“A further impetus toward teasing out the precise mechanism of how PV-10 can exert a systemic
immune response in patients,” said Dr. Sarnaik in an interview, “is to allow us to rationally combine PV-10 treatment with some of the exciting emerging immunotherapies for metastatic melanoma.” The focus at Moffitt, Dr. Sarnaik continued, is on discerning the presence of immune cell infiltrate in untreated tumors after PV-10 injections into other lesions. “We are really interested in harnessing immune cell infiltrate as a form of treatment,” he said, noting also that while creating cancer vaccines has been thought of traditionally as one of the Holy Grails of cancer research, cancer vaccines have turned out to be not strong enough to generate an adequate immune response. CANCER WATCH, VOL. 22, FEBRUARY 2013
I kept coming back to this portion of the article for two reasons. First, was Sarnaik's quote rationally combining PV-10 with other exciting emerging immunotherapies. What, PV-10 isn't exciting? Second, was his quote about harnessing immune cell infiltrate as a form of treatment. A better cancer vaccine because of PV-10?
The crux of the matter is that PV-10, as a local agent, needs to show systemic potential for life science investors, Big Pharma and the FDA to view the drug as clinically relevant. This is the local agent "burden of proof." There must be systemic potential to be relevant for treating cancer after surgery, where cancer is viewed by definition as a systemic disease. PV-10 is an drug with tremendous local efficacy and before unseen systemic benefit (even more so than systemic agents).
But PV-10 still is a local agent.
The mindset of the industry -- medical oncologists and hematologist-oncologists, but not surgeons -- is that a local agent has to be combined with a systemic agent to be relevant. The industry does not yet realize how much of an impact PV-10 will be just as a local agent. All it can currently understand is that PV-10 works, and harnesses the immune system in novel manner. It is obvious to industry PV-10 will get even better systemic results when combined with other agents, or, other agents will get better results when combined with PV-10.
I am getting hung up about trying to understanding PV-10's potential as a monotherapy, but that's not where the industry's mindset currently resides (i.e., as I wrote above, it's in combination for now). That should come, however, and probably quickly.
A tumor's core is an anaerobic necrotic area where more dangerous cancer cells reside, compared to the outer layers and exterior of the tumor where cancerous cells are less abnormal and virulent. Standard chemotherapy or radiation kills the "easy" cancerous targets on the periphery of the tumor. The "hard" targets in the anaerobic core are difficult for drugs and radiation to destroy. A very Darwinian natural selection process occurs. The more hardy and vicious variants in the core survive, feeding off of and expanding into the room provided by the destruction of the easier-to-kill cancer cells at the periphery. Tumors re-occur and the next generation of them is drug- and radiation-resistant, having selected for the more virulent population. If you miss with the first round, everything is made worse for the patient.
The historic and current work on cancer vaccines faces the challenge of not being strong enough to generate an adequate immune response. Like with failed chemotherapy or radiation, where the treatment miss makes everything worse for the patient, vaccines are inducing tolerance by repeated exposure to antigens, convincing the immune not to react to tumor-associated antigens.
Recall Craig et al.'s conclusion at SITC:
PV-10 in situ vaccination. Let the the antigen presenting cells (APCs) pick the antigen, rather than the other way around (as others are doing) and kill the tumor in situ. Let the APCs do their job and present antigens to T cells.
Moffitt, however, through their focus on adoptive T cell immunotherapy, believes PV-10 induces better T cells. T cell activation to very specific tumor targets. The ability to transfer immunity. Notice Moffitt's AACR abstract about PV-10 inducing a systemic anti-tumor immune response in murine models of melanoma and, now, breast cancer. More cancer indications validated by Moffitt on the way?
Moffitt, it seems to me, thinks PV-10 is the pathway to a more potent (i.e., generates a much stronger immune response), more effective (i.e., it heals, it cures), more broad (i.e., multi-indication) cancer vaccine.
I admit this post is a work in progress. The much greater point is understanding what PV-10 does to the immune system. PV-10: The Holy Grail? The cure for cancer? Perhaps...
Showing posts with label Moffitt. Show all posts
Showing posts with label Moffitt. Show all posts
March 2, 2013
February 28, 2013
$PVCT: The #Holy #Grail of #Cancer Treatment is...
Some believe Provectus has the most unusual opportunity in the history of drug development thus far, and that no one has ever seen a drug work like Rose Bengal in PV-10 and PH-10.
[Emphasis below all mine.]
The Cancer Watch article establishes context with historical PV-10 clinical trial success: Back in October, 2012, Phase 2 metastatic melanoma data on use of intralesional PV-10 presented at ESMO (European Society for Medical Oncol- ogy) 2012 Congress in Vienna, Austria, showed an objective response rate (ORR) of 51%, and a disease control rate of 69% in target melanoma lesions. The other finding, which lies behind some of the intensifying interest in PV-10, was a 61% ORR in bystander (uninjected) lesions among patients who had complete or partial responses in their target lesions. The bystander lesion ORR in patients with non responsive target lesions was 18%. Of deep interest, as well, were case studies showing potential stasis or regression of untreated visceral lesions in patients following PV-10 treatment of their cutaneous lesions.
The author further establishes governing thought: A bystander effect was clearly apparent in the first study in that PV-10-treated mice had 3 or fewer lung metastases as compared with more than 250 in each of the untreated mice.
In further elucidating the work at the cancer center, the author wrote: The focus at Moffitt, Dr. Sarnaik continued, is on discerning the presence of immune cell infiltrate in untreated tumors after PV-10 injections into other lesions. "We are really interested in harnessing immune cell infiltrate as a form of treatment," he said, noting also that while creating cancer vaccines has been thought of traditionally as one of the Holy Grails of cancer research, cancer vaccines have turned out to be not strong enough to generate an adequate immune response. Is Dr. Sarnaik implying PV-10 is the Holy Grail?
In response to the weakness of cancer vaccines: The strategy of adoptive cell transfer potentially overcomes the weak vaccine response...While adoptive cell transfer offers the advantage that enough T cells can be obtained for infusion in all patients, the T-cell receptors transfected into the T cells have a limited antigen specificity.
PV-10, however: ...Shari Pilon-Thomas, PhD, also a Moffitt researcher, demonstrated that T-lymphocytes recovered from mice treated with PV-10 do appear to be of a higher quality, as evidenced by stronger tumor reactivity...
Sarnaik's study will test Pilon-Thomas' conclusions in humans: "This is a straightforward study that will give a yes or no answer," Dr. Sarnaik said.
The Cancer Watch article about Moffitt may be the information/PR prelude to the cancer center's long-awaited high profile conference presentation and contemporaneous publication of its work on the immunological MOA characterization of PV-10 work.
When I think of appropriate "high profile" conferences, two come to mind: the annual meetings of the American Association for Cancer Research ("AACR") and the American Society of Clinical Oncology ("ASCO"). The former runs April 6-10, while the latter runs May 31-June 4. While research is presented at both conferences, ASCO is more focused on clinical trial results and updates. As such, and since Moffitt's work to date (excluding the recently initiated human trial) has been murine research, I'd guess the target conference for Moffitt is AACR. If AACR is indeed where Moffitt presents, then I'd also guess the target peer-reviewed publication is Cancer Research.
February 26, 2013
$PVCT: It's the #immune system, #stupid.
"It's the economy, stupid" is a slight variation of the phrase "The economy, stupid" which James Carville had coined as a campaign strategist of Bill Clinton's successful 1992 presidential campaign against sitting president George H. W. Bush. (Source: Wikipedia).
To understand PV-10 is to understand the relationship between chemoablation and immune-mediated signaling. It's what Craig means when he says the immune system responds in direct proportion to the degree of insult. Think of PV-10 chemoablation as the proxy for the degree of insult, which is rapid, complete and durable in the case of PV-10. Moffitt should say the same thing in its conference presentation(s) and contemporaneous peer-reviewed publication.
A good amount of work -- creative, innovative, pragmatic, historical, scientific, medical literature-based, etc. -- went and a continues to go into dosing, of historical and upcoming clinical trials, of certain indications, of pre-clinical work on newer indications.
Yes, management doesn't talk openly about dosing, nor do they talk openly about several other topics of import and value.
The thing that makes you go hmmmm... Rose Bengal has an established safety history, a short half-life in the bloodstream, and is excreted via the liver and kidneys. The half-life is measured in minutes, like 15-30 or thereabouts. The drug is gone from the body almost immediately, so there is never enough of it to kill remote (untreated) tumors like what injected (treated) tumors receive via intratumoral delivery. PV-10 is long gone before the immune system removes the untreated ones. Hmmmm...
To understand PV-10 is to understand the relationship between chemoablation and immune-mediated signaling. It's what Craig means when he says the immune system responds in direct proportion to the degree of insult. Think of PV-10 chemoablation as the proxy for the degree of insult, which is rapid, complete and durable in the case of PV-10. Moffitt should say the same thing in its conference presentation(s) and contemporaneous peer-reviewed publication.
A good amount of work -- creative, innovative, pragmatic, historical, scientific, medical literature-based, etc. -- went and a continues to go into dosing, of historical and upcoming clinical trials, of certain indications, of pre-clinical work on newer indications.
Yes, management doesn't talk openly about dosing, nor do they talk openly about several other topics of import and value.
The thing that makes you go hmmmm... Rose Bengal has an established safety history, a short half-life in the bloodstream, and is excreted via the liver and kidneys. The half-life is measured in minutes, like 15-30 or thereabouts. The drug is gone from the body almost immediately, so there is never enough of it to kill remote (untreated) tumors like what injected (treated) tumors receive via intratumoral delivery. PV-10 is long gone before the immune system removes the untreated ones. Hmmmm...
February 21, 2013
$PVCT Who? Rose Bengal What?
I found what I believe are circa 2008 figures of the number of public companies on major and minor U.S. stock exchanges. Thousands and thousands... Of those, there are 400 to 450 public biotechnology companies.
It's easy to rail against the universe and management in the echo chamber of blogs like mine and online stock boards about lack of awareness of the company. Some of it is warranted. Most of it is not. We certainly can argue all day about this. I find myself on each side of the argument, depending on the day or month. The hard truth, and really what it comes down to, is most folks, investors-at-large at a minimum, do not know about the company.
Management has routinely said the company's primary customers are the FDA and Big Pharma; the former as it relates to the regulatory path, and the latter as it relates to the eventual buyer of the company. When it comes to creating an actual product and traveling the path to bring it to market (or seeking the right pharma partner to do so), management's approach is smart, particularly given the novelty and innovation of PV-10, its new pathway, a long-desired approach to treat cancer in a different, more effective manner, etc.
It is hyperbole for anyone outside of the medical community to even think about calling PV-10 a cure for cancer or the closest thing to it.
The rule for treating cancer is to treat it as early as one can. PV-10 is positioned very well in the treatment ladder (we await Moffitt to explain the drug's clinical relevance), from a diagnostic to pre-surgery application to post-surgery application -- collectively the vast portion of the cancer market -- to application in combination with radiotherapy and systemic chemotherapies and immunotherapies for very late stage disease-afflicted patients -- a very important but small portion of the market.
As I wrote previously, Provectus is approaching catalytic milestone events that should move the share price substantially (that is what management believes); as a result, it is now or in short order fair to management and shareholders to measure the resulting movement or lack of movement in share price after these events (the SPA's receipt, Moffitt's explanations, a China deal).
I did a quick thought experiment:
After one or more of the SPA, Moffitt and China, what happens when more people become aware of the drug, company and stock?
What's the outcome of a 10x increase in PVCT SeekingAlpha subscribers, for example and as a proxy for greater awareness by investors-at-large?
It's easy to rail against the universe and management in the echo chamber of blogs like mine and online stock boards about lack of awareness of the company. Some of it is warranted. Most of it is not. We certainly can argue all day about this. I find myself on each side of the argument, depending on the day or month. The hard truth, and really what it comes down to, is most folks, investors-at-large at a minimum, do not know about the company.
Management has routinely said the company's primary customers are the FDA and Big Pharma; the former as it relates to the regulatory path, and the latter as it relates to the eventual buyer of the company. When it comes to creating an actual product and traveling the path to bring it to market (or seeking the right pharma partner to do so), management's approach is smart, particularly given the novelty and innovation of PV-10, its new pathway, a long-desired approach to treat cancer in a different, more effective manner, etc.
It is hyperbole for anyone outside of the medical community to even think about calling PV-10 a cure for cancer or the closest thing to it.
The rule for treating cancer is to treat it as early as one can. PV-10 is positioned very well in the treatment ladder (we await Moffitt to explain the drug's clinical relevance), from a diagnostic to pre-surgery application to post-surgery application -- collectively the vast portion of the cancer market -- to application in combination with radiotherapy and systemic chemotherapies and immunotherapies for very late stage disease-afflicted patients -- a very important but small portion of the market.
As I wrote previously, Provectus is approaching catalytic milestone events that should move the share price substantially (that is what management believes); as a result, it is now or in short order fair to management and shareholders to measure the resulting movement or lack of movement in share price after these events (the SPA's receipt, Moffitt's explanations, a China deal).
I did a quick thought experiment:
- Came up with 15 so-called cancer immunotherapy companies.
- Went to SeekingAlpha and typed in their ticker symbols in order to find out how many SA members had subscribed to alerts about each company.
- Graphed the results, and they are telling (see below).
After one or more of the SPA, Moffitt and China, what happens when more people become aware of the drug, company and stock?
What's the outcome of a 10x increase in PVCT SeekingAlpha subscribers, for example and as a proxy for greater awareness by investors-at-large?
February 20, 2013
$PVCT: It doesn't get more definitive.
It doesn't get more simple than this. I think management's position on two key value drivers clearly is definitive.
#1. The SPA is key to life sciences investors getting into the stock because of the very specific regulatory path clarity and the management of such it provides. Get the SPA, and these investors will buy Provectus shares.
#2. Life sciences investors need to understand PV-10's clinical relevance. Substantive data explaining the "bystander effect" and PV-10's immune mediated signaling, a new pathway, among other things, will be presented at a major conference and contemporaneously published in a peer-reviewed publication for the first time (I am confident I figured out the names of the conference and journal). Moffitt explains the systemic and immune-mediated benefit of local agent PV-10, and these investors will buy stock.
#3. China needs no comment from management. Close at least a good regional license deal with good upfront, milestone and royalty payments, and investors of all stripes will buy Provectus shares.
I looked at these three near-term value drivers -- the SPA, Moffitt's work and a China deal -- and their respective importance to key Provectus constituents: the FDA, Big Pharma, life sciences investors, and investors at large (of which I of course am one). This assessment is illustrated in the table below.
The FDA is the constiuent most relevant to PV-10's regulatory path for (a) the SPA so Provectus ultimately may achieve approval for the focused label of local treatment of Stage III MM cancer patients, (b) accelerated approval for MM, which could have a one-in-two chance of being achieved after Moffitt's conference presentation and journal publication, and (c) to a lesser extent only because management is mum on the topic, breakthrough therapy designation for liver cancer.
Big Pharma cares about the SPA, but it seems they care much more about Moffitt's story and explanation of PV-10's clinical relevance. Randomized data, interim or otherwise, is very meaningful to Big Pharma. Should the pivotal MM trial start at the end of March or in April, it is conceivable an interim dataset could be made available to Pfizer and others to peruse in late-Q3 or Q4 2013. Nevertheless, the new pathway discovered explains how PV-10 facilitates the death of cancer, and it could be novel and important enough to spur Big Pharma to act. Only time and the degree of Moffitt's reception by the global oncology community will tell.
Life Sciences Investors care about the SPA and Moffitt. Management comes across as definitive on these items: Get it and explain it, respectively, and these investors will buy Provectus stock.
Investors at large care about the SPA, Moffitt and China. Portions of this constituency get in early -- before the SPA, Moffitt and China fully unfold -- because they like the story and think they understand the opportunity. Other portions jump in after one or more of these events occur. Still others enter the fray as the share price moves higher.
It is fair, now, to both management and shareholders, to measure the resulting movement or lack of movement in share price after the SPA's receipt and Moffitt's explanations, whether you're an investor in Denmark, Germany, Switzerland, Saudi Arabia, Singapore, Hong Kong, New York, Illinois, Texas, Tennessee, California, or in one of many other places in North America, Europe and Asia.
In truth, no one really knows what the company's share price will do until it does it. After the SPA and Moffitt, let's take stock (pardon the pun) of Provectus, management and the share price.
#1. The SPA is key to life sciences investors getting into the stock because of the very specific regulatory path clarity and the management of such it provides. Get the SPA, and these investors will buy Provectus shares.
#2. Life sciences investors need to understand PV-10's clinical relevance. Substantive data explaining the "bystander effect" and PV-10's immune mediated signaling, a new pathway, among other things, will be presented at a major conference and contemporaneously published in a peer-reviewed publication for the first time (I am confident I figured out the names of the conference and journal). Moffitt explains the systemic and immune-mediated benefit of local agent PV-10, and these investors will buy stock.
#3. China needs no comment from management. Close at least a good regional license deal with good upfront, milestone and royalty payments, and investors of all stripes will buy Provectus shares.
I looked at these three near-term value drivers -- the SPA, Moffitt's work and a China deal -- and their respective importance to key Provectus constituents: the FDA, Big Pharma, life sciences investors, and investors at large (of which I of course am one). This assessment is illustrated in the table below.
The FDA is the constiuent most relevant to PV-10's regulatory path for (a) the SPA so Provectus ultimately may achieve approval for the focused label of local treatment of Stage III MM cancer patients, (b) accelerated approval for MM, which could have a one-in-two chance of being achieved after Moffitt's conference presentation and journal publication, and (c) to a lesser extent only because management is mum on the topic, breakthrough therapy designation for liver cancer.
Big Pharma cares about the SPA, but it seems they care much more about Moffitt's story and explanation of PV-10's clinical relevance. Randomized data, interim or otherwise, is very meaningful to Big Pharma. Should the pivotal MM trial start at the end of March or in April, it is conceivable an interim dataset could be made available to Pfizer and others to peruse in late-Q3 or Q4 2013. Nevertheless, the new pathway discovered explains how PV-10 facilitates the death of cancer, and it could be novel and important enough to spur Big Pharma to act. Only time and the degree of Moffitt's reception by the global oncology community will tell.
Life Sciences Investors care about the SPA and Moffitt. Management comes across as definitive on these items: Get it and explain it, respectively, and these investors will buy Provectus stock.
Investors at large care about the SPA, Moffitt and China. Portions of this constituency get in early -- before the SPA, Moffitt and China fully unfold -- because they like the story and think they understand the opportunity. Other portions jump in after one or more of these events occur. Still others enter the fray as the share price moves higher.
It is fair, now, to both management and shareholders, to measure the resulting movement or lack of movement in share price after the SPA's receipt and Moffitt's explanations, whether you're an investor in Denmark, Germany, Switzerland, Saudi Arabia, Singapore, Hong Kong, New York, Illinois, Texas, Tennessee, California, or in one of many other places in North America, Europe and Asia.
In truth, no one really knows what the company's share price will do until it does it. After the SPA and Moffitt, let's take stock (pardon the pun) of Provectus, management and the share price.
February 17, 2013
$PVCT: "The irony of the situation was apparent to everyone."
 |
| Click to enlarge figure |
If what I think will come to pass does, the irony of the sequence of events will not be lost on long-time Provectus shareholders. Could the 6-year chart to the right go vertical (on a relative basis to the y-axis describing a share price in the 60-cent range) as a result, finally? After years of patience and impatience, it is hard not to chuckle about the potential for an inexplicable congruence of events.
The SPA: A PR around the week of March 18th?
A China deal: Peter leaves the week of February 25th. A PR during the week of March 4th (with an 8-K filing of an MOU identifying the Chinese strategic partner or an LOI detailing key terms and provisions of a deal with said partner), followed by a closing and funding as early as the week of April 1st (i.e., 30 days later)?
Moffitt: Moffitt's first snippets emerge, followed by the full presentation and contemporaneous peer-reviewed publication. Snippets start sometime in March (a PR or two by Provectus or Moffitt, or both), followed by the full monty in early-April (multiple PRs by either or both parties)?
February 6, 2013
$PVCT: Clinical Relevance
A reader of the blog led me to the twitter feed below. Thank you.
I asked Pete for his perspective on it in the context of management's work with the FDA. Regulatory approval is a complex topic, especially as it relates to local agent PV-10 versus systemic ones. PV-10 as a local agent is a reality that has driven management's MM Phase 3 trial design more than anything else. The key to the FDA, according to Peter (and which was not specifically addressed in the Twitter feed above), is proving "clinical relevance." This certainly means meeting endpoints, but it also means whether a drug should be approved or not. PV-10 needs to be clinically relevant to be approved. Provectus' Phase 3 trial design discussed with the FDA would support PV-10 as being "clinically relevant." Moffitt's work also would further augment the case for PV-10's clinical relevance.
I asked Pete for his perspective on it in the context of management's work with the FDA. Regulatory approval is a complex topic, especially as it relates to local agent PV-10 versus systemic ones. PV-10 as a local agent is a reality that has driven management's MM Phase 3 trial design more than anything else. The key to the FDA, according to Peter (and which was not specifically addressed in the Twitter feed above), is proving "clinical relevance." This certainly means meeting endpoints, but it also means whether a drug should be approved or not. PV-10 needs to be clinically relevant to be approved. Provectus' Phase 3 trial design discussed with the FDA would support PV-10 as being "clinically relevant." Moffitt's work also would further augment the case for PV-10's clinical relevance.
February 5, 2013
$PVCT: Please Vote on the Blog's Poll!
Below the blog's title bar and above where my posts begin, you will see a new feature of the blog, a poll. This poll asks you: What company achievement will have the greatest impact on share price in Q1?
Please vote! And share your opinion with fellow blog readers.
I chose "A China deal."
I think the SPA will have a material but limited impact on share price. Over time, I have heard the SPA was (i) for the benefit of investors and the stock market, (ii) necessary to clarify the regulatory path of PV-10 and Provectus' lead indication of melanoma for Big Pharma, and (iii) of critical importance to serious life sciences investors' decision to get off the sidelines and into the stock for the aforementioned reason of regulatory clarity. Now, it seems, regulatory clarity is the residual reason for continuing to pursue the SPA in the absence of business decisions that honestly could only have been made with the benefit of hindsight (and, thus, now are somewhat irrelevant). In the absence of anything else happening at the time it arrives, perhaps by or before mid-March, I think the SPA could push the share price to $1.25 or higher.
Please vote! And share your opinion with fellow blog readers.
I chose "A China deal."
Dermatology needs more time. I do not think a first term sheet will be extended to the company for Provectus' dermatology business before quarter-end to trigger the formal hiring of the financial adviser/investment banker and start of the auction process to out-license PH-10, even though the annual meeting of the American Academy of Dermatology (where management and its world-class dermatology advisory team should be) occurs in early-March. I think a couple of additional steps are needed, such as (i) the completion and writing up of the immunologic mechanism of action and toxicity characterization work by the investigative dermatology laboratory of a renowned university and (ii) the setting of an end-of-Phase 2 meeting with the FDA.
China, China, China. While I don't think ultimately not consummating a China deal is the end of Provectus' world, I very strongly believe management must close a substantial regional license transaction such as the one being contemplated in China (in the absence of a global oncology license being consummated in its place). China is first up; India and Japan will take time and very likely not be completed this quarter. A China deal (i) provides the company with needed cash for pivotal and key clinical trials without equity dilution, (ii) helps management regain a substantial amount of credibility it lost from the sub-par execution and unfortunate outcome of the ill-fated preferred share PVCTP "IPO," and (iii) dramatically increases management's credibility, to nearly match their credibility as innovators, because of the size, scope and quality of the contemplated China deal. On balance, I am impressed with management's China deal process. Process comprises both transaction steps and outcome.
Adam Feuerstein tweeted the following today:
The Celsion (NASDAQ: CLSN) deal earlier in January was a call option-license transaction, with the option owned by Hisun. The option premium was $5MM. CLSN jumped 10-15% on the announcement of this pseudo-deal (about $25-30MM on a $250MM market cap). After poor ThermoDox Phase 3 results, Hisun walked away having lost the premium it paid.
Opexa Therapeutics (NASDAQ: OPXA) entered into a similar call option-licensing transaction with Merck Serono. Opexa receives a $5MM upfront payment for granting Merck the option to exclusively license Tcelna (imilecleucel-T) for the treatment of multiple sclerosis. OPXA jumped 160% today (about $10-11MM on a $7-8MM market cap).
Thus far, the substance of the contemplated China does not appear to have wavered. Structure seems customary, with no ifs, ands or buts. In the absence of anything else happening at the time a deal may be concluded, perhaps in late-February, I think the China deal could push the share price well above $2 (an increase of more than 200%, or about $150-160MM on a $70MM market cap) and keep it there.
The overhang of warrants at $1 (11MM), $1.12 (4MM+), $1.25 (5MM+) and $1.50 (~3MM) may create downward pressure on and several ceilings for the stock. Upward pressure may come from the SPA's arrival shortly after a China deal is inked, more than a hint of Moffitt's data and opinion around the same time, and the greater audience and buying that comes from a shift to a major stock exchange like the NASDAQ (5 consecutive days of greater than a $2 share price). Competing pushes and pulls will be resolved somehow, and perhaps we end the quarter closer to $5 than 60 cents.
Adam Feuerstein also wrote today about ZIOPHARM Oncology and the Feuerstein-Ratain Rule, and the likely outcome of this company's pending phase 3 sarcoma trial. With a market cap of $350MM, historical microcap oncology company data suggests a 17% chance of success, which is better than 0%, but a far cry from an ~80% success rate for $1B+ market cap companies.
Feuerstein's greater message is this: "The logic behind the F-R Rule borrows a bit from Occam's Razor. Good cancer drugs are scarce and valuable commodities, and potential Big Pharma partners and institutional investors are forever scouting for opportunities to buy, license or invest in companies with drugs that have a high probability of success. Said another way, there are very few, if any, cancer drugs that come out of nowhere to be big sellers."
A China-Moffitt combination can be very powerful, increasing Provectus' market value to the point where it "...can be a reasonably accurate proxy for the future success of a cancer drug in a phase III clinical trial."
I asked Pete, in his many meetings since SSO and, later, ESMO with Big Pharma, key opinion leaders, serious life sciences investors, etc., whether anyone had expressed skepticism about PV-10's efficacy, safety and benefit. He replied that literally no one has been skeptical, and attributed this lack of skepticism to the undeniable safety history of Rose Bengal as well as its obvious data sets of its use.
The issue for management from the beginning, I think, has been the necessity to show Rose Bengal delivered intralesionally or locally provides a systemic benefit. I also think no one initially and for some time believed Craig when he described his philosophy to curing cancer, the approach as manifested in the action of the drug itself, and the work he had done to demonstrate a Rose Bengal cure. Many more believe him today, after successful clinical trials, mouse work that is filling in more portions of his canvas' painting, and various and varied work by investigators and researchers around the world.
Adam Feuerstein tweeted the following today:
The Celsion (NASDAQ: CLSN) deal earlier in January was a call option-license transaction, with the option owned by Hisun. The option premium was $5MM. CLSN jumped 10-15% on the announcement of this pseudo-deal (about $25-30MM on a $250MM market cap). After poor ThermoDox Phase 3 results, Hisun walked away having lost the premium it paid.
Opexa Therapeutics (NASDAQ: OPXA) entered into a similar call option-licensing transaction with Merck Serono. Opexa receives a $5MM upfront payment for granting Merck the option to exclusively license Tcelna (imilecleucel-T) for the treatment of multiple sclerosis. OPXA jumped 160% today (about $10-11MM on a $7-8MM market cap).
Thus far, the substance of the contemplated China does not appear to have wavered. Structure seems customary, with no ifs, ands or buts. In the absence of anything else happening at the time a deal may be concluded, perhaps in late-February, I think the China deal could push the share price well above $2 (an increase of more than 200%, or about $150-160MM on a $70MM market cap) and keep it there.
The overhang of warrants at $1 (11MM), $1.12 (4MM+), $1.25 (5MM+) and $1.50 (~3MM) may create downward pressure on and several ceilings for the stock. Upward pressure may come from the SPA's arrival shortly after a China deal is inked, more than a hint of Moffitt's data and opinion around the same time, and the greater audience and buying that comes from a shift to a major stock exchange like the NASDAQ (5 consecutive days of greater than a $2 share price). Competing pushes and pulls will be resolved somehow, and perhaps we end the quarter closer to $5 than 60 cents.
Adam Feuerstein also wrote today about ZIOPHARM Oncology and the Feuerstein-Ratain Rule, and the likely outcome of this company's pending phase 3 sarcoma trial. With a market cap of $350MM, historical microcap oncology company data suggests a 17% chance of success, which is better than 0%, but a far cry from an ~80% success rate for $1B+ market cap companies.
Feuerstein's greater message is this: "The logic behind the F-R Rule borrows a bit from Occam's Razor. Good cancer drugs are scarce and valuable commodities, and potential Big Pharma partners and institutional investors are forever scouting for opportunities to buy, license or invest in companies with drugs that have a high probability of success. Said another way, there are very few, if any, cancer drugs that come out of nowhere to be big sellers."
A China-Moffitt combination can be very powerful, increasing Provectus' market value to the point where it "...can be a reasonably accurate proxy for the future success of a cancer drug in a phase III clinical trial."
I asked Pete, in his many meetings since SSO and, later, ESMO with Big Pharma, key opinion leaders, serious life sciences investors, etc., whether anyone had expressed skepticism about PV-10's efficacy, safety and benefit. He replied that literally no one has been skeptical, and attributed this lack of skepticism to the undeniable safety history of Rose Bengal as well as its obvious data sets of its use.
The issue for management from the beginning, I think, has been the necessity to show Rose Bengal delivered intralesionally or locally provides a systemic benefit. I also think no one initially and for some time believed Craig when he described his philosophy to curing cancer, the approach as manifested in the action of the drug itself, and the work he had done to demonstrate a Rose Bengal cure. Many more believe him today, after successful clinical trials, mouse work that is filling in more portions of his canvas' painting, and various and varied work by investigators and researchers around the world.
February 2, 2013
$PVCT: A Blogger's Failing Grade
About a month ago, I speculated quite a bit in my post Let's Make A Deal? How did I do? Not very well.
My self-graded report card shows an overall grade of C-minus. Ugh!
Mea maxima culpa.
My self-graded report card shows an overall grade of C-minus. Ugh!
Mea maxima culpa.
January 30, 2013
$PVCT: The @adamfeuerstein-Ratain Rule
A good read is Oncology Micro-Cap Stocks: Caveat Emptor!, a September 2011 article in the Journal of the National Cancer Institute authored and researched by Adam Feuerstein, Senior Columnist at TheStreet, and Dr. Mark J. Ratain, MD, Leon O. Jacobson Professor of Medicine at the University of Chicago Medicine.
The "Feuerstein-Ratain Rule:" If a publicly traded company has a market capitalization under $300MM 120 days before the issuance of public announcements (e.g., press releases, etc.) of its oncology Phase 3 clinical trials results, the trial will fail.
A key paragraph in the authors' article summarizes the essence of their rule:
The rule was tested, for the first time, in 2012 on Keryx Biopharmaceuticals (NASDAQ:KERX) and its drug Perifosine. On April 2, shares of Keryx fell by more than 60%.
0-for-22 (micro-cap).
Up next, in 2013, is Celsion (NASDAQ:CLSN) and ThermoDox. The company has scheduled a conference call tomorrow to present the top-line results from its pivotal Phase III HEAT Study with ThermoDox in combination with radiofrequency ablation (RFA) in patients with intermediate hepatocellular carcinoma versus those patients receiving RFA alone. Celsion had a sub-$200MM market cap about 4 months (120 days) ago. Last week, Celsion announced a development deal with China's Zhejiang Hisun Pharmaceutical.
0-for-23 (micro-cap), or 1-for-23?
It is too early to apply the rule to Provectus because the company is not close to the commencement of its pivotal MM Phase 3 trial and, thus, not close to the public release or announcement of trial data (i.e., interim, preliminary or final).
Nevertheless, it is informative to explore the Feuerstein-Ratain Rule, and keep it in mind as Provectus and its market capitalization approaches public pronouncement time.
For the MM Phase 3 trial, management thinks the company can make at least an April 1 start date should the SPA arrive on or around March 15. Further, management thinks interim data could be available in Q3 or Q4. Enrollment will take some time. The Dacarbazine control arm is expected to collapse within 1 to 2 months or less.
Assuming there is a public announcement of interim results by Provectus in late-Q3 (late-August to September) or Q4 (October to December) 2013 because, for this thought exercise, I assume results are available to announce, where would the company's share price have to be 4 months earlier in regards to the Feuerstein-Ratain Rule?
4 months earlier is early-May to early-September. At least a $300MM market cap is about $2.65 per share.
Between now, essentially the end of January, and early-May to early-September (illustratively), several events are needed or necessary to get Provectus' market cap safely above $300MM, and not have it become fodder for potentially another statistic for the lack of success of oncology micro-cap stocks and their Phase 3 trials:
The "Feuerstein-Ratain Rule:" If a publicly traded company has a market capitalization under $300MM 120 days before the issuance of public announcements (e.g., press releases, etc.) of its oncology Phase 3 clinical trials results, the trial will fail.
A key paragraph in the authors' article summarizes the essence of their rule:
The difference in the market capitalization at day −120 most likely reflects publically available information regarding the phase I and II clinical trials (as well as other factors, including competition and management), which has been incorporated into the market value of a stock. The stock market is known to anticipate future events, as opposed to reacting to the past. Thus, it is not surprising that sophisticated investors are able to judge the probability of success, which is reflected in the share price.The results of their analysis are stark:
...there were no positive trials among the 21 micro-cap companies (ie, companies with less than $300 million market capitalization..., whereas 21 of 27 studies reported by the larger companies analyzed (greater than $1 billion capitalization) were positive.0% (micro-cap) vs. 78% (larger cap).
The rule was tested, for the first time, in 2012 on Keryx Biopharmaceuticals (NASDAQ:KERX) and its drug Perifosine. On April 2, shares of Keryx fell by more than 60%.
0-for-22 (micro-cap).
Up next, in 2013, is Celsion (NASDAQ:CLSN) and ThermoDox. The company has scheduled a conference call tomorrow to present the top-line results from its pivotal Phase III HEAT Study with ThermoDox in combination with radiofrequency ablation (RFA) in patients with intermediate hepatocellular carcinoma versus those patients receiving RFA alone. Celsion had a sub-$200MM market cap about 4 months (120 days) ago. Last week, Celsion announced a development deal with China's Zhejiang Hisun Pharmaceutical.
0-for-23 (micro-cap), or 1-for-23?
It is too early to apply the rule to Provectus because the company is not close to the commencement of its pivotal MM Phase 3 trial and, thus, not close to the public release or announcement of trial data (i.e., interim, preliminary or final).
Nevertheless, it is informative to explore the Feuerstein-Ratain Rule, and keep it in mind as Provectus and its market capitalization approaches public pronouncement time.
For the MM Phase 3 trial, management thinks the company can make at least an April 1 start date should the SPA arrive on or around March 15. Further, management thinks interim data could be available in Q3 or Q4. Enrollment will take some time. The Dacarbazine control arm is expected to collapse within 1 to 2 months or less.
Assuming there is a public announcement of interim results by Provectus in late-Q3 (late-August to September) or Q4 (October to December) 2013 because, for this thought exercise, I assume results are available to announce, where would the company's share price have to be 4 months earlier in regards to the Feuerstein-Ratain Rule?
4 months earlier is early-May to early-September. At least a $300MM market cap is about $2.65 per share.
Between now, essentially the end of January, and early-May to early-September (illustratively), several events are needed or necessary to get Provectus' market cap safely above $300MM, and not have it become fodder for potentially another statistic for the lack of success of oncology micro-cap stocks and their Phase 3 trials:
- The receipt of the SPA around mid-March,
- The closing of the currently contemplated China deal (which should be much larger than Celsion's, assuming the Chinese pharma company exercises its option to do its Celsion deal) in late-February, and
- The release of highly anticipated Moffitt mouse and human data in early-April (e.g., "the closest thing we've seen to a cure for cancer," etc.).
January 27, 2013
$PVCT: March Madness 2013

March Madness 2013 begins on Tuesday, March 19 and ends on Monday, April 8. The Final Four will be played in the Georgia Dome in Atlanta, Georgia. I contend this period (including leading into it) will be an important time for the company, its more mainstream awareness, and the share price.
There is a lot of information to digest, from Craig's presentation at the Noble Financial Capital Markets Ninth Annual Equity Conference to Peter's trip to New York City.
PV-10
Most of Pete’s time lately, probably more than anything else it seems (save for another effort), is focused on communicating PV-10's unique immunotherapeutic characteristics in the context of global oncology. You see this manifested in discussions with Big Pharma (as Craig commented at Noble: "interactions with potential global license partners," and by that he does not include just Pfizer) and continued visiting with life sciences investors (trying to get them off the sidelines and into the stock).
PV-10 + “other stuff”
I think Craig et al.’s SITC work (PV-10 + systemic chemotherapy), their upcoming AACR work (PV-10 + systemic immunotherapy), Foote et al.’s expanded work (PV-10 + radiotherapy), and Craig’s skunk works work on more combinatory explorations (e.g., intra-tumoral GM-CSF, systemic interleukin, anti-PD-1 antibodies/agents, etc.) is opening a lot of eyes at Big Pharma and the FDA very wide.
Management is contemplating an MM Phase 1 trial combining PV-10 and ipilimumab (while Craig mentioned this in his Noble presentation, I also followed up). Perhaps the protocol might include the assessment of safety and efficacy in a number of patients with metastatic melanoma (Cohort 1 receives PV-10) and in a number of patients who are taking ipilimumab, an approved treatment for MM (Cohort 2).
As I wrote earlier this week, this combination work of PV-10 + ipi clearly targets and is in response to serious interest from Big Pharma: Bristol-Myers Squibb & ipilimumab/Yervoy and Pfizer/MedImmune-AstraZeneca & tremelimumab (MedImmune in-licensed tremi from Pfizer in 2011 for global development rights to the drug while Pfizer retained rights to specified types of combination therapies).
The liver trials
Let's segue from combination therapies to the expanded liver P1 trial, and what the likely results will mean for the design and very likely outcome of the liver P2/P3 trial. I think there is enough information to speculate (of course, the foundation of the speculation merely is a framework, and not overly substantial) or project success of PV-10 + sorafenib (a systemic drug, so see PV-10 + other stuff above) over the sorafenib-alone treatment arm. Interference studies and other work confirm PV-10 is orthogonal to sorafenib (and lots of other drugs, too): PV-10 does not interact negatively with sorafenib, and appears to enhance sorafenib's benefit by PV-10 first boosting the immune system.
When Provectus announces -- via an upcoming PR -- that patients have begun to be enrolled and treated in the expanded liver P1 trial, the timeline of results making their way to the FDA should not be lengthy. The same FDA group of folks reviewing Provectus’ pivotal MM Phase 3 trial design suitable for an SPA, Division of Oncology Products 2 (DOP2), are the same folks (i.e., DOP2) who will review the company’s liver Phase 2/Phase 3 trial design suitable for accelerated approval.
The hard work, time, energy, resources, expense, etc. exerted to get the SPA for MM should pay-off when it comes time for management to request AA for liver.
The SPA
Barring another eleventh hour request or issue, it appears the SPA should arrive around March 15.
While it certainly is possible that the SPA arrives earlier, I am setting my own expectations for the Ides.
Shelf filings
The pulling of the two $50MM common stock filings still are in process with Provectus’ attorneys. Management believes the act of pulling them is form over substance, since the company will not be using them. I think communicating their intended action to pull them and/or the actual act and notification to the market of pulling them is substantive.
Work continues, and the process of arriving at and consummating a deal progresses. Should Pete travel to China again (he visited there in late-November 2012), it would be to close the deal, the announcement of which should follow via PR and 8-K filing.
In terms of setting my own expectations for this item, a deal could get done by or around late-February. If Celsion's China deal is worth several hundreds of millions of dollars (per the analysts and others commenting on valuation), by comparison I think you're looking at a Provectus China worth at least $1 billion (perhaps as much as $2 billion) with higher upfront, milestone and royalty payments.
India & Japan
As a result of a completed China deal, Provectus’ visibility should be much more pronounced. Deals in India and Japan could follow thereafter, but more of the deal process must progress before I would speculate about timing. India could be accelerate given the level of interest of the top Bio-Pharma players in the country. For now, I’m not setting any expectations.
Moffitt & Reproducibility
Craig expanded in some detail about Moffitt's work in his Noble presentation: their reproduction of his work, his reproduction of their work, their upcoming data release and presentation(s), etc. According to Craig, Moffitt's immunological MOA characterization work results (mouse and human) will be revealed imminently. I think Craig's comments related to reproducibility, particularly in the context of creating and making a product (i.e., PV-10, PH-10), were very important and very true.
I think it is easy, at this point, to connect the dots so as to speculate (identify) about which conference Moffitt will present their highly anticipated results. I still expect forthcoming visibility about these Moffitt results in late-January, and some data released in stages in March, prior to the full dataset being released at the expected conference in early-April.
More valuation-raising work to be done
When I wrote my blog post entitled $PVCT: Immunologic Potential, and thus Value, I set a very lofty valuation for the company, particularly as it related to the expectation of management for an upfront payment ($3 billion at last check) at the end-game. You don't get from $67.65 million (Google Finance's market capitalization for the company as at 1/25/13) to $3 billion in one leap.
Rather, Provectus arrives there in several leaps and bounds, together with perhaps some end-game auctioning momentum and exuberance: China, India, Dermatology, momentum share buying, etc.
Peer-based management compensation proposal coming
As management noted in a previous filing, a peer company-based bonus compensation structure should be part of a new compensation plan that management should introduce with Provectus' next proxy filing likely in late-April.
PH-10
From a review of history, it appeared Provectus was on the cusp of securing a deal to license its dermatology business (i.e., inflammatory skin disorders) in early-2011. Up to that point, management's valuation expectations, on a net present value (NPV) basis, were about $500 million.
No term sheet materialized from the most serious prospective partner, which would have triggered the official hiring of the financial adviser (Bank of America Merrill Lynch) and an auction process involving the other prospective partners. I think the lack of certain desired information at the time, since fulfilled by the Psoriasis Phase 2c trial, created a valuation gap between the prospective lead and Provectus that prevented the parties from coming together on suitable top-line term sheet parameters. Hence, the prospective lead declined to extend a term sheet it knew would be turned down management.
As time progressed (i.e., as the psoriasis Phase 2c trial was completed), a process appears to have been established that paralleled Moffitt's work on PV-10. Namely, a world renowned cancer research center engaged in work, initially at their own expense, to explore and characterize the immunological mechanism of action of Provectus' oncology drug.
In addition to better understanding PH-10 immunologic MOA, and on a related note, more insight into the drug's distinct lack of toxicity is necessary to better inform the FDA and assist in the design of the eventual Phase 3 trial.
It is not unreasonable to analogize PV-10's path to PH-10's, and thus potentially explain the delay in getting to a dermatology license or sale transaction. Immunologic mechanism of action characterization work is being done on PH-10 by a world-class institution to complete the understanding of certain prospective dermatology licensees before they fully commit to jumping into the pool. I think management's NPV figure for the dermatology business has increased significantly to at least $750 million (perhaps as much as $1 billion).
As for expectations, Craig did note in his Noble Presentation that we should look for the company to request a end-of-Phase-II (EOP2) meeting with the FDA. Presumably this announcement, if one is made by the company, or step precedes or signals the extension of a term sheet for dermatology is imminent, inbound or at least very close at hand.
Labels:
$AZN,
$PFE,
$PVCT,
$PVCT.OB,
China,
DOP2,
dr. craig dees,
FDA,
India,
ipilimumab,
Japan,
March Madness,
Moffitt,
Peter Culpepper,
PH-10,
Provectus Pharmaceuticals,
PV-10,
share price,
SPA,
Valuation
January 24, 2013
$PVCT: Provectus to Present Data on PV-10 at the American Association for Cancer Research Annual Meeting in April 2013
Provectus issued a PR yesterday announcing the acceptance of Craig et al.'s poster presentation -- Combination of PV-10 immuno-chemoablation and systemic anti-CTLA-4 antibody therapy in murine models of melanoma -- at the American Association for Cancer Research (AACR) Annual Meeting in April 2013 in Washington, D.C.
Management checked with AACR to see what they could and could not say about the abstract and work in the PR. Provectus, at this point, is allowed to say (i) the abstract is accepted and (ii) the title. They cannot release data until the embargo is lifted, which will be in stages and does not start until sometime in March.
As Craig mentioned during his presentation at the Noble Financial Capital Markets Ninth Annual Equity Conference (click the preceding link to view it) around about minute 14:00, there is very significant interest to see how PV-10 works with systemic treatments (e.g., chemotherapy, immunotherapy, etc.), because it has become clear these secondary treatments work better after an initial use or application of PV-10.
You may recall from my post Provectus Pharmaceuticals Announces H. Lee Moffitt Cancer Center Initiates Phase 1 Study of PV-10 to Elucidate Bystander Effect the notion of PV-10 making cancer treatment more effective in combination with other therapies also is important (very, actually); particularly for late stage patients (a group not targeted by Provectus' current registration pathway for PV-10, which is to facilitate the treatment of Stage III and early-Stage IV patients) and those with heavy tumor burden.
Craig et al.'s work announced today -- the combination of PV-10 and systemic anti-CTLA-4 antibody therapy -- clearly targets and is in response to serious interest from Big Pharma: Bristol-Myers Squibb & ipilimumab/Yervoy and Pfizer/MedImmune-AstraZeneca & tremelimumab (MedImmune in-licensed tremi from Pfizer in 2011 for global development rights to the drug while Pfizer retained rights to specified types of combination therapies).
PV-10 + systemic chemotherapy: Craig etl al.'s murine work -- Generation of an antitumor response and immunity using a small molecule drug (PV-10) -- at the Society for Immunotherapy of Cancer (SITC) 27th Annual Meeting on October 26 and 27, 2012 in North Bethesda, Maryland explored time to progression and tumor growth from PV-10 alone, 2 cycles of 5-FU, PV-10 and 2 cycles of 5-FU, and a saline control. Provectus concluded co-administration of PV-10 immuno-chemoablation with other systemic therapy, in this case, 5-FU, can yield potent synergy in uninjected tumors (Combination of PV-10 with systemic chemotherapy can yield synergistic benefit in untreated tumors supportive of ongoing clinical work; f/n: A study to assess PV-10 chemoablation of cancer of the liver; clinical trial NCT00986661). PV-10 did not interfere with the systemic chemotherapy agent, nor did it create or cause a toxic reaction. In fact, by boosting the immune system, PV-10 allowed the chemo agent to perform better.
PV-10 + radiotherapy: Foote et al.'s initial and ongoing work combining PV-10 and radiotherapy are showing dramatic improvement for patients with much later stage disease (Stage IV) and heavy tumor burden. At least 10 patients have been treated, a follow-up to the success of their initial work on 3 patients.
PV-10 + systemic immunotherapy: As I wrote earlier, there is very significant interest to see how PV-10 works with ipilimumab (an anti-CTLA-4 agent). Because ipi is human-specific, Craig used a mouse anti-CTLA-4 antibody. Craig et al.'s work is a necessary pre-cursor to human trials (one cannot simply run such experiments without sufficient data to show the FDA how the combination might work and that it is safe). This mouse study will be used to convince regulators that Provectus should be allowed to run a Phase 1 trial combining PV-10 and ipi (as Craig mentioned at the Noble conference).
Other combinations: As a treatment PV-10 lies in the "sweet spot" of the addressable market of patients afflicted with melanoma that comprises the vast majority of patients. Patients with very late stage disease and/or very heavy tumor burden, depending on whose numbers you use, probably comprises 2-5%. Nevertheless, as with today's announcement that heralds the successful demonstration of PV-10 and a systemic immunotherapeutic agent (as with the company's SITC-presented earlier work that successfully demonstrated the combination of PV-10 and a systemic chemotherapeutic agent) Provectus is seeking further combinations to make very late stage disease patient treatment options better (e.g., intra-tumoral GM-CSF, systemic interleukin, anti-PD-1 antibodies/agents, etc.).
Today's PR is not related to the anticipated Moffitt immunological MOA characterization work (mouse & human) that will be presented later this year at a high profile conference like AACR. Moffitt presumably will notify Provectus if the cancer center's abstract(s) has (have) been accepted. Then, management will work with Moffitt to coordinate news flow.
Management checked with AACR to see what they could and could not say about the abstract and work in the PR. Provectus, at this point, is allowed to say (i) the abstract is accepted and (ii) the title. They cannot release data until the embargo is lifted, which will be in stages and does not start until sometime in March.
As Craig mentioned during his presentation at the Noble Financial Capital Markets Ninth Annual Equity Conference (click the preceding link to view it) around about minute 14:00, there is very significant interest to see how PV-10 works with systemic treatments (e.g., chemotherapy, immunotherapy, etc.), because it has become clear these secondary treatments work better after an initial use or application of PV-10.
You may recall from my post Provectus Pharmaceuticals Announces H. Lee Moffitt Cancer Center Initiates Phase 1 Study of PV-10 to Elucidate Bystander Effect the notion of PV-10 making cancer treatment more effective in combination with other therapies also is important (very, actually); particularly for late stage patients (a group not targeted by Provectus' current registration pathway for PV-10, which is to facilitate the treatment of Stage III and early-Stage IV patients) and those with heavy tumor burden.
Craig et al.'s work announced today -- the combination of PV-10 and systemic anti-CTLA-4 antibody therapy -- clearly targets and is in response to serious interest from Big Pharma: Bristol-Myers Squibb & ipilimumab/Yervoy and Pfizer/MedImmune-AstraZeneca & tremelimumab (MedImmune in-licensed tremi from Pfizer in 2011 for global development rights to the drug while Pfizer retained rights to specified types of combination therapies).
PV-10 + systemic chemotherapy: Craig etl al.'s murine work -- Generation of an antitumor response and immunity using a small molecule drug (PV-10) -- at the Society for Immunotherapy of Cancer (SITC) 27th Annual Meeting on October 26 and 27, 2012 in North Bethesda, Maryland explored time to progression and tumor growth from PV-10 alone, 2 cycles of 5-FU, PV-10 and 2 cycles of 5-FU, and a saline control. Provectus concluded co-administration of PV-10 immuno-chemoablation with other systemic therapy, in this case, 5-FU, can yield potent synergy in uninjected tumors (Combination of PV-10 with systemic chemotherapy can yield synergistic benefit in untreated tumors supportive of ongoing clinical work; f/n: A study to assess PV-10 chemoablation of cancer of the liver; clinical trial NCT00986661). PV-10 did not interfere with the systemic chemotherapy agent, nor did it create or cause a toxic reaction. In fact, by boosting the immune system, PV-10 allowed the chemo agent to perform better.
PV-10 + radiotherapy: Foote et al.'s initial and ongoing work combining PV-10 and radiotherapy are showing dramatic improvement for patients with much later stage disease (Stage IV) and heavy tumor burden. At least 10 patients have been treated, a follow-up to the success of their initial work on 3 patients.
PV-10 + systemic immunotherapy: As I wrote earlier, there is very significant interest to see how PV-10 works with ipilimumab (an anti-CTLA-4 agent). Because ipi is human-specific, Craig used a mouse anti-CTLA-4 antibody. Craig et al.'s work is a necessary pre-cursor to human trials (one cannot simply run such experiments without sufficient data to show the FDA how the combination might work and that it is safe). This mouse study will be used to convince regulators that Provectus should be allowed to run a Phase 1 trial combining PV-10 and ipi (as Craig mentioned at the Noble conference).
 |
| Click to enlarge figure. |
Today's PR is not related to the anticipated Moffitt immunological MOA characterization work (mouse & human) that will be presented later this year at a high profile conference like AACR. Moffitt presumably will notify Provectus if the cancer center's abstract(s) has (have) been accepted. Then, management will work with Moffitt to coordinate news flow.
January 20, 2013
$PVCT: A Contrarian Indicator?
The post below was written last week by SG, a long-time poster on the Silicon Investor PVCT chat board.
He first started contributing to the board in May 2006, noting his buys around that time (1st orange oval), perhaps in the $1.50-2.00 per share range. SG later wrote about his buys when the stock made an all-time high (intraday: $3.05, closing: $3.00) in September 2007 (2nd orange oval), perhaps in the $2.50-3.00 per share range.
Last week, after holding Provectus shares for about 6.7 years, SG wrote he sold all but a 1,000 shares, perhaps in the $0.55-0.60 per share range (blue arrow), several cents above the stock's all-time closing low ($0.52). The intraday low was $0.43.
In reading his post, I was struck by its similarity to the magazine cover indicator, "which says that the cover story on the major business magazines, particularly BusinessWeek, Forbes and Fortune in the United States is often a contrary indicator."
Technical analysts often talk about a market washout -- a large share price plunge on above-average volume -- that clear the markets of bearish sentiment and help mark the/a major bottom.
It's quite possible Provectus' bottom, either in share price or market irrelevance or both, was quietly, perhaps a tad ignominiously, and most irrelevantly marked by the departure of SG, a retail investor who believed at one time but believed no more.
But no one really knows in the moment that a contrarian indicator (or any indicator for that matter) is indeed an indicator. With the benefit of hindsight, it always is.
Management has set several expectations for a busy calendar quarter (1Q13) that should have positive impacts on the share price. Let's see how the rest of this month and February shape up in that regard.
I continue to perform due-diligence with KOLs relative to Provectus' value proposition, and will have more to share on that topic in due course.
 |
| Click to enlarge figure. |
 |
| Click to enlarge figure. |
In reading his post, I was struck by its similarity to the magazine cover indicator, "which says that the cover story on the major business magazines, particularly BusinessWeek, Forbes and Fortune in the United States is often a contrary indicator."
Technical analysts often talk about a market washout -- a large share price plunge on above-average volume -- that clear the markets of bearish sentiment and help mark the/a major bottom.
It's quite possible Provectus' bottom, either in share price or market irrelevance or both, was quietly, perhaps a tad ignominiously, and most irrelevantly marked by the departure of SG, a retail investor who believed at one time but believed no more.
But no one really knows in the moment that a contrarian indicator (or any indicator for that matter) is indeed an indicator. With the benefit of hindsight, it always is.
Management has set several expectations for a busy calendar quarter (1Q13) that should have positive impacts on the share price. Let's see how the rest of this month and February shape up in that regard.
 |
| Click to enlarge figure. |
January 11, 2013
$PVCT @ #JPM13: Immunotherapeutic Potential
The conversation with Big Pharma has changed considerably after SSO in March 2012. Big Pharma and Big Biotech eyes are on Moffitt's pre-clinical and clinical work. Discussion of immunotherapeutic compound potential at the conference was prominent.
Provectus News today listed the attention the company's January 8 press release about the Moffitt human trial has garnered thus far:
Provectus News today listed the attention the company's January 8 press release about the Moffitt human trial has garnered thus far:
- Pharmaceutical Business Review
- Medical News Today
- News-Medical.net
- Therapeutics Daily
- Kate's Foundation
- BioSpace
- BioPortfolio
- I4U News
- Topix
Sign up for Provectus News here.
January 10, 2013
$PVCT @ #JPM13
After spending most of this week in San Francisco in meetings and, time permitting, attending the 31st Annual J.P. Morgan Healthcare Conference, when asked, Peter said "...what [Provectus is] doing at Moffitt is being very closely watched."
January 8, 2013
$PVCT: Provectus Pharmaceuticals Announces H. Lee Moffitt Cancer Center Initiates Phase 1 Study of PV-10 to Elucidate Bystander Effect
Provectus issued a PR today to announce the Moffitt human trial and immunological MOA characterization work.
Dr. Sarnaik's quote, below and approved by the cancer & research center, highlights two key points.
First, verifying through this human work the second set of Moffitt murine results, which [as I wrote yesterday] are highly anticipated and will be presented at a very high profile conference later this year, is both important and, I think, highly likely. To garner the use of "verifying" from Moffitt in the quote also is important.
Second, the notion of PV-10 making cancer treatment more effective in combination with other therapies also is important (very, actually); particularly for late stage patients (a group not targeted by Provectus' current registration pathway for PV-10, which is to facilitate the treatment of Stage III and early-Stage IV patients) and those with heavy tumor burden.
Craig's quote, below, highlights the same key point about PV-10 therapy combinations.
Dr. Sarnaik's quote, below and approved by the cancer & research center, highlights two key points.
First, verifying through this human work the second set of Moffitt murine results, which [as I wrote yesterday] are highly anticipated and will be presented at a very high profile conference later this year, is both important and, I think, highly likely. To garner the use of "verifying" from Moffitt in the quote also is important.
Second, the notion of PV-10 making cancer treatment more effective in combination with other therapies also is important (very, actually); particularly for late stage patients (a group not targeted by Provectus' current registration pathway for PV-10, which is to facilitate the treatment of Stage III and early-Stage IV patients) and those with heavy tumor burden.
Craig's quote, below, highlights the same key point about PV-10 therapy combinations.
He clearly is speaking to big Pharma. Foote et al.'s initial and ongoing work combining PV-10 and radiotherapy are showing dramatic improvement for patients with much later stage disease (Stage IV) and heavy tumor burden. Craig et al.'s SITC-published work provided additional data demonstrating the use of PV-10 in combination with systemic chemotherapy (Fluorouracil or 5-FU, and trademarked as Efudex).
I have to think Big Pharma was very excited by Craig's study (the data for which, again, was shown at SITC) showing the improvement of chemotherapy when combined with PV-10 and, specifically, the improvement of 5-FU, another "dirt cheap" drug (see the table to the right, and this link from 2004 regarding the cost of 5-FU).
It is growing clearer PV-10 can be used first (before surgery), second, last and everywhere in between for cancer patient treatment. Showing further effectiveness in combination for late stage disease patients and those with heavy tumor burden adds to eventual PV-10 treatment decision tree.
It is growing clearer PV-10 can be used first (before surgery), second, last and everywhere in between for cancer patient treatment. Showing further effectiveness in combination for late stage disease patients and those with heavy tumor burden adds to eventual PV-10 treatment decision tree.
January 7, 2013
$PVCT: Moffitt's Human Clinical Trial for Immunologic MOA Characterization
Moffitt (responsible party) posted its human PV-10 clinical trial on ClinicalTrials.gov. Provectus has not yet issued a PR related to this work due in large part to, I think, the Moffitt personnel-derived quotes for the PR not yet being approved by the cancer research center's administration. Once approved, the PR should then be issued (perhaps later this week).
This human trial work is the highly anticipated follow-up to Moffitt's second set of murine model work (the first set of results was presented at SSO in March 2012), the results of which also are highly anticipated and to be presented at a very high profile conference later this year. I think some of the results of this human work also could make their way into the same conference presentation(s) of the aforementioned second set of murine work results.
Recall the conclusions of Craig et al.'s poster at SITC (below) in October.
As an aside, based on a very recent reading of several data points, I could be (probably am) off in my speculation of when the SPA might arrive. It is very possible management may finally be done with the refinements of the trial design, various associated parameters, features and facets, and related discussions with the FDA. If the SPA does not arrive in January, I would next expect February in order for the MM Phase 3 trial to commence prior to quarter (Q1) end.
This human trial work is the highly anticipated follow-up to Moffitt's second set of murine model work (the first set of results was presented at SSO in March 2012), the results of which also are highly anticipated and to be presented at a very high profile conference later this year. I think some of the results of this human work also could make their way into the same conference presentation(s) of the aforementioned second set of murine work results.
Recall the conclusions of Craig et al.'s poster at SITC (below) in October.
As an aside, based on a very recent reading of several data points, I could be (probably am) off in my speculation of when the SPA might arrive. It is very possible management may finally be done with the refinements of the trial design, various associated parameters, features and facets, and related discussions with the FDA. If the SPA does not arrive in January, I would next expect February in order for the MM Phase 3 trial to commence prior to quarter (Q1) end.
January 4, 2013
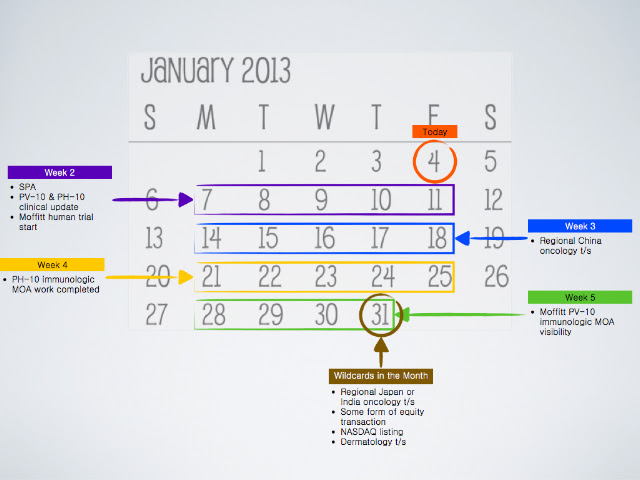
$PVCT: Let's Make A Deal?
Subscribe to:
Posts (Atom)














.jpeg)
.jpeg)


.jpeg)
.jpeg)
.jpeg)