly metastatic melanoma, and thus supporting traditional approval of the drug. See the blog's April 29, 2015 post The first guy through the wall. A key moment for T-Vec, and for the category of IL agents, was when the panel concluded the reduction of injected tumors equated to/was clinical benefit.
Amgen currently is engaged in two combination therapy trials of IL agent T-Vec and an immune checkpoint inhibitor in advanced (Stage IV) melanoma. See IL-CIB Combinations (September 26, 2015) below.
Given the overwhelming margin of the CTGTAC/ODAC vote above, one would imagine the FDA would approve T-Vec, despite potential concerns over T-Vec's pivotal Phase 3 trial's questionable primary endpoint of durable response rate and questionable trial comparator of GM-CSF, and the issue of viral shedding (T-Vec is a herpes simplex virus type 1).
But, is there something wrong with T-Vec/Keytruda combination therapy trial? Amgen did not report efficacy data from the trial at the 2015 European Cancer Congress. Only safety data was reported.
 |
| Click to enlarge. |
The data cutoff for the trial was 6 weeks after the last patient had his or her first pembrolizumab dose. Patient enrollment was completed by March 2015. The abstract submission deadline for this late-breaking abstract was August 5th. Why was no efficacy data presented? There should have been time the data cutoff to include some efficacy data and comments onto the poster.
 |
| Click to enlarge. |
Maybe T-Vec doesn't work in combination with Keytruda as well as it did in combination with Yervoy.
As an aside, Dr. Long, the presenter of the above abstract, is/was a consultant to Provectus.
 |
| Click to enlarge. Purple fuzzy underlined emphasis is mine. |
H/t a shareholder (a regular tipper of hats for this blog): It appears Provectus made a Trademark/Service Mark Application for PV-10 in June. See a screenshot of the webpage below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
"You must provide proof of use (Statement of Use or SOU) of the mark in the US within 3 years after the date the mark reaches Notice of Allowance (NOA) or becomes “accepted”. NOA is a status reserved for Intent to Use applications and means that the 1b classes on the mark are tentatively approved to be registered pending the filing of a proper and approved SOU." (Source: 1a, 1b, 44d, 44e, Intent to Use, Actual Use: Application Types, Macaria.com)IL-CIB Combinations (September 26, 2015)
The two late-stage intralesional compounds, Amgen's T-Vec and Provectus' PV-10, have been and are being combined with two of the three approved immune checkpoint inhibitors (Bristol-Myers' ipi, Merck's pembro and Bristol's nivo) in early-stage trials for patients with advanced melanoma:
- The Phase 1b/2 T-Vec & ipi trial protocol is here,
- The Phase 1b/3 T-Vec & pembro trial protocol is here (the second study originally was labelled as a Phase 2 trial), and
- The Phase 1b/2 PV-10 & pembro trial protocol is here.
A table comparing selected trial parameters of the above studies is below (I selectively noted certain parameters for comparison in yellowish-orange):
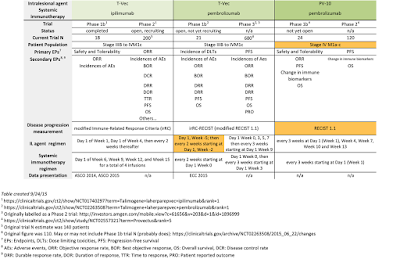 |
| Click to enlarge. |
- In the T-Vec/ipi trial (for which data also was presented at ESMO 2014), ipi is delayed by 5 weeks (see IL agent regimen [Day 1, Week 1] vs. Systemic immunotherapy regimen [Day 1, Week 6] above) in both the Phase 1b and 2 trials. Two things come to mind. First, to the extent that ipi is a standard of care (SOC), or that its proper prescription is for the drug to be given immediately (i.e., day 1) to patients with advanced melanoma, one would imagine this combination protocol (both 1b and 2 portions) is non-standard.
- Second, T-Vec requires 6 to 9 weeks to mount its priming or activation (immunologic signaling) function, (i.e., compared to PV-10, which requires 1-2 weeks).
 |
| Click to enlarge. |
- As expected (in terms of clinical "vector math" related to the combination of the two drugs), T-Vec/ipi Phase 1b investigators noted at ASCO 2014: "In consideration with published reports, these data, although preliminary, suggest higher CR and OR rates than either agent alone and earlier responses after ipi initiation during T-VEC+ipi than with ipi alone." ASCO 2015 comments are here.
- In the T-Vec/pembro trial (for which data will be presented at ECC 2015), pembro is delayed by 5 weeks (see IL agent regimen [Day 1, Week -5] vs. Systemic immunotherapy regimen [Day 1, Week 0] above) in the Phase 1b trial; however, this combination "complies" with pembro as standard of care by administering pembro "immediately" but following a lead-in period for T-Vec. The Phase 3 trial commences administration of both T-Vec and pembro at the same time (Day 1, Week 0).
- Provectus' CTO Dr. Eric Wachter, PhD designed the company's trial only to treat Stage IV patients, while T-Vec/ipi and T-Vec/pembro trials are treating/will treat Stage III and IV patients.
- In both Phase 1b and 2 portions, PV-10 and pembro will be administered at the same time, as is consistent with SOC (for pembro to be honestly administered immediately).
- Disease progression in Provectus' combination trials will be measured by RECIST 1.1, compared to modified measurements by Amgen, Bristol-Myers and Merck.
Updated below again.
Provectus filed its combination trial protocol on ClinicalTrials.gov, PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma.
Updated (9/23/15): Some initial thoughts (with more to follow):
- Provectus' CTO Dr. Eric Wachter, PhD beat expectations he set regarding the filing of the protocol. He had noted on the company's August 6th 2Q15 business update conference call that he anticipated completing the protocol before the end of Q3. Eric did. See Trying to Meet Expectations One Sets (September 21, 2015) below.
- On the same call, as on Provectus' prior May 7th 1Q15 business update call, Eric said the company could move ahead with a combination therapy trial for advanced melanoma with or without a Big Pharma partner. Could Provectus push forward down a regulatory path for a PV-10/pembrolizumab (Keytruda) pairing without the input or say so of Merck & Co. (U.S.)? It would appear it is quite common to pair an investigational therapy (like PV-10) with the standard of care (e.g., pivotal testing of Bristol-Myers' ipilimumab/Yervoy with dacarbazine [DTIC] vs. DTIC) that results in approval of the investigational therapy without requiring use in the combination. Since the SOC is routinely available, this does not require support from the owner of the SOC (i.e., in the case of PV-10 with pembro vs. pembro, and Merck).
- Thinking far too far ahead, how could pricing of the combination work? Once the above is accomplished, there may be situations where the combination is marketed together. This may be the case with Amgen's T-Vec, since the combination of it and ipilimumab appears to be synergistic (see ASCO 2014 and 2015). While it is of course hard to project pricing strategies, the current practice seems to be a la carte, although this may not be sustainable, which was a topic of discussion at ASCO 2015. In the case of PV-10, perhaps adding PV-10 could justify maintaining the high price of the immune checkpoint inhibitor.
- By virtue of Provectus' combination therapy patent (jointly issued to the company and Pfizer), does Provectus effectively "own" the combination of PV-10 and pembrolizumab? Provectus has patent claims covering the combination of course, so the company "owns" or controls any co-marketing. The compound (PV-10) and the drug (pembrolizumab) can be sold separately if PV-10 garners approval as a single agent but, for instance, Merck cannot suggest use of pembrolizumab with PV-10 unless Provectus allows it.
Updated (9/23/15): A key insight for me that derives from the process of the regulatory approval pathway for PV-10 in combination with an immune checkpoint inhibitor such as pembro (Keytruda) for patients with advanced melanoma (Stage IV), and that expands outward to include other pathways to approval (Stage III melanoma, HCC), is the notion of PV-10's use as a single agent (rather than referring to its use as a monotherapy). Thus, as it relates to Provectus' ongoing pivotal Phase 3 trial for unresected/unresectable locally advanced cutaneous melanoma, the company is seeking approval for single agent use of PV-10 for Stage III patients (I will move away from what is/was likely imprecise jargon by previously referring to the use of PV-10 as a monotherapy in this earlier disease stage setting).
The FDA's approval of ipilimumab in 2011 (based on their review of both the DTIC and gp100 studies that supported approval) is an example of a pivotal combination trial that supported single agent approval. While each drug approval certainly can be somewhat unique, the ipi examples reveal a very common strategy in the pharmaceutical industry for pivotal studies — that is, the trial of the investigational agent + the SOC vs. the SOC to gain single agent approval for the use of the investigational agent.
Noteably, this — a pivotal trial of the investigational agent + the SOC vs. the SOC to gain single agent approval for the use of the investigational agent — is exactly the same strategy Provectus appears to have delineated for hepatocellular carcinoma (HCC). In Asia, the SOC(s) more than likely will be local ablation technology(ies). In the U.S. and Western Europe the SOC would be sorafenib.
 |
| Image source |
"However, only recently this property of radiation has attracted the attention of immunologists seeking to induce or improve antitumor immunity. As immune checkpoint inhibitors are becoming mainstream cancer treatments, radiation oncologists have begun to observe unexpected out-of-the-field (abscopal) responses in patients receiving radiation therapy during immunotherapy. These unexpected responses were predicted by experimental work in preclinical tumor models and have clear biological bases. Accumulating experimental evidence that radiation induces an immunogenic cell death and promotes recruitment and function of T cells within the tumor microenvironment supports the hypothesis that radiation can convert the tumor into an in situ individualized vaccine." (Source: Combination of radiotherapy and immune checkpoint inhibitors, Pilones et al., Semin Radiat Oncol. 2015 Jan;25(1):28-33.) {Italicized emphasis is mine} The "abscopal effect" (above) refers "...distant tumor regression after localized irradiation."
What is the goal behind combining co-inhibitory blockage agents (i.e., immune checkpoint inhibitors) and radiation therapy? "[T]he tumor-antigen release achieved by localized radiation will promote specific tumor targeting by the adaptive immune system, which can be augmented further by systemic immune-stimulating agents. In this manner, clinicians hope to induce a phenomenon known as the abscopal effect, whereby localized radiation results in immune-mediated tumor regression in disease sites well outside of the radiation field." (Source: Combining Radiation and Immunotherapy: A New Systemic Therapy for Solid Tumors?, Tang et al., Cancer Immunol Res September 2014 2; 831) {Italicized emphasis is mine}
In order for immune checkpoint inhibitors like Keytruda and Opdivo to remain relevant, they will be combined with other therapies and therapeutics, with the resultant toxicity and cost having to both be managed in some way. See also Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer, Twyman-Saint Victor et al., Nature 520, 373–377 (16 April 2015). Note: Radiation was administered first, followed by co-inhibitory blockade. Is the idea, as noted above, that [localized] radiation reduces or destroys tumors, thus generating antigens/antigen fragements upon which the immune system may act, as the immune checkpoint inhibitor releases the breaks on the immune system?
PV-10 & Radiation Therapy. Radiation therapy has been combined with PV-10 in two situations. First, in 2010, Foote et al. reported on their combination work on 3 patients in A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series, Melanoma Research, 20:48, Jan 2010. All of the patients originally were enrolled in Provectus' metastatic melanoma Phase 2 trial (I believe). All patients achieved complete response (CR) (note detailed descriptions in the article's text); however, no discussion was provided regarding survival (that is, keep in the mind the difference or differentiation between [objective] response rate and overall survival). Note: PV-10 was administered first, followed by radiation.
 |
| Click to enlarge. Fuzzy purple underlined emphasis is mine. |
Note that in the contemplated combination therapy trial of PV-10 and co-inhibitory blockade (immune checkpoint inhibition), PV-10 would be administered first, several weeks before the checkpoint inihibitor would be given to the patient.
Provectus' founders Drs. Craig Dees, PhD, Tim Scott, PhD and Eric Wachter, PhD have long posited that all patients really need is PV-10, under the key assumptions that (i) most if not all of their disease is accessible to injection and (ii) tumor burden is manageable (i.e., not too heavy).
Injecting PV-10 into most or all of the patient's tumors harnesses the immune system via/because of the tumor cell, not inspite of it; see Allison vs/& Dees (September 11, 2015) below. From Provectus' 2013 white paper PV-10 Moves Forward by medical writer Walter Alexander:
"PV-10 murine research demonstrated unambiguously, Dr. Wachter noted, that tumor burden is a critical variable in predicting response to a combination therapy. It has been suggested that earlier research into therapeutic melanoma vaccines faltered because tumor burden grew beyond the immune system’s capacity for control before the vaccine could develop its full effect. “We think that the combination of PV-10 with something like a kinase-inhibitor has the potential to dial back or reduce tumor burden even better than an anti-CTLA-4 agent while the systemic PV-10 immunologic effect is developing. The kinase inhibitor would do the early work against visceral disease until PV-10 can catch up and take the baton across the finish line.”"
 |
| Image source, page 113 |
Eric reported on the progress of this Australian investigator-initiated study at ESMO 2012, noting 7 of up to 25 anticipated subjects were enrolled as of July of that year.
 |
| Image source, page 123 |
The Smither et al. trial continues, but another Foote trial/study appeared to commence in 2011: A phase I/II study of intralesional PV-10 followed by radiotherapy in the treatment of metastatic melanoma? See page 113 of the 2011 annual research report of Princess Alexandra Hospital in Brisbane, Australia. In 2012 Foote changed the objective of another trial/study of his entitled Radiotherapy followed by selective nodal dissection for bulky and/or inoperable nodal melanoma (REFORM) to read: A phase I/II study of intralesional PV-10 followed by radiotherapy in the treatment of metastatic melanoma. See page 103 of the 2012 annual report. The 2013 report reads to the right/above.
I asked Eric about the status update on the PV-10 + radiation therapy study by Foote/Smithers et al. on Provectus' March 12th 4Q14 business update conference call:
Me: Good afternoon, Dr. Wachter. Two questions, more broadly speaking, can you speak to the third-parties outside of Moffitt for example that may be undertaking preclinical and/or clinical work using PV-10? And in addition, could you comment on the 2010 paper by [Phillip Smitters] and all the Australian researchers and clinicians who conducted or examined three cases from your phase 2 study regarding PV-10 treatment followed by radiotherapy, as you have presented in past conferences, they were undertaking a study of up to 25 patients with that kind treatment regimen, and I was wondering if you can provide an update on that? {Italicized emphasis is mine}
Eric: Thank you for those questions, Dominic. I cannot comment on other third-party work that may or may not be underway with regard to non-clinical work with PV-10. Obviously that is something that may be of interest and if it is, and we haven't disclosed this, it’s probably a sensitive nature. But regarding the radiation therapy publication by Food that is the record as having led to an investigator initiated study that you mentioned, 25 patients, that study has been enrolling slowly, it had very specific eligibility criteria at a single center, we have had discussions over the last several months with the investigator about ways to get data from that study available publicly and we anticipate that sometime in the coming months, that is some time this year that there will be some presentation of interim data from that study. {Italicized emphasis is mine. Food = Foote}I received a status update (not from anyone at Provectus) about the trial, but am precluded from blogging about it.
There is a theme or narrative about PV-10 that might be accurate and appropriate. PV-10 may be agnostic to tumor type (histologic subtypes) or disease presentation. PV-10 may harness the immune system through/because of the tumor/tumor cell, not inspite of it/them. In a combination therapy setting, you might give PV-10 first (even when that setting includes surgery). Radiation therapy, kinase inhibitors, immune checkpoint inhibitors, etc. may merely help reduce tumor burden in cases where there is disease inaccessible to PV-10 or heavy tumor burden, so that PV-10 can help the immune system finish off the job.
Trying to Meet Expectations One Sets (September 21, 2015)
Provectus issued a press release today and filed an associated 8-K regarding enrollment in its PH-10 mechanism of action study, Completes Patient Accrual for PH-10 Phase 2 Clinical Study of Cellular and Immunologic Changes in the Skin. See PH-10 (September 17, 2015) below. Of note to me was the company's CTO Dr. Eric Wachter, PhD's quote in the PR:
"Given that the patients remain on the study for a total of 92 days to monitor their response to PH-10, Provectus anticipates that the originally projected completion date of December 2015 will be the actual date of completion for this 30 patient trial."Eric has been hilariously woeful in historically meeting his own expectations with respect to timing of deliverables, events, etc. That he would address his significant shortcoming in the release is not lost on me; however, one point a trend does not make. Eric has future opportunities to change his reputation, and develop a positive trend of meeting (let alone beating) expectations, such as:
- "Turning to the other primary component of our development plan for melanoma, we've continued to move towards commencement of a post-clinical study of PV-10 in combination with a new checkpoint inhibition in patients with advanced metastatic melanoma. After thorough consultation with leading investigators who will conduct this work, we have a study design that is undergoing final investigator review and anticipate completing the protocol before the end of the present quarter." {Eric, in reference to a Phase 1b/2 trial of the combination of PV-10 and an immune checkpoint inhibitor in advanced melanoma; August 6th business update conference call, underlined reference is mine}
- "We're optimistic that we can leverage existing investigator and site relationships to commence this study by the end of the calendar year." {Eric, in reference to the melanoma combination trial; August 6th business update conference call, underlined reference is mine}
- "As we continue learning about PV-10 in liver cancers from our ongoing Phase I study, this relationship with Boehringer is likely to play a pivotal role in finalizing plans for transition to Phase II in HCC in Asia and could impact companion work in the West. We expect substantial progress on this front throughout the remainder of the year." {Eric; August 6th business update conference call, underlined reference is mine}
More hilarity, related to the recent (and revisted) narrative of high drug prices as a result of actions by Turing Pharmeucticals:
 |
| Click to enlarge. |
- Yervoy: Earlier stage use in melanoma — a full regimen cost cost as much as $1.5 million per patient
- Keytruda: $150,000 per year, $12,500 per patient per month (a September 2014 price)
- Combination therapy: One immune checkpoint inhibitor + another checkpoint inhibitor — $x + $x = $2x
- CAR-T technogy companies: In excess of $500,000 per patient? (an October 2014 price estimate)
- PV-10: $30,000 per treatment regimen (a price last used in several year ago by equity research analysts covering the company
Relative price comparisons, such as % CR per thousand dollars of a 12-month treatment price? From a May 2014 blog presentation (using the above mentioned PV-10 price as a placeholder, since the acquiring company would determine the eventual price to market and sell the drug if approved):
 |
| Click to enlarge. |
SA published blog post Special Proxy Vote as Provectus Special Proxy Vote today, and also tweeted links to the article on their website.
 |
| Image sources: Here and here, respectively. |
Other items on this topic:
- Seeking Alpha, and Me (June 27, 2015), Archived News III page (blog)
- Returning to Seeking Alpha, sort of (January 21, 2015), Archived News III page (blog)
- Seeking Alpha, and this blog (August 26, 2014), Archived News II page (blog)
- "Connecting The Dots" (Seeking Alpha Instablog, October 29, 2013)
According to the study's ClinicalTrials.gov webpage, Provectus' Phase 2 Study of Cellular and Immunologic Changes in the Skin of Subjects Receiving PH-10 no longer is recruiting (i.e., the study completed recruiting its patients).
KOL update (September 16, 2015)
Dr. Paul Chapman, M.D., a medical oncologist at Memorial Sloan Kettering Cancer Center and professor of medicine at Weill Cornell Medical College now appears to be a consultant/advisor to Provectus. See a screenshot of disclosures from an August 2015 article below.
| Click to enlarge. |
H/t a shareholder (from September 10th): Moffitt Cancer Center's Dr. Jeffrey Weber, M.D., Ph.D. — he of the "PV-10 might offer the perfect way to prime the immune system" comment — will become Deputy Director of the Laura and Isaac Perlmutter Cancer Center at NYU Langone, Co-Director of its Melanoma Program and Head of Experimental Therapeutics in November.
Special Meeting/Proxy Vote, part 4 (Saturday version) (September 12, 2015)
 |
| Image source (edited) |
Provectus filed a preliminary Schedule 14A on August 21st and a definitive 14A on September 1st seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. Fully diluted shares outstanding at July 31st stood at ~294 million. The special meeting of stockholders the proxy requires takes place on October 1st. I previously wrote I would revisit this topic each Friday on the blog's Current News page until I cast our votes, which will be at least 48 hours before the meeting date and time.
I plan to vote our shares in favor of the proposal, and thus in favor of management. I'll blog our ballot and rationale in due course.
American entrepreneurship, the version where the rule proves the exceptions, is gritty, tough, dirty, full of ups and downs and highs and lows, persistent, consistent, gutwrenching, incoherent, resilient, inconsistent, replete with brilliant, befuddling, smart, dumb, arguably right and wrong decisions, and periodically presents multiple opportunities to quit. This version is especially circuitous when David knows (not thinks, not believes) he can beat Goliath, but has to learn some of how to do it correctly along the way.
Other news items on this topic:
- Special Meeting/Proxy Vote, part 3 (September 4, 2015)
- Tuesday (September 1, 2015), item 3
- Special Meeting/Proxy Vote, part 2 (August 28, 2015)
- Pfizer's Just Not That Into You (August 21, 2015), item Mo' shares.
Updated below.
Anti-CTLA-4 agent/mechanism discoverer and immunologist Dr. James Allison, PhD — he "...was the first to show that antibody blockade of a T-cell inhibitory molecule (known as CTLA-4) could lead to enhanced anti-tumor immune responses and tumor rejection" — provided a keynote address at Immune Profiling in Health and Disease conference today in Seattle, Washington. Luke Timmerman (Timmerman Report, @ldtimmerman) live tweeted several comments from Allison. Alison recently won the 2015 Lasker Award for clinical medical research for his anti-CTLA-4 work.
Generally speaking, Provectus' Chairman, CEO and co-founder Dr. Craig Dees, PhD, posits (as I see and understand it) (among other things) that one has to deal with the immune system upfront by confronting (attacking and completely killing) the tumor, doing it in vivo, and understanding one doesn't know what one doesn't know with respect to immune system mechanisms (including antigen hunting). In some and certain respects, Alison (and ipilimumab [as well as other earlier immunotherapy precursors]) and Amgen's intralesional immunotherapeutic agent T-Vec have paved the way for Craig and PV-10.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
Updated (9/11/15): See graph below.
 |
| Click to enlarge. |
 |
| Image source |
This would lend credence to the belief the additional shares merely are share tools should the company's COO/CFO Peter Culpepper require them for his business and corporate development efforts.
The assumption regarding raising cash would be that it was/is mandated by (i) Provectus' accounting firm BDO to maintain the firm's going concern opinion of the company and/or (ii) the New York Stock Exchange to maintain the company's listing on the NYSE MKT. I am sure there are gray and not-so gray criteria each of the BDO and NYSE utilize in their decision-making. I'm also sure there are black-and-white criteria.
Does Provectus have sufficient cash on hand — assuming no infusion(s) of non-dilutive monies from collaborations, co-developments or licenses — so as not to have to raise cash until after an interim analysis readout? I think so.
Consider the company's balance sheet as at June 30, 2015:
 |
| Click to enlarge. Page 2 of 2Q15 10-Q. |
 |
| Click to enlarge. |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Numbers (September 8, 2015)
Provectus management is preparing their annual CEO letter for 2015, in which I expect the company's CTO Dr. Eric Wachter, PhD to disclose the number of patients treated in the company's compassionate use program (CUP) or expanded access protocol (EAP) for PV-10 for cutaneous or subcutaneous tumors in calendar year 2014. A historical snapshot of the CUP/EAP is included in the company's investor and online corporate presentations (a screenshot of the one from the September 1, 2015 online version is below).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
No (0) or a small number (8) of new CUP patients. With only one PV-10 trial recruiting in 2014 (the since expanded Phase 1 trial for both HCC and liver metastases) and in the face of systemic immunotherapy approvals (Keytruda, Opdivo), a possible or potential takeaway could be PV-10 is not particularly relevant.
 |
| Click to enlarge. No increase in 2014. |
 |
| Click to enlarge. A 2012-like increase in 2014. |
 |
| Click to enlarge. A trend-like increase in 2014. |
 |
| Click to enlarge. A significant increase over 2014. |
 |
| Image source |
Provectus filed a preliminary Schedule 14A on August 21st and a definitive 14A on September 1st seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. Fully diluted shares outstanding at July 31st stands at ~294 million. The special meeting of stockholders this proxy requires takes place on October 1st.
I previously wrote I would revisit this topic each Friday on the blog's Current News page until I cast our votes, which will be at least 48 hours before the meeting date and time.
I haven't determined how I will vote our shares yet. If I had to vote today, I would reject the proposal because I don't believe management has done enough to explain why they deserve shareholder approval. They appear to be trying, including but not limited to amending the reasons for their proposal (the proxy statement's Proposed Amendment) to include some process description related to compensation. See Tuesday (September 1, 2015) below, item 3.
The reasons management has given for their proposal are possibilities. They may even be potentialities. Provectus requires more authorized shares (whether they issue them or not) for the company to remain viable and relevant to BDO and the New York Stock Exchange. Becoming unviable or irrelevant under this scenario, however, does not mean the end of the world for shareholders or the end of Provectus, or the end of the potential for a good investment return.
Voting no means communicating I'd settle for a few dollars per share (give or take) in the near- to short-term by hoping that stopping the increase in authorized shares would somehow force management into a sub-optimal deal or monetization (i.e., one that they don't want but have to do).
On the other hand voting yes means I believe there's the potential for garnering more-to-many-more dollars per share at some reasonable time into the future, whether management issued more shares to remain viable and relevant (and subsequently account for that dilution) or chose to do so for whatever intelligent and strategic reason until an interim transaction (or transactions) and/or the end-game could be achieved.
I don't believe my in-the-moment opposition is particularly principled. Given management's dismal record of shareholder value mismanagement, I'm just trying determine (as best I can) if voting yes might result in a higher share price than voting no.
It's been two weeks, and a few more weeks remain before the meeting.
Other blog posts or news items on this topic:
- Tuesday (September 1, 2015), item 3
- Special Meeting/Proxy Vote, part 2 (August 28, 2015)
- Pfizer's Just Not That Into You (August 21, 2015), item Mo' shares.
Updated below.
Compassionate use site and Phase 3 clinical trial site University of Louisville now is recruiting patients, about a month after appearing on the trial's ClinicalTrials.gov webpage. See Pivotal Melanoma Phase 3 Trial Site (August 3, 2015) below.
 |
Click to enlarge. |
One or more of our securities (September 2, 2015)
It doesn't make sense to me that Provectus would list a "direct" equity security of its on a Asia-Pacific stock exchange (even at an above market valuation). It's not simple to do. It's seems less than smart to do so, when a more straightforward and cost effective approach might be to communicate better, meet timelines, be more thoughtful about what information to release when, underpromise and overdeliver, etc. — in successfully doing so, it should make it more compelling for domestic and international institutional and retail investors to buy the company stock, on the New York Stock Exchange. It's probably not possible as well if the equity security is tied directly to Provectus stock (i.e., a Provectus common share listed on a foreign exchange denominated in that country's currency).
H/t InvestorVillage poster canis_star: A 2012 JP Morgan pamphlet of sorts entitled Strategic Thinking For Hong Kong IPOs is informative about the pros and cons, and requirements and issues to be addressed.
Thinking back at least a year ago, maybe even longer, I recall (perhaps incorrectly) management contemplating different models for a potential China transaction to maximize geography region value capture, and thus company valuation. One was the traditional license approach of upfront, milestone (regulatory and commercial) and royalty payments (e.g., teens to low-twenties percentages of gross revenues). Another was a joint venture (JV) with the prospective marketing/distribution partner (Provectus, while an independent company, was never going to turn over manufacturing to the Chinese), whereby the goal would be to garner half of [say] EBITDA (the local partner would receive the other half). Depending on JV expenses (COGS, SG&A), it probably the case the latter (the JV) has a higher net present value than the former (the license transaction).
If management is exploring an Asia-Pacific IPO, the equity security they could have in mind could be one of a Provectus subsidiary, which then could qualify as "one...of our securities" (the language from the Schedule 14A filing). Very, very loosely speaking, one might think about Yahoo! and Yahoo! Japan, which trades on the Nikkei and is a joint venture between Yahoo! and Japanese internet company SoftBank. There are other examples of publicly traded JVs. A successful outcome for Provectus — a successful IPO however it is formed or structured — probably has several key requirements, such as a complete and presumably filed Phase 1b/2 liver cancer (hepatocellular carcinoma) trial protocol for China, a complete and potentially filed Chinese investigational new drug (IND) application with the CFDA, and an agreeable partner and completed partnership in Sinopharm and/or Boehringer Ingelheim (China). More?
A successfully-IPO'ed JV means a high valuation for the entity, which then should drag up Provectus' NYSE-based market capitalization by virtue of the company's ownership of it. Potentially a lot of work, a less than simple deal structure, a different and non-trivial amount of incremental risk, etc. for what probability of and hoped for amount of success? It has the potential to be a home run, or the possibility of being a strikeout.
Tuesday (September 1, 2015)
Updated below.
 |
| Image source |
- -6% for # of unique visitors (2,373 v. 2,513),
- +3% for # of page views (30,557 v. 29,557),
- -6% for # of visits (9,020 v. 9,585),
- -2% for # of U.S. cities from where visitors came (638 v. 651),
- -13% for # of world cities (123 v. 142), and
- 0% for # of countries (54 v. 54).
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
H/t InvestorVillage poster Area51: "Defendants respectfully request that this Court extend the deadline for Defendants to file the Reply from August 19, 2015 to September 18, 2015. Undersigned counsel has discussed this motion with lead counsel for Plaintiffs and is authorized to represent that counsel for Lead Plaintiff agrees to the requested relief. A proposed agreed order is filed herewith."
3. Special meeting/proxy vote. I'd like to see management discuss, revise and/or specifically address future non-cash compensation, such as some variation on the below:
"These purposes may include: raising capital, which may be effectuated with a contemporaneous listing of one or more of our securities on one or more of the Singapore, Hong Kong, or Australia securities exchanges, although there can be no assurance that we will list any of our securities on any foreign securities exchange; providing equity incentives toNote: I view employee directors included as employees.employees, directors and consultants; establishing strategic relationships with other companies; the acquisition of any business, assets or technology; and other purposes." (Page 8, Schedule 14A)
Updated (9/1/15): Provectus filed its final (definitive) Schedule 14A today seeking shareholder approval to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million number. The prior 14A filing was a preliminary one; see Special Meeting/Proxy Vote, part 2 (August 28, 2015) below.
In recent days I asked management to consider variations of addressing the topic of non-cash compensation, including different compensation approaches and (almost as if not more importantly) their communication of such. I suggested they consider (i) not including themselves (as employees as noted in the above filing paragraph where the word is struck out) as eligible for equity security (i.e., stock options) issuances made possible and available by an increase in the authorized number of shares, (ii) putting their compensation consultant-based [x] compensation committee-approved [y] non-cash compensation packages up for shareholder approval (as and if/when appropriate) and/or (iii) better describing the process by which Provectus would determine the founders and officers' future compensation, particularly the non-cash component upon hoped for interim (from "now" until "whenever") and end-game success.
Management response thus far might be considered the changes in the definitive (final) proxy filing [right-hand side of the screenshot below], compared to the initial (preliminary) one [left-hand side]: "...as determined by our compensation committee in accordance with guidance from Pearl Meyer and Partners, our independent compensation consultants..."
 |
| Click to enlarge. |
[y] The compensation committee of Provectus' board of directors then would (more than likely) approve Pearl Meyer's recommendation (or "guidance"). I cannot find the explicit composition of the committee, save for its charter on the company's website. I imagine if the company calls it "independent," the committee would be comprised of the three directors who meet the independence requirements of the New York Stock Exchange (Smith, Koe, McMasters).
4. Branding. I communicated to management that if they're going to use vendor partners like PharmaHEALTHLabs (and Jeff Meehan) [1] combined with press releasing such use and also communicating it as a milestone [2], (i) don't in the first place unless you (ii) provide a timely update of the results of the event if you qualify the event as a milestone (e.g., essentially, why it is milestone worthy) and (iii) ensure the vendor's named website [3] is relevant, germane and serious as it relates to your own efforts, irrespective of his/its industry reputation (i.e., his/its brand as it relates to the service you utilized and your brand).
[1] The 2015 PM360 Elite, May 2015 (Entrepreneurs, Jeff Meehan, Cancer Action Now); ELITE Entrepreneur Jeff Meehan of Cancer Action Now, May 2015
 |
| LinkedIn screenshot 9/1/15 |
 |
| Slide 4, August 27th Online Corporate Presentation |
 |
| PharmaHealthLabs screenshot on 9/1/15. Website link |
 |
| Image source |
I haven't determined how I will vote our shares yet. If I had to vote today, I would reject the proposal because I don't believe management has done enough to more broadly explain and rationalize the approval they seek and, if approved, the potential/possible future outcome(s) for the company and shareholders.
To formulate my decision I asked (in my August 21st news item) and continue to ask three questions:
- Why is management seeking to raise the company's authorized share amount?
- Why are they seeking to raise it now?
- Why does management deserve shareholder approval?
"Reasons for the Proposed Amendment
The total number of shares of common stock (i) issued and outstanding, (ii) reserved for issuance pursuant to warrants to purchase common stock, and (iii) reserved for issuance pursuant to options to purchase common stock granted under the Provectus Pharmaceuticals, Inc. 2012 Stock Plan and the Provectus Biopharmaceuticals, Inc. 2014 Equity Compensation Plan totals 293,813,501 shares of common stock. In addition, on July 22, 2013, we entered into a purchase agreement (as amended on June 18, 2015, the “Alpha Capital Purchase Agreement”) with Alpha Capital Anstalt (“Alpha Capital”), pursuant to which we may, in our sole discretion, direct Alpha Capital to purchase up to $10,000,000 of our common stock over the 30-month term of the Alpha Capital Purchase Agreement, at a per share purchase price equal to the lesser of (i) the lowest sale price of our common stock reported on the NYSE MKT on the purchase date and (ii) the arithmetic average of the three lowest closing sale prices for our common stock during the 12 consecutive business days ending on the business day immediately preceding the purchase date; provided, however, that in no event may Alpha Capital purchase shares of our common stock for less than $0.75 per share. On April 30, 2014, we entered into a Controlled Equity OfferingSM Sales Agreement (the “Cantor Agreement”) with Cantor Fitzgerald & Co., as sales agent (“Cantor”), under which we may issue and sell shares of our common stock having an aggregate offering price of up to $50,000,000 from time to time through Cantor, acting as sales agent. Because the amount of shares we may issue and sell pursuant to the Cantor Agreement and the Alpha Capital Purchase Agreement depends on the value of our common stock at such time, we believe an increase in the number of shares of our common stock that we are authorized to issue is advisable to ensure that we have a sufficient number of shares of common stock available for issuance if and when we determine that it is in the Company’s best interest to issue and sell common stock pursuant to the Cantor Agreement or the Alpha Capital Purchase Agreement.
In addition, our Board of Directors believes that the amount of common stock we have available for issuance is insufficient for our future financing needs because it is likely that the sale of shares of common stock or securities convertible into shares of common stock will be the principal means by which we will raise additional capital until such time as we are able to generate earnings sufficient to finance our operations. Shares of common stock may be used for various purposes without further stockholder approval. These purposes may include: raising capital, which may be effectuated with a contemporaneous listing of one or more of our securities on one or more of the Singapore, Hong Kong, or Australia securities exchanges, although there can be no assurance that we will list any of our securities on any foreign securities exchange; providing equity incentives to employees, directors and consultants; establishing strategic relationships with other companies; the acquisition of any business, assets or technology; and other purposes. Although our Board of Directors has no current plan, arrangement or commitment to issue additional shares of common stock, our Board of Directors believes that it is in the best interest of us and our stockholders to have a sufficient number of authorized but unissued shares of common stock available for issuance in the future for such purposes.
Possible Anti-Takeover Effects of the Amendment
The proposed amendment to our Certificate of Incorporation is not being recommended in response to any specific effort of which our Board of Directors is aware to obtain control of the Company, and our Board of Directors does not intend or view the proposed increase of authorized common stock as an anti-takeover measure. However, the ability of our Board of Directors to authorize the issuance of the additional shares of common stock that would be available if the proposed amendment is approved and adopted could have the effect of discouraging or preventing a hostile takeover."Nearing the 300 million limit of authorized shares, management requires more shares for potential issuances in the future, via a private offering directly to a corporate purchaser [unstated above], an offering like June's Maxim Group-led one to financial investors [unstated above] or one of the vehicles represented by Cantor or Alpha Capital [stated above] to financial investors.
The circumstance of no shareholder-approved increase but yet the potential need for shares at some point more than likely causes Provectus' accounting firm BDO and/or the New York Stock Exchange to question the company's going concern and/or listing status, respectively. If no ability exists to issue shares BDO and/or the NYSE could question the company's future viability.
For management to try to monetize Provectus at a respectable valuation, let alone one that is commensurate with the company's worth (at least as I see it), CTO Dr. Eric Wachter, PhD and CFO/COO Peter Culpepper require the tools funded and/or made available by a positive shareholder vote. Eric and his team need to clinically develop the drug further, which may require money derived from the issuance of the new shares at some point in the future (especially if no money arrives by way of a transaction before then). Peter needs to translate this clinical development into license, collaboration, equity investment and/or sale-of-the-company transactions. In order to consummate a transaction, he may require new shares to issue to a prospective partner.
Management could (a) IPO some form of Provectus equity security on an Asian stock exchange (presumably at an above market valuation), (b) sell an equity security to a prospective partner with or without special rights or rights of first somethings (also presumably at an above market valuation), or (c) IPO and sell equity. Reason (a) alone does not make sense; IPOing on an Asian stock exchange for shits and giggles is obtuse, even by management's past standards. Reason (c) makes sense if it helps Provectus come to terms with Sinopharm, although it seems an overly complex solution to the problem of bridging differences between the parties. Reason (b) makes sense if a potential partner has expressed interest in a relationship with the company; however, the valuation of such a deal likely would have to be balanced against providing special rights or rights of first somethings to said partner.
Q2. Now could be as good a time as any for Provectus to bring this proposal to shareholders in light of the company essentially hitting its authorized share ceiling. Funding Eric is not a near-term issue given what should be a August-end cash balance of ~$19.5 million. Providing "share tools" for Peter may well be a near-term need if his business/corporate development efforts assume discussion(s) of the sale of Provectus equity to a prospective partner. Could the board and management have sought the proposal earlier? Sure. Could they seek it later? Maybe, but if current partner discussions require it in the here and now, then probably no.
It certainly would have been much more respectful to shareholders for Peter (and, in context, Eric) to discuss the proposal on the August 6th business update conference call. He of course would have to have filed the proxy statement prior to conducting the call. Putting voices (on the call) to words (in the filing) may have helped both shareholders and management.
Q3. Management hasn't done enough to this point (in terms of broad, substantive and effective communication) to deserve shareholder approval. They should be capable of rationalizing the approval they seek, and describing the possible/potential future outcome(s) for the company and shareholders if the proposal is approved. A few hundred words in an SEC filing don't cut it. Management continues to poorly communicate broadly to the very [retail] shareholders who have funded their circuitous path. The manner in which the proposal has been handled to date underscores each and every Provectus manager's continued ineffective handling of their responsibilities regarding shareholder value. That said, it's only been a week, and I continue to look forward to hearing Chairman and CEO Dr. Craig Dees, PhD et al.'s rationale/description.
I plan to revisit this topic each Friday morning on the blog's Current News page until I cast our votes, which will be at least 48 hours before the meeting date and time.
Not that good (August 27, 2015)
1. Some dialogue today on Twitter regarding side effects of anti-PD-1 therapeutics:
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
"Once the diagnosis of this disease is suspected, treatment of diabetic ketoacidosis must be started immediately, as in all other cases of type 1 diabetes. Otherwise, the death of the patient is likely to occur within 24 h. All medical practitioners must remember that this extremely rapidly progressing type of diabetes does exist, and they must pay special attention not to overlook it" (Source: Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners)2. Amgen filed a Phase 1 trial protocol in July 2015 for the use of T-Vec in patients with hepatocellular carcinoma (HCC) and liver metastases.
 |
| Click to enlarge. |
In Provectus' Phase 1 liver trial of both HCC and liver mets, note the presentation of disease PV-10 treated below from the ESMO-GI 2015 poster, and that life expectancy was greater than or equal to 12 weeks (3 months).
 |
| Click to enlarge. |
3. An earlier article I had meant to note: Science Isn’t Broken. It’s just a hell of a lot harder than we give it credit for.
 |
| Image source |
 |
| Click to enlarge |
The Doctor Is In (August 25, 2015)
 |
| Image source |
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source. Yellow fuzzy emphasis is mine |
 |
| Image source |
- A meaningful interim data readout of Provectus' pivotal melanoma Phase 3 trial around July 2016, or
- The potential for the trial's data monitoring committee to observe statistically significant progression-free survival (PFS) [f/n 1] curve separation between PV-10 and systemic chemotherapy (dacarbazine or temozolomide) before-to-well before July.
Second, let's say I also assume [f/n 2]:
Thus, Provectus' primary 12-month share price catalyst is an early positive outcome from its pivotal melanoma Phase 3.
Note: Although the company (specifically, COO/CFO Peter Culpepper) has indicated "[c]ash on hand supports planned operations until into late-2017," Provectus' accounting firm BDO may require the company to raise money in or by the end of 2Q16.
Friday's Schedule 14A filing, see Pfizer's Just Not That Into You (August 21, 2015) below, illustrates Peter's efforts to raise the share price in anticipation of an early positive Phase 3 trial result: attempting to IPO the stock on one or more Asian/Pacific stock exchanges, and/or selling a piece of the company to a pharmaceutical company. His goal of the former (if successfully executed) could be to hope for a higher Asian stock price would drag the U.S. stock price up. His goal for the latter (if possible) would be for an equity investment either as part of an Asian IPO at an upround valuation (to the then U.S. market capitalization) and/or separately at a well-above market valuation to have the same upward effect on NYSE MKT:PVCT. In both cases, he requires shareholder approval of the board recommendation and company proposal to raise the number of authorized shares.
[f/n 1] Progression-free survival is the trial's primary endpoint.
[f/n 2] Utilizing language and thinking derived from this article.
Pfizer's Just Not That Into You (August 21, 2015)
Pfizer. Provectus' joint patent with Pfizer was issued this past Tuesday (by the US Patent and Trademark office) without the former able to include any significant reference to the latter in its associated press release — see Intellectual Property (August 18, 2015) below. According to Provectus' investor relation firm Porter, LeVay & Rose's Michael Porter, Pfizer had a draft version of the company's proposed PR for several weeks leading up to the August 18th patent issuance date. This date was available on the U.S. Patent and Trademark Office's Patent Application Information Retrieval website as early as July 29th.
Michael said the draft PR included Pfizer's name in its title, much like Provectus' April PR related to the patent's allowance (among other planned or hoped for additional references to and/or placeholders related to Pfizer). At the proverbial eleventh hour (i.e., early morning Eastern Daylight Time of the August 18th) a lawyer in Pfizer's Legal Division rejected the use of Pfizer's name (and thus, presumably, most or all other references to it). The lawyer's explanation for her decision, according to Michael, was that [the issuances of] patent-related PRs were not important to Pfizer; they are potentially very important to small companies like Provectus.
Listening to Michael, it sounded like the lawyer's last minute timing was due to her being on vacation and not getting around to dealing with the matter until after her return. The situation, at least from Pfizer Legal's perspective, merely could be something like it's just not that into Provectus, a view that may or may not be consistent with operationally-focused parts of the organization. Only time will tell.
Ownership vs. Inventorship. The patent-related PR that Provectus ultimately issued contained a sole reference to the pharmaceutical company, "Pfizer Inc. is a joint owner of the patent," which was either Provectus' attempt at maintaining some association to Pfizer in the PR or a solitary concession by the pharmaceutical company. Pfizer's Dr. Craig Eagle, MD is a co-inventor of the patent, along with Provectus' Chairman and CEO Dr. Craig Dees, PhD, CTO Dr. Eric Wachter and primary consultant Jamie Singer. Pfizer itself is listed as a co-assignee; the other assignee is Provectus. Writing in the PR that Pfizer is a joint owner could suggest the pharmaceutical company enjoys economic benefit from all of the patent's claims, which I do not believe to be the case.
Based on the perspectives of several shareholders who have discussed the matter with management over time, it's much more likely Pfizer's economic benefit is limited to the outcome of buying Provectus — if Pfizer buys Provectus it thusly benefits from the sales of drug combinations that include PV-10, but if Pfizer does not buy the company it gains nothing by way of patent-derived benefit (i.e., royalties).
This view presumably reflected discussions between Eagle and Provectus "back when" regarding a better way of harnessing a patient's immune system:
Eagle may have thought there was promise in combining local delivery of an immunomodulator (PV-10) with systemic delivery of an immunomodulator (tremelimumab), and envisioned Pfizer eventually owning a workable PV-10/treme combination. Treme eventually was mostly packaged off to AstraZeneca/MedImmune on October 3, 2011. Craig et al. likely envisioned the larger combination set of PV-10 + fill-in-the-blank, and put this into practice at least preclinically.
By giving Provectus ownership of the patent under the scenario where Pfizer does not acquire it, which might have been construed as a fair trade at the time, Eagle and Pfizer (i) may not have thought much about PV-10 use and thus value outside of a combination with treme, (ii) likely did not envision the immuno-oncology landscape would evolve to where so-called back-end agents like the immune checkpoint inhibitors (e.g., Keytruda, Opdivo, etc.) need immune system priming or activation help to remain relevant, and (iii) PV-10 would be the robust, multi-faceted small molecule it now appears to be. If they had, Eagle and Pfizer might have pressed for true joint ownership of the patent.
Mo' shares. Provectus filed a Schedule 14A today seeking shareholder support to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million. The number of fully diluted shares outstanding currently stands at 293,813,501. The special meeting of stockholders this proxy requires would take place on October 1st in New York. The recommendation's primary explanation appears to be:
"[E]stablishing strategic relationships with other companies" means Peter would consider a strategic equity investment in the company by a pharmaceutical company. In order to facilitate such an investment or buying of Provectus shares Peter again would require more authorized shares than are currently available.
From a capital formation and shareholder value perspective I am not against increasing the number of authorized common shares — as long as these newly issued shares go toward a useful, specific, and clearly and sufficiently explained purpose (or purposes) that objectively could result in the enhancement of shareholder value. The paragraph above that I highlighted from today's Schedule 14A filing simply is not enough information upon which to base one's support of Provectus' board of directors' recommendation to vote for the proposal (to increase the number of authorized shares). Given Craig, Provectus' President Dr. Tim Scott, PhD, Eric and Peter's dismal record of shareholder value mismanagement, and in the absence of more specific information about the proposed or potential use(s) of the additional shares, I don't think management deserves another blank check.
My comments above have nothing to do with the clinical side of the business, the pursuit of value utilizing all of Provectus' clinical assets, and the maximization of shareholder value by developing these assets. Rather, my views have everything to do with management more fully explaining why they are seeking to raise Provectus' authorized share amount, why they are seeking to raise it now, and why they deserve such authorization by shareholders. I look forward to hearing Craig et al.'s explanation.
Intellectual Property (August 18, 2015)
Updated below.
Provectus' joint patent with Pfizer, Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer, which was allowed in April, issued today.
Updated (8/18/15): Provectus issued a press release today and filed an associated 8-K regarding the patent issuance, Awarded US Patent Protecting Use of PV-10 as Part of Combination Therapy for Cancer. In April when the combination therapy patent was allowed, the press release headline included Pfizer's name. Today's PR included a sentence at the bottom mentioning Pfizer, "Pfizer Inc. is a joint owner of the patent," which could call into question the veracity of inventorship (recognition of Pfizer's Dr. Craig Eagle, a Provectus strategic advisory board member - see quote below) and ownership (who owns what and under what scenario[s])).
Twofer (August 17, 2015)
Pfizer. Below are a couple of screenshots from Pfizer's Oncology Research Unit's (ORU's) partnering webpage (the main page would be that of Worldwide R&D).
Breast cancer. Regarding Moffitt Cancer Center's purported clinical use of PV-10 in breast cancer — see Breast Cancer (August 8, 2015) and More Notes (August 10, 2015) below — the shareholder who first informed me of it followed up to note the patient's treatment involved "regular scheduled injections" of PV-10.
Mostly a note to self (August 16, 2015)
Definitionally, it seems, the grail is anti-tumor immunity, while the myth is a pathological complete response (pCR).
Institutional (August 14, 2015)
13F filings (through today) for the period ending June 30, 2015 showed an increase in institutional share holdings of Provectus: 12.65 million share owned/held (up from 4.25 million as at 3/31/15, or about +200%), and 6.18% of shares outstanding (not a fully diluted figure) (up from 2.28%). See Downer (May 18, 2015) for the last institutional holding blog report on the Archived News III page.
New funds that may (or may not) have participated in the Maxim Group bookrun offering and that filed 13Fs were Peter B. Cannell & Co (90K shares), Cormorant Asset Management (a story about founder Bihua Chen) (2.2 million), Millennium Management (500K), Sabby Management (a description of founder Hal Mintz) (~3.28 million) and Susquehanna International Group (~2.45 million), totalling about half of the offering's 17.5 million shares (plus matching warrants).
More Notes (August 10, 2015)
Updated below.
1. Breast cancer. Since Provectus has no clinical programs currently at Moffitt Cancer Center (the mechanism of action study is finished), it would seem the PV-10 breast cancer activity is Moffitt's. See Breast Cancer (August 8, 2015) below. The cancer center notes an industry-sponsored Phase 0 trial examining novel agents in breast cancer, on which I can find no additional information (i.e., no easily or clearly associated trial on ClinicalTrials.gov).
Phase 0 clinical trials are also called exploratory IND (investigational new drug) studies (or micro-dosing studies), and covered by FDA guidance entitled Guidance for Industry, Investigators, and Reviewers, Exploratory IND Studies, Center for Drug Evaluation and Research (CDER), January 2006, Pharmacology/Toxicology. Another take:
2. Combination therapy for advanced melanoma. Eric noted on Provectus' August 6th conference call (downloadable transcript):
In contrast, the combination of Amgen's IL agent talimogene laherparepvec (T-Vec) and pembrolizumab is a Phase 1b/3 (the 3 is an RCT of pembrolizumab with or without T-Vec) using a cumulative trial N of 680 (20 in the Phase 1b and apparently 660 in the Phase 3), which is to say that's not much of an effect size. Additionally, one has to love the "language of the trial," where pembrolizumab will be administered on Day 1, Week 0, which would make one think it is given to the patient upfront, except that T-Vec is administered on Day 1, Week -5 (minus 5).
3. Tumor-specific. A tumor-specific response is important to the durability of the immunotherapeutic response of a drug. While T-Vec may activate "a systemic T cell mediated anti-tumor immune response,"* it does not appear to mediate or generate a tumor-specific one. Dendreon's Provenge and CAR-T companies like Cellectis, Juno Therapeutics, Kite Pharma, etc. generate tumor-specific T cell responses. PV-10 generates a tumor-specific T cell response too. See Tumor [immune] response vs. Tumor-specific [immune] response (July 31, 2015) below.
Interestingly Medimmune entered into strategic cancer vaccine collaboration and license agreement with Inovio Pharmaceuticals today, noting in its press release:
3b. Updated (8/10/15): Today Merck U.S. entered into a collaboration with Immune Design to test two Immune Design immuno-oncology agents in combination with Merck's pembrolizumab, where Immune Design noted in its press release:
Earlier work (reported in 2011) combining 4-1BB activation and CTLA-4 blockade led to enhanced tumor rejection: "This study shows that combining T-cell co-inhibitory blockade with αCTLA-4 and active co-stimulation with α4-1BB promotes rejection of B16 melanoma in the context of a suitable vaccine."
Ironically Kohrt and other wrote in a 2014 paper entitled Intratumoral Immunization: A New Paradigm for Cancer Therapy:
Breast Cancer (August 8, 2015)
H/t a shareholder (who approved the following sentence): A shareholder said he or she has first-hand knowledge of a breast cancer patient being treated with PV-10 at Moffitt Cancer Center.
Should the above be true, it would appear Moffitt may have moved on from its original preclinical work on PV-10 and this indication (below) to clinical investigation of it.
Moffitt's 2013 PLoS One paper on Rose Bengal (PV-10) concluded:
Meh (August 7, 2015)
Provectus held its 2Q15 business update conference call yesterday. For me the call evoked an immediate feeling of meh following it conclusion, perhaps because of the company's CTO Dr. Eric Wachter, PhD's continued Paul Masson-esque communications reticence, and COO/CFO Peter Culpepper's game show host-esque introduction this time. Upon further reflection the call contained some good, some bad, some nuggets of useful and insightful information, and some possible dots for potential connecting. In a few places the call was more notable for what wasn't said than for what was. I'll confirm my initial thoughts (and additional initial thoughts triggered by the call) below after listening to a replay and reading the call's transcript.
1. Pivotal melanoma Phase 3 trial site activation slippage. Eric seemed to confirm activation was going slower than the faster expectations management set in April when a company press release noted "...additional sites to be added in the coming weeks and months." Clinical trial sites, as Eric confirmed on the call, were more likely to be activated in July, August and September as I wrote in Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part I (July 13, 2015) on the blog's Current News page.
2. Phase 3 trial interim analysis slippage. Peter noted one could expect the interim analysis this time next year (i.e., August 2016), rather than initial expectations he and Eric set of sometime in the first half of 2016. His confirmation of a later planned interim analysis for efficacy and safety would be consistent with my later timing estimate of 2H16 in July 23, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV. Of course, I also think there is a smaller number of event threshold that would be triggered sooner than that. See August 4, 2015 blog post August 4, 2015
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part VI.
3. Asia (China) Phase 1b liver protocol slippage. It seems to me Provectus is moving ahead with Boehringer Ingelheim (China) (BI China) to finalize a suitable regional liver protocol and file an investigational new drug (IND) application directly with the Chinese FDA (or via Hong Kong). BI China, and BI more broadly, is a true pharmaceutical company while Sinopharm is a drug distributor (with whom BI also has a drug distribution relationship). If a regional deal is struck with Sinopharm one has to imagine it would be a drug distribution relationship, while if one is consummated with BI China one could surmise it would be a [traditional pharmaceutical] license transaction.
4. Asia/ROW liver clinical development program convergence. It struck me an Asian Phase 1b liver trial might converge with the existing U.S. expanded Phase 1 liver trial to eventually become a global or international Phase 2 liver randomized controlled trial (considering Eric's comments regarding sorafenib/Nexavar's regulatory approval path).
5. Authorized shares. As I noted in Notes (August 6, 2015) the end-of-2Q15 fully diluted shares outstanding figure appears to be ~294 million, leaving about 6 million available (but not addressing issuance of warrants for services going forward). Nearly 10 million warrants were issued in 2010, with about 5 million forfeited in 1H15. This could mean Peter has leeway under the authorized share ceiling of about 11 million shares. My note below that Provectus would have to raise money in 3 to 4 quarters (i.e., 8-11 months) also assumes he does and can not consummate a strategic equity investment by a Big Pharma. It also the share price remains the same such that he either does not receive some cash for warrant exercises (and/or cashless exercises provide more shares under the ceiling).
6. Moffitt, Part I. Eric noted the orthogonality of Merck's approved anti-PD-1 agent, Keytruda (pembrolizumab), and PV-10 (as explored by Moffitt Cancer Center). Orthogonality (as Provectus refers to it) not only means low or de minimis clinically relevant drug-drug interaction, it also means (I believe) the cumulative efficacy of the individual drugs in combination (in math, the magnitude of the combination vector is the magnitudes of the individual vectors adjusted by the sin of the degrees between the vectors, which is at its maximum when the vectors are orthogonal), and thus his (or Moffitt's) preference for use in a combination trial. What happened to Bristol-Myers' drugs, either anti-CTLA-4 ipilimumab or anti-PD-1 nivolumab?
7. Moffitt, Part II. There was no mention by Eric of Moffitt's work with anti-PD-L1 agents. Recall their initial murine model work on combinations of PV-10 and immune checkpoint inhibitors (Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma). One has to think Moffitt also undertook so-called orthogonality work of one or more of these agents, whose owners include Pfizer (via Merck KGaA), Roche/Genentech and AstraZeneca.
8. Moffitt, Part III. I (as did another shareholder) though I (we) heard Eric say Moffitt's mechanism of action work might be available (published/presented) before year-end, sooner than "later this year or early in 2016" noted on the company's May 7th business update conference call. If so, the venue and timing could be SITC's 30th Anniversary Annual Meeting (i.e., SITC 2015) on November 4-8. Moffitt presented their combination therapy murine model work referenced in 7. above at SITC 2014.
9. T-Vec. Eric said a combination therapy protocol (for patients with advanced melanoma) would (might) be completed this quarter, presumably leveraging key opinion leader and clinical site experience and feedback, and a review of T-Vec/ipilimumab Phase 1b trial results. But I also have to wonder how much more positive influence T-Vec may yet have on PV-10. Provectus finalized its pivotal melanoma Phase 3 trial protocol before T-Vec's advisory committees' review meeting at the end of April. The assembled panel concluded the reduction of injected tumors equated to/was clinical benefit (see April 29, 2015 blog post The first guy through the wall.) Aside from potentially having to incorporate T-Vec as a possible comparator in the Phase 3 trial, T-Vec could very positively influence the trial's protocol design.
Notes (August 6, 2015)
1. Provectus issued a press release today and filed an associated 8-K regarding its 2Q15 financial results, Reports Second Quarter 2015 Financial Results, in conjunction with filing its 10-Q.
2. The end-of-2Q15 cash balance (i.e., B/S item Cash and cash equivalents) was $23.1 million, compared to the prior end-of-Q's $14.2 million figure.
3. The company's monthly cash burn for the quarter appears to have been $1.34 million, -4% quarter-over-quarter.
4. Working in the gray — e.g., using a cash balance threshold of $12 million, estimating a monthly cash burn range of $1 million (a presumed "rough" projected figure for accounting firm BDO's purposes) to $1.4 million (on the current high end of recent actual), assuming the absence of non-dilutive deal-related cash inflows, making an assumption regarding an acceptable low watermark for the then pre-raise cash balance figure, assuming an increase in the authorized share number, etc. — Provectus would have to raise money in 3 to 4 quarters (i.e., 8-11 months).
5. Regarding the class action lawsuits (see the current Q's No. 7 under Notes on page 10): "On June 5, 2015, Provectus filed its Motion to Dismiss the Consolidated Complaint (the “Motion to Dismiss”). On July 20, 2015, the Lead Plaintiff filed his response in opposition to the Motion to Dismiss (the “Response”). Pursuant to order of the Court, Provectus must reply to the Response no later than August 19, 2015."
6. A derivative lawsuit naming the company as a nominal defendant was added in the quarter: the Donato Shareholder Derivative Lawsuit. See the current Q's No. 7 under Notes on page 12.
7. The end-of-2Q15 fully diluted shares outstanding figure appears to be ~294 million.
I was in the ballpark: ~293 million without assumptions for stock and warrants issued for services; see Offering, Part I (June 29, 2015) on the blog's Archived News III page.
8. One wonders if Maxim exercised its over-allotment of common stock, which would have expired on August 3rd. If exercised the transaction would have netted Provectus an additional ~$1.8 million. Given the company's COO/CFO Peter Culpepper's approach to reporting, if Maxim had exercised its option he more than likely would have reported it in the current Q (which it appears does not). As an aside, management previously noted the "cooling off period" for the warrant "IPO" as 90 days, which may suggest Maxim could initiate coverage of PVCTWS (which for all intents and purposes is covering PVCT) towards the end of September.
9. What would a Provectus fiscal quarter be without money raise via Network 1 Financial? The company appears to have received net proceeds of ~$589,000 in the quarter under the existing private placement (i.e., $1 unit of 1 share of common stock + half of a warrant with a $1.25 exercise price). See the current Q's Item 2 on page 20.
10. This breast cancer patient chat room/board comment from 2013 (huh?) about PV-10 and melanoma could suggest one or more Canadian sites are part of Provectus' melanoma Phase 3 trial.
The Calgary site referenced above may be Tom Baker Cancer Centre in Calgary, Alberta (postal code T2N 4N2), which is a clinical site for Amgen's talimogene laherparepvec (T-Vec). There are and have been other T-Vec sites in Canada (i.e., at least in Alberta, Ontario and Quebec).
11. It is possible the University of Pennsylvania's Abramson Cancer Center (Philadelphia, PA), which posted both Provectus' melanoma Phase 3 and liver Phase 1 clinical trial information before taking it down, is a satellite site of St. Luke's Cancer Center (Bethlehem, PA) for the Phase 3 trial. St. Luke's (Temple University), Abramson, Penn State's Hershey Melanoma Center, Thomas Jefferson University's Kimmel Cancer Center, the University of Pittsburgh, and The Wistar Institute are members of the Consortium of Melanoma Centers of PA. Consortium objectives include "Clinical trials on personalized medicine (Accrual of patients to personalized therapeutic trials spanning sites)."
Spending Alpha (August 4, 2015)
Each efficacy look into a randomized controlled trial (RCT) incurs a statistical ding (i.e., "costs alpha") — that is, looks at the data increase Type I error risk (safety looks should cost nothing). For drugs with low effect sizes (i.e., low measures of treatment effect when compared with a control) multiple looks can be problematic and may be mitigated by running trials with large Ns (i.e., large number of patients).
In Provectus' pivotal melanoma Phase 3 there should be a large treatment effect between the treatment arm of PV-10 and the control arm of systemic chemotherapy (i.e., dacarbazine or temozolomide). The accordant effect size should facilitate multiple planned or pre-specified (communicated or uncommunicated) efficacy looks into the data.
It seems to me, however, that the company's Phase 3 trial is more than just about PV-10's progression-free survival (PFS) curve separating as quickly as it can (by at least 70%) from the chemotherapy PFS curve. The fundamental hypothesis of the trial, according to Provectus' CTO Dr. Eric Wachter, PhD is that "local therapy can afford direct clinical benefit to melanoma patients in terms of eliminating lesions," which means "melanoma staying away [is] measured by progression free survival between the PV-10 arm and controlled arm." Melanoma staying away, or the hypothesis that eliminating lesions affords clinical benefit, is underscored by the trial's secondary endpoint of overall survival (OS).
The trial may or may not have an early efficacy look designed into it that PV-10 can afford for PFS, statistically speaking, but can the drug afford it for OS? I imagine so, but the real test of alpha spend affordability course will be when secondary endpoint OS data eventually is readout. I believe the trial will continue to run, chemotherapy patient crossover and crossover analysis notwithstanding, to collect OS data to definitively prove or disprove the trial's hypothesis — potentially much more so (if PV-10 OS is robustly lengthy and statistically significant) than Amgen's T-Vec, which barely missed achieved OS statistical significance.
Recall Eric's comments from the May 7th conference call:
H/t a shareholder: Compassionate use site University of Louisville was added (not yet recruiting).
“Most drugs are really German dyes” (August 3, 2015)
Forbes.com's Matthew Herper authored an article today entitled Lexicon's Quest For A New Blockbuster. In it he writes and also quotes:
In 1998 Provectus' founders, Drs. Craig Dees, PhD, Timothy Scott, PhD and Eric Wachter, PhD, "re-discovered"* Rose Bengal, a molecule with a long and diverse medical history. Ironically the compound had lain around in plain sight of the global pharmaceutical industry for nearly 85 years** before Dees et al. began their journey of demonstrating Rose Bengal’s cancer fighting potential.
[1] Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie 1887; 238:318–338
[2] High Responses in Melanoma for Wool Dye/Ocular Stain Rose Bengal, Walter Alexander, The Oncology Pharmacist, October 2010}
* "Re-discovered" to mean that Rose Bengal’s therapeutic benefits remained hidden until the 1986 when sufficient quantities were administered orally in preclinical studies carried out by Japanese researchers. Ironically, while investigating the tumorigenicity of red food dye No. 105 (also made from Rose Bengal) they observed dose-dependent survival increases in test mice. Provectus’ multidisciplinary team of founders — a molecular virologist, a chemical engineer, and a chemist — looking for drug candidates having antineoplastic activity through a commercial data search separately came across Rose Bengal in 1998.
** I compute "nearly 85 years" based on the first recorded medical use of Rose Bengal, added to safranin victoria yellow for ocular pneumococcal infection (Römer, 1914) [3].
[3] Feenstra RPG and Tseng CG. Arch Ophthalmol 1992; 110:984–993
One month does not a trend make, but.../July Blog Stats (August 1, 2015)
Bounce rate and pages per session changed on or around July 8th.
Bounce rate means "the percentage of visitors to a particular website who navigate away from the site after viewing only one page." Lower is better. Said another way, bounce rate is "the percentage of single-page sessions (i.e. sessions in which the person left your site from the entrance page without interacting with the page)." Blog visitors appeared to interact with more than just the immediate or initial visit or landing page upon visiting the blog (and thus lowered the bounce rate) after July 8th. Perhaps more visibility of and awareness about Provectus' liver data and the company's letter of intent with Boehringer Ingelheim (China) (and thus PV-10 and Provectus) was reflected in some of the blog's readership stats, which I would look to confirm or deny potential trends or trending of such in August and beyond.
Tumor [immune] response vs. Tumor-specific [immune] response (July 31, 2015)
Intralesional agents in development with the potential to generate or facilitate a tumor response include Amgen's talimogene laherparepvec ("T-Vec") (Phase 3 reported) and Provectus' PV-10 (Phase 3 ongoing).
A tumor-specific response is important to the durability of the immunotherapeutic response of a drug. See, for example, a screenshot of a Dendreon webpage below.
While T-Vec may activate "a systemic T cell mediated anti-tumor immune response,"* it does not appear to mediate or generate a tumor-specific one. Dendreon's Provenge and CAR-T companies like Cellectis, Juno Therapeutics, Kite Pharma, etc. generate tumor-specific T cell responses. PV-10 generates a tumor-specific T cell response too:
* Talimogene laherparepvec activates systemic T-cell-mediated anti-tumor immunity, AACR 2015
They who must not be named (July 30, 2015)
H/t a shareholder (and regular blog-contributing due diligencer) for the below. Provectus added its third Pfizer executive/employee (Deanna Angello, a commercial strategist) to its strategic advisory board (SAB) in late-2014, announcing the addition in a December 17, 2014 press release. At Pfizer [according to the Provectus PR] Ms. Angello was Director, Commercial Strategy and New Business Planning for the Global Established Pharma business, the business organization where longer-time and more Provectus relationship-tangled (as well as SAB member since 2011) Dr. Craig Eagle, MD then resided [I believe]. She appears to have left Pfizer in Q1 (to join Genentech) but also appears to still be working with Provectus.
Turning a pivotal trial on its head (July 27, 2015)
In Provectus' pivotal Phase 3 trial of PV-10 vs. systemic chemotherapy in patients with unresectable locally advanced cutaneous melanoma, patients are randomized to the two arms of the trial on a 2-to-1 basis — 2 patients enter the treatment arm (of PV-10) for each patient who enters the control arm (of dacarbazine [DTIC] or temozolomide [TMZ]). Thus, for a trial N of 225, 150 patients receive PV-10 and 75 patients receive chemotherapy. A stated single interim analysis of efficacy (and safety) would be triggered after 50% of the required events have occurred (a "prescribed number of events" clause), where an event is disease progression in a patient (thus, about 113 events are required). Given PV-10 and chemotherapy's historical performance results for events (progressive disease or PD) and progression-free survival (PFS), it's unlikely the trial could trigger an interim analysis based on a prescribed number of events. See blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV.
The effect size of PV-10 in comparison to systemic chemotherapy makes its unusual in the distribution of responses, specifically PV-10's complete (and partial) responses to systemic chemotherapy's PDs.
Playing with the math, a 1-to-2 basis — 1 patient enters the PV-10 arm for every two patients entering the chemotherapy arm — generates the required number of events (~113) to trigger an interim analysis for efficacy (and safety).
Potentially there may be additional pre-specified and unspecified triggers such as (i) a pre-specified interim analysis for efficacy (and safety) based on the prescribed time after which the required number of events are expected to be occurred and/or (ii) prespecified interim analyses for safety at which time, at its sole discretion, the trial's data monitoring committee also may review efficacy data based on PFS events. For (ii) see page 10 of FDA Briefing Document, Oncologic Drugs Advisory Committee Meeting, April 12, 2011, NDA 21938/S013, Sutent® (sunitinib malate), Applicant: Pfizer, Inc; however, these early and unspecified efficacy "looks" are discouraged because of the potential for overestimating the treatment effect magnitude at earlier analysis times.
Highlights (July 25, 2015)
Updated below.
H/t a shareholder: PMO Live 2015 - Highlights from Friday, July 24 (2015 Fourth Annual World Cutaneous Malignancies Congress)
Surgical and Locoregional Therapies of Melanoma
Unit pricing
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part V (July 24, 2015)
The fifth in a series of blog posts and news items assessing the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma (Stage III patients, and not metastatic [Stage IV] melanoma).
Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015)
A second location, Gabrail Cancer Center in Canton, Ohio, appears to be involved in the clinical study Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma. The study is not yet listed on ClinicalTrials.gov. See New Moffitt Cancer Center study related to PV-10? (July 21, 2015) below. Moffitt's investigator for the trial also was and is an investigator on Amgen's T-Vec clinical trials.
In October 2014 the FDA announced an opportunity for public comment related to FDA's patient-focused drug development initiative, and published a preliminary list of nominated disease areas for consideration in patient-focused drug development meetings during fiscal years 2016 and 2017, including "Melanoma, specifically unresectable loco-regional disease."
Why? (July 22, 2015)
Updated below.
Updated (7/22/15): ConvergEx Group is a brokerage and trading firm. The ConvergEx Cyclers are partnered up with The Jimmy Fund of the Dana-Farber Cancer Institute.
ConvergEx Cyclers in fund raising support of the Pan-Mass Challenge appear to periodically ring the NYSE and NASDAQ bells, such as in 2010, 2011 and 2013. Provectus COO/CFO Peter Culpepper's ringing of the NYSE bell probably was related to the recent "IPO" of the company's warrants (NYSE MKT: PVCTWS) issued as part of the Maxim Group-led public offering.
New Moffitt Cancer Center study related to PV-10? (July 21, 2015)
The indication of Provectus' pivotal Phase 3 trial is [unresectable] locally advanced cutaneous melanoma. Inclusion criteria include "Stage IIIB or IIIC recurrent, satellite or in-transit cutaneous or subcutaneous melanoma." Exclusion criteria include "Presence of active nodal metastasis." The trial's protocol on ClinicalTrials.gov is here.
Moffitt appears to have begun a study with the title of Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma, with the goal of understanding and documenting "...the signs and symptoms of the disease as well as its impact on patients' lives." The study's investigator is Dr. Jonathan Zager, MD, a member of the "Moffitt PV-10 team."
Moffitt's Phase 1 feasibility study of PV-10 (i.e., the molecules mechanisms of action) had the center identifier 17183.
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part III (July 20, 2015)
The third in a series of blog posts and news items assessing the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma (Stage III patients, and not metastatic [Stage IV] melanoma).
Entry 2: 3. Good or bad process:" Designing an interim analysis for efficacy into Provectus' pivotal melanoma Phase 3 trial. See July 16, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part II.
This blog news item, "3. Good or bad process:" Patient crossover
Provectus' pivotal Phase 3 trial for locally advanced cutaneous melanoma includes a crossover design where patients in the control arm on systemic chemotherapy (i.e., dacarbazine or temozolomide) may crossover to the treatment arm of PV-10 at any time during the study.
I imagine crossover after a trial is completed or stopped (in favor of the treatment arm). For example, in recent news regarding Bristol-Myers' Phase 3 trial of anti-PD-1 agent Opdivo in kidney cancer, the trial "...stopped early because an assessment conducted by the independent Data Monitoring Committee (DMC) concluded that the study met its endpoint, demonstrating superior overall survival in patients receiving Opdivo compared to the control arm;" as a result, "Bristol-Myers Squibb is working to ensure that eligible patients will be informed of the opportunity to continue or start treatment with Opdivo in an open-label extension as part of the company’s commitment to providing patient access to Opdivo, and characterizing long-term survival." This Opdivo trial did not have crossover designed into it (see the trial protocol here). With overall survival ("OS") as the trial's primary endpoint (progression-free survival ["PFS"] was/is a secondary endpoint), an estimated enrollment of 822 and early stoppage, there obviously is nothing wrong with a post crossover.
Interestingly, on the same day another company revealed pivotal Phase 3 kidney cancer data: Exelixis' cabozantinib beat its primary endpoint of PFS; however, the trial will continue (there cannot be patient crossover) because the secondary OS endpoint only shows a trend towards the treatment arm beating the control arm — but has not beat it, statistically speaking. Exelixis' trial had an estimated enrollment of 650, and utilized the same control drug as the Opdivo trial (everolimus) (see cabozantinib's trial protocol here).
Provectus had patient crossover at the end of the study removed from its trial design.
Crossover has pros and cons. An advantage is that "...it could yield a more efficient comparison of treatments" (i.e., fewer patients are required for the trial). I would imagine there is an ethical consideration as well. Crossover's primary disadvantage is the crossover effect, where "[s]ignificant carryover effects can bias the interpretation of data analysis..." Bias occurs in a trial where Drug B (in Provectus' trial, this would be chemotherapy) is given first, and then after crossover Drug A (PV-10) is given. Dacarbazine's half life is 0.5-2.5 hours. Overall survival can be measured in trials that include crossover in their design using one of several methods that may depend on a trial's individual circumstances, goals, etc.
Although BioVex/Amgen's intralesional agent OncoVEX/T-Vec's pivotal melanoma Phase 3 trial included a planned interim analysis for efficacy of secondary endpoint OS, no patient crossover was permitted because the trial needed to collect OS data. See blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part II. Vical's intralesional agent Allovectin-7's pivotal melanoma Phase 3 trial did not include either an interim analysis or a patient crossover.
On another note, h/t InvestorVillage Juggernaut noted the Abramson Cancer Center of the University of Pennsylvania identified itself as a site for Provectus' pivotal melanoma Phase 3 trial.
The timing of UPenn's addition may be consistent with the process and timing of site activations discussed in Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part I (July 13, 2015) on the blog's Current News page.
H/t a shareholder: UPenn's Abramson Cancer Center also appears to be a clinical site for Provectus' ongoing Phase 1 liver trial.
Eric noted on the company's May 7th 1Q15 conference call:
Following up further on the "commercially reasonable efforts" phrase in the Provectus-Boehringer Ingelheim (China) letter of intent — see the July 2nd press release and BioXcellence™ (July 9, 2015) below — particularly in the context of the pharmaceutical industry, note the selected (by me) slides from a June 2015 presentation by folks from JSB-Partners*, SRS Acquiom** and law firm McDermott Will & Emery LLP entitled Structuring Milestones and Contingent Payments in Biotech, Pharma and MedTech M&A and Collaborations.
Blog new items takeaway: The use of commercially reasonable efforts as a threshold in the pharmaceutical industry appears accepted, appropriate and commonplace.
Slides are below. Fuzzy purple emphasis, and "commercially" blue highlight are mine.
* "JSB-Partners offers specialized investment banking and advisory services to biotechnology and pharmaceutical companies"
** "...SRS Acquiom has become the undisputed expert at managing private M&A post-closing activity."
"...It’s Not All About PD-1" (July 17, 2015)
Caroline Helwick recently wrote an article in The ASCO Post entitled Oncolytic Immunotherapy in Melanoma: It’s Not All About PD-1, which profiled comments by Dr. Robert Andtbacka, MD, Associate Professor of Surgical Oncology at the Huntsman Cancer Institute at the University of Utah, Salt Lake City, and included a mention of PV-10. She previously wrote an article in The ASCO Post that profiled PV-10 (among other therapeutic agents) last year entitled Intralesional Injections Trigger Immune Responses in Melanoma.
Andtbacka's comments apparently derive from a presentation he gave to/at a/the the American Society of Gene and Cell Therapy meeting, where he discussed talimogene laherparepvec (T-VEC/Amgen), plasmid interleukin-12 (IL-12) electroporation (OncoSec), PV-10 (rose bengal) and coxsackievirus A21 (CVA21/Viralytics). The title of the article itself is interesting, and a "money quote" in it includes:
For me the point of this article is the building trend or theme that the immune checkpoint inhibitors — the so-called back-end or agents that help release the brakes of the immune system — are not "all that," and very likely require help from the so-called front-end or agents that start the engine of the car and/or help press on the gas pedal. Additionally, while some oncolytic virus companies (like Viralytics) are experimenting with delivering their agents intravenously, the likely better and much more effective way to deliver the front-end is via injection directly into cancerous lesions and tumors.
Helwick's article notes in regards to disclosure that Andtbacka reported no potential conflicts of interest. He is/has been a consultant to BioVex/Amgen, Provectus and Viralytics, and been involved in at least one recent event put on by OncoSec.
On another note — h/t InvestorVillage poster Juggernaut — Provectus updated its July calendar of events to include PMO Live, an annual joint meeting of the Global Biomarkers Consortium (GBC) and World Cutaneous Malignancies Congress (WCMC). Conference faculty includes Dr. Sanjiv Agarwala, MD of St. Luke’s Cancer Center (PV-10 investigator) (Conference Co-Chair), Andtbacka, Ross (PV-10 investigator [although the conference's associated title is different]), Dr. Grant McArthur, PhD (Provectus consultant) of Peter MacCallum Cancer Centre (PV-10 compassionate use program site) and Moffitt Cancer Center's Dr. Vernon Sondak, MD (Provectus consultant).
New SEC Filing (July 16, 2015)
Provectus filed a Form S-3 today, a $100 million mixed securities shelf offering of common stock, preferred stock and warrants (with prospectuses dated July 16, 2015). The registration statement appears to replace the prior Form S-3, a $100 million mixed securities shelf offering of common stock, preferred stock and warrants (with prospectuses dated June 29, 2012) filed on July 2. In 2012 the company was incorporated in Nevada. It currently is incorporated in Delaware.
The company drew down on the prior June 2012 shelf by issuing common stock and warrants via the Maxim Group public offering in the amount of gross proceeds of $13,313,750. The offering closed on June 24, 2015 and generated the issuance of 17.25 million shares of common stock and 17.25 million of warrants on common stock. A prospectus for the shelf itself is included in today's filing (the “Base Prospectus” in Ex. 5.1 below). The proceeds above then would have left $86,686,250 available in/on the shelf.
Maxim also exercised their 2.625 million over-allotment option of warrants (on common stock); thus, 20.125 million warrants were created or issued as a result of the Maxim offering. A prospectus for the warrants is included in today's filing (the “Warrant Shares Prospectus” in Ex. 5.1 below). Maxim has until August 3rd to exercise their over-allotment option to purchase 2.625 million of common stock, which if they do would generate gross proceeds of $1,968,750 (and reduce the approximately $87 million balance figure above to $84,717,500).
The Maxim public offering was a means of raising (a vehicle to raise) money. Two other means also are available to the company:
Exhibit (Ex.) 5.1 is Provectus' law firm Baker Donelson's opinion, and important to read.
As a result of the Maxim offering as well as depending on whether the additional shares are purchased by Maxim and what number of common shares and/or warrants (on common stock) may be issued to consultants, Provectus effectively is up against its limit of authorized shares of 300 million. See Offering, Part I (June 29, 2015) on the blog's Archived News III page.
Provectus cannot issue additional shares via another public offering like June's Maxim one or through either Cantor or Alpha Capital to exceed the 300 million authorized share ceiling, unless shareholders approve a proposal from management to increase the authorized share number.
You can use the following online web page comparison application to contrast the two Form S-3s. When I did so I noticed, among other things:
Updated 7/15/15 for additional writing at the end of the news item.
On Provectus' May 7th 1Q15 conference call the company's CTO Dr. Eric Wachter, PhD said in regards to combining PV-10 with an immune checkpoint inhibitor for patients with advanced (i.e., Stage IV) melanoma:
On the company's March 13th 4Q14 conference call Eric said:
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part I (July 13, 2015)
The first in a possible series of blog posts and/or news items assessing the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma (Stage III patients, and not metastatic [Stage IV] melanoma).
In a 2008 PharmaExec.com article entitled Site Activation: The Key to More Efficient Clinical Trials Gen Li wrote "Patient enrollment, at its simplest, consists of three steps: Site selection, Site activation, Patient recruitment."
Step one: Site selection. This step for Provectus could be mostly complete.
During Provectus' April 9th panel discussion in New York — comprised of the company's CTO Dr. Eric Wachter, PhD, lead Phase 3 principal investigator Dr. Sanjiv Agarwala, MD (St. Luke's Cancer Center), very likely but not yet officially confirmed Phase 3 trial investigator and former Phase 2 trial investigator Dr. Merrick Ross (MD Anderson Cancer Center), and Provectus COO/CFO Peter Culpepper — Eric said:
Li also wrote: "It takes several steps to bring a site to the point where it is ready to recruit patients. This process is called site activation, and it consists of a variety of tasks including: Negotiate a financial contract, Gain approval from Institutional Review Board (IRB) or, in Europe, Ethics Board (EB), Provide clinical supplies, Obtain other documents from site (CV, financial disclosure, etc)."
Provectus' April 15th press release Provectus Biopharmaceuticals Opens Patient Enrollment; Begins Phase 3 International FDA Comparative Clinical Trial of PV-10 for Melanoma noted:
Step 3: Patient recruitment. This Provectus step may be underway only at St. Luke's.
I examined the approaches of the three intralesional agents for melanoma that Huntsman Cancer Institute's (and likely PV-10 Phase 3 trial investigator) Dr. Robert Andtbacka often compared and contrasted (in chronological order of pivotal trial commencement) — Vical's Allovectin-7 (2007), BioVex/Amgen's OncoVEX/T-Vec (2009) and Provectus' PV-10 (2015) — which comprised reviewing the list of changes to or updates of each drug's trial's ClinicalTrials.gov webpage below.
All three companies submitted an initial trial protocol, registering the trial by listing it on ClinicalTrials.gov. Subsequent changes or updates of one category (type) or another were later made.
BioVex/Amgen issued a press release regarding the commencement of its Phase 3 trial. I could not find any mentions of when the trial's first patient visited or was treated. I also could not find any press releases or public statements regarding the addition of trial sites. Chicago's Rush University Medical Center did issue a press release early on noting it was leading the trial, which may suggest Dr. Howard Kaufman, MD was the lead investigator.
Vical appeared to be all over the place in regards to when and what it communicated about its Phase 3 trial, from (among other things) the commencement of the trial to when the first patient was treated to discussing site initiation activity.
Provectus issued its release regarding the commencement of the Phase 3 trial, unfortunately noting the underlined below:
On the same May 7th call Eric also said:
Is there something "wrong" with the startup of Phase 3 trial so as to warrant some sort of correction? I say "no," leaning on what appears to be good process by Eric for my answer. Alternatively, consider recent tweets from @johnhallnj on the topic at the end of June:
I asked John to expand on his comments, and he permitted me to blog about his explanation. The basis for his viewpoint is the assumption no patient has been enrolled/treated because Provectus has not issued a press release stating such:
In my June 26th blog post Eric's Process I wrote a certain author (Kamp) noted activation of a single center takes an average of 100 days. I continued to write: "If I assume activation requires the trial protocol to be finalized, and I further assume finalization is equivalent to posting the protocol on the ClinicalTrials.gov website, 100 days after April 16th (when it was first posted) is July 25th (the "average" date)." I should have started counting on March 12th (when the revised or final protocol was posted, rather than when the trial opened at St. Luke's), yielding June 20th. Irrespective of John's estimate or mine, other sites still have not been added to the trial's ClinicalTrials.gov webpage.
Later, I dug deeper into the topic of site activation (particularly for oncology trials) because the 100-day figure was not indication-specific. Activation, from a process workflow perspective, might comprise document submission to opening enrollment and patient accrual. See below, for example:
Site activations for cancer trials appear to take longer than the 100-day figure both Kamp and Li quoted. For example, Wang-Gillam et al. (2010) note median times (from submission to opening a trial) of 163 and 112.5 days for a U.S. academic center and a European one, respectively, for lung cancer trials. Dilts et al. (2006) note median times of 171 days and 191 days for a U.S. National Cancer Institute–designated comprehensive cancer center and its community practice sites, respectively (95% confidence intervals were 158 to 182 days and 119 to 269 days, respectively).
Measuring from when Eric filed the revised/final Phase 3 protocol, the range of "median" dates above to add other sites (into St. Luke's) might lead to:
It is likely Eric engaged some, many or all identified sites before, when or after the initial protocol was filed in November 2014, and/or before, when or after the Phase 3 protocol was updated on ClinicalTrials.gov in March 2015. For example, the initial protocol appears to be have been filed with and/or approved by St. Luke's in 2014 (see blog post Eric's Process). As such, counting down to site activation for each of the remaining 24 U.S. and the 10 Australian sites could have started before March 12th. Thus, some of these sites may appear on ClinicalTrials.gov in July, August and September, obviating the need for corrective action and re-confirming Eric's good process. The larger lesson for Provectus management, however, may be to better manage the expectations they themselves set for and communicate to shareholders and the market at large.
Asia Pivot (July 10, 2015)
On Provectus' 4Q14 conference call (March 12th) the company's CTO Dr. Eric Wachter, PhD said:
BioXcellence™ (July 9, 2015)
German pharmaceutical company Boehringer Ingelheim ("BI") has a subsidiary or organization called, Boehringer Ingelheim BioXcellence™, which is a biopharmaceutical contract manufacturer that can work with and assist clients as "...a strategic partner...from development to fill and finish."
BI BioXcellence™customer services include {fuzzy purple line emphasis is mine}:
BI BioXcellence™plies its services in China. See here, and below.
My takeaways for this blog news item:
On July 7th Medical writer Walter Alexander wrote an article that included comments about PV-10 (published today on DocGuide) relating to the Phase 1 liver results presented by Provectus at the World GI Congress in Barcelona: Trials Show Promise for Locoregional Treatment for Hepatocellular Carcinoma and Liver Metastases. His past work includes articles in 2010 (here, here and here), 2011, 2013 (here and here) and 2014. His current article about the company's Phase 1 liver cancer data included a quote by Dr. Chris Verslype, MD of University Hospital Gasthuisberg in Leuven, Belgium, who commented on trials across several phases presented at the meeting (Dr. Verslype was a faculty member of the World GI Congress):
If Provectus cannot get Sinopharm to agree to an acceptable [to Provectus] upfront payment (among other acceptable terms and conditions), which I believe has been the "snag" all along with the Chinese company, then [through the letter of intent relationship with Boehringer Ingelheim ("BI")] Provectus has pushed out commercial relationship discussions about China and liver cancer (primary and secondary) until more clinical data is available from an Asia/Pacific Phase 1b/2 trial (with BI having the first seat at the negotiating table when the time comes).
Updated (7/8/15): On the company's last conference call, CTO Dr. Eric Wachter, PhD said:
Comfort Level (July 7, 2015)
Medical writer Janet Fricker wrote another article about PV-10 (and Provectus) (published today on eCancer News), this time about the Phase 1 liver results presented by the company at the World GI Congress in Barcelona: PV-10 shows potential in hepatocellular carcinoma and metastatic liver disease. On eCancer News she has written about PV-10 at least seven times: October (ESMO) 2012, October (ECC) 2013, April (AACR), June (ASCO), October (ESMO) and November (SITC) 2014, and now July 2015 (ESMO World GI). She also has written about PV-10 on PharmiWeb.com four times: October (ECC) 2013, and June (ASCO), October (ESMO) and November (SITC) 2014. Her current article about Provectus' Phase 1 liver cancer data included the following notable items:
Provectus issued its press release regarding the company signing a letter of intent ("LOI") with German pharmaceutical company Boehringer Ingelheim's (BI's) China subsidiary (Boehringer Ingelheim (China) Investment Co. Ltd.) on July 2nd via BusinessWire. BI's China subsidiary issued its press release regarding the LOI on July 3rd via PR Newswire Asia.
Parent company Boehringer Ingelheim did not note the subsidiary's press release.
Ostensibly The Same (July 6, 2015)
Provectus issued a press release and filed an 8-K today regarding the company's poster presentation in Osaka, Japan, Phase 1 PV-10 Data on Liver Cancer Presented at 6th Asia-Pacific Primary Liver Cancer Expert Meeting. For all intents and purposes the APPLE poster (the link is here) is the same as the World GI one, with no new information. See ESMO World GI Poster (July 1, 2015) below. Provectus management did take the opportunity of the PR to note the liver poster presenter, St. Luke's Cancer Center's Dr. Argawala, was the lead investigator for the company's pivotal melanoma Phase 3 trial, and to remind folks the Phase 3 trial had begun: "He serves as a principal investigator of the phase 1 clinical trial that produced the data presented, and is the lead investigator for the phase 3 clinical trial of PV-10 as an investigational treatment for melanoma which recently began."
A Simple Enough Explanation (July 5, 2015)
Image source. Story source.
Clinical Trial (July 4, 2015)
[Updated 7/4/15: News item takeaways at the end of this post]
Provectus' poster at the ESMO 17th World Congress on Gastrointestinal Cancer in Barcelona included the below in the bottom right-hand corner {fuzzy green box emphasis is mine}:
In regards to an Asia/Pacific* clinical trial for hepatocellular carcinoma ("HCC," or primary liver cancer) and the above:
For example, on the 4Q14 conference call (March 13th) Eric said:
Nevertheless, there is enough information about and around, I think, to potentially frame the Phase 1b portion of an Asian (i.e., Chinese) Phase 1b/2 study:
transarterial chemoembolization: clinical data from a single teaching hospital, Int J Clin Exp Med 2013;6(5):367-371)
Clinical evidence for transarterial embolization (TACE):
Comparable, Consistent PV-10 Pharmacokinetics (July 3, 2015)
"Pharmacokinetics, sometimes described as what the body does to a drug, refers to the movement of drug into, through, and out of the body."
Pharmacokinetic variability: "Inter-individual variations of a drugs pharmacokinetic parameters, resulting in fairly different plasma concentration-time profiles after administration of the same dose to different patients," where inter-individual means among individuals (people or patients).
I wrote in my June 2015 blog post Reproducibility, the Hallmark of Western Science that PV-10's clinical value proposition had been repeated by Provectus' lead researcher (Chairman and CEO Dr. Craig Dees, PhD), independently reproduced by Moffitt Cancer Center and the University of Illinois, and demonstrated in multiple cancer indications across multiple species (mice, higher level animals like dogs and horses, humans). As a small molecule it is Rose Bengal's (PV-10's) physical chemistry that underscores its consistent capabilities, rather other cancer immunotherapies' bio-chemistry, which makes them prone to sub-par and inconsistent performance. All of this (and more) speaks very loudly to PV-10's fitness as a product as well, e.g., works under many different conditions, ships well, has a long shelf life, does not require refrigeration, etc. As I wrote in a recent post The Risk-Reward of Provectus Biopharmaceuticals: Management,
Thoughts about Barcelona (July 2, 2015)
Provectus issued two press releases and filed an associated 8-K filing today:
Multi-indication. Provectus first presented clinical melanoma data (interim Phase 2) at a medical conference (ASCO) in 2009. In the company's 2015 World Gastrointestinal Cancer Congress poster, one sees the demonstration of PV-10's efficacy on both primary or hepatocellular carcinoma or HCC, and secondary or liver metastases. With this preliminary evidence of efficacy, I think it is reasonable (and intellectually honest) to say PV-10 is or can be a multi-indication drug.
The following jumps out from the Barcelona liver cancer poster:
About two years ago I wrote a blog post about my take on Big Pharma game theory (see For $PVCT, it's the FDA's move), noting no one moves unless they have to move, and everyone moves when somebody moves. Perhaps this letter of intent might have two outcomes. First, it may encourage Sinopharm to move by the July 15th memorandum of understanding expiry date (if Sinopharm doesn't move, BI may well be an ideal early collaborator for Provectus). Second, it may encourage other Big Pharma to move too.
ESMO World GI Poster (July 1, 2015)
Selected portions of the poster are below.
Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver (July 1, 2015)
The 6th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2015), Evidence and Consensus on HCC Management (p. 217):
The abstract content appears to be the same as the PV-10-related one at the ESMO World Congress on Gastrointestinal Cancer 2015, which should mean the posters themselves are of course key. See Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver (June 24, 2015) on the blog's Archived News III page. The authors, however, are somewhat different. For the Barcelona conference (ESMO World Congress), the authors were Goldfarb, Low, Lyon, Agarwala, Rosemurgy and Wachter. For Osaka (APPLE) they are Goldfarb, Lyon, Low, Wachter and McMillan.
Goldfarb, Agarwala and Rosemurgy are clinical/principal investigators at Sharp Memorial Hospital (San Diego, CA), St Luke's University Health Network (Bethlehem, PA) and The Southeastern Center for Digestive Disorders & Pancreatic Cancer (Tampa, FL). Lyon is a Sharp interventional radiologist. Low is a Sharp diagnostic radiologist. McMillian is founder/President of Boston-based gRadiant Research, and "developing thermal therapy as an alternative to traditional surgery."
June Blog Stats (July 1, 2015)
- The Phase 3 trial was designed from the outset to support an approval filing, and
- The data are worthy of review under accelerated approval.
Thus, Provectus' primary 12-month share price catalyst is an early positive outcome from its pivotal melanoma Phase 3.
Note: Although the company (specifically, COO/CFO Peter Culpepper) has indicated "[c]ash on hand supports planned operations until into late-2017," Provectus' accounting firm BDO may require the company to raise money in or by the end of 2Q16.
Friday's Schedule 14A filing, see Pfizer's Just Not That Into You (August 21, 2015) below, illustrates Peter's efforts to raise the share price in anticipation of an early positive Phase 3 trial result: attempting to IPO the stock on one or more Asian/Pacific stock exchanges, and/or selling a piece of the company to a pharmaceutical company. His goal of the former (if successfully executed) could be to hope for a higher Asian stock price would drag the U.S. stock price up. His goal for the latter (if possible) would be for an equity investment either as part of an Asian IPO at an upround valuation (to the then U.S. market capitalization) and/or separately at a well-above market valuation to have the same upward effect on NYSE MKT:PVCT. In both cases, he requires shareholder approval of the board recommendation and company proposal to raise the number of authorized shares.
[f/n 1] Progression-free survival is the trial's primary endpoint.
[f/n 2] Utilizing language and thinking derived from this article.
Pfizer's Just Not That Into You (August 21, 2015)
Pfizer. Provectus' joint patent with Pfizer was issued this past Tuesday (by the US Patent and Trademark office) without the former able to include any significant reference to the latter in its associated press release — see Intellectual Property (August 18, 2015) below. According to Provectus' investor relation firm Porter, LeVay & Rose's Michael Porter, Pfizer had a draft version of the company's proposed PR for several weeks leading up to the August 18th patent issuance date. This date was available on the U.S. Patent and Trademark Office's Patent Application Information Retrieval website as early as July 29th.
Michael said the draft PR included Pfizer's name in its title, much like Provectus' April PR related to the patent's allowance (among other planned or hoped for additional references to and/or placeholders related to Pfizer). At the proverbial eleventh hour (i.e., early morning Eastern Daylight Time of the August 18th) a lawyer in Pfizer's Legal Division rejected the use of Pfizer's name (and thus, presumably, most or all other references to it). The lawyer's explanation for her decision, according to Michael, was that [the issuances of] patent-related PRs were not important to Pfizer; they are potentially very important to small companies like Provectus.
Listening to Michael, it sounded like the lawyer's last minute timing was due to her being on vacation and not getting around to dealing with the matter until after her return. The situation, at least from Pfizer Legal's perspective, merely could be something like it's just not that into Provectus, a view that may or may not be consistent with operationally-focused parts of the organization. Only time will tell.
Ownership vs. Inventorship. The patent-related PR that Provectus ultimately issued contained a sole reference to the pharmaceutical company, "Pfizer Inc. is a joint owner of the patent," which was either Provectus' attempt at maintaining some association to Pfizer in the PR or a solitary concession by the pharmaceutical company. Pfizer's Dr. Craig Eagle, MD is a co-inventor of the patent, along with Provectus' Chairman and CEO Dr. Craig Dees, PhD, CTO Dr. Eric Wachter and primary consultant Jamie Singer. Pfizer itself is listed as a co-assignee; the other assignee is Provectus. Writing in the PR that Pfizer is a joint owner could suggest the pharmaceutical company enjoys economic benefit from all of the patent's claims, which I do not believe to be the case.
Based on the perspectives of several shareholders who have discussed the matter with management over time, it's much more likely Pfizer's economic benefit is limited to the outcome of buying Provectus — if Pfizer buys Provectus it thusly benefits from the sales of drug combinations that include PV-10, but if Pfizer does not buy the company it gains nothing by way of patent-derived benefit (i.e., royalties).
This view presumably reflected discussions between Eagle and Provectus "back when" regarding a better way of harnessing a patient's immune system:
"The present invention includes immunotherapeutic procedures wherein large amounts of tumor antigen are exposed to a patient's immune system, for example upon intralesional delivery of an immunomodulator, including but not limited to intralesional rose bengal, in combination with one or more systemic immunomodulator, to enhance the immune-mediated antitumor response" (Source: Detailed Description of the Presently Preferred Embodiments, Combo Patent).As background the combination therapy provisional patent was filed on March 10, 2011, before the FDA's approval of anti-CTLA-4 agent ipilimumab for advanced melanoma on March 25, 2011 and after Pfizer discontinued its pivotal Phase 3 trial of anti-CTLA agent tremelimumab for advanced melanoma on April 1, 2008.
Eagle may have thought there was promise in combining local delivery of an immunomodulator (PV-10) with systemic delivery of an immunomodulator (tremelimumab), and envisioned Pfizer eventually owning a workable PV-10/treme combination. Treme eventually was mostly packaged off to AstraZeneca/MedImmune on October 3, 2011. Craig et al. likely envisioned the larger combination set of PV-10 + fill-in-the-blank, and put this into practice at least preclinically.
By giving Provectus ownership of the patent under the scenario where Pfizer does not acquire it, which might have been construed as a fair trade at the time, Eagle and Pfizer (i) may not have thought much about PV-10 use and thus value outside of a combination with treme, (ii) likely did not envision the immuno-oncology landscape would evolve to where so-called back-end agents like the immune checkpoint inhibitors (e.g., Keytruda, Opdivo, etc.) need immune system priming or activation help to remain relevant, and (iii) PV-10 would be the robust, multi-faceted small molecule it now appears to be. If they had, Eagle and Pfizer might have pressed for true joint ownership of the patent.
Mo' shares. Provectus filed a Schedule 14A today seeking shareholder support to increase the number of authorized number of shares from the current figure of 300 million to a proposed 400 million. The number of fully diluted shares outstanding currently stands at 293,813,501. The special meeting of stockholders this proxy requires would take place on October 1st in New York. The recommendation's primary explanation appears to be:
"In addition, our Board of Directors believes that the amount of common stock we have available for issuance is insufficient for our future financing needs because it is likely that the sale of shares of common stock or securities convertible into shares of common stock will be the principal means by which we will raise additional capital until such time as we are able to generate earnings sufficient to finance our operations. Shares of common stock may be used for various purposes without further stockholder approval. These purposes may include: raising capital, which may be effectuated with a contemporaneous listing of one or more of our securities on one or more of the Singapore, Hong Kong, or Australia securities exchanges, although there can be no assurance that we will list any of our securities on any foreign securities exchange; providing equity incentives to employees, directors and consultants; establishing strategic relationships with other companies; the acquisition of any business, assets or technology; and other purposes. Although our Board of Directors has no current plan, arrangement or commitment to issue additional shares of common stock, our Board of Directors believes that it is in the best interest of us and our stockholders to have a sufficient number of authorized but unissued shares of common stock available for issuance in the future for such purposes." {Underlined emphasis is mine}"[R]raising capital, which may be effectuated with a contemporaneous listing of one or more of our securities on one or more of the Singapore, Hong Kong, or Australia securities exchanges" means Provectus' COO/CFO Peter Culpepper is exploring listing Provectus stock on, perhaps, the SGX (the Singapore stock exchange), SEHK (the Hong Kong stock exchange) and/or ASX (the Australian stock exchange). In order to IPO the stock on one or more of these exchanges Peter would require more authorized shares than the company currently possesses.
"[E]stablishing strategic relationships with other companies" means Peter would consider a strategic equity investment in the company by a pharmaceutical company. In order to facilitate such an investment or buying of Provectus shares Peter again would require more authorized shares than are currently available.
From a capital formation and shareholder value perspective I am not against increasing the number of authorized common shares — as long as these newly issued shares go toward a useful, specific, and clearly and sufficiently explained purpose (or purposes) that objectively could result in the enhancement of shareholder value. The paragraph above that I highlighted from today's Schedule 14A filing simply is not enough information upon which to base one's support of Provectus' board of directors' recommendation to vote for the proposal (to increase the number of authorized shares). Given Craig, Provectus' President Dr. Tim Scott, PhD, Eric and Peter's dismal record of shareholder value mismanagement, and in the absence of more specific information about the proposed or potential use(s) of the additional shares, I don't think management deserves another blank check.
My comments above have nothing to do with the clinical side of the business, the pursuit of value utilizing all of Provectus' clinical assets, and the maximization of shareholder value by developing these assets. Rather, my views have everything to do with management more fully explaining why they are seeking to raise Provectus' authorized share amount, why they are seeking to raise it now, and why they deserve such authorization by shareholders. I look forward to hearing Craig et al.'s explanation.
Intellectual Property (August 18, 2015)
Updated below.
Provectus' joint patent with Pfizer, Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer, which was allowed in April, issued today.
 |
| Click to enlarge. |
"Dr. Wachter noted, “The forthcoming patent arose from discussions several years ago with Dr. Craig Eagle of Pfizer, and appropriately given his contribution he is named as the lead inventor for these claims. In addition to the claimed combinations of PV-10 with immunotherapy agents, the specification covers combination with other classes of agents, and Provectus will pursue these areas through one or more divisional application.”" Source: April press release regarding the patent's allowanceTwo other patent applications or continuation applications follow the patent issued today: 20130416494 and 20140748634. Neither of these have shown up yet on the US PTO's patent application website.
 |
| Click to enlarge. Screenshot above. Fuzzy purple emphasis is mine. |
Pfizer. Below are a couple of screenshots from Pfizer's Oncology Research Unit's (ORU's) partnering webpage (the main page would be that of Worldwide R&D).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Mostly a note to self (August 16, 2015)
Definitionally, it seems, the grail is anti-tumor immunity, while the myth is a pathological complete response (pCR).
Institutional (August 14, 2015)
| Image source |
New funds that may (or may not) have participated in the Maxim Group bookrun offering and that filed 13Fs were Peter B. Cannell & Co (90K shares), Cormorant Asset Management (a story about founder Bihua Chen) (2.2 million), Millennium Management (500K), Sabby Management (a description of founder Hal Mintz) (~3.28 million) and Susquehanna International Group (~2.45 million), totalling about half of the offering's 17.5 million shares (plus matching warrants).
 |
| Click to enlarge. |
Updated below.
 |
| Image source |
Phase 0 clinical trials are also called exploratory IND (investigational new drug) studies (or micro-dosing studies), and covered by FDA guidance entitled Guidance for Industry, Investigators, and Reviewers, Exploratory IND Studies, Center for Drug Evaluation and Research (CDER), January 2006, Pharmacology/Toxicology. Another take:
"ExpIND studies, often called as phase 0 clinical trials, are conducted prior to traditional phase I dose escalation, safety and tolerability studies with very limited human exposure (<30 patients, usually 10–15 patients for a period of ≤7 days) and have no therapeutic or diagnostic potential (e.g., microdose or screening studies). These studies assess feasibility for further clinical development of a drug or biological product regulated by Center for Drug Evaluation and Research (CDER). Bridging the gap between traditional preclinical studies and clinical development, phase 0 trials provide an opportunity to assess pharmacokinetics (PK) and pharmacodynamics (PD) of new molecules early in humans with reduced preclinical testing. ExpIND approach also allows investigators to conduct phase 0 studies of closely related agents under a single IND application. Phase 0 studies have the potential of identifying promising candidates more quickly and precisely." (Source: Gupta et al., Phase 0 clinical trials in oncology new drug development. Perspectives in Clinical Research, 2011)Assuming this is Moffitt-only work, Provectus' CTO Dr. Eric Wachter, PhD must have visibility into it. It could well be another example of work being done on and with PV-10 of which we are unaware.
2. Combination therapy for advanced melanoma. Eric noted on Provectus' August 6th conference call (downloadable transcript):
Turning to the other primary component of our development plan for melanoma, we've continued to move towards commencement of a post-clinical study of PV-10 in combination with a new checkpoint inhibition in patients with advanced metastatic melanoma. After thorough consultation with leading investigators who will conduct this work, we have a study design that is undergoing final investigator review and anticipate completing the protocol before the end of the present quarter. This includes comprehensive definition of patient population, dosing schedule for both agents, and the study endpoints. As I've indicated previously, to assess potential benefit of PV-10 for patients with advanced melanoma, this Phase Ib/II study will incorporate a modest-sized single arm Phase I key - Phase Ib component of 24 patients with expedited safety and efficacy endpoints. Completion of this initial phase is expected to support expansion to a larger randomized Phase II component having an estimated 120 patients. The actual size of the Phase II component will be determined by modeling and response data among Phase Ib participants, that is the so called observed effect size. Endpoints for Phase Ib will comprise assessment of acute safety of the combination regimen and objective response rate at three to four months. For the Phase II portion, endpoints will be overall survival, progression-free survival, and objective response rate. We anticipate using the anti-PD1 drug pembrolizumab, also known as Keytruda, as the checkpoint inhibitor. This class of drug has been shown to work favorably with PV-10 in mouse models with melanoma, as presented by our colleagues at Moffitt last November at the Annual Meeting of the Society for Immunotherapy Cancer, and as anticipated in our allowed drug patent application with Pfizer, the two drugs have largely unrelated or orthogonal side effect profiles. These factors provide justification for conducting the study. Also, since pembro is standard of care for the study of patient population, it is standard practice to conduct these kinds of studies in an add-on mode where all patients receive standard of care. We're optimistic that we can leverage existing investigator and site relationships to commence this study by the end of the calendar year. And since pembrolizumab is licensed in the US, we can commence this study with or without the assistance of a partner. If ongoing negotiations with prospective corporate partners lead to interest in testing PV-10 with a different checkpoint inhibitor, the study is designed to facilitate use with other drugs to enable such testing in a straightforward manner. {Underlined emphasis is mine}The above noted trial approach to the combination of an intralesional agent (IL) (PV-10) and an immune checkpoint inhibitor (pembrolizumab or Keytruda) is a Phase 1b/2 (the 2 would a randomized controlled trial [RCT] of pembrolizumab with or without PV-10) projecting a cumulative trial N of 144 patients (24 in the Phase 1b, and [for now] 120 in the Phase 2 RCT). As Eric noted the actual number of Phase 2 patients would be determined by observed effect size of the pairing in the Phase 1b trial.
In contrast, the combination of Amgen's IL agent talimogene laherparepvec (T-Vec) and pembrolizumab is a Phase 1b/3 (the 3 is an RCT of pembrolizumab with or without T-Vec) using a cumulative trial N of 680 (20 in the Phase 1b and apparently 660 in the Phase 3), which is to say that's not much of an effect size. Additionally, one has to love the "language of the trial," where pembrolizumab will be administered on Day 1, Week 0, which would make one think it is given to the patient upfront, except that T-Vec is administered on Day 1, Week -5 (minus 5).
 |
| Click to enlarge. Image source |
Interestingly Medimmune entered into strategic cancer vaccine collaboration and license agreement with Inovio Pharmaceuticals today, noting in its press release:
"Emerging evidence suggests that the benefits from immuno-oncology molecules, such as those in MedImmune's portfolio, can be enhanced when they are used in combination with cancer vaccines that generate tumour-specific T-cells." {Underlined emphasis is mine}"Immuno-oncology molecules" means immune checkpoint inhibitors.
3b. Updated (8/10/15): Today Merck U.S. entered into a collaboration with Immune Design to test two Immune Design immuno-oncology agents in combination with Merck's pembrolizumab, where Immune Design noted in its press release:
"LV305, in contrast, is designed to activate the immune system through the in vivo generation of cytotoxic T cells (CTLs), initially against a specific tumor-associated antigen, NY-ESO-1. Immune Design is studying LV305 primarily as part of CMB305, a prime boost approach currently in a Phase 1 expansion trial." {Underlined and bolded emphasis is mine}4. Immune checkpoint inhibitors need help. Last week Reuters (Bill Berkrot) wrote an article entitled Pfizer, Bristol revive cancer drugs that rev up immune system, which discussed immune system "accelerator" agent 4-1BB (also know as CD137) and highlighted early success by a Stanford physcian and researcher Dr. Holbrook Kohrt, MD, PhD. 4-1BB is a co-stimulatory agent or agonist (the "front-end") that helps to get the car moving (or helps to step on the gas pedal) — Step 3, Priming and Activation of the Cancer Immunity Cycle below — in the analogy of releasing the brakes of the car by the immune checkpoint inhibitors (the "back-end") — Step 7, Killling of Cancer Cells.
 |
| Click to enlarge. Image source |
Ironically Kohrt and other wrote in a 2014 paper entitled Intratumoral Immunization: A New Paradigm for Cancer Therapy:
"Immune cell infiltration in the tumor microenvironment is of prognostic and therapeutic import. These immune cell subsets can be heterogeneous and are composed of mature antigen-presenting cells, helper and effector cytotoxic T cells, toleragenic dendritic cells, tumor-associated macrophages, and regulatory T cells, among other cell types. With the development of novel drugs that target the immune system rather than the cancer cells, the tumor immune microenvironment is not only prognostic for overall patient outcome, but also predictive for likelihood of response to these immune-targeted therapies. Such therapies aim to reverse the cancer immunotolerance and trigger an effective antitumor immune response. Two major families of immunostimulatory drugs are currently in clinical development: pattern recognition receptor agonists (PRRago) and immunostimulatory monoclonal antibodies (ISmAb). Despite their immune-targeted design, these agents have so far been developed clinically as if they were typical anticancer drugs. Here, we review the limitations of this conventional approach, specifically addressing the shortcomings of the usual schedules of intravenous infusions every 2 or 3 weeks. If the new modalities of immunotherapy target specific immune cells within the tumor microenvironment, it might be preferable to deliver them locally into the tumor rather than systemically. There is preclinical and clinical evidence that a therapeutic systemic antitumor immune response can be generated upon intratumoral immunomodulation. Moreover, preclinical results have shown that therapeutic synergy can be obtained by combining PRRagos and ISmAbs to the local tumor site." {Underlined emphasis is mine}"Immunostimulatory monoclonal antibodies" include ipilimumab (Yervoy), nivolumab, pembrolizumab (Keytruda).
Breast Cancer (August 8, 2015)
H/t a shareholder (who approved the following sentence): A shareholder said he or she has first-hand knowledge of a breast cancer patient being treated with PV-10 at Moffitt Cancer Center.
Should the above be true, it would appear Moffitt may have moved on from its original preclinical work on PV-10 and this indication (below) to clinical investigation of it.
 |
| Click to enlarge. Image/paper source |
"These studies have demonstrated that intralesional PV-10, in addition to reducing the growth of a directly injected tumor, leads to the induction of a robust anti-tumor T cell response and supports the use of PV-10 to induce systemic anti-tumor immunity for the treatment of metastatic melanoma and breast cancer."Provectus concluded its recurrent breast cancer Phase 1 trial in 2008 (the ClinicalTrials.gov entry is here).
Meh (August 7, 2015)
 |
| Image source |
1. Pivotal melanoma Phase 3 trial site activation slippage. Eric seemed to confirm activation was going slower than the faster expectations management set in April when a company press release noted "...additional sites to be added in the coming weeks and months." Clinical trial sites, as Eric confirmed on the call, were more likely to be activated in July, August and September as I wrote in Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part I (July 13, 2015) on the blog's Current News page.
2. Phase 3 trial interim analysis slippage. Peter noted one could expect the interim analysis this time next year (i.e., August 2016), rather than initial expectations he and Eric set of sometime in the first half of 2016. His confirmation of a later planned interim analysis for efficacy and safety would be consistent with my later timing estimate of 2H16 in July 23, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV. Of course, I also think there is a smaller number of event threshold that would be triggered sooner than that. See August 4, 2015 blog post August 4, 2015
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part VI.
3. Asia (China) Phase 1b liver protocol slippage. It seems to me Provectus is moving ahead with Boehringer Ingelheim (China) (BI China) to finalize a suitable regional liver protocol and file an investigational new drug (IND) application directly with the Chinese FDA (or via Hong Kong). BI China, and BI more broadly, is a true pharmaceutical company while Sinopharm is a drug distributor (with whom BI also has a drug distribution relationship). If a regional deal is struck with Sinopharm one has to imagine it would be a drug distribution relationship, while if one is consummated with BI China one could surmise it would be a [traditional pharmaceutical] license transaction.
4. Asia/ROW liver clinical development program convergence. It struck me an Asian Phase 1b liver trial might converge with the existing U.S. expanded Phase 1 liver trial to eventually become a global or international Phase 2 liver randomized controlled trial (considering Eric's comments regarding sorafenib/Nexavar's regulatory approval path).
5. Authorized shares. As I noted in Notes (August 6, 2015) the end-of-2Q15 fully diluted shares outstanding figure appears to be ~294 million, leaving about 6 million available (but not addressing issuance of warrants for services going forward). Nearly 10 million warrants were issued in 2010, with about 5 million forfeited in 1H15. This could mean Peter has leeway under the authorized share ceiling of about 11 million shares. My note below that Provectus would have to raise money in 3 to 4 quarters (i.e., 8-11 months) also assumes he does and can not consummate a strategic equity investment by a Big Pharma. It also the share price remains the same such that he either does not receive some cash for warrant exercises (and/or cashless exercises provide more shares under the ceiling).
6. Moffitt, Part I. Eric noted the orthogonality of Merck's approved anti-PD-1 agent, Keytruda (pembrolizumab), and PV-10 (as explored by Moffitt Cancer Center). Orthogonality (as Provectus refers to it) not only means low or de minimis clinically relevant drug-drug interaction, it also means (I believe) the cumulative efficacy of the individual drugs in combination (in math, the magnitude of the combination vector is the magnitudes of the individual vectors adjusted by the sin of the degrees between the vectors, which is at its maximum when the vectors are orthogonal), and thus his (or Moffitt's) preference for use in a combination trial. What happened to Bristol-Myers' drugs, either anti-CTLA-4 ipilimumab or anti-PD-1 nivolumab?
7. Moffitt, Part II. There was no mention by Eric of Moffitt's work with anti-PD-L1 agents. Recall their initial murine model work on combinations of PV-10 and immune checkpoint inhibitors (Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma). One has to think Moffitt also undertook so-called orthogonality work of one or more of these agents, whose owners include Pfizer (via Merck KGaA), Roche/Genentech and AstraZeneca.
8. Moffitt, Part III. I (as did another shareholder) though I (we) heard Eric say Moffitt's mechanism of action work might be available (published/presented) before year-end, sooner than "later this year or early in 2016" noted on the company's May 7th business update conference call. If so, the venue and timing could be SITC's 30th Anniversary Annual Meeting (i.e., SITC 2015) on November 4-8. Moffitt presented their combination therapy murine model work referenced in 7. above at SITC 2014.
9. T-Vec. Eric said a combination therapy protocol (for patients with advanced melanoma) would (might) be completed this quarter, presumably leveraging key opinion leader and clinical site experience and feedback, and a review of T-Vec/ipilimumab Phase 1b trial results. But I also have to wonder how much more positive influence T-Vec may yet have on PV-10. Provectus finalized its pivotal melanoma Phase 3 trial protocol before T-Vec's advisory committees' review meeting at the end of April. The assembled panel concluded the reduction of injected tumors equated to/was clinical benefit (see April 29, 2015 blog post The first guy through the wall.) Aside from potentially having to incorporate T-Vec as a possible comparator in the Phase 3 trial, T-Vec could very positively influence the trial's protocol design.
Notes (August 6, 2015)
 |
| Image source |
2. The end-of-2Q15 cash balance (i.e., B/S item Cash and cash equivalents) was $23.1 million, compared to the prior end-of-Q's $14.2 million figure.
3. The company's monthly cash burn for the quarter appears to have been $1.34 million, -4% quarter-over-quarter.
4. Working in the gray — e.g., using a cash balance threshold of $12 million, estimating a monthly cash burn range of $1 million (a presumed "rough" projected figure for accounting firm BDO's purposes) to $1.4 million (on the current high end of recent actual), assuming the absence of non-dilutive deal-related cash inflows, making an assumption regarding an acceptable low watermark for the then pre-raise cash balance figure, assuming an increase in the authorized share number, etc. — Provectus would have to raise money in 3 to 4 quarters (i.e., 8-11 months).
5. Regarding the class action lawsuits (see the current Q's No. 7 under Notes on page 10): "On June 5, 2015, Provectus filed its Motion to Dismiss the Consolidated Complaint (the “Motion to Dismiss”). On July 20, 2015, the Lead Plaintiff filed his response in opposition to the Motion to Dismiss (the “Response”). Pursuant to order of the Court, Provectus must reply to the Response no later than August 19, 2015."
6. A derivative lawsuit naming the company as a nominal defendant was added in the quarter: the Donato Shareholder Derivative Lawsuit. See the current Q's No. 7 under Notes on page 12.
7. The end-of-2Q15 fully diluted shares outstanding figure appears to be ~294 million.
 |
| Click to enlarge. |
8. One wonders if Maxim exercised its over-allotment of common stock, which would have expired on August 3rd. If exercised the transaction would have netted Provectus an additional ~$1.8 million. Given the company's COO/CFO Peter Culpepper's approach to reporting, if Maxim had exercised its option he more than likely would have reported it in the current Q (which it appears does not). As an aside, management previously noted the "cooling off period" for the warrant "IPO" as 90 days, which may suggest Maxim could initiate coverage of PVCTWS (which for all intents and purposes is covering PVCT) towards the end of September.
9. What would a Provectus fiscal quarter be without money raise via Network 1 Financial? The company appears to have received net proceeds of ~$589,000 in the quarter under the existing private placement (i.e., $1 unit of 1 share of common stock + half of a warrant with a $1.25 exercise price). See the current Q's Item 2 on page 20.
 |
| Click to enlarge |
____________________
10. This breast cancer patient chat room/board comment from 2013 (huh?) about PV-10 and melanoma could suggest one or more Canadian sites are part of Provectus' melanoma Phase 3 trial.
 |
| Click to enlarge. Image source. Fuzzy purple emphasis is mine |
11. It is possible the University of Pennsylvania's Abramson Cancer Center (Philadelphia, PA), which posted both Provectus' melanoma Phase 3 and liver Phase 1 clinical trial information before taking it down, is a satellite site of St. Luke's Cancer Center (Bethlehem, PA) for the Phase 3 trial. St. Luke's (Temple University), Abramson, Penn State's Hershey Melanoma Center, Thomas Jefferson University's Kimmel Cancer Center, the University of Pittsburgh, and The Wistar Institute are members of the Consortium of Melanoma Centers of PA. Consortium objectives include "Clinical trials on personalized medicine (Accrual of patients to personalized therapeutic trials spanning sites)."
Spending Alpha (August 4, 2015)
 |
| Image source |
In Provectus' pivotal melanoma Phase 3 there should be a large treatment effect between the treatment arm of PV-10 and the control arm of systemic chemotherapy (i.e., dacarbazine or temozolomide). The accordant effect size should facilitate multiple planned or pre-specified (communicated or uncommunicated) efficacy looks into the data.
It seems to me, however, that the company's Phase 3 trial is more than just about PV-10's progression-free survival (PFS) curve separating as quickly as it can (by at least 70%) from the chemotherapy PFS curve. The fundamental hypothesis of the trial, according to Provectus' CTO Dr. Eric Wachter, PhD is that "local therapy can afford direct clinical benefit to melanoma patients in terms of eliminating lesions," which means "melanoma staying away [is] measured by progression free survival between the PV-10 arm and controlled arm." Melanoma staying away, or the hypothesis that eliminating lesions affords clinical benefit, is underscored by the trial's secondary endpoint of overall survival (OS).
The trial may or may not have an early efficacy look designed into it that PV-10 can afford for PFS, statistically speaking, but can the drug afford it for OS? I imagine so, but the real test of alpha spend affordability course will be when secondary endpoint OS data eventually is readout. I believe the trial will continue to run, chemotherapy patient crossover and crossover analysis notwithstanding, to collect OS data to definitively prove or disprove the trial's hypothesis — potentially much more so (if PV-10 OS is robustly lengthy and statistically significant) than Amgen's T-Vec, which barely missed achieved OS statistical significance.
Recall Eric's comments from the May 7th conference call:
"Last Wednesday, the 23-person advisory committee met all day here in Washington to lay the pros and cons for approval of T-VEC another intralesional therapy underdevelopment for melanoma. Such committees advise the FDA when there are technical questions about license application such as flaws in study design or where the risk benefit ratio is in doubt.
While the agency is not bound to follow advice of the committee, it's rare that they do not. After hearing the case for and against approval including review of a very detailed analysis of the study design and data by agency statisticians, the committee voted 22 to 1 in favor of following question. “Does T-VEC have an overall favorable benefit risk profile for the treatment of injectable regionally or distantly metastatic melanoma. In voting, please consider only whether the available evidence which support traditional approval and not accelerated approval.”
Despite the fact that the pivotal Phase 3 study of T-VEC did not show statistically significant survival benefit in its secondary endpoint of overall survival, the committee vote was resoundingly in favor of full approval. This means the committee believes that an agent with minimal side effects and it acts locally to reduce tumor burden is potentially a clinical benefit. And this is exactly the case we have been making for PV-10. Committee member Leisha Emens, M.D. of Johns Hopkins was quoted after vote, saying "The bulk of the data suggests there is clearly a favorable benefit risk ratio for this particular therapy; it represents an important new tool for patients."
And committee member, Brian Rini, M.D. of the Cleveland Clinic was quoted saying, "I thought the totality of evidence was there if there was a clinical benefit. And it seems to be beneficial even giving the evolving current landscape in melanoma.” We are grateful for the insight of the committee members and believe this vote is very good news for all intralesionally administered oncology drugs in development including PV-10.
In advance to the meeting, the agency prepared an extremely compressive 79-page briefing document describing and analyzing all available clinical data on T-VEC. This included analysis on a number of shortcomings in study design, study execution end-points and outcomes. Many of these shortcomings were familiar to us. And I was pleased to note that our Phase 3 study of single agent PV-10 is designed to avoid these shortcomings. For instance, enrollment of a carefully defined patient population and use of a standard primary end-point should minimize questions about clinical meaningfulness of study results while adjudication of outcomes that is for these progressions by an independent review committee serves to minimize potential discordance between sponsor reported results and those of the agency following their audit of the study.
The decision on approval is currently scheduled for the end of October. Since it is unknown whether T-VEC will be approved this year and if approved, when it will become available, this does not directly impact our Phase 3 study. In fact, the decision of the committee supports the fundamental hypothesis underlying our study that local therapy can afford direct clinical benefit to melanoma patients in terms of eliminating lesions. If T-VEC ultimately comes available during our study, it is likely that we would amend the protocol to allow its use as a competitor.Pivotal Melanoma Phase 3 Trial Site (August 3, 2015)
H/t a shareholder: Compassionate use site University of Louisville was added (not yet recruiting).
 |
| Click to enlarge. |
Forbes.com's Matthew Herper authored an article today entitled Lexicon's Quest For A New Blockbuster. In it he writes and also quotes:
"In a sense, the pharmaceutical industry has been working with a surprisingly repetitive set of compounds. “Most drugs are really German dyes,” says Brent Stockwell, a scientist at the MIT-affiliated Whitehead Institute for Biomedical Research. That’s because they’re based on what has worked before–mostly compounds that evolved from drugs developed at dye companies like F. Bayer & Co. a century ago."In a 2010 article by medical writer Walter Alexander: "In 1882, German patent No. 32584 [1] was granted to Ghnem for a new family of wool dyes. Ghnem took different halogens and added them to fluorescein to produce molecules with different colors. The name, Rose Bengal, arose presumably from the fact that the deep rosy red color was similar to that of the middle-of-the-forehead dot indicating marriage in women of the Bengali region of India." [2]
In 1998 Provectus' founders, Drs. Craig Dees, PhD, Timothy Scott, PhD and Eric Wachter, PhD, "re-discovered"* Rose Bengal, a molecule with a long and diverse medical history. Ironically the compound had lain around in plain sight of the global pharmaceutical industry for nearly 85 years** before Dees et al. began their journey of demonstrating Rose Bengal’s cancer fighting potential.
[1] Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie 1887; 238:318–338
[2] High Responses in Melanoma for Wool Dye/Ocular Stain Rose Bengal, Walter Alexander, The Oncology Pharmacist, October 2010}
* "Re-discovered" to mean that Rose Bengal’s therapeutic benefits remained hidden until the 1986 when sufficient quantities were administered orally in preclinical studies carried out by Japanese researchers. Ironically, while investigating the tumorigenicity of red food dye No. 105 (also made from Rose Bengal) they observed dose-dependent survival increases in test mice. Provectus’ multidisciplinary team of founders — a molecular virologist, a chemical engineer, and a chemist — looking for drug candidates having antineoplastic activity through a commercial data search separately came across Rose Bengal in 1998.
** I compute "nearly 85 years" based on the first recorded medical use of Rose Bengal, added to safranin victoria yellow for ocular pneumococcal infection (Römer, 1914) [3].
[3] Feenstra RPG and Tseng CG. Arch Ophthalmol 1992; 110:984–993
One month does not a trend make, but.../July Blog Stats (August 1, 2015)
 |
| Click to enlarge |
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
Intralesional agents in development with the potential to generate or facilitate a tumor response include Amgen's talimogene laherparepvec ("T-Vec") (Phase 3 reported) and Provectus' PV-10 (Phase 3 ongoing).
A tumor-specific response is important to the durability of the immunotherapeutic response of a drug. See, for example, a screenshot of a Dendreon webpage below.
 |
| Click to enlarge. Image source |
“The cells we took from mice treated with PV-10 were indeed activated against B16 melanoma tumors. We demonstrated that by showing that they could be transferred to untreated mice with the same tumor and produce an antitumor response. This is the definitive way to test for a tumor-specific T-cell response.” Against MC-38 adenocarcinoma tumors, the T-cells from treated B16 mice had no significant effect. “This is a very specific response to the B16 tumor,” Dr. PilonThomas said. {Source: Evidence for Systemic Effects Moves PV-10 Toward Further Clinical Trials, a white paper, June 2013}PV-10's tumor-specific T cell response may be broader and more robust than Provenge or any of the CAR-T companies' investigational agents.
* Talimogene laherparepvec activates systemic T-cell-mediated anti-tumor immunity, AACR 2015
They who must not be named (July 30, 2015)
H/t a shareholder (and regular blog-contributing due diligencer) for the below. Provectus added its third Pfizer executive/employee (Deanna Angello, a commercial strategist) to its strategic advisory board (SAB) in late-2014, announcing the addition in a December 17, 2014 press release. At Pfizer [according to the Provectus PR] Ms. Angello was Director, Commercial Strategy and New Business Planning for the Global Established Pharma business, the business organization where longer-time and more Provectus relationship-tangled (as well as SAB member since 2011) Dr. Craig Eagle, MD then resided [I believe]. She appears to have left Pfizer in Q1 (to join Genentech) but also appears to still be working with Provectus.
 |
| Click to enlarge. Edited from the version sent to me to remove her photo. |
 |
| Click to enlarge. Image source: A shareholder who sent it to me |
In Provectus' pivotal Phase 3 trial of PV-10 vs. systemic chemotherapy in patients with unresectable locally advanced cutaneous melanoma, patients are randomized to the two arms of the trial on a 2-to-1 basis — 2 patients enter the treatment arm (of PV-10) for each patient who enters the control arm (of dacarbazine [DTIC] or temozolomide [TMZ]). Thus, for a trial N of 225, 150 patients receive PV-10 and 75 patients receive chemotherapy. A stated single interim analysis of efficacy (and safety) would be triggered after 50% of the required events have occurred (a "prescribed number of events" clause), where an event is disease progression in a patient (thus, about 113 events are required). Given PV-10 and chemotherapy's historical performance results for events (progressive disease or PD) and progression-free survival (PFS), it's unlikely the trial could trigger an interim analysis based on a prescribed number of events. See blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV.
 |
| Click to enlarge. |
 |
| Click to enlarge. See blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV. |
 |
| Click to enlarge. |
Highlights (July 25, 2015)
Updated below.
H/t a shareholder: PMO Live 2015 - Highlights from Friday, July 24 (2015 Fourth Annual World Cutaneous Malignancies Congress)
Surgical and Locoregional Therapies of Melanoma
"Dr. Sanjiv Agarwala then presented an overview of intralesional therapy in melanoma, including the use of current and emerging agents. He provided an expert perspective on intralesional agents currently in development and showed clinical data on their safety and efficacy in stage III-IV injectable, but unresectable, melanoma. The goals of intralesional therapy include local disease control, systemic effects, and delaying or preventing the need for systemic therapy. Moreover, intralesional therapy presents a potential as neoadjuvant therapy. He showed results from the OPTiM trial with TVEC, where overall response rates and overall survival with TVEC was superior to granulocyte-macrophage colony-stimulating factor. Similarly, PV-10 (Rose Bengal disodium) was tested in a phase 2 clinical study in patients with locally advanced cutaneous melanoma that showed a 51% response in target lesions and a 33% response rate in nontarget lesions, suggesting a systemic effect. PV-10 is currently being tested in a randomized phase 3 trial. He also reviewed other intralesional agents that are in earlier stages of clinical development. Dr. Agarwala concluded that in the current era of melanoma therapy, intralesional approaches may have value (with a local direct effect and a systemic immune effect), that several agents in development appear promising, and that combination therapies of intralesional plus systemic therapies (eg, TVEC + ipilimumab or pembrolizumab) may be the best way to integrate the intralesional agents into clinical practice." {Underlined emphasis is mine}Updated (7/27/15): Presentations slides of Dr. Agarwala are here, including:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
The fifth in a series of blog posts and news items assessing the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma (Stage III patients, and not metastatic [Stage IV] melanoma).
- Win or lose: Will the trial succeed in meeting or fail to meet its primary and/or secondary endpoints?
- Time-to-success or -failure: How long might it take for the trial to succeed or fail?
- Good or bad process: Are there process steps and aspects thereof that may provide hints of potential future trial success or failure.
- 3. Good or bad process: Patient enrollment. See Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part I (July 13, 2015) on the blog's Current News page,
- 3. Good or bad process: Designing an interim analysis for efficacy into Provectus' pivotal melanoma Phase 3 trial. See July 16, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part II,
- 3. Good or bad process: Patient crossover. See Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part III (July 20, 2015) on the blog's Current News page, and
- 2. Time-to-success or -failure: Triggering the interim analysis. See July 23, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV.
This news item comprises: 3. Good or bad process: Patient-reported outcomes.
Blog news item takeaways:
Provectus announced concurrent with ESMO 2014 that the company had engaged ERT to assist with better quantifying symptom assessment and PROs. The poster noted:
Eric also said at the time (September 2014):
A comparable of the PRO study and work Provectus appears to be undertaking as part of its pivotal melanoma Phase 3 trial could be Incyte's PRO tool for identifying the symptoms of pancreatic cancer. The poster entitled Content Validity of a Patient-Reported Outcome Instrument for Pancreatic Cancer was presented at the ISPOR 20th Annual International Meeting in May 2015. Portions of it are pictured below.
The Incyte-ERT work saw 18 patients (out of an estimated trial N of 270) undergo concept elicitation interviews. If appropriate to use, that would suggest ~15 patients out of Provectus' pivotal melanoma trial's N of 225.
Moffitt and Gabrail are two identified sites for Provectus' PRO work. See New Moffitt Cancer Center study related to PV-10? (July 21, 2015) and Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015), respectively.
Finally, the IN VIVO blog discusses PFS, PRO and accelerated approval in the context of Genentech, Avastin, metastatic breast cancer, and the drug's withdrawal (and how I think the "inverse" of the situation applies to Provectus -- IN VIVO blog post also references Incyte’s Jakafi [ruxolitinib]):
Recall the FDA's BTD denial of PV-10: "This determination is based on the paucity of data on endpoints indicative of clinical benefit (e.g., pain, infection, significant bleeding) and our inability to determine the clinical significance of the reduction in the size in one to 10 target lesions in patients with locally advanced melanoma, who may have additional untreated cutaneous, subcutaneous, or visceral sites of disease" {Underlined emphasis is mine}.Blog news item takeaways:
- It would appear Provectus is continuing the process of its pivotal melanoma Phase 3 trial by beginning to collect patient-reported outcome ("PRO") data. This collection process could be the Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma study recently identified,
- PROs may be an exploratory or secondary endpoint or outcome measure of the Phase 3 trial. The presumed goals of the above noted study (as part of the trial itself), first, may be to develop a new instrument (that partially includes or exclusively comprises PRO items) to assess the key signs, symptoms and impacts experienced by patients with locally advanced cutaneous melanoma. Then, through the course of the study, second, collect data that would hopefully support the content validity of the instrument,
- The study might be sized for about 15 patients (given the trial's N of 225). The study sites conducting that work thus far appear to be Moffitt Cancer Center and Gabrail Cancer Center,
- The above speculation is influenced by the possibly comparable work of Incyte, which employed ERT (which also has been engaged by Provectus for its Phase 3 trial) to develop a PRO instrument for pancreatic cancer and had its drug Jakafi® (ruxolitinib) be the first to get symptom data on its approved label for pancreatic cancer since the FDA issued its draft PRO Guidance in 2006,
- The above interview centers may also recruit, enroll and treat patients for the Phase 3 trial, potentially bringing the number of sites that have been activated for at least one task or another to at least 4, and:
- St. Luke’s Cancer Center (Bethlehem, Pennsylvania),
- The University of Pennsylvania’s Abramson Cancer Center (Philadelphia, Pennsylvania),
- Moffitt Cancer Center (Tampa, Florida), and
- Gabrail Cancer Center (Canton, Ohio).
- Finally, the parallel and additive work of undertaking the PRO study may well be critical to the FDA’s expectations surrounding progression-free survival, the trial's primary endpoint, in the context of accelerated approval; that quality of life benefits could serve as evidence that the benefit is clinically meaningful.
"We have reviewed your request and while we have determined that treatment of “locally advanced cutaneous melanoma” meets the criteria for a serious or life-threatening disease or condition, the preliminary clinical evidence you submitted does not indicate that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Therefore, designation as a Breakthrough Therapy cannot be granted at this time.
The preliminary clinical data provided in your request for Breakthrough Therapy designation are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma; however, the preliminary clinical data do not demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. This determination is based on the paucity of data on endpoints indicative of clinical benefit (e.g., pain, infection, significant bleeding) and our inability to determine the clinical significance of the reduction in the size in one to 10 target lesions in patients with locally advanced melanoma, who may have additional untreated cutaneous, subcutaneous, or visceral sites of disease. The information provided on durability of response is also of unclear clinical significance given the modifications to RECIST. Finally, there was also insufficient information provided in the package to ascertain improvement in or relief of tumor-related symptoms of pain, bleeding, or tumor ulceration. We acknowledge that a subset of patients with pre-treatment pain was identified, with summary data presented for two post-treatment time-points (at 8 and 13 weeks). However, from the information provided using visual analogue scale (VAS) for assessment of pain is incomplete as it did not capture pain throughout the course of treatment, no information on concomitant pain medications were provided, and it is not clear that the results observed were clinically significant, as no information was provided on validation of the VAS instrument used." {Underlined emphasis is mine}On the company's May 23, 2014 conference call in the wake of BTD denial, Provectus' CTO Dr. Eric Wachter, PhD discussed the above issue (the transcript in part below is lightly edited to remove uhs, ums, etc.):
"We were able to show that the best overall response rate in the 28 patients where we treated all of their disease was exceptionally high. 71 percent with a 95 percent competence interval went from 51 to 87 percent. And most notably perhaps, a 50 percent complete response rate which is competence interval of 31 to 69 percent. We showed among the total of those 54 patients that is patients who had all of their disease monitored in the study complete response was achieved in 232 of 363 injected lesions. So, that was 64 percent per lesion complete response rate. Complete response is achieved quite quickly in the majority of those lesions, 121 occurring after a single injection of PV-10, 84 requiring two injections, 22 requiring three injections, and only five of those requiring four injections. We felt that that data provided a logical justification for the application in that it seemed clear to us that a complete response to all lesions implied resolution of any melanoma-related symptoms present at baseline. We had lengthy discussions with the Agency in our December 2013 meeting on the outcome in these patients, discussed the objective response parameters that I've just outlined. And the Agency asked us for additional evidence showing that those responses correlated with symptomatic improvement in the patients. That is, improvement in things such as pain, lesions, bleeding in the lesions, infection in the lesions, and so on. Unfortunately, the phase two study did not require patients to have baseline symptoms in these types outside, uh, the existence of biopsy proven melanoma for enrollment. And we did not design the study to collect the type of detailed information on symptoms that might be ideal for showing that correlation. Available symptom assessment instruments, uh, were used such as the EORTC- QL2-C30. That’s a lot of letters and numbers. But it's a Quality of Life Questionnaire that’s been validated for cancer. But unfortunately, that and other similar validated instruments are primarily used for evaluation of symptoms related to systemic disease and the effects of systemic therapies and have not proven to be particularly valuable in addressing and quantifying the kinds of local and regional symptoms that affect the patients in this 54 patient subgroup. So, we followed up on that meeting with the Agency by conferring with our biostatistician to analyze the objective response data, populated the competence intervals for us. And we also poured through Visual Analog Scale Pain Data. We mentioned of a Visual Analog Scale in the letter. We had used a standard visual analog scale designed implemented uniformly throughout the study to assess pain pretreatment and at various time points subsequent to PV-10 treatment. Because of the lack of requirements for patients to have pain symptoms upon enrollment, only a small fraction of patients had clinically significant pain at baseline. So, we analyzed those patients and presented them that analysis of those data in context of the objective response data. We found that there was a strong relationship between the two types of data, that there was simply not enough of the symptomatic, or symptomology data to show a statistical function. I have to conclude that that’s the principal basis for the rejection of the application. I'd say that it was our assumption going into the application that improvement in symptoms, if we made the patient’s symptoms go away was tantamount to -- I’m sorry -- if we make the patient’s lesions go away that’s tantamount to making the patient’s symptoms of that disease go away. We don’t seem to have been successful in convincing the Agency of that. {Underlined emphasis is mine.}Ironically Eric said of the Type C meeting with the FDA in December 2013:
"And so, in the meeting in December [2013], the Agency said, to paraphrase, hey, we like this ablative effect that you're showing in these patients with disease confined to the skin. But can you show us that that also improves symptoms that we've heard are important in melanoma, pain, bleeding, infection, for example. That would be a winning combination." {Underlined emphasis is mine.} See my May 30, 2014 blog post "Why did it take four years...to arrive at this point?"In subsequent presentations at ASCO 2014 and ESMO 2014 the company discussed this symptom topic.
 |
| Click to enlarge. Image source is the ASCO 2014 poster. |
 |
| Click to enlarge. Image source is the ESMO 2014 poster. |
 |
| Click to enlarge. Image source is the ESMO 2014 poster. |
"We are looking forward to initiation of the phase 3 trial and have assembled an experienced multi-disciplinary team to help us execute this important study. For example, we are thrilled to collaborate with ERT, Inc., experts in quantifying patient reported outcomes for pivotal oncology trials, and expect this collaboration to help place Provectus at the forefront in the study of cutaneous symptoms of locally advanced melanoma." {Underlined emphasis is mine.}In October ERT issued their own press release about the engagement noting:
"ERT’s [Clinical Outcome Assessment] consultants are working with Provectus to select a patient-reported outcome (PRO) measure of symptoms in this population that can be implemented in the Phase III clinical trial of PV-10 and ultimately support treatment benefit claims in the product label." {Underlined emphasis is mine.}According to the FDA's 2009 Guidance for Industry, Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims:
A PRO is any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else. The outcome can be measured in absolute terms (e.g., severity of a symptom, sign, or state of a disease) or as a change from a previous measure. In clinical trials, a PRO instrument can be used to measure the effect of a medical intervention on one or more concepts (i.e., the thing being measured, such as a symptom or group of symptoms, effects on a particular function or group of functions, or a group of symptoms or functions shown to measure the severity of a health condition).
Generally, findings measured by a well-defined and reliable PRO instrument in appropriately designed investigations can be used to support a claim in medical product labeling if the claim is consistent with the instrument’s documented measurement capability. The amount and kind of evidence that should be provided to the FDA is the same as for any other labeling claim based on other data. Use of a PRO instrument is advised when measuring a concept best known by the patient or best measured from the patient perspective. A PRO instrument, like physician-based instruments, should be shown to measure the concept it is intended to measure, and the FDA will review the evidence that a particular PRO instrument measures the concept claimed. The concepts measured by PRO instruments that are most often used in support of labeling claims refer to a patient’s symptoms, signs, or an aspect of functioning directly related to disease status. PRO measures often represent the effect of disease (e.g., heart failure or asthma) on health and functioning from the patient perspective.
Claims generally appear in either the Indications and Usage or Clinical Studies section of labeling, but can appear in any section."The PRO study being conducted by Moffitt and Gabrail have as its goal and approach the below:
 |
| Click to enlarge. Image source is New Moffitt Cancer Center study related to PV-10? (July 21, 2015) below. Fuzzy purple emphasis is mine. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Fuzzy purple emphasis is mine. |
| Click to enlarge. Fuzzy purple emphasis is mine. |
 |
| Click to enlarge. |
Moffitt and Gabrail are two identified sites for Provectus' PRO work. See New Moffitt Cancer Center study related to PV-10? (July 21, 2015) and Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015), respectively.
Finally, the IN VIVO blog discusses PFS, PRO and accelerated approval in the context of Genentech, Avastin, metastatic breast cancer, and the drug's withdrawal (and how I think the "inverse" of the situation applies to Provectus -- IN VIVO blog post also references Incyte’s Jakafi [ruxolitinib]):
"For other sponsors, the Avastin process has added some clarity on FDA’s expectations surrounding PFS – in particular, that quality of life benefits could serve as evidence that the benefit is clinically meaningful. So the Avastin withdrawal shouldn’t just dissuade sponsors from using PFS, it should encourage them to design trials with supportive measures that can themselves turn into additional claims. (Indeed, Incyte’s Jakafi (ruxolitinib) just cleared FDA with a patient-reported outcome based symptom claim.)" {Underlined emphasis is mine}
Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015)
A second location, Gabrail Cancer Center in Canton, Ohio, appears to be involved in the clinical study Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma. The study is not yet listed on ClinicalTrials.gov. See New Moffitt Cancer Center study related to PV-10? (July 21, 2015) below. Moffitt's investigator for the trial also was and is an investigator on Amgen's T-Vec clinical trials.
 |
| Click to enlarge. Image source. Fuzzy purple emphasis is mine. |
Why? (July 22, 2015)
Updated below.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source. |
New Moffitt Cancer Center study related to PV-10? (July 21, 2015)
The indication of Provectus' pivotal Phase 3 trial is [unresectable] locally advanced cutaneous melanoma. Inclusion criteria include "Stage IIIB or IIIC recurrent, satellite or in-transit cutaneous or subcutaneous melanoma." Exclusion criteria include "Presence of active nodal metastasis." The trial's protocol on ClinicalTrials.gov is here.
Moffitt appears to have begun a study with the title of Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma, with the goal of understanding and documenting "...the signs and symptoms of the disease as well as its impact on patients' lives." The study's investigator is Dr. Jonathan Zager, MD, a member of the "Moffitt PV-10 team."
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Moffitt's Phase 1 feasibility study of PV-10 (i.e., the molecules mechanisms of action) had the center identifier 17183.
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part III (July 20, 2015)
The third in a series of blog posts and news items assessing the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma (Stage III patients, and not metastatic [Stage IV] melanoma).
- Win or lose: Will the trial succeed in meeting or fail to meet its primary and/or secondary endpoints?
- Time-to-success or -failure: How long might it take for the trial to succeed or fail?
- Good or bad process: Are there process steps and aspects thereof that may provide hints of potential future trial success or failure.
Entry 2: 3. Good or bad process:" Designing an interim analysis for efficacy into Provectus' pivotal melanoma Phase 3 trial. See July 16, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part II.
This blog news item, "3. Good or bad process:" Patient crossover
Provectus' pivotal Phase 3 trial for locally advanced cutaneous melanoma includes a crossover design where patients in the control arm on systemic chemotherapy (i.e., dacarbazine or temozolomide) may crossover to the treatment arm of PV-10 at any time during the study.
 |
| Click to enlarge. Image source. Purple emphasis is mine |
Interestingly, on the same day another company revealed pivotal Phase 3 kidney cancer data: Exelixis' cabozantinib beat its primary endpoint of PFS; however, the trial will continue (there cannot be patient crossover) because the secondary OS endpoint only shows a trend towards the treatment arm beating the control arm — but has not beat it, statistically speaking. Exelixis' trial had an estimated enrollment of 650, and utilized the same control drug as the Opdivo trial (everolimus) (see cabozantinib's trial protocol here).
Provectus had patient crossover at the end of the study removed from its trial design.
 |
| Click to enlarge. Image source. Purple emphasis is mine. |
Although BioVex/Amgen's intralesional agent OncoVEX/T-Vec's pivotal melanoma Phase 3 trial included a planned interim analysis for efficacy of secondary endpoint OS, no patient crossover was permitted because the trial needed to collect OS data. See blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part II. Vical's intralesional agent Allovectin-7's pivotal melanoma Phase 3 trial did not include either an interim analysis or a patient crossover.
On another note, h/t InvestorVillage Juggernaut noted the Abramson Cancer Center of the University of Pennsylvania identified itself as a site for Provectus' pivotal melanoma Phase 3 trial.
 |
| Click to enlarge. Image source. Purple emphasis is mine. |
H/t a shareholder: UPenn's Abramson Cancer Center also appears to be a clinical site for Provectus' ongoing Phase 1 liver trial.
 |
| Click to enlarge. Image source. Purple emphasis is mine. |
"Additionally, our Phase 1 study of PV-10 for liver tumor has continued to recruit patients at our clinical sites in the U.S. especially those with tumors metastatic to liver and is providing valuable insight into potential additional areas for development, since a range of cancers metastasized to liver."Commercially Reasonable Efforts (July 18, 2015)
Following up further on the "commercially reasonable efforts" phrase in the Provectus-Boehringer Ingelheim (China) letter of intent — see the July 2nd press release and BioXcellence™ (July 9, 2015) below — particularly in the context of the pharmaceutical industry, note the selected (by me) slides from a June 2015 presentation by folks from JSB-Partners*, SRS Acquiom** and law firm McDermott Will & Emery LLP entitled Structuring Milestones and Contingent Payments in Biotech, Pharma and MedTech M&A and Collaborations.
Blog new items takeaway: The use of commercially reasonable efforts as a threshold in the pharmaceutical industry appears accepted, appropriate and commonplace.
Slides are below. Fuzzy purple emphasis, and "commercially" blue highlight are mine.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
** "...SRS Acquiom has become the undisputed expert at managing private M&A post-closing activity."
"...It’s Not All About PD-1" (July 17, 2015)
Caroline Helwick recently wrote an article in The ASCO Post entitled Oncolytic Immunotherapy in Melanoma: It’s Not All About PD-1, which profiled comments by Dr. Robert Andtbacka, MD, Associate Professor of Surgical Oncology at the Huntsman Cancer Institute at the University of Utah, Salt Lake City, and included a mention of PV-10. She previously wrote an article in The ASCO Post that profiled PV-10 (among other therapeutic agents) last year entitled Intralesional Injections Trigger Immune Responses in Melanoma.
Andtbacka's comments apparently derive from a presentation he gave to/at a/the the American Society of Gene and Cell Therapy meeting, where he discussed talimogene laherparepvec (T-VEC/Amgen), plasmid interleukin-12 (IL-12) electroporation (OncoSec), PV-10 (rose bengal) and coxsackievirus A21 (CVA21/Viralytics). The title of the article itself is interesting, and a "money quote" in it includes:
"Oncolytic immunotherapies work not only through a lytic effect but by activating the entire immune system, “not just in lesions we treat, but elsewhere in the body.” He noted that intralesional therapies are tailor-made for local and in-transit cutaneous lesions and the accessible lymph nodes of melanoma.
Intralesional oncolytic immunotherapies aim to achieve a local ablative effect as well as a systemic immune effect, the so-called bystander response. They may also help to prevent the development of visceral metastases, if given early, he predicted. Durable responses with limited toxicity are additional goals." {Underlined emphasis is mine}I don't get hung up on the label oncolytic immunotherapy, which appears to be used when describing [oncolytic] viruses like T-Vec, CVA21, Jedd Wolchok and James Allison's Newcastle Disease Virus, etc. MD Anderson Cancer Center's Dr. Merrick Ross, MD, for example, has referred to Provectus' PV-10 as an oncolytic virus. John Hopkins' Dr. Suzanne Topalian, MD has referred to PV-10 as a vaccine.
For me the point of this article is the building trend or theme that the immune checkpoint inhibitors — the so-called back-end or agents that help release the brakes of the immune system — are not "all that," and very likely require help from the so-called front-end or agents that start the engine of the car and/or help press on the gas pedal. Additionally, while some oncolytic virus companies (like Viralytics) are experimenting with delivering their agents intravenously, the likely better and much more effective way to deliver the front-end is via injection directly into cancerous lesions and tumors.
Helwick's article notes in regards to disclosure that Andtbacka reported no potential conflicts of interest. He is/has been a consultant to BioVex/Amgen, Provectus and Viralytics, and been involved in at least one recent event put on by OncoSec.
On another note — h/t InvestorVillage poster Juggernaut — Provectus updated its July calendar of events to include PMO Live, an annual joint meeting of the Global Biomarkers Consortium (GBC) and World Cutaneous Malignancies Congress (WCMC). Conference faculty includes Dr. Sanjiv Agarwala, MD of St. Luke’s Cancer Center (PV-10 investigator) (Conference Co-Chair), Andtbacka, Ross (PV-10 investigator [although the conference's associated title is different]), Dr. Grant McArthur, PhD (Provectus consultant) of Peter MacCallum Cancer Centre (PV-10 compassionate use program site) and Moffitt Cancer Center's Dr. Vernon Sondak, MD (Provectus consultant).
New SEC Filing (July 16, 2015)
Provectus filed a Form S-3 today, a $100 million mixed securities shelf offering of common stock, preferred stock and warrants (with prospectuses dated July 16, 2015). The registration statement appears to replace the prior Form S-3, a $100 million mixed securities shelf offering of common stock, preferred stock and warrants (with prospectuses dated June 29, 2012) filed on July 2. In 2012 the company was incorporated in Nevada. It currently is incorporated in Delaware.
The company drew down on the prior June 2012 shelf by issuing common stock and warrants via the Maxim Group public offering in the amount of gross proceeds of $13,313,750. The offering closed on June 24, 2015 and generated the issuance of 17.25 million shares of common stock and 17.25 million of warrants on common stock. A prospectus for the shelf itself is included in today's filing (the “Base Prospectus” in Ex. 5.1 below). The proceeds above then would have left $86,686,250 available in/on the shelf.
 |
| Click to enlarge. Image source: 2015 S-3 |
The Maxim public offering was a means of raising (a vehicle to raise) money. Two other means also are available to the company:
- A Controlled Equity Offering sales agreement with Cantor Fitzgerald for up to $50 million (that if utilized would result in the issuance of common stock) (a prospectus of which is included in the 2015 S-3 — the “Cantor Sales Agreement Prospectus” in Ex. 5.1 below). The company has not drawn on this facility, and
- An equity line of credit (as I've referred to it in the past) with Alpha Capital Anstalt (which replaced a similar vehicle with Lincoln Park Capital) for up to $10 million (that if utilized would result in the issuance of common stock) (a prospectus of which is included in the 2015 S-3 — the “Alpha Capital Purchase Agreement Prospectus” in Ex. 5.1 below). As a result of the Maxim offering the Alpha Capital vehicle was reduced to $10 million from $30 million. Provectus has not drawn on this facility.
Exhibit (Ex.) 5.1 is Provectus' law firm Baker Donelson's opinion, and important to read.
As a result of the Maxim offering as well as depending on whether the additional shares are purchased by Maxim and what number of common shares and/or warrants (on common stock) may be issued to consultants, Provectus effectively is up against its limit of authorized shares of 300 million. See Offering, Part I (June 29, 2015) on the blog's Archived News III page.
 |
| Click to enlarge. |
You can use the following online web page comparison application to contrast the two Form S-3s. When I did so I noticed, among other things:
- Verbiage related to developing ethical pharmaceuticals:
 |
| Click to enlarge. |
- As at June 30, 2015 warrants of 79,680,528 and stock options of 9,545,214, which are close to my estimates above.
Updated 7/15/15 for additional writing at the end of the news item.
On Provectus' May 7th 1Q15 conference call the company's CTO Dr. Eric Wachter, PhD said in regards to combining PV-10 with an immune checkpoint inhibitor for patients with advanced (i.e., Stage IV) melanoma:
"And in addition to the progress on the combination therapy patent front that Pete mentioned, we've also made substantial progress towards commencement of our proposed clinical study of PV-10 in combination with immune checkpoint inhibition. We have identified the investigators who will lead this work, the agent to be used in conjunction with PV-10, the patient population and the dosing schedule for both agents along with the study end-point. To assess potential benefit of PV-10 for patients with advance melanoma, this phase 1b/2 study will incorporate a modest sized single arm Phase 1b component with expedited safety and efficacy end point supporting expansion to a larger randomized Phase 2 component." {Underlined emphasis is mine}If Eric has identified (decided on) the investigators to undertake work, the clinical sites at which the work would be done, the immune checkpoint inhibitor for pairing, the patient population, the dosing schedule, and the study endpoint, then what might be "taking so long" for Provectus to [make a decision to] partner up on such work or to go it alone?
On the company's March 13th 4Q14 conference call Eric said:
Throughout our consultations with key clinical opinion leaders, we’ve also been attuned to the need to address the needs of patients with more advanced disease. With enrollment now complete in the mechanism of action study of PV-10 of Moffitt Cancer Center, and initial results reported on that study and on the companion nonclinical study to assess combination of PV-10 with immune checkpoint inhibition, we are making progress on design of our proposed study to assess PV-10 in combination with immune checkpoint inhibitions in patients with advanced melanoma.
We continue to expect this to be structured as a Phase 1b/2 study with a modest sized single arm Phase 1b component and expedited safety and efficacy endpoints supporting expansion to a larger randomized Phase 2 component. Endpoints for Phase 1b are likely to comprise acute safety as a combination regimen and objective response rate at three to four months. For Phase 2, we anticipate endpoints of progression free survival at overall survival. With three agents now approved in the US and also available in other key locations abroad, I expect to have very concrete details on this work to discuss at our next quarterly conference call. Importantly now that we have options for this systemic agent, this study can commence with or without the assistance of a partner." {Underlined emphasis is mine}On the same call Provectus' COO/CFO Peter Culpepper said in regards to a co-development relationship to undertake a combination trial for melanoma:
"We’re obviously because of the Moffitt data already talking to potential codevelopment partners. That’s why we put that in there. Now the question is how much data is enough data to support a transaction that makes sense? That’s the question, that’s the question everyone has in the life science community is what’s the meaningful amount data and in the codevelopment, in the whole immunotherapeutic arena, it does not take much data, clinical data in particular, and I am talking about 12 patient type data, it doesn’t take much to show the value of a relationship, or to support the value in the relationship. I would argue that you could see a 1b/2 go quick, so that, that would support a larger study. And that’s where the industry is focused. The industry is focused on PV-10 in combination with a number of these agents." {Underlined emphasis is mine}What do "data" mean, and does data sufficiency mean, if you'll indulge the exercise? Data include Moffitt Cancer Center's Society for Immunotherapy of Cancer ("SITC") 2014 annual meeting poster entitled Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma. The poster and abstract were "at odds" in that the former neither mentioned nor elucidated comments in the latter (the abstract is below):
"PV-10 is a 10% solution of Rose Bengal that is currently being examined as a novel cancer therapeutic. We have previously shown that intralesional (IL) injection of PV-10 into a single subcutaneous B16 melanoma tumor led to regression of both the injected tumor and uninjected B16 lung lesions. Tumor regression correlated with the induction of systemic anti-melanoma T cell immunity. In melanoma patients, IL injection of PV-10 has led to regression of treated lesions as well as untreated bystander lesions. In this study, we have examined whether IL PV-10 and co-inhibitory blockade could improve anti-tumor immunity and regression of melanoma. B16 cells were injected into C57BL/6 mice to establish one subcutaneous tumor. Treatment of this lesion with a single IL injection of PV-10 alone led to partial regression of the injected B16 lesion. Systemic administration of anti-CTLA-4 or anti-PD1 antibodies in combination with IL PV-10 resulted in increased tumor regression and improved survival in this model. Treatment with PV-10 also led to the induction of T cells that produced IFN-γ (495 ± 198 pg/ml) in response to B16 cells but not to irrelevant MC-38 cells. Combination therapy with IL PV-10 and anti-CTLA-4 led to increased IFN-γ responses to B16 (1235 ± 191 pg/ml, p < 0.05). In another experiment simulating heavy tumor burden using a bilateral model, systemic administration of anti-PD-L1 antibodies in combination with IL PV-10 led to regression of the injected B16 lesion as well as a bystander subcutaneous lesion on the opposite flank (p < 0.01 compared to mice treated with anti-PD-L1 antibodies or IL PV-10 alone). Together, these studies support the induction of increased tumor-specific immunity after co-inhibitory blockade in combination with IL PV-10 therapy." {Underlined emphasis is mine}How much data are enough for Big Pharma? Clearly the poster presentation was insufficient. Other data (in my view) include:
- Issuance [and not merely the allowance by the U.S. Patent and Trademark Office] of the Pfizer/Provectus patent application for the use of PV-10 in combination with systemic immunotherapy agents in treatment of cancer,
- The filing of a Phase 1b/2 trial protocol for combining PV-10 with a reimbursable immune checkpoint inhibitor on ClinicalTrials.gov,
- The presentation and/or publication of more of Moffitt's mechanism of action work to elucidate the workings of PV-10.
From a time, expense and resource perspective, Provectus should be able to undertake the Phase 1a portion of a combination study itself, and in a reasonable time generate results for Big Pharma to see. 2 above perhaps signals Provectus' intention to go it alone in order to generate the results Peter feels are necessary to support a co-development relationship (transaction). Could 2 above be sufficient?
 |
| Click to enlarge. Image source. |
Moffitt began publishing their Phase 1 feasibility study data at AACR 2014 and ASCO 2014. The ASCO abstract noted, which the poster repeated in a visual (see right): "Six of 8 patients had metastatic disease refractory to previous ipilimumab, anti-PD-1 and/or vemurafenib therapy." The PD-1s more than likely are Merck U.S.' Keytruda and Bristol-Myers' Opdivo. Patients in this study were to be ones diagnosed suspected to have metastatic (advanced) melanoma. There were no exclusion criteria regarding visceral disease, since it was a study and not a trial (as there is Provectus' pivotal Phase 3 trial for Stage III patients). It is possible the immune checkpoint inhibitors were in the study patients' blood long enough when PV-10 was administered, thus potentially rendering the study for the six patients refractory to ipilimumab, anti-PD-1 and/or vemurafenib therapy as a mini- or pseudo-Phase 1b combination therapy study. Could 3 above be sufficient? The company expects more of Moffitt's study work to be available later this year or in early 2016.
It is likely the outcome of or movement on the combination therapy for melanoma aspect of Provectus' clinical development program for PV-10 will based on the impact of some combination of 1, 2 and 3 above.
Updated 7/15/15: H/t InvestorVillage poster leave_the_gun, who specifically highlighted Provectus' pivotal Phase 3 trial exclusion criterion of "Immunotherapy for cancer within 4 weeks of initial study treatment." Moffitt's feasibility study had no such exclusion. Provectus' Phase 3 trial needs to demonstrate no additional benefit derived from patients having been on and subsequently failing immunotherapy (i.e., Yervoy, Keytruda, Opdivo). Note the half-lives of these immune checkpoint inhibitors: 15.4 days, 26 days and 26.7 days, respectively. Four weeks of course equals 28 days.
Updated 7/15/15: H/t InvestorVillage poster leave_the_gun, who specifically highlighted Provectus' pivotal Phase 3 trial exclusion criterion of "Immunotherapy for cancer within 4 weeks of initial study treatment." Moffitt's feasibility study had no such exclusion. Provectus' Phase 3 trial needs to demonstrate no additional benefit derived from patients having been on and subsequently failing immunotherapy (i.e., Yervoy, Keytruda, Opdivo). Note the half-lives of these immune checkpoint inhibitors: 15.4 days, 26 days and 26.7 days, respectively. Four weeks of course equals 28 days.
Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part I (July 13, 2015)
The first in a possible series of blog posts and/or news items assessing the company's pivotal Phase 3 trial for unresectable locally advanced cutaneous melanoma (Stage III patients, and not metastatic [Stage IV] melanoma).
- Win or lose: Will the trial succeed in meeting or fail to meet its primary and/or secondary endpoints?
- Time-to-success or -failure: How long might it take for the trial to succeed or fail?
- Good or bad process: Are there process steps and aspects thereof that may provide hints of potential future trial success or failure.
In a 2008 PharmaExec.com article entitled Site Activation: The Key to More Efficient Clinical Trials Gen Li wrote "Patient enrollment, at its simplest, consists of three steps: Site selection, Site activation, Patient recruitment."
Step one: Site selection. This step for Provectus could be mostly complete.
During Provectus' April 9th panel discussion in New York — comprised of the company's CTO Dr. Eric Wachter, PhD, lead Phase 3 principal investigator Dr. Sanjiv Agarwala, MD (St. Luke's Cancer Center), very likely but not yet officially confirmed Phase 3 trial investigator and former Phase 2 trial investigator Dr. Merrick Ross (MD Anderson Cancer Center), and Provectus COO/CFO Peter Culpepper — Eric said:
"...we continue to expect to require approximately twenty five centers in the U.S. for the Phase three melanoma study and an additional ten plus in Australia and we are in the process of expanding our reach to areas of the globe that we haven't worked in previously..." {quoted from a transcript of the discussion's audio, which I used an online transcription service to convert}Following Eric's April 9th comment, Peter noted during a May 19th BioTuesday interview article:
"The company is targeting 25 clinical sites in the U.S., 10 in Australia, and possibly one-or-two in San Paulo and Beijing."Step two: Site activation. This company process step appears to be a work in progress.
Li also wrote: "It takes several steps to bring a site to the point where it is ready to recruit patients. This process is called site activation, and it consists of a variety of tasks including: Negotiate a financial contract, Gain approval from Institutional Review Board (IRB) or, in Europe, Ethics Board (EB), Provide clinical supplies, Obtain other documents from site (CV, financial disclosure, etc)."
Provectus' April 15th press release Provectus Biopharmaceuticals Opens Patient Enrollment; Begins Phase 3 International FDA Comparative Clinical Trial of PV-10 for Melanoma noted:
"The Company is seeking 225 patients and enrollment has begun at St. Luke's University Hospital and Health Network, Bethlehem, PA, the first study site to be opened, with additional sites to be added in the coming weeks and months." {Underlined emphasis is mine}I assume but of course cannot be certain Provectus' definition of patient enrollment is consistent with Li's, and thus patients may be (or may not be) being treated at St. Luke's. Because no other sites are listed on the trial's ClinicalTrial.gov's webpage, I obviously assume the other 24 U.S. and 10 Australia sites have not been activated.
Step 3: Patient recruitment. This Provectus step may be underway only at St. Luke's.
I examined the approaches of the three intralesional agents for melanoma that Huntsman Cancer Institute's (and likely PV-10 Phase 3 trial investigator) Dr. Robert Andtbacka often compared and contrasted (in chronological order of pivotal trial commencement) — Vical's Allovectin-7 (2007), BioVex/Amgen's OncoVEX/T-Vec (2009) and Provectus' PV-10 (2015) — which comprised reviewing the list of changes to or updates of each drug's trial's ClinicalTrials.gov webpage below.
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
BioVex/Amgen issued a press release regarding the commencement of its Phase 3 trial. I could not find any mentions of when the trial's first patient visited or was treated. I also could not find any press releases or public statements regarding the addition of trial sites. Chicago's Rush University Medical Center did issue a press release early on noting it was leading the trial, which may suggest Dr. Howard Kaufman, MD was the lead investigator.
 |
| Click to enlarge. |
Provectus issued its release regarding the commencement of the Phase 3 trial, unfortunately noting the underlined below:
"The Company is seeking 225 patients and enrollment has begun at St. Luke's University Hospital and Health Network, Bethlehem, PA, the first study site to be opened, with additional sites to be added in the coming weeks and months."Despite the above, it would appear, according to Eric, Provectus will not comment on specifics about the trial going forward, such as when the first patient was or is treated or trial site initiation or activation activity, save for periodically updating the trial's ClinicalTrials.gov webpage and providing appropriate periodic study status updates. Highlighting when the first patient is treated may make it possible to project key trial milestones. Recall his comments from the company's May 7, 2015 conference call:
"I don’t anticipate that company will provide site by site or patient by patient announcements of study milestones since this will be highly unusual for our industry but we do anticipate providing periodic summaries of study status. And also as noted by Pete, when we open new centers, these will be shown on the NCI clinical trials registry website."It should be noted, however, that Provectus did make frequent site addition and patient accrual announcements during the company's Phase 2 metastatic melanoma trial (10 in all). There were two study updates provided by the Phase 2 trial's lead investigator (Dr. John Thompson, MD).
 |
| Click to enlarge. |
"Turning to status of the Phase 3 study of PV-10, we've opened our first site for enrollment of patients and are in final steps of opening a number of others. As is noted previously, these initial sites will be both in the U.S. and Australia. And as Pete noted, we're actively engaged in bringing additional sites, both in these countries and in a number of other strategically selected countries such as Brazil and China into the program." {Underlined emphasis is mine}The first underlined portion could suggest patients are being treated at St. Luke's; however, a subsequent press release by the company regarding the first patient treated would of course undermine my assumption. The second underlined portion above would seem to be consistent with Peter's May 19th interview comments.
Is there something "wrong" with the startup of Phase 3 trial so as to warrant some sort of correction? I say "no," leaning on what appears to be good process by Eric for my answer. Alternatively, consider recent tweets from @johnhallnj on the topic at the end of June:
 |
| Click to enlarge. |
"I'm making the assumption FP Enrolled/dosed has not occurred. Assuming that is correct, this window for study start up is broke on two levels. They need to get the other sites up and running ASAP (CRO change). PVCT needs to address the protocol barriers to enrollment that has screening open for close to two months and no one randomized (Amendment consideration). Until that happens, there will be no faith in timelines for data readout..."
On January 22nd following the company's December 2014 press release [T]o Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment that signalled a delay in the start of the trial and followed Provectus' November PR Protocol for Phase 3 Study Of PV-10 As Treatment for Melanoma Now Available Online that registered the trial on ClinicalTrials.gov and in my view begun the trial process (administrative as that function was), John asked when I thought the company would start screening/begin treating patients. I replied: "If I had to guess, I'd imagine they'd might receive guidance from the FDA in early-February at the earliest; Eric appears to habitually miss his deadlines or expectations with respect to timing." We learned in February that the meeting with the FDA to discuss potential changes to the Phase 3 trial protocol occurred at the end of January; see February 9th's PR Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study (byline: FDA allows Provectus to go forward on Phase 3).
On February 12th John continued our dialogue:
"Do you know if the [trial] sites in the U.S. are private or academic sites? Assuming private and they can use a central IRB my estimate is P3 start of screening mid April. If academic sites, May/June. This assumes final protocol by the end of this month and a favorable IRB submission/review and site contract execution." {Underlined emphasis is mine}
John's timeline, presumably from document submission (e.g, final trial protocol, etc.) submission to opening enrollment (and patient accrual), appeared to be 45 days for a trial site of a private hospital with a central institutional review board ("IRB") and 90-120 days for academic ones (presumably with local IRBs). With Eric filing the revised or final protocol around mid-March, John's timeline would extend from late-April to June/July. As an aside, "Tufts estimates it takes an average of 45 days to execute a [Master Clinical Trial Agreement ("CTA")] at a central IRB site and an average of 145 days to execute a CTA at a local IRB site." As a further aside, I think John's comment about a longer FPFE (which probably should be FPFV, first patient first visit) leading to a longer LPLV (last patient last visit) is largely irrelevant (as I see it) in the context of the trial's interim analysis as a key catalyst.
Comparing the startup of the OncoVEX/T-Vec and PV-10 pivotal trials in a cursory way, one might see:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Later, I dug deeper into the topic of site activation (particularly for oncology trials) because the 100-day figure was not indication-specific. Activation, from a process workflow perspective, might comprise document submission to opening enrollment and patient accrual. See below, for example:
 |
| Click to enlarge. Image source. |
Measuring from when Eric filed the revised/final Phase 3 protocol, the range of "median" dates above to add other sites (into St. Luke's) might lead to:
 |
| Click to enlarge. |
Asia Pivot (July 10, 2015)
On Provectus' 4Q14 conference call (March 12th) the company's CTO Dr. Eric Wachter, PhD said:
"In addition, our Phase 1 study of PV-10 for liver RECIST continuing to recruit patients, in particular those with tumorous metastatic liver. This work is continuing to provide valuable insight into potential additional areas of development since a range of cancers are metastasized to liver. It is also continuing to be supportive of our Pivot Asia for hepatocellular carcinoma or HCC and is a key driving force behind our partnering activities in China and elsewhere.
We expect to report initial data from this study at one or more international cancer conferences in the summer. We are also in continued dialogue with the key opinion leaders in Asia to guide – of our proposed phase 1b/2 study of PV-10 plus standard care for HCC." {Underlined emphasis is mine}On the company's 1Q15 conference call (May 7th) Eric said:
"Additionally, our Phase 1 study of PV-10 for liver tumor has continued to recruit patients at our clinical sites in the U.S. especially those with tumors metastatic to liver and is providing valuable insight into potential additional areas for development, since a range of cancers metastasized to liver.
We also expect this study to be instrumental in achieving our Pivot Asia for hepatocellular carcinoma or HCC and it is playing an important role in our partnering activities in China and elsewhere. As Pete noted, we will report initial data from the study at the ESMO Congress on Gastrointestinal Cancer in early July with additional data presentations possible during the second half of the year.
We've also begun the preparatory process for regulatory filing necessary to begin clinical work for this indication in China, in support of an anticipated corporate partnership as well as to begin enrollment for the Phase 3 melanoma study.
With regard to HCC planning and design of our post Phase 1b/2 study of PV-10 plus standard of care for HCC remains unchanged. Moving forward on the regulatory side now will allow us to move forward in Asia with or without the assistance of the corporate partner."Musings about Sinopharm, and a Phase 1b/2 liver cancer trial in Asia:
 |
| Click to enlarge. |
German pharmaceutical company Boehringer Ingelheim ("BI") has a subsidiary or organization called, Boehringer Ingelheim BioXcellence™, which is a biopharmaceutical contract manufacturer that can work with and assist clients as "...a strategic partner...from development to fill and finish."
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. Image source. |
- The initial portion of the letter-of-intent ("LOI") relationship for Provectus with BI appears to be the former engaging the latter as a contract manufacturing organization ("CMO") (and at this time the latter's registration experience in the region(s) in question) for an Asia/Pacific Phase 1b/2 liver trial.
- BI will provide commercially reasonable support, which is the lowest standard of “best efforts,” “reasonable efforts” and “commercially reasonable efforts.” Commercially reasonable "...is a term incapable of a precise definition and will vary depending on the context in which it is used...Commercially reasonable efforts should be consistent with good faith business judgments." So, while BI may expend some time and resources to assist Provectus in China and environs, it may not be a "huge" amount.
| Click to enlarge. Image source. |
- Provectus' granting BI priority rights in China (see More Pub (July 8, 2015) below) or first priority to collaborate on PV-10's commercialization in the country (see A Simple Enough Explanation (July 5, 2015) below) would appear commensurate with BI's potential time, resource and money expenditure.
 |
| Click to enlarge. Image source. |
- The Provectus-BI LOI may or may not encourage Sinopharm to move. There was no mention (as far as I can tell) of an exclusivity clause in the parties' memorandum of understanding (see here and here for the relevant Provectus press releases).
- As Provectus' COO/CFO Peter Culpepper has said, data will drive the deal(s). Engaging BI as their CMO for China, Hong Kong and Taiwan begins the process of generating hepatocellular carcinoma clinical data in those geographies (which also should be germane to other regions of the world). Sinopharm walking away from Provectus' terms and conditions doesn't preclude the Chinese drug distributor from returning to the table. It'll mean that for the time being it isn't willing to pay Provectus' price.
On July 7th Medical writer Walter Alexander wrote an article that included comments about PV-10 (published today on DocGuide) relating to the Phase 1 liver results presented by Provectus at the World GI Congress in Barcelona: Trials Show Promise for Locoregional Treatment for Hepatocellular Carcinoma and Liver Metastases. His past work includes articles in 2010 (here, here and here), 2011, 2013 (here and here) and 2014. His current article about the company's Phase 1 liver cancer data included a quote by Dr. Chris Verslype, MD of University Hospital Gasthuisberg in Leuven, Belgium, who commented on trials across several phases presented at the meeting (Dr. Verslype was a faculty member of the World GI Congress):
“We may expect some immunological effects with this kind of treatment, so locoregional treatment doesn’t necessarily mean that the effect of the therapy remains limited to the tumour itself in the liver. There may be effects on distant tumour sites,” Dr. Verslype concluded.FiercePharmaAsia noted the below on July 7th:
 |
| Click to enlarge. |
Updated (7/8/15): On the company's last conference call, CTO Dr. Eric Wachter, PhD said:
"I would like to make an additional comment on the topic of China. Of course as I mentioned it's a valuable corporate endeavor from the partnering and financial side, but from the clinical development side, it's a compelling story, both for our melanoma indication and for hepatocellular carcinoma. So we're doing an appropriate thing right now which is moving forward in a position that will allows us to get into China with or without a partner in China." {Underlined emphasis is mine}I take "with or without a partner" to mean a commercial license partner who has not reasonably met the terms and conditions (especially the upfront payment) Provectus seeks. Provectus may be in the position, with certain operational assistance of BI, to undertake the regulatory approval process until such time as the Phase 1b and/or 2 liver cancer data drive the deal. A Phase 1b trial should be able to generate data and data analysis measured in several months. The recent Phase 1 trial Provectus presented in Barcelona and Osaka measured objective response rate of injected tumors after at least 3 months. Interestingly, the trial protocol said Provectus also would measure bystander lesions if present.
Comfort Level (July 7, 2015)
Medical writer Janet Fricker wrote another article about PV-10 (and Provectus) (published today on eCancer News), this time about the Phase 1 liver results presented by the company at the World GI Congress in Barcelona: PV-10 shows potential in hepatocellular carcinoma and metastatic liver disease. On eCancer News she has written about PV-10 at least seven times: October (ESMO) 2012, October (ECC) 2013, April (AACR), June (ASCO), October (ESMO) and November (SITC) 2014, and now July 2015 (ESMO World GI). She also has written about PV-10 on PharmiWeb.com four times: October (ECC) 2013, and June (ASCO), October (ESMO) and November (SITC) 2014. Her current article about Provectus' Phase 1 liver cancer data included the following notable items:
- Provectus' CTO Dr. Eric Wachter, PhD's quote "The study suggests that PV-10 has moved beyond just melanoma and may be agnostic to tumour type" {Underlined emphasis is mine}. See my blog post The Ever Expanding Clinical Value Proposition of PV-10. In addition to clinical melanoma data from the company's Phase 1 and 2 trials and Moffitt Cancer Center's Phase 1 feasibility study, Fricker notes two other tumor types: "Two patients – one with hepatocellular carcinoma (HCC) and one with colorectal cancer (CRC) metastatic to the liver - had non-existent liver cancer at more than 40 months follow-up after a single injection of PV-10 to the liver" {Underlined emphasis is mine}. She also notes tumor agnosticism in preclinical: "In animal models the investigators have successfully ablated liver, renal, breast and pancreatic tumours with PV-10" {Underlined emphasis is mine}.
- Eric later was replaced in the interview by his doppelgänger, Bizarro Eric, who said: "Having liver cancer patients alive at up to 54 months of follow-up with no evidence of disease is remarkable. This is even more extraordinary when you consider these patients received just one or two intralesional injections" {Underlined emphasis is mine}.
- Fricker's reference of the sorafenib GIDEON study (first interim analysis is here, second interim analysis is here): "The recent GIDEON study showed sorafenib, currently the only approved first line treatment in HCC, delivers a median overall survival of 10 months in patients under 70 years, and 20 months in patients older than 70 years" {Underlined emphasis is mine}. In regards to Provectus' poster information Fricker wrote: "For the first analysis of five patients (six tumours) who had longer-term assessment, two patients showed no evidence of disease at more than 40 months follow-up according to RECIST and EASL criteria." {Underlined emphasis is mine}
- Interestingly, Fricker also wrote about commercials matters, which she hadn't done in ten previous PV-10/Provectus-related articles: "On the basis of these results Provectus Biopharmaceuticals, Inc. announced the signing of a Letter of Intent (LoI) with Boehringer Ingelheim China on July 2 to collaborate in bringing PV-10 to market in mainland China. Together the two companies are planning phase 1b/2 studies of PV-10 in combination with standard of care (SOC) in liver cancer."
Provectus issued its press release regarding the company signing a letter of intent ("LOI") with German pharmaceutical company Boehringer Ingelheim's (BI's) China subsidiary (Boehringer Ingelheim (China) Investment Co. Ltd.) on July 2nd via BusinessWire. BI's China subsidiary issued its press release regarding the LOI on July 3rd via PR Newswire Asia.
 |
| Click to enlarge. Boehringer Ingelheim China |
 |
| Click to enlarge. PR Newswire Asia |
 |
| Click to enlarge. Boehringer Ingelheim |
Provectus issued a press release and filed an 8-K today regarding the company's poster presentation in Osaka, Japan, Phase 1 PV-10 Data on Liver Cancer Presented at 6th Asia-Pacific Primary Liver Cancer Expert Meeting. For all intents and purposes the APPLE poster (the link is here) is the same as the World GI one, with no new information. See ESMO World GI Poster (July 1, 2015) below. Provectus management did take the opportunity of the PR to note the liver poster presenter, St. Luke's Cancer Center's Dr. Argawala, was the lead investigator for the company's pivotal melanoma Phase 3 trial, and to remind folks the Phase 3 trial had begun: "He serves as a principal investigator of the phase 1 clinical trial that produced the data presented, and is the lead investigator for the phase 3 clinical trial of PV-10 as an investigational treatment for melanoma which recently began."
A Simple Enough Explanation (July 5, 2015)
 |
| Click to enlarge. Fuzzy colored line emphasis is mine. |
Clinical Trial (July 4, 2015)
[Updated 7/4/15: News item takeaways at the end of this post]
Provectus' poster at the ESMO 17th World Congress on Gastrointestinal Cancer in Barcelona included the below in the bottom right-hand corner {fuzzy green box emphasis is mine}:
 |
| Click to enlarge. |
- SAT means a single-arm trial of the particular Asia sub-region's (e.g., China's) standard of care ("SOC") plus PV-10 for the Phase 1b portion of the study, and
- RCT means a randomized controlled trial of the above referenced SOC (i.e., SOC - PV-10) vs. the SOC plus PV-10 (i.e., SOC + PV-10) for the Phase 2 portion of the study.
For example, on the 4Q14 conference call (March 13th) Eric said:
"We expect to report initial data from this study at one or more international cancer conferences in the summer. We are also in continued dialogue with the key opinion leaders in Asia to guide – of our proposed phase 1b/2 study of PV-10 plus standard care for HCC. As with our melanoma combination study, I expect to have very concrete details to discuss at our next quarterly conference call." {Underlined emphasis is mine}On the 1Q15 call (May 7th) he said:
"With regard to HCC planning and design of our post Phase 1b/2 study of PV-10 plus standard of care for HCC remains unchanged. Moving forward on the regulatory side now will allow us to move forward in Asia with or without the assistance of the corporate partner." {Underlined emphasis is mine}Eric said nothing in the design had changed (as of May); however, he did not follow through with the expectation he set in regards to the "very concrete details" of the design (expectations set in March). The above lines on the Barcelona poster are not additive in terms of design details. There is no need to set expectations, and there certainly is no need to set expectations you can't, won't and/or don't meet.
Nevertheless, there is enough information about and around, I think, to potentially frame the Phase 1b portion of an Asian (i.e., Chinese) Phase 1b/2 study:
- 20-24 patients,
- SOC: Transcatheter arterial chemoembolization ("TACE")
- Endpoints: [acute] safety, objective tumor response rate (complete response + partial response), and
- Maybe (also?) time-to-progression, disease-control rate (complete response + partial response + stable disease)
- I would imagine progression-free survival and/or overall survival would be among the endpoints for the Phase 2 portion
- Interim readout: Likely after a couple of months of observation plus time to analyze and report.
"Until now TACE has made a main method in the therapy of liver tumors and is considered the gold standard for treating intermediate-stage HCC. Today sole use of TACE as a locoregional therapy gains a complete local tumor control of 25–35% and permits an increase of survival in patients with intermediate HCC according to Barcelona-Clinic Liver Cancer (BCLC) classification. TACE is currently mostly taken as a regional treatment of inoperable HCC, but more and more studies concluded that it is an alternative to resectable early-stage HCC. It is also indicated for patients with regional recurrence in the liver after previous resection of HCC. Another important use of TACE is to downstage HCC in patients who exceed the Milan criteria for liver transplantation. In other words, TACE makes the tumors shrink enough to lower the stage of the cancer. Selected patients with stage III/IV HCC can be downstaged to Milan criteria with TACE, and in patients with a complete response to TACE, excellent posttransplantation outcomes are expected with great disease-free and overall survival." (Source: Yong-Song Guan, Qing He, and Ming-Quan Wang, “Transcatheter Arterial Chemoembolization: History for More than 30 Years,” ISRN Gastroenterology, vol. 2012, Article ID 480650, 8 pages, 2012.)"TACE is the current standard of care for patients presenting with multinodular HCC and relatively preserved liver function, absence of cancer-related symptoms, and no evidence of vascular invasion or extrahepatic spread." (Source: Wang et al., Unresectable hepatocellular carcinoma treated with
transarterial chemoembolization: clinical data from a single teaching hospital, Int J Clin Exp Med 2013;6(5):367-371)
Clinical evidence for transarterial embolization (TACE):
"The most reliable way to confirm a survival benefit is large RCTs; however, initial small RCTs had failed to show a survival benefit of TACE treatment for HCC patients. In 2002, two RCTs from and Spain and Hong Kong investigated the survival benefits of conventional TACE compared to the best conservative treatment. These RCTs were followed by cumulative meta-analyses, showing that c-TACE significantly reduced the overall 2-year mortality rate compared to control patients who received conservative treatments. In 2003, Llovet et al. reported a meta-analysis, constructed from 7 RCTs including 545 HCC patients, comparing c-TACE or bland transarterial embolisation (TAE) vs. conservative management or other therapies (systemic chemotherapy or tamoxifen). Most patients had cirrhosis, with Okuda stages I-II, and lacked evidence of PVT. Doxorubicin was used in one study and cisplatin in three; Gelfoam® was used as the embolic agent in all the trials. Mean number of treatment ranged between 1 and 5 sessions. Survival benefits were identified in two studies. The two-year survival rate in the treated group was 41% (range, 19-63%) vs. 27% (range, 11-50%) in the control group (P=0.017). The significant survival benefit was for c-TACE with doxorubicin or cisplatin, but not for bland TAE alone. In 2007, Marelli et al. (38) also found similar results in a meta-analysis. In a recent Asian prospective cooperative study including 99 HCC patients, however, conventional TACE was associated with median OS of 3.1 years with 2-year OS of 75% (95% CI: 65.2-82.8%). O’Suilleabhain et al. evaluated the long-term survival of TACE in patients with unresectable HCC and suggested that a cure for unresectable HCC may be possible with TACE, although this is rare. TACE after radical excision of HHC can also destroy remnant cancer cells, decrease recurrence rate, and increase survival rate. A possible survival advantage has also been reported in patients treated with TACE before resection of HCC when compared with resection alone.
TACE has shown to be effective in the treatment of CRC metastases for unresectable patients. In one article, nineteen trials were reviewed. In these studies, TACE has been applied in 324 patients with CRC metastases with conventional method or its variants, with response rates varying from 25-100%. In a prospective study, 463 patients with unresectable liver metastases of CRC that did not respond to systemic chemotherapy were repeatedly treated with TACE in 4-week intervals. The anticancer drug was mixed with Lipiodol® and consisted of mitomycin C alone, mitomycin C with gemcitabine, or mitomycin C with irinotecan. Embolization was performed with starch microspheres. Partial response was achieved in 68 patients (14.7%), stable disease in 223 patients (48.2%), and progressive disease in 172 patients (37.1%). The 1-year survival rate after TACE was 62% and the 2-year survival rate was 28%." {Underlined emphasis is mine} (Source: Wáng Y-XJ, De Baere T, Idée J-M, Ballet S. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chinese Journal of Cancer Research. 2015;27(2):96-121.)My takeaways for this blog news item:
- I think Eric has completed the "high-level" design of his China liver trial, which would appear to be straightforward in design, standard of care selection, potentially cost, subsequent pathway to approval if and when successful, and capable of generating interim results in the short-term. Design framework aside, I would imagine the actual detail of the trial protocol is far, far from insubstantial,
- I also think the trial more than likely will take place in Hong Kong, see SAR (May 14, 2015) on the blog's Archived News III page: "In terms of the future in Hong Kong, early drug development is important. In China, recruitment is really fast, so you can easily do a big phase three trial there. So our role in Hong Kong is in phase one and two, and then you go to the mainland or wherever for phase three," and
- The potential Boehringer Ingelheim relationship, see Thoughts about Barcelona (July 2, 2015) below, may well facilitate this trial.
Comparable, Consistent PV-10 Pharmacokinetics (July 3, 2015)
"Pharmacokinetics, sometimes described as what the body does to a drug, refers to the movement of drug into, through, and out of the body."
Pharmacokinetic variability: "Inter-individual variations of a drugs pharmacokinetic parameters, resulting in fairly different plasma concentration-time profiles after administration of the same dose to different patients," where inter-individual means among individuals (people or patients).
I wrote in my June 2015 blog post Reproducibility, the Hallmark of Western Science that PV-10's clinical value proposition had been repeated by Provectus' lead researcher (Chairman and CEO Dr. Craig Dees, PhD), independently reproduced by Moffitt Cancer Center and the University of Illinois, and demonstrated in multiple cancer indications across multiple species (mice, higher level animals like dogs and horses, humans). As a small molecule it is Rose Bengal's (PV-10's) physical chemistry that underscores its consistent capabilities, rather other cancer immunotherapies' bio-chemistry, which makes them prone to sub-par and inconsistent performance. All of this (and more) speaks very loudly to PV-10's fitness as a product as well, e.g., works under many different conditions, ships well, has a long shelf life, does not require refrigeration, etc. As I wrote in a recent post The Risk-Reward of Provectus Biopharmaceuticals: Management,
The risk of an investment in Provectus (primarily from the vantage point of [July 1st's] share price) is mostly if not completely mitigated by the clinical value proposition of its investigational drug (PV-10/Rose Bengal).With the release of the company's first liver cancer poster, another example of PV-10's consistency or comparability presents itself: the drug's pharmacokinetics.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
- Data on PV-10 for Chemoablation of Liver Cancers Presented at ESMO 17th World Congress on Gastrointestinal Cancer (the poster is below, see ESMO World GI Poster (July 1, 2015)), with a sub-headline of "Preliminary Evidence of Efficacy Observed" and
- Signs Letter of Intent with Boehringer Ingelheim (China) to Collaborate in Bringing PV-10 to Market in China, with a sub-headline of "LOI Signed at ESMO GI Addresses Melanoma and Cancers of the Liver."
Multi-indication. Provectus first presented clinical melanoma data (interim Phase 2) at a medical conference (ASCO) in 2009. In the company's 2015 World Gastrointestinal Cancer Congress poster, one sees the demonstration of PV-10's efficacy on both primary or hepatocellular carcinoma or HCC, and secondary or liver metastases. With this preliminary evidence of efficacy, I think it is reasonable (and intellectually honest) to say PV-10 is or can be a multi-indication drug.
The following jumps out from the Barcelona liver cancer poster:
- Transient toxicity — recall that injections into cutaneous or subcutaneous melanoma lesions and tumors generated transient mild to moderate locoregional pain (with mostly Grade 1 and 2 AEs and rarely Grade 3s),
- Transient increase in liver enzymes ("Transient Elevation of Transaminases" on the poster) — consistent with another type of minimally invasive liver cancer ablation therapy called percutaneous ethanol injection or PEI ("EtOH," for ethanol, on the poster). Interestingly, and as an aside, anesthesia during surgical procedures, because it reduces arterial blood flow in the liver and the amount of oxygen to the liver, causes transient increases in liver enzymes.
- PV-10 is agnostic to disease presentation in the liver — The drug's ability to handle disease diversity was on display. Not only were different types of liver metastases treated (colorectal, melanoma, non-small cell lung, ovarian), but different "variants" of HCC (Hepatitis B and C, cirrhosis). To be fair, the poster only directly addressed successful treatment of an HCC tumor (subject 001), an HCC variant tumor (subject 005) and a colorectal met tumor (subject 006), and indirectly by virtue of the statement of how long patients were alive.
It will be interesting to compare, contrast and/or combine Provectus' Barcelona poster with the one being presented at Dr. Agarwala at the 6th Asia-Pacific Primary Liver Cancer Expert (APPLE 2015) Meeting.
Validation. If there was only one thing I took away from the letter of intent Provectus signed with Boehringer Ingelheim's ("BI's") China subsidiary, it was [in my view] validation by a Big Pharma (BI is ranked 19th in 2014 sales revenues) of PV-10's clinical value proposition.
Beyond that, the "deal" seems to be:
- BI providing material assistance to Provectus to get PV-10 registered and approved in China (co-development of a sort) (although Provectus at the current time would very likely bear the brunt of such China drug development expense), in exchange for
- BI receiving an option to be the first to negotiate an exclusive commercial relationship of some sort with Provectus should PV-10 be approved in China.
ESMO World GI Poster (July 1, 2015)
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
The 6th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2015), Evidence and Consensus on HCC Management (p. 217):
 |
| Click to enlarge. |
Goldfarb, Agarwala and Rosemurgy are clinical/principal investigators at Sharp Memorial Hospital (San Diego, CA), St Luke's University Health Network (Bethlehem, PA) and The Southeastern Center for Digestive Disorders & Pancreatic Cancer (Tampa, FL). Lyon is a Sharp interventional radiologist. Low is a Sharp diagnostic radiologist. McMillian is founder/President of Boston-based gRadiant Research, and "developing thermal therapy as an alternative to traditional surgery."
June Blog Stats (July 1, 2015)
 |
| Click to enlarge. |
 |
| Click to enlarge. |


No comments:
Post a Comment