♢♢ Note: I and others entered into a securities transaction with Provectus in March 2017.
News items from January to May 2016.
News items from January to May 2016.
Challenges & Opportunities (May 22, 2016)
1. Roles for intralesional (IL) therapy in cancer treatment — increasing in number
- Notable and lengthy tumor destruction and shrinkage upon injection; locoregional and systemic immune responses; minimal toxicity; use in earlier disease settings of cancer; priming of the immune system to allow other immunomodulatory drugs to boost and sustain its response.
- See Class of Drug (May 20, 2016) below
- Pipeline-focused
- Amgen's talimogene laherparepvec (Imylgic): melanoma (approved); currently in development (ongoing or planned) — neoadjuvant to surgery (melanoma), HCC/liver mets, breast, head & neck (in combination with pembrolizumab), soft tissue sarcoma (in combination with radiation), pediatric non-CNS tumors
- Provectus' Rose Bengal (PV-10): melanoma (ongoing pivotal trial); currently in development (ongoing or planned) — HCC/liver mets, melanoma (in combination with pembrolizumab), melanoma (in combination with radiation)
- Others: OncoSec's electroporation of plasmid interleukin-12 (ImmunoPulse), Viralytics' coxsackievirus A21 (CAVATAK, CVA21), Takara Bio's Herpes simplex virus type 1 (HF10), etc.
- See Class of Drug (May 20, 2016) below
- Quality/quantity-focused
- Amount/properness of tumor destruction upon injection: PV-10 > Imylgic
- Amount/quality/diversity of subsequent (to tumor destruction upon injection) immunologic signaling: PV-10 > Imylgic
Article: "Chemistry’s Age of Enlightenment," March 20, 2014, Jonathan Montagu, Life Sci VC
"Despite many decades of success, small molecule drug discovery appears to be increasingly challenged. The likelihood of approval of a small molecule at phase 1 is approximately half that of a biologic; pharma companies have shifted their pipelines dramatically towards large molecules over the past decade and have laid off thousands of medicinal chemists; and many of the advantages of small molecules – intracellular targeting and access to immune-privileged sites like the brain – are being eroded by new modalities and innovative delivery techniques.
This raises the inevitable question: is small molecule drug discovery a dying art, reminiscent of a golden age, but now destined for the antiquities section of the nearest molecular museum?"
Old-school drug discovery
For decades, “traditional” small molecule drug discovery has been dominated by compounds that obey Lipinski’s rule of five (for those of you that believe in it). These rules guided chemists in their pursuit of classical chemical beauty: molecules that are small and efficient – less than 500 molecular weight – reflecting the types of disease targets that fueled the industry’s historic success, i.e. those with a well-defined binding site or small allosteric pocket on an enzyme, receptor or ion channel.
Playing in this space has become increasingly difficult. Much of the low hanging fruit has been picked over after many decades of success and we now find ourselves taking on substantially more risk:
Chemical risk because we are left with targets that are seemingly intractable e.g. PTP1B, KRas, c-Myc and lack simple, obvious or precedented active sites; and
Biological risk as we have turned to exciting but emerging biologies e.g. epigenetics and cancer metabolism."
Article: Small Molecule Chemistry’s “Limited Utility”?, March 20, 2014, Derek Lowe, In The Pipeline
"Over at LifeSciVC, guest blogger Jonathan Montagu talks about small molecules in drug discovery, and how we might move beyond them. Many of the themes he hits have come up around here, understandably – figuring why (and how) some huge molecules manage to have good PK properties, exploiting “natural-product-like” chemical space (again, if we can figure out a good way to do that), working with unusual mechanisms (allosteric sites, covalent inhibitors and probes), and so on. Well worth a read, even if he’s more sanguine about structure-based drug discovery than I am. Most people are, come to think of it.
His take is very similar to what I’ve been telling people in my “state of drug discovery” presentations (at Illinois, most recently) – that we medicinal chemists need to stretch our definitions and move into biomolecule/small molecule hybrids and the like. These things need the techniques of organic chemistry, and we should be the people supplying them. Montagu goes even further than I do, saying that “...I believe that small molecule chemistry, as traditionally defined and practiced, has limited utility in today’s world.” That may or may not be correct at the moment, but I’m willing to bet that it’s going to become more and more correct in the future. We should plan accordingly."
- Montagu's utilizes the definition of small molecule as less than 500 MW; Rose Bengal's weight is approximately 1,000; immune checkpoint inhibitors like pembrolizumab may weigh about 150,000 g/mol
- Rose bengal relies on physical chemistry to work; see December 20, 2015 blog post "An incomplete thought"
- Rose bengal has good, consistent pharmacokinetics; see July 5, 2015 blog post "The Ever Expanding Clinical Value Proposition of PV-10"
- Yes, Rose Bengal appears to work in and exploit a “natural-product-like” chemical space
- Yes, Rose Bengal works by unusual mechanisms
- Etc. (= "and so on")
- Rose Bengal lacks a reliance on a single immunologic signaling pathway to work; lacks a focus on a single cell receptor to work; lacks cancer drug resistance
- Is the above because it may be implicated in different kinds of cancer cell death: apoptosis, autophagy (autophagic cell death), immunogenic cell death, and necrosis?
- See May 21, 2016 blog post "Could PV-10 (Rose Bengal) be implicated in different kinds of cell death?"
5. The technology that is PV-10 — scientific postulation and investigation manifested
- Safe (spares healthy tissue); both local and systemic efficacy; multi-indication viability (agnostic to disease presentation); synergistic in combination with and orthogonal to other cancer treatments
6. The product that is PV-10 — solving problems, creating value
- Ease of physician use; supportive of patient compliance; easy to use, re-use, ship, store and handle – all at room temperature; globally affordable
There are a couple of good reasons for the pharmaceutical industry to be skeptical of the class of drugs called intralesional (IL) therapies. First, until very recently, there has been no history of clinical success, and thus regulatory approval. Second, what promising, approved therapy there has been has turned out to be a poor product.
Before Amgen's IL drug talimogene laherparepvec (T-Vec) was approved in October 2015 (as Imlygic) for advanced melanoma, failure preceded it more than two year before in the form of Vical's IL agent velimogene aliplasmid (Allovectin-7) in August 2013 for the same indication. Before Vical, there was the failure of IL agent bacillus Calmette-Guérin (BCG) in 1978, also for advanced melanoma. Taken together, IL therapies would have modest to no history of regulatory validation for nearly 40 years; how many generations of medical practitioners, and pharmaceutical industry R&D and corporate leadership would that represent?
 |
| Image source |
The clinical value proposition of IL therapies is nicely summarized in an article this month by St. Luke's Cancer Center's Dr. Sanjiv Agarwala, MD entitled "The Role of Intralesional Therapies in Melanoma." Agarwala has been involved with IL therapies for some time (e.g., Vical, T-Vec, PV-10, HF10). The class proposition comprises:
- Notable and lengthy tumor destruction and shrinkage upon injection,
- Locoregional and systemic immune responses,
- Minimal toxicity,
- Use in earlier disease settings of cancer, and
- Priming of the immune system to allow other immunomodulatory drugs to boost and sustain its response.
Amgen's goal for T-Vec, product deficits notwithstanding, would be to broaden the IL drug's applicability, in terms of solid tumor cancer indications, patient populations, and pairing with other classes of drugs. The other late-stage cancer asset is of course PV-10. Amgen's work on T-Vec benefits PV-10 because the latter has the same applicability as the former, but is more safe, results in greater tumor destruction, and causes stronger immunologic signalling.
Behind Rose Bengal there are a few more assets, none of which appears to have a pivotal trial (as a monotherapy) designed and all of which, like T-Vec and PV-10, are engaged in combination trials with checkpoint inhibitors.
 |
| Click to enlarge. |
Updated below.
There are several major themes, and observations potentially bordering on conclusions (collectively, "takeaways") that one could draw from Foote et al., A phase 2 study of intralesional PV-10 followed by radiotherapy for localized in transit or recurrent metastatic melanoma, J Clin Oncol 34, 2016 (suppl; abstr e21072). See May 18th blog post "Rose Bengal (PV-10) at ASCO 2016." Before going into them, let me frame the rest of this blog news item by writing the following.
Context, as I see it. Successful combination of two (or three or however many) cancer treatments, whether therapy (e.g., radiation) or therapeutic (e.g., chemotherapy, targeted therapy, immunotherapy), should require each member of the pair or group to be synergistic with and orthogonal to each other:
- Successful synergism means the pair or triplet (or...) work well together, presumably and hopefully producing efficacy results better than that of when each component or treatment is offered to a patient as a monotherapy, and
- Successful orthogonality means the combination creates no more safety issues or no more frequent adverse effects than when the individual components or treatments are used as monotherapies; that is, there is little or minimal risk of therapeutic-therapy (i.e., drug-drug) interaction.
I drew the following takeaways from Foote et al.'s work:
- Foote et al.'s data standalones on their merits. An overall response rate (ORR) of 87% (33% complete response [CR], 53% partial response) and disease control rate (DCR) of 93% ('clinical benefit' in the abstract), both on an intent-to-treat basis, and melanoma specific survival 65.5 months. I think that's impressive, plain and simple.
- Comparison of this work initially could be made to Provectus' melanoma Phase 1 study. If we're going to compare results, I think it would be more appropriate to compare the Phase 2 PV-10 + radiation study with the company's early-stage trial where (a) PV-10 was injected once into patient lesions/tumors and (b) patients had Stage III disease. Stage IIIB-C patients in Foote's study received one shot of PV-10 into their lesions before receiving radiotherapy. The study's peer-reviewed paper is Thompson et al., "Chemoablation of metastatic melanoma using intralesional Rose Bengal," Melanoma Research, 18:405, Dec 2008. Using Provectus' September 2007 press release figures, and as noted above in regards to combination synergy, the PV-10 + radiation Phase 2 results bested the PV-10 Phase 1 data: 33% vs. 20% CR, 86% v. 40% ORR, 93% vs. 75% DCR.
 |
| Click to enlarge. |
I loosely offer this comparison because the timeframe of the two studies overlap or are close. The preparatory work upon which Foote et al.'s Phase 2 study was built comprises experiences applying radiotherapy to patients from Provectus' Phase 1 work: "A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series," Melanoma Research, 20:48, Jan 2010.
Subsequent comparison might include the subgroup of the company's melanoma Phase 2 trial who had all of their disease treated. Here, all disease would be treated with PV-10. The study's peer-reviewed paper is Thompson et al., "Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma," Annals of Surgical Oncology, (Epub ahead of print), Oct 2014. Using the paper's figures, the PV-10 + radiation Phase 2 results were somewhat better than the PV-10 Phase 2 sub-group data: 33% vs. 50% CR, 86% v. 71% ORR, 93% vs. 82% DCR. Stage IIIB-C patients in the sub-group (and patients in the trial) received up to 4 injections of their lesions/tumors.
A better comparison might be the eventual pivotal melanoma Phase 3 trial results, where patients would regularly receive PV-10 to all of their disease. Treating all Stage III disease to prevent or forestall it's spread to Stage IV, one of the hypotheses of the Phase 3 trial, may or may not be wholly realistic for every patient (because there could well be occult melanoma cells out of reach of or too small for injection with PV-10.
- Foote et al.'s PV-10 + radiation results could bode well for Provectus' Phase 1b/2 study combination of PV-10 + pembrolizumab. Again, consider the rationale of combination in regards to synergy. Successful combination would require successful synergy of the combination's component parts. Moffitt Cancer Center already has provided synergy-based observations of PV-10 and co-inhibitory blockade in their murine (mouse) model work at SITC 2014, "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma," and AACR 2016, "T Cell Mediated Immunity after Combination Therapy with Intralesional PV-10 and Co-Inhibitory Blockade in a Melanoma Mode."
Updated (5/19/16): Provectus issued press releases today (here — trials-in-progress — and here —) investigator-initiated PV-10 + radiation) and filed associated 8-Ks regarding PV-10-related ASCO 2016 abstracts (here and here, respectively). Of note the this blog news item was the second PR, "Announces Abstract Available on PV-10 Plus Radiotherapy in Melanoma."
As is the case with the company, most relevant in PRs are Provectus' CTO Dr. Eric Wachter's associated commentary. First, with my bolded and underlined emphasis:
"This abstract describes initial analysis of data from an investigator-initiated study of PV-10 followed by regional radiotherapy for refractory melanoma. Patients received a single course of PV-10, and if not immediately achieving a complete response they received a modest dose of radiation 6-10 weeks later. This combination yielded an 87% response rate with a third of patients achieving complete response. The response was durable (12.2 months mean duration of complete response), however, the protocol did not allow for subsequent retreatment, and the authors report that 80% of patients eventually had recurrence. Nonetheless, the melanoma specific survival of almost 5 and a half years is encouraging, and I agree with the authors' conclusion that these results justify expanded evaluation in a randomized trial."See my bolded point above "Comparison of this work initially could be made to Provectus' melanoma Phase 1 study." I also would re-emphasize the durability of PV-10's response (bolded above), even after only one injection.
Second:
"This work arose from observations by these same researchers several years ago that, as they stated at the time, melanoma patients treated with PV-10 followed by external beam radiotherapy 'had an impressive response.' Also as observed in those early cases, the data reported in this ASCO abstract show that there appears to be minimal potential for increased side effects when PV-10 is used in combination with radiotherapy."These comments relate to synergy ('impressive response' = efficacy in combination better than individual treatment outcomes) and orthogonality ('minimal potential for increased side effects' = no greater safety issues in combination than when incurred as individual treatments); see "Context, as I see it" above.
Third:
"The primary purpose of presenting research data at international meetings such as ASCO is to share knowledge within the medical community. An important implication of this is fostering dialog within the community regarding appropriate use of such data. In this case, I hope this initial report will help foster relationships that facilitate advancement of these findings into advanced studies, as suggested by the authors."Radiation seemed to experience a resurgence of thought and dialog within the medical research community. See, for example, Wednesday (March 2, 2016) and AACR Tweets so far (April 19, 2016) below. And, for example, Demaria et al., "Can abscopal effects of local radiotherapy be predicted by modeling T cell trafficking?," Journal for ImmunoTherapy of Cancer 20164:29 or Poleszczuk et al., "Abscopal Benefits of Localized Radiotherapy Depend on Activated T-cell Trafficking and Distribution between Metastatic Lesions," Cancer Res; 76(5); 1009–18.
Updated below.
A. The Role of Intralesional Therapies in Melanoma, May 15, 2016, Oncology Journal, Melanoma, Dr. Sanjiv Agarwala, MD
 |
| Click to enlarge. Image source |
"Likely Future and Unresolved Questions
If the ongoing and planned clinical trials confirm initial trends observed in early studies, combinations of systemic immunotherapies with intralesional agents may find a place in the ever-expanding therapeutic toolbox available to clinicians who treat patients with melanoma. As long as a lesion amenable to intralesional injection is available, it may allow clinicians to exploit the potential synergy and nonoverlapping toxicities of these approaches.
Another interesting area for ongoing and future research is in the neoadjuvant setting. Would it be justifiable to consider T-VEC, PV-10, or CVA21 as an upfront strategy in patients with surgically resectable disease before surgery, with the intent of making the tumor our ally? If ablating the patient’s autologous tumor can stimulate a tumor-specific immune response that persists after the tumor is removed, then creating an ally is what we would be doing. However, would the benefits be substantial enough to warrant incurring the risk inherent in delaying surgery?...
Through the emergence of new immunotherapies, treatment of melanoma is undergoing a long-awaited revolution. Ongoing research will clarify the outlines of the place that intralesional therapies will occupy in the therapeutic armamentarium in the years ahead."
 |
| Click to enlarge. Image source |
B. Above article's sidebar: Dr. Robert Andtbacka, MD, CM, Intralesional Therapies: An Old Treatment Is Reborn
"In the late 1800s, William Coley described the use of an intralesional bacteria-free extract, known as “Coley toxins,” in the treatment of sarcomas. Coley’s data showed an over 60% remission rate of sarcomas. Intralesional therapies are especially well suited to the treatment of melanoma, in particular in-transit and lymph node metastases. Intralesional bacillus Calmette-Guérin and interleukin-2 have both shown good responses in injected lesions; however, they have had minimal to no effect on noninjected lesions, likely due to inadequate activation of the adaptive immune system. Yet over the past 15 years, an improved understanding of immune activation has led to the development of intralesional therapies with enhanced immune activation and improved effects in noninjected metastases. More than 20 different intralesional therapies, ranging from viruses and cytokines to glycolipids, plasmids, antibodies, and small molecules, have been tested in clinical trials for metastatic melanoma alone."
"Early results from combination studies of intralesional therapies with other immunotherapies have indicated no increased toxicity and possibly an additive improvement in response rates above that of single-agent use; these results will likely cement the role of intralesional therapies as important immunomodulatory agents in combination therapies for metastatic melanoma. These agents may also have a future role in neoadjuvant treatment for resectable metastatic melanoma. To date, most of the intralesional agents have been administered in palpable dermal, subcutaneous, and lymph node metastases. There are ongoing studies with injection of T-VEC into visceral lesions, and also of intravenous administration of intralesional therapies, such as coxsackievirus A21. If these new routes of administration are found safe and responses are seen, it will certainly expand the role of these agents, not only in metastatic melanoma, but also in many other cancers. Indeed, intralesional therapies have the potential, in the near future, to transform the way we treat metastatic cancer."N.B. "Financial Disclosure: Dr. Andtbacka has received honoraria and/or served on advisory boards for Amgen, Merck, and Provectus; his institution has received research support from Amgen, Provectus, Takara, and Viralytics."
C. A Review of Novel Intralesional Therapies for Melanoma, With an Emphasis on a Potential Combination Approach, May 15, 2016, Oncology Journal, Melanoma, Drs. Lawrence Chen, MS and Adil Daud, MD
"Perhaps the greatest attraction and chief benefit of intratumoral therapies is their ability to synergize with systemic checkpoint therapies and accelerate the development of a lymphoid infiltrate and perhaps secondary lymphoid structures in vivo, which in turn can result in systemic mobilization of a T-cell response: the local injection–global effect model. While data are still preliminary at this point, there are hints that this model may in fact be valid, as noted in the review. The model may be valid for both viral and nonviral immunotherapy approaches."N.B. "Financial Disclosure: Dr. Daud owns intellectual property licensed to OncoSec, and owns stock in OncoSec; he receives research funding from Bristol-Myers Squibb, Incyte, Merck, and Pfizer. Dr. Chen has no significant financial interest in or other relationship with the manufacturers of any products or providers of any service mentioned in this article."
Updated (5/18/16): Takeaways:
- Intralesional (IL) therapies (aka locoregional therapies), as a class or categories, are growing in importance and gaining in awareness because they are able to generate greater tumor destruction more safely than other drug classes or categories (e.g., radiation, chemotherapy, targeted therapies, immunotherapies, etc.).
- The class includes Amgen's approved T-Vec, and wholly owned cancer assets PV-10 (Provectus), electroporation with plasmid IL-12 (epIL-12) (OncoSec), coxsackievirus A21 (CVA21) (Viralytics) and, not mentioned in Dr. Agarwala's article, HF10 (Takara Bio) — to name the approved drugs and more advanced investigational drug compounds.
- IL therapy treatment modalities may comprise use as a monotherapy, and in combination with other cancer treatments like immunotherapies.
- Monotherapy: Aside from T-Vec, which was approved in 2015 for advanced melanoma, only PV-10 is engaged in a pivotal melanoma trial (registration study). epIL-12 and CVA21 have completed Phase 2 trials but no pivotal study designs have been revealed as yet; their sole opportunities, possibly along with HF-10, only may be in combination with another cancer treatment.
- Combination therapy: T-Vec, PV-10, epIL-12, CVA21 and HF-10 are all in studies combining them with other immunomodulatory agents.
- Nitpicking: The mechanism of action figure in Agarwala's article is not of T-Vec, but of PV-10. See, for example, October 4, 2014 blog post “We should see a difference very quickly between PV-10 and chemotherapy responses.” Amgen could only hope T-Vec could work so fast, and possess so robust immunologic signalling.
- Agarwala's article spent about 15-18% of its words on PV-10 (a total of five IL agents are discussed); Chen and Daud, who owns OncoSec stock (both appear to be epIL-12 clinical investigators), spend more than 60% of their words discussing their "client."
- Most funny [to me] is Chen and Daud's statement "Dr. Agarwala points out that there are two main types of intralesional therapy: direct lytic and immune-activating. The principal direct lytic agent is PV-10 (rose bengal), which is a dye that is selectively taken up by melanoma cells. Other direct lytic approaches include laser light and hyfrecation, as well as surgical resection and radiation therapy. Immune approaches are more interesting due to their potential synergy with checkpoint inhibitors and novel immunotherapies." To be clear, PV-10 is an immunotherapy, and a unique one at that. It is not without a rather copious amount of irony that Chen and Daud seem confused about what may or may not activate, prime or otherwise harness the immune system. You may recall that Dr. Sally Church, MD recently asked if chemotherapy was immunotherapy; see May 3, 2016 blog post "Catching On." Then, there is Moffitt Cancer Center's paper of PV-10's mechanism of action: Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1.
Tell me more... (May 17, 2016)
Updated below.
So, you're saying these TIL things are important?
 |
| Click to enlarge. Tweet image source |
 |
| Click to enlarge. |
"In addition, Moffitt's team found that PV-10 was cytotoxic to B16 mouse melanoma cells with minimal cytotoxicity to normal skin cells (fibroblasts). This cytotoxicity occurred via necrosis with minimal evidence of apoptosis. The PV-10 treatment of B16 tumors in mice led to release of HMGB1, a soluble Damage Associated Molecule Pattern (DAMP) that is important in activation of dendritic cells; such dendritic cells from these mice were selectively active against B16 tumor cells. PV-10 treatment of B16 tumors in mice also led to infiltration of dendritic cells into the lymph nodes draining the treated tumors; no infiltration was observed in non-draining nodes."Moffitt, ASCO 2014, "Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions"
 |
| Click to enlarge. |
'The Moffitt abstract provided interim results of a pilot clinical trial designed to investigate the local and immunologic effects of tumor ablation with PV-10. Lead author, Dr. Sarnaik, noted "In the peripheral blood of patients after PV-10 injection, we saw a significant increase in circulating T-cells, including CD3+ and cytotoxic CD8+ cells. This suggests an immunologic-mediated antitumor response is engendered by PV-10. We are hoping to undertake combination trials that combine PV-10 with the promising systemic immunotherapies being developed by our medical oncology colleagues."'Moffitt, Oncotarget 2016, "Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1"
 |
| Click to enlarge. |
"The percentage of infiltrating immune cells in PV-10 treated and bystander lesions were compared before and after treatment with IL PV-10. However, very few infiltrates were detected in the lesions that completely regressed, and no significant changes were measured. Thus an alternative method was used to compare the presence of immune subsets in peripheral blood mononuclear cells (PBMCs) before and after treatment. There was a statistically significant increase in circulating CD8+ T cells, CD4+ T cells, and NKT cells after PV-10 treatment (Supplementary Figure S2). There was no difference in circulating NK cells, MDSC, CD4+FOXP3+ regulatory T cells or plasmacytoid DCs before and after treatment (data not shown)."So, you're saying you want to see in humans what you saw in mice? And, maybe, what you saw in humans you'd also like to see in mice?
 |
| Click to enlarge. Tweet image source |
"In this study, we have shown a mechanism of tumor-specific immune response induced by IL PV-10. In melanoma-bearing mice, IL PV-10 induced necrosis of tumor cells leading to the release of HMGB1, which is crucial for DC activation. This resulted in DC maturation and infiltration into draining LNs for the activation of tumor-specific T cells. Additionally, increased HMGB1 levels measured in sera of patients treated with IL PV-10 suggests that HMGB1 may be involved in eliciting a systemic immune response in patients. We have shown that circulating T cell populations and tumor-specific CD8+ T cells are increased in melanoma patients after IL PV-10 therapy. Together these results support the design of additional clinical studies to measure anti-tumor immune responses after IL injection of PV-10 in patients with melanoma."May 2016 Provectus press release:
"Eric Wachter, CTO of Provectus, observed, "The Moffitt researchers have systematically documented each of the key steps in the immuno-oncology cycle described by Chen and Mellman in their landmark review article (Oncology Meets Immunology: the Cancer-Immunity Cycle. Immunity 2013; 39: 1-10). In an exemplary demonstration of translational medicine, this team identified important immunologic markers in model systems and verified key facets of these in clinical trial participants, and similarly identified other markers in clinical trial participants and substantiated these in mouse models. While a number of their main observations were previously reported at scientific meetings, these are presented here in detailed, integrated fashion for the first time."So, you're saying independent production/reproduction are hallmarks of Western science?
 |
| Image source |
- A decrease in institutional share holdings of Provectus: 9.04 million shares (down from 9.83 million as at 12/31/15, or about -8%) and 4.3% of shares outstanding (not a fully diluted figure) (down from 4.8%), and
- An increase in the number of filers to 48 (up from 46).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
See Institutional (February 16, 2016) below for the last blog news item on institutional holdings.
Institutional holdings (i.e., 13-F-based reporting) of publicly traded warrants remained essentially the same quarter-over quarter.
New institutional holdings (common stock) for the quarter ending June 30, 2015 of, presumably, the firms and funds that participated in the Maxim-led June 2015 offering — see, for example, Offering, Part I (June 29, 2015) and Offering, Part II (June 30, 2015) on the blog's Archived News III page — are shown in the table below.
 |
| Click to enlarge. WhaleWisdom website |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Tweet image source |
 |
| Click to enlarge. Tweet image source |
Re: David Miller's tweet above: It has struck me (some time ago) that Provectus' Dr. Eric Wachter, PhD's research & development strategy, in part perhaps, may have been for Moffitt Cancer Center to:
- Independently validate, reproduce, repeat, etc. Provectus' original work on the clinical value proposition of PV-10 and Rose Bengal (i.e., cancer fighting step #1 of tumor ablation, step #2 of a tumor-specific immune response) — the antithesis of Theranos, if you will, and, in reality (it would seem to me), most other biotechnology companies,
- Elucidate the investigational compound's mechanism of action, and
- Demonstrate PV-10's synergy and orthogonality in combination with different classes of drugs and drug compounds (e.g., immunotherapies and the different groupings therein, targeted therapies).
I wonder if Eric's approach with the University of Illinois at Chicago includes or comprises having the Maker Laboratory address PV-10 and Rose Bengal's effectiveness with all solid tumor cancers, whether the tumor is "hot" or "cold;" that PV-10-induced immune system components can infiltrate all manner of cancer tumor, irrespective of "temperature."
Re: Adam Friedman's tweet above: Synergy. Orthogonality. Key components that in my view enable the definition of success for combining therapeutics and therapies. Foote et al.'s ASCO 2016 abstract, A phase 2 study of intralesional PV-10 followed by radiotherapy for localized in transit or recurrent metastatic melanoma, J Clin Oncol 34, 2016 (suppl; abstr e21072), begins the presentation of PV-10's value as a combination partner. The next installment would be the combination of PV-10 and pembrolizumab in patients with advanced (Stage IV) melanoma.
Foote et al.: An overall response rate (ORR) of 87% (33% complete response [CR], 53% partial response) and disease control rate (DCR) of 93% ('clinical benefit' in the abstract), both on an intent-to-treat basis, and melanoma specific survival 65.5 months.
Rare CRs, scattered PRs? Not so much...
Sites matter (May 15, 2016)
Updated below.
Provectus' CTO Dr. Eric Wachter, PhD said on the May 10th 1Q16 business update conference call in regards to revised guidance about the timing of pivotal melanoma Phase 3 trial data readout triggers {bolded and underlined emphasis is mine}:
"I must unfortunately report that we are behind with regard to our initial schedule for reaching the interim and final analysis triggers for the study. I’m confident that we are implementing the proper study to support licensure of PV-10, but our present estimate that we are approximately six months behind schedule with regard to site start-up, patient recruitment and eventual data read-out."Eric referred primarily to data readout triggers, not data readout timing. You may interpret or form an opinion of the following based on your own view of when enough data are enough. You think:
- "Scenario A:" Provectus requires enough data to show statistical significance of progression-free survival (PFS) curve separation between the treatment (PV-10) and control (chemotherapy, T-Vec) arms — my guess, 40-60 (maybe as high as 80) patients — a prescribed time [interim data readout] trigger,
- "Scenario B:" The trial must collect 50% of the events required for disease progression to have occurred — 112.5 patients — a prescribed event [interim data readout] trigger, and
- "Scenario C:" The trial must collect 100% of events — silly, but nevertheless, 225 patients.
I believe Scenario A is germane.
Part I. Travel back to the April 9, 2015 panel session Provectus held in New York — see Audiophile (April 19, 2015) and Kick-off (June 22, 2015) on the blog's Archived News III page — Eric said pivotal trial would comprise 25 sites in the U.S. and 10 in Australia (not including sites Provectus could have in other countries, such as China, India and Brazil).
Clinical trial design literature would suggest the average enrollment rate of Provectus' trial is about 0.4 patients per site per month {225 patients ÷ 35 sites ÷ an 18-month enrollment period}. This figure appears to be consistent with, albeit slower than, three datasets: the company's metastatic melanoma Phase 2 trial experience, and the experiences of the Australian Princess Alexandra Hospital and Peter MacCallum <see, for example, Remarkably consistent (May 14, 2016) below>.
 |
| Click to enlarge. |
Diving a little deeper into Provectus' Phase 2 trial experience, the actual enrollment rate — where one considers the actual length of time sites actually recruited —is noticeably higher than the average rate.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Takeaway: It's about running the trial from here on out, and documenting the expected results —assuming "the constantly changing playing field in oncology and the evolving process for starting and executing clinical trials on a global scale" don't change further. Run the trial, Eric. Run the trial.
It's not about winning. I don't believe it is about whether PV-10 (and Provectus) will win its pivotal trial or registration study. Medical literature is replete with clinical data showing one of the comparators, chemotherapy (either dacarbazine or temozolomide), should lead to disease progression in patients (a trial event) within one treatment cycle (i.e., 4 weeks) and prior to a clinical assessment, or at most two.
The other comparator, oncolytic viral therapy (T-Vec), should lead to disease progression within short order but probably would take longer than chemotherapy (and ultimately should cause a trial event) because melanoma tumors or lesions injected with T-Vec become larger or progress before they shrink, which is why T-Vec's pivotal melanoma Phase 3 trial used durable response rate (DRR) as its primary endpoint — DRR, which is complete response plus partial response, needed to be "maintained continuously for at least 6 months from the time the objective response was first observed and initiating within 12 months of starting therapy." PV-10's Phase 3 trial's tumor measurement criteria is RECIST 1.1, which is not as lenient as T-Vec's Phase trial's modified World Health Organization (WHO) criteria.
 |
| Click to enlarge. Image source |
- Being Stage IIIB-C and Stage IV M1a,
- Or, said another way, recurrent, satellite or in-transit locally advanced cutaneous or subcutaneous melanoma metastases,
- Having failed immunotherapy, and
- Having failed targeted therapy.
It's not about geography, per se. I don't believe it's about where PV-10 recruits for its pivotal trial. Interestingly, Provectus' metastatic melanoma Phase 2 trial experience showed that U.S. and Australian sites (of the seven sites, four were the former and three were the latter) recruited roughly the same number of patients per site per month (or about 1 patient per site per month).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
It's not about the number of sites. I don't believe it's about having a very large number of trial sites. Eric's April 9th panel comments about using 35 U.S. and Australian sites, which corresponds to an average patient per site per month figure of ~0.4, was made in the breath, so to speak, as working with investigators who and sites that were familiar with PV-10 treatment, and investigators who and sites that were familiar to Eric. If you hold steady the number of patients in the trial (given) and the enrollment period (in months) (fixed), and then assume/utilize a well understood and actual enrollment rate higher than its average counterpart, you don't need nearly as many trial sites.
 |
| Click to enlarge. |
It's not different this time, per se. Medical times have undoubtedly changed since Provectus' metastatic melanoma Phase 2 trial (c. 2007-2009). Five monotherapy and combination immunotherapies have been approved for advanced melanoma since then (ipi, pembro, nivo, T-vec, ipi + nivo). Five monotherapy and combination targeted therapies also have been approved for advanced melanoma since then (vemurafenib, dabrafenib, trametinib, t + d, v + cobimetinib). But many patients' melanoma still recur after failing treatment by these approved drugs, and the NCCN guidelines for melanoma still recommend clinical trial for Provectus' pivotal trial's patient population.
It's about opening sites. Since I believe it is about Scenario A, a smaller but necessarily sufficient number of trial patients to show statistically significant separation of treatment and control arm PFS curves, Eric has to open enough sites recruiting about 1 patient per site per month to arrive at his "number," or trigger.
I speculate, or hazard a rather wild-a@# guess the pivotal melanoma Phase 3 trial has recruited about 12 patients, which may well be on the high side.
 |
| Click to enlarge. |
If I believe or speculate Eric needs about 60 patients (i.e., 40-60, or maybe as high as 80) to get to his "number" — remember, who the heck knows! — at 0.9 patients per site per month (the Phase 2 trial experience, assumed for all surgical onc-based sites), the study would require about 53 recruitment months more. Using the currently active sites above, a trigger could be reached in about 11 months. If Eric's trigger is six months behind schedule, it would seem he needs a handful more sites opened and recruiting very soon (i.e., assumes he needs 48 more patients, 11 sites recruiting at a blended ~0.8 rate to account for medical vs. surgical onc practices).
My main point here, trying not to be precise above but rather just as accurate as I can, is that Eric just has to run the trial. Run the trial, Eric. Run the trial.
My main point here, trying not to be precise above but rather just as accurate as I can, is that Eric just has to run the trial. Run the trial, Eric. Run the trial.
Remarkably consistent (May 14, 2016)
 |
| Image source |
- (i) PV-10 as a single agent or monotherapy for patients with locally advanced cutaneous melanoma,
- (ii) PV-10 in combination with pembrolizumab, an immune checkpoint inhibitor, for patients with advanced melanoma, and
- (iii) PV-10 for hepatocellular carcinoma (primary liver cancer).
That said, like with Moffitt Cancer Center's recent paper PV-10 mechanism of action paper Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1, further emerging data from third parties like two Australian expanded access/compassionate use program (EAP/CUP) sites (called special access scheme [SAS] locations in the country) continue to show the remarkable consistency (repeatability, reproducibility) of the investigational drug compound's performance with respect to safety and efficacy. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) below.
These Australian data/results might put Provectus in the position for early or preliminary regulatory approval down under, or more of PV-10 and the company may be required by the Therapeutic Goods Administration. Nevertheless, these data/results still point to a consistent performer in PV-10.
On the May 10th 1Q16 business update conference call, Provectus' CTO Dr. Eric Wachter, PhD said {underlined emphasis is mine}:
"So there was a meeting of the Royal Australian College of Surgery last week in Brisbane, where there was a session on melanoma and two of our expanded access investigators presented summaries of their history of using PV-10 in their practice. In the one case, the patients were exclusively under the expanded access protocol and in the second case about half of the patients were from the Phase II study, they called the patient records for all of those patients and analyzed what happened to patients in their wake of having PV-10. Both abstracts – I’ve seen the – presentation as of now, but the abstracts showed remarkably similar story that is quite consistent with what we’ve reported in definitive journal article on the Phase II study. So I think that it represents something of a real world case, what could be expected of PV-10."Takeaways:
- Perhaps analogous to the Energizer Bunny, PV-10 keeps working and working and... Provectus' metastatic melanoma Phase 2 trial data reported in July 2015 Annals of Surgical Oncology journal article "Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma" noted of the intent-to-treat (ITT) population of 80 patients, among other endpoints and observations, 26% complete response and 69% disease control (complete response + partial response + stable disease).
- The first RACS abstract from Peter MacCallum noted about its 21-patient EAP/CUP/SAS experience 26% complete response and 68% disease control — essentially the same result.
- Eric clearly is saying the results achieved by Australia's Princess Alexandra Hospital (Phase 2 and EAP/CUP/SAS patients) and Peter MacCallum (only EAP/CUP/SAS patients) teams are remarkably consistent with Provectus' Phase 2 observations.
- Release of the other clinical benefit, response and survival data collected by the two medical institutions would further verify Eric's position.
- I believe he also is saying the hypothesis of the company's pivotal melanoma Phase 3 trial, noted in Provectus' press release about the Moffitt mechanism of action paper, that PV-10 alone can produce a systemic immune response that translates to longer progression free survival, the trial's primary endpoint, essentially has been proven at least twice over by the two Australian sets of data.
Updated below.
Provectus form Form S-4 (registration statement) and Schedule TO (tender offer statement) today, reopening the original warrant exchange program at a higher exchange price of $0.75, which was the original price of the original program but was reduced to $0.50 for the transaction that ultimately closed on March 28th. Private/non-tradable ("existing") warrants were and now also may be exchanged for publicly-traded ("replacement") warrants having an $0.85 exercise price and a June 19, 2020 expiration.
The first transaction, where exercising private warrants for $0.50, irrespective of their respective, original exercise prices, gained the transactor one common share and one publicly-traded warrant, saw 7.798 million existing securities out of an outstanding amount of 59.861 million, or 13%, tendered. 51.149 million of these existing warrants remain. The higher number (52.063 million) I noted in New SEC filing (May 9, 2016) below indicates some existing warrants expired in the interim.
The current contemplated exchange or second transaction has a completion date of June 28, 2016.
Updated (6/28/16): Provectus extended the warrant exchange program to July 28th at the same price of $0.75. The associated 8-K and tender offering filings are here and here, respectively.
Clinical Trial Status Update: Australia Recruiting (May 13, 2016)
Updated below.
H/t Investor Village poster canis_star here and here: Lead Australian clinical site Princess Alexandra Hospital of Brisbane now is recruiting for Provectus' two melanoma trials:
- PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma (6 sites now are recruiting; a screenshot of some, including the lead Australian site, are shown below), and
 |
| Click to enlarge. |
- PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma (3 sites now are recruiting).
 |
| Click to enlarge. |
 |
| Click to enlarge. Change information is here. |
A quick 'n dirty comparison and analysis of various dates and changes:
 |
| Click to enlarge. |
In the literature (May 12, 2016)
It would appear the convention for medical journal articles (and abstracts) and poster presentations (and abstracts) is to use the non-proprietary/generic/non-brand name of a drug compound. Proprietary names may be used but, for example, not in the title of an article or poster.
E.g., The New England Journal of Medicine:
E.g., Journal of Clinical Oncology:
PV-10 is Provectus' proprietary name for the company's investigational oncology drug, and of course PH-10 for its investigational dermatology one. Rose Bengal is the non-proprietary name; the IUPAC name of course is 4,5,6,7-Tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H- spiro[isobenzofuran-1,9'-xanthen]-3-one. While Rose Bengal's original and first medicinal use was/is as a diagnostic, Provectus own's its second medicinal use as a therapeutic. Hence, in Moffitt's recent Oncotarget article, the title is Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1.
10-Q Notes (May 10, 2016)
Updated below, again, once more (for the conference call)
1. Provectus issued a press release today and filed an associated 8-K regarding its 1Q16 financial results, Reports First Quarter 2016 Financial Results, in conjunction with filing its 10-Q. I plan to update this blog news item frequently as I further review and analyze the filing, and eventually the transcript of the 1Q16 business update conference call held today.
2. 3/31/16 cash and cash equivalents were $9.8 million, compared to a 12/31/15 amount of $14.2 million. Cash at 4/30/16 was approximately $8 million.
3. The company's monthly cash burn for the quarter appears to have been $2.7 million, +62% quarter-over-quarter (QoQ) (the prior quarter-over-quarter change was +18%).
5. Language regarding runway remain unchanged from the prior quarterly filing: "into 2017."
6. Research and development expense for the quarter was $2.4 million, -34% QoQ (prior QoQ: +36%).
7. Lab supplies and pharmaceutical preparations expense for the quarter was $18K, -5% QoQ (prior QoQ: -96%).
8. Note under Other Regulatory Matters (p. 11): "We have received a subpoena from the staff of the Securities and Exchange Commission related to the travel expense advancements and reimbursements received by H. Craig Dees, our former Chairman and Chief Executive Officer."
Updated (5/10/16): As of this writing, the transcript of the call is not yet available. I have two speculations. First, I speculate the lead investigator of the European portion of the pivotal melanoma Phase 3 trial is (is going to be) Dr. Axel Hauschild, MD, PhD, Professor and Head of the Interdisciplinary Skin Cancer Center at the Department of Dermatology, University Hospital Schleswig-Holstein in Kiel, Germany. Hauschild also is one of the two course directors of the Sixth Post-Chicago Meeting on Melanoma/Skin Cancer (June 30-July 1, 2016 in Munich, Germany), and to which Provectus COO and interim CEO made reference on today's conference call.
Second, I speculate PV-10 plus radiotherapy data could be presented at ASCO 2016.
Updated (5/11/16): The conference call transcript is now available (from Seeking Alpha). I'd like to organize my thoughts on it in the following sequence or manner:
Eric's plan to address being behind schedule was:
I thought it was helpful for Eric to contextualize the significance of and significant changes in the amendment to Phase 3 trial protocol that (i) added intralesional (IL) talimogene laherparepvec (T-Vec, Imlygic) as another comparator, (ii) extended eligibility to include Stage IV M1a patients with no active nodal or distant metastatic disease, (iii) clarified eligibility for patients not having access to immune checkpoint inhibitors, (iv) clarified eligibility for patients not having access to targeted therapy, (v) extended eligibility to patients failing targeted therapy, and (vi) relaxed crossover (to the PV-10 treatment arm) eligibility criteria:
Melanoma Phase 1b/2 study of PV-10 and pembrolizumab. First, enrollment of up to 24 patients in the Phase 1b portion may be completed this year:
Third, and unrelated to the conference call, Provectus updated its Phase 1b/2 study on ClinicalTrials.gov on May 9th to include principal investigator Dr. Victoria Atkinson, MD of Australia's Princess Alexandra Hospital and Gallipoli Medical Research Foundation.
See a January 2016 article featuring her entitled Nivolumab Continues to Improve Outcomes in Advanced Melanoma.
Moffitt Cancer Center work. First, I believe Moffitt's peer-reviewed publication of their work on PV-10's mechanism of action should be available prior to ASCO, maybe as early as this week. Peter referred to Moffitt several times on the call:
Compassionate use/expanded access program. Provectus said it would close down it's 7-year expanded access or compassionate use program (CUP) that offered PV-10 patients in the U.S. and Australia for cutaneous or subcutaneous tumors. Designed for 115 patients but having an initial target of 25-30, according to Eric, it treated 160 patients through the end of 2015, and an undisclosed number year-to-date in 2016:
Updated below.
For the sixth time the anticipated completion date (and anticipated last follow-up date) of Moffitt Cancer Center's PV-10 mechanism of action work, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study, has been pushed out -- now to December 2016 (the change was made on ClinicalTrials.gov on May 6th). The original completion (last follow-up) date was March 2014, or about 14 months from the opening of recruitment. A current completion date of December 2016 would mean an approximately 48-month study period, or nearly 3.5 times longer than initially contemplated.
A summary record of the above changes are provided in the table below:
In the context and construct of this study, why has the completion/last follow-up date been moved so frequently?
Recall that in 2014 Moffitt, after enrolling and treating 7 patients for their study, PV-10-treated (and possibly untreated) tumors went away too quickly -- faster than the study protocol's allotted 7-14 days before tumor biopsies were to be taken from patients {underlined emphasis is mine}
Moffitt showed new data from clinical study specimens at SITC (i.e., HMGB1 in human plasma) that corroborated observations from their ongoing non-clinical work. Final reporting of study data — that is, completing the clinical study report, which is the formal end of the study — may have been delayed as new things that were learned from mice were then tested in the human samples from the study.
Updated (5/10/16): A tweet reply by @johnhallnj was a reminder to note in this blog news item that Moffitt's feasibility study above was fully enrolled in 2014, perhaps by April of that year. The enrollment figure of Moffitt's study was 15. See "...it’s a bona fide immunological response" (April 17, 2014) on the blog's Archived News I page, which references an April 2014 article by ecancer reporter Janet Fricker entitled "PV-10 decreases melanoma cells in tumours" {underlined emphasis and bolded verbiage below is mine}:
Provectus filed a Form POS AM (Post-effective amendment to a registration statement that is not immediately effective upon filing) today, related to the warrant exchange of private/non-tradable ("existing") warrants it completed on March 28th for publicly-traded ("replacement") warrants having an $0.85 exercise price and a June 19, 2020 expiration.
The above transaction, where exercising private warrants for $0.50, irrespective of their respective, original exercise prices, gained the transactor one common share and one publicly-traded warrant, saw 7.798 million existing securities out of an outstanding amount of 59.861 million, or 13%, tendered. 52.063 million of these existing warrants remain.
Could this filing pave the way for a reopening of the warrant exchange?
Questions for the 1Q16 CC (May 9, 2016)
Of the questions shareholder would hope are answered by Provectus on the company's 1Q16 business update conference call (e.g., this InvestorVillage post), mine include (primarily asked of Provectus' CTO Dr. Eric Wachter, PhD):
Updated below.
For Provectus' pivotal melanoma Phase 3 trial (a registration study), the company achieved consensus (agreement) with the FDA about a defined patient population per National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines (e.g., in-transit melanoma or ME-5, etc.), recognized and appropriate endpoints (e.g., progression-free survival [PFS], complete response, overall survival, etc.), and standard comparators (e.g., systemic chemotherapy, T-Vec).
I believe most folks would agree PV-10 should win the trial, and achieve better results phase-over-phase (solely because patients' lesions would have been treated more with PV-10 from early-stage to late-stage trial).
In trying to understand the company's process of undertaking the global pivotal trial and registration study in advance of any potential update and/or explanation on Provectus' 1Q16 business update conference call on May 10th, I decided to revisit the company's January 2014 press release PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes. Specifically, the company noted {underlined emphasis is mine}:
For approval by the FDA in the U.S., Provectus requires some number of patients (presumably from the U.S.) for the pivotal trial (registration study) to meet -- to document -- the primary endpoint of PFS, and to establish -- to document -- the relationship between, say, complete response and patient-reported-outcomes (i.e., if the disease's tumors go away, so do the symptoms of the disease).
The submission of the company's investigational medicinal product dossier (IMPD) to the European Union (EU) -- see below -- would permit the running of the trial (a bridging study of sorts?) in that geographic region for eventual approval.
A shareholder noted the April 2016 date of guidance provided by Australia's Therapeutics Goods Administration for clinical trials involving unapproved drugs.
The idea of a so-called bridging study is to generate data on pharmacokinetics, efficacy, safety and dosage in geographic populations in order to enable the extrapolation of international clinical trial data to a relevant domestic population (adapted from here).
The pivotal trial is an international one ("an international multicenter, open-label, randomized controlled trial (RCT) of single-agent intralesional PV-10 versus systemic chemotherapy or intralesional oncolytic viral therapy to...") that presumably requires certain geographic steps for each geography in order to gain approval -- approval, that is, once Provectus documents certain things.
Updated 5/9/16): Provectus appears to be using the Clinical Trial Notification (CTN) scheme in Australia for the company's pivotal melanoma Phase 3 trial, which expects to enroll patients at 10 Aussie sites. See the webpage from the TGA entitled Clinical trials at a glance (the image immediately above) dated April 29th.
Multicenter clinical trials, like Provectus' Phase 3, are now being implemented under the National Ethics Application Form (NEAF) system where one trial site serves as the central institutional review board (IRB) review and oversight for all Australia centers. This central site for the company's pivotal trial might be Princess Alexandra Hospital in Brisbane (see the table under Monday (May 2, 2016) below). This centralized approach has advantages (e.g., one IRB to keep informed) and disadvantages (e.g., an additional administrative load for the lead site because it must collect and distribute information from and to all other sites). It would appear the overall effect of the above has been slower study initiation for Provectus' Phase 3 trial than perhaps otherwise expected or anticipated, at least maybe until the lead site is prepared and ready to address the extra administrative load.
Rose Bengal in children (hepatoblastoma, radiopharmaceutical) (May 6, 2016)
Of note:
Provectus Biopharmaceuticals, Inc. v. Dees et al (May 6, 2016)
Updated below.
H/t a shareholder:
I've asked another shareholder to see if they can access additional information on this.
Updated (5/6/16): H/t InvestorVillage poster joeytakasugi: A link to the suit is here.
Nothing to see here folks (May 6, 2016)
Murine model work: "Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1," Chiba et al., Nat Immunol. 2012 Sep;13(9):832-42:
"Regression/CR of lesions in locally advanced melanoma may be an approvable endpoint" (May 5, 2016)
H/t @JSwatercooler:
Keytruda: 'a “substantial mismatch” between the public’s expectations for the so-called “breakthrough drug” and the supporting scientific data' (May 5, 2016)
June 2015: 'Revolutionary' melanoma drug worth $150,000 a year listed on PBS, saving Australian patients thousands
Growth Capital Expo: I sat in on Provectus' interim CEO and COO Peter Culpepper's presentation at Growth Capital Expo 2016 in Las Vegas Wednesday morning (PDT). His slides may be found here. I thought I heard, among other things, in no particular order:
Relevant blog news items below:
In the rough or anecdotal analysis of MD Anderson Phase 3 trial IRB review and approval timeframes above, do we measure time for Provectus' situation at that which elapsed between the filing of the final protocol and IRB approval (i.e., ~13.5 months), or do we somehow adjust and credit the situation for the positive T-Vec AdComm meeting and its subsequent drug approval, and thus measure time from the final protocol's amendment (i.e., <1.5 months)?
Is oncology a moving playing field? The facts and so-called truth probably are all of the above, more, and then some.
Monday (May 2, 2016)
1. Provectus-associated speaker (at the Vatican's April Conference on Regenerative Medicine) Dr. Grant McArthur from Melbourne, Australia's Peter MacCallum Cancer Centre's noted in conference media materials that traditional cancer treatments do not kill enough cells; however, immunotherapy can leverage the immune system's memory to fight cancer. See the video under Steps 1-3 (April 30, 2016) below.
Wikipedia's Adaptive immune system webpage: "Adaptive immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to subsequent encounters with that pathogen." There are different types of memory T cells (e.g., stem, central, effector), among them CD8+ cells. See, for example, "Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells," Klebanoff et al., Proc Natl Acad Sci USA. 2005 Jul 5; 102(27): 9571–9576.
Provectus' plan for its clinical development program (CDP) for melanoma is two-fold: PV-10 as single agent for locally advanced disease, and PV-10 in combination with another agent for widely metastatic disease.
Moffitt Cancer Center has shown the meaningful involvement of CD8+ cells in (a) human work for PV-10 as a single agent, and (b) murine model work when PV-10 is combined with co-inhibitory blockade (i.e., immune checkpoint inhibitors) {bolded and underlined emphasis is mine}:
Some anecdotal case series include (anecdotal because I only provide a few somewhat random/not so random ones here):
After working with this partner/vendor for perhaps a few years, I still do not understand why Eric does not say or disclose the "major research university" is Rockefeller University (of course I am not completely certain it is), and why he does not disclose the investigator/entity within the university doing the work (which I believe to be Dr. James Krueger, MD, PhD's Laboratory for Investigative Dermatology, although I could be wrong). As an aside, the company's July 2016 calendar of events contains a psoriasis conference, 5th Congress of the Psoriasis International Network - Psoriasis 2016.
April blog readership stats (May 1, 2016)
Revised (5/3/16): H/t a shareholder who told me my labels for Current Month and Prior Month were dated 2015.
Steps 1-3 (April 30, 2016)
Updated below, again.
See also Episode 11: Cancer Immunity Cycle, and the original work, "Oncology meets immunology: the cancer-immunity cycle," Chen and Mellman, Immunity. 2013 Jul 25;39(1):1-10. When I asked Dr. Chen where Dr. Mellman and he would introduce or place the category of intralesional (IL) or intratumorally-delivered agents (e.g., Imylgic, Rose Bengal, other oncolytic viruses like NDV, etc.) on the cancer immunity cycle (i.e., what step or steps), he replied step numbers 1 to 3 (purple edits below to the original image are mine):
In 2014 (prior to Moffitt Cancer Center's work presented at SITC 2014), Dr. Chen provided a broad, thoughtful response to questions of mine regarding combination therapy, and co-stimulation and co-inhibition (paraphrasing a portion, with slight editing for better presentation):
Consider:
Provectus as a Vatican conference sponsor:
It would appear the convention for medical journal articles (and abstracts) and poster presentations (and abstracts) is to use the non-proprietary/generic/non-brand name of a drug compound. Proprietary names may be used but, for example, not in the title of an article or poster.
E.g., The New England Journal of Medicine:
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Image source |
10-Q Notes (May 10, 2016)
Updated below, again, once more (for the conference call)
1. Provectus issued a press release today and filed an associated 8-K regarding its 1Q16 financial results, Reports First Quarter 2016 Financial Results, in conjunction with filing its 10-Q. I plan to update this blog news item frequently as I further review and analyze the filing, and eventually the transcript of the 1Q16 business update conference call held today.
2. 3/31/16 cash and cash equivalents were $9.8 million, compared to a 12/31/15 amount of $14.2 million. Cash at 4/30/16 was approximately $8 million.
3. The company's monthly cash burn for the quarter appears to have been $2.7 million, +62% quarter-over-quarter (QoQ) (the prior quarter-over-quarter change was +18%).
- Adjusting for the expense to Provectus of the class action lawsuit settlement (i.e., moving money into escrow) of $1.85 million, and carefully noting (but not necessarily assuming these are one-offs) the increased investor and public relations ($800K) and legal costs associated with the audit committee’s investigation ($450K), monthly burn would have been $1.65 million, essentially flat QoQ.
5. Language regarding runway remain unchanged from the prior quarterly filing: "into 2017."
6. Research and development expense for the quarter was $2.4 million, -34% QoQ (prior QoQ: +36%).
7. Lab supplies and pharmaceutical preparations expense for the quarter was $18K, -5% QoQ (prior QoQ: -96%).
8. Note under Other Regulatory Matters (p. 11): "We have received a subpoena from the staff of the Securities and Exchange Commission related to the travel expense advancements and reimbursements received by H. Craig Dees, our former Chairman and Chief Executive Officer."
Updated (5/10/16): As of this writing, the transcript of the call is not yet available. I have two speculations. First, I speculate the lead investigator of the European portion of the pivotal melanoma Phase 3 trial is (is going to be) Dr. Axel Hauschild, MD, PhD, Professor and Head of the Interdisciplinary Skin Cancer Center at the Department of Dermatology, University Hospital Schleswig-Holstein in Kiel, Germany. Hauschild also is one of the two course directors of the Sixth Post-Chicago Meeting on Melanoma/Skin Cancer (June 30-July 1, 2016 in Munich, Germany), and to which Provectus COO and interim CEO made reference on today's conference call.
Second, I speculate PV-10 plus radiotherapy data could be presented at ASCO 2016.
Updated (5/11/16): The conference call transcript is now available (from Seeking Alpha). I'd like to organize my thoughts on it in the following sequence or manner:
- Pivotal melanoma Phase 3 trial of PV-10,
- Melanoma Phase 1b/2 study of PV-10 and pembrolizumab,
- Moffitt Cancer Center work,
- Compassionate use/expanded access program,
- The possibility or potential of drug approval in Australia,
- Liver cancer clinical development, and
- PH-10.
I thought the message was substantive, having several high points and a few low points, but was delivered noticeably weakly, again, in places. Underlined emphasis below is mine.
Pivotal melanoma Phase 3 trial of PV-10. Provectus' CTO Dr. Eric Wachter, PhD noted a 6-month delay:
"I must unfortunately report that we are behind with regard to our initial schedule for reaching the interim and final analysis triggers for the study. I’m confident that we are implementing the proper study to support licensure of PV-10, but our present estimate that we are approximately six months behind schedule with regard to site start-up, patient recruitment and eventual data read-out."Prior guidance had been an estimated primary completion date (per ClinicalTrials.gov) of September 2017, and an interim assessment of safety and efficacy of June 2016 (halfway between an April 2015 study start date and the estimated completion date). Adding six months, purely on the basis of math, would push the interim and complete data read-outs to December 2016 and February 2018, respectively.
Eric's plan to address being behind schedule was:
"First, we’ve enlisted the support of a very prominent clinical investigator in Germany to lead the European portion of our study. He joins similarly selective leads for each of the geographic regions, North America, Oceana, Brazil and China that the study stands. Second, we’ve been working with our lead site in Australia to assure that it can fulfill a nationwide regulatory role under the National Ethics Application System, or NEAS, as additional Australian sites join the study. Third, we’ve begun exploring expansion of our study to additional regions including Argentina, a country with demographics and melanoma incidents similar to the U.S. And four, we’ve begun streamlining clinical operations to show that human and capital resources are focused on our core mission."I remain frustrated and confused about Eric not being able to bring himself to name folks or institutions he is working with while saying he is working with them. As I noted in the prior update above, I believe he is referring to Dr. Axel Hauschild, MD, PhD, Professor and Head of the Interdisciplinary Skin Cancer Center at the Department of Dermatology, University Hospital Schleswig-Holstein in Kiel, Germany.
I thought it was helpful for Eric to contextualize the significance of and significant changes in the amendment to Phase 3 trial protocol that (i) added intralesional (IL) talimogene laherparepvec (T-Vec, Imlygic) as another comparator, (ii) extended eligibility to include Stage IV M1a patients with no active nodal or distant metastatic disease, (iii) clarified eligibility for patients not having access to immune checkpoint inhibitors, (iv) clarified eligibility for patients not having access to targeted therapy, (v) extended eligibility to patients failing targeted therapy, and (vi) relaxed crossover (to the PV-10 treatment arm) eligibility criteria:
"So we did in effect reset the clock on a number of sites with that major protocol upgrade...it really was a substantial change that necessitated renegotiation of certain aspects of contracts, IRB approvals and we’re about to -- we’re looking forward."Peter's takes on the delayed timing of interim data read-out the included:
"This year is shaping up to be a year of significant change for Provectus. Among other things, we expect to have interim data from our phase 3 study of PV-10 as a treatment for locally advanced cutaneous melanoma."
"We were projecting mid-year previously and now that would occur mid end of the year or early next year with current projections."
"It is conceivable that interim data will be available prior to one of our upcoming conference calls and if so, it is possible that we will hold a special update call, separate from the filing related call." Upcoming business update conference calls would be August for 2Q16 results, November for 3Q16 results, and March 2017 for 4Q16/CY16 results.The number of patients required to show (document) statistically significant progression-free survival curve separation should be much lower than the number of patients for 50% of the events required for the primary endpoint to have occurred (i.e., 40-80 vs. 112.5). Unfortunately, management's track record with respect to timing is far from certain, and uncertainty is bad for institutional investors (it's not good of course for investors of all stripes).
Melanoma Phase 1b/2 study of PV-10 and pembrolizumab. First, enrollment of up to 24 patients in the Phase 1b portion may be completed this year:
Eric: "Turning to our combination study, we began enrollments in the fourth quarter of 2015 and continue to expect enrollment in this portion of the study will be completed this year. We’ve been working with the sites listed on clinicaltrials.gov to each one open for enrollment of patients and working on several additional sites in U.S. and Australia, still with a goal of seven sites participate in Phase 1b. We’ve also been working with our global CRO to prepare for smooth transition of the study to Phase II and expansion of study to include sites in Europe when that phase commences. We’ll hold an investigator meeting in Germany to gather input as we have multiple times with our Phase 3 study to allow us to assure that the study addresses needs across all regions when it expands to Phase 2."Second, data, likely safety, PFS (primary endpoint) and objective response (secondary, along with overall survival) may be presented this year:
Provectus' COO and interim CEO Peter Culpepper: This year is shaping up to be a year of significant change for Provectus...Key initial data from our phase 1b/2 trial of PV-10 in combination with pembrolizumab for metastatic melanoma’ expand our clinical program in hepatic cancers’ and advance our interactions with prospective corporate partners.
Eric: "Turning to our combination study, we began enrollments in the fourth quarter of 2015 and continue to expect enrollment in this portion of the study will be completed this year...The mechanism data on PV-10 both non-clinical and clinical including that reported by our collaborators from Moffitt Cancer Center last month at the AACR Annual Meeting, suggest that this should be the case. But of course, we can’t be sure until we see initial data expected later this year."From a timing perspective, the two remaining major medical conference venues for potential presentation would be SMR (Society for Melanoma Research) (November 6th to 9th) and SITC (9th to 13th).
Third, and unrelated to the conference call, Provectus updated its Phase 1b/2 study on ClinicalTrials.gov on May 9th to include principal investigator Dr. Victoria Atkinson, MD of Australia's Princess Alexandra Hospital and Gallipoli Medical Research Foundation.
 |
| Click to enlarge. Image source |
Moffitt Cancer Center work. First, I believe Moffitt's peer-reviewed publication of their work on PV-10's mechanism of action should be available prior to ASCO, maybe as early as this week. Peter referred to Moffitt several times on the call:
"While I am on the subject of the mechanism of action, let me provide some insight on developments there. Dr. Shari Pilon-Thomas of the Moffitt Cancer Center recently presented some of her team’s non-clinical research at the American Association for Cancer Research, AACR, Annual Meeting 2016 in New Orleans. She said, “Our results show that combining intralesional PV-10 with anti-PD-1 co-inhibitory blockade not only suppresses tumor growth vs. either agent alone but also yields marked increases in tumor-specific T cell activation against injected tumor.” This helps illustrate the potential value of PV-10 in combination therapy, constructs and may delineate possible new strategies that could harness additional targets in T-cell signaling. The work on the mechanism of action continues, but I firmly believe the important point to investors to note is that there is an identifiable mechanism for PV-10 already characterized, in that, PV-10 has potential growth as a standalone treatment and as all other combination treatment."
"In addition, we recently attended The American Association for Cancer Research Annual Meeting, where we supported the Moffitt Cancer Center in unveiling the research on PV-10 in combination therapy via poster presentation. We remain committed to building these valuable relationships and ultimately securing more news coverage to continue telling the powerful story of Provectus and the implications of PV-10. We expect heightened interest in PV-10 and Provectus to continue this month as we close in on ASCO in Chicago in our publicly known presentation on June 4th, a peer reviewed new publication is anticipated and other evidences of progress through data communication such as the two recent effects Compassionate Use data from two separate sites in Australia."Second, while one key aspect of Moffitt's work on PV-10 has been on its mechanism (and role as a single agent), the other (or another) key aspect has been and is the combination of PV-10 with other therapeutic compounds, like anti-CTLA-4, anti-PD-1, anti-PD-L1 and targeted therapies.
Eric: "Turning to our combination study, we began enrollments in the fourth quarter of 2015 and continue to expect enrollment in this portion of the study will be completed this year. We’ve been working with the sites listed on clinicaltrials.gov to each one open for enrollment of patients and working on several additional sites in U.S. and Australia, still with a goal of seven sites participate in Phase 1b. We’ve also been working with our global CRO to prepare for smooth transition of the study to Phase II and expansion of study to include sites in Europe when that phase commences. We’ll hold an investigator meeting in Germany to gather input as we have multiple times with our Phase 3 study to allow us to assure that the study addresses needs across all regions when it expands to Phase 2.
The study was designed to demonstrate the potential benefits of combining the ablated immunotherapy with an immune checkpoint inhibitor and if successful, should pave the way for potential combination with many other agents. The mechanism data on PV-10 both non-clinical and clinical including that reported by our collaborators from Moffitt Cancer Center last month at the AARC Annual Meeting, suggest that this should be the case. But of course, we can’t be sure until we see initial data expected later this year."
Eric: "Certainly for PV-10 the path forward looks really favorable to me for combination with multiple immunotherapies.
We’re looking as the first examples with combining with anti-PD-1 antibodies in the form of pembrolizumab or Keytruda. As Pete mentioned, we could equally contemplate using another anti-PD-1 Opdivo. There are anti-PD-L-1 drugs on the verge of approval. We’ve exchanged from mechanisms of action studies that PV-10 may work very well with PD-L-1 drugs and then we have data reported at AACR last month by a group at Moffitt showing that there are even other classes of immunotherapies that PV-10 may be compatible with. So that being said, with both compounds we’re trying to establish check boxes with each of those items that would be enumerated by the Pfizer executive that you mentioned."I don't see why Eric cannot appropriately discuss or expand upon what other classes of immunotherapies PV-10 may be compatible with. This "dancing around the edges" or "dropping breadcrumbs," or at worst being obtuse, is unnecessary, much like saying the company is working with "a very prominent clinical investigator in Germany" for Provectus' pivotal melanoma Phase 3 trial and then refusing to name said person.
Compassionate use/expanded access program. Provectus said it would close down it's 7-year expanded access or compassionate use program (CUP) that offered PV-10 patients in the U.S. and Australia for cutaneous or subcutaneous tumors. Designed for 115 patients but having an initial target of 25-30, according to Eric, it treated 160 patients through the end of 2015, and an undisclosed number year-to-date in 2016:
"As an example of the last item, after very careful consideration we have recently announced to our clinical investigators that we will be winding down our expanded access program for PV-10 during the remainder of 2016. Our expanded access protocol was opened in May 2009 to provide access to PV-10 for cancer patients with cutaneous or sub-cutaneous lesions who are not eligible for another PV-10 trial. At that time, we were completing our Phase II study and beginning design of what would become the current Phase III study. In the interval between then and now, we commenced the Phase III study, open to patients with Stage 3 melanoma and started our Phase 1b/2 combination study open to melanoma patients with Stage 4 disease. While these studies were in the formative state, the expanded access protocol was expanded from an initial target of 25 to 30 patients to eventually provide for enrollment of up to approximately 115 patients.
As of the end of 2015, the expanded access protocol had accrued 160 patients in the U.S. and Australia with more accrued to-date. We are required to closely track all patients receiving PV-10 under all protocols to meet global reporting obligations with regard to document in use and safety of our investigational product. Since we are now in Phase III, this tracking requires markedly greater diligence. We initiated the expanded access protocol and needs not addressed within the context of formal clinical trials. Now that we have two active trials underway opened to a substantial fraction of Stage 3 and 4 melanoma patients and have reached the accrual target for the expanded access protocol, it is necessary and appropriate that we wrap up enrollment under the EA-O2 protocol.
To minimize disruption, we will continue to allow enrollment of new patients until the end of June and will continue to supply PV-10 until at least the end of December for patients commencing PV-10 on or before our June update. We appreciate the enthusiasm our investigators have shown for PV-10 under this program, and I’m sure you will appreciate that it is of most importance that use is conductive within the context of clinical trials needed to support approval of PV-10 in the USA, Australia and the rest of the world. There will be trials such as our Phase 3 melanoma trial and our Phase 1b/2 combination therapy trial."Updated (5/12/16): The possibility or potential of drug approval in Australia. Eric said on the last business update conference call on March 16th:
"In regard to the TGA, yes, definitely moving forward with approval in Australia is something that we have been considering, we are currently considering, and we will continue to consider, especially given that roughly half of the melanoma patients that received PV-10 throughout the history of our development have been from Australia. So--and we have a significant base of data now on patients in Australia."On this call, he said in regards to regulatory approval, it would seem:
"Okay, Pete mentioned that early accelerated or expedited, we can use all sorts of terms, may be in a big fashion processes for approval in Australia, we are assessing, we’ve been assessing them since inception of our clinical work in Australia. One of the aspects of closing the expanded access protocol is that we now have an opportunity to collate all of the data from that process to be able to actually use it for at least supportive purposes if not better higher purposes in terms of regulatory approvals. So we’ll be continuing to review that opportunity in Australia on an ongoing basis. And as the case dictates, we may elect to move forward in Australia on a regulatory stance that’s faster or slower than in the U.S."
"So there was a meeting of the Royal Australian College of Surgery last week in Brisbane, where there was a session on melanoma and two of our expanded access investigators presented summaries of their history of using PV-10 in their practice. In the one case, the patients were exclusively under the expanded access protocol and in the second case about half of the patients were from the Phase II study, they called the patient records for all of those patients and analyzed what happened to patients in their wake of having PV-10. Both abstracts – I’ve seen the – presentation as of now, but the abstracts showed remarkably similar story that is quite consistent with what we’ve reported in definitive journal article on the Phase II study. So I think that it represents something of a real world case, what could be expected of PV-10.
With that being said, it is a limited patient population in both cases certainly in an adequate number of patients to support regulatory filing and most of the work was done in the context of expanded access protocol. So the details in terms of the data from these treatments are not of the same level that we would have say for example pivotal study. The reference to standard of care comes from some information that one of the investigators with regard to describing their practice and how PV-10 has been used to address the needs of patients in their practice. And I think it’s a very loose use of term standard of care. Standard of care comes from typically from a very formal decision among a group of experts. I referenced in my scripted comments at the National Comprehensive Cancer Network which defines standard of care in the U.S. and this is a panel of 30 or 40 very prominent oncologists that get together, meet regularly and determine how to treat cancer in a standard fashion.
So they define standard of care. But that being said, there are less formal standards of care and that example from Australia was representative of practice or an institutional standard of care. That does not confer that this drug is widely available, in fact it is available outside the context of that expand access protocol and we’ll have some discussion presumably in the coming months with that particular group on how to address the needs for the standard of care."Liver cancer clinical development. Eric said several things of note, I believe:
"For tumors of the liver, we have continued to add patients to our Phase I study of hepatocellular carcinoma and metastases to the liver and have been actively engaged with the investigator community throughout Asia to expand this program to this important region."
"The problem with that design in the west is finding clinical trial participants that are appropriate for -- sorafenib and PV-10 is relatively rare when we go to parts of world where there are 10 times or 100 times as many patients with this disease then it becomes easier to connect those dots. It is anticipated that we will start our work with dedicated study with PV-10 plus sorafenib in Asia either this year or early next year."Peter added:
"As part of these commercialization considerations, we continue to work with our partners in China, both Boehringer as I mentioned with the HCC phase1b/2 study design and execution and with Sinopharm. We believe China-based partners are crucial for success in China and we believe that our interim data are ready, things in China can move very quickly because of the groundwork we’ve done there. India and Brazil remain areas of interest for us and we continue to develop ties in both of those markets. Based on the recent trip Eric and I made to Mumbai, we believe we have potential partners not just in India but also the Indian subcontinents in MENAT as well. In each of these markets in the U.S. and – as well, protecting our intellectual property is vital to our success."In regards to PH-10, Eric said:
"...we’ve also been busy on the mechanism of action for topical PH-10 and completed clinical work for our mechanism of action study in psoriasis patients in December. We’re finalizing compilation of clinical data from the study with immunohistopathologic analysis of tissue collected from study participants now also complete. And we are reviewing a data together to assess changes in skin biopsies collected pre- and post-PH-10. The study was designed to allow us to probe possible immunologic, structural, and hyperproliferative changes in psoriatic plaque and detect any evidence of cellular atypia upon application of PH-10. It will also allow us to assess concordance of any such changes with clinical observations in those plaques.
We continue to expect these data to inform decisions on an anticipated request to meet with FDA to assess strategies for advancing the program from Phase II into Phase III including whether any additional clinical or non-clinical safety data are necessary to advance to Phase III."
"We expect to have analysis of PH-10 data set completed this quarter. So by the end of June we haven’t determined yet when and how that will be reported, presumably try to report that in some sort of conference fashion in the second half of the year as is our typically with the case for these type of data leading if it looks favorable publication. I’ve only seen portions of the data as stated in previous conference calls are expected to be quite interesting. What I’ve seen looks quite interesting, but until we’ve done a full analysis, we can’t make any quantitative statements.""Delay" may not be a bad thing (May 9, 2016)
Updated below.
For the sixth time the anticipated completion date (and anticipated last follow-up date) of Moffitt Cancer Center's PV-10 mechanism of action work, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study, has been pushed out -- now to December 2016 (the change was made on ClinicalTrials.gov on May 6th). The original completion (last follow-up) date was March 2014, or about 14 months from the opening of recruitment. A current completion date of December 2016 would mean an approximately 48-month study period, or nearly 3.5 times longer than initially contemplated.
A summary record of the above changes are provided in the table below:
 |
| Click to enlarge. |
Recall that in 2014 Moffitt, after enrolling and treating 7 patients for their study, PV-10-treated (and possibly untreated) tumors went away too quickly -- faster than the study protocol's allotted 7-14 days before tumor biopsies were to be taken from patients {underlined emphasis is mine}
'Dr. Pilon-Thomas of Moffitt stated, “These data are exciting and illustrate successful translation of our pre-clinical work in mice to clinical results in melanoma patients. With only 8 patients we've been able to clearly observe statistically significant increases in beneficial T cell populations in peripheral blood. Ironically, the original aim of the trial to assess tumor-infiltrating lymphocytes was thwarted when biopsies of patient tumors collected just 7-14 days after PV-10 injection no longer contained viable tumor tissue. We are following up both the human data and continuing to design more experiments in mice to better explain the systemic immune effects elicited by PV-10 ablation.”' (Source: Provectus April 2014 press release)Perhaps the simplest answer to the question above is that Moffitt's study is part of a translational research program on PV-10. As Moffitt reported at AACR in April, T cell Mediated Immunity After Combination Therapy with Intralesional PV-10 and Co-Inhibitory Blockade in a Melanoma Model, and SITC in November 2015, Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1, they learned new features of the immunologic signaling that occur upon PV-10 tumor ablation (i.e., what happens to the cancer tumor or lesion after it is injected with PV-10).
Moffitt showed new data from clinical study specimens at SITC (i.e., HMGB1 in human plasma) that corroborated observations from their ongoing non-clinical work. Final reporting of study data — that is, completing the clinical study report, which is the formal end of the study — may have been delayed as new things that were learned from mice were then tested in the human samples from the study.
Updated (5/10/16): A tweet reply by @johnhallnj was a reminder to note in this blog news item that Moffitt's feasibility study above was fully enrolled in 2014, perhaps by April of that year. The enrollment figure of Moffitt's study was 15. See "...it’s a bona fide immunological response" (April 17, 2014) on the blog's Archived News I page, which references an April 2014 article by ecancer reporter Janet Fricker entitled "PV-10 decreases melanoma cells in tumours" {underlined emphasis and bolded verbiage below is mine}:
"The investigators presented data on biopsies sampled from injected tumours and uninjected bystander tumours taken from eight melanoma patients seven to 14 days after PV-10 injection. [n_1 = 8]
The studies showed that immunohistochemical staining of biopsy specimens for mel A (an immunohistochemical marker of melanoma viability) demonstrated the complete disappearance of viable melanoma cells in both the injected and bystander tumours.
The team found that these changes in tumours were accompanied by increased populations of CD3 , CD4 and CD8 T cells along with NKT cells in peripheral blood...
“Ironically, the original aim of the trial to assess tumour-infiltrating lymphocytes was thwarted when biopsies of patient tumours collected just seven to 10 days after PV-10 injection no longer contained viable tumour tissue,” said Pilon-Thomas...
Studies are now underway in an additional seven patients to take biopsies and blood samples at more frequent time intervals after PV-10 injection to elucidate the pathways more clearly." [n_2 = 7; N = n_1 + n_2 = 8 + 7 = 15]New SEC filing (May 9, 2016)
Provectus filed a Form POS AM (Post-effective amendment to a registration statement that is not immediately effective upon filing) today, related to the warrant exchange of private/non-tradable ("existing") warrants it completed on March 28th for publicly-traded ("replacement") warrants having an $0.85 exercise price and a June 19, 2020 expiration.
The above transaction, where exercising private warrants for $0.50, irrespective of their respective, original exercise prices, gained the transactor one common share and one publicly-traded warrant, saw 7.798 million existing securities out of an outstanding amount of 59.861 million, or 13%, tendered. 52.063 million of these existing warrants remain.
Could this filing pave the way for a reopening of the warrant exchange?
Questions for the 1Q16 CC (May 9, 2016)
Of the questions shareholder would hope are answered by Provectus on the company's 1Q16 business update conference call (e.g., this InvestorVillage post), mine include (primarily asked of Provectus' CTO Dr. Eric Wachter, PhD):
- What non-pivotal clinical studies have you undertaken and/or completed in support of PV-10's new drug application (NDA) as a single agent for locally advanced cutaneous melanoma (i.e., Human Pharmacokinetics and Bioavailability)?
- How does the clinical study Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma, conducted or being conducted at such places as Moffitt Cancer Center, Gabrail Cancer Center, and Huntsman Cancer Institute, fit into PV-10's NDA as a single agent for locally advanced cutaneous melanoma?
- Do you expect clinical data of [the Australian investigator-initiated study* of] the combination of PV-10 and radiotherapy to be presented at ASCO 2016? *This study may be entitled Intralesional (IL) PV-10 and hypofractionated radiotherapy in patients with recurrent and or metastatic malignant melanoma or A Phase I/II study of intralesional PV-10 followed by radiotherapy in the treatment of metastatic melanoma.
- When do you expect information from the recent Royal Australasian College of Surgeons (RACS) conference's Princess Alexandra Hospital, single-center experience** with PV-10 for patients with cutaneous melanoma metastases to be made available -- since Provectus (Eric) was named on the abstract and poster's co-author line? ** This work was entitled Intralesional PV-10 Chemoablation Therapy for the Treatment of Cutaneous Melanoma Metastases - Results of a Prospective, Non-Randomised, Single Centre Study.
Updated below.
For Provectus' pivotal melanoma Phase 3 trial (a registration study), the company achieved consensus (agreement) with the FDA about a defined patient population per National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines (e.g., in-transit melanoma or ME-5, etc.), recognized and appropriate endpoints (e.g., progression-free survival [PFS], complete response, overall survival, etc.), and standard comparators (e.g., systemic chemotherapy, T-Vec).
I believe most folks would agree PV-10 should win the trial, and achieve better results phase-over-phase (solely because patients' lesions would have been treated more with PV-10 from early-stage to late-stage trial).
 |
| Click to enlarge. |
"The Agency may yet recommend and it may be in the best interest of Provectus to undertake a small, short bridging study in patients where all tumor burden can be injected. This would allow more frequent dosing than was permitted in the Phase 2 study, presumably akin to the dosing schedule currently used to treat nearly 100 patients under our expanded access protocol, and allow symptomatic endpoints to be prospectively correlated with objective response criteria...If such a study is conducted, it also fits with needs for an international study supportive of licensure in Australia, Europe, China and India."Later, consider the comments from Provectus' June 19, 2014 conference call:
 |
| Click to enlarge. |
The submission of the company's investigational medicinal product dossier (IMPD) to the European Union (EU) -- see below -- would permit the running of the trial (a bridging study of sorts?) in that geographic region for eventual approval.
A shareholder noted the April 2016 date of guidance provided by Australia's Therapeutics Goods Administration for clinical trials involving unapproved drugs.
 |
| Click to enlarge. Image source |
The pivotal trial is an international one ("an international multicenter, open-label, randomized controlled trial (RCT) of single-agent intralesional PV-10 versus systemic chemotherapy or intralesional oncolytic viral therapy to...") that presumably requires certain geographic steps for each geography in order to gain approval -- approval, that is, once Provectus documents certain things.
Updated 5/9/16): Provectus appears to be using the Clinical Trial Notification (CTN) scheme in Australia for the company's pivotal melanoma Phase 3 trial, which expects to enroll patients at 10 Aussie sites. See the webpage from the TGA entitled Clinical trials at a glance (the image immediately above) dated April 29th.
Multicenter clinical trials, like Provectus' Phase 3, are now being implemented under the National Ethics Application Form (NEAF) system where one trial site serves as the central institutional review board (IRB) review and oversight for all Australia centers. This central site for the company's pivotal trial might be Princess Alexandra Hospital in Brisbane (see the table under Monday (May 2, 2016) below). This centralized approach has advantages (e.g., one IRB to keep informed) and disadvantages (e.g., an additional administrative load for the lead site because it must collect and distribute information from and to all other sites). It would appear the overall effect of the above has been slower study initiation for Provectus' Phase 3 trial than perhaps otherwise expected or anticipated, at least maybe until the lead site is prepared and ready to address the extra administrative load.
Rose Bengal in children (hepatoblastoma, radiopharmaceutical) (May 6, 2016)
 |
| Click to enlarge. Image source |
- 6 patients: 5 boys and 1 girl, and
- Discussion: "It is concluded that, if conventional therapy of hepatoblastoma has failed and liver transplantation is not feasible, it seems worthwhile to consider 131I-rose bengal as a potential radiopharmaceutical for tumour targeted radiation therapy, in the hope of making the tumour resectable." {bolded and underlined emphasis is mine}
Starting in 1961, 131-I Rose Bengal routinely was used to diagnose neonatal jaundice. Since the compound was excreted via bile, this was a simple way to determine whether jaundice was due to bile duct abnormality. Safety standards today of course are not what they were in 1961, but the topic of safety, and the potential safety of Rose Bengal as a therapeutic for both adults and children, certainly cast the article above in the proper context.
Provectus Biopharmaceuticals, Inc. v. Dees et al (May 6, 2016)
Updated below.
H/t a shareholder:
 |
| Click to enlarge. Image source |
Updated (5/6/16): H/t InvestorVillage poster joeytakasugi: A link to the suit is here.
Nothing to see here folks (May 6, 2016)
 |
| Click to enlarge. Tweet image source |
 |
| Image source |
"The mechanisms by which tumor microenvironments modulate nucleic acid-mediated innate immunity remain unknown. Here we identify the receptor TIM-3 as key in circumventing the stimulatory effects of nucleic acids in tumor immunity. Tumor-associated dendritic cells (DCs) in mouse tumors and patients with cancer had high expression of TIM-3. DC-derived TIM-3 suppressed innate immune responses through the recognition of nucleic acids by Toll-like receptors and cytosolic sensors via a galectin-9-independent mechanism. In contrast, TIM-3 interacted with the alarmin HMGB1 to interfere with the recruitment of nucleic acids into DC endosomes and attenuated the therapeutic efficacy of DNA vaccination and chemotherapy by diminishing the immunogenicity of nucleic acids released from dying tumor cells. Our findings define a mechanism whereby tumor microenvironments suppress antitumor immunity mediated by nucleic acids."Human work: "Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1," Liu et al., Journal for Immunotherapy of Cancer 2015, 3(Suppl 2):P408, November 2015:
"Intralesional (IL) therapy is under investigation to treat dermal and subcutaneous metastatic cancer. Rose Bengal (RB) is a staining agent that was originally used by ophthalmologists and in liver function studies. Previously, IL injection of RB induced regression of injected and uninjected tumors in murine models. However, the relevant mechanism is yet unknown. In this study, we used an OVA-expressing B16 melanoma murine model and found that IL RB treatment led to increased tumorspecific T cells with memory characteristics. IL RB therapy also increased antigen-specific T cell proliferation and enhanced tumor regression. In addition, IL RB facilitated dendritic cells (DCs) infiltrating lymph nodes draining from tumor. Incubation of melanoma cells with RB led to necrosis and the release of High Mobility Group Box 1 (HMGB1), which activated DCs. The blockade of HMGB1 significantly reduced the antigen-presenting ability of DCs. To determine whether this mechanism was relevant in patients treated with IL RB, we performed a pilot clinical study in melanoma patients (NCT01760499). IL RB led to tumor regression in both RB-injected and uninjected lesions, associated with an increase in circulating T cells. Increased tumor-specific response was found from those circulating T cells of 5 out of 7 tested patients after IL RB treatment. HMGB1 levels in patient sera were also elevated. Together, these results reveal a clinically relevant immunoadjuvant pathway triggered by tumor cell death secondary to ablation with RB."And, I laugh...
"Regression/CR of lesions in locally advanced melanoma may be an approvable endpoint" (May 5, 2016)
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source above |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
June 2015: 'Revolutionary' melanoma drug worth $150,000 a year listed on PBS, saving Australian patients thousands
'Oncologist Professor Grant McArthur, from the Peter MacCallum Cancer Centre, said Australia had the highest rates of melanoma in the world, and added that the drug was "revolutionary".
"Australians will be amongst the first in the world to get access to this new treatment," he said.
"The listing of Keytruda on the PBS shows us what can happen when everyone works together to get these drugs listed quickly - so that's patients, industry, clinicians, researchers and government - if we work together we can speed up access to cancer drugs."'April 2016: Melanoma drug Keytruda denied full listing on PBS
'A much-hyped melanoma drug credited with saving the life of businessman Ron Walker has been denied a full listing on the Pharmaceutical Benefits Scheme after an advisory group ruled there was not enough evidence to justify taxpayers footing the bill.
The drug, Keytruda, which can cost about $120,000 a year unsubsidised, has been available at a reduced price on the government-funded PBS for the past seven months.
However, conditions of a managed entry scheme, including a risk-share arrangement, struck in May last year have meant that drugmaker Merck Sharp & Dohme has been unable to charge full price...
But notes from the PBAC’s March meeting, which have only now been made public, show that the committee opted against lifting the conditions on Keytruda’s availability, which would have enabled Merck to raise the price.
The knock-back comes less than a year after the PBAC flagged concerns about a “substantial mismatch” between the public’s expectations for the so-called “breakthrough drug” and the supporting scientific data...
In Merck’s latest submission to the PBAC, it asked that new data from a previously submitted clinical trial comparing Keytruda with rival drug Yervoy be assessed, along with a new economic evaluation.
However, the committee ruled that neither were “considered to provide sufficient basis to justify a change in the initial managed entry scheme conditions or risk-share arrangements or an increase in price over that of … (Yervoy)”.
Managed entry schemes typically provide the public with accelerated, subsidised access to urgently needed drugs in cases where there remains some uncertainty about the extent or value of their effect. Instead of footing the entire bill, the government requires a drug’s sponsor to agree to a lower price until such time that it provides further data to support a full PBS listing.May 2016: "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool with an acceptable side effect profile for the management of unresectable in transit and locally recurrent melanoma." See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) below.
Growth Capital Expo: I sat in on Provectus' interim CEO and COO Peter Culpepper's presentation at Growth Capital Expo 2016 in Las Vegas Wednesday morning (PDT). His slides may be found here. I thought I heard, among other things, in no particular order:
- The company's investigational medicinal product dossier (IMPD) had been completed, and may have been submitted to the European Union (EU),
- PV-10 is an ablative immunotherapy, with a "chemical" reference to ablative compared with a "virus" reference to oncolytic in oncolytic immunotherapy (e.g., T-Vec/Imylgic, Cavatak, etc.),
- One needs to destroy the tumor specifically and enough in order to sufficiently alert (signal) the immune system, and
- There is evidence of immunological signalling from local liver tumor injections (as previously has been shown that there is immunological signalling from local melanoma lesion or tumor injections).
Relevant blog news items below:
- Updated Clinical Trial Information (March 28, 2016), and
- IRB review & approval (February 17, 2016).
MD Anderson's Institutional Review Board (IRB) review and approval dates for Provectus' PV-10 Intralesional Injection vs Systemic Chemotherapy or Intralesional Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma without Distant Metastases (Study #2015-0773) and A Phase 1b/2 Study of PV-10 Intralesional Injection in Combination with Systemic Immune Checkpoint Inhibition for Treatment of Metastatic Melanoma (Study #2015-0793) was April 26, 2016.
With the above IRB review and approval date for MD Anderson now in hand, I explored the "first received date" on ClinicalTrials.gov for 5 and 10 Phase 3 trials at the cancer center (not including Provectus'), compared them with their respective MD Anderson IRB dates, and calculated the number of days (and months) that elapsed between the two dates (i.e, first received, and IRB review and approval). I compared this data set to Provectus' dates: (i) the date the .gov website received Provectus' CTO Dr. Eric Wachter, PhD's initial protocol (11/4/14), (ii) the date the final protocol was received (3/12/15), and (iii) the date the final protocol was amended to include intralesional agent T-Vec and broaden the patient population from melanoma Stage IIIB-C to include Stage IV M1a (3/15/16).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
While it took ~13.5 months for MD Anderson's IRB to review and approve Provectus' final Phase 3 trial protocol (i.e., March 12, 2015 to April 26, 2016), if that is what happened, it took less than one-and-a-half months to approve the company's amended trial protocol (i.e., March 15 to April 26).
In Provectus' annual CEO letter of 2013, the company's CTO Dr. Eric Wachter, PhD noted via contribution to the missive, specifically my underlined portion below:
"Provectus is finalizing details for submission of a pivotal Phase 3 randomized controlled trial ("RCT") of PV-10 for metastatic melanoma, suitable for Special Protocol Assessment ("SPA"), to the Food and Drug Administration ("FDA"). While preparation for submission of our SPA has taken longer than expected, it is crucial to remember that oncology presents a moving playing field. Fine tuning of the study design is expected to mitigate clinical efficacy risk, optimize patient accrual, and increase FDA's confidence that the study design and protocol will ensure the best possible outcome for our pivotal trial. We have every reason to believe this key milestone will be achieved in 2013."In April 2015, prior to the FDA's Joint Meeting of the Cellular, Tissue and Gene Therapies Advisory Committee and Oncologic Drugs Advisory Committee (AdComm) for T-Vec, many folks (including the company, I believe) did not believe intralesional agent (IL) T-Vec would advance in the drug approval process. Provectus initiated its pivotal Phase 3 program on April 15th. AdComm members determined on April 29th that tumor reduction was a clinical benefit. T-Vec was approved on October 27th. As such, Eric determined and decided he had to include T-Vec in the Phase 3 trial protocol.
In the rough or anecdotal analysis of MD Anderson Phase 3 trial IRB review and approval timeframes above, do we measure time for Provectus' situation at that which elapsed between the filing of the final protocol and IRB approval (i.e., ~13.5 months), or do we somehow adjust and credit the situation for the positive T-Vec AdComm meeting and its subsequent drug approval, and thus measure time from the final protocol's amendment (i.e., <1.5 months)?
Is oncology a moving playing field? The facts and so-called truth probably are all of the above, more, and then some.
Monday (May 2, 2016)
1. Provectus-associated speaker (at the Vatican's April Conference on Regenerative Medicine) Dr. Grant McArthur from Melbourne, Australia's Peter MacCallum Cancer Centre's noted in conference media materials that traditional cancer treatments do not kill enough cells; however, immunotherapy can leverage the immune system's memory to fight cancer. See the video under Steps 1-3 (April 30, 2016) below.
Wikipedia's Adaptive immune system webpage: "Adaptive immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to subsequent encounters with that pathogen." There are different types of memory T cells (e.g., stem, central, effector), among them CD8+ cells. See, for example, "Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells," Klebanoff et al., Proc Natl Acad Sci USA. 2005 Jul 5; 102(27): 9571–9576.
Provectus' plan for its clinical development program (CDP) for melanoma is two-fold: PV-10 as single agent for locally advanced disease, and PV-10 in combination with another agent for widely metastatic disease.
Moffitt Cancer Center has shown the meaningful involvement of CD8+ cells in (a) human work for PV-10 as a single agent, and (b) murine model work when PV-10 is combined with co-inhibitory blockade (i.e., immune checkpoint inhibitors) {bolded and underlined emphasis is mine}:
- Single agent, AACR 2014: "The Moffitt researchers presented clinical data on 8 melanoma patients that demonstrated significant decreases in melanoma cells in injected tumors and uninjected bystander tumors 7-14 days after PV-10 injection as evidenced by pathologic evaluation confirmed with immunohistochemical staining of biopsy specimens for melA (a marker of melanoma). The researchers showed that these changes in tumors were accompanied by increased populations of CD3+, CD4+ and CD8+ T cells along with NKT cells in peripheral blood. T cells from one patient were purified and exhibited increased interferon-gamma expression when exposed to the patient's pre-treatment melanoma cells." (Source: Provectus press release, April 2014)
- In combination, AACR 2016: "In the poster, authors Amy M Weber, Hao Liu, Krithika Kodumudi, Amod A Sarnaik and Shari Pilon-Thomas state that "treatment with IL PV-10 and anti-PD-1 antibody results in a delay in tumor growth and enhanced T cell activation in the M05 tumor model." They also conclude that "the effect of combination therapy with IL PV-10 and PD-1 blockade is mediated by CD8+ T cells, and depletion of either CD4+ T cells or CD25+ Tregs enhances anti-tumor immunity in the M05 melanoma model." (Source: Provectus PR, April 2016)
3. So, what exactly is going on in Australia? There are at least 8 PV-10-related programs (schemes), studies and trials run in Australia, of which 7 pertain to melanoma; half of these were/are run entirely in the country (3 for melanoma, 1 for [secondary] liver cancer). A very rough guess could be that more than 40% of patients treated with PV-10 for melanoma came or come from Australia.
 |
| Click to enlarge. |
Currently, one Australia medical center, Princess Alexandra is listed on ClinicalTrials.gov as a participant in Provectus' pivotal melanoma Phase 3 trial, but is "not yet recruiting" as of this writing -- more than 6 months after it was added (in October 2015). No other Australian sites are listed -- more than a year after the trial program was initiated (in April 2015), and about 6 weeks after the protocol was amended (in March 2016 to broaden the trials' patient population and to add oncolytic virus T-Vec as an investigator's choice comparator).
4. Should PV-10 "win" it's pivotal melanoma Phase 3 trial by meeting its primary endpoint, and its various secondary endpoints, would it eventually have the opportunity to become a first-line treatment option for its applicable/appropriate patient population? T-Vec is a treatment option for the following NCCN melanoma guideline categories or patient populations. It's unlikely PV-10, based on the design of its Phase 3 trial would be appropriate for distant metastatic disease (ME-10); however, is Stage III (ME-4) open or more open?
 |
| Click to enlarge. |
 |
| Click to enlarge. |
5. As noted below under "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016), Princess Alexandra is presenting an abstract about the center's experience treating melanoma patients in Provectus' Phase 2 trial and as part of a compassionate use program (special access theme in Australia) there:
"The results of this investigation...form the basis of the world’s largest single centre case series...With the incorporation of phenotypic subgroup considerations, this strategy facilitates appropriate patient selection in an evidence-based manner."
What is a case series? According to the National Cancer Institute:
 |
| Click to enlarge. |
See, for example, "Improving the quality of therapeutic reports of single cases and case series in oncology--criteria and checklist," Kienle et al., Altern Ther Health Med. 2004 Sep-Oct;10(5):68-72:
"Single cases, case series and retrospective reviews are usually the only possibility for physicians and therapists to conduct their own clinical research, to communicate their therapeutic experiences to the medical community and thus contribute to the body of scientific knowledge. Reports of single cases and case series can preserve and disseminate the knowledge of successful, ingenious and passionate therapists, which has been the main source for dramatic progress of clinical innovations and effective therapies in medical history. At their best, high quality reports of single cases and case series represent the art of cultivating therapeutic experiences and clinical judgment. Although single cases and case series are graded low in evidence-based medicine (and often not considered at all), even in conventional medicine spectacular and well-presented case series, eg, on tumor remissions following an experimental therapy can arouse tremendous public attention."The Princess Alexandra Hospital work explored several clinical endpoints like complete clinical response of treated lesions (determined using RECIST), overall response (OR), clinical benefit, time-to-best response, disease-free survival (DFS) and overall survival (OS).
Some anecdotal case series include (anecdotal because I only provide a few somewhat random/not so random ones here):
- "The Results of Pancreatic Resections and Long-Term Survival for Pancreatic Ductal Adenocarcinoma: A Single-Institution Experience" (April 2016) at Helsinki University Hospital
 |
| Click to enlarge. |
- "Adenocarcinomas of the sinonasal tract: a case series from an Oncology Centre in Northern Portugal" (September 2014/August 2015)
 |
| Click to enlarge. |
- "Atypical Metastatic Presentations in Colorectal Cancer: A Case Series" (Nove,ber 2013/March 2014)
 |
| Click to enlarge. |
 |
| Click to enlarge. |
While understanding the strengths and weaknesses of case studies (as contrasted with and compared to clinical trials), note that the case studies above observe and comment on overall survival-related measures.
6. On Provectus' March 16th 4Q15/CY15 business update call, Eric answered a question regarding PH-10:
Question: Are we still expecting PH-10 data by the end of the month is my first question.
Eric's reply: As I mentioned in my remarks, we have completed all of the data collection from the patients. We are in the process of compiling the clinical data. So, this is the numerical data that's collected when a patient visits the clinic.
We are working with a major research university on analyzing skin biopsies that were collected three times during the course of the study for each patient; at the beginning of the study pretreatment, after four weeks of application of vehicle, and then four weeks after application of PH-10.
That work is essentially complete. We are in the process of receiving that this week. And I think that it's possible that we will have a preliminary analysis of that completed this month. Certainly if not this month, early into the next month, the month of April.The above noted conference call was held in March. March then turned into April. Now, it is a few days into May.
After working with this partner/vendor for perhaps a few years, I still do not understand why Eric does not say or disclose the "major research university" is Rockefeller University (of course I am not completely certain it is), and why he does not disclose the investigator/entity within the university doing the work (which I believe to be Dr. James Krueger, MD, PhD's Laboratory for Investigative Dermatology, although I could be wrong). As an aside, the company's July 2016 calendar of events contains a psoriasis conference, 5th Congress of the Psoriasis International Network - Psoriasis 2016.
 |
| Click to enlarge. |
Revised (5/3/16): H/t a shareholder who told me my labels for Current Month and Prior Month were dated 2015.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below, again.
See also Episode 11: Cancer Immunity Cycle, and the original work, "Oncology meets immunology: the cancer-immunity cycle," Chen and Mellman, Immunity. 2013 Jul 25;39(1):1-10. When I asked Dr. Chen where Dr. Mellman and he would introduce or place the category of intralesional (IL) or intratumorally-delivered agents (e.g., Imylgic, Rose Bengal, other oncolytic viruses like NDV, etc.) on the cancer immunity cycle (i.e., what step or steps), he replied step numbers 1 to 3 (purple edits below to the original image are mine):
 |
| Click to enlarge. Original image source |
"I believe that for some cancers, the anti-cancer immune response is already present and is just inhibited by PD-L1. For these tumors, one may not need more than a therapy like anti-PD-L1. These can be identified by PD-L1 expression in the tumor microenvironment. For other tumors, the anti-cancer immune response may be weak or nonexistent. These may be best treated with an approach that helps generate an immune response (if this is possible)...The idea is generate the immune response and allow it to do its job."Updated (4/30/16): Moffitt's AACR 2016 poster noted PD-L1 expression.
 |
| Click to enlarge. Image source |
"Tumoral PD-L1 expression status has been shown to be prognostic in multiple tumor types, including melanoma (MEL), renal cell carcinoma (RCC), and non–small-cell lung cancer (NSCLC). In addition, tumoral PD-L1 expression appears to correlate closely with response to anti–PD-1 antibodies." See "Prospects for Targeting PD-1 and PD-L1 in Various Tumor Types" by Drs. Kim and Eder, MD in cancernetwork in November 2014.From the conference on regenerative medicine at the Vatican (April 28-30):
Provectus-associated speaker, Dr. Grant McArthur from Melbourne, Australia's Peter MacCallum Cancer Centre's: "Grant believes that traditional cancer treatments do not kill enough cells. However, immunotherapy can leverage the memory of our immune system in order to fight cancer."
Provectus as a Vatican conference sponsor:
 |
| Click to enlarge. Image source |
Updated (4/30/16): From the conference on regenerative medicine at the Vatican (April 28-30):
With Dr. McArthur sitting next to him (and saying nothing in this snippet), I imagine there is more such media available.
New auditors, Proxy filing (April 29, 2016)
Updated below.
Provectus made an 8-K filing today regarding the company's dismissal of its long-time auditors BDO and the hiring of new auditors Marcum, noting:
The Vatican (April 27, 2016)
Updated below.
Provectus issued a press release and made a related 8-K filing regarding the company's presentation at the Vatican's healthcare conference, to Participate in Panel at Third International Conference on the Progress of Regenerative Medicine and Its Cultural Impact. Presenting on behalf of Provectus is Melbourne, Australia's Peter MacCallum Cancer Centre's Dr. Grant McArthur -- Vatican conference bio is here, and PeterMac's McArthur Translational Research Laboratory description is here. See Il Papa (April 2, 2016) and Ridiculousness (April 7, 2016) below. He is a long-time clinical investigator, and a consultant to the company. Dr. McArthur will be on a panel discussing combination therapies. PeterMac is a PV-10 special access scheme/compassionate use program site in Australia. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) immediately below.
Updated (4/27/16): The panel of which Dr. McArthur is a part is shown below.
"PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016)
Updated below, again, once more.
Takeaways:
There are two PV-10-related abstracts from two different Australian PV-10 expanded access/compassionate use program/special access scheme sites, Peter MacCallum Cancer Centre (PeterMac) in Melbourne and Princess Alexandra Hospital in Brisbane. Both are scheduled for May 3rd.
The abstract for PeterMac's Intralesional PV-10 For In-Transit Melanoma - A Single Centre Experience is below.
Princess Alexandra's abstract Intralesional PV-10 Chemoablation Therapy For The Treatment Of Cutaneous Melanoma Metastases – Results Of A Prospective, Non-Randomised, Single Centre Study is below. I'm going to guess this work, "the world’s largest single centre case series," may form a compassionate use program-focused peer-reviewed publication.
Updated (4/25/16): The PeterMac study achieved locoregional disease control (CR + PR + SD) of 68% and complete response (CR) of 26%. PR is partial response. SD is stable disease. This compares with Provectus' metastatic melanoma Phase 2 trial results of 67% disease control and 24% CR (preliminary full study results, November 2010). In the trial subjects with cutaneous or nodal disease only achieved locoregional disease control of 78%, while subjects with visceral metastases achieved 56% disease control.
Interestingly, the hospital weighed in on treatment cost as well.
The Princess Alexandra study, aretrospective analysis, collected a wide array of interesting endpoints:
A useful and helpful conversation (April 24, 2016)
Updated below, again.
Several weeks ago I communicated with someone who could be a possible CEO candidate. "Possible" because, while (s)he has expressed interest in the position to the company, I do not believe (s)he has interviewed with Provectus' board of directors' CEO search committee yet.
Intelligent, knowledgeable, suitably experienced and reasonably qualified, this individual possesses a PhD in a relevant medical sciences subject area. (S)he has more than 25 years of increasingly senior roles and responsibilities at global pharmaceutical companies (n.b. Top 10, both annual overall and oncology revenues) in business development, corporate development, operations, and strategic planning.
(S)he has an awareness and understanding of PV-10, and is known to both COO and interim CEO Peter Culpepper and CTO Dr. Eric Wachter, PhD. I hope the board would interview this individual to, at the very least:
This individual believes (s)he could maintain continuity at the company with Peter and Eric, energizing the team and them in the process. Cash compensation expectations seemed reasonable-to-high given her or his experience (we did not discuss equity compensation). Her or his drawbacks include:
New [Interim] CFO (April 22, 2016)
Updated below.
Provectus issued an 8-K filing today to announce the hiring of an interim CFO, John R. Glass, CPA, who would replace the CFO role the company's CFO/COO and interim CEO Peter Culpepper held and filled. Glass' bio, from the 8-K:
Everything's up (April 21, 2016)
Updated below.
Understanding that Moffitt Cancer Center's feasibility study (see April 21, 2016 blog post Burning Down The House) and other R&D work on PV-10 has been multi-faceted and dimensional, how much of proving the hypothesis that PV-10 produces a better immune cell infiltrate is the crux of (crucial to) bringing a co-development partner (for combining PV-10 with another drug) to the table?
Current approaches to harnessing the immune system are predicated on affecting it largely at a single point (e.g., CTLA-4, PD-1, PD-L1, OX-40, Tim-3, CD[pick a number], etc.) because we do not understand how to efficiently harness the complexity that resides within the immune system itself. Provectus' emphasis, via Moffitt's R&D efforts, on various recognized touch points such as TILs and CD8+ may be potential triggers for investment. As has been shown multiple times since Moffitt's early work, the impact of tumor ablation with PV-10 affects or has an impact on multiple immunologic signaling pathways (e.g., CD8+, CD4+, CD3+, NK, HMGB1, etc.). Human work includes:
I'm looking forward to reading Moffitt Cancer Center's American Association for Cancer Research ("AACR") annual meeting abstract next month, viewing the associated poster in April, and learning about what if anything they present at the American Society of Clinical Oncology ("ASCO") annual meeting in May/June. I presume they'll present something at ASCO because of Sondak's already scheduled PV-10 presentation at the 4th European Post-Chicago Melanoma/Skin Cancer Meeting. See Moffitt @ ASCO 2014? (February 10, 2014) under the blog's News tab.
There are three topics of their possible work I hope their poster presentation at AACR and any potential presentation(s) at ASCO would elucidate:
While Provectus pursues the initial local path to approval for PV-10 of locally advanced cutaneous melanoma, Moffitt is more interested in PV-10's systemic properties and benefit, and more than likely its application to patients with metastatic melanoma and other late-stage solid tumor cancer indications. Some observations of the above, which was first presented at AACR 2013 and discussed more in the paper:
- First, in murine model work, Moffitt confirmed PV-10 generated an anti-tumor immune response that shrunk or destroyed untreated tumors (red underlined portions 1, 2 and 3 in paragraphs 1 and 2). They observed the systemic potential of PV-10 for both breast cancer and melanoma.
- Second, they hypothesized that large amounts of tumor debris created by the rapid ablation that followed PV-10's injection into tumors, debris containing antigens (thus, large amounts of debris should mean large numbers of antigens), are taken up by dendritic cells ("DCs") (red underlined portion 4 in paragraph 3). DCs, antigen-presenting cells, present (display) antigens they've taken up to the body's immune system's T-cells.
- Third, Moffitt suggested combining PV-10 with other immunotherapies may lead to better results in patients with metastatic melanoma (red underlined portion 5 in paragraph 4).
- Finally, fourth, they demonstrated immunity could be transferred from mice with PV-10 treated tumors to mice with untreated tumors, hypothesizing (I think) that more and better T-cells could be created using PV-10 (better ones) together with ACT (more of these better ones) to then use on more than one patient (red underlined portions 5 and 6 in paragraph 4).
There are T-cells, and then there are T-cells. There are different types of T-cells, each with a distinct function: helper, cytotoxic, memory, regulatory, natural killer, and mucosal associated invariant. "Cytotoxic T cells ("CTLs") destroy virally infected cells and tumor cells." "Regulatory T cells, formerly known as suppressor T cells, are crucial for the maintenance of immunological tolerance." "Natural killer T cells ("NKT cells")...are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses."
CTLA-4 and PD-1 cell receptors are the so-called brakes of the immune system, limiting its response. Taking the brakes off by inhibiting these blockade receptors (with anti-CTLA-4/PD-1 agents) allows for a more active and vigilant immune system. CTLA-4 and PD-1 agents, I think, help out with or activate these regulatory T-cells.
PV-10, on the other hand, I think, helps out with or activates CTLs, by virtue of the drug's rapid ablation causing tumor debris containing antigens to be taken up by DCs that then display them to this type of T-cell. "For long it has been discussed whether ablated tumor debris is able to induce a systemic immune response."
So, while activating regulatory T-cells and CTLs (cytotoxic T-cells) both may result in tumor destruction and shrinkage, regulatory T-cell activation by checkpoint inhibitors is not permanent and eventually resisted or overcome, whereas CTL activation by PV-10 may result in [permanent?] immunity.
"CTLs specific for tumor antigens play a major role in the immunity against cancer." Will Moffitt show PV-10 specifically activates these CTLs?
If the checkpoint inhibitors remove the brakes of the immune system, allowing it to shrink tumors but not activate the specific T-cells responsible for destroying tumor cells, the utility of combining them with PV-10 would be to have these inhibitors reduce tumor burden and thus the "weight" on the immune system, thereby allowing it to be better stimulated by PV-10. Such combination use would be very helpful for late stage metastatic melanoma patients where tumor burden is high.
Moffitt has conducted trial work on both MK-3475 (Merck) and nivolumab (Bristol-Myers) PD-1 agents. Will Moffitt present combination work with PV-10 involving one of these agents?
"Adoptive T cell therapy involves the isolation and ex vivo expansion of tumor specific T cells to achieve greater number of T cells than what could be obtained by vaccination alone. The tumor specific T cells are then infused into patients with cancer in an attempt to give their immune system the ability to overwhelm remaining tumor via T cells which can attack and kill cancer. There are many forms of adoptive T cell therapy being used for cancer treatment; culturing tumor infiltrating lymphocytes or TIL, isolating and expanding one particular T cell or clone, and even using T cells that have been engineered to potently recognize and attack tumors" (Source: Tumor Vaccine Group, UW Medicine). The current approaches for ACT seem to focus on ex vivo (out of the tumor/body) improvements to T-cells; that is, endeavoring to make more of them better external to the patient.
But, as Moffitt first introduced in the February Cancer Watch article, per Dr. Sarnaik, PV-10 appears to improve the quality of T lymphocytes (cytotoxic?). It does this in vivo (in the tumor/body), of course. Perhaps Moffitt's novel approach is to utilize T-cells taken directly from the patient’s blood after they have received a PV-10 injection, which primes the tumor antigen specific T-cells first. Then, with active immunization already established by PV-10, they expand the number of those T-cells in the lab to greater numbers for therapeutic infusion, and thus even greater response success.
March (abstract), April (presentation), May (abstract(s)?), June (presentation(s)?)...
PV-10 at ASCO: Trials In Progress (April 19, 2016)
Presumably Provectus' pivotal melanoma Phase 3 trial:
AACR Tweets so far (April 19, 2016)
Of note to me, from @JSwatercooler's Twitter feed at AACR 2016:
Above: Stress, death, immunity, and radiation therapy. Lorenzo Galluzzi, Paris, France
Above: Sloan Kettering's Dr. Jedd Wolchok is a co-author of a combination therapy patent involving Newcastle disease virus (NDV). See In the conversation (April 14, 2016) below. See also "Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy," Zamarin et al. Sci Transl Med. 2014.
Above: The interplay of radiotherapy with the tumour microenvironment: Novel opportunities to overcome adaptive resistance, Tim M. Illidge. University of Manchester, Manchester, United Kingdom {underlined emphasis is mine}
Above: The future of combinations of immunomodulators with local radiation: toward a personalized immunotherapy approach, Sandra Demaria. Weill Cornell Medical College, New York, NY {underlined and bolded emphasis is mine}
Above: From foe to friend: Transforming tumors into personalized vaccines
As noted below under [Australian] Standard of Care? (April 11, 2016), PV-10 was referenced (in a trial of another compound) as an "existing standard of care" (SOC) (along with isolated limb infusion, or ILI) at Brisbane, Australia's Princess Alexandra Hospital (PAH) based on data derived from hospital records between 1996 and 2014. PAH was a Provectus melanoma Phase 2 trial site, will be a pivotal melanoma Phase 3 and melanoma combination therapy (of PV and pembrolizumab) Phase 1b site, and is an expanded access/compassionate use program (EAP/CUP) (U.S. terminology) site. The U.S. EAP/CUP appears to be called a Special Access Scheme (SAS) in Australia.
PAH replied to me (today) on the topic of SOC; I had e-mailed the hospital (on April 11th) for clarification of the use of SOC. The hospital staff member said {underlined emphasis is mine}:
"Hepatocellular Carcinoma from an Immunologic Perspective" (April 17, 2016)
Updated below.
While waiting for (a) Provectus' Dr. Eric Wachter, PhD to unveil his "Phase 1b/2 Combination Strategy" for hepatocellular carcinoma (HCC) and standard of care pairings (e.g., sorafenib, transarterial chemoembolization [TACE], radiofrequency ablation [RFA], etc.) and (b) Godot, I read "Hepatocellular Carcinoma from an Immunologic Perspective," Greten et al., Clin Cancer Res; 19(24); 6678–85 (2013).
The December 2013 article's abstract notes {underlined emphasis is mine}:
At AACR Novartis tweeted "The three-step immune response to cancer exposes new lines of attack on tumors," which implicated the multiple checkpoint roles of Tim-3 {purple arrows below are mine}.
In the Tweet stream for this project, Paul Rennert tweeted in response that because Tim-3 was implicated in more than one step, mechanism of action was unclear.
We know less about the immune system than we think we know. When something is implicated in multiple steps, or is implicated in having multiple roles, why is mechanism unclear? What is it that you have to know or understand in order for it to be clear? I think it's fair that skepticism exists about the multiplicity of involvement.
PV-10 is potentially implicated in each and every step of Chen & Mellman's Cancer Immunity Cycle. The investigational drug is as much about “starting the engine” or “stepping on the gas pedal” of the immune system, as it is about “releasing the brakes,” as shown below.
Managed entry agreements/Managed access programmes (April 16, 2016)
In February Provectus' CFO/COO and interim CEO Peter Culpepper discussed an "expense reimbursement scheme in Australia" during his presentation at the 19th Annual BIO CEO & Investor Conference in New York {underlined emphasis is mine}:
In April it was discovered that Brisbane, Australia's Princess Alexandra Hospital considered PV-10 a standard of care (SOC) for melanoma patients with in-transit disease (i.e., Stage III). See [Australian] Standard of Care? (April 11, 2016) below. The hospital appeared to have used clinical data from patients from its institution that participated in Provectus' metastatic melanoma Phase 2 trial and the company's expanded access/compassionate use program to arrive at the SOC determination.
My questions about this would be, and I hope to ask these of the company's CTO Dr. Eric Wachter, PhD on Provectus' 1Q16 business update conference call in May:
Precursor (April 15, 2016)
Updated below.
An abstract at AACR 2016 about a retrospective analysis -- derived from a Phase 2 study of intralesional (IL) electroporation of plasmid interleukin-12 (epIL12) -- of the combination of epIL-12 and immune checkpoint inhibitors (i.e., anti-PD-1/PD-L1 agents) revealed itself today, Intratumoral electroporation of plasmid IL-12 can prime response to anti-PD1/PD-L1 blockade in patients with Stage III/IV-M1a melanoma.
OncoSec's epIL-12 is shown below as IL-12 in the pipeline chart from a presentation by melanoma, IL and PV-10 key opinion leader Dr. Sanjiv Agarwala (the original chart was prepared by melanoma, IL and T-Vec KOL Dr. Robert Andtbacka);
The AACR abstract of epIL12 and anti-PD-1/PD-L1 blockade noted:
Although intratumoral or intralesional epIL12 -- electroporation of IL-12 -- requires special equipment, causes patient discomfort, and incurs higher toxicity, the investigator's observation of the potential to prime the immune system provides a potential precursor of possible results from Provectus' melanoma Phase 1b study combining PV-10 and pembrolizumab.
Note in the table above that the epIL12 "combos" have higher complete responses (CRs) (and also objective responses) than the T-Vec combos. This may be due to epIL12 having the ability to (i) generate comparable tumor destruction to T-Vec and (ii) potentially have better immunologic signalling. Consider the exploratory analyses Provectus, Amgen and OncoSec each did on complete responses achieved on treated lesions:
While OncoSec started presenting preliminary data of epIL12's melanoma Phase 2 trial in 2012, it has not yet begun a pivotal Phase 3 trial. This company began a melanoma Phase 2 trial combining epIL12 and pembrolizumab in 2014 (trial protocol is here, company press release is here), which should build on the retrospective analysis presented at AACR this year.
The "jargon trend" appears to be that in the age of the tumor microenvironment, large amounts or greater levels of tumor destruction are necessary to begin the antigen cascade that kicks off the cancer immunity cycle, and to generate more and greater infiltration of T-cells into distant (in the case of IL agents, uninjected or "bystander") tumors.
Updated (4/16/16): H/t a shareholder. OncoSec's combination trial, a Phase 2 single-arm trial (SAT), with no discussion in the protocol of transitioning to a later-stage randomized controlled trial (RCT), is recruiting both Stage III and IV melanoma patients (i.e., metastatic melanoma), like Amgen and Merck's combination trial of T-Vec and pembrolizumab (i.e., Stage IIIB to Stage IV M1c). Provectus' combination study only is recruiting Stage IV patients. One would expect Stage III patients to have better response rates and overall survival than Stage IV patients.
OncoSec's trial is an investigator-initiated trial, and as I noted above, there is no protocol component transitioning to an RCT. Provectus' trial would be expected to transition into an expanded phase 2 RCT of PV-10 + pembrolizumab vs. pembrolizumab alone (i.e., PV-10 + standard of care [SOC] vs. SOC).
In the conversation (April 14, 2016)
Whether you refer to them as more popularly as intralesional (IL) agents, or as locoregional immunomodulatory agents, investigational drug PV-10 is routinely mentioned by key opinion leaders working with Provectus and KOLs not working with the company together with other IL agents, such as approved oncolytic virus-based drug talimogene laherparepvec (T-Vec, or trade name Imylgic), other investigational oncolytic virus-based drugs (e.g., Viralytics' Coxsackievirus A21, Newcastle disease virus patented by ipilimumab creator Dr. James Allison, PhD and others, Takara Bio's HF10, etc.), and OncoSec's plasmid IL-12 with electroporation.
T-Vec has been approved. PV-10 is a late-stage asset (i.e., in a pivotal Phase 3 trial). The others have not had their Phase 3 trials finalized yet.All appear to be exploring early-stage combination trials with immune checkpoint inhibitors.
At December's Melanoma Bridge 2015 conference in Naples, Italy, IL KOLs Drs. Sanjiv Agarwala (PV-10 KOL) and Igor Puzanov (T-Vec KOL), MD both discussed PV-10 among other IL agents. The former is from St. Luke's Cancer Center in Bethlehem, Pennsylvania, while the latter is from Vanderbilt University in Nashville, Tennessee.
Dr. Agarwala's presentation included the following slides:
Dr. Puzanov's presentation included the following slides:
The slide below is Provectus' CTO Dr. Eric Wachter, PhD's PowerPoint presentation theme (and thus, I believe, his slide or a variation thereof).
It is interesting that Dr. Puzanov included a PV-10 liver trial patient slide in his presentation.
Tumor infiltrating T-cells are a biomarker of PV-10, too. Moffitt Cancer Center's mechanism of action work was entitled Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study.
Chinese Guidelines on the Diagnosis and Treatment of Melanoma (April 13, 2016)
From Chinese Guidelines on the Diagnosis and Treatment of Melanoma (2015 Edition), published in Chinese Clinical Oncology in November 2015. Purple emphasis below is mine.
Another update to the 2013 guidelines in the 2015 version:
Also:
And:
PeterMac (April 12, 2016)
Updated below.
#1. Australia's Peter MacCallum Cancer Institute (Melbourne) will present the inaugural Multidisciplinary Management of Locoregionally Advanced Melanoma - From Surgery to Immunotherapy conference in August 2016. Sponsors are Bristol-Myers (platinum) and Amgen (gold). PV-10 should be discussed in the presentation Injectable therapy versus limb infusion for intransit disease: Which treatment for which patient? by Professor/Dr. Mark Smithers, MD.
#2. If PV-10 is a standard of care (SOC) for in-transit melanoma at one Australian hospital, is it SOC for the said disease presentation at more Australian hospitals?
#3. A pharmaceutical company may have accessed the blog from a non-corporated named router in the city where it has its headquarters. As such, I revised my table -- see Visitors (April 6, 2016) below -- in the 1-5 rank grouping, from C to A.
#5. A Fortune 100, non-traditional pharmaceutical company accessed the blog (from 4 different IP addresses). Over the last 12 months it has acquired and licensed biotechnology assets.
#6. I was struck by the Princess Alexandra Hospital study's protocol, which in part said: "Data for these control treatments were derived from institutional records between 1996 - 2014." "These control treatments" refer to isolated limb infusion and PV-10.
Princess Alexandra became a metastatic melanoma Phase 3 trial site in December 2007, Adds Second Center to Phase 2 Clinical Trial of PV-10. It, along with other Australian clinical trial (and possibly other sites that only wanted to treat patients), became a compassionate use program (CUP) in June 2009, to Offer Compassionate Use of PV-10 for Non-Visceral Indications in Cancer Patients. I would presume the data set comprised (i) Phase 2 patients from 2007 to perhaps 2009, when the trial completed accrual, and (ii) CUP patients from perhaps 2009 to perhaps 2014. This situation might also suggest Princess Alexandra is one of the places from where a CUP-oriented peer-reviewed journal article might originate.
If Princess Alexandra deemed the data to be dispositive on the issue of PV-10's safety as well as clinical activity and benefit (in order to determine it to be an SOC for in-transit melanoma), would it be reasonable to believe the FDA might as well once the Agency has pivotal melanoma Phase 3 data in hand?
#7. CNN.com: Aspirin a day may push death away, says study, Taking an aspirin a day might help prevent heart attacks, stroke and colon cancer. {bolded emphasis is mine}
See As pervasive as aspirin (October 11, 2015) on the blog's Archived News IV page. Several years ago, Provectus' CTO Dr. Eric Wachter, PhD extemporaneously said (paraphrasing) he'd liked to see PV-10 eventually be used as pervasively as aspirin. As such, it was with no small amount of irony to initially read the title of Moffitt Cancer Center's PV-10-related SITC 2015 abstract title, Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1. See on the same blog news page SITC 2015 Preview: Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1 (October 5, 2015) and HMGB1 (October 6, 2015).
A Google News search of HMGB1 (as of this writing) yields several articles about a recent study of salicylic acid targeting HMGB1. Salicylic acid is the active ingredient of aspirin. The study, entitled Aspirin′s Active Metabolite Salicylic Acid Targets High Mobility Group Box 1 to Modulate Inflammatory Responses, noted in its abstract:
Updated below.
Note the following from the section entitled Comparator / control treatment of a 2015 Australian trial conducted at Princess Alexandra Hospital in Brisbane entitled Topical Imiquimod or Diphenylcyclopropenone for the Management of Cutaneous In Transit Melanoma Metastases – A Phase II Single Centre Prospective Randomised Pilot Study {underlined and bolded emphasis is mine}:
The hospital was/is a trial site for the following Provectus clinical studies:
"Isolated limb perfusion (ILP) is a method of drug delivery that is designed to deliver high local
doses of chemotherapeutic agents to isolated anatomic regions while avoiding systemic toxicity." "Isolated Limb Perfusion/Infusion for Malignant Melanoma" is reimbursable in the U.S. (Note: Thompson below: link)
Updated (4/11/16): I erred, in that the two trials of topical imiquimod or diphenylcyclopropenone (DPCP) I wrote about above are one in the same. Some thoughts about this trial:
Updated below.
It would seem for some number of sites (e.g., some or all of the projected 25 U.S. sites and 10 Australian sites) that the original pivotal melanoma Phase 3 trial protocol was sent to them at the same time, or maybe at least in 2014 when the protocol was listed on ClinicalTrials.gov as "First Received" on 11/4/14. St. Luke's Cancer Center had a 2014-based identification (ID) number of 2014-117. Reviewing ID numbers at Huntsman Cancer Institute, the number assigned to the Phase 3 trial (81139) appears to indicate this site also may have received the protocol in 2014. See the table below. The trial protocol was finalized on 3/12/15, and then amended on 3/15/16.
If a highly qualified clinical site has requested PV-10 to treat its patients under expanded access (i.e., compassionate use) but does not wish to recruit patients for a Provectus clinical trial, the site would not be listed in public registries. How many such sites exist (i.e., what is the total number of compassionate use program locations)?
As difficult as it might be for me as an investor in the company to fully appreciate all of the time, Provectus (i.e., CTO Dr. Eric Wachter, PhD) does not publicize all of its R&D activities for presumably a number of reasons, such as not wanting to provide a road map for competitors. I might not agree with all of the reasons, however. It is interesting to note that Amgen developed a Phase 2 trial protocol (First Received: 1/14/16) for Talimogene Laherparepvec (T-VEC) for Breast Cancer Local Recurrence.
On a business update conference call or during an investor conference presentation (I currently cannot [for the life of me] locate the source so as to provide it a link here), Provectus' CFO/COO and interim CEO Peter Culpepper had said the company "owned" the work being done in the Phase 1b/2 program combining PV-10 and pembrolizumab in patients with advanced (Stage IV) melanoma. Since Provectus is sponsoring and conducting the trial work PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma, all data from it are owned by the company. Typical of any sponsored clinical trial, while top line data customarily may be reported at conferences and in journals, the full data set remain under the trial sponsor's control. Importantly, the full data set that global regulators review remain the property of the sponsor, unless it enters into a deal that affords access or rights to the other party.
In June 2015 Dynavax Technologies Corporation (NASDAQ: DVAX) and Merck & Co. entered into a collaboration combining the former's intratumorally administered SD-101 and pembrolizumab. See Merck and Dynavax Announce New Collaboration Investigating the Combination of Immuno-Oncology Therapies. Under the terms revealed, Dynavax would sponsor and fund the SD-101/pembro study (Merck would sponsor and fund the SD-101/MK-1966 work). SD-101 is a toll-like receptor 9 (TLR9) agonist, which appears to have a relationship with HMGB1. While UCLA's Dr. Antoni Ribas, MD is the principal investigator of the combination study work A Phase 1b, Open-label, Multicenter, Dose Escalation Trial of Intratumoral Injections of SD-101 in Combination With Pembrolizumab in Patients With Metastatic Melanoma, St. Luke's Cancer Center's Dr. Sanjiv Agarwala, MD also is involved.
Provectus' initial intellectual property surrounding combination therapy was awarded in August 2015, Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (the so-called "parent" award [formerly application]). Subsequently, in October, three so-called "daughter" (continuation) applications were made that addressed, among other things combined therapeutic regimens, and targeted therapies (the aforementioned patent award explicitly covered among other things immunotherapies -- here, here and here). These three continue to proceed through the US Patent and Trademark Office (USPTO) process. For complicated filings the USPTO often requests an application be broken up into smaller units to facilitate examination, which may lead to multiple daughter applications from the original parent one as noted above. Sometimes the applicant "traverses" this request -- register a formal disagreement with the examiner's findings the requested breakup is inappropriate. In this case the applicant specifies an exemplary topic for the examiner to focus on, presumably allowing the examiner to review pertinent prior art without requiring that he or she look into all possible permutations. If there are problems later, such as other prior art coming to light that would invalidate the outcome of the examination, it is the applicant’s responsibility to defend this shortcoming. An example of this in Provectus' case would be when the parent above originally was filed; the examiner issued a restriction requirement that resulted in the cleaving out of the checkpoint inhibitor portion of the application (the relevant daughters above). There is another restriction requirement in the process, and thus another round of activity.
Updated (4/10/16): #1. The Huntsman information above would lend further credence to the belief Eric probably sent or did send the pivotal melanoma Phase 3 protocol to all U.S. and Australia sites he originally planned to be in the trial. See also June 26, 2015 blog post Eric's Process.
#2. There very likely are more than one CUP site that are not public because all such sites want to do is treat patients with PV-10. They do not want to recruit patients for clinical trials.
#3. There very likely is R&D work going on involving PV-10 that is not visible.
#4. Owning the Phase 1b/2 PV-10-pembrolizumab combination trial results for advanced melanoma means that while regulators may see the full data set, Merck & Co. (and/or any other company) doesn’t get access to the dataset unless they do a deal with Provectus.
#5. I think Merck may have decided, in part, to use a PV-10 "comparable" in SD-101, an intratumoral (intralesional) drug, when Provectus said it wanted to get paid to combine PV-10 with pembrolizumab.
#6. Provectus is proceeding with expanding its combination therapy intellectual property. The current activity with the US PTO may require them to potentially split up the daughter combo patent applications into granddaughter patent apps (maybe).
#7. Disease control rate, aka locoregional disease control, the primary endpoint of the Amgen T-Vec breast cancer trial, equals complete response (CR) + partial response (PR) + stable disease (SD). Provectus reported lororegional disease control in its melanoma Phase 2 trial. The idea behind this approach was, in part, to demonstrate that injection of PV-10 into cancerous lesions could destroy (i.e., CR), prevent (i.e., PR) or forestall (i.e., SD) melanoma from spreading from Stage III disease to Stage IV -- which is one of the hypothesis (or thesis) or the pivotal melanoma Phase 3 trial underway. I think Amgen is following Provectus' thesis, and explicitly incorporated Provectus' observation as a design parameter in the breast cancer trial (including "Photographs taken of lesions on chest at baseline, at Cycle 2, Cycles 4, 8, and 10 if the doctor thinks it is needed, Day 1 of Cycle 5, Day 1 of Cycle 7, and then every odd numbered cycle after that").
Consider the following slides from melanoma, intralesional (IL) agent and PV-10 key opinion leader St. Luke's Cancer Center's Dr. Sanjiv Agarwala, MD's presentation at SMR 2010 (purple arrows are mine):
Ridiculousness (April 7, 2016)
I track a variety of Twitterers and their tweets for this project. For example, Paul Rennert was tweeting today from the Jefferies Immuno-Oncology Summit in Boston (April 7-9).
Provectus issued a PR on March 16th, Amends Protocol for Phase 3 Study of PV-10 in Treatment of Locally Advanced Cutaneous Melanoma, that appeared [to me] to broaden the applicable patient population of the company's pivotal melanoma Phase 3 trial (a registration study), PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma, which if successful could (should) lead to a broader label for the investigational drug PV-10 if/when approved.
The patient population increased in several ways, and thus the potential label for PV-10 broadened (or could provide Provectus with "a label advantage") because, in the amended protocol:
Over the last four months there were several visits by at least 14 U.S. and international pharmaceutical and biotechnology companies (that I could identify) to the blog. Visits comprised multiple visits on multiple days by multiple IP addresses, multiple visits on multiple days by a single IP address, a single visit on multiple days by multiple IP addresses, and a single visit on a single day by a single IP address. These data (not comprehensive) are summarized in the table below.
Risk Factors (April 5, 2016)
(aka 10-K Notes, part 2)
"Peter Culpepper believes that local injectibles such as PV-10 provide the answer to many of the challenges of cancer treatment"
With Dr. McArthur sitting next to him (and saying nothing in this snippet), I imagine there is more such media available.
New auditors, Proxy filing (April 29, 2016)
Updated below.
Provectus made an 8-K filing today regarding the company's dismissal of its long-time auditors BDO and the hiring of new auditors Marcum, noting:
"During the fiscal years ended December 31, 2015 and 2014, and the subsequent interim period through April 26, 2016, the date of BDO USA, LLP’s dismissal, there were no “disagreements” (as defined in Item 304(a)(1)(iv) of Regulation S-K and related instructions) with BDO USA, LLP on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of BDO USA, LLP, would have caused BDO USA, LLP to make reference to the subject matter of the disagreements in connection with its reports on the Company’s consolidated financial statements for such periods." {underlined emphasis is mine}And:
"The Company has provided BDO USA, LLP with a copy of the foregoing disclosures and requested that BDO USA, LLP furnish the Company with a letter addressed to the Securities and Exchange Commission (“SEC”) stating whether or not it agrees with the statements in the above paragraphs. BDO USA, LLP’s letter to the SEC stating whether it agrees with such statements is filed as Exhibit 16.1 to this Current Report on Form 8-K."To which, BDO replied:
"We have been furnished with a copy of the response to Item 4.01 of Form 8-K for the event that occurred on April 26, 2016, to be filed by our former client, Provectus Biopharmaceuticals, Inc. We agree with the statements made in response to that Item insofar as they relate to our Firm."Several thoughts come to mind thus far:
- Counsel's recommendations to Provectus' audit committee included the hiring of an interim CFO,
- Such recommendations presumably also included the removal and replacement of "the independent consulting group previously utilized by management to aid in its documentation and testing of internal controls over financial reporting," and
- Given the removal and replacement of the above firm (in context and given the circumstances of the situation), BDO also presumably could not continue its involvement.
The company also filed its proxy statement (Schedule 14A) today. Of note to me thus far, aside from the questions being put to a vote:
- Overall compensation in 2015, including cash bonuses, that total nearly $1 million per management principal. Contrast this with the annualized salary of the new interim CFO of approximately $208K. See New [Interim] CFO (April 22, 2016) below.
 |
| Click to enlarge. |
- As a follow-up to the above point, what did Provectus' President and a co-founder Dr. Tim Scott, PhD do vis a vis (a) his specific contribution to the company's commercial and operational performance in 2015 and (b) his specific contribution to Provectus' achievement of scientific, medical and clinical milestones during the year to warrant an approximately $1 million compensation package?
- The Hurtado, Montiminy, Donato, and Foley derivative lawsuits appear to have been settled (or settled on a preliminary or proposed basis: "The Company agreed to implement certain corporate governance changes, including the adoption of a Disclosure Controls and Procedures Policy, and to use its best efforts to replace one of its existing directors with an independent outside director by June 30, 2017. The Company agreed to pay from insurance proceeds the amount of $300,000 to plaintiffs’ counsel in the Derivative Litigation."
Updated (6/13/16): Provectus filed an 8-K related to entering into a "Stipulation of Settlement," where the parties "resolved the derivative claims to their mutual satisfaction:"
"The Individual Defendants have not admitted the validity of any claims or allegations, and the settling plaintiffs have not admitted that any claims or allegations lack merit or foundation. Under the terms of the Settlement, the Company has agreed to implement certain corporate governance changes, including adopting a Disclosure Controls and Procedures Policy and using its best efforts to replace one of its existing directors with an independent, outside director by June 30, 2017. The Individual Defendants and the Company also agreed to pay from insurance proceeds $300,000 to plaintiffs’ counsel, as an award of attorneys’ fees and reimbursement of expenses in connection with their role in this litigation, subject to Court approval. The insurance carrier will pay the award directly to the plaintiffs’ counsel’s trust account, and the payment will not pass through the Company." The settlement hearing is scheduled for August 26th.
I expect to post my proxy vote before the end of May. My 2015 vote was described in May 30, 2015 blog post Proxy Vote.
The Vatican (April 27, 2016)
Updated below.
Provectus issued a press release and made a related 8-K filing regarding the company's presentation at the Vatican's healthcare conference, to Participate in Panel at Third International Conference on the Progress of Regenerative Medicine and Its Cultural Impact. Presenting on behalf of Provectus is Melbourne, Australia's Peter MacCallum Cancer Centre's Dr. Grant McArthur -- Vatican conference bio is here, and PeterMac's McArthur Translational Research Laboratory description is here. See Il Papa (April 2, 2016) and Ridiculousness (April 7, 2016) below. He is a long-time clinical investigator, and a consultant to the company. Dr. McArthur will be on a panel discussing combination therapies. PeterMac is a PV-10 special access scheme/compassionate use program site in Australia. See "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool..." (April 25, 2016) immediately below.
Updated (4/27/16): The panel of which Dr. McArthur is a part is shown below.
 |
| Click to enlarge. Image source |
Updated below, again, once more.
Takeaways:
- PeterMac: "PV-10 is an effective, durable, well-tolerated and cost effective treatment tool with an acceptable side effect profile for the management of unresectable in transit and locally recurrent melanoma."
- Princess Alexandra: "The results of this investigation...form the basis of the world’s largest single centre case series...With the incorporation of phenotypic subgroup considerations, this strategy facilitates appropriate patient selection in an evidence-based manner." [phenotypic = observable physical or biochemical characteristics]
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source, p. 292 |
 |
| Click to enlarge. Image source, p. 158 |
 |
| Click to enlarge. Image course, p. 158 |
 |
| Click to enlarge. Image source, as above |
Interestingly, the hospital weighed in on treatment cost as well.
The Princess Alexandra study, a
- Primary: complete clinical response of treated lesions (determined using RECIST), and
- Secondary: overall response (OR), clinical benefit, time-to-best response, disease-free survival (DFS) and overall survival (OS).
I wrote retrospective, which means {underlined emphasis is mine} -- "A retrospective study looks backwards and examines exposures to suspected risk or protection factors in relation to an outcome that is established at the start of the study."
But, as the abstract notes, the work was a prospective study, which means {underlined emphasis is mine} -- "A prospective study watches for outcomes, such as the development of a disease, during the study period and relates this to other factors such as suspected risk or protection factor(s). The study usually involves taking a cohort of subjects and watching them over a long period. The outcome of interest should be common; otherwise, the number of outcomes observed will be too small to be statistically meaningful (indistinguishable from those that may have arisen by chance). All efforts should be made to avoid sources of bias such as the loss of individuals to follow up during the study. Prospective studies usually have fewer potential sources of bias and confounding than retrospective studies."
Updated (4/26/16): Princess Alexandra Hospital began recruiting patients in December 2007 for Provectus' metastatic melanoma Phase 2 trial, Adds Second Center to Phase 2 Clinical Trial of PV-10. By July 2008 it, and the Sydney Melanoma Unit (Dr./Prof. John Thompson, MD) had treated 20 patients, First Twenty-Five Percent of Subjects in Phase 2 Melanoma Clinical Trial Treated. Princess Alexandra, along with other clinical sites, became PV-10 expanded access/compassionate use program (CUP, in the U.S.) and special access scheme (in Australia) sites in 2009, to Offer Compassionate Use of PV-10 for Non-Visceral Indications in Cancer Patients (June in Australia) and Initiates Compassionate Use Program of PV-10 for Non-Visceral Indications in Cancer Patients in U.S. (October).
The Princess Alexandra work, an open-label, single-arm, non-randomised, prospective study, appears to be about the same size or larger than most intralesional agent melanoma Phase 2 studies, and almost comparable to several Phase 1 and 2 ipilimumab studies.
Some readers cannot open the link to the above abstracts.
Updated (4/28/16): Provectus issued a press release and made an 8-K filing today related to the above abstracts, Announces Publication of Two Abstracts on Research into IL PV-10 for Melanoma in Special Issue of ANZ Journal of Surgery.
Updated (4/26/16): Princess Alexandra Hospital began recruiting patients in December 2007 for Provectus' metastatic melanoma Phase 2 trial, Adds Second Center to Phase 2 Clinical Trial of PV-10. By July 2008 it, and the Sydney Melanoma Unit (Dr./Prof. John Thompson, MD) had treated 20 patients, First Twenty-Five Percent of Subjects in Phase 2 Melanoma Clinical Trial Treated. Princess Alexandra, along with other clinical sites, became PV-10 expanded access/compassionate use program (CUP, in the U.S.) and special access scheme (in Australia) sites in 2009, to Offer Compassionate Use of PV-10 for Non-Visceral Indications in Cancer Patients (June in Australia) and Initiates Compassionate Use Program of PV-10 for Non-Visceral Indications in Cancer Patients in U.S. (October).
The Princess Alexandra work, an open-label, single-arm, non-randomised, prospective study, appears to be about the same size or larger than most intralesional agent melanoma Phase 2 studies, and almost comparable to several Phase 1 and 2 ipilimumab studies.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. |
 |
| Image source |
Several weeks ago I communicated with someone who could be a possible CEO candidate. "Possible" because, while (s)he has expressed interest in the position to the company, I do not believe (s)he has interviewed with Provectus' board of directors' CEO search committee yet.
Intelligent, knowledgeable, suitably experienced and reasonably qualified, this individual possesses a PhD in a relevant medical sciences subject area. (S)he has more than 25 years of increasingly senior roles and responsibilities at global pharmaceutical companies (n.b. Top 10, both annual overall and oncology revenues) in business development, corporate development, operations, and strategic planning.
(S)he has an awareness and understanding of PV-10, and is known to both COO and interim CEO Peter Culpepper and CTO Dr. Eric Wachter, PhD. I hope the board would interview this individual to, at the very least:
- Gain more pharmaceutical and specifically more immuno-oncology (IO) industry insight to augment the board's independent directors' knowledge,
- Potentially better evaluate/assess Eric's clinical development program (CDP) efforts and process
- Potentially better evaluate/assess Peter's business/corporate development efforts and process,
- Re-assess, with the goal of potentially re-affirming, PV-10's worth and valuation (i.e., the drug compound's industry calculation of net present value, and assumptions therein), and
- Delve into, among other topics, re-assessing and potentially re-affirming or augmenting the board's understanding of competitive pricing (i.e., pricing of PV-10 if/when approved, and the pricing action/reaction by Big Pharma of already approved IO drugs and compounds in the approval pipeline) and its possible and potential impact on valuation, and pharmaceutical industry deal transactions (i.e., possible and potential terms and conditions).
- Rose bengal/PV-10 is an attractive oncology asset,
- PV-10 could be a driver of immune checkpoint inhibitor performance when these drugs are used in combination with it (PV-10),
- As a monotherapy PV-10 has a place in the treatment of melanoma and hepatocellular carcinoma (liver cancer), and
- Exit valuations for PV-10 and the company would be driven if/when the drug compound is proven to be a potentiator of immune response across multiple solid tumor cancers.
There are several things and ways this individual believes (s)he would do differently than current management, including:
- Positioning PV-10 data differently with potential strategic partners,
- Providing potential partners with a better understanding of how the drug can be used to solve current problems for patients who may be candidates for immunotherapy, and
- Simply having more pharmaceutical transactional experience (and a larger deal sheet) than Peter and Eric (and the board's independent directors).
- This individual's lack of name recognition (to non-life sciences investors like me, which of course isn't hugely important) in the biotechnology or pharmaceutical industry (the industry experience (s)he has certainly is notable to me),
- The CEO position being her or his first [very] senior organizational leadership opportunity, and
- The propensity to think like Big Pharma (duh), which isn't necessarily a "bad" thing (e.g., some time ago suggesting Provectus position PV-10 as a checkpoint inhibitor*).
As I noted immediately below regarding the nature and structure of the hire of Provectus' interim CFO John Glass, it strikes me the board does not want to disrupt in the short-term the current execution of Eric's CDP plans nor Peter's business and corporate development activities. I think that is appropriate and prudent for the time being. There must be, however, a well defined and tight timeframe and, more importantly, a better, well defined and more robust process in place, for assessing and evaluating whether their (Eric and Peter's) efforts can, will and do yield tangible success (i.e., data generation, presentation and publication, and deal making that could lead to a much higher share price) before changes could or should be made.
Updated (4/24/16): * See, for example, Everything's up (April 21, 2016) and Implication, Skepticism, MOA, Show Me... (April 17, 2016) below. Current approaches to harnessing the immune system are predicated on affecting it largely at a single point (e.g., CTLA-4, PD-1, PD-L1, OX-40, Tim-3, CD[pick a number], etc.). PV-10 affects or has an impact on multiple immunologic signaling pathways (e.g., CD8+, CD4+, CD3+, NK, HMGB1, etc.). Additionally, it's administration path is intralesional (IL) or intratumoral (IT). PV-10 is potentially implicated in each and every step of Chen & Mellman's Cancer Immunity Cycle. The investigational drug is as much about “starting the engine” or “stepping on the gas pedal” of the immune system, as it is about “releasing the brakes,” as shown below.
So, I get where this individual was coming from regarding the potentially challenging but very novel, unique, multi-faceted positioning of PV-10 in IO, a physician's medical bag, and thus the pharmaceutical industry itself.
Updated (4/25/16): The above possible candidate is not someone I have in mind, nor someone I don't have in mind. Part of a CEO search process presumably is the board getting smarter about what it knows and doesn't know about the strengths, weaknesses and blindspots of PV-10, the opportunities and challenges, the total addressable market, the competitive landscape, Provectus, current management, itself, etc. As implied above, one of the potential strengths of this individual could be her or his purported Big Pharma corporate development experience (e.g., licensing, M&A, negotiations, transaction execution, etc.); see "more pharmaceutical transactional experience (and a larger deal sheet)" above.
Updated (4/24/16): * See, for example, Everything's up (April 21, 2016) and Implication, Skepticism, MOA, Show Me... (April 17, 2016) below. Current approaches to harnessing the immune system are predicated on affecting it largely at a single point (e.g., CTLA-4, PD-1, PD-L1, OX-40, Tim-3, CD[pick a number], etc.). PV-10 affects or has an impact on multiple immunologic signaling pathways (e.g., CD8+, CD4+, CD3+, NK, HMGB1, etc.). Additionally, it's administration path is intralesional (IL) or intratumoral (IT). PV-10 is potentially implicated in each and every step of Chen & Mellman's Cancer Immunity Cycle. The investigational drug is as much about “starting the engine” or “stepping on the gas pedal” of the immune system, as it is about “releasing the brakes,” as shown below.
 |
| Click to enlarge. |
Updated (4/25/16): The above possible candidate is not someone I have in mind, nor someone I don't have in mind. Part of a CEO search process presumably is the board getting smarter about what it knows and doesn't know about the strengths, weaknesses and blindspots of PV-10, the opportunities and challenges, the total addressable market, the competitive landscape, Provectus, current management, itself, etc. As implied above, one of the potential strengths of this individual could be her or his purported Big Pharma corporate development experience (e.g., licensing, M&A, negotiations, transaction execution, etc.); see "more pharmaceutical transactional experience (and a larger deal sheet)" above.
New [Interim] CFO (April 22, 2016)
Updated below.
Provectus issued an 8-K filing today to announce the hiring of an interim CFO, John R. Glass, CPA, who would replace the CFO role the company's CFO/COO and interim CEO Peter Culpepper held and filled. Glass' bio, from the 8-K:
Mr. Glass, 72, is the President of J.R. Glass & Associates, a consulting firm he founded in 1990 to assist clients in the financial, operational and marketing segments of their business. In this role, his responsibilities have included, among others, preparation of periodic reports to be filed with the Securities and Exchange Commission and Sarbanes-Oxley compliance documentation. From January 2007 to May 2014, Mr. Glass served as controller for CytoCore, Inc. (OTCBB: CYOE) (now known as Medite Cancer Diagnostics Inc.), a late development stage bio molecular diagnostics company. His prior chief financial officer experience includes serving as Chief Financial Officer of U. S. RealTel, Inc., a publicly traded company in the telecommunications industry, Vice President and Chief Financial Officer of Health Charge Corporation, a financial services company in the health care industry, and Vice President and Chief Financial Officer of Aluminum Distributors, Inc., a metal processor and distributor. He also previously served as Vice President of Fulton Manufacturing Industries, Inc. and as a Manager at Grant Thornton LLP, a registered public accounting firm. Mr. Glass is chairman of the Plan Commission of Elk Grove Village, a member of the Illinois CPA Society and past chairman and member of the board of directors for the Greater O’Hare Service Corporation. He received his B.B.A. in Accounting from Loyola University."Of note to me, he will be:
- Filling the interim CFO role under an independent contractor agreement,
- Paid $100 per hour and a per diem (when rendering services), and reimbursed for all reasonable and necessary expenses (again, when rendering services),
- Indemnified for claims made against him based upon the performance of his services, and will be named as an additional named insured under Provectus' general liability and directors and officers liability insurance policies, and
- Having an initial term of April 19 to December 1, 2016, and would go month-to-month thereafter.
Updated (4/22/16): Glass' hire and the disclosure of his hourly wage (annualized to a figure of $208K) strike at least three chords with me:
- First, his work experience combined with his contractual arrangement suggest [to me] Provectus' board of directors — particularly the independent directors (Chairman Al Smith, Dr. Kelly McMasters, MD, PhD and Jan Koe — don't want a new executive or C-suite voice at this time that could disrupt the execution of the company's CTO Dr. Eric Wachter, PhD's clinical development program (CDP) plans at least in the short-term (and perhaps for the remainder of 2016). The board's approach may extend to the CEO search, potentially suggesting it could be prepared to be measured and patient in its search, at least in the short-term and until independent board members can see tangible success from Eric's CDP efforts (i.e., data generation and deal making that lead to a much higher share price),
- Second, Glass taking over Peter's CFO role and responsibilities should provide him (Peter) the time to focus more on business and corporate development activities in the short-term, and also provide him (Peter) the opportunity to also show the board tangible success from his efforts, and
- Third, Glass' compensation calls into question President and a co-founder Dr. Tim Scott, PhD's role and responsibility at Provectus, and his $500K annual salary (more than double the interim CFO's annualized comp) in said context — is what he is doing as President commensurate with his compensation (see Provectus 2015 Schedule 14A).
Updated below.
Understanding that Moffitt Cancer Center's feasibility study (see April 21, 2016 blog post Burning Down The House) and other R&D work on PV-10 has been multi-faceted and dimensional, how much of proving the hypothesis that PV-10 produces a better immune cell infiltrate is the crux of (crucial to) bringing a co-development partner (for combining PV-10 with another drug) to the table?
Current approaches to harnessing the immune system are predicated on affecting it largely at a single point (e.g., CTLA-4, PD-1, PD-L1, OX-40, Tim-3, CD[pick a number], etc.) because we do not understand how to efficiently harness the complexity that resides within the immune system itself. Provectus' emphasis, via Moffitt's R&D efforts, on various recognized touch points such as TILs and CD8+ may be potential triggers for investment. As has been shown multiple times since Moffitt's early work, the impact of tumor ablation with PV-10 affects or has an impact on multiple immunologic signaling pathways (e.g., CD8+, CD4+, CD3+, NK, HMGB1, etc.). Human work includes:
- AACR 2014: Induction of anti-melanoma immunity after intralesional ablative therapy,
- ASCO 2014: Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions, and
- SITC 2015: Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1.
Updated (4/22/16): Provectus issued a press release about Moffitt's AACR 2016 data, Reports Data On PV-10 in Combination Therapy and T Cell Mediated Immunity Presented at American Association for Cancer Research (AACR) Annual Meeting 2016, and filed an associated 8-K.
Of note were quotes by Moffitt's Dr. Shari Pilon-Thomas, PhD and Provectus' CTO Dr. Eric Wachter, PhD shown below, respectively.
Pilon-Thomas: "Our results show that combining intralesional PV-10 with anti-PD-1 co-inhibitory blockade not only suppresses tumor growth vs. either agent alone but also yields marked increases in tumor-specific T cell activation against injected tumor."
Eric: "The nonclinical data reported by our collaborators at Moffitt reaffirm the crucial role T cells play in response to tumor ablation with intralesional PV-10, and further demonstrate the potential value of combining PV-10 with T cell directed checkpoint inhibition, such as the anti-PD-1 agent pembrolizumab. Intriguingly, these data also highlight possible strategies for augmenting this paradigm by harnessing additional targets in T cell signaling." {My underlined emphasis}It's all about the T cells. See February 21, 2014 blog post It's all about the T-cells from more than two years ago below:
I'm looking forward to reading Moffitt Cancer Center's American Association for Cancer Research ("AACR") annual meeting abstract next month, viewing the associated poster in April, and learning about what if anything they present at the American Society of Clinical Oncology ("ASCO") annual meeting in May/June. I presume they'll present something at ASCO because of Sondak's already scheduled PV-10 presentation at the 4th European Post-Chicago Melanoma/Skin Cancer Meeting. See Moffitt @ ASCO 2014? (February 10, 2014) under the blog's News tab.
There are three topics of their possible work I hope their poster presentation at AACR and any potential presentation(s) at ASCO would elucidate:
- PV-10's "mechanism of immune response," or the mechanism by which the drug induces systemic immunity (I think PV-10's primary mechanism of action, rapid tumor ablation following injection, is understood),
- Improved responses (i.e., tumor destruction and/or shrinkage) from combining PV-10 and other immunotherapies,
- PV-10's role and responsibility with respect to adoptive cell therapy ("ACT").
 |
| Click on the figure to enlarge it: Discussion, portion 1 |
 |
| Click on the figure to enlarge it: Discussion, portion 2 |
- First, in murine model work, Moffitt confirmed PV-10 generated an anti-tumor immune response that shrunk or destroyed untreated tumors (red underlined portions 1, 2 and 3 in paragraphs 1 and 2). They observed the systemic potential of PV-10 for both breast cancer and melanoma.
- Second, they hypothesized that large amounts of tumor debris created by the rapid ablation that followed PV-10's injection into tumors, debris containing antigens (thus, large amounts of debris should mean large numbers of antigens), are taken up by dendritic cells ("DCs") (red underlined portion 4 in paragraph 3). DCs, antigen-presenting cells, present (display) antigens they've taken up to the body's immune system's T-cells.
- Third, Moffitt suggested combining PV-10 with other immunotherapies may lead to better results in patients with metastatic melanoma (red underlined portion 5 in paragraph 4).
- Finally, fourth, they demonstrated immunity could be transferred from mice with PV-10 treated tumors to mice with untreated tumors, hypothesizing (I think) that more and better T-cells could be created using PV-10 (better ones) together with ACT (more of these better ones) to then use on more than one patient (red underlined portions 5 and 6 in paragraph 4).
There are T-cells, and then there are T-cells. There are different types of T-cells, each with a distinct function: helper, cytotoxic, memory, regulatory, natural killer, and mucosal associated invariant. "Cytotoxic T cells ("CTLs") destroy virally infected cells and tumor cells." "Regulatory T cells, formerly known as suppressor T cells, are crucial for the maintenance of immunological tolerance." "Natural killer T cells ("NKT cells")...are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses."
CTLA-4 and PD-1 cell receptors are the so-called brakes of the immune system, limiting its response. Taking the brakes off by inhibiting these blockade receptors (with anti-CTLA-4/PD-1 agents) allows for a more active and vigilant immune system. CTLA-4 and PD-1 agents, I think, help out with or activate these regulatory T-cells.
PV-10, on the other hand, I think, helps out with or activates CTLs, by virtue of the drug's rapid ablation causing tumor debris containing antigens to be taken up by DCs that then display them to this type of T-cell. "For long it has been discussed whether ablated tumor debris is able to induce a systemic immune response."
So, while activating regulatory T-cells and CTLs (cytotoxic T-cells) both may result in tumor destruction and shrinkage, regulatory T-cell activation by checkpoint inhibitors is not permanent and eventually resisted or overcome, whereas CTL activation by PV-10 may result in [permanent?] immunity.
"CTLs specific for tumor antigens play a major role in the immunity against cancer." Will Moffitt show PV-10 specifically activates these CTLs?
If the checkpoint inhibitors remove the brakes of the immune system, allowing it to shrink tumors but not activate the specific T-cells responsible for destroying tumor cells, the utility of combining them with PV-10 would be to have these inhibitors reduce tumor burden and thus the "weight" on the immune system, thereby allowing it to be better stimulated by PV-10. Such combination use would be very helpful for late stage metastatic melanoma patients where tumor burden is high.
Moffitt has conducted trial work on both MK-3475 (Merck) and nivolumab (Bristol-Myers) PD-1 agents. Will Moffitt present combination work with PV-10 involving one of these agents?
"Adoptive T cell therapy involves the isolation and ex vivo expansion of tumor specific T cells to achieve greater number of T cells than what could be obtained by vaccination alone. The tumor specific T cells are then infused into patients with cancer in an attempt to give their immune system the ability to overwhelm remaining tumor via T cells which can attack and kill cancer. There are many forms of adoptive T cell therapy being used for cancer treatment; culturing tumor infiltrating lymphocytes or TIL, isolating and expanding one particular T cell or clone, and even using T cells that have been engineered to potently recognize and attack tumors" (Source: Tumor Vaccine Group, UW Medicine). The current approaches for ACT seem to focus on ex vivo (out of the tumor/body) improvements to T-cells; that is, endeavoring to make more of them better external to the patient.
But, as Moffitt first introduced in the February Cancer Watch article, per Dr. Sarnaik, PV-10 appears to improve the quality of T lymphocytes (cytotoxic?). It does this in vivo (in the tumor/body), of course. Perhaps Moffitt's novel approach is to utilize T-cells taken directly from the patient’s blood after they have received a PV-10 injection, which primes the tumor antigen specific T-cells first. Then, with active immunization already established by PV-10, they expand the number of those T-cells in the lab to greater numbers for therapeutic infusion, and thus even greater response success.
March (abstract), April (presentation), May (abstract(s)?), June (presentation(s)?)...
Presumably Provectus' pivotal melanoma Phase 3 trial:
 |
| Click to enlarge. Image source (search) |
Of note to me, from @JSwatercooler's Twitter feed at AACR 2016:
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
"Radiation Therapy (RT) is a highly effective anti-cancer treatment delivered to approximately 50 - 60 % of all cancer patients, In addition to the direct tumor cell kill, emerging evidence suggests that RT has important effects on the tumor microenvironment and can generate anti-tumor immunity. RT induced tumor cell death is known to lead to increased ecto-calreticulin and tumor antigen expression as well as the release of several damage-associated molecular patterns (DAMPs). These “danger signals” include High Mobility Group Box 1 (HMGB1) and ATP which can lead to recruitment and activation of antigen presenting cells (APCs) and priming of tumor antigen-specific T cell responses. Despite these immunomodulatory properties of RT, systemic anti-tumor immune responses leading to clinically meaningful anti-tumor responses outside of the irradiated tumor field the so called the “abscopal effect” are rare in routine clinical practice. This lack of clinically meaningful abscopal effect is thought to be secondary to the immunosuppressive nature of the tumor microenvironment (TME), including Myeloid Derived Suppressor Cells (MDSC), Foxp3 T regulatory cells (Tregs) or blocking inhibitory molecules such as Programmed cell death protein 1 (PD-1), Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) ; (LAG-3) Lymphocyte-activation gene 3, T-cell immunoglobulin and mucin-domain containing-3 (Tim-3) ,receptors or inhibitory cytokines including Transforming Growth Factor ( TGF-β) and IL-13 . Therefore therapeutic strategies designed to overcome these inhibitory immunosuppressive networks may provide an opportunity to enhance anti-tumor immunity by promoting the generation of T cell priming in combination with RT."
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. Image source |
"We have shown in multiple preclinical tumor models that ionizing radiation therapy can convert tumors refractory to immune checkpoint inhibitors and other immune modulators into responsive ones. Importantly, the elicited antitumor T cells rejected not only the irradiated tumor but also nonirradiated spontaneous metastases or synchronous palpable tumors (abscopal effect), demonstrating the potential of radiation to generate systemically effective antitumor immune responses.
Our data also suggest that generation of an in situ tumor vaccine by radiation is regulated by the intrinsic tumor immunogenicity, the host immune system, and the radiation regimen employed. The interaction of these three compartments determines the levels of immune modulating mediators in the tumor microenvironment, including interferon type I, transforming growth factor-b, and adenosine, which in turn control the number and state of activation of intratumoral dendritic cells, and the magnitude of the induced T-cell response. Thus, while radiation offers the promise of a simple treatment to achieve individualized vaccination against each patient’s own tumor, success is likely to be achieved by a personalized combination of the most effective radiation regimen and immune modulator(s)."
 |
| Click to enlarge. Image source |
"Radiation has long been a pillar of cancer care, alongside surgery and chemotherapy. But what if it could be used in a new way - to trigger a set of immune responses that transform tumors into killing machines, a type of in situ vaccine?
Silvia Formenti, M.D., has re-visited radiation as a tool that turns tumor from foe to friend. She uses it to help activate the immune system and prime the tumor microenvironment so that the cancer is more responsive to other treatments. In some cases, she has even been able to kill one tumor by irradiating another, inducing what is called the “abscopal effect.”"Correction (April 19, 2016)
As noted below under [Australian] Standard of Care? (April 11, 2016), PV-10 was referenced (in a trial of another compound) as an "existing standard of care" (SOC) (along with isolated limb infusion, or ILI) at Brisbane, Australia's Princess Alexandra Hospital (PAH) based on data derived from hospital records between 1996 and 2014. PAH was a Provectus melanoma Phase 2 trial site, will be a pivotal melanoma Phase 3 and melanoma combination therapy (of PV and pembrolizumab) Phase 1b site, and is an expanded access/compassionate use program (EAP/CUP) (U.S. terminology) site. The U.S. EAP/CUP appears to be called a Special Access Scheme (SAS) in Australia.
PAH replied to me (today) on the topic of SOC; I had e-mailed the hospital (on April 11th) for clarification of the use of SOC. The hospital staff member said {underlined emphasis is mine}:
"PV-10 is certainly not considered SOC at our hospital. The drug is only available either through a clinical trial or via the Special Access Scheme. For patients accessing the drug through our SAS scheme, the company, Provectus, has been willing to supply drug on a case by case application, free of charge to the patient. There has been a strict criteria that has needed to be followed. Our preference is to treat patients with PV-10 over ILI where the disease is appropriate to do so. We find this to be better for the patients, and more cost effective for the hospital. I want to highlight that PV-10 is NOT standard of care. I was not aware that the below study stated that PV-10 was SOC. I will highlight this incorrect statement to the PI. This study has yet to open for recruitment and I am unsure when it will open."The study referenced in the last sentence would appear to be Topical Imiquimod or Diphenylcyclopropenone for the Management of Cutaneous In Transit Melanoma Metastases – A Phase II Single Centre Prospective Randomised Pilot Study, where PV-10 was referenced as an SOC along with ILI.
"Hepatocellular Carcinoma from an Immunologic Perspective" (April 17, 2016)
Updated below.
While waiting for (a) Provectus' Dr. Eric Wachter, PhD to unveil his "Phase 1b/2 Combination Strategy" for hepatocellular carcinoma (HCC) and standard of care pairings (e.g., sorafenib, transarterial chemoembolization [TACE], radiofrequency ablation [RFA], etc.) and (b) Godot, I read "Hepatocellular Carcinoma from an Immunologic Perspective," Greten et al., Clin Cancer Res; 19(24); 6678–85 (2013).
The December 2013 article's abstract notes {underlined emphasis is mine}:
Hepatocellular carcinoma is the third most common cancer worldwide. It is an inflammation-associated cancer. Multiple investigators have demonstrated that analysis of the tumor microenvironment may be used to predict patient outcome, indicating the importance of local immune responses in this disease. In contrast with other types of cancer, in which surgery, radiation, and systemic cytotoxic chemotherapies dominate the treatment options, in hepatocellular carcinoma locoregional treatments are widely applied. Such treatments induce rapid tumor cell death and antitumor immune responses, which may favor or impair the patients’ outcome. Recent immunotherapeutic studies demonstrating promising results include trials evaluating intratumoral injection of an oncolytic virus expressing granulocyte macrophage colonystimulating factor, glypican-3 targeting treatments, and anti-CTLA4 treatment. Although some of these novel approaches may provide benefit as single agents, there is a clear opportunity in hepatocellular carcinoma to evaluate these in combination with the standard modalities to more effectively harness the immune response.
From the journal above, transition to an April 2016 Knoxville News Sentinel online newspaper article entitled "UTMC enrolls first US patient in liver cancer study," which discussed the ongoing Phase 3 Randomized, Open-Label Study Comparing Pexa Vec (Vaccinia GM CSF / Thymidine Kinase-Deactivated Virus) Followed by Sorafenib Versus Sorafenib in Patients With Advanced Hepatocellular Carcinoma (HCC) Without Prior Systemic Therapy. UTMC is The University of Tennessee Medical Center. Trial endpoints include:
Implication, Skepticism, MOA, Show Me... (April 17, 2016)- Primary: Overall survival (OS),
- Secondary: Time-to-progression (TTP), Progression-free survival (PFS), Overall response rate (ORR), Disease control rate (DCR), Proportion of patients whose best overall response during their participation in the study is either CR, PR, or stable disease (SD), Incidence of Adverse Events (AE) and Serious Adverse Events (SAE), and Time-to-Symptomatic Progression (TSP).
Pexa-Vec is the former JX-594, an oncolytic poxvirus modified by insertion of b-galactosidase. See SillaJen and Jennerex's intertwined and eventually combination of histories. The Greten paper above notes {underlined emphasis is mine}:
"Although initial studies using a JX-594 prototype were done in patients with melanoma, intratumoral application of JX-594 was shown to be safe in a phase I study in hepatocellular carcinoma. Results from a second study in 30 patients with advanced hepatocellular carcinoma were recently reported. In this trial, patients were randomized for treatment with low-dose (108 PFU) or high-dose (109 PFU) JX-594, and safety, patient outcome, and induction of immunity against both cancer and vaccinia were studied. JX-594 was administered by imaging-guided intratumoral injection on days 1, 15, and 29. The objective response rate was 15%, and an intrahepatic disease control rate of 50% was achieved."
Objective response [rate] = complete response (CR) + partial response (PR) + stable disease. Disease control [rate] = CR + PR + SD. Articles on the above noted JX-594 trials can be found here:
At this point reconsider Provectus' initial liver cancer data presented in 2015 (the same data, different poster presentations): "Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver," 6th Asia-Pacific Primary Liver Cancer Expert Meeting, Abstract #P1-84, and "Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver," ESMO 17th World Congress on Gastrointestinal Cancer, Abstract #P-116. I had to reread the posters to remind myself of how to better understand the data therein. Use the ESMO World GI 2015 poster abstract as the starting point:
 |
| Click to enlarge. |
First, "[a]ll injected tumors were stable in size at 28 days..." means disease control of 100%, which did not strike me until reading the materials above on the abovementioned oncolytic virus and HCC. Second, which I had digested when the Provectus HCC abstracts came out, the patient group had an "...objective response of 50%." Third, note the patient survival observations below from the four patients noted in Provectus abstract (the patients are 0001, 0004, 0005 and 0006), where patients were alive at July 2015 for 42 to 54 months:
 |
| Click to enlarge. |
Now compare to the Pexa-Vec/JX-594 clinical data collected thus far: 15% objective response (cf. PV-10 of 50%), 50% disease control (100%), and median patient or subject survival of 6.7 months (low dose) to 14.1 months (up to 54 months).
The takeaway for me, more broadly speaking, is that intralesional (IL) therapies (e.g., oncolytic viruses, PV-10, etc.) -- or intratumoral (IT) therapies...therapies that are directly injected into cancer patients' tumors and lesions -- are here to stay, and not just for melanoma. They are here to stay because of their two-step approach to fighting cancer. Step 1 is tumor or lesion ablation, the more and better destruction the better. Step 2 is to kick-off, via Step 1's ablation or tumor destruction, an antigen cascade that generates immunologic signalling and an immune response. That immune response might then help the checkpoint inhibitors do their job better.
How better to deal with the so-called tumor microenvironment than with a needle injecting and hopefully destroying the microenvironment of the tumor -- in fact and hopefully, the complete environment of the tumor, and in so doing educate and train the immune system to do the same thing elsewhere and around the body.
Updated (4/18/16): To be fair to the comparison of PV-10 and Pexa-Vec/JX-594, one should not compare clinical trial or study data too much. It's instructive, but perhaps not much more than that. PV-10's response rate the Phase 1 study of the first six liver cancer patients probably is better represented as 2 out of the initial N of 6, rather than 2 out of the 4 who had long-term assessment (although maybe that, the fact that some [4] had long-term assessment and some presumably did not [2] indeed is better and appropriate).
To be fair to Eric, and as I wrote under [Possible] HCC and Liver Mets Phase 1b/2 "Combination" Clinical Trial Strategy (April 2, 2016) below, I'm pretty sure he has the HCC trial protocol effectively done, but of course I am impatient as to when he'll raise the curtain on that work.
Updated (4/18/16): To be fair to the comparison of PV-10 and Pexa-Vec/JX-594, one should not compare clinical trial or study data too much. It's instructive, but perhaps not much more than that. PV-10's response rate the Phase 1 study of the first six liver cancer patients probably is better represented as 2 out of the initial N of 6, rather than 2 out of the 4 who had long-term assessment (although maybe that, the fact that some [4] had long-term assessment and some presumably did not [2] indeed is better and appropriate).
To be fair to Eric, and as I wrote under [Possible] HCC and Liver Mets Phase 1b/2 "Combination" Clinical Trial Strategy (April 2, 2016) below, I'm pretty sure he has the HCC trial protocol effectively done, but of course I am impatient as to when he'll raise the curtain on that work.
At AACR Novartis tweeted "The three-step immune response to cancer exposes new lines of attack on tumors," which implicated the multiple checkpoint roles of Tim-3 {purple arrows below are mine}.
 |
| Click to enlarge. Unedited image source |
 |
| Click to enlarge. Image source |
PV-10 is potentially implicated in each and every step of Chen & Mellman's Cancer Immunity Cycle. The investigational drug is as much about “starting the engine” or “stepping on the gas pedal” of the immune system, as it is about “releasing the brakes,” as shown below.
 |
| Click to enlarge. Image source |
One would have to think Moffitt Cancer Center's mechanism of action work, whenever it is published, is necessary and vital for anyone trying to wrap his or head around PV-10.
Managed entry agreements/Managed access programmes (April 16, 2016)
In February Provectus' CFO/COO and interim CEO Peter Culpepper discussed an "expense reimbursement scheme in Australia" during his presentation at the 19th Annual BIO CEO & Investor Conference in New York {underlined emphasis is mine}:
"We’re also, on the third bullet point, looking at strategic activity. These can take different forms, the regional licenses and collaborations, equity stake with big pharma. We’re also now looking at, on the fourth point which is a new development, there’s large grants available through the EU, Singapore, expense reimbursement scheme in Australia, to enable companies like Provectus, a development company, to continue to operate clinically, have a benefit to support that work in particular jurisdictions, not only because the patients have a need but because these are forward-looking entities that want to develop the life science activity. So we’re doing this now in the EU, Singapore and Australia as examples."The associated presentation slide is below {purple edit is mine}:
 |
| Click to enlarge. Image source |
My questions about this would be, and I hope to ask these of the company's CTO Dr. Eric Wachter, PhD on Provectus' 1Q16 business update conference call in May:
- #1: If PV-10 is considered an SOC at an Australian hospital (and if there's one hospital, I have to believe [and I do believe] there are more), has it been, is it being or will it be offered to suitably staged (diagnosed) Australian melanoma patients in this SOC capacity in the country as a whole?
- #2: If the answer to #1 is yes, under what government or other program is PV-10 being made available to Australians? What are the costs and benefits to Provectus? What are the pros and cons to the company of doing this?
- #3: If the answer to #1 is yes, what impact does this potential or possible activity (i.e., use as an SOC) and program (i.e., a MAP) have on (a) Provectus' pivotal Phase 3 trial, given that management identified the importance of Australian clinical sites to the registration study and its enrollment (and enrollment rate), and (b) potential marketing approval by the TGA?
Consider the article "Access to new cancer medicines in Australia: dispelling the myths and informing a public debate" by Vitry et al., J Pharm Policy Pract. 2016; 9: 13, which describes at least two approaches to registration and funding of cancer medicines in Australia:
- Managed entry agreement (MEA): "Most MEAs to date in Australia have been financial agreements that involve price or volume rebates, or agreements that link the continuation of funding to evidence of benefit documented at the individual patient level."
- Managed access program (MAP): MAP "...is conditional on the subsequent provision of favourable scientific evidence of population-level efficacy. In most cases, the manufacturer would be expected to pay a rebate to the Government should these medicines fail to deliver on their claimed benefits."
Does Provectus have or is it trying to attain an MEA or enter into a MAP or sum such approach/relationship with the Therapeutic Goods Administration (TGA), Pharmaceutical Benefit Advisory Committee (PBAC) and/or Pharmaceutical Benefits Scheme (PBS)?
A March 2015 working document entitled "Framework for the Managed Access Programme for submissions to the PBAC" notes {underlined emphasis is mine}:
The Managed Access Programme (MAP) is a mechanism that enables the Pharmaceutical Benefits Schedule (PBS) listing of products, under special circumstances of high unmet clinical need, on terms that allow for the resolution of otherwise unacceptable clinical or economic uncertainty for the Pharmaceutical Benefits Advisory Committee (PBAC). It seeks to enhance the quality and strength of evidence provided to decision-makers in reimbursement applications...
In most cases where there are not sufficient high quality data to clarify the efficacy and safety of a drug, randomisation in a Human Research Ethics Committee approved clinical trial is an appropriate standard of care. Such trials are not subsidised via the PBS. The application of the MAP mechanism is thus restricted to submissions where the PBAC agrees that, based on the data available, the proposed drug would provide a significant net clinical benefit, but the PBAC is uncertain about the magnitude of that benefit and therefore the price that should be paid, and when:
-- there is a high and urgent unmet clinical need for the drug;
-- the PBAC would not otherwise recommend the listing of the drug at the proposed price because the extent of the clinical effect is uncertain and/or there is a high cost to the PBS overall and/or on a per patient basis; and
-- there is evidence that can reliably be reported and evaluated within a reasonable timeframe, which the PBAC is satisfied would resolve the identified area of uncertainty.
Updated below.
An abstract at AACR 2016 about a retrospective analysis -- derived from a Phase 2 study of intralesional (IL) electroporation of plasmid interleukin-12 (epIL12) -- of the combination of epIL-12 and immune checkpoint inhibitors (i.e., anti-PD-1/PD-L1 agents) revealed itself today, Intratumoral electroporation of plasmid IL-12 can prime response to anti-PD1/PD-L1 blockade in patients with Stage III/IV-M1a melanoma.
OncoSec's epIL-12 is shown below as IL-12 in the pipeline chart from a presentation by melanoma, IL and PV-10 key opinion leader Dr. Sanjiv Agarwala (the original chart was prepared by melanoma, IL and T-Vec KOL Dr. Robert Andtbacka);
 |
| Click to enlarge. |
Patients who received IT-pIL12-EP and later went on to receive a PD-1/PD-L1 inhibitor demonstrated a high PD-1/PD-L1-associated response rate (64% CR+PR). Interestingly, patients who received a PD-1/PD-L1 inhibitor with no intervening therapy post-IT-pIL12-EP had a BOR of 75% (CR+PR). These data suggest that IT-pIL12-EP may prime for responses to PD-1 blockade. Patient tumor samples are being evaluated for TIL status and gene expression analysis to determine the mechanism of IT-pIL12-EP priming. A prospective clinical trial combining IT-pIL12-EP and pembrolizumab in advanced melanoma is ongoing at the University of California San Francisco.This retrospective analysis would have generated some of the highest response rate data of the melanoma combination work being done or analyzed.
 |
| Click to enlarge. |
Note in the table above that the epIL12 "combos" have higher complete responses (CRs) (and also objective responses) than the T-Vec combos. This may be due to epIL12 having the ability to (i) generate comparable tumor destruction to T-Vec and (ii) potentially have better immunologic signalling. Consider the exploratory analyses Provectus, Amgen and OncoSec each did on complete responses achieved on treated lesions:
While OncoSec started presenting preliminary data of epIL12's melanoma Phase 2 trial in 2012, it has not yet begun a pivotal Phase 3 trial. This company began a melanoma Phase 2 trial combining epIL12 and pembrolizumab in 2014 (trial protocol is here, company press release is here), which should build on the retrospective analysis presented at AACR this year.
The "jargon trend" appears to be that in the age of the tumor microenvironment, large amounts or greater levels of tumor destruction are necessary to begin the antigen cascade that kicks off the cancer immunity cycle, and to generate more and greater infiltration of T-cells into distant (in the case of IL agents, uninjected or "bystander") tumors.
Updated (4/16/16): H/t a shareholder. OncoSec's combination trial, a Phase 2 single-arm trial (SAT), with no discussion in the protocol of transitioning to a later-stage randomized controlled trial (RCT), is recruiting both Stage III and IV melanoma patients (i.e., metastatic melanoma), like Amgen and Merck's combination trial of T-Vec and pembrolizumab (i.e., Stage IIIB to Stage IV M1c). Provectus' combination study only is recruiting Stage IV patients. One would expect Stage III patients to have better response rates and overall survival than Stage IV patients.
OncoSec's trial is an investigator-initiated trial, and as I noted above, there is no protocol component transitioning to an RCT. Provectus' trial would be expected to transition into an expanded phase 2 RCT of PV-10 + pembrolizumab vs. pembrolizumab alone (i.e., PV-10 + standard of care [SOC] vs. SOC).
Although PV-10 achieved a 53% CR in all lesions treated in Provectus' Phase 2 trial (as noted above), the CR rate of treated lesions in the subgroup of patients who had all their disease treated -- the patient population of the company's pivotal melanoma Phase 3 trial -- was 74%.
 |
| Click to enlarge. Image source |
What should be key to PV-10-pembrolizumab study would be the proportion of disease treated with PV-10 in order to kick-off the antigen cascade that pembrolizumab could take advantage of.
"Eleven of 70 pts (16%) have been treated with MOXR0916 for > 6 months (≥9 cycles) with a best response of stable disease per RECIST v1.1."
OX40 is a stimulatory agent found in Step 3 below.
 |
| Click to enlarge. Image source |
Whether you refer to them as more popularly as intralesional (IL) agents, or as locoregional immunomodulatory agents, investigational drug PV-10 is routinely mentioned by key opinion leaders working with Provectus and KOLs not working with the company together with other IL agents, such as approved oncolytic virus-based drug talimogene laherparepvec (T-Vec, or trade name Imylgic), other investigational oncolytic virus-based drugs (e.g., Viralytics' Coxsackievirus A21, Newcastle disease virus patented by ipilimumab creator Dr. James Allison, PhD and others, Takara Bio's HF10, etc.), and OncoSec's plasmid IL-12 with electroporation.
T-Vec has been approved. PV-10 is a late-stage asset (i.e., in a pivotal Phase 3 trial). The others have not had their Phase 3 trials finalized yet.All appear to be exploring early-stage combination trials with immune checkpoint inhibitors.
At December's Melanoma Bridge 2015 conference in Naples, Italy, IL KOLs Drs. Sanjiv Agarwala (PV-10 KOL) and Igor Puzanov (T-Vec KOL), MD both discussed PV-10 among other IL agents. The former is from St. Luke's Cancer Center in Bethlehem, Pennsylvania, while the latter is from Vanderbilt University in Nashville, Tennessee.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
| Click to enlarge. |
 |
| Click to enlarge. |
From Chinese Guidelines on the Diagnosis and Treatment of Melanoma (2015 Edition), published in Chinese Clinical Oncology in November 2015. Purple emphasis below is mine.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Also:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below.
#1. Australia's Peter MacCallum Cancer Institute (Melbourne) will present the inaugural Multidisciplinary Management of Locoregionally Advanced Melanoma - From Surgery to Immunotherapy conference in August 2016. Sponsors are Bristol-Myers (platinum) and Amgen (gold). PV-10 should be discussed in the presentation Injectable therapy versus limb infusion for intransit disease: Which treatment for which patient? by Professor/Dr. Mark Smithers, MD.
#2. If PV-10 is a standard of care (SOC) for in-transit melanoma at one Australian hospital, is it SOC for the said disease presentation at more Australian hospitals?
#3. A pharmaceutical company may have accessed the blog from a non-corporated named router in the city where it has its headquarters. As such, I revised my table -- see Visitors (April 6, 2016) below -- in the 1-5 rank grouping, from C to A.
 |
| Click to enlarge. |
#6. I was struck by the Princess Alexandra Hospital study's protocol, which in part said: "Data for these control treatments were derived from institutional records between 1996 - 2014." "These control treatments" refer to isolated limb infusion and PV-10.
Princess Alexandra became a metastatic melanoma Phase 3 trial site in December 2007, Adds Second Center to Phase 2 Clinical Trial of PV-10. It, along with other Australian clinical trial (and possibly other sites that only wanted to treat patients), became a compassionate use program (CUP) in June 2009, to Offer Compassionate Use of PV-10 for Non-Visceral Indications in Cancer Patients. I would presume the data set comprised (i) Phase 2 patients from 2007 to perhaps 2009, when the trial completed accrual, and (ii) CUP patients from perhaps 2009 to perhaps 2014. This situation might also suggest Princess Alexandra is one of the places from where a CUP-oriented peer-reviewed journal article might originate.
If Princess Alexandra deemed the data to be dispositive on the issue of PV-10's safety as well as clinical activity and benefit (in order to determine it to be an SOC for in-transit melanoma), would it be reasonable to believe the FDA might as well once the Agency has pivotal melanoma Phase 3 data in hand?
 |
| Image source |
See As pervasive as aspirin (October 11, 2015) on the blog's Archived News IV page. Several years ago, Provectus' CTO Dr. Eric Wachter, PhD extemporaneously said (paraphrasing) he'd liked to see PV-10 eventually be used as pervasively as aspirin. As such, it was with no small amount of irony to initially read the title of Moffitt Cancer Center's PV-10-related SITC 2015 abstract title, Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1. See on the same blog news page SITC 2015 Preview: Intralesional Rose Bengal in melanoma elicits tumor immunity via HMGB1 (October 5, 2015) and HMGB1 (October 6, 2015).
A Google News search of HMGB1 (as of this writing) yields several articles about a recent study of salicylic acid targeting HMGB1. Salicylic acid is the active ingredient of aspirin. The study, entitled Aspirin′s Active Metabolite Salicylic Acid Targets High Mobility Group Box 1 to Modulate Inflammatory Responses, noted in its abstract:
"Salicylic acid (SA) and its derivatives have been used for millennia to reduce pain, fever and inflammation. In addition, prophylactic use of acetylsalicylic acid, commonly known as aspirin, reduces the risk of heart attack, stroke and certain cancers. Because aspirin is rapidly de-acetylated by esterases in human plasma, much of aspirin’s bioactivity can be attributed to its primary metabolite, SA. Here we demonstrate that human high mobility group box 1 (HMGB1) is a novel SA-binding protein. SA-binding sites on HMGB1 were identified in the HMG-box domains by nuclear magnetic resonance (NMR) spectroscopic studies and confirmed by mutational analysis. Extracellular HMGB1 is a damage-associated molecular pattern molecule (DAMP), with multiple redox states. SA suppresses both the chemoattractant activity of fully reduced HMGB1 and the increased expression of proinflammatory cytokine genes and cyclooxygenase 2 (COX-2) induced by disulfide HMGB1. Natural and synthetic SA derivatives with greater potency for inhibition of HMGB1 were identified, providing proof-of-concept that new molecules with high efficacy against sterile inflammation are attainable. An HMGB1 protein mutated in one of the SA-binding sites identified by NMR chemical shift perturbation studies retained chemoattractant activity, but lost binding of and inhibition by SA and its derivatives, thereby firmly establishing that SA binding to HMGB1 directly suppresses its proinflammatory activities. Identification of HMGB1 as a pharmacological target of SA/aspirin provides new insights into the mechanisms of action of one of the world’s longest and most used natural and synthetic drugs. It may also provide an explanation for the protective effects of low-dose aspirin usage." {Underlined emphasis is mine}Updated 4/11/16: #8. A sort of recent article on intralesional (IL) agents (published in January 2016, accepted in July 2015, and submitted in March): Developments in Intralesional Therapy for Metastatic Melanoma. Authors with relevant disclosures include Moffitt Cancer Center's Drs. Amod Sarnaik (Provectus) and Jonathan Zager (Amgen, Provectus), MD. It appears to be a follow-up of a 2014 article entitled Intralesional therapy for metastatic melanoma. The newer article's conclusion is {underlined emphasis is mine}:
The standard of care for patients with locoregional advanced or metastasized melanoma is to render a patient free of disease as long as the disease is sufficiently limited. When this is no longer feasible, intralesional therapy is a possible option due to its good local response and tolerable adverse-event profile, as well as the option to provide outpatient treatment. A bystander effect observed in various agents adds to its appeal. During the last few decades, other agents have been tested for intralesional therapy with varying success. Many intralesional compounds now available produce a broad range of local response rates. The ideal agent should have a low toxicity profile, be easy to administer, lead to fast responses, and trigger a systemic immune response, thereby creating a bystander effect. These criteria were predominantly met in the results of trials using 10% rose bengal and talimogene laherparepvec in up to 40% of study patients.
Most agents (Bacille-Calmette-Guerin, interferon, granulocyte macrophage colony-stimulating factor) demonstrated inconsistent rates of efficacy, but the treatment field changed when velimogene aliplasmid, 10% rose bengal, and talimogene laherparepvec were introduced. Velimogene aliplasmid did not meet its primary end point in a phase 3 trial, but talimogene laherparepvec did meet its phase 3 trial objectives, demonstrating a survival benefit in select study patients. The results of phase 2 results of 10% rose bengal trials are also promising and a phase 3 is still recruiting (NCT02288897). Other options include combinations of intralesional therapies and systemic therapies, including ipilimumab/talimogene laherparepvec and pembrolizumab/rose bengal (NCT02557321).
Our treatment approach should be individualized per patient, based on the extent of disease, tumor characteristics, and disease-free interval, as well as patient characteristics such as age, performance status, and comorbidities, and work to maintain quality of life for as long as possible. An appropriate approach is often not a single therapy but rather a combination of injectable treatments, regional perfusion therapies, and systemic therapies."[Australian] Standard of Care? (April 11, 2016)
Updated below.
Note the following from the section entitled Comparator / control treatment of a 2015 Australian trial conducted at Princess Alexandra Hospital in Brisbane entitled Topical Imiquimod or Diphenylcyclopropenone for the Management of Cutaneous In Transit Melanoma Metastases – A Phase II Single Centre Prospective Randomised Pilot Study {underlined and bolded emphasis is mine}:
"Historical control. Following completion of the TIDAL Melanoma Study a comparison to retrospective data will be made for patients treated with isolated limb infusion and PV-10 treatments. These modalities represent the existing standard of care at our institution. Data for these control treatments were derived from institutional records between 1996 - 2014.
PV-10 treatment involves an intra-lesional injection of Rose Bengal solution into in transit melanoma metastases and has been investigated as a part of another phase II trial completed at our centre. For the formal treatment results of this investigation please refer to: Thompson JF, et al. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann Surg Oncol. 2014.
Isolated limb infusion involves isolating the vascular supply to a limb affected by in transit melanoma metastases and instilling a loco regional chemotherapeutic agent, melphalan. For the formal institutional treatment results of from therapy please refer to: Barbour AP, et al. Isolated Limb Infusion for Malignant Melanoma: Predictors of Response and Outcome. Ann Surg Oncol. 2009."
 |
| Click to enlarge. |
- Phase 2 Study of Intralesional PV-10 for Metastatic Melanoma,
- Expanded Access Protocol for PV-10 for Cutaneous or Subcutaneous Tumors,
- PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma, and
- PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma.
"Isolated limb perfusion (ILP) is a method of drug delivery that is designed to deliver high local
doses of chemotherapeutic agents to isolated anatomic regions while avoiding systemic toxicity." "Isolated Limb Perfusion/Infusion for Malignant Melanoma" is reimbursable in the U.S. (Note: Thompson below: link)
 |
| Click to enlarge. Image source |
- In-transit disease presents in a number of different ways. For patients with small dermal deposits, physicians are unable to treat them with PV-10 because the lesions are too small to be injected. These patients have been treated with DPCP.
- The proposed study of imiquimod and DPCP would compare the two.
- The control in the study is a historical control using the historical data of ILI and PV-10 collected from 1996 to 2014.
- While ethics approval was attained for the study in November 2015, the trial has not started yet. The "Anticipated date of first participant enrolment" had been february 2016. As of this writing the status of the trial is "not yet recruiting."
Note the placement of ILI, topical imiquimod and other treatments for in-transit disease on the NCCN guidelines for melanoma (purple emphasis is mine):
 |
| Click to enlarge. |
Provectus' pivotal melanoma Phase 3 trial inclusion criteria is (underlined emphasis is mine):
"Recurrent, satellite or in-transit locally advanced cutaneous or subcutaneous melanoma metastases (i.e. AJCC Stage IIIB, IIIC or Stage IV M1a with no active nodal or distant cutaneous or subcutaneous metastases); subjects whose Stage IV M1a disease has been treated must be free of disease at distant cutaneous, subcutaneous and nodal sites for at least 30 days prior to randomization."A List of Things (April 9, 2016)
Updated below.
It would seem for some number of sites (e.g., some or all of the projected 25 U.S. sites and 10 Australian sites) that the original pivotal melanoma Phase 3 trial protocol was sent to them at the same time, or maybe at least in 2014 when the protocol was listed on ClinicalTrials.gov as "First Received" on 11/4/14. St. Luke's Cancer Center had a 2014-based identification (ID) number of 2014-117. Reviewing ID numbers at Huntsman Cancer Institute, the number assigned to the Phase 3 trial (81139) appears to indicate this site also may have received the protocol in 2014. See the table below. The trial protocol was finalized on 3/12/15, and then amended on 3/15/16.
 |
| Click to enlarge. |
As difficult as it might be for me as an investor in the company to fully appreciate all of the time, Provectus (i.e., CTO Dr. Eric Wachter, PhD) does not publicize all of its R&D activities for presumably a number of reasons, such as not wanting to provide a road map for competitors. I might not agree with all of the reasons, however. It is interesting to note that Amgen developed a Phase 2 trial protocol (First Received: 1/14/16) for Talimogene Laherparepvec (T-VEC) for Breast Cancer Local Recurrence.
On a business update conference call or during an investor conference presentation (I currently cannot [for the life of me] locate the source so as to provide it a link here), Provectus' CFO/COO and interim CEO Peter Culpepper had said the company "owned" the work being done in the Phase 1b/2 program combining PV-10 and pembrolizumab in patients with advanced (Stage IV) melanoma. Since Provectus is sponsoring and conducting the trial work PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma, all data from it are owned by the company. Typical of any sponsored clinical trial, while top line data customarily may be reported at conferences and in journals, the full data set remain under the trial sponsor's control. Importantly, the full data set that global regulators review remain the property of the sponsor, unless it enters into a deal that affords access or rights to the other party.
In June 2015 Dynavax Technologies Corporation (NASDAQ: DVAX) and Merck & Co. entered into a collaboration combining the former's intratumorally administered SD-101 and pembrolizumab. See Merck and Dynavax Announce New Collaboration Investigating the Combination of Immuno-Oncology Therapies. Under the terms revealed, Dynavax would sponsor and fund the SD-101/pembro study (Merck would sponsor and fund the SD-101/MK-1966 work). SD-101 is a toll-like receptor 9 (TLR9) agonist, which appears to have a relationship with HMGB1. While UCLA's Dr. Antoni Ribas, MD is the principal investigator of the combination study work A Phase 1b, Open-label, Multicenter, Dose Escalation Trial of Intratumoral Injections of SD-101 in Combination With Pembrolizumab in Patients With Metastatic Melanoma, St. Luke's Cancer Center's Dr. Sanjiv Agarwala, MD also is involved.
Provectus' initial intellectual property surrounding combination therapy was awarded in August 2015, Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer (the so-called "parent" award [formerly application]). Subsequently, in October, three so-called "daughter" (continuation) applications were made that addressed, among other things combined therapeutic regimens, and targeted therapies (the aforementioned patent award explicitly covered among other things immunotherapies -- here, here and here). These three continue to proceed through the US Patent and Trademark Office (USPTO) process. For complicated filings the USPTO often requests an application be broken up into smaller units to facilitate examination, which may lead to multiple daughter applications from the original parent one as noted above. Sometimes the applicant "traverses" this request -- register a formal disagreement with the examiner's findings the requested breakup is inappropriate. In this case the applicant specifies an exemplary topic for the examiner to focus on, presumably allowing the examiner to review pertinent prior art without requiring that he or she look into all possible permutations. If there are problems later, such as other prior art coming to light that would invalidate the outcome of the examination, it is the applicant’s responsibility to defend this shortcoming. An example of this in Provectus' case would be when the parent above originally was filed; the examiner issued a restriction requirement that resulted in the cleaving out of the checkpoint inhibitor portion of the application (the relevant daughters above). There is another restriction requirement in the process, and thus another round of activity.
Updated (4/10/16): #1. The Huntsman information above would lend further credence to the belief Eric probably sent or did send the pivotal melanoma Phase 3 protocol to all U.S. and Australia sites he originally planned to be in the trial. See also June 26, 2015 blog post Eric's Process.
#2. There very likely are more than one CUP site that are not public because all such sites want to do is treat patients with PV-10. They do not want to recruit patients for clinical trials.
#3. There very likely is R&D work going on involving PV-10 that is not visible.
#4. Owning the Phase 1b/2 PV-10-pembrolizumab combination trial results for advanced melanoma means that while regulators may see the full data set, Merck & Co. (and/or any other company) doesn’t get access to the dataset unless they do a deal with Provectus.
#5. I think Merck may have decided, in part, to use a PV-10 "comparable" in SD-101, an intratumoral (intralesional) drug, when Provectus said it wanted to get paid to combine PV-10 with pembrolizumab.
#6. Provectus is proceeding with expanding its combination therapy intellectual property. The current activity with the US PTO may require them to potentially split up the daughter combo patent applications into granddaughter patent apps (maybe).
#7. Disease control rate, aka locoregional disease control, the primary endpoint of the Amgen T-Vec breast cancer trial, equals complete response (CR) + partial response (PR) + stable disease (SD). Provectus reported lororegional disease control in its melanoma Phase 2 trial. The idea behind this approach was, in part, to demonstrate that injection of PV-10 into cancerous lesions could destroy (i.e., CR), prevent (i.e., PR) or forestall (i.e., SD) melanoma from spreading from Stage III disease to Stage IV -- which is one of the hypothesis (or thesis) or the pivotal melanoma Phase 3 trial underway. I think Amgen is following Provectus' thesis, and explicitly incorporated Provectus' observation as a design parameter in the breast cancer trial (including "Photographs taken of lesions on chest at baseline, at Cycle 2, Cycles 4, 8, and 10 if the doctor thinks it is needed, Day 1 of Cycle 5, Day 1 of Cycle 7, and then every odd numbered cycle after that").
Consider the following slides from melanoma, intralesional (IL) agent and PV-10 key opinion leader St. Luke's Cancer Center's Dr. Sanjiv Agarwala, MD's presentation at SMR 2010 (purple arrows are mine):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Image source |
Provectus issued a PR on March 16th, Amends Protocol for Phase 3 Study of PV-10 in Treatment of Locally Advanced Cutaneous Melanoma, that appeared [to me] to broaden the applicable patient population of the company's pivotal melanoma Phase 3 trial (a registration study), PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma, which if successful could (should) lead to a broader label for the investigational drug PV-10 if/when approved.
The patient population increased in several ways, and thus the potential label for PV-10 broadened (or could provide Provectus with "a label advantage") because, in the amended protocol:
- The applicable disease stage increased from Stage III B-C to include Stage IV M1a,
- Applicable patients included those who failed immune checkpoint inhibitor drugs (at least one), and
- Applicable patients included those who failed targeted therapy with BRAF or combined BRAF/MEK inhibitors.
To be clear, Rennert only was referencing or referring to Lion Biotechnologies in his tweet below. Lion has not yet entered a pivotal Phase 3 trial (for metastatic melanoma) yet. This company utilizes tumor infiltrating lymphocytes (TILs) for Stage IV disease ("In Phase 2 clinical trials, patients treated with this product demonstrated objective response rates of 49%"). Lion expects to "initiate a Phase 3 trial in 2016 in refractory metastatic melanoma patients."
 |
| Click to enlarge. Tweet image source. |
 |
| Click to enlarge. Tweet image source. |
On another note, a cursory examination of current or recent clinical/medical consultants includes:
- Dr. Paul Chapman, MD, Memorial Sloan Kettering Cancer Center; and Weill Cornell Medical College, New York,
- Dr. Grant McArthur, MD, Peter MacCallum Cancer Centre, Melbourne, Australia, and
- Dr. Georgina Long, MD, PhD, University of Sydney, Sydney, Australia.
Over the last four months there were several visits by at least 14 U.S. and international pharmaceutical and biotechnology companies (that I could identify) to the blog. Visits comprised multiple visits on multiple days by multiple IP addresses, multiple visits on multiple days by a single IP address, a single visit on multiple days by multiple IP addresses, and a single visit on a single day by a single IP address. These data (not comprehensive) are summarized in the table below.
 |
| Click to enlarge. |
Included in the list visitors to the blog thus far today could have been a [Boehringer Ingelheim] investigational site in Kaohsiung, Taiwan (search ClinicalTrials.gov for "Kaohsiung, Taiwan HCC:" 57 studies).
Risk Factors (April 5, 2016)
(aka 10-K Notes, part 2)
As I noted under #10 in 10-K Notes, part 1 (March 30, 2016) below, I wrote I would address language changes in the K related to Risk Factors, "material weakness," etc. in a subsequent blog news item. One way to undertake this is to compare Risk Factors (K item 1A) language year-over-year. Using an online text comparison tool like Text Compare!, these changes include:
- Existing CY15 10-K Risk Factor: We are subject to securities class action lawsuits and shareholder derivative lawsuits that could adversely affect our business. This litigation, and potential similar or related litigation, could result in substantial damages and may divert management’s time and attention from our business {new language: underlined emphasis},
- The language of the risk headline changed; original (10-K for CY14) language follows: We are subject to securities class action lawsuits that could adversely affect our business. This litigation, and potential similar or related litigation, could result in substantial damages and may divert management’s time and attention from our business.
- Additionally, language changed underneath this item to reflect a status update. It is worthwhile to read this section. See pp. 24-55 of the CY15 10-K filing.
- New Risk: Our former Chief Executive Officer and Chairman of the Board of Directors received travel expense advancements, which may be deemed a violation of Section 402 of the Sarbanes-Oxley Act of 2002 and/or other federal securities laws,
- See p. 26.
- New Risk: We have identified a material weakness in our internal control over financial reporting, and our management has concluded that our disclosure controls and procedures are not effective. We cannot assure you that additional material weaknesses or significant deficiencies do not exist or that they will not occur in the future. If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results or prevent fraud, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price,
- See p. 26.
- New Risk: The resignation of our Chief Executive Officer and Chairman of the Board of Directors, the appointment of our Interim Chief Executive Officer and any search for, and appointment of, a long-term Chief Executive Officer and an Interim Chief Financial Officer creates uncertainties and could have a material adverse impact on our business, and
- See p. 31.
- Existing CY15 10-K Risk Factor: If we lose any of our key personnel, we may be unable to successfully execute our business plan.
- Language changed underneath this item to reflect former Chairman, CEO and a co-founder Dr. Craig Dees, PhD's resignation and departure. See pp. 32.
PV-10's competition in melanoma (April 4, 2016)
Medical writer Walter Alexander wrote an article entitled The Checkpoint Immunotherapy Revolution, What Started as a Trickle Has Become a Flood, Despite Some Daunting Adverse Effects; New Drugs, Indications, and Combinations Continue to Emerge in the March edition of Pharmacy & Therapeutics (h/t InvestorVillage poster rmgillis). The article discussed among other things:
[Possible] HCC and Liver Mets Phase 1b/2 "Combination" Clinical Trial Strategy (April 2, 2016)
Let me try to establish a couple of baseline parameters or points. First, to get an investigational drug approved as a single agent for a cancer disease indication, it would appear the process comprises undertaking a clinical trial program of the drug compound + the standard of care (SOC) vs. SOC. In Provectus' case, for advanced (Stage IV disease) melanoma for example, the approach based on the above construct -- investigational drug + SOC vs. SOC -- would be PV-10 + pembrolizumab vs. pembrolizumab.
Second, in more recent times, as the FDA and pharmaceutical industry have endeavored to improve clinical development, and make it more efficient and effective in the pursuit of quicker trial failures and shorter approval paths (if early-stage trial data bear out), the clinical trial progression of the above could be:
Il Papa (April 2, 2016)
Updated below.
The Vatican's upcoming Third International Regenerative Medicine Conference (April 28-30), a conference that appears to occur every three years, includes several immunotherapy (oncology) Provectus and PV-10 touch points:
Me and my [toffee nut, whole milk] steamer (April 1, 2016)
Director & officer warrant tenders. Form 4s were filed yesterday for Provectus' CFO/COO and interim CEO Peter Culpepper, CTO Dr. Eric Wachter, PhD, and member of the company's board of director Jan Koe. In total they tendered approximately 774K of their 1.28 million non-tradable warrants, representing about 10% of all warrants exchanged by shareholders (and approximately 2% of all non-tradable warrants). A table of their transactions and additional tender information is below.
4Q15/CY15 business update conference call review, part 1. Peter made several quizzical, interesting, curious, etc. comments on the March 30th call. The transcript is here.
A. Keytruda and Opdivo and...
Moffitt's newest work at AACR 2016 is "T cell mediated immunity after combination therapy with intralesional PV-10 and co-inhibitory blockade in a melanoma model," where co-inhibitory blockade covers (like with SITC 2014) anti-CTLA-4, -PD-1 and -PD-L1 antibodies (agents).
B. And liver begins...(when exactly?)
In July 2015, Provectus and Boehringer Ingelheim (BI) China made announcements of their entering into/signing a letter of intent: Provectus Biopharmaceuticals Signs Letter of Intent with Boehringer Ingelheim (China) to Collaborate in Bringing PV-10 to Market in China (Provectus' PR on July 2nd) and Boehringer Ingelheim and Provectus biopharmaceutical company signed a letter of intent - the two sides will cooperate to promote melanoma and liver cancer research new drugs listed in the Chinese mainland, Hong Kong and Taiwan (BI's PR, translated into English, on July 3rd).
On July 10th, Jialing Dai wrote a LinkedIn article entitled Boehringer's Ambition In Liver Cancer. The article's byline read: "Eye On Oncology Market: Boehringer Ingelheim In-Licenses China Rights For A Provectus Late-Stage Oncology Compound." The article was edited by Howard Fields. Dai describes his some of his work as "PharmaDJ is online portal in English to provide tailored services and intelligence of China market for pharma executives. our goal is to build up a bridge to China market for foreign companies doing business in China." In his article Dai wrote:
What did Provectus mean when the company wrote in its July LOI PR (see my underlined emphasis)?
C. More indications into the clinic?
b. ASCO 2016, abstract titles available April 20 (conference June 3-7): Trials in progress?, Phase 1b melanoma combination therapy data?
c. The Vatican, April 28-30: ?
F. Delay in the pivotal melanoma Phase 3 interim assessment?
1Q16 blog readership stats. See below. See also the blog's Blog Readership Statistics page.
Updated Clinical Trial Information (March 31, 2016)
H/t a shareholder: PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma: MD Anderson, recruiting
10-K Notes, part 1 (March 30, 2016)
1. Provectus issued a press release today and filed an associated 8-K regarding its 4Q15/CY15 financial results, Reports Fourth Quarter and Year End 2015 Financial Results, in conjunction with filing its 10-K.
2. 12/31/15 cash and cash equivalents were $14.2 million, compared to a 9/30/15 amount of $18.9 million.
3. The company's monthly cash burn for the quarter appears to have been $1.66 million, +18% quarter-over-quarter (QoQ) (the prior quarter-over-quarter change was +5%).
4. Total stockholders' equity (TSE) was $2.1 million, -62% QoQ (prior QoQ: -21%). The drop primarily comprised a reduction in assets related to "Long-term receivable – settlement, net of discount and reserve for uncollectibility" and an increase in liabilities related to "Accrued settlement expense," which should/could be the company's portion of the class action lawsuit settlement amount.
5. Language regarding runway remain unchanged from the prior quarterly filing: "into 2017."
6. Research and development expense for the quarter was $3.7 million, +36% QoQ (prior QoQ: +31%).
7. Lab supplies and pharmaceutical preparations expense for the quarter was $19K, -96% QoQ (prior QoQ: +58%).
8. A trend graph of the above (starting 1Q14):
9. Provectus should have sufficient cash and sufficiently high enough TSE through 2Q16. The company potentially could have high enough TSE through 9/30/16 (assuming no money raised via dilutive and/or non-dilutive sources), but it may not.
10. I'll address language changes in the K related to Risk Factors, "material weakness," etc. in a subsequent blog news item.
Jargon, Terminology, Themes, Narratives (March 30, 2016)
H/t the Twitter feed of @bradloncar: the agenda of the 6th Annual Cancer Immunotherapy: A Long-Awaited Reality (yellow highlights/edits are mine):
From Provectus' SITC 2012 poster "Generation of an Antitumor Response and Immunity Using a Small Molecule Drug (PV-10)" (yellow edits/highlights are mine):
PV-10 potentially implicated in each and every step of the Cancer Immunity Cycle (March 28, 2016)
From Provectus' 2016 BioPharma Asia Convention presentation (Suntec City, Singapore) entitled "Altering T cells and induced tumor-specific immunity via PV-10 ablation."
Step #5, Infiltration of T cells into Tumors also compares to the name of Moffitt Cancer Center's mechanism of action work entitled Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study.
Updated Clinical Trial Information (March 28, 2016)
Updated below.
PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma: MD Anderson, recruiting
Updated (3/28/16): Assuming (for the sake of quick and easy calculation) the date MD Anderson Cancer Center's Institutional Review Board (IRB) reviewed and approved Provectus' pivotal melanoma Phase 3 trial protocol was the date MD Anderson's recruitment status on ClinicalTrials.gov was changed (if very likely was not; it should be earlier), time taken would have been:
Trick park (March 23, 2016)
Updated below.
MRSA-related. In abstract from the American Journal of Ophthalmology that I believe is current (today?), "Rose bengal and riboflavin mediated photodynamic therapy to inhibit methicillin-resistant Staphylococcus aureus keratitis isolates:"
Tipping point. I was struck by certain of the company's CTO Dr. Eric Wachter, PhD's comments on the 4Q15/CY15 business update conference call:
Visitor. I previously wrote someone from the headquarters of a Big Pharma has regularly visited since Monday, January 11th. See Breadcrumbs (January 27, 2016) below. An update is below:
Updated (3/28/16): I updated the graph and table above to include a second IP address (to the first pictured above) and correct some prior incorrect math.
The [PD-1] core (March 22, 2016)
Updated below, again, once more.
For the moment, set aside Bristol-Myers suing Merck & Co. over the latter's anti-PD-1 drug Keytruda for infringing on the patent of the latter's drug Opdivo. It would seem most of the pharmaceutical industry believes an anti-PD-1 (or an anti-PD-L1) drug compound is required table stakes. A quick 'n dirty analysis indicates 65% of the top 20 oncology-focused pharmaceutical companies (in yellow) have or have access to the next generation (anti-PD-1) or next-next generation (anti-PD-L1) of immune checkpoint inhibitor. The figure rises to ~70% after adjusting for the acquisition of Pharmacyclics (#19) by AbbVie (#18).
In an industry that appears to currently believe a suitable combination therapy for late-stage cancer patients is a checkpoint inhibitor plus something else, owning an anti-PD-1/PD-L1 then would appear to be a necessity to play the game (i.e., table stakes). That's one way to look at the competitive landscape and one's business strategy.
Another way to look at it is to have a/the "something else." Agenus, for example, has illustrated this in one of its investor presentation slides:
In Agenus' depiction, one adds the something else -- a checkpoint modulator or CPM (a stimulatory agent by yet another name) -- to "the core."
So, while Provectus' pivotal melanoma Phase 3 trial is the company's immediate potential ticket to a drug approval, the initial results of Provectus' Phase 1b/2 melanoma combination therapy trial program (combining the something else of PV-10 with the core of Keytruda) inform the respective business strategies of each of the companies on the list above:
In his presentation at the HemOnc Today Melanoma and Cutaneous Malignancies on March 18th/19th (Current Trials with Oncolytic Agents; slides have not yet been published as of this writing) and in a March 19th article (Intralesional therapy ‘here to stay’ for melanoma), melanoma, intralesional agent and PV-10 key opinion leader (KOL) Dr. Sanjiv Agarwala referred to Provectus' melanoma combination trial as a Phase 2 study. Provectus initiated the Phase 1b study (of the Phase 1b-Phase 2 program) in October 2015 (PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma).
My sense is any advancement into the [randomized] Phase 2 portion (pembrolizumab vs. pembrolizumab + PV-10) has been planned or is being planned pending pending data review team recommendations.* So, while a projected Phase 1b enrollment of 24 patients likely is far from complete, safety and efficacy data from whatever enrollment already has been achieved to date might have been or is sufficient to encourage planning for the Phase 2 portion.
In particular, although Agarwala's slide was titled "PV-10 Phase II Combination With Pembrolizumab," there was no mention of the Phase 2's N -- whether the originally planned or estimated 120, or some number higher or lower than 120 that would reflect the preliminary efficacy of the Phase 1b and thus the potential treatment effect size of the combination over the standard of care). It is possible more patients might be required to fully establish the treatment effect and thus the Phase 2's N or, then again, the observed treatment effect may be good enough to establish this calculation.
* For example, consider the process described for the T-Vec + ipilimumab work: Phase 1 results of a phase 1b/2, multicenter, open-label trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) vs ipi alone in previously untreated, unresected stage IIIB-IV melanoma, Puzanov et al., J Immunother Cancer. 2013; 1(Suppl 1): P84 -- see Conclusions.
Agarwala's presentation noted the timeframe for the T-Vec + pembro Phase 1b trial's enrollment and data cutoff; see below: enrollment of about 90 days, and a data cutoff about 100 days after the last patient was enrolled.
Preliminary safety and efficacy (response) data from the Phase 1b were presented at SMR 2015 in November. A trials-in-progress poster was presented at SITC 2015, which preceded SMR by a few weeks; the SITC poster listed investigators from 14 clinical sites (where 16 patients of the 21 patients were assessed or evaluable).
More on the "Intralesional therapy ‘here to stay’ for melanoma" article. I thought the article provided a nice timeline and evolution of the increasing efficacy of an IL agent and an immune checkpoint inhibitor (my underlined emphasis).
Given that PV-10 is a much stronger (i.e., quicker and more frequent complete responses or destruction of injected tumors) IL agent than T-Vec, and also displaying much greater immunological signalling, one should see PV-10 + pembro >> T-Vec + pembro > T-Vec + ipi.
I'm not sure why Agarwala included HF-10 in his presentation, other than the fact that Huntsman Cancer Institute's Dr. Robert Andtbacka, MD and him are investigators in the HF-10-ipi trial.
The Phase 1 clinical trial of HF-10 alone generated 1 partial response and no complete responses in head and neck cancer, malignant melanoma, and other malignancies. That kind of low level or amount of tumor destruction is unlikely to generate a notable antigen cascade, which then might not lead to generation of a robust immune response.
Let's make them all our friends (March 20, 2016)
(aka Business update conference call review, part 3)
G. #3. Stage III melanoma Phase 3 trial
There did not sound like additional information on site activations save for Eric's comments of:
(aka Business update conference call review, part 2)
Tomorrow, Friday, March 18th, St. Luke's Cancer Center's Dr. Sanjiv Argawala will make a presentation entitled Current Trials with Oncolytic Agents at the HemOnc Today Melanoma and Cutaneous Malignancies in New York.
On the March 13th 4Q15, CY2015 business update conference call, Provectus' CFO/COO and interim CEO Peter Culpepper said:
Peter and Eric's conference call comments addressed in part:
The transcript of the call may be found here (via Provectus) and here (courtesy of Seeking Alpha). I'll also reference March 13, 2016 blog post Fourth Quarter, Year-End 2015 Business Update/Conference Call Prep.
A. #7. CUP data: How many patients were treated in the compassionate use program (CUP) (aka the expanded access program) in CY2015? What guidance is there on the timing of the publication or publications of CUP clinical results and data?
Eric said:
B. Marketing approval in Australia
The last guidance on this topic could come from Provectus' November 2010 press release Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia, which noted (my bolded and underlined emphasis):
On the call Peter said:
Eric said:
Progression Prior to Response (PPR) (March 17, 2016)
One of the sources underlying my comments under #3 of To Dos (March 16, 2016) was Amgen's sponsor material in support of the FDA's AdComm meeting for T-Vec in 2015. In T-Vec's pivotal melanoma Phase 3 trial, PPR was defined as "the appearance of a new lesion or >25% increase in baseline tumor burden." See, from ASCO 2014, Patterns of Durable Response (DR) With Intralesional Talimogene Laherparepvec (T-VEC) From a Phase 3 Trial in Patients With Stage IIIB-IV Melanoma, Ross et al., J Clin Oncol 32:5s, 2014 (suppl; abstr 9026). Ross et. al. concluded:
And here, from the same poster:
Progression after initial and potentially early subsequent treatment cycles of T-Vec appear to be in the period of time of 1-3 months (4-12 weeks).
Provectus' pivotal melanoma Phase 3 trial, now named PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma, utilizes T-Vec treatment per the drug's prescribing information (i.e., initial treatment, second treatment 3 weeks (21 days) after the initial treatment, subsequent treatments 2 weeks (14 days) after previous treatment).
For the purposes of ascertaining progression-free survival (PFS), patients would have a comprehensive assessment every 12 weeks (3 months) with measurement per the RECIST v 1.1. framework. Clinical assessments to determine progression and for the purposes of determining crossover eligibility post-treatment, however, would be undertaken every cycle completion, or every 4 weeks (1 month).
Thus, it is quite possible that at least half of T-Vec patients in PV-10's Phase 3 trial, if T-Vec's Phase 3 trial observations are any indication (that is, those who experience PPR), might be determined to progress (PD = progression of disease) and therefore generate a trial event.
Class action lawsuit update. As this InvestorVillage poster (Area51) routinely does, here is a link to an update on the suit's settlement process.
To Dos (March 16, 2016)
Note to self: Things I have to write about.
1. The initial goal of Moffitt Cancer Center's mechanism of action work -- Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study -- was to elucidate what is Step #5 of Chen and Mellman's Cancer-Immunity Cycle: Infiltration of T cells into tumors.
2. Moffitt's abstract at AACR 2016 is out. See March 16, 2016 blog post PV-10 at AACR 2016: T cell mediated immunity after combination therapy with intralesional PV-10. It’s pretty clear now that combining PV-10 with anti-PD-1 is just the beginning of ways the drug might be used.
3. Amgen did not report progression-free survival (PFS) for talimogene laherparepvec's (T-Vec's) pivotal melanoma Phase 3 trial. Rather, they report data on progression and durability of response in a number of other ways: a primary endpoint of durable response rate (DRR), and secondary endpoints that included duration of response, and time to treatment failure.
Amgen (or BioVex, which originally designed the Phase 3 trial prior to its acquisition by Amgen) used a modified WHO assessment method (i.e., tumor response criteria) that allowed for progression prior to response (PPR). In Amgen's advisory committee meeting materials prior to T-Vec's approval, the Big Biotech noted: "Progression-free survival was not chosen as a secondary endpoint because an increase in lesion size and/or development of new lesion(s) prior to response was expected based on results from the phase 2 study (Study 002/03)." {my underlined emphasis} T-Vec's Phase 2 study (when it was called OncoVex) used a modified RECIST tumor response measurement to allow progression before response. Interestingly, in T-Vec's Phase 3 trial, more than half (54%) of responders "experienced an increase in overall lesion size of ≥ 25% and/or developed at least one new lesion prior to ultimately achieving a response...." This progression apparently is consistent with a delayed immune response.
Assessment of progression in Provectus' pivotal Phase 3 trial that now will include T-Vec as a comparator (investigator's choice of T-Vec or chemotherapy), however, will be RECIST 1.1 criteria -- where disease progression is defined in lesion size of > 20%.
The Ugly, the Bad and the Good (March 16, 2016)
There's a good amount of information, both on company internals and externals, to digest and discuss, and opinion(s) to be formed, made and conveyed. I plan to write more on several topics here under Current News and as blog posts. I'd also like the benefit of hearing comments by Provectus' CTO Dr. Eric Wachter, PhD (clinical development program progress), mostly, and interim CEO Peter Culpepper (an explanation).
The Ugly: Provectus issued a press release and made an 8-K filing today regarding an internal review surrounding the resignation of former Chairman, CEO and a co-founder Dr. Craig Dees, PhD, Announces Results of Internal Investigation. Of note, Craig received nearly $2.5 million in travel expense advances from CY 2013 to CY 2015 (to travel where and to do what?), and provided receipts for less than 15% of these expenses (~$350K), most of which the Audit Committee indicated "did not appear to be authentic." See a summary table below.
Questions include:
What's in a name? (March 12, 2016)
By now the pharmaceutical industry understands immune checkpoint inhibitors are limited, in terms of the fraction of patients they can help. Very generally speaking, anti-PD agents like Keytruda or Opdivo may help treat only about 20% of cancer patients. This limitedness could apply to all checkpoint inhibitors or inhibitory agents:
But as you dig deeper into stimulation, what's the difference between the cancer immunity cycle's left-hand side? Steps 1 and 2 appeal to the creation and presentation of antigens after you've directly done something to tumor (i.e., making your tumor your friend).
Some call it an "antigen cascade;" see for example a tweet by @ChrisHeery (discussing the same presentation that included immunogenic intensification by National Cancer Institute's Dr. James Gulley, MD, PhD:
It's 2016. Several years ago, Provectus and Pfizer's Dr. Craig Eagle, MD were discussing another name for antigen cascade, referring to the notion or concept as "antigenization" or an "antigen storm."
Antigenization. See for example March 10, 2013 blog post $PVCT: Florey, Chain & Heatley
Chemoablation via PV-10 Ablation Causes Antigenization & Antigenization Causes Immunization. I wrote:
I noted making cold tumors hot under the news item immediately below. A shareholder sent me a weblink to a March P&T Community article by Walter Alexander entitled The Checkpoint Immunotherapy Revolution (my underlined emphasis below):
Updated below.
Class action lawsuit update. H/t InvestorVillage poster Area51, who often posts information regarding Provectus' on-going class action lawsuit; see the latest information under Section 7, Class Action Lawsuits starting on page 10 of the third quarter 2015 10-Q, which was mediation between the parties. Area51 gave me a heads up regarding this recent information, which I encouraged to be put out now: There is a pending settlement, which you can access via the TNED (Tennessee Eastern District) PACER (Public Access to Court Records) website.
Area51 notes a $3.5 million figure subject to court approval. Cut and pastes from the court filings, according to Area51:
At AACR 2014 Moffitt noted in their poster presentation entitled Induction of anti-melanoma immunity after intralesional ablative therapy (Provectus PR source of the below):
Variable tumor immunogenicity. In the same vein, the following two slides from a recent Agenus presentation are interesting (purple edits are mine).
See February 18, 2015 blog post The Early Obsolescence of Checkpoint Inhibitors, for example:
In writing about Provectus's new clinical study of a new indication, neuroendocrine tumors (NET) metastatic to the liver -- see February 27th blog post A new article, a new clinical trial, and Our logic seemed clear (February 29, 2016) below -- I now realize that I did not address an additional measure or trial endpoint (a secondary one) of efficacy, specifically objective response rate (after 6 months).
Thus, taking into account, among other comparators to discuss the topic, either Novartis' Afinitor (everolimus) that was recently approved for NET* or its pivotal trial's comparator (a placebo), Provectus CTO Dr. Eric Wachter, PhD's measurement of better patient outcome would address (i) reduction in the size or complete elimination of the tumor (upon injection with PV-10) and (ii) diminishment or elimination of critical symptoms like diarrhea and flushing.
As noted below, Afinitor achieved a tumor response of 2% (the placebo achieved 1%). As for symptom improvement, the drug (compared to the placebo) achieved for infections (11.0% vs 2.0%), diarrhea (9.0% vs 2.0%), stomatitis (9.0% vs 0.0%), fatigue (5.0% vs 1.0%) and hyperglycemia (5.0% vs 0.0%).
PV-10 previously has been used to treat at least one NET tumor, so one would presume Eric has experience or observable data on both tumor and symptom reduction (tumor reduction in PV-10 lingo also is known as tumor ablation).
Interestingly, the FDA's approval for this new NET indication was Afinitor's sixth. There currently are 428 open clinical studies of the drug listed on ClinicalTrials.gov.
* The treatment of adult patients with progressive, well-differentiated, nonfunctional neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin that are unresectable, locally advanced or metastatic
Moneyness redux (March 7, 2016)
Updated below.
As I wrote on Friday, March 4th, see Warrant tender offer under Late-night Boba (March 4, 2016) below, Provectus made an 8-K filing today (among other related filings: a prospectus supplement, the tender offer) to disclose:
"you cannot buy Rose Bengal off the shelf and use it in a patient" (March 6, 2016)
There's a recent article that includes a blurb about Rose Bengal/PV-10 by The Australian's banking reporter Tim Boreham entitled Biotech player CogState has plenty of credibility behind it:
* Until if challenged, and such challenge is lost by Provectus and/or its acquirer.
Boreham previously wrote an article about PV-10 in October 2014 entitled Food dye threatens cancer biotechs’ business model:
For the last few years I have taught a CFA Level 1 exam prep course to seniors of UNLV's Lee Business School. The course is integrated into the curriculum for finance majors, but it is not offered to industry or other professionals sitting for the exam.
Over roughly the same period I've also coached student teams from the university competing in the CFA Institute Research Challenge, where they reprise the role of an equity research analyst to provide and defend an investment recommendation and 12-month price target of a public company.
It's a privilege and a blast to do both activities, although the latter is so much more of an emotional endeavor for me (because I'm heavily invested in the student teams and whatever success they achieve).
UNLV students have represented themselves well in the Research Challenge, competing in a local/first round with teams of seniors and MBA students from (depending on the year) Arizona State University, Grand Canyon University, Northern Arizona University, Thunderbird School of Global Management, the University of Arizona, and the University of New Mexico. Teams are graded on an equity research report (i.e., initiation of coverage) and an oral defense (including Q&A) of their recommendation (i.e., buy, sell or hold) and price target.
Over the last five years UNLV teams, comprised only of seniors, have won or tied for first in the local round three times (2012-13, 2016), advanced to the semi-finals of the regional Americas (second) round (2012), and placed second in the Global Final (third round) (2013). Our students are incredible people trying to do their best to achieve great things (I, on the other hand, am a biased homer; see footnotes at the end of this blog news item).
Both of these activities heavily emphasize financial analysis and valuation, and, to some extent, conviction. In particular, with Research Challenge teams, I strongly encourage them to think like hedge fund analysts rather than equity research analysts. The not so nuanced difference, in my view, is that the former drives towards a potentially executable trade or investment recommendation and hoped for action, with possible profit or loss thereafter, while the latter (irrespective of whether they are buy- or sell-side analysts) has no skin in the game, can be somewhat-to-very conflicted, or simply is uninvested in the outcome. Thus, the difference may or should influence both the quality of analysis and the amount of conviction.
Just like my students and student teams, I try to grow and learn, always striving to be better at my tradecraft. I often feel a sell recommendation is easier to construct because an overvaluation thesis can be made more compelling by honest, accurate identification of substantial mis-, weak or poor management (but harder to deliver to most audiences because it makes people feel uncomfortable).
I have found my long Provectus thesis, while firmly anchored to a unique small molecule with an increasingly compelling clinical value proposition and led by a management team that remains supportable and a net positive, is from time-to-time buffeted by initially inexperienced managers who have grown on the job (to varying degrees depending on the manager; some not at all) and made poor decisions by choice or out of necessity (but sometimes forced by choice).
In the social media experiment that is this blog, I offer up near real-time due diligence, analysis, opinion and, occasionally, rumor in hopes of finding out as much as I can with the almost daily goal of wanting to reaffirm or trying to undermine my investment thesis. Not a day goes by, or so it seems, that hasn't garnered opposition or criticism from some side of this trade/investment (e.g., management, short-term longs, long-term longs, undecideds, quiet shorts, loud shorts, skeptics, neutrals, observers, etc.).
I guess the experiment is still working.
E/n 1: The 2012 team missed out on advancing to the regional Americas second round because of a poorly framed judge's question that caused the students to hesitate before answering very well (their target public company: MGM Resorts International, NYSE:MGM). The price target was hit within 12 months.
E/n 2: The 2013 team fell just short in the Global Final, losing to an MBA team from a Polish university. I wholly blame time zones and jet lag (Kona Grill, NASDAQ:KONA). The price target was hit within 12 months.
E/n 3: The 2014 team had the daunting challenge of defending a sell recommendation, and did not deliver an oral defense commensurate with their compelling research report (Meritage Homes, NASDAQ:MTH). The price target was hit within 12 months.
E/n 4: The 2015 team could not coalesce, and did not submit a report (nor compete in the oral portion of the first round). I believe they learned some lessons that I hope they took with them upon graduation.
E/n 5: The 2016 team did not advance out of the local round after losing a second tiebreaker to an ASU MBA team. I blame two of the three judges who did not credit UNLV students with properly and thoughtfully employing three different valuation techniques (i.e., DCF, price multiples, a qualitative method), compared to ASU's use of DCF only (Sprouts Farmers Market, NASDAQ:SFM). The price target, finalized on January 22nd, was $30.50 (when the share price closed at $22.77) (the March 3rd closing price was $28.21).
Late-night Boba (March 4, 2016)
Making the [cancer] tumor your friend. H/t @bradpalm1 for the heads up on an article entitled Tumours contain the seeds of their own destruction:
For more, see October 15, 2015 blog post Still Standing.
Checkpoint inhibitors need help to help the immune system. Consider an article entitled Next-Gen Immunotherapy Offers New Hope for Beating Brain Cancer:
A thought for small molecule people. H/t again @bradpalm1's link to a blog post by Dr. Derek Lowe, PhD entitled Where Cancer Immunotherapy Works (and Doesn’t):
But the larger thought is PV-10's potential ability to make non-immunogenic tumors immunogenic, and immunogenic tumors more so. Achieving T cell immunity almost if not actually by definition should mean overcoming resistance to cancer, thus overcoming checkpoint blockade and mitigating the need to artificially release the brakes.
Should stimulation via stimulatory therapeutics and therapies start the engine and enables the gas pedal to be stepped on sufficiently and appropriately (i.e., with minimal or manageable side effects or adverse events) so as to achieve T cell immunity, brakes may not be necessary once the car is moving (in context, and given the car [the immune system] can drive itself and not careen off the road because it then should know what it is doing).
Over time, however, road friction may start slowing the car down to the point where waning immunosurveillance (the immune system recognizing and eliminating continuously arising cancerous cells) no longer can protect the patient from relapse (analogous to how waning varicella zoster antibody titers may result in a bout of shingles). Keeping the brakes disengaged, especially with non-immunogenic tumors, should have some role going forward, making Merck, Bristol-Myers, Roche, AstraZeneca, Pfizer and other companies’ checkpoint inhibitors not necessarily obsolete as much as persnickety. And we haven't even begin to talk about steering wheels...
For more, see October 10, 2015 blog post Brakes, Gas Pedals, Steering Wheels, Roads, Planes, Trains and Automobiles.
Warrant tender offer. H/t a shareholder (I believe) who wrote [to me] that [he was aware/heard] there is some consideration being given to changing or advocating for a change in the terms of the exchange to make the economics of tendering more attractive to non-tradable warrant holders.
Wednesday (March 2, 2016)
Business update conference call. In retrospect, considering Tuesday's call on its own, Provectus' CFO/COO and now interim CEO Peter Culpepper said nothing that would explicitly suggest the company would not hire a new CEO (irrespective of time frame). The company's Monday press release on leadership changes did note "[t]he Board also formed a Search Committee, to be chaired by Board Chairman Smith, to immediately begin work to identify a permanent CEO." And while Peter's prepared comments on the call did not address the search process, his answers to a question on the topic did. I suppose my takeaway under "...we have now begun to win" (March 1, 2016) below was colored by my inclusion of other facts, thoughts and opinions.
I don't think the company would be sold or acquired in the near- and perhaps medium-term; however, I do believe core management (the company's CTO Dr. Eric Wachter, PhD and Peter) think they have the opportunity to and can lead Provectus towards this end given their projections of clinical data generation, presentation and publication efforts this year.
De novo antitumor immune response. Reading a tweet from this project's curated Twitter feed, I noted the continued consideration of radiation or radiotherapy's role in generating and/or contributing to an anti-tumor immune response.
Radiation has been implicated in immunogenic cell death, as has PV-10. PV-10's promise as an immune system primer complements the same thinking in regards to radiation. The abstract of the article referenced in the above tweet follows, Role of Local Radiation Therapy in Cancer Immunotherapy, Demaria et al., JAMA Oncol. 2015;1(9):1325-1332:
Updated below.
Provectus held the business update conference it scheduled for today to discuss changes in company leadership that stemmed from the resignation of Chairman, CEO and one of Provectus' co-founders Dr. Craig Dees, PhD. See Resignation (February 29, 2016) below. The transcript of the call is here.
My primary takeaway from the call is this:
While I thought Peter did overdo the analogies and popular references, I believe I understand why he said the last sentence of the following portion of his statement:
His saying "we have now begun to win" probably refers to (as Eric and he now see and hear it) the growing acceptance of and desire to use PV-10 by global key opinion leaders in oncology studies and trials in more and more solid tumor indications, whether as a single agent or in combination with another therapeutic or therapy.
Shots and wins have not yet translated into the share price management and shareholders want, let alone need. Clinical and other data generation/presentation/publication in 2016 may change that.
Updated (3/1/16): I changed "near-term" above in the bullet point paragraph to "immediate, near-term or even perhaps medium-term."
Synthesis (March 1, 2016)
Provectus issued a press release and made an associated 8-K filing today regarding the award of a new patent related to the synthesis of Rose Bengal and other halogenated xanthenes (Rose Bengal being the first of its class, with the other halogenated xanthenes as members of that same class), Awarded Patent Extending Protection of the PV-10 Manufacturing Process. The original patent was awarded on September 10, 2013; the patent awarded today was a continuation of it. Also see "...clear that our protection of Rose Bengal for therapeutic use is in fact ours" (February 11, 2016) below.
The press release noted:
The PR also provided a quote by Provectus' Dr. Eric Wachter, PhD.:
Cambrex is "...a generics and biosimilar manufacturer that provides small molecule pharmaceutical ingredients and finished dose products for the innovator and generic pharmaceutical markets." Some slides from a November 2015 investor presentation of Cambrex (purple emphasis is below):
February Blog Readership Stats (March 1, 2016)
Changes in blog readership statistics were mixed for the month of February. Month-over-month (February 2016 v. January) percent differences included:
The continuation of the original synthesis patent was awarded: Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2', 4', 5'7'-tetraiodo-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (Rose Bengal) and related xanthenes (9,273,022, Singer et al., March 1, 2016).
Our logic seemed clear (February 29, 2016)
In the wake of being denied breakthrough therapy designation for PV-10 in patients with locally advanced cutaneous melanoma (Stage III disease), Provectus' CTO Dr. Eric Wachter, PhD had said:
I noted in February 27, 2016 blog post A new article, a new clinical trial that the FDA approved Novartis' everolimus (Afinitor) for the treatment of a version of neuroendicrine tumors (NET), but the drug generated an overall response rate of 2%. That is, the tumor was not made to go away by Afinitor; it stayed pretty much the same size.
Eric's quote in today's NET Phase 1 trial PR was interesting to me:
Resignation (February 29, 2016)
Updated below.
Provectus issued a press release and made an associated 8-K filing today regarding the resignation of the company's Chairman, CEO and one of its three co-founders, Dr. Craig Dees, PhD, Announces Leadership Changes. According to Provectus, there will be a conference call tomorrow at 4 pm EST related to this topic.
My main takeaways thus far, in no particular order, are:
Updated (2/29/16): A shareholder called me to say he met Craig this afternoon (East Coast time), and that they spent time talking about his illness. The shareholder offered the opinion that he thought Craig was very ill.
Epidemiology of neuroendocrine tumors/cancers (February 28, 2016)
Provectus' new neuroendocrine tumors metastatic to the liver Phase 1 trial, thus far with a single clinical site in Australia, appeared today on the Australian New Zealand Clinical Trials Registry.
The anticipated date of when the first patient would be enrolled is March 1st (Commonwealth countries report dates as day/month/year, compared to our month/day/year).
The question of the activation of, and the enrollment and recruitment at Australian clinical sites for Provectus' pivotal melanoma Phase 3 trial comes to mind. According to the trial's ClinicalTrials.gov webpage, the sole Australian site listed thus far, Brisbane's Princess Alexandra Hospital, is not yet recruiting.
So, the company's CTO Dr. Eric Wachter, PhD expects to begin enrolling a Phase 1 trial at the beginning of March (i.e., two days from now), but none of the 10 ten sites he previously guided that would participate in the Phase 3 trial are recruiting yet, and only one of them currently is listed as a site?
The NET Phase 1 trial investigator is Australia's Dr. Timothy Price, MD, who has notable professional interest in and experience with NET. See, for example, his paper Epidemiology of neuroendocrine cancers in an Australian population, Cancer Causes Control (2010) 21:931–938:
Another paper, also related to NET but not focused on Australia, is Epidemiology of Neuroendocrine Tumours, Neuroendocrinology 2004;80(suppl 1):3–7:
New clinical study (February 26, 2016)
Updated below.
A Phase 1 Study of PV-10 Chemoablation of Neuroendocrine Tumours (NET) Metastatic to the Liver
Clinical site & investigator: The Queen Elizabeth Hospital, Woodville, Australia & Dr. Timothy Price, MD
Updated (2/26/16): I previously had written about neuroendocrine tumor (NET) treatment with PV-10 on the blog in July 2013 (see the Archived News I page).
Provectus' trial's endpoints include:
"Old red dye shows promise as new cancer foe" (February 25, 2016)
Updated below.
A Reuters' article by Bill Berkrot on Rose Bengal, PV-10 and Provectus was published: Old red dye shows promise as new cancer foe. Quotes include:
T cells (February 24, 2016)
Updated below.
Intralesional immunotherapy as a strategy to treat melanoma. See below:
Medical writer Walter Alexander wrote an article entitled The Checkpoint Immunotherapy Revolution, What Started as a Trickle Has Become a Flood, Despite Some Daunting Adverse Effects; New Drugs, Indications, and Combinations Continue to Emerge in the March edition of Pharmacy & Therapeutics (h/t InvestorVillage poster rmgillis). The article discussed among other things:
- Immune checkpoint inhibitors (aka co-inhibitory blockade): anti-CTLA-4 drug ipilimumab (Bristol-Myers; approved in 2011 for advanced melanoma), and anti-PD-1 drugs pembrolizumab and nivolumab (Merck & Co. and Bristol-Myers, respectively; both approved in 2014),
- The role of intralesional (IL) injections (and agents): talimogene laherparepvec or T-Vec (Amgen; approved in 2015), PV-10, and coxsackievirus A21 or Cavatak (Viralytics),
- Biomarkers, and
- Combination therapies.
Of note [to me]:
- Dr. Jeffrey Weber, MD, PhD, NYU Langone Medical Center: "Finally, Dr. Weber pointed to the locally injected or intralesional therapies, such as talimogene laherparepvec (T-VEC), PV-10 (Rose Bengal 10% disodium, Provectus), and coxsackievirus A21 (Cavatak, Viralytics). Not only do they generally share the virtue of being devoid of dose-limiting toxicities, but they also may be able to “prime” the immune response, Dr. Weber said, so that “beyond trying to diminish the Treg and MDSC activity, you could augment T-cell activity, causing an influx of them to the tumor and making a cold tumor hot.”
- Dr. Merrick Ross, MD, MD Anderson Cancer Center: "...suggested that the programmed cell death induced by the PD-1/PD-L1 agents may not cause the tumor to express antigens in a manner that evokes an immune response.24 On the other hand, viral vectors and a chemoablative agent like PV-10 actually rupture the tumor, releasing and presenting intact antigens. Such an effect, he said, could be synergistic with ipilimumab or other targeted immunotherapies, because it occurs at a different place in the immune system."
- On the topic of biomarkers, which for PV-10 would be one of the outcome measures of Moffitt Cancer Center's feasibility study (mechanism of action) work, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study -- PV-10's biomarker should be greater amounts of circulating immune cells in a patient's peripheral blood mononuclear cells:
- "Biomarker testing has been a particular focus of Dr. Herbst’s research. “The data from the pembrolizumab trial suggests that using a biomarker can help you to identify patients who are more likely to benefit from the drug. With pembrolizumab it’s the immunohistochemical (IHC) staining assay using the 22C3 antibody—the higher the expression, the more likely the patient is to respond and to survive longer,” he said...The ability to use biomarkers to identify patients more likely to benefit from treatment is important because the checkpoint inhibitors, Dr. Herbst predicted, will be used in earlier, more curative settings among patients with previously untreated metastatic disease, as well as for maintenance or adjuvant therapy."
- "A biomarker analysis from a phase 1b/2 trial of T-VEC plus ipilimumab versus T-VEC alone found increases from baseline after treatment with T-VEC and further after ipilimumab in total and activated CD8+ T cells in peripheral blood, plus increases in CD4 T cells expressing inducible T-cell costimulator. This indicates up-regulated CTLA-4 blockade following ipilimumab treatment. Patients with disease control after T-VEC had 1.4 times as many activated CD8 T cells."
- Provectus' pivotal Phase 3 trial addresses patients with Stage III disease. The company's trial of PV-10 + pembrolizumab addresses patients with Stage IV disease. Earlier disease settings, like Stage I/II would be addressed on the introductory basis of T-Vec's "multinational, phase 2 neoadjuvant study comparing T-VEC plus surgery to surgery alone..."
- Dr. Robert Andtbacka, MD, University of Utah School of Medicine: "...the FDA approval of T-VEC did not support the claim of distant effects..."
- "Research with PV-10,26,27 for example, has indicated that immune responses are tumor specific and associated with increases in CD8+ T cells in both animal and human research."
Setting aside T-Vec's approval, there should be no immediate (and potentially no long-term competition) from other investigational IL agents/drug compounds. In his presentation at the HemOnc Today Melanoma and Cutaneous Malignancies on March 18th/19th (Current Trials with Oncolytic Agents), melanoma, intralesional agent and PV-10 key opinion leader (KOL) Dr. Sanjiv Agarwala presented the slide below:
 |
| Click to enlarge. |
Cavatak are HF-10 are virus-based oncolytics, like T-Vec, and suffer from concern about viruses as well as require special handling. IL-12 uses electroporation (OncoSec Medical), which requires special equipment, causes patient discomfort, and incurs higher toxicity. Efficacy is comparable to PV-10 but is not better (Cavatak -- 28% response rate in Phase 2; HF-10 -- 0% response rate in Phase 1; and pIL-12 EP --31% response rate in Phase 2; cf. PV-10: 51% response rate in Phase 2). Furthermore, Phase 3 registration trials for these other IL agents have not yet been designed.
Let me try to establish a couple of baseline parameters or points. First, to get an investigational drug approved as a single agent for a cancer disease indication, it would appear the process comprises undertaking a clinical trial program of the drug compound + the standard of care (SOC) vs. SOC. In Provectus' case, for advanced (Stage IV disease) melanoma for example, the approach based on the above construct -- investigational drug + SOC vs. SOC -- would be PV-10 + pembrolizumab vs. pembrolizumab.
Second, in more recent times, as the FDA and pharmaceutical industry have endeavored to improve clinical development, and make it more efficient and effective in the pursuit of quicker trial failures and shorter approval paths (if early-stage trial data bear out), the clinical trial progression of the above could be:
- (a) a Phase 1b single-arm trial (SAT) of the investigational drug + SOC primarily focused on safety and dosing (with an eye on potential dose limiting toxicities, and potential adverse events), while at the same time collecting some key efficacy data,...
- (b) a potentially immediate transition to a Phase 2 randomized controlled trial (RCT) of the investigational drug + SOC vs. SOC focused on collecting appropriate efficacy data that could be used for drug approval -- if a safety assessment of the Phase 1b is successful (and presumably if the efficacy data proves promising).
Provectus' liver Phase 1 trial program comprised:
- (x) An initial cohort or group of 13 patients (15 patient tumors) receiving a single injection of PV-10 to one hepatic tumor -- hepatocellular carcinoma (HCC) or liver metastases. Two patients of the original 13 re-enrolled so as to receive a single injection to a second hepatic tumor, and
- An expansion phase comprised of two expansion cohorts or subgroups:
- (y) Additional HCC and liver mets patients who could receive a single injection of PV-10 to multiple tumors,
- (z) HCC patients on U.S. (and some parts of the rest of the world) SOC of sorafenib who could receive a single injection of PV-10 to a single HCC tumor.
Provectus' Dr. Eric Wachter, PhD could have sufficient clinical data on safety, dosing and some efficacy (preliminary efficacy is focused on response rate [i.e., tumor reduction or destruction] while approvable efficacy is focused on progression-free survival and overall survival). He also could have sufficient clinical data to design a clinical trial program that, if successful, would provide the argument to approval PV-10 for HCC and liver mets.
I obviously don't have enough regulatory affairs and clinical trial design experience of course to understand his rationale for his process. For example, what are the pros and cons of undertaking two separate, staggered regional programs for HCC, or a global HCC program with one arm for each region?
Nevertheless, what could be Provectus' approach to advancing its liver clinical development program in the U.S. and China (and potentially elsewhere)? I believe there are two perspectives to this -- the design of the trials comprising the trial program itself (first, the safety, dosing and preliminary efficacy collection study, and second, the pivotal trial), and the process of trial design approval in different geographic regions.
The strategy for HCC should mimic (or be very similar to) the melanoma combination strategy, in order to potential attain approval:
- (m1) a U.S. Phase 1b SAT arm of PV-10 + sorafenib (SOC), and
- Dosing of 6 + 6 subjects (i.e., low-dose and high-dose PV-10),
- Sorafenib dose stable and tolerable for some fixed period of time, and
- Potential treatment and retreatment of multiple lesions with PV-10
- (m2) a China or Asian Phase 1b SAT arm of PV-10 + the region's SOC
- Dosing of 6 + 6 subjects (i.e., low-dose and high-dose PV-10),
- SOC dose or treatment stable and tolerable for some fixed period of time, and
- Potential treatment and retreatment of multiple lesions with PV-10.
Followed by:
- (n) a potentially immediate transition to a Phase 2 RCT of PV-10 + SOC arm vs. SOC arm focused on collecting appropriate efficacy data that could be used for drug approval if safety assessments of the respective Phase 1b arms are successful, and presumably if the efficacy data proves promising.
In terms of region, Provectus' CFO/COO and interim CEO Peter Culpepper's comments on the March 30th 4Q15/CY15 business update conference call might have indicated the trial design is in the process of being reviewed by the Chinese FDA (underlined emphasis below is mine):
"We have other sites--because of the addition of Imlygic as a comparator, other sites in the U.S. that are wanting to join in the existing Phase 3 quickly. We have--for, say, E.U. and Brazil and China, we have completed an international conversion of the file, the IND file with the FDA, the analogous file for rest of world, for regulatory bodies. That's now available. So, that facilitates the other sites becoming active."
Updated below.
The Vatican's upcoming Third International Regenerative Medicine Conference (April 28-30), a conference that appears to occur every three years, includes several immunotherapy (oncology) Provectus and PV-10 touch points:
- Immunotherapy (cancer)-related conference speakers include Dr. James Allison, PhD (ipilimumab), Dr. Ronald DePinho, MD (President of MD Anderson), Dr. Axel Hoos, MD, PhD (GlaxoSmithKline Pharmaceuticals), Dr. Carl June, MD (chimeric antigen receptor [CAR] T-cell therapy; Novartis), and Dr. Patrick Soon‐Shiong, MD (Abraxane, NantKwest).
- Conference steering committee member Todd Aydelotte is Provectus' media relations conact from Allison+Partners,
- Provectus is a Gold Key sponsor, and
- A conference speaker is a long-time consultant to the company, and has played and I believe still does plays another clinical role.
Updated (4/3/16): Chuckle.
 |
| Click to enlarge. Tweet/image source |
 |
| Image source |
 |
| Click to enlarge. |
A. Keytruda and Opdivo and...
"We believe we can successfully combine PV-10 with Bristol's Opdivo, another anti-PD-1 systemic immunotherapy just like Merck’s Keytruda. For that matter, as we disclosed earlier in the week on Monday from our presentation last week at the BioPharma Asia Convention 2016 in Singapore, we expect to be able to combine PV-10 with many different agents due to the unique mechanism of actions and delivery approach of PV-10.
We chose to start with Merck’s Keytruda because it aligns up well for purposes of our Phase 1b/2 clinical study design and as a leading new drug in melanoma. The use of PV-10 with any immune checkpoint blockade is possible we believe. Our joint patent that is co-owned with Pfizer is basis for this approach to combine locally administered PV-10 with systematically administered immunomodulatory agents such as Keytruda and Opdivo."Moffitt Cancer Center's PV-10 work related to combining the drug compound with other therapeutics was first presented at SITC 2014 as "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma."
Moffitt's newest work at AACR 2016 is "T cell mediated immunity after combination therapy with intralesional PV-10 and co-inhibitory blockade in a melanoma model," where co-inhibitory blockade covers (like with SITC 2014) anti-CTLA-4, -PD-1 and -PD-L1 antibodies (agents).
B. And liver begins...(when exactly?)
"Our Phase 1 study of PV-10 has as a hepatocellular carcinoma, HCC, and cancers metastatic to the liver has demonstrated preliminary evidence of efficacy in treatment of cancers of the liver with PV-10. We have therefore been developing a Phase 1b/2 protocol to pursue developments of PV-10 to treat HCC and the Phase 1b portion of the study will commence in Asia based on inputs from KOL under the offices of our collaboration with Boehringer Ingelheim."As I wrote under Network 1, Maxim, China, etc. (January 3, 2016) below:
In July 2015, Provectus and Boehringer Ingelheim (BI) China made announcements of their entering into/signing a letter of intent: Provectus Biopharmaceuticals Signs Letter of Intent with Boehringer Ingelheim (China) to Collaborate in Bringing PV-10 to Market in China (Provectus' PR on July 2nd) and Boehringer Ingelheim and Provectus biopharmaceutical company signed a letter of intent - the two sides will cooperate to promote melanoma and liver cancer research new drugs listed in the Chinese mainland, Hong Kong and Taiwan (BI's PR, translated into English, on July 3rd).
On July 10th, Jialing Dai wrote a LinkedIn article entitled Boehringer's Ambition In Liver Cancer. The article's byline read: "Eye On Oncology Market: Boehringer Ingelheim In-Licenses China Rights For A Provectus Late-Stage Oncology Compound." The article was edited by Howard Fields. Dai describes his some of his work as "PharmaDJ is online portal in English to provide tailored services and intelligence of China market for pharma executives. our goal is to build up a bridge to China market for foreign companies doing business in China." In his article Dai wrote:
"PV-10, an injectable formulation of rose bengal, also known as Provecta, is the leading compound of the Knoxville, Tennessee-based company. The firm received orphan-drug designation from the U.S. FDA for treating of hepatocellular carcinoma (HCC) in 2011 and melanoma in 2007. Currently, the drug is in a U.S. Phase III clinical trial for melanoma and Phase I trial for HCC.
“We need to wait on the outcomes of these studies before deciding to file or not with CFDA, but we certainly have an interest in developing this drug in HCC in China, as there is a large unmet medical need,” BI China told PharmaDJ in an interview." {Underlined emphasis is mine}Dai also noted in his article the below:
"The German pharma giant doesn't have a footprint in the China oncology market so far. However, BI is intent on building its oncology platform in the country, where it filed an approval application in 2013 for its first oncology product, Giotrif (afatinib).
“We are now awaiting CFDA’s decision regarding our application, but hope to have a positive opinion from CFDA very soon so that we can provide Giotrif to NSCLC patients in China,” BI said. Giotrif was approved in the U.S. and Europe in 2013." {Underlined emphasis is mine}In December Sanofi and BI announced "...they were in “exclusive negotiations” on a potential asset swap that would make Sanofi one of the world’s largest manufacturers of nonprescription medicines. Under the terms of the deal, Sanofi would send its animal health business to Boehringer and Sanofi would receive 4.7 billion euros, or about $5.2 billion, in cash and Boehringer’s consumer health care business, excluding its operations in China."{Underlined emphasis is mine}
What did Provectus mean when the company wrote in its July LOI PR (see my underlined emphasis)?
"Under the terms of the LOI, Boehringer will provide certain commercially reasonable support in the aspects of product registration with the China Food and Drug Administration ("CFDA"), communication preparation, market intelligence and other assistance to Provectus in China to the extent that is within Boehringer's approved business scope and permissible by Chinese laws.
In return, Provectus will grant Boehringer the first priority to be the exclusive collaborator of Provectus in China for PV-10 in the event that PV-10 is successfully registered and approved by the CFDA. The exclusive collaboration may take the form of exclusive distribution and promotion, exclusive licensing or other agreement, subject to both parties' mutual agreement. At the appropriate time, Provectus and Boehringer will enter into a definitive agreement, including a non-compete provision, for PV-10 to be exclusively developed, distributed and promoted through the collaboration within China, although there can be no assurance that the parties will enter into a definitive agreement."One could imagine "registered" may refer to a filing of a Investigational New Drug (IND) application in China. Does "approved by the CFDA" refer to drug approval, or the approval of a clinical trial to be conducted in the country like the below?
- December 16, 2015, Celsion Receives CFDA Approval to Conduct the OPTIMA Study in China,
- December 10, 2015, Sinovac Obtains Clinical Trial Approval for Sabin-IPV Candidate, and
- March 30, 2015, CASI receives CFDA approval for ENMD-2076 Phase 2 clinical trial in ovarian clear cell carcinoma.
C. More indications into the clinic?
"Results of an in vitro testing of PV-10 on colon cancer, murine CT-26 cells, that has been presented by the University of Illinois, Chicago, researchers are consistent with immunologic cell death caused by PV-10 on melanoma.
We are continuing to work on protocols for further developments with PV-10 to treat colon cancers as a result. We intend to move forward with PV-10 as a treatment for other indications as the data supports, such as in prostate, breast, bladder, pancreas and other solid tumors."D. Into the future?
"We are continuing to work on protocols for further developments with PV-10 to treat colon cancers as a result. We intend to move forward with PV-10 as a treatment for other indications as the data supports, such as in prostate, breast, bladder, pancreas and other solid tumors. In tandem, we are determined to fully harness our Compassionate Use program for the benefit of patients now and in the future. As we know, as we have trademarks, when patients win, we all win."E. Key conference attendance and presentations?
We are making preparation for an aggressive media push in the coming months aligned with several key prestigious healthcare conferences. These conferences include American Association for Cancer Research, AACR, American Society of Clinical Oncology, ASCO, and the Third International Conference on Regenerative Medicine at the Vatican. We are looking forward to telling the story of PV-10 and positioning it among the top investigational cancer therapies in the industry."a. AACR 2016, April 16-20: Moffitt Cancer Center, abstract above
b. ASCO 2016, abstract titles available April 20 (conference June 3-7): Trials in progress?, Phase 1b melanoma combination therapy data?
c. The Vatican, April 28-30: ?
F. Delay in the pivotal melanoma Phase 3 interim assessment?
"The key important point to underscore is that we have expanded patients’ eligibility in our Phase 3 study, which should accelerate patients’ recruitment. The first patient had been enrolled and we are assessing the impacts these changes in eligibility and comparators they have on the study timelines. We will complete this assessment by the time of the next quarterly investor call...
We are assessing the study timeline we do have on clinicaltrials.gov that we expect the full study to be completed at the end of Q3, beginning Q4 of 2017. We also have said that we expect interim data this year. We are assessing that. We are trying at this juncture to add as many sites in the U.S., Australia. We have sites in process in the EU, Brazil, China, throughout the world that are going through the process to come on line. So certainly we’ll communicate status of Phase 3 in our next call, which would be when we file the 10-Q in May. However I should also point out that we do not expect to comment on patient enrollment details until the interim data is communicated when 50% of the events had been triggered."March blog readership stats. See below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
H/t a shareholder: PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma: MD Anderson, recruiting
 |
| Click to enlarge. |
1. Provectus issued a press release today and filed an associated 8-K regarding its 4Q15/CY15 financial results, Reports Fourth Quarter and Year End 2015 Financial Results, in conjunction with filing its 10-K.
2. 12/31/15 cash and cash equivalents were $14.2 million, compared to a 9/30/15 amount of $18.9 million.
3. The company's monthly cash burn for the quarter appears to have been $1.66 million, +18% quarter-over-quarter (QoQ) (the prior quarter-over-quarter change was +5%).
4. Total stockholders' equity (TSE) was $2.1 million, -62% QoQ (prior QoQ: -21%). The drop primarily comprised a reduction in assets related to "Long-term receivable – settlement, net of discount and reserve for uncollectibility" and an increase in liabilities related to "Accrued settlement expense," which should/could be the company's portion of the class action lawsuit settlement amount.
5. Language regarding runway remain unchanged from the prior quarterly filing: "into 2017."
6. Research and development expense for the quarter was $3.7 million, +36% QoQ (prior QoQ: +31%).
7. Lab supplies and pharmaceutical preparations expense for the quarter was $19K, -96% QoQ (prior QoQ: +58%).
8. A trend graph of the above (starting 1Q14):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Jargon, Terminology, Themes, Narratives (March 30, 2016)
H/t the Twitter feed of @bradloncar: the agenda of the 6th Annual Cancer Immunotherapy: A Long-Awaited Reality (yellow highlights/edits are mine):
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
"We hypothesize that production of a vaccine-like immune response using a small molecule drug is possible and requires: 1) an intralesional route of injection that generates rapid, durable tumor destruction via autolysis; 2) rapid clearance of drug from normal tissue; and 3) antitumor effects targeted only to tumor tissue...Co-administration of PV-10 immuno-chemoablation with other systemic therapy can yield potent synergy in uninjected tumors."
 |
| Click to enlarge. |
"This immuno-chemoablative response to PV-10 is tantamount to “in situ vaccination.”
 |
| Click to enlarge. |
From Provectus' 2016 BioPharma Asia Convention presentation (Suntec City, Singapore) entitled "Altering T cells and induced tumor-specific immunity via PV-10 ablation."
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated Clinical Trial Information (March 28, 2016)
Updated below.
PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma: MD Anderson, recruiting
Updated (3/28/16): Assuming (for the sake of quick and easy calculation) the date MD Anderson Cancer Center's Institutional Review Board (IRB) reviewed and approved Provectus' pivotal melanoma Phase 3 trial protocol was the date MD Anderson's recruitment status on ClinicalTrials.gov was changed (if very likely was not; it should be earlier), time taken would have been:
- 16.6 months since the initial protocol first was received by the .gov site, which would have been on the high side of the sample set in the table below,
- 12.4 months since Provectus' CTO Dr. Eric Wachter, PhD submitted a revised protocol to ClinicalTrials.gov, which very close to the median of the sample set, and
- About a third of a month since he submitted an amended protocol (to include, for example, T-Vec as a comparator) to the .gov site.
See also IRB review & approval (February 17, 2016) below.
 |
| Click to enlarge. |
After 5 sites started recruiting, it has been taking 2 (median) to less than 3 (mean) months for a Phase 3 site to change its status from "not yet recruiting" to "recruiting" on the study's ClinicalTrials.gov webpage.
At the April 9th panel session Provectus held in New York -- see Audiophile (April 19, 2015) on the blog's Archived News III page -- the company said its pivotal melanoma Phase 3 trial would comprise 25 sites in the U.S. and 10 in Australia (not including sites Provectus may or may not have in other countries and regions, such as China, India, Brazil, Mexico and Western Europe).
Of the 7 sites currently listed on ClinicalTrials.gov (5 recruiting, 2 not yet recruiting), 6 are U.S. locations and 1 is Australian. Sites in international locations, in addition to providing patient numbers to reach the interim analysis "N," also would facilitate eventual approval in those geographic regions should the trial be successful and PV-10 be approved by the FDA. Since it is reasonably well understood that the Phase 3 trial requires less than 112.5 (113) treated patients for the interim assessment of safety and efficacy to undertaken, does Eric really need that many U.S sites? I suppose the bigger question might be -- to reach the lower trial N for the interim assessment, how many sites does Eric really need?
 |
| Click to enlarge. |
Of the 7 sites currently listed on ClinicalTrials.gov (5 recruiting, 2 not yet recruiting), 6 are U.S. locations and 1 is Australian. Sites in international locations, in addition to providing patient numbers to reach the interim analysis "N," also would facilitate eventual approval in those geographic regions should the trial be successful and PV-10 be approved by the FDA. Since it is reasonably well understood that the Phase 3 trial requires less than 112.5 (113) treated patients for the interim assessment of safety and efficacy to undertaken, does Eric really need that many U.S sites? I suppose the bigger question might be -- to reach the lower trial N for the interim assessment, how many sites does Eric really need?
Trick park (March 23, 2016)
 |
| Image source |
Updated below.
MRSA-related. In abstract from the American Journal of Ophthalmology that I believe is current (today?), "Rose bengal and riboflavin mediated photodynamic therapy to inhibit methicillin-resistant Staphylococcus aureus keratitis isolates:"
"Rose bengal and riboflavin mediated photodynamic therapy demonstrated complete growth inhibition in vitro of two multidrug resistant MRSA strains. Rose bengal was also effective in dark and ambient conditions. These results may have implications for in vivo therapy."Patents. I found an article by Adam Feuerstein from today entitled "Gilead, Biogen Troubles Remind Investors Why Strong Drug Patents Are Vital" interesting. Provectus gained award (by the US PTO) of its key synthesis patent Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes in September 2013. The company award of its key combination therapy patent Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer in August 2015. Provectus' intellectual property portfolio owns all halogenated xanthenes and protects their therapeutic use as single agents and in combination with (or as part of a combination regimen of) other therapies and therapeutics.
Tipping point. I was struck by certain of the company's CTO Dr. Eric Wachter, PhD's comments on the 4Q15/CY15 business update conference call:
"It appears to me that we are rapidly approaching a tipping point in terms of clinical trial data, in terms of mechanisms of action data, in terms of regulatory path forward certainly for PV-10. And as I said, I can understand frustration in the efforts to capitalize the company, but we're not at that tipping point yet. We're approaching that tipping point. We can anticipate when and where that will happen with some degree of understanding. But, until we reach that tipping point we haven't reached that tipping point, if you understand. Once we've passed that, I think that things get to be very easy for us."Key clinical trial data generation/presentation events in 2016 should be:
- More from the expanded liver Phase 1 trial (July venues: ESMO World GI 2016 and/or Apple 2016?),
- Preliminary safety and response data from the melanoma Phase 1b trial of PV-10 and Keytruda (June: ASCO 2016?, November: SMR 2016?), and
- The interim assessment of the pivotal melanoma Phase 3 trial (2H16: press release?).
Disruptive. What is more likely? A Big Pharma with a checkpoint inhibitor that potentially sees PV-10 as a disruptive complement, or one w/o a checkpoint inhibitor that sees PV-10 simply as potentially disruptive?
Comments await. I want to read the 10-K filing before opining further on the topic of former Chairman, CEO and a co-founder Dr. Craig Dees, PhD, CFO/COO and interim CEO Peter Culpepper, what may have happened, the internal investigation, remediation, etc.
Chicken or the egg. I noted the comments made from last weekend's Hemonc Today Melanoma and Cutaneous Malignancies meeting: e.g., “intralesional therapies are here to stay,” “IL therapies are the ideal candidates for combination therapy,” etc. These recognitions are due in no small part to Amgen's regulatory progress (i.e., T-Vec's approval) and Provectus. I am curious, however: could an argument be made that the regulatory groundwork behind T-Vec's approval was laid by PV-10 (and Provectus)?
Light bulb. The recognition of intralesional (IL) agents clearly contributed to by the realization of the strengths of IL approaches; that is, they direct attack tumor burden while educating or awakening the immune system.
It ain't over till it's over. As checkpoint inhibitors begin to become mature, does there seem to be a realization the war on melanoma (and cancer) is far from over?
Comments await. I want to read the 10-K filing before opining further on the topic of former Chairman, CEO and a co-founder Dr. Craig Dees, PhD, CFO/COO and interim CEO Peter Culpepper, what may have happened, the internal investigation, remediation, etc.
Chicken or the egg. I noted the comments made from last weekend's Hemonc Today Melanoma and Cutaneous Malignancies meeting: e.g., “intralesional therapies are here to stay,” “IL therapies are the ideal candidates for combination therapy,” etc. These recognitions are due in no small part to Amgen's regulatory progress (i.e., T-Vec's approval) and Provectus. I am curious, however: could an argument be made that the regulatory groundwork behind T-Vec's approval was laid by PV-10 (and Provectus)?
Light bulb. The recognition of intralesional (IL) agents clearly contributed to by the realization of the strengths of IL approaches; that is, they direct attack tumor burden while educating or awakening the immune system.
It ain't over till it's over. As checkpoint inhibitors begin to become mature, does there seem to be a realization the war on melanoma (and cancer) is far from over?
Visitor. I previously wrote someone from the headquarters of a Big Pharma has regularly visited since Monday, January 11th. See Breadcrumbs (January 27, 2016) below. An update is below:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below, again, once more.
For the moment, set aside Bristol-Myers suing Merck & Co. over the latter's anti-PD-1 drug Keytruda for infringing on the patent of the latter's drug Opdivo. It would seem most of the pharmaceutical industry believes an anti-PD-1 (or an anti-PD-L1) drug compound is required table stakes. A quick 'n dirty analysis indicates 65% of the top 20 oncology-focused pharmaceutical companies (in yellow) have or have access to the next generation (anti-PD-1) or next-next generation (anti-PD-L1) of immune checkpoint inhibitor. The figure rises to ~70% after adjusting for the acquisition of Pharmacyclics (#19) by AbbVie (#18).
 |
| Click to enlarge. |
Another way to look at it is to have a/the "something else." Agenus, for example, has illustrated this in one of its investor presentation slides:
 |
| Click to enlarge. Image source |
So, while Provectus' pivotal melanoma Phase 3 trial is the company's immediate potential ticket to a drug approval, the initial results of Provectus' Phase 1b/2 melanoma combination therapy trial program (combining the something else of PV-10 with the core of Keytruda) inform the respective business strategies of each of the companies on the list above:
- For those with an immune checkpoint inhibitor, PV-10 might be the something of the something elses, and maybe must have addition to their inhibitor.
- For those without a checkpoint inhibitor, and it's a short list, having the something else might be better in a world with potentially indistinguishable checkpoint inhibitors (save for one's marketing department).
Updated (3/22/16): Provectus made an 8-K filing today to disclose an extension to the warrant exchange tender offer, from the second extension date of March 21st to a revised date of March 28th.
Updated (3/22/16): As I wrote about the warrant exchange below under Moneyness redux (March 7, 2016), I see Provectus CFO/COO and interim CEO Peter Culpepper's approach comprising:
It is quite possible another extension is in the cards. At this point, however, I'd hazard the guess there won't be a fourth extension. One would have fully expected Provectus officers and directors who own non-tradable warrants (Peter, CTO Dr. Eric Wachter, PhD, board member Jan Koe) to tender them upon completion of the program. One also might expect Network 1 and/or Maxim Group to tender their own non-tradable warrants. Until one hears or reads these officers, directors and/or financial services firms have tendered, one would have to believe the program isn't over till it's over. In addition, one also would have fully expected any warrants tendered by each of the extensions would have received the program's final terms. As such, I've heard from several sources -- keeping in mind this comment is rumor/hearsay -- that Provectus management and Network 1 names are tendering their warrants as part of this extension. It shouldn't be too hard for company shareholders to confirm the above.
Updated (3/22/16): I updated the table above.
Also, J&J announced a collaboration with Roche/Genentech combining the former's anti-CD38 antibody with the latter's PD-L1 agent. Is CD38 from Step 2 or 3 of the Cancer Immunity Cycle?
Updated (3/22/16): As I wrote about the warrant exchange below under Moneyness redux (March 7, 2016), I see Provectus CFO/COO and interim CEO Peter Culpepper's approach comprising:
- Trying the warrant exchange, extending it and bettering the economics while knowingly risking further potential scorn and derision from existing and prospective shareholders,
- Seeing what amount of warrants are tendered over whatever timeframe he deems necessary,
- Seeing what information of progress emerges,
- Continuing to pursue non-dilutive funding options,
- Eventually, if necessary, tapping the capital markets, and
- Above all else, hopefully allowing the enormity of PV-10's promise to materialize in a manner that helps to alleviate, mitigate and explain the past.
It is quite possible another extension is in the cards. At this point, however, I'd hazard the guess there won't be a fourth extension. One would have fully expected Provectus officers and directors who own non-tradable warrants (Peter, CTO Dr. Eric Wachter, PhD, board member Jan Koe) to tender them upon completion of the program. One also might expect Network 1 and/or Maxim Group to tender their own non-tradable warrants. Until one hears or reads these officers, directors and/or financial services firms have tendered, one would have to believe the program isn't over till it's over. In addition, one also would have fully expected any warrants tendered by each of the extensions would have received the program's final terms. As such, I've heard from several sources -- keeping in mind this comment is rumor/hearsay -- that Provectus management and Network 1 names are tendering their warrants as part of this extension. It shouldn't be too hard for company shareholders to confirm the above.
Updated (3/22/16): I updated the table above.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
In his presentation at the HemOnc Today Melanoma and Cutaneous Malignancies on March 18th/19th (Current Trials with Oncolytic Agents; slides have not yet been published as of this writing) and in a March 19th article (Intralesional therapy ‘here to stay’ for melanoma), melanoma, intralesional agent and PV-10 key opinion leader (KOL) Dr. Sanjiv Agarwala referred to Provectus' melanoma combination trial as a Phase 2 study. Provectus initiated the Phase 1b study (of the Phase 1b-Phase 2 program) in October 2015 (PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma).
My sense is any advancement into the [randomized] Phase 2 portion (pembrolizumab vs. pembrolizumab + PV-10) has been planned or is being planned pending pending data review team recommendations.* So, while a projected Phase 1b enrollment of 24 patients likely is far from complete, safety and efficacy data from whatever enrollment already has been achieved to date might have been or is sufficient to encourage planning for the Phase 2 portion.
In particular, although Agarwala's slide was titled "PV-10 Phase II Combination With Pembrolizumab," there was no mention of the Phase 2's N -- whether the originally planned or estimated 120, or some number higher or lower than 120 that would reflect the preliminary efficacy of the Phase 1b and thus the potential treatment effect size of the combination over the standard of care). It is possible more patients might be required to fully establish the treatment effect and thus the Phase 2's N or, then again, the observed treatment effect may be good enough to establish this calculation.
* For example, consider the process described for the T-Vec + ipilimumab work: Phase 1 results of a phase 1b/2, multicenter, open-label trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) vs ipi alone in previously untreated, unresected stage IIIB-IV melanoma, Puzanov et al., J Immunother Cancer. 2013; 1(Suppl 1): P84 -- see Conclusions.
Agarwala's presentation noted the timeframe for the T-Vec + pembro Phase 1b trial's enrollment and data cutoff; see below: enrollment of about 90 days, and a data cutoff about 100 days after the last patient was enrolled.
 |
| Click to enlarge. Slide from Agarwala's Current Trials with Oncolytic Agents presentaton |
 |
| Click to enlarge. |
"“Combinations will be the future,” Agarwala said. “Why not find a way to combine modalities that have different mechanisms of action and have, very importantly, nonoverlapping toxicities?”Talimogene laherparepvec, also known as T-VEC, is under evaluation in combination with ipilimumab (Yervoy, Bristol-Myers Squibb). A small trial of 17 patients has shown the combination has the promise to yield long-term responses in patients, Agarwala said.T-VEC also is being studied with pembrolizumab (Keytruda, Merck) in patients with previously untreated, unresected, stage IIIB to IVM1c melanoma.So far, safety results have shown a low adverse event profile for this combination, Agarwala said. Preliminary efficacy results showed an overall response rate of 56.3%, which suggests improvement over the T-VEC–ipilimumab combination.“We’ve been able to now make an intralesional therapy applicable to not only patients with M1a disease, but also to patients with M1b and M1c disease. So, patients with multiple metastatic sites might be able to benefit,” Agarwala said.The phase 3 MASTERKEY-265 study is comparing T-VEC plus pembrolizumab to placebo plus pembrolizumab, which should definitively prove or disprove the theory that a viral oncolytic agent plus a checkpoint inhibitor will improve outcomes, Agarwala said.Further, a phase 2 trial is ongoing evaluating PV-10 with pembrolizumab, and another phase 2 trial is evaluating HF10 with ipilimumab."
I'm not sure why Agarwala included HF-10 in his presentation, other than the fact that Huntsman Cancer Institute's Dr. Robert Andtbacka, MD and him are investigators in the HF-10-ipi trial.
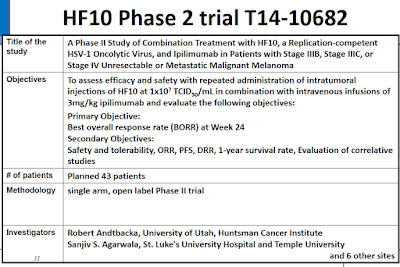 |
| Click to enlarge. |
The Phase 1 clinical trial of HF-10 alone generated 1 partial response and no complete responses in head and neck cancer, malignant melanoma, and other malignancies. That kind of low level or amount of tumor destruction is unlikely to generate a notable antigen cascade, which then might not lead to generation of a robust immune response.
 |
| Click to enlarge. |
(aka Business update conference call review, part 3)
 |
| Tweet/Image source |
In an apparently continuing medical research and pharmaceutical industry trend directly related to the tumor microenvironment, and tumor heterogeneity, consider a March 10th article (research brief) by Amy Blum entitled Tumor Heterogeneity Influences the Immune System's Ability to Fight Cancer (from the National Institutes of Health's The Cancer Genome Atlas).
The brief notes:
"A new study led by Charles Swanton of the Francis Crick Institute in the United Kingdom found that activation of the immune system against cancer depends on the number and variety of mutations in the tumor. The findings, published on March 3,2016, in Science, provide insight into immune surveillance and suggest that variation, or heterogeneity, within a tumor may be an important predictor of whether patients could benefit from immunotherapy. "
See also:
- Late-night Boba (March 4, 2016) below, where I posted an article entitled Tumours contain the seeds of their own destruction, and
- February 26, 2013 blog post $PVCT: #Tumor Heterogeneity.
It's not unreasonable to think tumor heterogeneity ultimately may be one of the principal strengths of intralesional (IL) approaches for treating cancer. If you destroy numerous tumors, you teach the immune system about a heterogeneous range of tumor cell populations. Let's make all of a patient's tumors our friends.
Turning to the recent 4Q15/CY15 business update conference call:
F. #1. Cash balance, Cash burn, and Stockholders' Equity -- and -- #2. Certain disclosures (among others)
Questions listed on March 13, 2016 blog post Fourth Quarter, Year-End 2015 Business Update/Conference Call Prep, and any others, will have to wait until the filing of Provectus' 10-K. Management noted on the March 16th call that another investor call would be held together with/after the filing. This subsequent conference call should provide a further forum for questions directly related to the company financial results, the internal investigation related for former Chairman, CEO and a co-founder Dr. Craig Dees, PhD*, and the tender offer. Provectus' CFO/COO and interim CEO Peter Culpepper said on the call:
"That's our purpose for having the next conference call when we file the 10-K and when we expect to complete and close that warrant exchange transaction, yes."And:
"Thank you for your time this afternoon. And we look forward to being with you again when we file the Form 10-K this month and in May when we file the Form 10-Q."* The company noted in the related March 16th 8-K filing that "[t]he Company is currently proceeding as quickly as possible to complete, but has not yet completed, its quantification and evaluation of the specific impact of the issues identified in the Audit Committee’s report on the Company’s financial statements, but the Company does not expect the Audit Committee’s findings to have a material impact on the Company’s financial statements." In addition, in regards to the CEO search, Peter said on the 4Q15/CY15 call "I have been asked to apply by the Board and plan to do so."
G. #3. Stage III melanoma Phase 3 trial
- Is the timing of the trial's interim data readout/analysis still [early] August 2016? Where is the version of the trial protocol that would include Amgen's intralesional agent (IL) talimogene laherparepvec (T-Vec, or Imlygic) as a comparator? What is the status of trial site activations in the U.S., Australia, Western Europe (Germany, Poland), South and Central America (Brazil, Mexico), and Asia (China, Hong Kong, Singapore)?
In regards to the interim analysis Peter said on the call:
"Beginning with our Phase 3 study, we have a protocol that calls for 225 patients at our first site with St. Luke’s University Hospital and Health Network, Bethlehem, Pennsylvania. We have announced that our first patient was dosed last year. As stated in our previous call, our estimated primary completion date remains September 2017 and an estimated study completion date of October 2017. When 50 percent of the events required for the primary endpoint have occurred, the Impendent Data Monitoring Committee will issue an interim assessment of efficacy and safety. So, meaningful clinical data could come this year. I stress the word “could.” And as we get closer to the 50 percent level, we will refine that target date. Eric Wachter will discuss this further in his remarks in a few minutes."Provectus' CTO Dr. Eric Wachter, PhD said:
"I expect we will be able to refine study time lines by the time we have the next quarterly investor call. Importantly, all of the amendments are expected to enhance patient eligibility and enrollment in this global study. "The amended protocol for the pivotal Phase 3 trial was filed on March 14th as PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma.
"It’s also important to note that many of these changes will facilitate opening the study efficiently on a truly global scale, incorporating advice from regional leading investigators and institutions to assuredly address differences within and between regions."And
"As that currently amended protocol has been developed, as I mentioned in my remarks, we began the process of distributing that to investigators and their sites about a month ago. And so, there's no expected interruption in the process of moving the study forward."H. #5. Phase 1 liver cancer data presentation/publication
- What guidance is there on the presentation and/or publication of more and/or initial [expanded] Phase 1 trial data?
"Moving onto tumorous liver, we continue to add patients to our Phase I study of hepatocellular carcinoma and metastases of liver and are working to add one or more additional centers in the U.S. to facilitate completion of enrollment. We expect to report additional data this year as a followup to our initial reporting in mid-2015.
Because HCC is a major health issue in Asia, we’ve been actively engaged with an investigator community there to expand this program into this important region. Following up on a productive meeting with key investigators from Greater China in October, we’re meeting with a similar group in Singapore this month to review our strategy and assure that our plans meet the needs for development of PV-10 for HCC in Asia.
As noted previously this work is expected to lead to commencement of one or more Phase I/II studies of PV-10 or PV-10 plus standard of care. This effort has been facilitated in large measure by our relationship with Boehringer Ingelheim, who have provided crucial advice and contacts throughout Asia."I. #6. Follow-on hepatic cancer trials, including NET Phase 1 study
- Why did the NET hepatic cancer trial commence before any other hepatic cancer trial, early- or late-stage?
"We also announced recently that we have initiated a companion study assessing the potential of PV-10 in symptomatic neuroendocrine tumors, or NETs, metastatic liver. This study builds on what we’ve learned so far in our initial study, PV-10 [unintelligible] I, looking at HCC and other metastatic tumors. In addition to standard objective response assessments, since they are well established quality of life instruments specific to these patients, this study will allow us to assess topics concerning symptomatic benefit that are increasingly important to regulators.
And they are excellent biomarkers to allow us to test concordance of objective response and biological response. The single center study is designed to have two successive cohorts of patients. And if initial results appear encouraging, we may elect to expand to additional sites to accelerate study completion."PV-10 Phase II Combination With Pembrolizumab (March 17, 2016)
(aka Business update conference call review, part 2)
Tomorrow, Friday, March 18th, St. Luke's Cancer Center's Dr. Sanjiv Argawala will make a presentation entitled Current Trials with Oncolytic Agents at the HemOnc Today Melanoma and Cutaneous Malignancies in New York.
 |
| Click to enlarge. |
"Next, we have our Phase 1b/2 testing the PV-10 in combination with Merck’s Keytruda in patients with Stage 4 melanoma. In September, we announced completion of the protocol for Phase 1b/2 testing of PV-10 in combination with pembrolizumab, which sells as Keytruda, in patients with Stage 4 melanoma. This study will approximately 144 patients with 24 of these in the initial Phase 1b portion of the study and the remainder in the Phase 2 portion. We expect completion in of enrollment in the Phase 1b portion this year, and if all goes as expected to commence enrollment in Phase 2 about four months after the last patient enters Phase 1b. We announced early--earlier the Phase 1b portion is expected to be completed this year. The first patients have been treated in this important study."And:
"The Phase 1B, II, study of PV-10, in combination with Keytruda, establishes a strong position in an area of very active scientific and commercial interests in our patent that protects PV-10 when used in combination--is held jointly with Pfizer."The company's CTO Dr. Eric Wachter, PhD said:
"Actually, a very informative overview, Pete. I’ll start my remarks with a brief synopsis of major technical aspects of our business before going into detail on key elements. As Pete noted earlier, in the fourth quarter Provectus started a combination therapy trial in latestage cancer patients where the safety and preliminary efficacy of PV-10 in combination with pembrolizumab, also known as Keytruda, is being assessed."And:
In our Phase IB combination study, we began enrollment in the fourth quarter, and we expect enrollment in this portion of the study to be completed this year. We’ve been working with the sites listed on the clinictrials.gov listing for the study to get each one open for enrollment to patients and working to add several additional sites in the U.S. and Australia with the goal of having five to seven sites participate in Phase IB. We are also working with our global CRO to prepare for transition of the study to Phase II and to expand the study to include sites in Europe when that work commences.
The study was designed to demonstrate the potential additive benefits of combining the ablative and immunological aspects of PV-10 with an immune checkpoint inhibitor. And a success could pave the way for potential combination with many other agents. The mechanism data on PV-10, both nonclinical and clinical, suggests that this should be the case. But, of course, we can’t be sure until we begin to see initial data, expected later this year."H/t a regular hatter: Dr. Argawala's presentation includes (but is not limited) to the following slides:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
- E. #4. Advanced melanoma (Stage IV), combination therapy (PV-10 + Keytruda), Phase 1b trial:
- What guidance is there on the timing, and possibly a potential venue (medical conference), of the presentation of interim clinical trial data?
- What is the status of trial site activations in the U.S. and Australia?
- Would these sites, in addition to the single recruiting site to date (long-time PV-10 investigator and medical oncologist Dr. Sanjiv Agarwala, MD's St. Luke's Cancer Center), be part of the Phase 1b portion of the combination therapy program, or the eventual Phase 2 portion?
- At this point in recruiting and treatment, what guidance do you have of the Phase 1b's actual or potential impact factor or effect size or treatment effect?
The transcript of the call may be found here (via Provectus) and here (courtesy of Seeking Alpha). I'll also reference March 13, 2016 blog post Fourth Quarter, Year-End 2015 Business Update/Conference Call Prep.
A. #7. CUP data: How many patients were treated in the compassionate use program (CUP) (aka the expanded access program) in CY2015? What guidance is there on the timing of the publication or publications of CUP clinical results and data?
Eric said:
- "As a final note on melanoma, I mentioned in the November call that approximately 140 patients had received PV-10 at that time under our expanded access protocol. And as of the end of the fourth quarter this number has risen to 160, with the vast majority having melanoma. As Pete noted, we currently plan to keep this protocol open to patients who are not eligible for other PV-10 studies."
- "I noted that we have, as of the end of 2015, enrolled 160 patients, the majority of those being melanoma. We are working with investigators at several of our larger higher-enrolling centers to release data on their individual sets of patients, which looks to be very interesting. And I would anticipate that some of that will come out in the first half of this year."
The program treated 40 patients in CY2014 (a cumulative figure of 140). Therefore, 20 patients would have been treated in CY2015, a 14% year-over-year (YOY) increase for a year in which Provectus initiated two melanoma-related clinical trials.
 |
| Click to enlarge. |
The last guidance on this topic could come from Provectus' November 2010 press release Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia, which noted (my bolded and underlined emphasis):
"The recent meeting focused on manufacturing, characterization and specifications for PV-10, along with a review of clinical data and anticipated Phase 3 study design and endpoints. The proposed primary endpoint of progression free survival, which Provectus proposed to the U.S. Food and Drug Administration (FDA) earlier this year in its first end-of-Phase-2 meeting with FDA, was deemed appropriate for assessment of efficacy in light of established European Medicines Agency (EMEA) standards adopted by TGA. Use of interim data from the first half of Phase 3 study subjects, in conjunction with safety data collected in earlier studies of PV-10 for melanoma, was discussed to allow early evaluation for marketing approval for metastatic melanoma, and TGA agreed that these data should be sufficient for this review if the analysis confirmed efficacy."It strikes me, like with some other matters (like licensing or acquisition interest) that the pivotal melanoma Phase 3 trial's interim assessment is a potential catalyst or trigger, including [early] marketing approval by the TGA. And it would seem to me such marketing approval is within site, or perhaps even expected.
On the call Peter said:
- "As the trial progresses over the course of 2016, we will assess the paths for expedited development, fast track accelerated approval, market approval with the FDA in the U.S. and the Therapeutic Good Administration in Australia."
Eric said:
- "In regard to the TGA, yes, definitely moving forward with approval in Australia is something that we have been considering, we are currently considering, and we will continue to consider, especially given that roughly half of the melanoma patients that received PV-10 throughout the history of our development have been from Australia. So--and we have a significant base of data now on patients in Australia."
C. #8. PH-10 MOA data (psoriasis Phase 2 study): What are the results? What guidance is there on the timing of the revelation of clinical results and data?
Peter said on the call:
"Touching briefly on PH-10, as Eric will provide much more information, I want to make three points about 2016. First, we expect to report on our Phase 2C mechanism results--and mechanism results. Second, we expect to have an end of Phase 2 meeting with the FDA regarding toxicology and further clinical development. And third, we will be seeking a licensing agreement based on the strength of the mechanism in other PH-10 Phase 2 studies."Provectus has been seeking a license agreement since at least 2010.
Eric said:
- "In addition, there’s our Phase 2 study of the cellular and immunologic changes in the skin of patients receiving PH-10, our investigational topical treatment for atopic dermatitis and psoriasis. Other research is going on with PV-10 and other cancers, but these are the most advanced studies and, therefore, the most likely to result in marketable treatments in the near future."
- "And the topical PH-10 program has been moving ahead with completion of clinical work in our recent mechanism of action study in December. Analysis of tissue collected from study participants is underway, and we are awaiting review of the results of skin biopsies collected pre- and post-PH-10."
- As I noted earlier, we’ve been busy on the mechanisms of action for topical PH-10 as well and reported completion of clinical work in our mechanism of action study in psoriasis patients in December. We are finalizing compilation of clinical data from the study, and analysis of tissue collected from study participants is nearly complete. We expect to review results of skin biopsies collected pre- and post-PH-10 in the very near future.
- "As I mentioned in my remarks, we have completed all of the data collection from the patients. We are in the process of compiling the clinical data. So, this is the numerical data that's collected when a patient visits the clinic. We are working with a major research university on analyzing skin biopsies that were collected three times during the course of the study for each patient; at the beginning of the study pretreatment, after four weeks of application of vehicle, and then four weeks after application of PH-10. That work is essentially complete. We are in the process of receiving that this week. And I think that it's possible that we will have a preliminary analysis of that completed this month. Certainly if not this month, early into the next month, the month of April." {my underlined emphasis}
Interestingly, Vitae Pharmaceuticals recently achieved proof-of-concept with an RORyt inhibitor in moderate to severe psoriasis. In this company's press release Vitae noted Dr. James Krueger, MD, PhD, Head of the Laboratory for Investigative Dermatology at Rockefeller University would join the related conference call, as he did for Vitae in November 2015.
 |
| Click to enlarge. November 2015 conference call. |
The "major research university" and the individual (team) with whom Provectus is working are...
D. #9. Psoriasis and atopic dermatitis Phase 3 trials: What guidance is there regarding additional (if any) regulatory and/or commercial licensing (if applicable or appropriate) steps before the commencement of one or both of these pivotal studies?
Eric said on the call:
Eric said on the call:
- "The study was designed to allow us to probe possible immunologic, structural, and hyperproliferative changes in psoriatic plaque and detect any evidence of cellular atypia upon application of PH-10--will also allow us to assess concordance of any such changes with clinical observations in those plaques. We expect these data to inform decisions on an anticipated request to meet with FDA to address strategies for advancing the program from Phase II into Phase III"
One of the sources underlying my comments under #3 of To Dos (March 16, 2016) was Amgen's sponsor material in support of the FDA's AdComm meeting for T-Vec in 2015. In T-Vec's pivotal melanoma Phase 3 trial, PPR was defined as "the appearance of a new lesion or >25% increase in baseline tumor burden." See, from ASCO 2014, Patterns of Durable Response (DR) With Intralesional Talimogene Laherparepvec (T-VEC) From a Phase 3 Trial in Patients With Stage IIIB-IV Melanoma, Ross et al., J Clin Oncol 32:5s, 2014 (suppl; abstr 9026). Ross et. al. concluded:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Provectus' pivotal melanoma Phase 3 trial, now named PV-10 vs Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma, utilizes T-Vec treatment per the drug's prescribing information (i.e., initial treatment, second treatment 3 weeks (21 days) after the initial treatment, subsequent treatments 2 weeks (14 days) after previous treatment).
For the purposes of ascertaining progression-free survival (PFS), patients would have a comprehensive assessment every 12 weeks (3 months) with measurement per the RECIST v 1.1. framework. Clinical assessments to determine progression and for the purposes of determining crossover eligibility post-treatment, however, would be undertaken every cycle completion, or every 4 weeks (1 month).
 |
| Click to enlarge. Image source |
Class action lawsuit update. As this InvestorVillage poster (Area51) routinely does, here is a link to an update on the suit's settlement process.
To Dos (March 16, 2016)
Note to self: Things I have to write about.
1. The initial goal of Moffitt Cancer Center's mechanism of action work -- Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study -- was to elucidate what is Step #5 of Chen and Mellman's Cancer-Immunity Cycle: Infiltration of T cells into tumors.
 |
| Click to enlarge. Figure 1, Chen and Mellman. Colored edit is mine. |
3. Amgen did not report progression-free survival (PFS) for talimogene laherparepvec's (T-Vec's) pivotal melanoma Phase 3 trial. Rather, they report data on progression and durability of response in a number of other ways: a primary endpoint of durable response rate (DRR), and secondary endpoints that included duration of response, and time to treatment failure.
Amgen (or BioVex, which originally designed the Phase 3 trial prior to its acquisition by Amgen) used a modified WHO assessment method (i.e., tumor response criteria) that allowed for progression prior to response (PPR). In Amgen's advisory committee meeting materials prior to T-Vec's approval, the Big Biotech noted: "Progression-free survival was not chosen as a secondary endpoint because an increase in lesion size and/or development of new lesion(s) prior to response was expected based on results from the phase 2 study (Study 002/03)." {my underlined emphasis} T-Vec's Phase 2 study (when it was called OncoVex) used a modified RECIST tumor response measurement to allow progression before response. Interestingly, in T-Vec's Phase 3 trial, more than half (54%) of responders "experienced an increase in overall lesion size of ≥ 25% and/or developed at least one new lesion prior to ultimately achieving a response...." This progression apparently is consistent with a delayed immune response.
Assessment of progression in Provectus' pivotal Phase 3 trial that now will include T-Vec as a comparator (investigator's choice of T-Vec or chemotherapy), however, will be RECIST 1.1 criteria -- where disease progression is defined in lesion size of > 20%.
The Ugly, the Bad and the Good (March 16, 2016)
There's a good amount of information, both on company internals and externals, to digest and discuss, and opinion(s) to be formed, made and conveyed. I plan to write more on several topics here under Current News and as blog posts. I'd also like the benefit of hearing comments by Provectus' CTO Dr. Eric Wachter, PhD (clinical development program progress), mostly, and interim CEO Peter Culpepper (an explanation).
The Ugly: Provectus issued a press release and made an 8-K filing today regarding an internal review surrounding the resignation of former Chairman, CEO and a co-founder Dr. Craig Dees, PhD, Announces Results of Internal Investigation. Of note, Craig received nearly $2.5 million in travel expense advances from CY 2013 to CY 2015 (to travel where and to do what?), and provided receipts for less than 15% of these expenses (~$350K), most of which the Audit Committee indicated "did not appear to be authentic." See a summary table below.
 |
| Click to enlarge. |
- Where were Provectus' accounting firm and auditors, BDO, in this situation and process?
- What will Peter's explanation be for this gross lack of oversight?
- Given the magnitude and duration of this activity, he would be hard pressed to remain in the role of CFO. To that end, the 8-K on this topic noted "The Audit Committee is also in the process of implementing the recommendations made by counsel to the Audit Committee to remediate these, including but not limited to the appointment of an interim Chief Financial Officer to assist in the organization and strategic operation of the Company as to its procedures and daily operations of the Company." {my underlined emphasis}
The Bad: The company issued a Form 12b-25, Notification of Late Filing, to communicate Provectus' 2015 10-K would be late. The filing noted "The Company currently anticipates that its Form 10-K for the year ended December 31, 2015 will be filed as soon as practicable and no later than fifteen days calendar days following its prescribed due date." {my underlined emphasis}
Questions include:
- What will the filing say, read in its entirety?
- What was the 12/31/15 cash balance? What could the 3/31/16 figure be? Essentially, am I correct in my estimation(s) of cash burn and burn rates? See item 1, Cash balance, Cash burn, and Stockholders' Equity on March 13, 2016 blog post Fourth Quarter, Year-End 2015 Business Update/Conference Call Prep.
The Good: Provectus issued a press release and made an 8-K filing regarding changes to the company's ongoing pivotal melanoma Phase 3 trial, Amends Protocol for Phase 3 Study of PV-10 in Treatment of Locally Advanced Cutaneous Melanoma. The protocol on ClinicalTrials.gov is here, PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma.
 |
| Click to enlarge. Trial Purpose Before (left) and After (right) |
Notable changes (or non-changes), aside from the addition of Amgen's intralesional agent talimogene laherparepvec (T-Vec, or trade name Imylgic), primarily address the expansion of the trial's applicable patient population across at least a couple of dimensions:
- To include Stage IVM1a melanoma patients
| Click enlarge. Before |
| Click to enlarge. After |
- To more easily include patients having immunotherapy and targeted therapy options, or lack thereof
| Click to enlarge. Before |
| Click to enlarge. After |
Approved drugs for metastatic melanoma include:
- Immunotherapies: (1) Ipilimumab, (2) Pembrolizumab, (3) Nivolumab, (4) Ipilimumab + Nivolumab,
- Targeted therapies: (5) Vemurafenib, (6) Dabrafenib, (7) Trametinib , (8) Dabrafenib + Trametinib
- Intralesional therapies: (9) T-Vec.
Provectus' Phase 3 trial now addresses all of these.
Takeaways and Questions:
- The trial's "label" appears to have grown bigger.
- When does a pivotal trial, already in process, expand its patient population?
- There is no change in the trial's N of 225.
- How much faster could the trial now enroll?
- How long could the potential timing of the interim analysis be pushed out, if at all? What could T-Vec's inclusion as a comparator do to the required number of patients (between the treatment arm of PV-10 and the control arm of either chemotherapy or T-Vec) to show statistically significant progress-free survival curve separation?
- Did the FDA see any Phase 3 data as part of their deliberation and determination to accept the patient population expansion into Stage IV disease?
By now the pharmaceutical industry understands immune checkpoint inhibitors are limited, in terms of the fraction of patients they can help. Very generally speaking, anti-PD agents like Keytruda or Opdivo may help treat only about 20% of cancer patients. This limitedness could apply to all checkpoint inhibitors or inhibitory agents:
- "First generation" ones like anti-PD-1 agents (e.g., Merck & Co.'s Keytruda, Bristol-Myers' Opdivo, China's BeiGene's BGB-A317, etc.),
- Second generation ones like anti-PD-L1 agents (e.g., Roche/Genentech's atezolizumab, Pfizer/Merck KGaA's avelumab, etc.), and
- Third and later generations, like anti-Tim-3 or anti-Lag-3 agents...or whatever.
Takeaways: Releasing the brakes on the immune system clearly is not enough. Some or many pharmaceutical companies may believe table stakes requires them to have a checkpoint inhibitor.
Recently, or thereabouts, we have been hearing and reading about the pharmaceutical industry exploring stimulation -- that is, agents that step on the gas pedal [of the immune system] or help turn on its engine. Investigating stimulation is an entirely different side or step(s) of the cancer immunity cycle -- left-hand side, steps no. 1-3, my orange edit -- than inhibition -- right-hand side, step 7, my purple edit. I've added notations to Chen and Mellman's Figures 1 and 3 below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Stimulation, or approaches to stimulation, could have many names, such as:
- Turning a cold tumor hot, and
- Given variable tumor immunogenicity, making non-immunogenic tumors immunogenic or making immunogenic tumors more so.
Other names or labels of approaches could include:
- Inflamed tumors, and so, making tumors inflamed or more inflamed), and
- Immunogenic intensification, which originally was/is meant to be the concept of combining a checkpoint inhibitor with another inhibitor (inhibition+inhibition) or a vaccine (stimulation+inhibition). I think the idea has evolved into proper context of combining a stimulatory agent with an inhibitory agent.
For inflamed tumors, see for example a retweet from @ChrisHeery (my orange edits):
 |
| Click to enlarge. |
For immunogenic intensification, see for example a tweet by @ChrisHeery:
 |
| Click to enlarge. |
Some call it an "antigen cascade;" see for example a tweet by @ChrisHeery (discussing the same presentation that included immunogenic intensification by National Cancer Institute's Dr. James Gulley, MD, PhD:
 |
| Click to enlarge. |
Antigenization. See for example March 10, 2013 blog post $PVCT: Florey, Chain & Heatley
Chemoablation via PV-10 Ablation Causes Antigenization & Antigenization Causes Immunization. I wrote:
"PV-10 causes antigenization. Antigenization causes immunization. Antigenization is the expression of antigens, in a tumor into which PV-10 has been injected, in context. Immunization is "the process by which...[the] immune system becomes fortified against an agent." PV-10 facilitates the relationship between antigenization and immunization.See also October 14, 2014 blog post The Immune Checkpoint Inhibitor Global 4 (or 5), where I wrote:
"PV-10 kills tumors far better than T-Vec. PV-10 produce higher complete responses than T-Vec. The medical community has understood for a while the more antigens produced and presented as a result of tumor destruction (antigenization) the more likely the potential of a greater immune response by the body."Antigen storm. See June 19, 2013 blog post $PVCT & $PFE's Hisun-Pfizer Pharmaceuticals Co., Ltd., I wrote:
"...the injection of PV-10 and its subsequent chemoablative action creates lots of antigens. The creation of lots of antigens is the key to the successful, sustainable treatment of cancer and, thus, its cure. Antigen presentation is a process in the body's immune system by which macrophages, dendritic cells and other cell types capture antigens and then enable their recognition by T-cells. PV-10 creates an "antigen storm," the creation of many antigens, much more than than any other antigen-creating material currently available.""...you could augment T-cell activity, causing an influx of them to the tumor and making a cold tumor hot" (March 10, 2016)
I noted making cold tumors hot under the news item immediately below. A shareholder sent me a weblink to a March P&T Community article by Walter Alexander entitled The Checkpoint Immunotherapy Revolution (my underlined emphasis below):
"Many clinical trials are evaluating PD-1 inhibitors in combination with one or more agents, Dr. Weber said. “If any of these combinations can be shown to be less toxic and anywhere near as effective as nivolumab plus ipilimumab, ipilimumab plus nivolumab will go the way of the dodo,” he said. “Maybe everyone will use OX40 plus nivolumab. We’ll see if it’s well tolerated; maybe even nivolumab plus OX40 plus ipilimumab.”
If a clinical trial of PD-1/PD-L1 inhibition plus the indoleamine 2,3-dioxygenase inhibitor epacadostat (Incyte Corporation) demonstrates a 50% response rate with median survival in the 35 to 40 month range, Dr. Weber speculated, people will lose interest in combinations with ipilimumab. “But until then, that is the combination to beat.”
Preliminary results of a phase 1/2 study of epacadostat in combination with pembrolizumab, presented at the Society for the Immunotherapy of Cancer annual meeting in November 2015, may be a foreshadowing. In 54 patients with a variety of tumor types, the overall response rate was 53%, with only 2% of patients discontinuing the drug because of treatment-related adverse events. Survival results will need another year or two to mature, Dr. Weber said. “This would be kind of nice, but right now the ipilimumab/nivolumab combination is a very effective regimen with long-term survival that’s very impressive.”
Finally, Dr. Weber pointed to the locally injected or intralesional therapies, such as talimogene laherparepvec (T-VEC), PV-10 (Rose Bengal 10% disodium, Provectus), and coxsackievirus A21 (Cavatak, Viralytics). Not only do they generally share the virtue of being devoid of dose-limiting toxicities, but they also may be able to “prime” the immune response, Dr. Weber said, so that “beyond trying to diminish the Treg and MDSC activity, you could augment T-cell activity, causing an influx of them to the tumor and making a cold tumor hot” (Figure 1).
Merrick Ross, MD, Professor of Surgery at MD Anderson Cancer Center in Houston, Texas, has suggested that the programmed cell death induced by the PD-1/PD-L1 agents may not cause the tumor to express antigens in a manner that evokes an immune response. On the other hand, viral vectors and a chemoablative agent like PV-10 actually rupture the tumor, releasing and presenting intact antigens. Such an effect, he said, could be synergistic with ipilimumab or other targeted immunotherapies, because it occurs at a different place in the immune system.Thursday evening expressos (March 10, 2016)
Updated below.
 |
| Image source |
Area51 notes a $3.5 million figure subject to court approval. Cut and pastes from the court filings, according to Area51:
Statement of Class Recovery
Pursuant to the Settlement described herein, a $3.5 million Settlement Fund has been established. Based on Lead Plaintiff’s estimate of the number of shares of Provectus common stock damaged during the Class Period, the average distribution per share under the Plan of Allocation is roughly $0.072 per share before deduction of any taxes on the income earned on the Settlement Amount thereof, notice and administration costs, and the attorneys’ fees, costs, and expenses as determined by the Court. Class Members should note, however, that these are only estimates. A Class Member’s actual recovery will be a proportion of the Net Settlement Fund determined by that claimant’s claims as compared to the total claims of all Class Members who submit acceptable Proofs of Claim. An individual Class Member may receive more or less than this estimated average amount. See Plan of Allocation set forth and discussed at pages 11 through 13 below for more information on the calculation of your claim.
Reasons for the Settlement
Lead Plaintiff’s principal reason for entering into the Settlement is the benefit to the Class now, without further risk or the delays inherent in continued litigation. The cash benefit under the Settlement must be considered against the significant risk that a smaller recovery – or, indeed, no recovery at all – might be achieved after contested motions, trial, and likely appeals, a process that could last several years into the future. For the Settling Defendants, who have denied and continue to deny all allegations of liability, fault, or wrongdoing whatsoever, the principal reason for entering into the Settlement is to eliminate the uncertainty, risk, costs, and burdens inherent in any litigation, especially in complex cases such as this Litigation. Settling Defendants have concluded that further conduct of this Litigation could be protracted and distracting. {my underlined emphasis}Takeaways & Questions:
- Assuming the settlement is consummated (i.e., the court approves its), this would be the second legal black mark on management (under the leadership of Provectus' former Chairman, CEO and a co-founder Dr. Craig Dees, PhD). The first was a compensation-related lawsuit settlement arrived at in June 2014.
- How much of the $3.5 million would be paid by D&O insurance, and how much would be paid by Provectus? Speaking with some folks experienced in such general matters, depending on the circumstances, insurance could cover most or all of the amount.
- What could be the outcome of the remaining derivative lawsuit (see Section 7 starting on page 9 of the 10-Q noted above)?
Updated (3/10/16): Hot Tumors vs. Cold Tumors. H/t InvestorVillage poster canis_star who sent me a weblink to a panel discussion that included NYU Langone Medical Center's Dr. Jeffrey Weber, MD, PhD entitled Hot Versus Cold Tumors: Predictive Markers in Melanoma. Weber notes:
Tom Gajewski has taken it to another step to try to understand the idea of the hot tumor and the cold tumor. The cold tumor has no PD-L1 expression, no T-cell infiltrate...versus a hot tumor, which is maybe 30%, 40% of all the melanoma patients where you have T-cells infiltrating. They’re in the tumor. They get out or into the tumor itself, and there’s PD-L1 positivity throughout the tumor, and those are the patients who are going to do well...So the question is, as you’re implying, how do you get from a cold tumor to a hot tumor?...I think that’s the new frontier, and I think we’ll see real progress in melanoma when we can make a cold tumor into a hot one." {my underlined emphasis}Recall that Moffitt Cancer Center's Phase 1 feasibility study (mechanism of action study) was entitled Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study.
At AACR 2014 Moffitt noted in their poster presentation entitled Induction of anti-melanoma immunity after intralesional ablative therapy (Provectus PR source of the below):
"The PV-10 treatment of B16 tumors in mice led to release of HMGB1, a soluble Damage Associated Molecule Pattern (DAMP) that is important in activation of dendritic cells; such dendritic cells from these mice were selectively active against B16 tumor cells. PV-10 treatment of B16 tumors in mice also led to infiltration of dendritic cells into the lymph nodes draining the treated tumors; no infiltration was observed in non-draining nodes." {my underlined emphasis}Can PV-10 turn cold tumors hot?
Variable tumor immunogenicity. In the same vein, the following two slides from a recent Agenus presentation are interesting (purple edits are mine).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
"Possibly over-using the car analogy further, with the potential risk of over-simplifying it inappropriately, consider T cell immunity as a car at rest. More immunogenic tumors and their associated cancers like melanoma are like a car sitting on a slight incline. Release its brakes by treating the tumors (and thus the cancer) with checkpoint inhibitors, and the car may roll forward move some distance, notable or otherwise. With other less or non-immunogenic cancers, think of the car as sitting on a flat surface. Releasing the brakes does not enable the car to move any meaningful distance, if at all."I whiffed (March 8, 2016)
In writing about Provectus's new clinical study of a new indication, neuroendocrine tumors (NET) metastatic to the liver -- see February 27th blog post A new article, a new clinical trial, and Our logic seemed clear (February 29, 2016) below -- I now realize that I did not address an additional measure or trial endpoint (a secondary one) of efficacy, specifically objective response rate (after 6 months).
Thus, taking into account, among other comparators to discuss the topic, either Novartis' Afinitor (everolimus) that was recently approved for NET* or its pivotal trial's comparator (a placebo), Provectus CTO Dr. Eric Wachter, PhD's measurement of better patient outcome would address (i) reduction in the size or complete elimination of the tumor (upon injection with PV-10) and (ii) diminishment or elimination of critical symptoms like diarrhea and flushing.
As noted below, Afinitor achieved a tumor response of 2% (the placebo achieved 1%). As for symptom improvement, the drug (compared to the placebo) achieved for infections (11.0% vs 2.0%), diarrhea (9.0% vs 2.0%), stomatitis (9.0% vs 0.0%), fatigue (5.0% vs 1.0%) and hyperglycemia (5.0% vs 0.0%).
PV-10 previously has been used to treat at least one NET tumor, so one would presume Eric has experience or observable data on both tumor and symptom reduction (tumor reduction in PV-10 lingo also is known as tumor ablation).
Interestingly, the FDA's approval for this new NET indication was Afinitor's sixth. There currently are 428 open clinical studies of the drug listed on ClinicalTrials.gov.
 |
| Click to enlarge. |
Moneyness redux (March 7, 2016)
Updated below.
As I wrote on Friday, March 4th, see Warrant tender offer under Late-night Boba (March 4, 2016) below, Provectus made an 8-K filing today (among other related filings: a prospectus supplement, the tender offer) to disclose:
- An extension to the warrant exchange's tender offer, from the first extension date of March 10th to the revised-again date of March 21st,
- A reduction in the price-to-paid for receiving the tradable $0.85 exercise priced warrant, from the original price of $0.75 to a revised price of $0.50,
- ~323K of non-tradable warrants had been tendered as of March 4th (Friday).
As of this writing, when the PVCT share price appears to be $0.45 and the PVCT.WS warrant price appears to be ~$0.20, a total "unit" price of ~$0.65, the tender offer would be in-the-money (i.e., ~$0.65 > $0.50).
See Moneyness (January 2, 2016) below. I told Provectus' CFO/COO and interim CEO Peter Culpepper, presumably presumptuously and for whatever it is/was worth, that I thought the approach was acceptable. The in-the-moneyness (ITMness) will of course depend on the share and warrant prices from here on out up until March 21st.
Updated (3/7/16): In deeming the changes to the tender exchanges acceptable:
"After" -- which, for convenience, could be post-2015 -- injectable (locally delivered) oncolytic [virus] immunotherapy and stimulatory agent T-Vec was approved. And stimulation first (e.g., antigen presentation to the immune system, activation and priming of the immune system), rather than inhibition first, has begun to come to the fore.
Take, for example, today's collaboration announcement between AbbVie and Boehringer Ingelheim regarding the former's license of the latter's anti-CD-40 antibody or agent. Note: While the compound is a stimulatory agent (see purple oval below), it is delivered systemically.
For this company and its share price, only regulatory and/or commercial validation -- the prospect of regulatory approval, a material geographic license deal and/or a meaningful collaboration arrangement with Big Pharma -- matters. What will matter should be, among a few critical things in the hopper, a successful interim analysis of the pivotal melanoma Phase 3 trial (strictly Stage III disease) and notable data from the advanced melanoma Phase 1b study combining PV-10 and Keytruda (strictly Stage IV).
Updated (3/7/16): In deeming the changes to the tender exchanges acceptable:
- I observe the increasingly favorable changes to both the macro and micro clinical and regulatory backdrops for intralesional (IL) PV-10 (Rose Bengal). Specifically, local delivery is growing increasingly acceptable, and delivery of a stimulatory agent instead of and/or with an inhibitory agent is gaining traction relatively quickly, and
- I do not absolve management of their historical behavior, including the approach to this tender offer by and in consultation with their "investment advisors," but given the challenges and time to arrive at the point above (and what remains to achieve, little-to-more as may be), this is the least worst choice of a small list of bad ones, and one they should pursue.
Increasingly favorable "macro" environment. "Before" -- that is, pre-2015 (going as far back as, say, the start of T-Vec's pivotal melanoma Phase 3 trial and maybe even further) -- was marked by no Big Pharma wanting to develop and/or own an injectable (locally administered) stimulatory oncology drug compound. In fact, only one Big Biotech (Amgen) was willing to take the plunge, by buying Biovex, T-Vec's creator, in 2011. Systemic delivery and inhibition (the opposite of stimulation) has been the industry's almost sole focus.
"After" -- which, for convenience, could be post-2015 -- injectable (locally delivered) oncolytic [virus] immunotherapy and stimulatory agent T-Vec was approved. And stimulation first (e.g., antigen presentation to the immune system, activation and priming of the immune system), rather than inhibition first, has begun to come to the fore.
Take, for example, today's collaboration announcement between AbbVie and Boehringer Ingelheim regarding the former's license of the latter's anti-CD-40 antibody or agent. Note: While the compound is a stimulatory agent (see purple oval below), it is delivered systemically.
 |
| Click to enlarge. Image source: Figure 2, The Cancer Immunity Cycle |
The least worst choice. It's clear Peter has few choices in regards to raising money from the capital markets at least-punitive (let alone favorable) prices. Years of a variety of weak management practices -- despite arguably considerable known and apparently unknown progress -- have atrophied at best and destroyed at worst potentially what could have been a very positive reputation to Wall Street and the larger traditional professional investor class.
But one cannot ignore the previous "macro" environment in which Provectus has had to operate, irrespective of current or hypothetical strong new/different managers. In the absence of the right non-dilutive deals, presumably driven by the generation/presentation/publication of necessary clinical data, Peter has two choices.
First, he can tap the existing shareholder base holding non-tradable warrants now -- based on what they know now, what they will know by March 21st, and cajoling from Network 1 Financial. He does this reasonably well in advance of requiring money (i.e., a threshold of the balance sheet's stockholders' equity of $6 million). Second, he could wait and risk insufficient information on progress to emerge before turning to what likely would be a very costly capital raise.
First, he can tap the existing shareholder base holding non-tradable warrants now -- based on what they know now, what they will know by March 21st, and cajoling from Network 1 Financial. He does this reasonably well in advance of requiring money (i.e., a threshold of the balance sheet's stockholders' equity of $6 million). Second, he could wait and risk insufficient information on progress to emerge before turning to what likely would be a very costly capital raise.
There is a third choice or option, of course, which is the path he is taking:
- Try the warrant exchange, extending it and bettering the economics while knowingly risking further potential scorn and derision from existing and prospective shareholders,
- See what amount of warrants are tendered over whatever timeframe he deems necessary,
- See what information of progress emerges,
- Continue to pursue non-dilutive funding options,
- Eventually, if necessary, tap the capital markets, and
- Above all else, hopefully allow the enormity of PV-10's promise to materialize in a manner that helps to alleviate, mitigate and explain the past.
As minor a point as I believe it to be for this situation (i.e., Friday versus other days), it was "good" Provectus notified the market of the tender offer changes on Monday, rather than on a Friday.
Although the tender offer temporarily is ITM -- as of this writing the share price is $0.41 and the tradable warrant price is ~$0.25, for a "unit" price of ~$0.66 -- Peter and Chief Technology Officer Dr. Eric Wachter, PhD must endeavor to meaningfully contribute to the market's understanding of the Provectus' progress. ITMness matters not today, but by or before March 21st.
Although the tender offer temporarily is ITM -- as of this writing the share price is $0.41 and the tradable warrant price is ~$0.25, for a "unit" price of ~$0.66 -- Peter and Chief Technology Officer Dr. Eric Wachter, PhD must endeavor to meaningfully contribute to the market's understanding of the Provectus' progress. ITMness matters not today, but by or before March 21st.
I have believed officers and directors (i.e., Peter, Eric and director Jan Koe) would consummate their participation in this tender exchange when the tender was completed. I suppose they could've paid the original price of $0.75, but it only would make sense to pay the final price (which is what I would expect those who tendered on Friday ultimately to pay) at the final time when everything was and had been set.
If the warrant exchange were an option, the changes to the tender offer have increased its optionality (a lowered strike price and increased time to expiration have increased it's "value" to non-tradable warrant holders). Potential dilution hasn't changed, since it was bounded and still is bound by the minimum and maximum number of warrants tendered (i.e., zero, or ~60 million). What may have changed, as a function of the amount of moneyness at the time of decision-making, is the potential amount of dilution.
Prior to the changes announced today, Network 1 Financial, and to a lesser extent Maxim Group (because Network 1 holds about 7 times as many of these private warrants for clients and itself) were unable to convince clients to tender their warrants. Irrespective of what news management and they believed would or could come prior to February 15th (the original expiration date) and then March 10th, the lack of moneyness presumably was the overriding factor in a client's decision of whether to tender or not. I still remain curious how much of the firm and firm employee holdings of non-tradable warrants Network 1 will tender.
There's a recent article that includes a blurb about Rose Bengal/PV-10 by The Australian's banking reporter Tim Boreham entitled Biotech player CogState has plenty of credibility behind it:
"Speaking of clinical trials, billions of dollars are being poured into immuno-oncology research, which means finding ways of encouraging the body itself to attack cancerous cells.
Even if a successful drug emerges, the huge development costs could mean prohibitive costs for sufferers.
But does the solution lie with a red food dye that in theory would sell for a couple of cents a dose?...
Rose bengal is not patented, so the product shouldn’t cost anything like the $100,000 a pop incurred by Walker (who, to his credit, successfully pushed for Keytruda to be listed on the Pharmaceutical Benefits Scheme)."Takeaways:
- When Boreham writes above "...a red dye that in theory would sell for a couple of cents a dose..." (my underlined emphasis), he converts the price of Rose Bengal by PV-10 concentration (i.e., 10% by concentration) to arrive at a "price" of a few cents -- as he did below.
- Although Rose Bengal has been used in other applications for close to 150 years -- patented for use as a wool dye, used as a food coloring, used as a diagnostic agent for both the liver and eye, etc. -- it's use as a therapeutic agent was not patented until Provectus' co-founders did so.
- Provectus has not publicly discussed a price for PV-10, whether as a course of treatment or per vial. Equity research analysts that have provided coverage for the company in the past used as an assumption in their financial models a treatment price of $30,000.
- Intellectual property is critical to Provectus. The company's initial synthesis patent and its first continuation patent, Process for the Synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (Rose Bengal) and Related Xanthenes, protect* Rose Bengal's use in, for example, cancer patients. Only Provectus' manufactured Rose Bengal, the drug substance, which then would be turned into PV-10, the drug product, can be used because it (Provectus' Rose Bengal) and it alone meets ICH globally-recognized guidelines. ICH stands for the "International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use." The guidelines are here.
- Provectus' CFO/COO Peter Culpepper, and recently named interim CEO, addressed this point at the 2016 BIO CEO & Investor Conference earlier this year (my underlined emphasis):
"Now the intellectual property is important on Page 28. The synthesis patent, the fourth one down, that’s the new one that expires in 2031. That was necessary so you cannot buy Rose Bengal off the shelf and use it in a patient. It does not meet ICH specifications. So the new synthesis is the way that we can protect the manufacturing of Rose Bengal to the specifications necessary. However, we’ve been notified by the US PTO that there is a new patent coming, so we have a Notice of Allowance of a new synthesis patent. We’ll be sure to announce that. That’s very important as well and will make it very clear that our protection of Rose Bengal for therapeutic use is in fact ours.
- Provectus owns both the therapeutic use of all of Rose Bengal's immediate relatives, the halogenated xanthines, and the manufacturing processes (per ICH guidelines) to both make them and turn them into drugs. It's sort of like Bristol-Myers owning its own immune checkpoint inhibitor Opdivo, Merck's version Keytruda, and anybody else's version (now, and in the future) of anti-PD-1 agent to treat cancer (sort of).
- The halogen family on the periodic table comprises fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
- Consider PV-12, for example, which swaps out the Chlorine atoms with Bromine ones, was briefly mentioned by management several years ago: "PV-12’s photodynamic yield is greater than that of PV-10, as is its radiodensity with ionizing radiation. Therefore, PV-12 may have more utility as a topical agent activated by ambient light and as a diagnostic agent." Source: Drug Delivery Technology, January 2007.
 |
| Click to enlarge. Purple emphasis is mine. Image source |
* Until if challenged, and such challenge is lost by Provectus and/or its acquirer.
Boreham previously wrote an article about PV-10 in October 2014 entitled Food dye threatens cancer biotechs’ business model:
"Using the body’s defences to fight cancer is a popular notion, pursued in various guises by locally listed biotechs including Viralytics, Prima BioMed, Virax and Imugene.
There’s strengthening evidence rose bengal — a red food dye that has been around for more than a century — has a similar vaccine effect. Rose bengal sells for about $8 a gram and when diluted to a 10 per cent formulation equates to a few cents a dose. As a water-soluble xanthene dye, it has been used in liver function studies and is still used by ophthalmologists as a stain to detect eye damage.
“It’s something very simple and very cheap, and its shining the light on the immunology mechanism in the cancer patient,’’ says University of Melbourne research fellow Martin Ashdown."Analysis, Valuation and Conviction (March 5, 2016)
 |
| Image source. Saturday morning. |
Over roughly the same period I've also coached student teams from the university competing in the CFA Institute Research Challenge, where they reprise the role of an equity research analyst to provide and defend an investment recommendation and 12-month price target of a public company.
It's a privilege and a blast to do both activities, although the latter is so much more of an emotional endeavor for me (because I'm heavily invested in the student teams and whatever success they achieve).
UNLV students have represented themselves well in the Research Challenge, competing in a local/first round with teams of seniors and MBA students from (depending on the year) Arizona State University, Grand Canyon University, Northern Arizona University, Thunderbird School of Global Management, the University of Arizona, and the University of New Mexico. Teams are graded on an equity research report (i.e., initiation of coverage) and an oral defense (including Q&A) of their recommendation (i.e., buy, sell or hold) and price target.
Over the last five years UNLV teams, comprised only of seniors, have won or tied for first in the local round three times (2012-13, 2016), advanced to the semi-finals of the regional Americas (second) round (2012), and placed second in the Global Final (third round) (2013). Our students are incredible people trying to do their best to achieve great things (I, on the other hand, am a biased homer; see footnotes at the end of this blog news item).
Both of these activities heavily emphasize financial analysis and valuation, and, to some extent, conviction. In particular, with Research Challenge teams, I strongly encourage them to think like hedge fund analysts rather than equity research analysts. The not so nuanced difference, in my view, is that the former drives towards a potentially executable trade or investment recommendation and hoped for action, with possible profit or loss thereafter, while the latter (irrespective of whether they are buy- or sell-side analysts) has no skin in the game, can be somewhat-to-very conflicted, or simply is uninvested in the outcome. Thus, the difference may or should influence both the quality of analysis and the amount of conviction.
Just like my students and student teams, I try to grow and learn, always striving to be better at my tradecraft. I often feel a sell recommendation is easier to construct because an overvaluation thesis can be made more compelling by honest, accurate identification of substantial mis-, weak or poor management (but harder to deliver to most audiences because it makes people feel uncomfortable).
I have found my long Provectus thesis, while firmly anchored to a unique small molecule with an increasingly compelling clinical value proposition and led by a management team that remains supportable and a net positive, is from time-to-time buffeted by initially inexperienced managers who have grown on the job (to varying degrees depending on the manager; some not at all) and made poor decisions by choice or out of necessity (but sometimes forced by choice).
In the social media experiment that is this blog, I offer up near real-time due diligence, analysis, opinion and, occasionally, rumor in hopes of finding out as much as I can with the almost daily goal of wanting to reaffirm or trying to undermine my investment thesis. Not a day goes by, or so it seems, that hasn't garnered opposition or criticism from some side of this trade/investment (e.g., management, short-term longs, long-term longs, undecideds, quiet shorts, loud shorts, skeptics, neutrals, observers, etc.).
I guess the experiment is still working.
E/n 1: The 2012 team missed out on advancing to the regional Americas second round because of a poorly framed judge's question that caused the students to hesitate before answering very well (their target public company: MGM Resorts International, NYSE:MGM). The price target was hit within 12 months.
E/n 2: The 2013 team fell just short in the Global Final, losing to an MBA team from a Polish university. I wholly blame time zones and jet lag (Kona Grill, NASDAQ:KONA). The price target was hit within 12 months.
E/n 3: The 2014 team had the daunting challenge of defending a sell recommendation, and did not deliver an oral defense commensurate with their compelling research report (Meritage Homes, NASDAQ:MTH). The price target was hit within 12 months.
E/n 4: The 2015 team could not coalesce, and did not submit a report (nor compete in the oral portion of the first round). I believe they learned some lessons that I hope they took with them upon graduation.
E/n 5: The 2016 team did not advance out of the local round after losing a second tiebreaker to an ASU MBA team. I blame two of the three judges who did not credit UNLV students with properly and thoughtfully employing three different valuation techniques (i.e., DCF, price multiples, a qualitative method), compared to ASU's use of DCF only (Sprouts Farmers Market, NASDAQ:SFM). The price target, finalized on January 22nd, was $30.50 (when the share price closed at $22.77) (the March 3rd closing price was $28.21).
Late-night Boba (March 4, 2016)
 |
| Image source |
"The genetic complexity of cancer*, which is flagged by tumour antigens, arises when cancers evolve in a branched manner. The earliest faults are found in all cells, forming the ‘trunk’ of the disease, while later mutations arise in some cells but not all. It is these ‘branches’ that allow the disease to adapt and become drug resistant."The cancer tumor is not so much a patient’s enemy as it could be his or her “frenemy” (both friend and enemy). Provectus views the tumor as essential to making good on the promise of anti-tumor immunity, believing tumors are repositories of a cancer patient’s known knowns, known unknowns and unknown unknowns. The immune system needs to gain access to this information (in antigenic structure and biological context) in order to effectively fight back. There arguably is more we don’t know about the immune system than we know about it. The company's philosophy in regards to Mother Nature’s creation (the immune system), as a result, is to help rather than change or tinker with it.
For more, see October 15, 2015 blog post Still Standing.
Checkpoint inhibitors need help to help the immune system. Consider an article entitled Next-Gen Immunotherapy Offers New Hope for Beating Brain Cancer:
"The major goal of any anticancer treatment is to kill all cancer cells and prevent any remaining malignant cells from growing or spreading again", Professor Agostinis continues. "This goal, however, is rarely achieved with current chemotherapies, and many patients relapse. That's why the co-stimulation of the immune system is so important for cancer treatments. Scientists have to look for ways to kill cancer cells in a manner that stimulates the immune system. With an eye on clinical studies, our findings offer a feasible way to improve the production of vaccines against brain tumours." {Underlined emphasis is mine}
Take as a starting point Inman et al.’s 2007 article entitled Costimulation, coinhibition and cancer, and their statement therein: (underlined emphasis is mine):
“If sufficient co-stimulation is provided in the presence of adequate tumor-associated antigenic stimulation, the immune system will act against tumor antigen and, thus, destroy early tumors before they become fully established. Contrarily, if co-inhibitory signaling dominates, the immune system will be tolerized to tumor antigens, and the tumor will be permitted to grow unfettered and unmolested by the immune system. If neither co-stimulatory nor co-inhibitory signals dominate, the adaptive immune system may remain in a tenuous state of equilibrium, militating against tumor outgrowth with varying degrees of success.”
The essence of the authors’ view might be that the immune system is capable of decisively acting against cancer only in the situation where or circumstance that co-stimulation dominates co-inhibition. Take also as context to this starting point, however, that what we don’t know about the immune system probably dwarfs what we know about it.
The notion of “releasing the brakes” in the medical literature and mainstream press describes the approach of inhibiting cancer’s ability to suppress or block the body’s immune system from acting, and thus to evade attack. Although possibly coined in the early-2000s (see, for example, Tirapu et al.’s 2002 article entitled Effective tumor immunotherapy: start the engine, release the brakes, step on the gas pedal,...and get ready to face autoimmunity), use of the releasing-the-brakes phrase may have grown more widespread starting in the late-2000s and around the time of Dr. James Allison, Ph.D’s seminal work of blocking (inhibiting) the CTLA-4 protein receptor (using Bristol-Myers’ ipilimumab) and, later, the follow-up scientific exploration of blocking (inhibiting) PD-1 and PD-L1 ligands too (and associated PD-1 therapeutics pembrolizumab and nivolumab, for example, from Merck and Bristol-Myers, respectively).
Medical literature has more sparsely touched on, and mainstream press much less so, the other two components of the get-the-car-moving analogy (where the car is the immune system), “starting the engine” and “stepping on the gas pedal,” where these phrases relate to different aspects of stimulating the body’s immune system.
Possibly over-using the car analogy further, with the potential risk of over-simplifying it inappropriately, consider T cell immunity as a car at rest. More immunogenic tumors and their associated cancers like melanoma are like a car sitting on a slight incline. Release its brakes by treating the tumors (and thus the cancer) with checkpoint inhibitors, and the car may roll forward move some distance, notable or otherwise. With other less or non-immunogenic cancers, think of the car as sitting on a flat surface. Releasing the brakes does not enable the car to move any meaningful distance, if at all.
If you want to get the car to really move, you have to start its engine, and then step on its gas pedal. Releasing the brakes might help the car move farther and faster, but it also is quite possible the car may be able to move sufficiently without the need for further action other than to start its engine and/or stepping on its gas pedal.
The continued use of get-the-car-moving analogy of course requires the assumption the car can drive by itself; that is, the immune system can handle its own business once it has been started, and is appropriately up and running from stepping on the gas pedal.
For more, see February 18, 2015 blog post The Early Obsolescence of Checkpoint Inhibitors.
Financial obligations to Craig. InvestorVillage poster PVCT123 notes potential financial obligations related to the resignation of Provectus Chairman, CEO and a co-founder Dr. Craig Dees, PhD.For more, see February 18, 2015 blog post The Early Obsolescence of Checkpoint Inhibitors.
A thought for small molecule people. H/t again @bradpalm1's link to a blog post by Dr. Derek Lowe, PhD entitled Where Cancer Immunotherapy Works (and Doesn’t):
"And this brings up something for us small-molecule people to think about: as the march of the immunotherapies continues, it’s possible that we’re going to have to modify our own targets. It could well be that the unmet medical need is going to concentrate more in the tumor types that are not good candidates for immune approaches (such as the squamous cell example above), and that for maximum clinical impact we should be deliberately looking for these areas. Thoughts?"Rose Bengal is a small molecule. How's that for a thought?
But the larger thought is PV-10's potential ability to make non-immunogenic tumors immunogenic, and immunogenic tumors more so. Achieving T cell immunity almost if not actually by definition should mean overcoming resistance to cancer, thus overcoming checkpoint blockade and mitigating the need to artificially release the brakes.
Should stimulation via stimulatory therapeutics and therapies start the engine and enables the gas pedal to be stepped on sufficiently and appropriately (i.e., with minimal or manageable side effects or adverse events) so as to achieve T cell immunity, brakes may not be necessary once the car is moving (in context, and given the car [the immune system] can drive itself and not careen off the road because it then should know what it is doing).
Over time, however, road friction may start slowing the car down to the point where waning immunosurveillance (the immune system recognizing and eliminating continuously arising cancerous cells) no longer can protect the patient from relapse (analogous to how waning varicella zoster antibody titers may result in a bout of shingles). Keeping the brakes disengaged, especially with non-immunogenic tumors, should have some role going forward, making Merck, Bristol-Myers, Roche, AstraZeneca, Pfizer and other companies’ checkpoint inhibitors not necessarily obsolete as much as persnickety. And we haven't even begin to talk about steering wheels...
For more, see October 10, 2015 blog post Brakes, Gas Pedals, Steering Wheels, Roads, Planes, Trains and Automobiles.
Warrant tender offer. H/t a shareholder (I believe) who wrote [to me] that [he was aware/heard] there is some consideration being given to changing or advocating for a change in the terms of the exchange to make the economics of tendering more attractive to non-tradable warrant holders.
Wednesday (March 2, 2016)
Business update conference call. In retrospect, considering Tuesday's call on its own, Provectus' CFO/COO and now interim CEO Peter Culpepper said nothing that would explicitly suggest the company would not hire a new CEO (irrespective of time frame). The company's Monday press release on leadership changes did note "[t]he Board also formed a Search Committee, to be chaired by Board Chairman Smith, to immediately begin work to identify a permanent CEO." And while Peter's prepared comments on the call did not address the search process, his answers to a question on the topic did. I suppose my takeaway under "...we have now begun to win" (March 1, 2016) below was colored by my inclusion of other facts, thoughts and opinions.
I don't think the company would be sold or acquired in the near- and perhaps medium-term; however, I do believe core management (the company's CTO Dr. Eric Wachter, PhD and Peter) think they have the opportunity to and can lead Provectus towards this end given their projections of clinical data generation, presentation and publication efforts this year.
De novo antitumor immune response. Reading a tweet from this project's curated Twitter feed, I noted the continued consideration of radiation or radiotherapy's role in generating and/or contributing to an anti-tumor immune response.
 |
| Click to enlarge. Tweet source |
"The recent success of cancer immunotherapy has demonstrated the power of the immune system to clear tumors, generating renewed enthusiasm for identifying ways to induce antitumor immune responses in patients. Natural antitumor immune responses are detectable in a fraction of patients across multiple malignant neoplasms and can be reactivated by targeting rate-limiting immunosuppressive mechanisms. In most patients, however, interventions to induce a de novo antitumor immune response are necessary. We review growing evidence that radiation therapy targeted to the tumor can convert it into an in situ tumor vaccine by inducing release of antigens during cancer cell death in association with proinflammatory signals that trigger the innate immune system to activate tumor-specific T cells. In addition, radiation’s effects on the tumor microenvironment enhance infiltration of activated T cells and can overcome some of the barriers to tumor rejection. Thus, the complementary effects of radiation on priming and effector phases of antitumor immunity make it an appealing strategy to generate immunity against a patient’s own individual tumor, that through immunological memory, can result in long-lasting systemic responses. Several anecdotal cases have demonstrated the efficacy of combining radiation with available immunotherapies, and results of prospective trials are forthcoming." {Underlined emphasis is mine}Neural crest cells. I'm still struck that Eric decided to drop the protocol related to "A Phase 1 Study of PV-10 Chemoablation of Neuroendocrine Tumours (NET) Metastatic to the Liver" before advancing the company's clinical work in hepatocellular carcinoma (HCC) (in Asia, and/or compared to sorafenib) and other cancers metastatic to the liver. Speaking with a regular hatter (who also is a physician and Provectus shareholder) about the topic, I am curious about Eric's decision-making, wondering among other things whether his rationale included that:
- Malignant melanoma and NET may share the same origin of neural crest cells (e.g., "[m]alignant melanoma is a neuroendocrine tumor"),
- Measuring changes in symptoms and quality of life (QoL) for this indication of NET can be quite objective, and
- Symptoms and QoL could be viewed as much as biomarkers for patient response and the potential for predicting treatment success as tumor response, peripheral blood mononuclear cell (PMBC), etc.
Updated below.
Provectus held the business update conference it scheduled for today to discuss changes in company leadership that stemmed from the resignation of Chairman, CEO and one of Provectus' co-founders Dr. Craig Dees, PhD. See Resignation (February 29, 2016) below. The transcript of the call is here.
My primary takeaway from the call is this:
- There very likely will be no immediate, near-term or even perhaps medium-term hiring of a CEO to replace interim CEO Peter Culpepper (also Provectus' long-time CFO/COO) because core management, the company's CTO Dr. Eric Wachter, PhD and Peter, believe they are sufficiently close enough to seeing Provectus sold/acquired.
After not presenting/publishing new clinical data since 2010, save for seemingly an appetizer's worth of liver data in July 2015, Provectus appears to be poised to both generate and present/publish much more in 2016. See February 15, 2016 blog post Data-driven.
 |
| Click to enlarge. |
"At the expense of overdoing the analogy, I believe all these stakeholders are making more and more beautiful music together. There are 4 movements in a symphony: sonata, adagio, minuet and allegro. And this is analogous to our story at Provectus. From popular reference to this: First they ignored us, then they laughed at us, then they fought us, and just recently, we have now begun to win."When Moffitt Cancer Center presented their AACR 2013 work, Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma, Peter called it "the shot heard round the world" at the time (much to the confusion of some shareholders) because Moffitt had validated and reproduced PV-10's ability to generate an immune response -- that is, its potential as an immunotherapy.
His saying "we have now begun to win" probably refers to (as Eric and he now see and hear it) the growing acceptance of and desire to use PV-10 by global key opinion leaders in oncology studies and trials in more and more solid tumor indications, whether as a single agent or in combination with another therapeutic or therapy.
Shots and wins have not yet translated into the share price management and shareholders want, let alone need. Clinical and other data generation/presentation/publication in 2016 may change that.
Updated (3/1/16): I changed "near-term" above in the bullet point paragraph to "immediate, near-term or even perhaps medium-term."
Synthesis (March 1, 2016)
Provectus issued a press release and made an associated 8-K filing today regarding the award of a new patent related to the synthesis of Rose Bengal and other halogenated xanthenes (Rose Bengal being the first of its class, with the other halogenated xanthenes as members of that same class), Awarded Patent Extending Protection of the PV-10 Manufacturing Process. The original patent was awarded on September 10, 2013; the patent awarded today was a continuation of it. Also see "...clear that our protection of Rose Bengal for therapeutic use is in fact ours" (February 11, 2016) below.
The press release noted:
"The patent extends the scope of protection of the manufacturing process conferred initially by U.S. Patent No. 8,530,675, issued in 2013, to include coverage of the use of an alternative raw material in manufacturing the active ingredient (API) in PV-10."The new patent (No. 9,273,022) covers a variant on the starting material phthalic anhydride by adding provision for use of phthalic acid.
The PR also provided a quote by Provectus' Dr. Eric Wachter, PhD.:
"It is a pleasure to have Cambrex team members as co-inventors on this process patent. Although the scientists and engineers working behind the scenes aren't always visible to patients or shareholders, these professionals work tirelessly to enable manufacturing the active ingredient in PV-10 on a commercial scale. Chemistry, Manufacturing and Controls (CMC) is a critical part of any investigational new drug (IND) application and subsequent new drug application (NDA), and this aspect of our PV-10 submission is built on a firm foundation, due in no small part to the efforts of the Cambrex team." {Underlined emphasis is mine}It would seem Cambrex is or might be the API (drug substance) manufacturer. A question is, who is the manufacturer of PV-10 (drug product); is Cambrex one, or the one?
Cambrex is "...a generics and biosimilar manufacturer that provides small molecule pharmaceutical ingredients and finished dose products for the innovator and generic pharmaceutical markets." Some slides from a November 2015 investor presentation of Cambrex (purple emphasis is below):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Changes in blog readership statistics were mixed for the month of February. Month-over-month (February 2016 v. January) percent differences included:
- +0.4% for # of unique visitors (2,269 v. 2,259),
- -11% for # of page views (33,243 v. 37,297),
- -2% for # of visits (10,632 v. 10,834),
- +2% for # of U.S. cities from where visitors came (619 v. 608),
- -8% for # of world cities (131 v. 143),
- -7% for # of countries (51 v. 55), and
- -25% for # of blog posts and news items (24 v. 32).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Our logic seemed clear (February 29, 2016)
In the wake of being denied breakthrough therapy designation for PV-10 in patients with locally advanced cutaneous melanoma (Stage III disease), Provectus' CTO Dr. Eric Wachter, PhD had said:
"...our logic seemed clearer that if we were making the patients' tumors disappear in 50 percent of the patients that was a very large effect size, and that was tantamount to making any symptoms that they might have been suffering from the tumor burden disappear." {Underlined emphasis is mine} See November 15, 2014 "This determination is based on the paucity of data..."Provectus issued a press release and made an associated 8-K filing today regarding a new clinical trial, Initiating Phase 1 Study of PV-10 in Neuroendicrine Tumors Metastatic to Liver.
I noted in February 27, 2016 blog post A new article, a new clinical trial that the FDA approved Novartis' everolimus (Afinitor) for the treatment of a version of neuroendicrine tumors (NET), but the drug generated an overall response rate of 2%. That is, the tumor was not made to go away by Afinitor; it stayed pretty much the same size.
Eric's quote in today's NET Phase 1 trial PR was interesting to me:
"This protocol is a natural complement to work ongoing in our initial study of hepatic cancers under protocol PV-10-LC-01, which has allowed us to assess PV-10 in a number of tumor types using the method of administration that will be used in this new study. In addition to providing further data on the overall safety of this approach, this study is tailored to NET patients and will allow us to assess potential clinical benefit in terms of objective response and changes in biomarkers and symptoms of these tumors. Patients with metastatic NET tumors are often plagued by persistent diarrhea, flushing, breathing difficulties, abdominal cramping and swelling of the arms and legs. It will be very useful to determine whether PV-10 ablates NET tumors and if ablation has a positive impact on quality of life for patients." {Underlined emphasis is mine}So, to borrow a phrase from his past, I'd imagine Eric is saying that if PV-10 can make a patient's NET tumors go away, that could be tantamount to making his or her symptoms go away too.
Resignation (February 29, 2016)
Updated below.
Provectus issued a press release and made an associated 8-K filing today regarding the resignation of the company's Chairman, CEO and one of its three co-founders, Dr. Craig Dees, PhD, Announces Leadership Changes. According to Provectus, there will be a conference call tomorrow at 4 pm EST related to this topic.
My main takeaways thus far, in no particular order, are:
- The inclusion of the below was notable:
"The Board added that in this transition it has designated the company's Audit Committee to review a number of company procedures, policies and practices, including executive compensation and expenses. Smith said results of that review will be released when completed."
The results of this review are very important to existing and prospective investors' short- and long-term views of Provectus. First, as CEO Craig was one of the two officers who certified the company's quarterly and annual financial statements; the other was CFO/COO Peter Culpepper. I imagine initiating an audit committee review following Craig's resignation is prudent in the context of ensuring continued certification of these statements (and the process by which certification is undertaken and established).
Second, shareholders and potential investors need to understand what specifically Provectus' board of directors meant by referencing "executive compensation and expenses." Any board and/or board subcommittee review, particularly an audit committee one, bears serious and detailed watching.
- The company's approach to splitting Craig's roles also is notable:
- Independent director Al Smith, IV becomes Chairman,
- Peter becomes interim CEO, and
- The company's CTO Dr. Eric Wachter, PhD fills Craig role as an inside director.
First, while resuming his former role as a company director may add to his other work for Provectus, Eric's return to the board is a good thing. He should never have been the insider to step off the board when he did in May 2012.
Second, Craig's resignation does emphasize the leadership changes that already had taken place at the company. Both Eric and Peter have been transitioning PV-10 from Provectus' laboratory to a global laboratory and worldwide stage for some time. Clinical, regulatory, business and corporate development already were being handily led by them, and it was clear Craig's titles as Chairman and CEO for some time were not commensurate with his actual role and responsibilities.
- The press release noted the following, perfunctorily and customarily attributed to Smith:
"He has helped bring us to this stage of the company's development. We are optimistic about the company's current position and confident that the team which Craig helped build will continue his work."
I believe Craig's vision has been achieved for the most part.
In his view properly destroying cancer tumors meant killing only tumors and doing so completely, quickly and, very importantly, safely (that is, leaving healthy tissue unharmed). He believed this approach was the only effective way of sustainably stimulating a person’s natural anti-cancer defenses. Instead of bathing the entire body or even parts of it with radiation, or filling the bloodstream with oral or intravenous chemotherapies or present-day immunotherapies, he firmly held the position that stimulating the immune system was best achieved through treating tumor tissue by injecting into it a drug capable of destroying the entire tumor as quickly as possible without damaging surrounding healthy cells. Completely also meant everything from visible tumor tissue to occult or hidden cells in and immediately around the injection site. Quickly meant having the drug processed through and excreted from the body in short order. Antigens generated from the tumor destruction caused by drug injection then could be presented to the body’s cells responsible for selecting the best and most relevant antigens in order to encourage cancer-killing cells to replicate themselves throughout the body. Importantly, tumor antigens had to be viewed in context; physical tumor destruction techniques such as heating or freezing tissue destroyed fragile antigens and disrupted their relevant contextual structures. Disruption of cell membranes and removal of lipids, proteins, and complex carbohydrates destroyed the antigens’ context, which is to what immune system cells responded. Thermal destruction denatured potential antigens, changing their chemical structure so that they were no longer representative of the tumor cell. In order to work rapid destruction of tumors had to preserve both antigenic structure and biological context. See July 27, 2015 blog post Provectus Biopharmaceuticals: Advancing a New Front in the War against Cancer.
Craig often framed Provectus' approach to treating cancer in the following way: the harder you punch the immune system, the greater the response. He would reference a lecture by a veterinary virologist who showed a picture of a cow with a basketball-sized papilloma hanging from its stomach. The professor said, paraphrasing: "Cut that off aseptically and cleanly, and it will grow back every time. Tear it off, make it bleed, kick dirt into the wound, and it will never come back." Shock the heck out of the immune system with tissue destruction (wake it up), break tolerance and encourage a large-scale release of tumor antigens into the system so they can be seen in context.
What remains of course is the required clinical data to cement his clinical vision and views about how cancer should be treated, and defeated.Shareholders hopefully may get necessary color about Craig's abrupt resignation and departure from the company he co-founded than just limited commentary regarding his health and health problems, which long-time shareholders certainly would have known, and tracked.
Updated (2/29/16): A shareholder called me to say he met Craig this afternoon (East Coast time), and that they spent time talking about his illness. The shareholder offered the opinion that he thought Craig was very ill.
Epidemiology of neuroendocrine tumors/cancers (February 28, 2016)
Provectus' new neuroendocrine tumors metastatic to the liver Phase 1 trial, thus far with a single clinical site in Australia, appeared today on the Australian New Zealand Clinical Trials Registry.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
So, the company's CTO Dr. Eric Wachter, PhD expects to begin enrolling a Phase 1 trial at the beginning of March (i.e., two days from now), but none of the 10 ten sites he previously guided that would participate in the Phase 3 trial are recruiting yet, and only one of them currently is listed as a site?
The NET Phase 1 trial investigator is Australia's Dr. Timothy Price, MD, who has notable professional interest in and experience with NET. See, for example, his paper Epidemiology of neuroendocrine cancers in an Australian population, Cancer Causes Control (2010) 21:931–938:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below.
A Phase 1 Study of PV-10 Chemoablation of Neuroendocrine Tumours (NET) Metastatic to the Liver
Clinical site & investigator: The Queen Elizabeth Hospital, Woodville, Australia & Dr. Timothy Price, MD
Updated (2/26/16): I previously had written about neuroendocrine tumor (NET) treatment with PV-10 on the blog in July 2013 (see the Archived News I page).
 |
| Click to enlarge. |
- Number of Participants with Adverse Events (primary),
- Objective Response Rate (ORR) (secondary) [response of injected target and measurable bystander lesions (if present)], and
- Change in Neuroendocrine Tumor Biomarkers (secondary) [change in Chromogranin A (CgA) and/or 5-Hydroxyindole Acetic Acid (5-HIAA)].
"Liver metastases occur in 75% to 80% of patients with neuroendocrine tumors (NETs), and are considered significant adverse prognostic indicators. Management of NETs liver metastases is challenging and requires aggressive therapy. Currently, there are many therapeutic options for metastatic NETs. However, there is considerable controversy regarding the optimal management. Although complete surgical resection remains the optimal therapy, a variety of other minimally invasive surgical and medical options are available, this includes thermal ablative techniques (e.g., radiofrequency ablation, microwave ablation, cryotherapy), embolization using transcatheter embolization, chemoembolization, or radioembolization, and medical therapy (e.g., chemotherapy, biotherapy with somatostatin analogues and interferon). Currently there is no evidence-based data directly comparing surgical versus alternative liver-directed treatment options. An aggressive surgical approach, coupled with additional liver-directed procedures is often recommended as it extends the overall survival. Optimal patient care should be directed by a multidisciplinary team to assure that all treatment options are explored for decision-making while treating this aggressive disease." See Surgical treatment of liver metastases in patients with neuroendocrine tumors, Saeed et al., Annals of Translational Medicine (2013)Some addressable market information (the below from a 2012 Delcath investor presentation):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below.
 |
| Click to enlarge |
- "This is one of the really neat examples of what we call repurposing, taking drugs that been around for years ... and suddenly realizing that they may have an oncologic value," said Dr. Vernon Sondak, head of cutaneous oncology at the Moffitt Cancer Center in Tampa, Florida. Sondak has been running clinical trials for Provectus.
- Dr Sondak also is a consultant to the company.
- "We've come to the conclusion that it is immune based," said Dr. Sanjiv Agarwala, chief of medical oncology and hematology at St. Luke's Cancer Center in Bethlehem, Pa. Agarwala, who has been conducting PV-10 trials funded by Provectus, also has run several cancer immunotherapy studies, including on Keytruda.
- St. Luke's MD Anderson currently is a PV-10 compassionate use program (CUP) site, and a clinical site for Provectus' pivotal melanoma Phase 3 trial of PV-10 vs. chemotherapy, its melanoma combination therapy Phase 1b/2 study of PV-10 and Keytruda, and its cancers of the liver Phase 1 trial. Dr. Agarwala is the St. Luke's principal investigator for all of this work, and was a clinical investigator for Provectus' metastatic melanoma Phase 2 trial.
- Dr. Patrick Hwu, an immunotherapy expert from MD Anderson Cancer Center, said PV-10 is one of several interesting tumor ablation techniques in the works. "The biggest value will be if it can affect distant disease," said Hwu, who is not involved in PV-10 testing.
- According to his MD Anderson bio, Dr. Hwu "is considered one of the leading tumor immunologists in the country, and a primary force in the development of novel vaccine and adoptive T-cell therapies."
- MD Anderson currently is a PV-10 CUP site, and will be a clinical trial site for the melanoma Phase 3 and Phase 1b/2 studies. The MD Anderson principal investigator for all of this work is/will be Dr. Merrick Ross, MD, who was a clinical investigator for the melanoma Phase 2 trial.
Other items of interest [to me] include, with my underlined emphasis:
- "Pfizer holds a co-patent on PV-10 for use in combination therapies, and Boehringer Ingelheim has secured right of first refusal on use of the drug against liver cancer in China, the companies told Reuters."
- "Provectus executives say the small development tab - along with relatively low manufacturing costs and easy handling requirements - could make PV-10 a less expensive new treatment. But the final decision on price is likely to be made by a bigger drugmaker, as Provectus plans to put itself up for sale once its drug is approved."
Updated (2/26/16): The Reuters article was picked up by the following media organizations:
- The New York Times, see the link here,
- Fortune, see the link here, and
- Fox News Health, see the link here.
Updated below.
Intralesional immunotherapy as a strategy to treat melanoma. See below:
 |
| Click to enlarge. Image source |
The bio of co-author Dr. Claus Garbe, MD, PhD is below:
 |
| Click to enlarge. Image source |
Updated (2/25/16): H/t a regular hatter who provided me with a copy of the Garbe et al. paper above today.
A good portion of this paper on intralesional therapies and metastatic melanoma addresses PV-10 (Rose Bengal). Overall, it seemed like a reasonably balanced review. This certainly is an evolving story, however, as Amgen's T-Vec combo data (with Merck & Co.'s Keytruda) dribbles out, and Provectus expands its single agent and combination therapy efforts. Several excerpts follow.
Factors appear to include:
Updated below, again.
Moffitt Cancer Center: T cell mediated immunity after combination therapy with intralesional PV-10 and co-inhibitory blockade in a melanoma model
Updated (2/23/16): Takeaways
Consistent (February 22, 2016)
I previously referenced a paper written in 1976 in Klaassen titled Pharmacokinetics of rose bengal in the rat, rabbit, dog, and guinea pig when noting that very small amounts of rose bengal cross the blood-brain barrier. See December 1, 2014 blog post November Blog Stats, and other bits and bobs. I had written that some patients in Provectus' melanoma Phase 2 trial had visceral disease, including brain metastases. How many patients with brain mets was unclear. Provectus had publicly discussed one such patient at medical conferences (Subject 0907 with Stage IV M1c disease), noting "“Near complete resolution” of pulmonary nodules observed at Week 12" during an ASCO 2010 clinical development update presentation. If the brain is on the other side of the blood-brain barrier (and the immune system is on the other, so to speak), and PV-10 is injected into visible lesions on the skin, how were these brain tumors (nodules) positively impacted? The small amounts of rose bengal that may have permeated the barrier were very unlikely to be able to reduce or destroy tumors, and the drug was not systemically administered (just injected into the skin at the location of a cutaneous or subcutaneous lesion). As Provectus' Chairman and CEO Dr. Craig Dees, PhD would say, Mother Nature's immune system knows how to get to brain tumors without destroying the normal brain tissue. But how?
As or more importantly, Klaassen's paper discusses Rose Bengal's pharmacokinetics, the consistency of which I observed when comparing the pharmacokinetics of PV-10's use in clinical melanoma work, and that in clinical liver work. See Comparable, Consistent PV-10 Pharmacokinetics (July 3, 2015) on the blog's Archived News IV page. The results are comparable and consistent, such as biological half-life, measured in hours, and terminal half-life, measured in days. On the topic of pharmacokinetics, Klaassen's paper's abstract noted:
Klaassen did administer Rose Bengal intravenously. Craig previously presented murine model work (January 2013) that included oral administration, and compared it to intratumoral administration.
I note and write the above, because of the comparison between intralesional or intratumoral delivery of Rose Bengal (PV-10), consistent with Craig's clinical vision of treating cancer, and non-intratumoral administration (in this case, oral, which is how Ito et al. (1986) used Rose Bengal in their murine model work). I also note and write the above because of a story Craig tells regarding a request from some folks from Orbimed Advisors (he referred to them as MDs) who has him about systemic delivery of the compound. I have wondered whether that request came leading up to the failed PVCTP "IPO" in (October) 2012, when Provectus' CFO/COO Peter Culpepper insinuated or fostered the implication Orbimed and Aisling Capital were potential leads of that investment round. See October 29, 2012 blog post $PVCT.OB: Did Some Shareholders Sabotage the $PVCTP "IPO," and Other Stories Stranger Than Fiction and July 13, 2013 blog post The Beginning of the End[-game] for $PVCT. My larger point here is that there was (and presumably less so, but still is) skepticism and questions from the "smart" money about different, at-the-time-non-mainstream, now-being-validated approaches to treating cancer.
I previously noted a March 2013 reference from someone in Calgary, Alberta, Canada to Rose Bengal (PV-10). See #10 under Notes (August 6, 2015) on the blog's Archived News IV page.
A good portion of this paper on intralesional therapies and metastatic melanoma addresses PV-10 (Rose Bengal). Overall, it seemed like a reasonably balanced review. This certainly is an evolving story, however, as Amgen's T-Vec combo data (with Merck & Co.'s Keytruda) dribbles out, and Provectus expands its single agent and combination therapy efforts. Several excerpts follow.
 |
| Click to enlarge. |
Excerpt #1 (Introduction): "Advanced cutaneous melanoma is a rare but highly dangerous form of skin cancer. It has a poor prognosis, with a median survival time of approximately 9 months in the era of chemotherapy, and a high death rate of over 90%. Despite all recent advances in the field of melanoma, many patients do not respond to treatment. Some patients are not able to tolerate some of the drugs for a variety of reasons, and so any agent that has some activity is important. Therefore, there is a medical need for the development of local treatment approaches or limited metastasis in order to achieve more cures.
The domain for intralesional immunotherapies are multiple, inoperable loco regional metastases and distant soft-tissue metastases. Locoregional therapies like Interleukin-2 and Rose Bengal or electrochemotherapy have very high response rates and may also produce bystander effects, which means that non-injected metastases also respond to the treatment. The magnitude of these bystander effects are variable between the different treatments."
Excerpt #2 (Method): "A systematic literature research has been performed in PubMed with the following search strategy: “Melanoma [ti] AND (intralesional [ti] OR local [ti] or topical [ti]) And (therapy [ti] OR treatment [ti])”. With these specific topics 168 publications were identified. Further publications were identified from the reference lists of the reviews among the above mentioned 168 publications. Intralesional Injections with talimogene laherparepvec, velimogene aliplasmid (allovectin-7) and interleukin-2 have been classified as direct immunotherapies. Additionally, intralesional therapies with Rose Bengal and with electrochemotherapy have been included, because bystander effects have been described for both therapeutic approaches. Intralesional and topical treatment approaches with BCG and with dinitrochlorobenzene as well as with diphencyprone were judged to be obsolete and not included into this review."
Excerpt #3 (Discussion): "The main indication for intralesional immunotherapy in the past was treatment and cure of multiple cutaneous and soft tissue metastases, particularly in stages IIIB, IIIC and IV A of melanoma. These are stages of limited tumor burden and long-term cure is achievable in a rather high percentage of patients.
The highest efficacy for the remission of locoregional and soft-tissue metastases has intralesional IL-2 treatment and electrochemotherapy. They may be the best options, when only these types of metastases are present and no other distant metastases are recognizable. The exact mechanism of action is not known for IL-2 intralesional treatment. It obviously attracts a dense T-cell infiltration, and the tumor cells transit into apoptosis. Electrochemotherapy is more a cytotoxic treatment, based on the effects of bleomycin and electrical pulses which enhance the intracellular uptake of bleomycin. Treatment with talimogene laherparepvec and with Rose Bengal have been described to elicit bystander effects. Therefore, these treatment options may be most interesting for the combination with systemic immune therapies. Indeed, the efficacy of talimogene laherparepvec is now tested in combination with ipilimumab and also in combination with pembrolizumab in phase III studies. Also, the efficacy of Rose Bengal will be tested in combination with immune therapy.
The role of all intralesional treatments described in this review will have to be tested in combination with new immune therapies, particularly with immune checkpoint blockade. This has to be performed in double blind, randomized phase III trials. Such trial are ongoing with T-VED and are under consideration for Rose Bengal. There are no experiences with the costs of the new drugs T-VEC (Imlygic™, Amgen) and Rose Bengal (PV-10™, Provectus) so far. The company Amgen announced that the cost of Imlygic will be $ 65,000 on an average per treatment. There is not yet a price proposed for Rose Bengal. Taking into account the high price for T-VEC, use of this drug will only be justified if there is convincing evidence that overall survival of patients is improved.
In conclusion, intralesional therapies have a high potential for inducing complete remissions of injected tumors. However, there are not yet data for their impact on overall survival. In the era of systemic immunotherapies with checkpoint inhibitors, their place has to be determined in this new environment. If an improvement of overall survival of treated patients cannot be shown in combination with checkpoint inhibitors, the future role of intralesional therapies may become marginal."
Excerpt #4 (Expert Opinion): "There are two main questions of future research in the field of intralesional therapies: (a) Does intralesional therapies stimulate the specific anti-tumor immunity or is the effect based on innate immunity? The answer of this question is important for finding the right combination therapies for intralesional approaches.(b) Can intralesional therapies successfully be combined with checkpoint blockade? Intralesional therapies may only find place in the landscape of future treatments, if they contribute to better responses to a checkpoint inhibition with PD-1-antibodies or to the combination of PD-1- and CTLA-4-antibodies.These questions can only be answered in controlled phase III trials, comparing checkpoint inhibition therapy plus intralesional therapies compared to a checkpoint inhibition therapy plus placebo. Such trials are on the way or talimogene laherparepvec and in preparation for Rose Bengal. Smaller trials have lready been performed for interleukin-2 plus ipilimumab (results not yet published).
The decisive question is, whether the combination of checkpoint inhibition plus intralesional therapy improves overall survival. Substances like talimogene laherparepvec and Rose Bengal may achieve a place in immuno-therapy of metastatic melanoma when they succeed to improve overall survival. If this cannot be shown, they will play only a marginal role in future treatment of metastatic melanoma. The key question will be whether intralesional therapies stimulate specific antitumor T-cell responses, and thus can supplement the unspecific effects of checkpoint blockade in an additive or synergistic fashion."Many factors limit efficacy of anti-tumor T cells. See the image below:
 |
| Click to enlarge. Image source via @luddyka |
- Limit innate cell activation (Step #2 of the cancer immunity cycle),
- Limit T cell activation (#3),
- Limit T cell access/recognition (#5), and
- T cell exhaustion (#7).
Updated below, again.
Moffitt Cancer Center: T cell mediated immunity after combination therapy with intralesional PV-10 and co-inhibitory blockade in a melanoma model
 |
| Image source |
- This still is murine (mice) model [pre-clinical] work,
- The AACR 2016 abstract (and eventual poster) about combination therapy (PV-10 + immune checkpoint inhibitors [aka co-inhibitory blockade agents]) is a follow-up to Moffitt's SITC 2014 abstract and poster presentation, Efficacy of intralesional injection with PV-10 in combination with co-inhibitory blockade in a murine model of melanoma,
- All of the authors of this medical research are from Moffitt (no Provectus person is listed): "Amy M. Weber, Hao Liu, Krithika N. Kodumudi, Amod A. Sarnaik, Shari Pilon-Thomas,"
- One word: immunity,
- This preclinical work dovetail with Provectus' clinical work of a Phase 1b study in patients with advanced melanoma receiving the combination of PV-10 and a co-inhibitory blockade agent (pembrolizumab).
In regards to Moffitt's process, it would seem there's an observable pattern. Let's consider Moffitt's initial work on PV-10, where the cancer center reproduced, and thus validated via this arm's length reproducibility, Provectus' original work of the drug compound's two-prong approach to fighting cancer -- prong #1, tumor ablation (upon injection), and prong #2, an anti-tumor immune response (by the immune system).
To set the stage a little further, Provectus CTO Dr. Erich Wachter, PhD said on the company's November 12, 2015 3Q15 business update conference call:
"The way this works with Moffitt, they are experts at something called translational medicine. And what translational medicine means is understanding therapeutic processes based on investigations in both model systems, typically either in-vitro, so in test tube, or in animals and in humans. And the translational part means that you frequently go back and forth between the two types of systems as you learn.
So, in this case, it was a very interesting process where some things that were elucidated in mice were then discovered to be occurring in humans. And the opposite actually happened in the clinical studies they've completed looking at human patients. And they went back to the bench and confirmed those in mice.
So, this is normal operating procedure for translational medicine groups. It allows you to benchmark what you're seeing in model systems and humans and efficiently understand what's going on in humans in a high throughput system; that is, the models.
Step #A1, March 2012 — Moffitt reproduced pre-clinically that PV-10 (Rose Bengal) ablates (shrinks, destroys, etc.) melanoma tumors into which it is injected: Intralesional Injection of Melanoma with Rose Bengal Induces Regression of Untreated Synchronous Melanoma In a Murine Model, SSO 2012.
Step #A2, April 2013 — The cancer center then reproduced pre-clinically that PV-10 causes or generates an anti-tumor immune response as a result of its injection into melanoma tumors: Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma, AACR 2013.
Step #A3, April 2014 — Moffit then showed pre-clinically, and thus concluded, that injection of PV-10 into melanoma tumors, which subsequently leads to an anti-tumor immune response, culminates in immunity against melanoma: Induction of anti-melanoma immunity after intralesional ablative therapy, AACR 2014.
Step #A4, June 2014 — The cancer center then transitioned clinically to results in humans that reflected Moffitt's preclinical work, injected tumors were completely destroyed (pathologic complete response) and anti-tumor immune responses were generated: Assessment of immune and clinical efficacy after intralesional PV-10 in injected and uninjected metastatic melanoma lesions, ASCO 2014.
Step #A5, November 2015 — Moffitt then began to explain clinically how anti-melanoma immunity is attained: Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1, SITC 2015.
Takeaway: Tumor ablation in mice for melanoma (Step #A1) → Anti-tumor immune response in mice for melanoma (Step #A2) → Immunity in mice from melanoma (Step #A3) → Growing confirmation in humans for melanoma (Step #A4) → Beginning to explain how (Step #A5).
Now, consider Moffitt's translational work on the combination of PV-10 and checkpoint inhibitors.
Step #B1, November 2014 — The cancer center showed pre-clinically that the combination of PV-10 and these inhibitors improved both tumor shrinkage/destruction and survival for melanoma, "Systemic administration of anti-CTLA-4 or anti-PD1 antibodies in combination with IL PV-10 resulted in increased tumor regression and improved survival in this model:" Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma, SITC 2014.
I would imagine "improved survival" above should mean a better anti-tumor immune response.
Step #B2, April 2016 — Moffitt's abstract title then appears to indicate or suggest pre-clinically that immunity from melanoma can be achieved via the combination of PV-10 and checkpoint inhibitors.
Takeaway: Better tumor destruction and survival from combination therapy (Step #B1) → Immunity in mice from melanoma via combination therapy (Step #B2) → Growing confirmation in humans for melanoma (Step #B3 -- ??? ) → Beginning to explain how (Step #B4 -- ???).
The distinction between the two sets of Moffitt work (Step #s A1-5 and B1-???) is mirrored by ongoing Provectus clinical trials (and their respective trial hypotheses/theses):
- Step #s A1-5: The company's pivotal melanoma Phase 3 trial, PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma, might see patients achieve anti-melanoma immunity if all of their disease is treated, and
- Step #s B1-???: Provectus' melanoma Phase 1b combination therapy trial, PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma, might see patients whose disease cannot all be treated with PV-10 (i.e., they have, for example, visceral disease unreachable with a needle of PV-10) also achieve anti-melanoma immunity.
Updated (2/24/16): Provectus issued a press release and made an associated 8-K filing today regarding Moffitt's upcoming pre-clinical work at AACR 2016, Announces Data on PV-10 and Co-Inhibitory Blockade to Be Presented at American Association for Cancer Research Annual Meeting 2016.
For some understanding of t-cell mediated immunity see, for example, Wikipedia's cell-mediated immunity entry, a screenshot of which is below:
Or see, for example, from F.P. Nijkamp and M.J. Parnham (eds.), Principles of Immunopharmacology: 3rd revised and extended edition (2011):
For some understanding of t-cell mediated immunity see, for example, Wikipedia's cell-mediated immunity entry, a screenshot of which is below:
 |
| Click to enlarge. Orange emphasis above is mine. |
 |
| Click to enlarge. |
I previously referenced a paper written in 1976 in Klaassen titled Pharmacokinetics of rose bengal in the rat, rabbit, dog, and guinea pig when noting that very small amounts of rose bengal cross the blood-brain barrier. See December 1, 2014 blog post November Blog Stats, and other bits and bobs. I had written that some patients in Provectus' melanoma Phase 2 trial had visceral disease, including brain metastases. How many patients with brain mets was unclear. Provectus had publicly discussed one such patient at medical conferences (Subject 0907 with Stage IV M1c disease), noting "“Near complete resolution” of pulmonary nodules observed at Week 12" during an ASCO 2010 clinical development update presentation. If the brain is on the other side of the blood-brain barrier (and the immune system is on the other, so to speak), and PV-10 is injected into visible lesions on the skin, how were these brain tumors (nodules) positively impacted? The small amounts of rose bengal that may have permeated the barrier were very unlikely to be able to reduce or destroy tumors, and the drug was not systemically administered (just injected into the skin at the location of a cutaneous or subcutaneous lesion). As Provectus' Chairman and CEO Dr. Craig Dees, PhD would say, Mother Nature's immune system knows how to get to brain tumors without destroying the normal brain tissue. But how?
As or more importantly, Klaassen's paper discusses Rose Bengal's pharmacokinetics, the consistency of which I observed when comparing the pharmacokinetics of PV-10's use in clinical melanoma work, and that in clinical liver work. See Comparable, Consistent PV-10 Pharmacokinetics (July 3, 2015) on the blog's Archived News IV page. The results are comparable and consistent, such as biological half-life, measured in hours, and terminal half-life, measured in days. On the topic of pharmacokinetics, Klaassen's paper's abstract noted:
"The biological half-life for excretion of rose bengal into the bile of rats and rabbits was similar (30 min), that of the guinea pig shorter (17 min), and that of the dog longer (46 min). rose bengal clearance was slower in the bile duct-ligated rat, the rat with two-thirds of its liver removed, and in newborn rats, conditions which are known to alter hepatic excretory function. The blood concentration of rose bengal was higher in the two-thirds hepatectomized rats only during the first 30 min after administration and not at later times; thus, if rose bengal is used as a measure of hepatic function and if only one blood sample is taken, the time interval chosen is critical."Interestingly, Klaassen noted:
"Blood disappearance curves of rose bengal after doses between 0.01 and 10 mg/kg in the rat were almost identical, having an initial biological half-life of 2 min and a terminal half-life of 100 min."With regard to the immediately above, Provectus' ASCO 2010 poster and the company's ESMO GI 2015 poster seem to suggest doses in or around the same range. The inter-species comparison from Klaassen's work is below:
 |
| Click to enlarge. |
 |
| Click to enlarge. Orange edits are mine. |
I previously noted a March 2013 reference from someone in Calgary, Alberta, Canada to Rose Bengal (PV-10). See #10 under Notes (August 6, 2015) on the blog's Archived News IV page.
 |
| Click to enlarge. |
I found another reference (from a breast cancer patient chat board) from Calgary dated January 2014.
 |
| Click to enlarge. Image source |
Process (February 21, 2016)
In keeping track of various company processes as best I can, I note Memorial Sloan Kettering Cancer Center's Dr. Paul Chapman, MD still is (was) [it would appear] a consultant to Provectus. The takeaways here are (i) Chapman likely is (would have been) involved in the company's Phase 1b/2 study program of the combination of PV-10 and pembrolizumab in advanced (metastatic) melanoma (Stage IV disease) and (ii) Sloan Kettering likely will be a clinical site of this study. See also KOL update (September 16, 2015) on the blog's Archived News IV page.
Chapman's involvement in combination therapies for melanoma include, for example, ipilimumab (Yervoy) and nivolumab (Opdivo) -- Combination Immunotherapy Produces Striking Results against Melanoma, May 2015.
I recently noticed Professor/Dr. Georgina Long, PhD of Melanoma Institute Australia (MIA) (University of Sydney) also is a consultant to Provectus. Longtime PV-10 clinical investigator Professor/Dr. John Thompson is Executive Director of the Institute. Dr. Long's involvement goes back to at least May 2015. MIA was a clinical site of Provectus' metastatic melanoma Phase 2 trial, is a PV-10 compassionate use program site, and should be a site of the company's pivotal melanoma (Stage III disease) Phase 3 trial. The takeaways here are (i) Long likely is involved in the melanoma combination program and (ii) MIA likely will be a clinical site of this study.
Dr. Long recently presented data on Amgen and Merck & Co.'s Phase 1b study of the combination of T-Vec and pembro in Stage III/IV melanoma patients, and data on the combination of pembro and ipi (e.g., KEYNOTE-029: Pembrolizumab (pembro) + low-dose ipilimumab (ipi) for advanced melanoma).
Processes:
In the conversation (February 19, 2016)
H/t InvestorVillage poster Juggernaut who pointed me to a table in recently published paper Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation by Garg et al. (Cell Death & Differentiation, February 19, 2016), which mentions Rose Bengal as one of several drugs that causes immunogenic or tolerogenic cell death (ICD or TCD, respectively). See a screenshot of this table, Table 1, Summarization of ‘eat me’ signals, antibody-dependent cell phagocytosis (ADCP) and inhibitory effect on phagocytic activity associated with major anticancer therapies, below.
The cancer therapy is Rose Bengal acetate-based photodynamic therapy, which means the reference likely (because I can't access the reference list to know exactly) is Rose Bengal Acetate PhotoDynamic Therapy (RBAc-PDT) Induces Exposure and Release of Damage-Associated Molecular Patterns (DAMPs) in Human HeLa Cells by Panzarini et al. (2014, PLoS ONE 9(8): e105778).
The Garg paper is a review one, and doesn't include either of Moffitt Cancer Center's SITC 2015 or University of Illinois at Chicago's (UIC's) ASC 2016 poster presentations, the underlying work of which has not yet been published, that identify immunogenic cell death with Rose Bengal.
Two triggers (February 18, 2016)
A big thanks to InvestorVillage poster Hombre77 for finding comments by Provectus' CTO Dr. Eric Wachter, PhD on the company's March 12, 2015 4Q15/year-end business update conference call about a second trigger of an interim analysis of Provectus' pivotal melanoma Phase 3 trial (bolded and underlined emphasis below is mine):
Updated below, again.
InvestorVillage poster canis_star anecdotally observed the time taken for a Phase 3 clinical trial to achieve Institutional Review Board (IRB) review and approval at MD Anderson Cancer Center. The poster provided a sample of five recent or current Phase 3 trials there.
MD Anderson, a metastatic melanoma Phase 2 trial site of Provectus', and also a PV-10 compassionate use/expanded access program site, is a pivotal melanoma (Stage 3 disease) Phase 3 trial site of the company's; however, the cancer center currently is not yet recruiting.
I explored the "first received date" on ClinicalTrials.gov for each trial, compared them with their respective MD Anderson IRB dates, and calculated the number of days (and months) that elapsed between the two dates (i.e, first received, and IRB review and approval). I compared this data set to Provectus' dates: (i) the date the .gov website received Provectus' CTO Dr. Eric Wachter, PhD's initial protocol (11/4/14) and (ii) the date the final protocol was received (3/12/15). See the associated company press releases below.
Initial: 11/6/14, Submits PV-10 Phase 3 Melanoma Protocol to FDA
Final: 12/22/14, to Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment; 2/9/15, Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study; 3/16/15, Amended Protocol of PV-10 for Phase 3 Study as Treatment for Melanoma Now Available Online
Updated (2/17/16): canis_star's original observations and my follow-up analysis above, while interesting at least to me, cannot capture, among other things, the uniqueness or nuances of each clinical trial as well as the changing administrative climate at large research institutions like MD Anderson Cancer Center. The roughly 12-month average or thereabouts to open a Phase 3 trial at the cancer center must reflect, at a minimum, the obvious complexity of the undertaking itself.
13F filings (through today) for the period ending December 31, 2015 showed:
See Institutional (November 16, 2015) on the blog's Archived News IV page for the last blog news item on institutional holdings.
Expounding (February 12, 2016)
Updated below, again, once more.
I could envision Provectus' exchange offer (tender offer) to exchange/replace existing warrants (non-tradable ones, various exercise prices and expiration dates) for replacement warrants (tradable ones as PVCT.WS or PVCT/WS) being extended such that the current end of the offer period (February 15th) conforms with the March 1st date (the withdrawal/completion date).
At prior and current shares plus tradable warrant prices, the tender offer only should be a real decision, generally speaking, to holders of non-tradable warrants expiring through the tender offer/completion date, and to holders of non-tradable warrants with high exercise prices (i.e., in excess of $1.75-2.00). I could make the case for tendering for these tranches or series. I don't believe there currently is a case for lower exercise prices. In addition, there is some squishiness in the language of the offering (in my view) as it relates to the delivery of paperwork and money, and the respective dates of these deliveries.
Conforming the dates on the surface shouldn't be anything other than potentially accommodative. Nevertheless, one would've wanted a cleaner, crisper process in regards to the tender.
Updated (2/12/16): Non-tradable warrants ordered by expiration date (see pp. 13-15 of the S-4):
Ordered by exercise price:
Updated (2/12/16): As I mostly but not completely expected, Provectus extended the offer period of the tender offer from February 15th to March 10th (at 4 pm ET), which coincides with the company's fourth quarter/year-end business update conference call. The 8-K filing related to the extension is here.
In keeping track of various company processes as best I can, I note Memorial Sloan Kettering Cancer Center's Dr. Paul Chapman, MD still is (was) [it would appear] a consultant to Provectus. The takeaways here are (i) Chapman likely is (would have been) involved in the company's Phase 1b/2 study program of the combination of PV-10 and pembrolizumab in advanced (metastatic) melanoma (Stage IV disease) and (ii) Sloan Kettering likely will be a clinical site of this study. See also KOL update (September 16, 2015) on the blog's Archived News IV page.
 |
| Click to enlarge. Reference date: January 2016 |
I recently noticed Professor/Dr. Georgina Long, PhD of Melanoma Institute Australia (MIA) (University of Sydney) also is a consultant to Provectus. Longtime PV-10 clinical investigator Professor/Dr. John Thompson is Executive Director of the Institute. Dr. Long's involvement goes back to at least May 2015. MIA was a clinical site of Provectus' metastatic melanoma Phase 2 trial, is a PV-10 compassionate use program site, and should be a site of the company's pivotal melanoma (Stage III disease) Phase 3 trial. The takeaways here are (i) Long likely is involved in the melanoma combination program and (ii) MIA likely will be a clinical site of this study.
 |
| Click to enlarge. Reference date: January 2016 |
Processes:
 |
| Click to enlarge. |
H/t InvestorVillage poster Juggernaut who pointed me to a table in recently published paper Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation by Garg et al. (Cell Death & Differentiation, February 19, 2016), which mentions Rose Bengal as one of several drugs that causes immunogenic or tolerogenic cell death (ICD or TCD, respectively). See a screenshot of this table, Table 1, Summarization of ‘eat me’ signals, antibody-dependent cell phagocytosis (ADCP) and inhibitory effect on phagocytic activity associated with major anticancer therapies, below.
 |
| Click to enlarge. |
The Garg paper is a review one, and doesn't include either of Moffitt Cancer Center's SITC 2015 or University of Illinois at Chicago's (UIC's) ASC 2016 poster presentations, the underlying work of which has not yet been published, that identify immunogenic cell death with Rose Bengal.
- Moffitt: Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1
- UIC: Data on Immunology Effects of Provectus Biopharmaceuticals PV-10 in Colon Cancer Presented at 11th Annual ASC Meeting (company press release)
Two triggers (February 18, 2016)
A big thanks to InvestorVillage poster Hombre77 for finding comments by Provectus' CTO Dr. Eric Wachter, PhD on the company's March 12, 2015 4Q15/year-end business update conference call about a second trigger of an interim analysis of Provectus' pivotal melanoma Phase 3 trial (bolded and underlined emphasis below is mine):
"That being said, as I said in my opening comments, the study design from the statistical perspective is predicated on an 18-month accrual process with a 30-month overall duration. To the extent that we can exceed enrolment projections, that would be implicit in those numbers, we can expedite the study. The study does have an interim assessment, as I mentioned during my introductory comments, when half of the required events have occurred; that’s basically close to half of the patients have had disease progression. Because it’s a 2 to 1 randomization, we have language in the protocol that will allow us to close the study early if the number of events don’t occur in the projected number. That number again is based on the Null Hypothesis which is patients in both arms of the study will have progression at the same rate. Obviously the goal with any clinical trial is to disprove the Null Hypothesis and so as the sponsor we expect that there will be a difference in the rate of progression, the number of progression events in the two study arms. That’s the basis for that additional opportunity to terminate the study if those events don’t occur. So that’s the fundamental design of the study. Again, 18 months since last summer, 30-month enrolment—sorry, 30-month overall. We will do our best to exceed that accrual rate. We can’t force the patients to have disease progression but we have done everything we can to make sure that in the event that there is disparity in disease progression between the two study arms that we are allowed to end the study at an appropriate time. Again, most importantly perhaps to the investment community and to prospective partners is that we do have that interim assessment which allows to examine and report interim data once the study is halfway through."The above would validate Eric's comments to me in 2014 (first underlined section); see Updated Clinical Trial Information (February 5, 2016) below:
"If there is a non-normal distribution (e.g., a substantial fraction of patients in one arm do not incur progression within the projected timeframe) or an unexpected distribution (e.g., patients in one arm fare much better than predicted) of events, the time to accumulate the necessary number of events may be delayed. To address this possibility, the trial may be designed to trigger review of the data upon the first of (i) accumulating the necessary number of events or (ii) reaching a prescribed period of time after which the necessary number of events would be expected (such as 2 or 3 times the predicted PFS for the last patient in the treatment arm).
Consider another hypothetical study, this time with 300 patients and 2:1 randomization (i.e., 200 in the treatment arm and 100 in the control arm). Study readout is triggered when half of the patients incur the prescribed event. Thus, 150 events are needed. Because of the 2:1 randomization, 100 of these would be expected to occur in the treatment arm and 50 in the control. For an interim analysis, 75 events are needed (i.e., 50 and 25, respectively) once 150 patients have begun the trial (i.e., 100 and 50, respectively). All of the above observations apply. Again, if there is a non-normal distribution of events, the prescribed time clause might be required to trigger prompt data readout.
The above examples assume a normal distribution of events. The performance of DTIC and TMZ are well documented and generally yield a normal distribution of events. In the hypothetical time between trigger (i, prescribed event) and trigger (ii, prescribed time), it is likely the required events would occur through accumulation of events from both arms (albeit perhaps taking longer than expected). Trigger (ii) is likely to precede (i) only when there is a very unusual response in one or both of the arms. Thus, safeguards exist to compensate for non-normal or unexpected distribution of events and assure a timely readout of trial results."Eric's comments also would appear to foreshadow Provectus' CFO/COO Peter Culpepper's comments regarding the mid-year timing of an interim analysis (second underlined section); see Wrongness (February 7, 2016) below. Peter also said essentially the same thing on the same 4Q15/year-end call too:
"Our estimated primary completion date is September 2017, and an estimated study completion date of October 2017. When 50 percent of the events required for the primary endpoint have occurred, the Independent Data Monitoring Committee will report an interim assessment of efficacy and safety. So, meaningful clinical data could come as early as the middle of next year, which is halfway through the study, as documented on clinicaltrials.gov. I stretch the word could, and we will continue to make every effort possible to keep our stockholders and the market updated."Hombre77 concluded the post with: "[b]ut regardless of what may trigger an interim assessment, the trial would obviously need to have some minimum number of participants..." The crux of the matter is this minimum number, which should be the minimum number of treated patients to show statistically significant separation between the PV-10 arm's progression-free survival curve and the chemotherapy curve. So, as I wrote under Wrongness below:
"Rather than ask how long will it for take the trial to accumulate the necessary number of events or query with emphasis its enrollment rate, the critical question (for me) is will the pivotal melanoma Phase 3 trial have accumulated enough events (i.e., treated enough patients) to have achieved statistically significant progression-free survival curve separation by July 2016, which is management's current guidance to the biotechnology industry and stock market of the timing of the interim assessment of efficacy and safety."IRB review & approval (February 17, 2016)
Updated below, again.
InvestorVillage poster canis_star anecdotally observed the time taken for a Phase 3 clinical trial to achieve Institutional Review Board (IRB) review and approval at MD Anderson Cancer Center. The poster provided a sample of five recent or current Phase 3 trials there.
MD Anderson, a metastatic melanoma Phase 2 trial site of Provectus', and also a PV-10 compassionate use/expanded access program site, is a pivotal melanoma (Stage 3 disease) Phase 3 trial site of the company's; however, the cancer center currently is not yet recruiting.
I explored the "first received date" on ClinicalTrials.gov for each trial, compared them with their respective MD Anderson IRB dates, and calculated the number of days (and months) that elapsed between the two dates (i.e, first received, and IRB review and approval). I compared this data set to Provectus' dates: (i) the date the .gov website received Provectus' CTO Dr. Eric Wachter, PhD's initial protocol (11/4/14) and (ii) the date the final protocol was received (3/12/15). See the associated company press releases below.
Initial: 11/6/14, Submits PV-10 Phase 3 Melanoma Protocol to FDA
Final: 12/22/14, to Meet with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study with Aim to Maximize Speed of Enrollment; 2/9/15, Met with FDA on Operational Aspects of PV-10 Phase 3 Melanoma Study; 3/16/15, Amended Protocol of PV-10 for Phase 3 Study as Treatment for Melanoma Now Available Online
 |
| Click to enlarge. |
The analysis also could continue to caution that Eric usually takes longer than expected (expectations he often sets for himself, and thus for the company) to do things for whatever specific (and potentially good) reason(s) at the time for whatever workflow item in question.
If 12 months (give or take), which is far from a magic number of course, is what it takes for some, many or all of the remaining clinical locations of Provectus' pivotal melanoma Phase 3 trial to come on-line, a larger or full slate of sites could recruit in or by April. Could that still permit an interim analysis (a prescribed time analysis, let alone a prescribed event one) by mid-year? I remain skeptical.
A counter to my skepticism, otherwise known as the view through Rose Bengal-colored glassess would go something like this:
- First, control arm patients on chemotherapy should progress after one cycle on dacarbazine or temozolomide, or around 30 days (round number). Thus, patients enrolled in April would generate disease progression events in May or June (they'd crossover to the PV-10 arm as soon as progression was identified),
- Second, treatment arm patients on PV-10 only have to show a differential in progression (or lack thereof) of at least 30% compared to the control arm (unit of measured equals weeks or months), if my assumption of the trial's projected hazard ratio is correct,
- Third, if sufficient numbers of patients are not enrolled so as to generate statistical significance (i.e., p < 0.05), a good "fall back" would be to demonstrate a trend towards significance (if achievement has not been attained), and
- Fourth, slippage of the analysis by a few months into September or thereabouts should be no big deal if, a big if, the company has grown its balance sheet with cash at a decent dilutive price or in a non-dilutive fashion to lengthen the runway to conduct a later readout.
As an aside, Eric updated the World Health Organization (WHO) International Clinical Trials Registry Platform version of the Phase 3 trial a couple of days ago. Unfortunately, one cannot ascertain the change.
Updated (2/17/16): A shareholder e-mailed me yesterday writing that he had run a Black-Scholes calculation on the tradable warrants: "Based on stock price of $0.45, and tradable warrant price of $0.22, the implied volatility is 75%." My own quick 'n dirty historical vol calculations for various terms are below (second column from the right):
Institutional (February 16, 2016)Updated (2/17/16): A shareholder e-mailed me yesterday writing that he had run a Black-Scholes calculation on the tradable warrants: "Based on stock price of $0.45, and tradable warrant price of $0.22, the implied volatility is 75%." My own quick 'n dirty historical vol calculations for various terms are below (second column from the right):
 |
| Click to enlarge. |
13F filings (through today) for the period ending December 31, 2015 showed:
- A decrease in institutional share holdings of Provectus: 9.83 million shares (down from 10.68 million as at 9/30/15, or about -8%) and 4.8% of shares outstanding (not a fully diluted figure) (down from 5.22%), and
- An increase in filers to 46 (up from 42).
 |
| Click to enlarge |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Expounding (February 12, 2016)
Updated below, again, once more.
I could envision Provectus' exchange offer (tender offer) to exchange/replace existing warrants (non-tradable ones, various exercise prices and expiration dates) for replacement warrants (tradable ones as PVCT.WS or PVCT/WS) being extended such that the current end of the offer period (February 15th) conforms with the March 1st date (the withdrawal/completion date).
At prior and current shares plus tradable warrant prices, the tender offer only should be a real decision, generally speaking, to holders of non-tradable warrants expiring through the tender offer/completion date, and to holders of non-tradable warrants with high exercise prices (i.e., in excess of $1.75-2.00). I could make the case for tendering for these tranches or series. I don't believe there currently is a case for lower exercise prices. In addition, there is some squishiness in the language of the offering (in my view) as it relates to the delivery of paperwork and money, and the respective dates of these deliveries.
Conforming the dates on the surface shouldn't be anything other than potentially accommodative. Nevertheless, one would've wanted a cleaner, crisper process in regards to the tender.
Updated (2/12/16): Non-tradable warrants ordered by expiration date (see pp. 13-15 of the S-4):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
The tender offer makes sense for Provectus and existing shareholders (both those who have non-tradable warrants to potentially exchange, and those like me who simply hold common stock). See, for example, Love Me Tender under Cheers (January 1, 2016):
In the end, the extension would appear "no harm, no foul," but with a lowered bar for certain management processes/sub-processes: e.g., after filing the original tender offer documents on December 31st, the filing of the extension is done on a Friday before a long weekend, or including in the filing "While no Existing Warrants have been tendered to date, the Company understands that numerous Existing Warrant holders intend to participate in the Warrant Exchange Offer and are in the process of preparing their documentation for potentially tendering their Existing Warrants." (defensive, amateurish and unnecessary). Who writes this stuff? More importantly, who approves the writing of this stuff as well as its inclusion?
One continues to hope for better, however, as much more important execution opportunities present themselves in the near future.
Updated (2/12/16): H/t a regular hatter who reminded me of a comment Peter made during his 2016 BIO CEO & Investor Conference presentation, a screenshot of which (from the transcript) is below:
In 2014, Provectus' compassionate use program (CUP) treated approximately 40 patients. So, the 2015 figure may or should be greater than 40 (>40). Tallying up the total number of patients treated with PV-10, one could arrive at various figures depending on the method or approach: 265 (Provectus alone; 290 including third parties), to possibly or potentially more than 327-347 (the company alone; >367-387 including others).
In 2010, Provectus issued press release Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia, where the company noted (underlined emphasis is mine):
"In no particular order, the rationale for the tender/deal structure seems to be (i) doing a good one for some long-time existing shareholders/warrant holders (the higher the exercise price of their existing non-tradable warrants, the larger the do), (ii) enabling a "least toxic financing as possible," and (iii) deepening the market for tradable warrants, and hoping to prevent PVCT.WS's price from dictating the price of PVCT."I also wrote under Moneyness (January 2, 2016):
I contend Provectus' tender offer is OTM [out-of-the-money]...As such, derivative theory suggests a warrant holder would neither exercise nor exercise early -- despite the company's CTO Dr. Eric Wachter, PhD, CFO/COO Peter Culpepper, and board of directors member Jan Koe's intention to exercise all of their non-tradable warrants (~1.3 million) at a cumulative dollar cost to them of ~$960K, which of course would be an investment by them in and a cash flow into Provectus...As a result, I would imagine the tender actually has to become ITM [in-the-money] from a quantitative perspective; that is, the price of a common share (and, thus, the tradable warrant, which should move in tandem with the stock) has to rise above the tender's strike price before its expiration. If one assume's management is given no benefit of the doubt by the capital markets, then the share price only should move because of news (e.g., pre-clinical and/or clinical development, regulatory, business development, corporate development, media, etc.), and that news would have to materialize before February 15th."There has been some news of import, Announces Immunology Data on PV-10 in Colon Cancer to Be Presented at 11th Annual ASC Meeting (press release title), which I don't think was fully anticipated but very critical -- see Doppio Espresso Con Panna (January 21, 2016):
"1. Immunogenic cell death. I think InvestorVillage poster canis_star today said it better and more precisely in regards to the mechanism of immune action work undertaken by Moffitt Cancer Center (pre-clinical and clinical) and the University of Illinois at Chicago (UIC) (pre-clinical): 'Both research labs independently showed that PV-10 induce the immunogenic cell death (ICD) in multiple cancer[s]...'"But, I believe it would be reasonable to say no anticipated news materialized, leading to the extension of the tender offer -- giving more time for the moneyness of a non-tradable warrant holder's decision to become more compelling. Thus, one could imagine news could materialize by March 10th.
In the end, the extension would appear "no harm, no foul," but with a lowered bar for certain management processes/sub-processes: e.g., after filing the original tender offer documents on December 31st, the filing of the extension is done on a Friday before a long weekend, or including in the filing "While no Existing Warrants have been tendered to date, the Company understands that numerous Existing Warrant holders intend to participate in the Warrant Exchange Offer and are in the process of preparing their documentation for potentially tendering their Existing Warrants." (defensive, amateurish and unnecessary). Who writes this stuff? More importantly, who approves the writing of this stuff as well as its inclusion?
One continues to hope for better, however, as much more important execution opportunities present themselves in the near future.
Updated (2/12/16): H/t a regular hatter who reminded me of a comment Peter made during his 2016 BIO CEO & Investor Conference presentation, a screenshot of which (from the transcript) is below:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
"The recent meeting focused on manufacturing, characterization and specifications for PV-10, along with a review of clinical data and anticipated Phase 3 study design and endpoints. The proposed primary endpoint of progression free survival, which Provectus proposed to the U.S. Food and Drug Administration (FDA) earlier this year in its first end-of-Phase-2 meeting with FDA, was deemed appropriate for assessment of efficacy in light of established European Medicines Agency (EMEA) standards adopted by TGA. Use of interim data from the first half of Phase 3 study subjects, in conjunction with safety data collected in earlier studies of PV-10 for melanoma, was discussed to allow early evaluation for marketing approval for metastatic melanoma, and TGA agreed that these data should be sufficient for this review if the analysis confirmed efficacy."
Discussion around that time centered on having at least 300 patients (melanoma, and/or potentially otherwise) treated with PV-10 as a key threshold for achieving [evaluation of] marketing approval. Are they there yet?
"...clear that our protection of Rose Bengal for therapeutic use is in fact ours" (February 11, 2016)
Updated below.
Regarding the continuation patent application (14/447,063 or 20140343296/November 20, 2014) of Provectus's initial/original synthesis patent, Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes (8,530,675/September 10, 2013), the new synthesis patent should be issued on Tuesday, March 1st.
During Provectus CFO/COO Peter Culpepper's 2016 BIO CEO & Investor Conference presentation, he displayed and said:
On or after March 1st (when Provectus should PR the award), I would imagine management attempts to explain in greater detail the import of and strategy behind this portion of Provectus' intellectual property protection and portfolio.
Updated (2/29/16): The USPTO's PAIR site notes an award date of March 1st.
Reading above, below and between the lines, and the lines themselves (February 10, 2016)
Provectus provided copies of CFO/COO Peter Culpepper's 2016 BIO CEO & Investor Conference presentation's slide deck and transcript. Further thoughts include the below (first, the slide(s); second, the related transcript portion; and third, my commentary).
Takeaway: Peter repeatedly emphasized data readouts in 2016 from clinical trials, pre-clinical studies, medical conferences and publications.
Notes:
Notes:
Notes:
Notes:
Notes:
Notes:
Notes:
Notes:
Outright grants, Expense reimbursement (February 8, 2016)
Updated below.
Provectus' 2016 BIO CEO & Investor Conference slide presentation may be found here (scroll right only for now).
Updated below.
Regarding the continuation patent application (14/447,063 or 20140343296/November 20, 2014) of Provectus's initial/original synthesis patent, Process for the synthesis of 4,5,6,7-tetrachloro-3',6'-dihydroxy-2',4',5',7'-tetraiodo-3H-spiro[isoben- zofuran-1,9'-xanthen]-3-one (rose bengal) and related xanthenes (8,530,675/September 10, 2013), the new synthesis patent should be issued on Tuesday, March 1st.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Page/Slide #28 |
 |
| Click to enlarge. |
Updated (2/29/16): The USPTO's PAIR site notes an award date of March 1st.
 |
| Click to enlarge. |
Provectus provided copies of CFO/COO Peter Culpepper's 2016 BIO CEO & Investor Conference presentation's slide deck and transcript. Further thoughts include the below (first, the slide(s); second, the related transcript portion; and third, my commentary).
Takeaway: Peter repeatedly emphasized data readouts in 2016 from clinical trials, pre-clinical studies, medical conferences and publications.
 |
| Click to enlarge. Page/Slide #3 |
 |
| Click to enlarge. |
- Interim data from Provectus' pivotal melanoma Phase 3 trial, this year (mention #1)
- PH-10 (clinical) mechanism of action study data, this quarter (mention #1)
 |
| Click to enlarge. Page/Slide #4 |
 |
| Click to enlarge. |
- Arrows moved again, presumably establishing the "final" ordering of how clinical trials are advancing -- leading with melanoma Phase 3 and followed by melanoma combo Phase 1b/2, liver Phase 1 (in essence, now a "Phase 1b" of sorts), and, to come, I guess, a breast cancer trial. See Mechanics of the Process (February 2, 2016) for the last iteration (same ordering, but with breast farther to the left).
- The placement of the arrows, particularly the melanoma combo one, probably is no mistake. Peter is trying to convey what he knows/has learned; however, the question is, what does it really mean, and how accurate (if at all) is it?
 |
| Click to enlarge. Page/Slide #7 |
 |
| Click to enlarge. |
- There's really no need to highlight any of this verbiage in yellow. The clinical value proposition of PV-10 is both very broad and deep. Broad in terms of utility for different cancer indications and tumor types. Deep in terms of utility for different disease stages and presentations.
 |
| Click to enlarge. Page/Slide #8. |
 |
| Click to enlarge. |
- PV-10 mechanism of action study data, this year (mention #1)
 |
| Click to enlarge. Page/Slide #9 |
 |
| Click to enlarge. |
- With a local treatment and active/specific immunotherapy like PV-10, where the entry point or target is the tumor or lesion itself, Peter's reminder that thousands of tumors have been treated with PV-10 is a good visual.
 |
| Click to enlarge. Page/Slide #10 |
 |
| Click to enlarge. Page/Slide #11 |
 |
| Click to enlarge. |
- Complete response
- "Alter T cell immunity:" Peter's BioPharma Asia presentation in March in Singapore is entitled Altering T cells and induced tumour-specific immunity via PV-10 ablation.
- NYU Langone Medical Center and former Moffitt Cancer Center's Dr. Jeffrey Weber, MD, PhD is the expert who viewed PV-10 as the perfect immune system primer.
 |
| Click to enlarge. Page/Slide #12 |
 |
| Click to enlarge. Page/Slide #14 |
 |
| Click to enlarge. |
- Interim data from Provectus' pivotal melanoma Phase 3 trial, this year (mention #2)
- Combo melanoma Phase 1b study data, this year (mention #1)
- Opening liver sites/expanding liver trial work (mention #1)
- Advancing another cancer indication into the clinic (mention #1)
 |
| Click to enlarge. Page/Slide #15 |
 |
| Click to enlarge. Page/Slide #16 |
 |
| Click to enlarge. Page/Slide #17 |
 |
| Click to enlarge. |
Notes:
- Complete response
- Durable response
 |
| Click to enlarge. Page/Slide #23 |
 |
| Click to enlarge. Page/Slide #24 |
 |
| Click to enlarge. |
Notes:
- Liver data at ESMO World Congress on Gastrointestinal Cancer 2016 (Barcelona, June 29th-July 2nd)?
- Combo melanoma data at ASCO 2016 (Chicago, June 3rd-7th)?
- Phase 1 liver data, this year (together with opening liver sites/expanding liver trial work (mention #2)
- PV-10 mechanism of action study data (mention #2)
 |
| Click to enlarge. Page/Slide #25 |
 |
| Click to enlarge. |
Notes:
- Interim data from Provectus' pivotal melanoma Phase 3 trial, this year (mention #3)
- Combo melanoma Phase 1b study data, this year (mention #2)
- PV-10 clinical mechanism of action study data, this year (mention #1, #3)
- Advancing another cancer indication into the clinic (mention #2)
 |
| Click to enlarge. Page/Slide #26 |
 |
| Click to enlarge. |
Notes:
- Compassionate use program data, this year (mention #1)
 |
| Click to enlarge. Page/Slide #28 |
 |
| Click to enlarge. |
Notes:
- Synthesis patent continuation award, see Advancing (January 24, 2016) below
- Limited Pfizer ownership of Provectus' combination therapy patent
- Orthogonality: PV-10 + immunotherapy, PV-10 + targeted therapies, PV-10 + radiation (radiotherapy), etc.
 |
| Click to enlarge. Page/Slide #31 |
 |
| Click to enlarge. |
Notes:
- Together with PV-10's safety profile, pricing (pharmacoeconomics) often also is overlooked.
 |
| Click to enlarge. Page/Slide #33 |
 |
| Click to enlarge. |
- Interim data from Provectus' pivotal melanoma Phase 3 trial, this year (mention #4)
- PV-10 clinical mechanism of action study data, this year (mention #2, #4)
- Compassionate use program data, this year (mention #2)
- Advancing another cancer indication into the clinic (mention #3)
- Provectus' new trademark: When patients win, we all win; see A new tag line under Breadcrumbs (January 27, 2016)
- How well capitalized?
Updated below.
Provectus' 2016 BIO CEO & Investor Conference slide presentation may be found here (scroll right only for now).
Of note was the mention of the company's search for non-dilutive financing by way of outright grants and expense reimbursements in different jurisdictions where clinical work is underway or would be carried out.
Additionally, Provectus' CFO/COO Peter Culpepper mentioned the potential for an additional indication to make its way to the clinic this year. I would assume that indication is breast cancer. He also reiterated the interim analysis of the pivotal melanoma Phase 3 trial would be readout mid-2016, still consistent with previous management guidance, which was the point of the comments written below under Wrongness (February 7, 2016) and Updated Clinical Trial Information (February 5, 2016). "One way or another," there should be an interim assessment of efficacy and safety this summer.
Updated (2/8/16): Peter discussed the concept or idea of PV-10's agnosticism or indifference (my words) to solid tumor cancer or disease presentation. It, PV-10/Rose Bengal, ablates all form and manner of tumor into which it is injected. I've discussed agnosticism, and more broadly orthogonality, most recently under Breadcrumbs (January 27, 2016) below. See also July 5, 2015
blog post The Ever Expanding Clinical Value Proposition of PV-10.
In this project's curated Twitter feed I noted @bradloncar's tweet about targets.
The image in the tweet is bigger, below.
My reason for blogging about the above is to further extend the idea of PV-1's agnosticism, or orthogonality, or indifference, or flexibility, or diversity, or...; that PV-10 does not target a specific receptor or pathway -- which may be hard for the pharmaceutical/biotechnology industry at large to wrap their individual and collectives heads around.
Management's general comments about pivotal trial designs from the summer of 2014 are paraphrased by me below:
It's complicated (February 4, 2016)
Updated below, below.
H/t a regular hatter, who also in an internal medicine doctor: GNA11 Mutation in a Patient With Cutaneous Origin Melanoma: A Case Report, Patel et al., Medicine, January 2016, Volume 95, Issue 4, p e2336.
Takeaways:
H/t InvestorVillage poster canis_star: NYU Langone Medical Center's Dr. Jeffrey Weber, MD, PhD on topics to be covered at the above mentioned conference, including PV-10 (comments starting at 0:30).
Mechanics of the Process (February 2, 2016)
Updated below, again, once more.
Provectus currently is undertaking a Phase 1b study of the combination of intralesional agent (IL) PV-10 ("turn on the immune system's engine," "step on the immune system's gas pedal") and checkpoint inhibitor pembrolizumab/Keytruda ("release its brakes") in patients with Stage IV melanoma. The trial is entitled PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma ("PV-10/pembro"). As of this writing, one site is recruiting (St Luke's University Hospital and Health Network in Easton, Pennsylvania) and two currently are not (MD Anderson Cancer Center in Houston, Texas and Princess Alexandra Hospital in Brisbane, Australia) are not. The enrollment goal is for up to 24 patients. I have previously written that I believe the current enrollment estimate for the Phase 2 study portion could be less than 120 patients. See "FPFV" (January 12, 2016) below.
Generally speaking, while all combination trials would be of interest in terms of comparing their pairings' safety and efficacy, at least two are of particular note [to me]: the combination of IL agent T-Vec/Imylgic and checkpoint inhibitors (i) ipilimumab/Yervoy and (ii) pembrolizumab in patients with Stage III/IV melanoma. See Ipilimumab With or Without Talimogene Laherparepvec in Unresected Melanoma ("T-Vec/ipi") and Pembrolizumab With or Without Talimogene Laherparepvec or Talimogene Laherparepvec Placebo in Unresected Melanoma ("T-Vec/pembro"), respectively. These trials are of particular interest because I believe the set-up of the Amgen/Bristol-Myers and Amgen/Merck & Co. trials provides some insight into Provectus'.
T-Vec/ipi, which "began" in November 2012 when the trial protocols (Phase 1b/2) initially were filed, reported preliminary data at ASCO 2014, Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma, and 2015, Survival, safety, and response patterns in a phase 1b multicenter trial of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma.
Safety and efficacy data were presented on 17 assessed/evaluable patients (19 were enrolled, of which 18 were treated). Investigators from 6 clinical sites comprised non-company ASCO 2014 and 2015 poster authors. Data cut-off dates were October 15, 2013 and December 22, 2014 respective. A trials-in-progress poster at ESMO 2014, A phase 1b/2, multicenter, open label trial to evaluate the safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) versus ipi alone in previously untreated, unresected stage IIIB, IIIC, and IV melanoma, included in its authorship line investigators from 13 sites. The ESMO poster also noted the Phase 2 portion of the Phase 1b/2 opened in August 2013.
Projected enrollment of T-Vec/ipi's Phase 2 trial was increased from 140 to 200 (in December 2014). Randomization is 1:1. 40 sites are recruiting for this trial.
T-Vec/pembro, which "began" in September 2014, reported preliminary data at SMR 2015, Primary analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. A trials-in-progress poster was presented at SITC 2015, which preceded SMR by a few weeks. Safety and efficacy data were presented on 16 assessed/evaluable patients (21 were enrolled). Investigators from 14 clinical sites comprised non-company SITC poster authors.
The Phase 3 portion of T-Vec/pembro has not yet begun recruiting. Projected enrollment is 660. Randomization is 1:1. As background and context, T-Vec's pivotal melanoma Phase 3 trial recruited 436 patients (2:1 randomization).
Takeaways & Questions:
I emailed management with canis_star's observation (timestamp ~12:06 pm EDT); they replied shortly thereafter saying the Company would address the situation, and followed up a little later saying the trial's webpage was updated and that it would take a day or two for the change to be made effective. The webpage this morning showed the change on ClinicalTrials.gov.
January Blog Readership Stats (February 1, 2016)
Changes in blog readership statistics were all positive in January 2016. Month-over-month (January 2016 v. December 2015) percent differences included:
"Making cancer visible – Dyes in surgical oncology" (January 29, 2016)
H/t a regular hatter (also an internist, entrepreneur and shareholder).
Article highlights:
Also see “Most drugs are really German dyes” (August 3, 2015) on the blog's Archived IV News page: Underlined emphasis below is mine. In an August 3rd article by Forbes.com's Matthew Herper entitled Lexicon's Quest For A New Blockbuster, he quotes Brent Stockwell, a scientist at the MIT-affiliated Whitehead Institute for Biomedical Research (who now appears to be at Columbia University):
Updated below, again.
Biomarkers. H/t a regular hatter for a discussion about a PV-10 biomarker. Pharmafile's December 23, 2015 article 100 year old chemical could be the future of cancer treatment noted Provectus' CFO/COO Peter Culpepper as saying:
Filed in August 2015:
Updated (1/27/16): Provectus previously filed a trademark for both PV-10 and PH-10 in June 2015, about two months before the filing of the above slogan. See "Intent to use" (September 26, 2015) on the blog's Archived IV News page.
As reference, Provectus' pivotal Phase 3 trial commenced in April 2015.
 |
| Click to enlarge. Slide #30 at BIO CEO & Investor Conference 2016 |
Updated (2/8/16): Peter discussed the concept or idea of PV-10's agnosticism or indifference (my words) to solid tumor cancer or disease presentation. It, PV-10/Rose Bengal, ablates all form and manner of tumor into which it is injected. I've discussed agnosticism, and more broadly orthogonality, most recently under Breadcrumbs (January 27, 2016) below. See also July 5, 2015
blog post The Ever Expanding Clinical Value Proposition of PV-10.
In this project's curated Twitter feed I noted @bradloncar's tweet about targets.
 |
| Click to enlarge. Image source. |
 |
| Click to enlarge. |
Wrongness (February 7, 2016)
Updated below.
Takeaway: Am I wrong in my attempt to analyze this? Rather than ask how long will it take for the trial to accumulate the necessary number of events or query with emphasis its enrollment rate, it would seem to me the critical question is will the pivotal melanoma Phase 3 trial have accumulated enough events to have achieved statistically significant progression-free survival curve separation by July 2016, which is management's current guidance in regards to the timing of the interim assessment of efficacy and safety.
As for Updated Clinical Trial Information (February 5, 2016) below, I used Huntsman Cancer Institute's change of status as a Provectus pivotal melanoma Phase 3 site from not recruiting to recruiting to revisit the timing of the clinical study's interim assessment of efficacy and safety.
Why revisit the topic? Despite Huntsman and MD Anderson (which has not yet come online as a Phase 3 site) purportedly being the top 2 places where Stage 3 melanoma patients are treated in the U.S., I remain skeptical that the company can achieve an interim assessment in July/summer 2016, or halfway between the trial's study start (April 2015) and estimated primary/study completion (September/October 2017) dates.
Why could I be skeptical? Let me count the ways:
Updated below.
PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma: Huntsman Cancer Institute, recruiting.
Updated (2/5/16): I have been told, but cannot independently confirm as yet, that Huntsman Cancer Institute, an NCI-designated Comprehensive Cancer Center, treats more Stage 3 patients than any other center or location in the U.S. MD Anderson, also an NCI-designated Comprehensive Cancer Center, ranks second (in terms of the number of Stage 3 patients treated there).
Updated (2/6/16): As of Friday's close, for the first time in 3 months (last, November 6, 2015: $0.755), seemingly almost or actually to the day, the unit price of one share of Provectus common stock (PVCT) and one tradable warrant (PVCT.WS) exceeded the unit price's public offering (June 23, 2015) price of $0.75: $0.7549.
At a combined price of more than $0.75, the upcoming tender offer (with a current deadline of February 15th) is in-the-money, but (in my view) far from compellingly and potentially sustainably so for a holder of a non-tradable warrant to consider transacting.
Updated (2/6/16): With Huntsman's change in recruiting status for Provectus' pivotal melanoma Phase 3 trial, it's worthwhile revisiting the timing of the trial's overt Interim Assessment of Efficacy and Safety, to be performed by the study's independent review committee (IRC) when 50% of the events required for the primary endpoint (progression-free survival) have occurred (a so-called Prescribed Event Clause).
With no change to the trial's Estimated Study Completion Date (or Estimated Primary Completion Date), I continue to think (not just believe) there currently is no change to the timing of the interim assessment (in this form a so-called Prescribed Time Clause), which management previously guided as halfway between the Study Start Date and study completion. Halfway between April 2015 and October 2017 is July 2016 (primary completion would suggest June).
Updated below.
 |
| Image source |
As for Updated Clinical Trial Information (February 5, 2016) below, I used Huntsman Cancer Institute's change of status as a Provectus pivotal melanoma Phase 3 site from not recruiting to recruiting to revisit the timing of the clinical study's interim assessment of efficacy and safety.
Why revisit the topic? Despite Huntsman and MD Anderson (which has not yet come online as a Phase 3 site) purportedly being the top 2 places where Stage 3 melanoma patients are treated in the U.S., I remain skeptical that the company can achieve an interim assessment in July/summer 2016, or halfway between the trial's study start (April 2015) and estimated primary/study completion (September/October 2017) dates.
Why could I be skeptical? Let me count the ways:
- When has Provectus' CTO Dr. Eric Wachter, PhD met a deadline, self imposed or otherwise? (albeit paraphrasing, but not my words...) -- in part, yuck yuck; in part, deadly serious.
- Comparatively and cumulatively, trial arm responses of chemotherapy, DTIC or TMZ (normal distribution of events) and PV-10 (non-normal or unexpected distribution) should be vastly different -- so, how can an interim assessment be reached, based on an accumulation of 50% events, irrespective of time (but based on a trial N of 225) if, generally speaking, all control arm patients progress and no treatment arm patients progress?
- Assuming events occur in some form or fashion (n.b., not a proper assumption), how can an interim assessment be readout in July/summer 2016 that somehow accumulates 112.5 (=113) events (I believe one patient per event or one event per patient, so 113 patients) when, as of this writing, Provectus' pivotal trial only has 4 sites recruiting and about 5-6 months to go before the trial's so-called halfway point?
There are two triggers of an interim assessment. One (the prescribed event clause) is overt, designed to trigger review when the necessary number of events have been accumulated. The other (the prescribed time clause) is a safety valve, "relieving the system of pressure" if the time to accumulate the necessary number is delayed (and thus the first trigger is not pulled) and triggering review upon reaching the prescribed period of time after which the necessary number of events is expected.
It should be very hard to trigger the prescribed event clause. If most or all of the control arm patients progress on chemotherapy, and most of the treatment arm patients achieve complete response after one or two injections of PV-10 (and thus do not progress), the study should be hard pressed to accumulate 113 events (given a trial N of 225).
The response profiles of the trial's arms should be vastly different. The design of a clinical trial assumes both arms respond equivalently (the so-called null hypothesis). The base or common response is that of the control arm, which in Provectus' pivotal melanoma Phase 3 trial's case is chemotherapy, which is dacarbazine or temozolomide, which as noted in the news item below generally yield a normal distribution of events. PV-10 does not yield a normal distribution curve of response events. It should yield a non-normal (a substantial fraction of patients in one arm do not incur progression within the projected timeframe) or unexpected (patients in one arm fare much better than predicted), left or negative skewed, distribution of events.
PV-10's effect size over chemotherapy should be very large. As a result, the alternate hypothesis (the arms respond differently) should be very different than the null hypothesis (the arms respond the same). Given the likely response profiles above, and thus effect size, a relatively smaller number of patients (compared to less effective drug compounds in their respective pivotal trials) are needed to run the trial in the first place, and a relatively smaller number of patients to meet or exceed the predicted hazard ratio.
Large effect sizes and small hazard ratios mean fewer patients required to achieve statistical significance. The hazard ratio of the trial likely has been designed to be around or about 0.545. See June 23, 2014 blog post Trial Math: Meeting the Primary Endpoint, Pt. 1 or February 12, 2015 blog post Hazard ratio, and other stat stuff. As noted in the news item below, hazard ratio (HR) essentially is the ratio of response of the control arm divided by that of the treatment arm. The smaller the HR, the larger the effect size and clinical relevance of the observed difference. If the response of Stage III melanoma patients to DTIC/TMZ (chemotherapy) is probably 4 weeks (just over one cycle) but "officially" ~6 weeks (1.5 months) from the biomedical literature's perspective, then an HR_predicted of 0.545 means the PV-10 treatment arm must achieve a response of at least 11-12 weeks (2.8-3 months). HR_actual, when the trial is completed, will be 1.5 months of PFS under chemotherapy divided by many, many months of PFS under PV-10, or potentially a very small number. That is, HR_actual should be >> HR_predicted. I believe the corollary of this would be fewer patients are required for HR_actual to equal HR_predicted.
I'm not saying I come close to understanding Eric's pivotal Phase 3 trial subprocess (of his clinical development program process). The below (and the above) is my attempt to try and overcome my own skepticism.
A prescribed time clause by definition requires a set of assumptions. Time is taken. A prescribed event clause does not. Events happen. I assume, based on guidance from and comments by management, and calculate:
The key question: Rather than ask how long will it for take the trial to accumulate the necessary number of events or query with emphasis its enrollment rate, the critical question (for me) is will the pivotal melanoma Phase 3 trial have accumulated enough events (i.e., treated enough patients) to have achieved statistically significant progression-free survival curve separation by July 2016, which is management's current guidance to the biotechnology industry and stock market of the timing of the interim assessment of efficacy and safety.
If not, not only would it be yet another example of Eric's deadline missing malady, it would harm yet again Provectus' CFO/COO Peter Culpepper's process to strengthen the company's valuation (although I wouldn't absolve Peter of not understanding and thus mis-setting expectations). If so, the trial win would be a major catalyst for the drug and Provectus' share price.
Updated (2/7/16): An earlier version of the news item above (curse you Blogger...) contained wording of mine that read "Am I wrong (the "other side of the trade"), and if so, who, what, where, when, why, how? From a top-down perspective, is my investment thesis wrong? From a bottom-up perspective, are management's processes wrong?" I removed it because the sentences generated some consternation and phone calls. My writing isn't always clear, and I continue to strive to be better. I had intended for the sentences above to be a generalized set-up to the remainder of the news item, questions asked rhetorically with no meaning given to them other than the generic process from which they derive.
When doing a valuation of a public company or another asset, one can take a top-down approach (economy down) or view, a bottom-up approach (company specifics up), or both to see what if any gap occurs between the outputs of the two approaches. In doing ongoing due diligence, I tried to analogize my process to the same. My top-down "approach" is developing and continually testing and stress testing my investment thesis. My bottom-up "approach" is to, as best as I can, given that I am not a professional biotechnology investor and only have modest life sciences industry work experience, understand Eric's various processes.
Understanding Peter's corporate development process is no big thing; his business development process does require good amounts of the industry experience he has built up over time. More than determining if Eric is right (who am I, for the most part but not that much, to judge?) is exploring and examining Eric's whos, whats, wheres, whens, whys and hows.
By writing the above and below news items, it's clear I remain anxious, from time to time because of Eric's history of missed somethings, about the timing of the interim readout. But I'm not that anxious because I found him to be clear about his subprocess, believe I understand his examples and explanation, and think I can calculate and model the situation to arrive at the answer currently given by management. I could be wrong, but I don't think or believe I am. There are two triggers of an interim assessment of efficacy and safety. One is overt, and the other is a safety valve. The former would be strongly influenced by the trial's enrollment rate. The amount of enrollment, and not enrollment rate, is relevant to the latter (in order to show either statistical significance, or a trend towards such, as it relates to the trial's primary endpoint).
Updated Clinical Trial Information (February 5, 2016)It should be very hard to trigger the prescribed event clause. If most or all of the control arm patients progress on chemotherapy, and most of the treatment arm patients achieve complete response after one or two injections of PV-10 (and thus do not progress), the study should be hard pressed to accumulate 113 events (given a trial N of 225).
The response profiles of the trial's arms should be vastly different. The design of a clinical trial assumes both arms respond equivalently (the so-called null hypothesis). The base or common response is that of the control arm, which in Provectus' pivotal melanoma Phase 3 trial's case is chemotherapy, which is dacarbazine or temozolomide, which as noted in the news item below generally yield a normal distribution of events. PV-10 does not yield a normal distribution curve of response events. It should yield a non-normal (a substantial fraction of patients in one arm do not incur progression within the projected timeframe) or unexpected (patients in one arm fare much better than predicted), left or negative skewed, distribution of events.
PV-10's effect size over chemotherapy should be very large. As a result, the alternate hypothesis (the arms respond differently) should be very different than the null hypothesis (the arms respond the same). Given the likely response profiles above, and thus effect size, a relatively smaller number of patients (compared to less effective drug compounds in their respective pivotal trials) are needed to run the trial in the first place, and a relatively smaller number of patients to meet or exceed the predicted hazard ratio.
Large effect sizes and small hazard ratios mean fewer patients required to achieve statistical significance. The hazard ratio of the trial likely has been designed to be around or about 0.545. See June 23, 2014 blog post Trial Math: Meeting the Primary Endpoint, Pt. 1 or February 12, 2015 blog post Hazard ratio, and other stat stuff. As noted in the news item below, hazard ratio (HR) essentially is the ratio of response of the control arm divided by that of the treatment arm. The smaller the HR, the larger the effect size and clinical relevance of the observed difference. If the response of Stage III melanoma patients to DTIC/TMZ (chemotherapy) is probably 4 weeks (just over one cycle) but "officially" ~6 weeks (1.5 months) from the biomedical literature's perspective, then an HR_predicted of 0.545 means the PV-10 treatment arm must achieve a response of at least 11-12 weeks (2.8-3 months). HR_actual, when the trial is completed, will be 1.5 months of PFS under chemotherapy divided by many, many months of PFS under PV-10, or potentially a very small number. That is, HR_actual should be >> HR_predicted. I believe the corollary of this would be fewer patients are required for HR_actual to equal HR_predicted.
I'm not saying I come close to understanding Eric's pivotal Phase 3 trial subprocess (of his clinical development program process). The below (and the above) is my attempt to try and overcome my own skepticism.
 |
| Click to enlarge. |
- An 18-month enrollment period,
- 12.5 patients enrolled per month (a trial N of 225),
- 9 months to enroll 112.5 (113) patients (1 event per patient to accumulate ~113 events),
- Predicted enrollment of this figure to be reached by January 2016 (a study start of April 2015),
- A predicted PFS of 2 months (the null hypothesis requires the arms to respond the same),
- 3 times the predicted PFS for the last patient in the treatment arm, and
- An interim assessment in July 2016.
Am I wrong in my understanding of or attempt to analyze and calculate this subprocess?
If not, not only would it be yet another example of Eric's deadline missing malady, it would harm yet again Provectus' CFO/COO Peter Culpepper's process to strengthen the company's valuation (although I wouldn't absolve Peter of not understanding and thus mis-setting expectations). If so, the trial win would be a major catalyst for the drug and Provectus' share price.
Updated (2/7/16): An earlier version of the news item above (curse you Blogger...) contained wording of mine that read "Am I wrong (the "other side of the trade"), and if so, who, what, where, when, why, how? From a top-down perspective, is my investment thesis wrong? From a bottom-up perspective, are management's processes wrong?" I removed it because the sentences generated some consternation and phone calls. My writing isn't always clear, and I continue to strive to be better. I had intended for the sentences above to be a generalized set-up to the remainder of the news item, questions asked rhetorically with no meaning given to them other than the generic process from which they derive.
When doing a valuation of a public company or another asset, one can take a top-down approach (economy down) or view, a bottom-up approach (company specifics up), or both to see what if any gap occurs between the outputs of the two approaches. In doing ongoing due diligence, I tried to analogize my process to the same. My top-down "approach" is developing and continually testing and stress testing my investment thesis. My bottom-up "approach" is to, as best as I can, given that I am not a professional biotechnology investor and only have modest life sciences industry work experience, understand Eric's various processes.
Understanding Peter's corporate development process is no big thing; his business development process does require good amounts of the industry experience he has built up over time. More than determining if Eric is right (who am I, for the most part but not that much, to judge?) is exploring and examining Eric's whos, whats, wheres, whens, whys and hows.
By writing the above and below news items, it's clear I remain anxious, from time to time because of Eric's history of missed somethings, about the timing of the interim readout. But I'm not that anxious because I found him to be clear about his subprocess, believe I understand his examples and explanation, and think I can calculate and model the situation to arrive at the answer currently given by management. I could be wrong, but I don't think or believe I am. There are two triggers of an interim assessment of efficacy and safety. One is overt, and the other is a safety valve. The former would be strongly influenced by the trial's enrollment rate. The amount of enrollment, and not enrollment rate, is relevant to the latter (in order to show either statistical significance, or a trend towards such, as it relates to the trial's primary endpoint).
Updated below.
PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma: Huntsman Cancer Institute, recruiting.
 |
| Click to enlarge. |
Updated (2/6/16): As of Friday's close, for the first time in 3 months (last, November 6, 2015: $0.755), seemingly almost or actually to the day, the unit price of one share of Provectus common stock (PVCT) and one tradable warrant (PVCT.WS) exceeded the unit price's public offering (June 23, 2015) price of $0.75: $0.7549.
 |
| Click to enlarge. |
Updated (2/6/16): With Huntsman's change in recruiting status for Provectus' pivotal melanoma Phase 3 trial, it's worthwhile revisiting the timing of the trial's overt Interim Assessment of Efficacy and Safety, to be performed by the study's independent review committee (IRC) when 50% of the events required for the primary endpoint (progression-free survival) have occurred (a so-called Prescribed Event Clause).
With no change to the trial's Estimated Study Completion Date (or Estimated Primary Completion Date), I continue to think (not just believe) there currently is no change to the timing of the interim assessment (in this form a so-called Prescribed Time Clause), which management previously guided as halfway between the Study Start Date and study completion. Halfway between April 2015 and October 2017 is July 2016 (primary completion would suggest June).
Management's general comments about pivotal trial designs from the summer of 2014 are paraphrased by me below:
Let's assume we design a study with 200 patients, randomized 1:1, so there will be 100 patients in each arm. We are going to assess progression-free survival (PFS) (although it doesn't matter, the math is the same for any time-to-event endpoint, such as overall survival, distant metastasis-free survival, etc.) as the primary endpoint in this hypothetical study.
Such studies are designed assuming a null hypothesis (H0, the arms have equivalent response), and are powered to detect the alternate hypothesis (Ha, the arms have different responses). Hence, enough patients are enrolled to allow detection of a difference in response at least as large as Ha with a defined statistical power. If the power is 90% and statistics are two-sided with an alpha of 0.05, this means there is a 5% chance the true response is above or below a 90% confidence interval (CI). The study meets its endpoint when the CI for response in the treatment or test arm does not overlap the response of the control or comparator arm.
When H0 and Ha are close (i.e., a small effect size of the treatment drug over the control drug) the 90% CI for the treatment arm must be small or it will overlap the control arm, and thus a large number of patients are needed. Conversely, if the effect size is anticipated to be large, a relatively small number of patients are needed (i.e., the CI of the treatment arm can be large without overlapping the control arm). The fact the example above only has 200 patients signals this study assumes a relatively large effect size.
For PFS, the difference in response described by Ha often is expressed as a hazard ratio (HR), which essentially is the ratio of response of the control arm divided by that of the test arm. The smaller the HR, the larger the effect size and clinical relevance of the observed difference.
As this hypothetical study is underway, we assume H0 prevails (i.e., both arms respond equally). For the primary endpoint, we enroll and follow patients until half of them incur the prescribed event, which for PFS would be disease progression (i.e., 100 patients are reported to have progressed). The study sponsor is not allowed to peek at the trial results (there are severe statistical penalties each time this occurs, and peeking can introduce bias into the study), so one just counts reported event until this number is reached.
Once the required 100 progressions have occurred, the data are examined (typically by an independent review committee) to ascertain what happened. The trial is a success if the necessary differential occurs in the events of the two arms (i.e., Ha is demonstrated). For PFS, assuming a normal distribution of events, this analysis would show there is at least the predicted differential in PFS for the two arms. Since there may be differences in response of actual patients in both arms versus that used to design the study in the first place, this actual differential must meet or exceed that predicted by the HR used to design the study (i.e., observed HR must be greater than or equal to predicted HR).
If there is an interim analysis, this process occurs once 50 progressions have occurred. If the results support demonstration of Ha at that point, the study can be stopped (although in some cases it might continue so that data supportive of secondary endpoints can be acquired). Conversely, if the results show there is little chance of demonstrating Ha, even at full accrual, the study is stopped for futility. It might also be stopped here if there is a safety concern evident in either arm.
If there is a non-normal distribution (e.g., a substantial fraction of patients in one arm do not incur progression within the projected timeframe) or an unexpected distribution (e.g., patients in one arm fare much better than predicted) of events, the time to accumulate the necessary number of events may be delayed. To address this possibility, the trial may be designed to trigger review of the data upon the first of (i) accumulating the necessary number of events or (ii) reaching a prescribed period of time after which the necessary number of events would be expected (such as 2 or 3 times the predicted PFS for the last patient in the treatment arm).
Consider another hypothetical study, this time with 300 patients and 2:1 randomization (i.e., 200 in the treatment arm and 100 in the control arm). Study readout is triggered when half of the patients incur the prescribed event. Thus, 150 events are needed. Because of the 2:1 randomization, 100 of these would be expected to occur in the treatment arm and 50 in the control. For an interim analysis, 75 events are needed (i.e., 50 and 25, respectively) once 150 patients have begun the trial (i.e., 100 and 50, respectively). All of the above observations apply. Again, if there is a non-normal distribution of events, the prescribed time clause might be required to trigger prompt data readout.
The above examples assume a normal distribution of events. The performance of DTIC and TMZ are well documented and generally yield a normal distribution of events. In the hypothetical time between trigger (i, prescribed event) and trigger (ii, prescribed time), it is likely the required events would occur through accumulation of events from both arms (albeit perhaps taking longer than expected). Trigger (ii) is likely to precede (i) only when there is a very unusual response in one or both of the arms. Thus, safeguards exist to compensate for non-normal or unexpected distribution of events and assure a timely readout of trial results.
Updated below, below.
H/t a regular hatter, who also in an internal medicine doctor: GNA11 Mutation in a Patient With Cutaneous Origin Melanoma: A Case Report, Patel et al., Medicine, January 2016, Volume 95, Issue 4, p e2336.
Takeaways:
- A patient had (began with) Stage IIIc melanoma in 2009. She advanced to metastatic liver cancer in 2011. New metastatic lesions in the liver and the lung appeared in 2014.
- The patient of this case report received PV-10 injections to two metastatic liver tumors in 2014 without benefit.
- It would appear she received treatment as part of Provectus' expanded Phase 1 liver trial, but not at MD Anderson, which is not a liver trial site. Participation in the company's liver trial would have permitted at most two lesions to be treated. Updated (2/4/16): Patients in the liver trial were limited to the treatment of one lesion at a time, and at most two. Thus, the patient would've had one of her two metastatic liver tumors treated (injected) with PV-10, and then later, the second would've been treated. No liver study-related patient received more than two injections of PV-10. The number of injection sites and the number of injections both need to increase, like melanoma patients now experience, until the injected lesions or tumors are completely ablated.
- The patient received a number of treatments since 2009, including:
- Surgery (2009),
- Radiation (2009?),
- Bio-chemotherapy of cisplatin, dacarbazine, interferon, interleukin-2, and vinblastine (2011),
- The combination of ipilmumab and nivolumab (2011?),
- Nivolumab (2011?),
- Surgery (2013),
- PV-10 (2014),
- IL-2 (2014),
- Pembrolizumab (2014), and
- The combination of adoptive cell therapy and IL-2 (2015).
- The authors of the case report are from MD Anderson, which is not a liver trial site. The Houston cancer center was a metastatic melanoma Phase 2 trial site, and is a compassionate use/expanded access site, a pivotal Stage III melanoma site, and a PV-10/pembrolizumab combination therapy Phase 1b trial site.
- See the hatter's observations and comments at the conclusion of this news item.
- "The obvious point of this is not to point out a PV-10 failure, but to illustrate how difficult Stage 4 melanoma is to treat, how toxic these treatments are (particularly the checkpoint inhibitors), and show the hell oncologists and patients really go through when fighting this very tough disease."
- "Why did these two doses of PV-10 fail? Who can really tell except the patient’s tumor burden at the time may have been too high, the dosing of PV-10 too low, and the extensive prior treatments (particularly the mycophenolate, an immunosuppressant which reduces CD4+ T cells) too immunocompromising to allow PV-10 to adequately work."
- "This presentation also underscores, however, how impressive Moffitt’s earlier observation of significant response rates in patients in those end-stage patients in their ongoing MOA study."
Patel et al. paper:
"Abstract: The rapid advances in the molecular biology and genetics have improved the understanding of molecular pathogenesis of v-Raf murine sarcoma viral oncogene homolog B (BRAF), feline sarcoma viral oncogene v-kit (KIT), and neuroblastoma v-Ras oncogene homolog (NRAS) mutant melanomas with the subsequent development of targeted therapeutic agents. However, only limited data are available for melanoma harboring other somatic than BRAF, KIT, and NRAS mutations. Mutations in guanine nucleotide-binding protein Q polypeptide (GNAQ) and guanine nucleotide-binding protein alpha-11 (GNA11), alpha subunits of heterotrimeric G proteins, constitutively activate mitogen-activated protein kinase (MAPK) pathway in uveal melanoma. However, there are no reports of GNA11 mutations in cutaneous melanomas.
A 48-year-old woman was diagnosed with cutaneous nodular melanoma on the left scalp. Mutation analysis of the tumor revealed a GNA11 Q209L mutation. There was no evidence of uveal melanoma or malignant blue nevus in ophthalmologic exam, imaging studies, and pathology review.
To our knowledge, this is the first case report to demonstrate cutaneous origin melanoma harboring a GNA11 Q209L mutation."
"Case Presentation: A healthy 48-year-old Caucasian woman with no significant past medical history was diagnosed with a 4.4 mm, Clark level IV, nodular melanoma without ulceration on the left scalp in August of 2009. Subsequently, she underwent a wide local excision of the primary melanoma and sentinel node biopsy in the left neck, which revealed 4 positive lymph nodes for metastatic melanoma. A left neck node dissection was performed along with a left superficial parotidectomy. Zero of 49 lymph nodes dissected contained melanoma and her final stage was classified as T4aN3M0, Stage IIIC. She received adjuvant radiation to the left neck with 30 Gray (Gy) in 5 fractions without adjuvant systemic therapy. She remained in her usual state of health, with no significant comorbidities until August of 2011, when she was found to have a new 2 cm metastatic lesion in the inferior right liver, for which she received 2 doses of biochemotherapy (cisplatin, dacarbazine, interferon, interleukin-2, and vinblastine) without significant shrinkage of the metastatic lesion. Subsequently, she received 4 doses of combination of ipilmumab (3 mg/kg) and nivolumab (1 mg/kg), followed by 4 doses of nivolumab (1 mg/kg) with near complete response. Unfortunately, the treatment was complicated by autoimmune hypophysitis, grade IV liver toxicity, and grade III colitis. The highest alanine aminotransferase (ALT) level was 1197 Units/L and aspartate aminotransferase (AST) was 727 Units/L with normal bilirubin level. Initially, her symptoms and liver enzymes improved with high-dose intravenous steroid for 2 days, followed by oral prednisone 120 mg daily. When she started steroid taper, liver enzymes were elevated again. Thus, she restarted high-dose intravenous steroid and mycophenolate 1000 mg twice a day. After 3 months of steroid taper, she was placed on a maintenance physiologic dose of steroid. She was completely off steroid after 8 months. The liver lesion remained stable until August of 2013, when she had progression on positron emission tomography-computed tomography (PET-CT) (Figure 1), for which she underwent a wedge resection with harvest of tumor infiltrating lymphocytes in November of 2013. The pathology of the resected liver lesion confirmed metastatic melanoma (Figure 1). A molecular analysis of the liver lesion revealed a GNA11 mutation with wild-type BRAF, wild-type KIT, and wild-type NRAS genes. A GNA11 mutation was detected in codon 209, exon 5 of the gene that would change the encoded amino acid from glutamine to leucine (Q209L) (Figure 2). As GNA11 mutations have been reported in uveal melanoma frequently, extensive ophthalmology exams and a magnetic resonance imaging (MRI) of the brain were performed, which revealed no evidence of uveal melanoma (Figure 3). A follow-up computed tomography (CT) of the body revealed new metastatic lesions in the liver and the lung in January of 2014, and she received intratumoral injection of intralesional Rose Bengal disodium 10% (PV-10) on a clinical trial into 2 of the metastatic liver lesions with further progression of the liver and lung lesions. Subsequently, she received 4 cycles of high-dose interleukin-2 treatment from April to July of 2014 without significant hepatotoxicity or colitis. Unfortunately, a CT scan of the body revealed progression of lung lesions and a new subcutaneous mass in the right deltoid muscle area. She started treatment with pembrolizumab in September of 2014. Due to further disease progression of lung and liver lesions and multiple new subcutaneous, peritoneal, and retroperitoneal nodules after 3 doses of pembrolizumab, she received adoptive T cell therapy with 2 cycles of high-dose interleukin-2 on a clinical trial. Her treatment was complicated by grade III hepatotoxicity ([ALT]: 510). However, the toxicity completely resolved after discontinuation of interleukin-2 without any steroid. At the time of this manuscript draft, she has stable disease status.Underlined emphasis above is mine.
Hatter comments, slightly edited by me, with my bolded and underlined emphasis (the hatter is an internal medicine doctor whose patients have included those with afflicted with cancer):
Provectus thinks the Patel et al. case report is a very good study, so much so that the company's CFO/COO Peter Culpepper tweeted [indirectly] its addition to Provectus' list of Additional Peer Reviewed Literature (indirect) mentions of PV-10.
Provectus' position on the case report comprises the following:
12th Annual International Symposium on Melanoma and Other Cutaneous Malignancies (February 3, 2016)"This article is a detailed case presentation of a 48-year old woman, who originally presents with a scalp melanoma in 2009, who then proceeds to receive what’s been considered the “standard of care” over the years up to the present. Interestingly, she received intratumoral PV-10 into two metastatic liver lesions in 2014 without benefit. The obvious point of this is not to point out a PV-10 failure, but to illustrate how difficult Stage 4 melanoma is to treat, how toxic these treatments are (particularly the checkpoint inhibitors), and show the hell oncologists and patients really go through when fighting this very tough disease.
Why did these two doses of PV-10 fail? Who can really tell except the patient’s tumor burden at the time may have been too high, the dosing of PV-10 too low, and the extensive prior treatments (particularly the mycophenolate, an immunosuppressant which reduces CD4+ T cells) too immunocompromising to allow PV-10 to adequately work. This presentation also underscores, however, how impressive Moffitt’s earlier observation of significant response rates in patients in those end-stage patients in their ongoing MOA study. I think people forget that behind all these drug trials, recruitment of patients and variable responses with many adverse side effects is real human suffering and life and death consequences to decisions patients and their doctors make."Updated (2/5/16): A further comment from the hatter, slightly edited by me:
"There should be a better appreciation of what Stage 4 melanoma is and means, a better understanding of what PV-10 is really up against [when treating this disease stage], and how the drug will be measured in trials in the future."Regarding uveal melanoma survival rates: "Five-year survival rates for uveal melanoma ranges from 69% to 81.6% and ten-year survival rates from 57% to 62%. After detection of metastases 80% of patients die within 1 year, and 92% within 2 years." The patient should be dead, based on survival statistics for this disease.
Provectus thinks the Patel et al. case report is a very good study, so much so that the company's CFO/COO Peter Culpepper tweeted [indirectly] its addition to Provectus' list of Additional Peer Reviewed Literature (indirect) mentions of PV-10.
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source |
- Management does not plan to comment on unpublished details of specific patients in the ongoing Phase 1 liver trial.
- Provectus is very gratified that at the time of the publication of this case report, this patient, with an aggressive type of refractory melanoma, was still alive with stable disease.
- According to the case report, this patient progressed after surgery, radiation, chemotherapy, IL-2, ipiimumab, nivolumab, pembrolizumab, and PV-10. Her last treatment was adoptive T-cell therapy. The majority of these treatments were given in clinical trials.
- This case report illustrates the importance of clinical trial participation and MD Anderson’s important work in banking and evaluating patient tumor biopsies to ascertain genetic mutations.
- There is no prohibition to the enrollment of melanoma patients with this gene signature in any of Provectus' open clinical trials.
- This is why Provectus is working so hard -- in the hopes that a patient may benefit in one of the company's open clinical trials, or if they progress, PV-10 intervention will not jeopardize further treatment options.
H/t InvestorVillage poster canis_star: NYU Langone Medical Center's Dr. Jeffrey Weber, MD, PhD on topics to be covered at the above mentioned conference, including PV-10 (comments starting at 0:30).
Mechanics of the Process (February 2, 2016)
Updated below, again, once more.
Provectus currently is undertaking a Phase 1b study of the combination of intralesional agent (IL) PV-10 ("turn on the immune system's engine," "step on the immune system's gas pedal") and checkpoint inhibitor pembrolizumab/Keytruda ("release its brakes") in patients with Stage IV melanoma. The trial is entitled PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma ("PV-10/pembro"). As of this writing, one site is recruiting (St Luke's University Hospital and Health Network in Easton, Pennsylvania) and two currently are not (MD Anderson Cancer Center in Houston, Texas and Princess Alexandra Hospital in Brisbane, Australia) are not. The enrollment goal is for up to 24 patients. I have previously written that I believe the current enrollment estimate for the Phase 2 study portion could be less than 120 patients. See "FPFV" (January 12, 2016) below.
Generally speaking, while all combination trials would be of interest in terms of comparing their pairings' safety and efficacy, at least two are of particular note [to me]: the combination of IL agent T-Vec/Imylgic and checkpoint inhibitors (i) ipilimumab/Yervoy and (ii) pembrolizumab in patients with Stage III/IV melanoma. See Ipilimumab With or Without Talimogene Laherparepvec in Unresected Melanoma ("T-Vec/ipi") and Pembrolizumab With or Without Talimogene Laherparepvec or Talimogene Laherparepvec Placebo in Unresected Melanoma ("T-Vec/pembro"), respectively. These trials are of particular interest because I believe the set-up of the Amgen/Bristol-Myers and Amgen/Merck & Co. trials provides some insight into Provectus'.
T-Vec/ipi, which "began" in November 2012 when the trial protocols (Phase 1b/2) initially were filed, reported preliminary data at ASCO 2014, Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma, and 2015, Survival, safety, and response patterns in a phase 1b multicenter trial of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma.
Safety and efficacy data were presented on 17 assessed/evaluable patients (19 were enrolled, of which 18 were treated). Investigators from 6 clinical sites comprised non-company ASCO 2014 and 2015 poster authors. Data cut-off dates were October 15, 2013 and December 22, 2014 respective. A trials-in-progress poster at ESMO 2014, A phase 1b/2, multicenter, open label trial to evaluate the safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) versus ipi alone in previously untreated, unresected stage IIIB, IIIC, and IV melanoma, included in its authorship line investigators from 13 sites. The ESMO poster also noted the Phase 2 portion of the Phase 1b/2 opened in August 2013.
Projected enrollment of T-Vec/ipi's Phase 2 trial was increased from 140 to 200 (in December 2014). Randomization is 1:1. 40 sites are recruiting for this trial.
T-Vec/pembro, which "began" in September 2014, reported preliminary data at SMR 2015, Primary analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. A trials-in-progress poster was presented at SITC 2015, which preceded SMR by a few weeks. Safety and efficacy data were presented on 16 assessed/evaluable patients (21 were enrolled). Investigators from 14 clinical sites comprised non-company SITC poster authors.
The Phase 3 portion of T-Vec/pembro has not yet begun recruiting. Projected enrollment is 660. Randomization is 1:1. As background and context, T-Vec's pivotal melanoma Phase 3 trial recruited 436 patients (2:1 randomization).
Takeaways & Questions:
- Provectus very likely won't (won't need to) recruit 24 patients into its Phase 1b trial, and maybe some number closer to 16 or 17.
- The second leg (Phase 2 or 3) of a combination study can begin before data from the first leg (Phase 1b) is presented.
- Is St. Luke's exclusively recruiting patients for PV-10/pembro's Phase 1 trial, leaving MD Anderson, Princess Alexandra and others to join it when the Phase 2 trial commences?
- Trials have large Ns (patient enrollment and treatment number) to ensure the treatment arms are sufficiently big enough size-wise to top control arms when it comes to meeting/beating primary endpoints.
- T-Vec/ipi increased its N.
- T-Vec/pembro's N is 3.3 times T-Vec/ipi's. Pembro is of course standard of care for Stage IV melanoma.
- Did Provectus' CTO Dr. Eric Wachter, PhD revise his Phase 2 study enrollment (1:1 randomization) from at least (>) 120 patients to no more than (<) 120? If so, why? I assume he's seen some combination study data.
Updated (2/2/16): Provectus' CFO/COO Peter Culpepper has the habit (more out of necessity than choice, if you know and understand Eric and certain behaviors of his) of distributing actual, potential, possible, less than useful, etc. breadcrumbs via the company's investor presentation available on Provectus' website. These crumbs typically come from interactions with Eric and, thus, back in the day, information contained in medical conference presentations that may or may not have made its way to see the light of day. See, for example, November 5, 2013 blog post Expect More.
While I might think there are more effective and efficient ways to communicate such and other critical information to shareholders and the stock market that would add to/enhance rather than detract from management's credibility, we have what we have by way of this aspect or facet of Peter and Eric's "process." I'll take at face value that Peter is striving to more accurately state matters.
H/t InvestorVillage poster Juggernaut: A comparison of two slides in the most recent version of Provectus' investor presentation on the company's website (as of this writing, February 2nd) (right below) and Peter's January 13th Biotech Showcase 2016 presentation (left below) follows:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Question:
- How far advanced is Provectus' Phase 1b/2 study of PV-10 in combination with pembro for Stage IV melanoma?
Updated (2/3/16): Provectus updated its investor presentation on the company's website again.
Updated (2/3/16): InvestorVillage poster canis_star noted yesterday (timestamp ~11:43 am EDT) that Provectus' expanded Phase 1 liver trial on ClinicalTrials.gov, A Study to Assess PV-10 Chemoablation of Cancer of the Liver, had an "Unknown" status.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Screenshot of Original vs. Changed. |
 |
| Click to enlarge. Screenshot of current. |
Changes in blog readership statistics were all positive in January 2016. Month-over-month (January 2016 v. December 2015) percent differences included:
- +34% for # of unique visitors (2,259 v. 1,692),
- +60% for # of page views (37,297 v. 23,313),
- +54% for # of visits (10,834 v. 7,047),
- +11% for # of U.S. cities from where visitors came (608 v. 549),
- +70% for # of world cities (143 v. 84),
- +25% for # of countries (55 v. 44), and
- +52% for # of blog posts and news items (32 v. 21).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
The correlations between monthly percent changes in the number of (a) blog posts and news items and (i) unique visitors, (ii) page views and (iii) visits are somewhat positive (all data: 21-31%; 2015: 26%-63%). Correlations for all data (2012 to 2016 year-to-date [YTD]) and 2015 are below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Following up on a readership statistics topic from 3Q15 -- see One month does not a trend make, but.../July Blog Stats (August 1, 2015) and Tuesday (September 1, 2015) from the blog's Archived IV News page -- the step function-esque increase in pages per session and decrease in bounce rate that began on July 9th (around the time of the first communication of Phase 1 liver trial data and the collaboration announcement between Provectus and Boehringer Ingelheim [China]) continued into January. See selected blog statistics graphs from Google Analytics below:
 |
| Click to enlarge. Pages / Session (Pages per session) |
 |
| Click to enlarge. Bounce Rate |
H/t a regular hatter (also an internist, entrepreneur and shareholder).
 |
| Click to enlarge. |
- Dyes have roles in the aetiology, diagnosis, and treatment of cancer.
- Synthetic dyes particularly coal tar dyes can be carcinogenic.
- Dyes are used to identify cancer, and are especially useful in intra-operative imaging.
- Dyes inspired the birth of modern chemotherapy, and can be used as chemo-ablative agents.
- Theranostics is a new field where the same agent (such as a dye) can be used for both diagnosis and therapy.
"One compound that retains its roots as a true dye is Rose Bengal, a deep rosey-red dye derived from fluorescein. Initially used as a wool dye, it was then used medically to treat ocular infections and as an eye stain, and subsequently in liver function assays. Its association with cancer began serendipitously, when safety tests evaluating its carcinogenicity found that Rose Bengal paradoxically prolonged the survival of mice with cancers. Subsequently the selective chemo-toxicity of Rose Bengal towards cancer cells was confirmed, and recently it has been used to treat a variety of human cancers such as advanced unresectable metastatic melanoma resistant to current treatments, as well as refractory scalp sarcoma. Additional trials are underway for other cancers including breast and liver. Rose Bengal preferentially accumulates in the lysosome of cancer cells with subsequent release of lysosomal enzymes, triggering rapid autophagy. Interestingly, non-treated “bystander” lesions also respond to treatment due to activation of a systemic tumour-specific immune response by lysed cells from the treated lesions.Co-author Dr. Susan Neuhaus, MD (University of Adelaide and Royal Adelaide Hospital) was a clinical investigator in Provectus' metastatic melanoma Phase 2 trial. See Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma.
Also see “Most drugs are really German dyes” (August 3, 2015) on the blog's Archived IV News page: Underlined emphasis below is mine. In an August 3rd article by Forbes.com's Matthew Herper entitled Lexicon's Quest For A New Blockbuster, he quotes Brent Stockwell, a scientist at the MIT-affiliated Whitehead Institute for Biomedical Research (who now appears to be at Columbia University):
"In a sense, the pharmaceutical industry has been working with a surprisingly repetitive set of compounds. “Most drugs are really German dyes. That’s because they’re based on what has worked before–mostly compounds that evolved from drugs developed at dye companies like F. Bayer & Co. a century ago."Breadcrumbs (January 27, 2016)
Updated below, again.
"In Asia, PV-10 is seen as the ideal intralesional agent for liver cancer, and it comes back to the bystander effect. What we are seeing so far is that when we ablate the lesion in the liver—and it’s not just primary liver cancer, but any cancer metastatic to the liver—we can ablate the tumour directly, and are seeing evidence of immunologic signalling. We are tracking that now, with biomarkers in the current liver study, and will be tracking that in the Ib/II." {Underlined emphasis is mine}
Moffitt Cancer Center's clinical work on Rose Bengal, Detection of Immune Cell Infiltration Into Melanomas Treated by PV-10, a Feasibility Study, has the goals of ascertaining the feasibility of detecting immune cell infiltrates in injected melanoma tumors and also measuring circulating immune cells in peripheral blood post-PV-10 treatment (see also Provectus' website for this work's description).
Reference for the below is Immune Responses to Cancer: Are They Potential Biomarkers of Prognosis? by Whiteside (Front Oncol. 2013; 3: 107.). Underlined emphasis below is mine.
1. "Human solid tumors are nearly always infiltrated by immune cells." (Whiteside)
2. "Typing of tumor-infiltrating T lymphocyte (TIL) cells by immuno-histochemistry (IHC) and microscopic enumeration of these cells have been initially utilized to establish correlations between CD3+CD8+ T-cell infiltrations and prognosis (Naito et al., 1998)" [i]
3. ''In addition to scoring T cells at tumor sites, the frequency and functions of T cells circulating in the peripheral blood of cancer patients have been examined as potential biomarkers."
[i] Naito et al.: CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58, 3491–3494
Takeaways: Moffitt had identified infiltration early on in their murine (mouse) model work, when a 2014 press release by Provectus highlighting the cancer center's presented work at AACR 2014 noted: "PV-10 treatment of B16 tumors in mice also led to infiltration of dendritic cells into the lymph nodes draining the treated tumors; no infiltration was observed in non-draining nodes." -- See 1. above.
The same release also noted: "The researchers showed that these changes in tumors were accompanied by increased populations of CD3+, CD4+ and CD8+ T cells along with NKT cells in peripheral blood." -- See 2. above.
The question then would be, has Moffitt identified a biomarker for PV-10, and if so what is it? -- See 3. above.
Orthogonality. A theme I would expect Provectus to highlight more this year is PV-10's orthogonality with other drugs. Orthogonal refers to the the idea of perpendicular, non-overlapping, independently varying or uncorrelated items. I believe PV-10 orthogonality [to other drugs] relates to both safety and efficacy.
I was reminded of this because of Monday's Relypsa press release Relypsa Announces Results From Veltassa Drug-Drug Interaction Studies in Healthy Volunteers.
Orthogonality in regards to PV-10 and safety would suggest that combining PV-10 with another drug does not lead to greater or increased toxicity.
Orthogonality in regards to PV-10 and efficacy would suggest that combining PV-10 with another drug leads to greater or increased efficacy where all disease is not accessible to (cannot be injected with) Rose Bengal.
The relevant foundational work was discussed by Provectus in its January 28, 2014 press release Announces PV-10's Assessment for Drug-Drug Interaction Potential is Subject of Article Published by Xenobiotica: The byline was "Demonstrates Risk of Clinically Relevant Drug-Drug Interactions with Rose Bengal is Low."
Key statements included:
Updated (1/27/16): A new tag line. H/t a shareholder: Provectus has a new tag line, adding to "Advancing A New Front In The War Against Cancer" with and trademarking:Takeaways: Moffitt had identified infiltration early on in their murine (mouse) model work, when a 2014 press release by Provectus highlighting the cancer center's presented work at AACR 2014 noted: "PV-10 treatment of B16 tumors in mice also led to infiltration of dendritic cells into the lymph nodes draining the treated tumors; no infiltration was observed in non-draining nodes." -- See 1. above.
The same release also noted: "The researchers showed that these changes in tumors were accompanied by increased populations of CD3+, CD4+ and CD8+ T cells along with NKT cells in peripheral blood." -- See 2. above.
The question then would be, has Moffitt identified a biomarker for PV-10, and if so what is it? -- See 3. above.
Orthogonality. A theme I would expect Provectus to highlight more this year is PV-10's orthogonality with other drugs. Orthogonal refers to the the idea of perpendicular, non-overlapping, independently varying or uncorrelated items. I believe PV-10 orthogonality [to other drugs] relates to both safety and efficacy.
I was reminded of this because of Monday's Relypsa press release Relypsa Announces Results From Veltassa Drug-Drug Interaction Studies in Healthy Volunteers.
 |
| Click to enlarge. Image source. Red underlining is mine. |
Orthogonality in regards to PV-10 and efficacy would suggest that combining PV-10 with another drug leads to greater or increased efficacy where all disease is not accessible to (cannot be injected with) Rose Bengal.
The relevant foundational work was discussed by Provectus in its January 28, 2014 press release Announces PV-10's Assessment for Drug-Drug Interaction Potential is Subject of Article Published by Xenobiotica: The byline was "Demonstrates Risk of Clinically Relevant Drug-Drug Interactions with Rose Bengal is Low."
Key statements included:
- "The published research indicated that the risk of PV-10 causing clinically relevant drug-drug interactions is likely minimal."
- "Sorafenib is a competitive inhibitor of cytochrome P450 (CYP) drug metabolism enzymes and is reliant on the UDP-glucuronosyltransferase (UGT) pathway for efficient clearance. CYP and UGT enzymes help to biotransform small lipophilic drugs like sorafenib into water-soluble excretable metabolites."
- "As we discuss our clinical results with regulatory authorities, we continue to be intensely committed to building all sections of the prescribing information for a future package insert for PV-10."
Since this pre-clinical combination of PV-10 and targeted therapy sorafenib, Moffitt presented work at SITC 2014 about the combination of PV-10 and three categories of immunotherapies (anti-CTLA-4, anti-PD-1 and anti-PD-L1): "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma."
Takeaway: Provectus has shown, itself and via Moffitt, that PV-10 is orthogonal to targeted therapies and immunotherapies.
Agnostic. Another theme related to the "diversity" of PV-10, besides orthogonality (where PV-10 works with multiple drugs), is PV-10's agnosticism to solid tumor cancers. The pre-clinical and clinical dataset of PV-10 ablating a cancerous tumor or lesion continues to grow, while also demonstrating the drug's agnosticism to disease presentation: e.g., clinically in potentially different melanomas (mutations or variations, but lesions don't appear to be categorized by Provectus), hepatocellular carcinoma (Hepatitis B and C, cirrhosis), breast cancer, and liver metastases (colorectal, non-small cell lung, melanoma, ovarian), and pre-clinically in at least melanoma, liver, renal, breast and pancreatic tumors. Destroying the tumor -- ablating it -- is the first prong of PV-10's two-prong approach to fighting cancer. PV-10's ablation is agnostic to indication; it destroys all manner (indication, presentation) of solid tumor cancer.
But, as I wrote below under Doppio Espresso Con Panna (January 21, 2016), InvestorVillage poster canis_star today said it better and more precisely in regards to the mechanism of immune action work undertaken by Moffitt Cancer Center (pre-clinical and clinical) and the University of Illinois at Chicago (UIC) (pre-clinical) -- the second prong [the immune response prong] of PV-10's two-prong approach to fighting cancer: "Both research labs independently showed that PV-10 induce the immunogenic cell death (ICD) in multiple cancer[s]..."
Takeaway: Provectus has shown, via Moffitt and UIC, that PV-10 is agnostic to cancer indication (it is an equal opportunity destroyer of cancer), by (i) destroying all manner of solid tumor cancer when injected into it and (ii) generating the type and kind immune response as a result.
Big Pharma. Someone(s) from the headquarters of a Big Pharma has regularly visited the blog beginning on Monday, January 11, the start of the annual JP Morgan Healthcare Conference (January 11th to 14th). As of this writing, they'd have dropped in eight of 17 days, averaging 2.5 visits per day (median = 2). They hadn't visited the blog in some time prior to these visits. It's quite possible these are horribly bored employees. Or maybe they're waiting for something?
I'm all about that combo. The addition of ASCO 2016 to Provectus' Calendar of Events for June*, in my view, would continue to lend credence to the rumor that, pending data availability, trial investigators and the company would present initial preliminary Phase 1b study data of PV-10 in combination with pembrolizumab (Keytruda) in Stage IV melanoma patients. See "FPFV" (January 12, 2016) below.
* H/t InvestorVillage poster Juggernaut.
innovative or groundbreaking. H/t a regular hatter: At least one Provectus non-physician vendor/consultant with what appears to be substantial and substantive oncology therapy/therapeutic experience appears to have termed at least PV-10's application to liver cancer as "disruptive." While I share the same view of course, it was interesting (and, in no small part, funny) to observe the descriptor.
You know that we are living in a systemic world. I was reminded of the following when I read about the FDA placing a partial clinical hold on a Medivation trial while the company tries to better explain the trial compound's mechanism of action. The drug compound, pidilizumab, was thought to have been an anti-PD-1 agent, like Merck's Keytruda and Bristol-Myers' Opdivo (nivolumab) -- but it appears Medivation's compound does not inhibit PD-1 (and, hence, is not an anti-PD-1 agent).
I wonder how much of a role, if any, did Moffitt's mechanism of action work on PV-10 in melanoma play in the value proposition and thinking of Provectus' Phase 1b/2 combination study with Keytruda in Stage IV disease with the FDA. First, at AACR 2013, there was Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma. Second, at SITC 2014, there was Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma. Third and finally, at SITC 2015, there was Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1.
In a similar vein, if you buy the above, a question, what role, if any, does UIC's mechanism of action/mechanism of immune action work in colon cancer play, and for what clinical study? One of the early patients in Provectus' liver Phase 1 trial had a colorectal cancer tumor on his or her liver. The tumor (at the secondary site of/on the liver) presumably was ablated. But, what happened to the cancer at the primary site of the colon?
Breast cancer. I believe Provectus is trying to advance work on PV-10 in breast cancer, in addition to Peter's previous comments on the topic a while back. The company's CTO Dr. Eric Wachter, PhD's comfort zone and process sees him utilize trial investigators, medical institutions, contract research organizations, etc. from Provectus' melanoma work in other indication areas. For example, Sharp Memorial Hospital's Dr. Paul Goldfarb, MD was the lead investigator of the first six patients in the company's liver Phase 1 trial. Sharp's Dr. Kristen Rice, MD is an investigator in Provectus' pivotal melanoma Phase 3 trial. Sharp was a compassionate use program (CUP) site.
Note Dr. Goldfarb's breast cancer-related work from his LinkedIn bio:
Dr. Rice has personal and professional interest in breast cancer, noting the below from her Sharp bio:
The above is aside from Moffitt's preclinical work on breast cancer, Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma. It is possible, under Eric's approach and process, that Sharp Memorial Hospital and Moffitt Cancer Center are two places where breast cancer work is being done, or would be done.
GI cancer. I'm going to guess we may eventually hear Northwestern University and Dr. Riad Salem, MD are involved with PV-10 and one or more of colon cancer, colorectal cancer, liver cancer or rectal cancer.
China & cancer rates. From the South China Morning Post today, Cancer in China kills more than 7,500 people a day, new figures show. The source material is research by Chen et al., Cancer Statistics in China, 2015, CA: A Cancer Journal for Clinicians, 2016. Some examples of PV-10 solid tumor cancer applicability is noted below in fuzzy blue emphasis.
Takeaway: Provectus has shown, itself and via Moffitt, that PV-10 is orthogonal to targeted therapies and immunotherapies.
Agnostic. Another theme related to the "diversity" of PV-10, besides orthogonality (where PV-10 works with multiple drugs), is PV-10's agnosticism to solid tumor cancers. The pre-clinical and clinical dataset of PV-10 ablating a cancerous tumor or lesion continues to grow, while also demonstrating the drug's agnosticism to disease presentation: e.g., clinically in potentially different melanomas (mutations or variations, but lesions don't appear to be categorized by Provectus), hepatocellular carcinoma (Hepatitis B and C, cirrhosis), breast cancer, and liver metastases (colorectal, non-small cell lung, melanoma, ovarian), and pre-clinically in at least melanoma, liver, renal, breast and pancreatic tumors. Destroying the tumor -- ablating it -- is the first prong of PV-10's two-prong approach to fighting cancer. PV-10's ablation is agnostic to indication; it destroys all manner (indication, presentation) of solid tumor cancer.
But, as I wrote below under Doppio Espresso Con Panna (January 21, 2016), InvestorVillage poster canis_star today said it better and more precisely in regards to the mechanism of immune action work undertaken by Moffitt Cancer Center (pre-clinical and clinical) and the University of Illinois at Chicago (UIC) (pre-clinical) -- the second prong [the immune response prong] of PV-10's two-prong approach to fighting cancer: "Both research labs independently showed that PV-10 induce the immunogenic cell death (ICD) in multiple cancer[s]..."
Takeaway: Provectus has shown, via Moffitt and UIC, that PV-10 is agnostic to cancer indication (it is an equal opportunity destroyer of cancer), by (i) destroying all manner of solid tumor cancer when injected into it and (ii) generating the type and kind immune response as a result.
 |
| Click to enlarge. |
I'm all about that combo. The addition of ASCO 2016 to Provectus' Calendar of Events for June*, in my view, would continue to lend credence to the rumor that, pending data availability, trial investigators and the company would present initial preliminary Phase 1b study data of PV-10 in combination with pembrolizumab (Keytruda) in Stage IV melanoma patients. See "FPFV" (January 12, 2016) below.
* H/t InvestorVillage poster Juggernaut.
innovative or groundbreaking. H/t a regular hatter: At least one Provectus non-physician vendor/consultant with what appears to be substantial and substantive oncology therapy/therapeutic experience appears to have termed at least PV-10's application to liver cancer as "disruptive." While I share the same view of course, it was interesting (and, in no small part, funny) to observe the descriptor.
You know that we are living in a systemic world. I was reminded of the following when I read about the FDA placing a partial clinical hold on a Medivation trial while the company tries to better explain the trial compound's mechanism of action. The drug compound, pidilizumab, was thought to have been an anti-PD-1 agent, like Merck's Keytruda and Bristol-Myers' Opdivo (nivolumab) -- but it appears Medivation's compound does not inhibit PD-1 (and, hence, is not an anti-PD-1 agent).
I wonder how much of a role, if any, did Moffitt's mechanism of action work on PV-10 in melanoma play in the value proposition and thinking of Provectus' Phase 1b/2 combination study with Keytruda in Stage IV disease with the FDA. First, at AACR 2013, there was Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma. Second, at SITC 2014, there was Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma. Third and finally, at SITC 2015, there was Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via High Mobility Group Box 1.
In a similar vein, if you buy the above, a question, what role, if any, does UIC's mechanism of action/mechanism of immune action work in colon cancer play, and for what clinical study? One of the early patients in Provectus' liver Phase 1 trial had a colorectal cancer tumor on his or her liver. The tumor (at the secondary site of/on the liver) presumably was ablated. But, what happened to the cancer at the primary site of the colon?
Breast cancer. I believe Provectus is trying to advance work on PV-10 in breast cancer, in addition to Peter's previous comments on the topic a while back. The company's CTO Dr. Eric Wachter, PhD's comfort zone and process sees him utilize trial investigators, medical institutions, contract research organizations, etc. from Provectus' melanoma work in other indication areas. For example, Sharp Memorial Hospital's Dr. Paul Goldfarb, MD was the lead investigator of the first six patients in the company's liver Phase 1 trial. Sharp's Dr. Kristen Rice, MD is an investigator in Provectus' pivotal melanoma Phase 3 trial. Sharp was a compassionate use program (CUP) site.
Note Dr. Goldfarb's breast cancer-related work from his LinkedIn bio:
 |
| Click to enlarge. Image source. Blue emphasis is mine. |
 |
| Click to enlarge. Image source. Blue emphasis is mine. |
GI cancer. I'm going to guess we may eventually hear Northwestern University and Dr. Riad Salem, MD are involved with PV-10 and one or more of colon cancer, colorectal cancer, liver cancer or rectal cancer.
China & cancer rates. From the South China Morning Post today, Cancer in China kills more than 7,500 people a day, new figures show. The source material is research by Chen et al., Cancer Statistics in China, 2015, CA: A Cancer Journal for Clinicians, 2016. Some examples of PV-10 solid tumor cancer applicability is noted below in fuzzy blue emphasis.
 |
| Click to enlarge. page 6 of image source |
 |
| Image source |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Coverage gap? (January 25, 2016)
Updated below, again.
Maxim's coverage universe as at December 7, 2015:
Coverage as at January 4, 2016, the most recent list from the firm's website (as of this writing):
Updated (1/25/16): While I believe the drop in coverage (i.e., Provectus not being listed as a name in Maxim's coverage universe) is temporary and potentially related to ongoing tender offer (i.e., holders of non-tradable warrant of varying exercise prices having the opportunity to exercise these warrants for $0.75 [irrespective of their original exercise price] and receive a tradable one as a replacement) and Maxim's position as lead dealer manager of the tender, the greater question in my mind is whether the investment bank will merely add Provectus' name to the coverage list or re-initiate coverage with a substantive research note on the company.
Maxim's 2015/2016 version of their relationship with Provectus comprises:
Updated (1/25/16): I believe I understand the importance of equity research coverage to a public company, the value of a good to great analyst both to the target company and to his or her firm, the historical challenge of research and analyst independence, etc. I also understand the role a research analyst and his or her firm can play as a sponsor of a research-covered (client) company (e.g., "pound the table," defender of the share price, price targets, etc.).
I also understand Provectus management's decision making by choice at times and by necessity at others does not follow most algorithms of valuation building. What I wrote in September 2013 still stands, both by observation and in regards to support:
And, at this point, it's not that big of a question. As I wrote above, I was hoping for a check-the-box moment, but in reality the exercise is part of a question/determination of whether management is up to the task of maximizing asset value of Provectus at the end game.
The last Maxim research note was provided by former analyst Dr. Echo He, PhD in February 2014, but only to institutional clients. See Lavender Flowers under Potpourri (May 1, 2015) on the blog's Archived III News page. Historical hearsay would suggest Maxim's Jason Kolbert, Head of Healthcare Research and Senior Biotechnology Analyst, does not like Provectus' CFO/COO Peter Culpepper and does not think much of PV-10.
Neither observation or opinion, if true, matter to me. It's not like I think Kolbert, or the median Wall Street analyst, is exactly principled in how he or she undertakes their work at a sell-side investment bank. I'd say I already conveyed my view of the situation under Extension(s) (December 9, 2015) on the blog's Archived IV News page.
Advancing (January 24, 2016)
Previous related blog news item on the blog's Archived News IV page:
Revisiting BTD (January 23, 2016)
When the FDA denied Provectus' breakthrough therapy designation ("BTD") in May 2014 for patients with locally advanced cutaneous melanoma (Stage III disease), the Agency noted its decision was based on a paucity of data:
At the time, I wrote:
I thought it was worthwhile revisiting the topic of BTD because of the question by some at the time (understanding the "why" matters, and understanding why someone doesn't comment on the "why" matters too) why Provectus publicly disclosed the contents of the FDA's BTD denial letter. Carroll wrote:
What spurred me to write this blog news item was finding the following comment in Kidney Cancer Journal, Winter 2014, Volume 12, Number 4 (see page 90):
Provectus undertook a post hoc analysis of its previously completed metastatic melanoma Phase 2 trial data to establish an association between between objective response ("OR," which equals complete response [CR] + partial response [PR]) rate ("ORR") and patient symptoms. Post hoc means "occurring or done after the event." This guidance was noted in the company's January 24, 2014 PR, PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes:
But, in truth, while there were no requirements for measuring symptom data at enrollment, Provectus had data on less than 13 patients in the subgroup of patients with all disease treated (n = 28, trial N = 80) that presumably formed the basis for its BTD application. From ASCO 2014, Efficacy of intralesional rose bengal in patients receiving injection in all existing melanoma in phase II study PV-10-MM-02:
On the other hand, Amgen's T-Vec's pivotal melanoma Phase 3 trial collected quality of life data, on which the FDA requested Amgen to undertake a post hoc analysis to determine if there was an association between durable response, T-Vec's Phase 3 trial's primary endpoint, and quality of life data. During the FDA's Cellular, Tissue, and Gene Therapies Advisory Committee and
Oncologic Drugs Advisory Committee meeting on April 29, 2015 that would form the basis for approving Amgen's talimogene laherparepvec (Imylgic), Amgen-submitted materials noted:
Patient-reported outcome started out as a secondary endpoint in Provectus' pivotal Stage III melanoma Phase 3 trial. See August 10, 2014 blog post Juxtaposition. It later was moved to an exploratory one.
Takeaways:
1. Immunogenic cell death. I think InvestorVillage poster canis_star today said it better and more precisely in regards to the mechanism of immune action work undertaken by Moffitt Cancer Center (pre-clinical and clinical) and the University of Illinois at Chicago (UIC) (pre-clinical): "Both research labs independently showed that PV-10 induce the immunogenic cell death (ICD) in multiple cancer[s]..."
2. Meeting expectations. Related to the aforementioned mechanism of action work, Provectus' Dr. Erich Wachter, PhD set an expectation on the company's November 5th 3Q15 business update conference call: "We are also continuing to sponsor mechanism studies at Moffitt and the University of Illinois at Chicago, and anticipate further data from both groups to be released in coming months." In Below expectations. under Cheers (January 1, 2016) below, I had written "The benefit of the doubt pushes this expectation into at least very early-2016." With the UIC ASC 2016 abstract "PV-10 Induces Potent Immunogenic Apoptosis in Colon Cancer Cells," I think he's begun to meet this expectation.
3. Arm's length. Does the company derive or get "credit" for the arm's length and independently reproduced work undertaken by Moffitt and UIC?
Biotechnology and pharmaceutical companies produce and publish non-clinical work exclusively of their employees:
4. PV-10 + radiation. The discussion about immunogenic cell death, which can be caused by radiation, makes me wonder about the results of the investigator-initiated trial combining PV-10 and radiotherapy. Eric, from the November 2015 3Q15 conference call: "We also continued to work with the team in Australia conducting the investigator initiated trial of PV-10 in combination with radiation to report ongoing results in a suitable venue."
5. Not currently compelling. With closing prices for the common stock and tradable warrant today of ~$0.39 and $0.25, respectively (for a total unit price of ~$0.64), I am hard pressed, tonight, to see a compelling reason or reasons for holders of non-tradable warrants to tender. Yet, if this is to be believed, according to a source who normally would not have knowledge of the situation, but presumably spoke to someone who does and gain said or such knowledge, approximately 3-4 million warrants already have been tendered.
"We will sell no wine before its time." (January 21, 2016)
On January 27, 2011, Provectus issued press release Completes Patient Accrual and Treatment for Phase 1 Trial of PV-10 for Liver Cancer, noting:
Prior the January 2011 PR, the company had treated the first three liver cancer patients by September 14, 2010, Completes Initial Enrollment in Phase 1 Trial of PV-10 for Liver Cancer.
Bell et al. note in Cancer Survivorship: Why Labels Matter (JCO February 1, 2013 vol. 31 no. 4 409-411):
In April 2015 St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala, MD observed the following about clinical liver overall survival data:
Seven of 10 patients were alive after up to 50 months. Of the 10 patients, 6 patients had hepatocellular carcinoma (HCC, or primary liver cancer) and 4 had metastatic liver cancer (liver mets, or secondary liver cancer). The causes of the three deaths were noted at the bottom of Dr. Argawala's second slide above. See Initial Clinical Testing: HCC and Liver Mets (April 10, 2015) and "Oops" (April 10, 2015) on the blog's Archived News III page.
Going way back to earlier versions of Provectus' investor presentation, it appeared the make-up of the first six liver Phase1 trial patients comprised five HCC and one liver mets patients (based on the assumption of one treated tumor per patient).
In September 2012 the liver Phase 1 study was expanded to treat up to 24 additional patients with HCC or liver mets (Expansion Cohort 1) and up to 12 patients with HCC who were on a stable dose of sorafenib, a standard treatment for HCC (Expansion Cohort 2). The PR was Expands Protocol for Phase 1 Liver Cancer Study. A running tally of the trial could look like the table below.
In July 2015 data on 13 patients this time were presented at Barcelona and Osaka. See ESMO World GI Poster (July 1, 2015) and Ostensibly The Same (July 6, 2015) on the blog's Archived News IV page.
10 of 13 patients were alive after up to 54 months. The calendar difference between Agarwala's April presentation (or when the data in his presentation were tallied) and Provectus' July presentations could be 3-4 months. Three more patients, same number of deaths, no change in the causes of deaths.
I assume, because there was no mention of sorafenib in any of the three presentations (particularly the two presentations at the two medical conferences), that none of the 4-7 patients above the initial 6 were on stables doses of sorafenib. Thus, now, the 7 additional patients were part of Expansion Cohort 1.
It's not clear what Provectus' CTO Dr. Eric Wachter, PhD's numbering system was for presenting patient data. For example, does 0001 refer to the first patient enrolled or treated? Or did he use another convention? Consider the table below.
Assuming 30-day months, ~53-54 months would have elapsed between the Barcelona conference in 2015 and the patient accrual & treatment PR of 2011 (for all six initial patients). There are ~60-61 months between the PR and today.
58 months would have elapsed between the Barcelona conference in 2015 and the accrual and treatment PR of 2010 (for the first three patients).
Updated below, again.
Maxim's coverage universe as at December 7, 2015:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Maxim's 2015/2016 version of their relationship with Provectus comprises:
- Exercising their (Maxim's) over-allotment option with respect to warrants to purchase an additional 2,625,000 shares of common stock, which I think means they issued themselves that amount of those tradable warrants,
- Not exercising an over-allotment option to purchase up to 2,625,000 shares of common stock,
- Representing, I think, the company in discussions for a regional license in the geography of India.
Updated (1/25/16): I believe I understand the importance of equity research coverage to a public company, the value of a good to great analyst both to the target company and to his or her firm, the historical challenge of research and analyst independence, etc. I also understand the role a research analyst and his or her firm can play as a sponsor of a research-covered (client) company (e.g., "pound the table," defender of the share price, price targets, etc.).
I also understand Provectus management's decision making by choice at times and by necessity at others does not follow most algorithms of valuation building. What I wrote in September 2013 still stands, both by observation and in regards to support:
Management historically has relied on the input of a stable of high and low quality advisors and consultants in its decision-making, making many good and several questionable decisions and choices along the way. Provectus has eschewed playing Wall Street's game for the most part, and has suffered to some degree because of it. Some company wounds, non-fatal as they are, however, have been self-inflicted. Management possesses a sufficient threshold level of competence and integrity to warrant my support, and seeks to sufficiently protect the economics of Provectus's innovation for shareholders to warrant my continued holding of Company stock. -- Why I'm Long Provectus PharmaceuticalsSo, returning to the primary question in my mind regarding research coverage of Provectus by Maxim, will the firm merely add Provectus' name to the coverage list or re-initiate coverage with a substantive research note on the company?
And, at this point, it's not that big of a question. As I wrote above, I was hoping for a check-the-box moment, but in reality the exercise is part of a question/determination of whether management is up to the task of maximizing asset value of Provectus at the end game.
The last Maxim research note was provided by former analyst Dr. Echo He, PhD in February 2014, but only to institutional clients. See Lavender Flowers under Potpourri (May 1, 2015) on the blog's Archived III News page. Historical hearsay would suggest Maxim's Jason Kolbert, Head of Healthcare Research and Senior Biotechnology Analyst, does not like Provectus' CFO/COO Peter Culpepper and does not think much of PV-10.
Neither observation or opinion, if true, matter to me. It's not like I think Kolbert, or the median Wall Street analyst, is exactly principled in how he or she undertakes their work at a sell-side investment bank. I'd say I already conveyed my view of the situation under Extension(s) (December 9, 2015) on the blog's Archived IV News page.
Aeterna Zentaris. The company, which (i) entered into a collaboration agreement with Sinopharm (Sinopharm A-Think) in December 2014 but did not disclose deal terms save for a non-refundable $1 million fee to it*, (ii) did a 100-to-1 reverse split in October/November 2015 of ~633 million shares outstanding (building on a 6-to-1 reverse split in October 2012 of ~112 million shares outstanding) (iii) had a Maxim Group/Jason Kolbert price target of $2 in October 2015 (pre-reverse split) when the share price was less than 10 cents, (iv) issued a PR about its first patient enrolled in a confirmatory Phase 3 in November 2015, and (v) received a new price target from Maxim/Kolbert of $11 ($200 split adjusted) immediately after the reverse split was effective and the share price opened at $4.58 and closed at $8.52, raised money today through Maxim (as sole book runner).
 |
| Click to enlarge. Image source |
After Provectus' COO/CFO Peter Culpepper's Maxim fundraising in June, I wrote under Offering, Part I (June 29, 2015) on the blog's Archived News III page: "He better get full throated research coverage from Maxim's Jason Kolbert; existing shareholders paid dearly for it (although, research coverage from lower tier investment banking firms like Maxim are akin to "the moon does not exist if nobody is looking at it")."
I'm going to guess by this point research coverage initiation of/a new price target on Provectus from Maxim/Kolbert (or McCarthy or [fill in the blank] analyst) will not happen in a timely or meaningful way, and doesn't really matter at this point (I don't believe it matters or mattered to Peter, frankly). I had figured and still figure as much but was disappointed no quo materialized for the quid he paid (on behalf of existing shareholders at the time) to Maxim.I've not yet been satisfied with management's response regarding coverage emanating from/paid by the June 2015 public offering. Before, it seemed to be the implication of pending coverage. Now, it seems Maxim hasn't been able to update or actively cover Provectus since the summer offering. Either way, it isn't the outcome that matters to me; it's the process that does.
Advancing (January 24, 2016)
Previous related blog news item on the blog's Archived News IV page:
- Drug substance/product synthesis (December 8, 2015)
 |
| Click to enlarge. Continuation award. |
 |
| Click to enlarge. Example #1 |
 |
| Click to enlarge. Example #2 |
When the FDA denied Provectus' breakthrough therapy designation ("BTD") in May 2014 for patients with locally advanced cutaneous melanoma (Stage III disease), the Agency noted its decision was based on a paucity of data:
"This determination is based on the paucity of data on endpoints indicative of clinical benefit (e.g., pain, infection, significant bleeding) and our inability to determine the clinical significance of the reduction in the size in one to 10 target lesions in patients with locally advanced melanoma, who may have additional untreated cutaneous, subcutaneous, or visceral sites of disease."Paucity means not enough, but doesn't mean not good enough. But, at the time, not enough of what? Although both FierceBiotech's John Carroll and TheStreet.com's Adam Feuerstein hit upon the notion of insufficient data, neither articulated the kind of data, which of course is critical. See Provectus shares crushed in rout after FDA spurns tumor drug (Carroll, May 27, 2014). Insufficient efficacy or safety data are one thing. Insufficient symptom data are another. In the end, Carroll and Feuerstein's presumed larger journalistic observation, and what mattered to investors and shareholders at the time, was the impact on Provectus' share price as a result of BTD denial.
At the time, I wrote:
The FDA's choice of the word paucity is interesting and, I believe, nuanced: the presence of something only in small or insufficient quantities or amounts.
The Agency recognized the applicability of PV-10 to treat locally advanced cutaneous melanoma (which it also deemed a serious or life-threatening disease or condition). It recognized the drug worked. The FDA, however, said Provectus did not provide enough data -- from the 54 patient sub-group where all disease was followed and the 28 patient sub-group where all disease was treated -- regarding symptom control (e.g., pain, infection or significant bleeding) to warrant BTD. BTD's generalized two-fold criteria are (i) a serious condition and (ii) preliminary clinical evidence of substantial improvement over available therapy on a clinically significant endpoint(s). Robust complete response (which Eric believed was tantamount to symptom control) and some pain data from the aforementioned subgroups was not enough to establish the correlation between complete response and symptom control.
While the amount of data did not meet the FDA's threshold for BTD, the Agency agreed Provectus submitted appropriate data (albeit in small or insufficient quantities or amounts). -- May 24, 2014 blog post The FDA ResponseBut as a shareholder, then and now, the question was and is whether PV-10 is initially approvable, which the current design of Provectus' pivotal melanoma Phase 3 trial answers. In my view, the drug is approvable as a single agent for Stage III melanoma. What remains is for the investigational drug to "win" the trial, which the subset of patients in the company's metastatic melanoma Phase 2 trial who had all of their disease treated by (injected with) PV-10 unequivocally demonstrated.
I thought it was worthwhile revisiting the topic of BTD because of the question by some at the time (understanding the "why" matters, and understanding why someone doesn't comment on the "why" matters too) why Provectus publicly disclosed the contents of the FDA's BTD denial letter. Carroll wrote:
"Unlike quite a few biotechs, which wouldn't breathe a word about a rejection like this, Provectus included the FDA's reasoning. "The preliminary clinical evidence you submitted does not indicate that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints," regulators wrote. "Therefore, designation as a Breakthrough Therapy cannot be granted at this time."Compare the statement I extracted from the FDA response to Carroll's selection. His would fall in the bucket of lack of [comparative] efficacy. Mine would fall into the category of not enough symptom data. That juxtaposition of course is what underscores the cliche "that's what makes a market," or Feuerstein's caution to investors of "to know what the other side of the trade is thinking." Carroll isn't really the other side of the trade. The larger point is understanding whether the FDA was commenting on PV-10's efficacy, or lack thereof, or on symptom data. Carroll is not vested in what happens to the stock and share price, whereas I [obviously] am.
What spurred me to write this blog news item was finding the following comment in Kidney Cancer Journal, Winter 2014, Volume 12, Number 4 (see page 90):
"QOL as a Trials Endpoint
In a much different plenary presentation, Jennifer Beaumont of Northwestern spoke about using quality of life as a clinical trials end-point. She explained that traditional efficacy and safety end-points don’t measure a patient’s symptom burdens. FDA guidance now strongly encourages the use of patient-focused end-points, including data directly obtained from patients. Beaumont cited the FDA’s recent rejection of Provectus Pharmaceuticals’ request for breakthrough status for PV-10 (rose Bengal disodium in 0.9% saline) for use against locally advanced cutaneous melanoma as an example of the agencies’ drive to incorporate these measures."Dr. Beaumont, PhD (biostatistics) is a statistician at Northwestern University, and the work noted above was presented at the 2014 Expert Dialogues meeting in London, England (for which I as yet cannot find a link or information on).
Provectus undertook a post hoc analysis of its previously completed metastatic melanoma Phase 2 trial data to establish an association between between objective response ("OR," which equals complete response [CR] + partial response [PR]) rate ("ORR") and patient symptoms. Post hoc means "occurring or done after the event." This guidance was noted in the company's January 24, 2014 PR, PV-10 Path to Initial Approval in U.S. Now Clear Per FDA Meeting Minutes:
"The meeting and official meeting minutes provided valuable guidance on a number of issues surrounding the approval path of PV-10:
- The Agency agreed with Provectus that treatment of cutaneous and subcutaneous tumors in patients with locally advanced cutaneous melanoma (i.e., recurrent, in-transit or satellite melanoma that has not yet spread from the skin to distant sites) could provide clinical benefit to such patients, particularly if the measured objective responses in patients' disease correlated to a demonstrated treatment effect on one or more symptoms of their disease (e.g., pain, infection or significant bleeding).
- The Agency agreed to work with Provectus to quantify symptom control in this patient population.
The company's Phase 2 trial contained some data, but ultimately not enough for the Agency to establish an association between OR/ORR and symptom control (i.e., quality of life). Provectus' CTO Dr. Eric Wachter, PhD said afterwards,
- In reference to discussions on the potential for breakthrough therapy designation, 'FDA advised Provectus to provide objective response rates with adequate information to evaluate the symptomatic treatment effects (e.g. pain, infection, bleeding) in patients presenting with locally advanced cutaneous melanoma who received PV-10 to all lesions.'"
"...our logic seemed clearer that if we were making the patients' tumors disappear in 50 percent of the patients that was a very large effect size, and that was tantamount to making any symptoms that they might have been suffering from the tumor burden disappear."OR/ORR was a primary endpoint of Provectus' Phase 2 trial.
But, in truth, while there were no requirements for measuring symptom data at enrollment, Provectus had data on less than 13 patients in the subgroup of patients with all disease treated (n = 28, trial N = 80) that presumably formed the basis for its BTD application. From ASCO 2014, Efficacy of intralesional rose bengal in patients receiving injection in all existing melanoma in phase II study PV-10-MM-02:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Oncologic Drugs Advisory Committee meeting on April 29, 2015 that would form the basis for approving Amgen's talimogene laherparepvec (Imylgic), Amgen-submitted materials noted:
(p. 44) "Clinical Benefit
At FDA’s request, outcomes describing clinical benefits such as a decrease in pain, an improvement in Quality of Life, and prolonged survival were tabulated for the subjects with a durable response per EAC."And:
(p. 54-55) "Association Between Durable Response and Trial Outcome Index
In a post-hoc landmark analysis, a significant association between the achievement of a durable response and improvement in TOI (a measure of quality of life) was observed (p= 0.025), with an odds ratio of 2.8 (95% CI: 1.1, 7.0). A greater proportion of subjects who achieved a durable response per the EAC reported improvements in TOI (58.1%) when compared with those who did not achieve a durable response (30.0%)."It appeared the number of patients Amgen used for its post-trial analysis work was about 50.
Patient-reported outcome started out as a secondary endpoint in Provectus' pivotal Stage III melanoma Phase 3 trial. See August 10, 2014 blog post Juxtaposition. It later was moved to an exploratory one.
Takeaways:
- Loco-regional disease in melanoma, as a patient population, is/was new to the FDA.
- The Agency asked both Amgen and Provectus to undertake post hoc analyses (analysis after their respective trials were completed) to investigate and determine the association, if any, between the trials' respective primary endpoints and quality of life measures.
- For Amgen, durable response (i.e., all patients who achieved CR or PR after 6 months) and the improvement in total outcome index (a measure of quality of life).
- For Provectus, objective response (i.e., all patients who achieved CR or PR) and symptoms like pain, infection or significant bleeding.
- Clinical benefit, in FDA jargon, not only means efficacy and safety benefits, but it also means quality of life benefit.
- Amgen had more quality of life data (perhaps ~50) than Provectus (maybe <13).
- Provectus is collecting patient-reported outcomes (quality of life) as part of its pivotal melanoma Phase 3 trial by conducting the study Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma, and the trial's exploratory endpoint of "Change in domain scores from baseline using the patient reported Skindex-16 instrument."
- If PV-10 wins its pivotal trial by showing the clinical benefit (efficacy and safety) it previously demonstrated in its Phase 2 trial and for patients who had all of their disease treated by (injected with) the drug compound, it should win approval (for Stage III melanoma patients).
- Finally, for what it's worth, it would appear to me Provectus faced the FDA's post hoc analysis request of establishing an association between its trial's primary endpoint (say, 1H14) and patient quality of life before Amgen did (say, 2H14-1H15).
 |
| Image source |
2. Meeting expectations. Related to the aforementioned mechanism of action work, Provectus' Dr. Erich Wachter, PhD set an expectation on the company's November 5th 3Q15 business update conference call: "We are also continuing to sponsor mechanism studies at Moffitt and the University of Illinois at Chicago, and anticipate further data from both groups to be released in coming months." In Below expectations. under Cheers (January 1, 2016) below, I had written "The benefit of the doubt pushes this expectation into at least very early-2016." With the UIC ASC 2016 abstract "PV-10 Induces Potent Immunogenic Apoptosis in Colon Cancer Cells," I think he's begun to meet this expectation.
3. Arm's length. Does the company derive or get "credit" for the arm's length and independently reproduced work undertaken by Moffitt and UIC?
Biotechnology and pharmaceutical companies produce and publish non-clinical work exclusively of their employees:
- At AACR 2015, for example, Amgen produced a couple of internally generated posters (i.e., the author line was comprised exclusively of Amgen employees) for intraslesional agent talimogene laherparepvec ("T-Vec") -- see Talilmogene laherparepvec increases the anti-tumor efficacy of the anti-PD-1 immune checkpoint blockade and Talimogene laherparepvec activates systemic T-cell-mediated anti-tumor immunity.
- Provectus has generated those "internal" posters as well too; see Generation of an Antitumor Response and Immunity Using a Small Molecule Drug (PV-10) (SITC 2012) and Combination of PV-10 Immuno-chemoablation and Systemic anti-CTLA-4 Antibody Therapy in Murine Models of Melanoma (AACR 2013).
- NewLink published preclinical combination study work (their drug + anti-PD1/PD-L1 antibodies) at AACR 2014 (April), co-conducted by NewLink employees and Georgia Regents University Research Institute researchers/employees.
- In another example, at an ASCO 2014 presentation of a Phase 1/2 melanoma study, principal investigators (that included Moffitt Cancer Center's Dr. Jeffrey Weber, M.D., Ph.D.) noted "[p]reclinical data support antitumor synergy for INCB024360 when administered with an antibody antagonist to checkpoint receptors," referencing a October 2013 (submitted)/February 2014 (published) SITC journal paper* (the paper, however, does not present preclinical combo work on the subject drug but another related Incyte compound), co-conducted by Celldex employees and the University of Chicago researchers/employees.
4. PV-10 + radiation. The discussion about immunogenic cell death, which can be caused by radiation, makes me wonder about the results of the investigator-initiated trial combining PV-10 and radiotherapy. Eric, from the November 2015 3Q15 conference call: "We also continued to work with the team in Australia conducting the investigator initiated trial of PV-10 in combination with radiation to report ongoing results in a suitable venue."
5. Not currently compelling. With closing prices for the common stock and tradable warrant today of ~$0.39 and $0.25, respectively (for a total unit price of ~$0.64), I am hard pressed, tonight, to see a compelling reason or reasons for holders of non-tradable warrants to tender. Yet, if this is to be believed, according to a source who normally would not have knowledge of the situation, but presumably spoke to someone who does and gain said or such knowledge, approximately 3-4 million warrants already have been tendered.
"We will sell no wine before its time." (January 21, 2016)
On January 27, 2011, Provectus issued press release Completes Patient Accrual and Treatment for Phase 1 Trial of PV-10 for Liver Cancer, noting:
"The Phase 1 study of PV-10 for liver cancer involved six subjects with cancer metastatic to the liver or with recurrent liver cancer. The primary objective of the open-label study is to determine the safety and tolerability of a single intralesional injection of PV-10 in patients with cancer of the liver. Additional objectives include assessing the distribution and retention of PV-10 in the injected lesion, tumor response and viability, and plasma pharmacokinetics of PV-10 following intralesional injection. In each of two dose cohorts there were three subjects." {Underlined emphasis is mine}Next Wednesday, January 27, 2016 would make the fifth "anniversary" of the PR.
Prior the January 2011 PR, the company had treated the first three liver cancer patients by September 14, 2010, Completes Initial Enrollment in Phase 1 Trial of PV-10 for Liver Cancer.
Bell et al. note in Cancer Survivorship: Why Labels Matter (JCO February 1, 2013 vol. 31 no. 4 409-411):
"The concept of cancer survivorship has been widely debated over the past few decades. In biomedical usage, the term survivor has a distinct clinical meaning, referring to individuals who have had a life-threatening disease but have remained disease free for a minimum of 5 years.They also note as well, however:
"However, in recent years, a variety of other definitions have been outlined. Proposed definitions differ primarily around the scope of populations covered; some refer only to those diagnosed with cancer, whereas others extend to family, friends, and caregivers. Complicating the picture is the distinction drawn between the terms cancer survivor and cancer survivorship. Although the former is used to encapsulate individuals throughout the cancer trajectory, the latter refers to a distinct phase in the cancer trajectory between primary treatment and cancer recurrence or end of life."Nevertheless, my question is how many original liver Phase 1 patients (i.e., of the six) are alive after 5 years, and thus [depending on the appropriate terminology] are cancer survivors or "cancer-free?"
In April 2015 St. Luke's Hospital and Health Network's and Provectus principal investigator Dr. Sanjiv Argawala, MD observed the following about clinical liver overall survival data:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Going way back to earlier versions of Provectus' investor presentation, it appeared the make-up of the first six liver Phase1 trial patients comprised five HCC and one liver mets patients (based on the assumption of one treated tumor per patient).
 |
| Click to enlarge. Screenshot of presentation from February 14, 2012 |
 |
| Click to enlarge. |
 |
| Click to enlarge |
 |
| Click to enlarge. |
I assume, because there was no mention of sorafenib in any of the three presentations (particularly the two presentations at the two medical conferences), that none of the 4-7 patients above the initial 6 were on stables doses of sorafenib. Thus, now, the 7 additional patients were part of Expansion Cohort 1.
It's not clear what Provectus' CTO Dr. Eric Wachter, PhD's numbering system was for presenting patient data. For example, does 0001 refer to the first patient enrolled or treated? Or did he use another convention? Consider the table below.
 |
| Click to enlarge. |
| Click to enlarge. |
| Click to enlarge. |
Now, I think two of the six patients died or expired.
 |
| Click to enlarge. |
~33-34 months would have elapsed between the Barcelona conference in 2015 and the PR noting the expansion of the liver Phase 1 study in 2012.
| Click to enlarge. |
Questions?
* Armageddon (1998)
InvestorVillage poster canis_star's post today reminded me of a blog news item I had written when Moffitt Cancer Center's first introduced its view on Rose Bengal/PV-10's HGMB1-based mechanism of immune action, SITC 2015, HMGB1, Anti-tumor immunity, Immunogenic cell death-(?) (November 3, 2015) on the blog's Archived News IV page. canis_star referenced the same article I did in the news item, Bianchi, ME, Killing cancer cells, twice with one shot, Cell Death and Differentiation (2014) 21, 1–2. In searching the blog for this article, I came across what I had written because of another article.
In the news item I noted HMGB1 plays a key role in immunogenic cell death, and referenced an article by Gebremeskel et al. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: Impact on clinical studies and considerations for combined therapies., Oncotarget. 2015 Oct 14. I marked up a figure in the article in purple, with the mark-up related to HMGB1 release.
As you can see, ER stress (endoplasmic reticulum (ER) stress) on the left, now marked up by me in blue, also plays a role in immunogenic cell death (or ICD), which the University of Illinois at Chicago observed/concluded/hypothesized in its recent PV-10 abstract. See "PV-10 Induces Potent Immunogenic Apoptosis in Colon Cancer Cells" (January 19, 2016) below.
Moffitt Cancer Center and the University of Illinois at Chicago independently, and independently from each other, reproduced Provectus' original work, which first demonstrated PV-10’s two-prong approach to successfully fighting cancer in multiple indications: Tumor ablation, the local effect of destroying injected tumors; a tumor-specific immune response, the systemic effect of destroying non-injected tumors; tumor-specific IFN-gamma/γ production; and multi-indication viability in at least melanoma, breast cancer and colorectal cancer.
Both medical research organizations now have an/some understanding of Rose Bengal/PV-10's mechanism of immune action.
"PV-10 Induces Potent Immunogenic Apoptosis in Colon Cancer Cells" (January 19, 2016)
Updated below, again.
H/t InvestorVillage poster Juggernaut: The University of Illinois at Chicago (UIC) appears to have submitted a PV-10 (Rose Bengal) abstract to the 11th Annual Academic Surgical Congress, which will take place on February 2-4 in Jacksonville, Florida.
Underlined emphasis below is mine.
Abstract introduction:
When is a tea leaf just a leaf of tea? (January 18, 2016)
Takeaway:
Looking ahead (January 18, 2016)
H/t regular hatter AL:
Time (January 16, 2016)
Updated below.
Updated (1/16/16): In Rose Bengal, PV-10’s active pharmaceutical ingredient , Provectus’ co-founders discovered a small molecule lying around in plain sight of Big Pharma for more than 130 years – from Rose Bengal’s creation by Gnehm in the 1880s [1], to American doctor G.D. Delprat’s anecodatal patient-reported outcomes in the 1920s [2], to Japanese researchers Ito et al.’s preclinical observations of dose-dependent increases in survival in the 1980s [3], to the co-founders’ formal discovery of Rose Bengal’s tumor inhibiting properties in the 1990s [4].
Provectus’ outsiders have spent the well over 15 years [5] trying to convince the FDA, the medical community, Big Pharma, healthcare-focused Wall Street, biotechnology investors, and the media of the veracity of their claims about PV-10 and its potential groundbreaking therapeutic benefits for treating solid tumor cancer.
Sometimes I miss the mark in trying to communicate the kernel of the thought (the takeaway, as it were) that sparks a blog post or news item. In this case, for Time, ignore @JohnTuckerPhD's goal for his tweet above and a subsequent one that relate to antibody-based drug development programs that purportedly have intracellular ("located or occurring within a cell or cells") drug target locations.
The kernel of my thought for this news item was at least two-fold. First, I wanted to remind myself that most folks (particularly chemists) who yearn for the day when a small molecule rises up and provides robust immunotherapeutic benefits to cancer patients don't know (understandable), don't understand (less understandable, but not particularly egregious) or dismiss the fact (egregious) that Rose Bengal is a small molecule. The upshot of Rose Bengal's small molecule-ness is that part of the benefit a patient derives is from PV-10/Rose Bengal as a chemical cancer therapy, and not a biological therapy like anti-CTLA-4 (Yervoy), anti-PD-1 (Keytruda, Opdivo) and anti-PD-L1 approved drugs and investigational agents. See December 20, 2015 blog post An incomplete thought.
Second, the anti-CTLA-4s, anti-PD-1s, anti-PD-L1s, etc. target receptors on the outside of a cell -- extracellular ("situated or taking place outside a cell or cells") drug target locations. For Rose Bengal, a small molecule, all evidence points to activity inside the cell (i.e., lysosomes = intracellular). That, intracellular drug targeting (aka Rose Bengals' specificity), in part, explains the robustness of PV-10's multi-indication immunotherapeutic response.
[1] Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie, 1887; 238: 318-338
[2] The Rose Bengal test for liver function, Delprat, G.D. et al., The Journal of Laboratory and Clinical Medicine, Volume 16, Issue 9, 923-925
[3] Induction of thyroid tumors in (C57BL/6N x C3H/N) F1 mice by oral administration of 9-3',4',5',6'-tetrachloro-o-carboxy phenyl-6-hydroxy-2,4,5,7-tetraiodo-3-isoxanthone sodium (Food Red 105, rose bengal B), Ito et al., J Natl Cancer Inst. 1986 Jul;77(1):277-81
[4] American Society of Clinical Oncology, 2010 Annual Meeting and Rose Bengal: From a Wool Dye to a Cancer Therapy, Alexander, W., Pharmacy & Therapeutics. 2010 Aug; 35(8): 469-474, 478
[5] Provectus' Chairman and CEO Dr. Craig Dees, PhD starts counting at the inception of Provectus in 2002 (which is going on about 14 years). I don't. Rather, I start counting when they re-discovered Rose Bengal in 1998 as an antineoplastic candidate (which elongates the story to about 18 years).
Intracellular drug target locations & small molecules? Ask Genentech.
Il meglio è nemico del bene* (January 14, 2016)
Updated below, again.
* Perfect is the enemy of good (Wikipedia entry).
I suppose there might've been at least two reactions to Provectus' January 13th press release, Confirms First Patients Dosed in Trials of PV-10 for Melanoma. One, yawn. Two, the company began dosing in 2016. Three, Provectus confirmed patients already were/had been treated (in both melanoma trials).
In fact, one entity/individual had the second and third reactions, in that order. BioPharmCatalyst is a website by a random person on the Internet (kind of like this blog and me) tabulating and collating FDA approval, PDUFA and clinical trial catalysts. After the PR came out BioPharmCatalyst or @crusadernz tweeted:
Going to the main website (Company Pipeline Database), one would have seen in regards to Provectus (PVCT) -- the pivotal melanoma Phase 3 trial (that started in April 2015) began treating patients in January 2016:
Later, after I informed Provectus' CFO/COO Peter Culpepper of the above (more so to make a point, rather than as a nod to randomness), someone (likely someone at the company's investor relations firm Porter, LeVay & Rose) spoke to BioPharmCatalyst, who changed the information in the tweet's embedded link and on the main website, respectively below -- the pivotal melanoma trial started dosing patients in 2015:
My point (as part of my telling Provectus' CTO Dr. Eric Wachter, PhD that sometimes in the pursuit of the laudable goal of seeking preciseness, one falls short of achieving at least accuracy) was [what I believed to be] management's multiple messages to existing and prospective shareholders and investors, and to Big Pharma, were not completely clear (as evidenced by a simple read by one somewhat relevant and not so random datapoint). When you're short on credibility, I imagine you try harder to address areas where you're not good, or where you're dismissive of their relative importance or value.
To be clear, I like and embrace Eric's approach to the company's clinical development program's process. He is the right person in the right place at the right time, both among Provectus' principals, and from an industry perspective in regards to intralesional (IL) agents and IL agent experience in oncology. I draw the distinction between developed expertise and experience in the pursuit of generating meaningful data, and the communication of it. I think Eric places different levels of importance on things than other members of management do, and certainly most investors do.
One message might've been that Provectus has been treating patients in its pivotal Phase 3 trial (for Stage III melanoma). Although it's spilt milk/water under the bridge by now, management clearly was being reactive in addressing the topic of whether the first patient, and patients in generally, already was/were treated. Other than acquiescing to internal and external pressures, what was Eric's intellectually consistent rationale to put out this PR 9 months late?
I thought a more important message might have been in regards to the Phase 1b study (for Stage IV melanoma patients). Here, I believe, Eric has seen or been told of preliminary data from this clinical trial. Those very early results, building on pre-clinical combination work undertaken by both Provectus (SITC 2012, AACR 2013) and Moffitt Cancer Center (SITC 2014), may well have informed his statements and comments in the PR -- that the effect size of adding pembrolizumab to PV-10 is potentially much larger (and perhaps much larger than expected) than pembrolizumab alone in this patient population. These comments were:
New SEC filing. Provectus' amended S-4 (for the tender offer related to non-tradable warrants with varying exercise prices) notes the following primary changes (on the right, when compared to the initial S-4 filed on December 31st) (I used Text Compare!):
According to the company, the SEC required the date change from March 15th to March 1st in order for the stock (resulting from the exercise of non-tradable warrants) and tradable warrants (as replacements for the aforementioned exercised non-tradable ones) to be issued in a more timely fashion. I believe the next quarterly business update conference call is scheduled for March 10th.
Updated (1/14/16): Management's guidance in regards to an interim assessment of efficacy and safety (i.e., interim data readout) of Provectus' pivotal melanoma Phase 3 trial (underlined emphasis below is mine):
"FPFV" (January 12, 2016)
Updated below, again.
Oz (January 11, 2016)
MIT > Wisconsin (January 11, 2016)
No abscopal effect with checkpoint inhibitors (January 9, 2016)
Promise (January 9, 2016)
Updated below.
H/t AL, a shareholder, blog reader and regular hatter for this news item.
In an article entitled International Symposium on Melanoma May Set Foundations for Clinical Decision Making by Sandra Kear and published online in TargetedOncology on January 5th, Dr. Omid Hamid, MD*, co-chair of the 12th Annual International Symposium on Melanoma and Other Cutaneous Malignancies (February 20th) notes:
At the 11th Annual International Symposium on Melanoma and Other Cutaneous Malignancies® Hamid made the following disclosures:
Updated (1/11/16): Dr. Hamid noted investigational strategies in late-stage development with the promise of changing melanoma treatment that included IDO inhibitors (and PV-10). Note the below from an article by FierceBiotech's John Carroll today entitled Merck buys out IOmet, adding new immuno-oncology tech:
No change (January 8, 2016)
Updated below.
Because no change was made to the estimated primary completion date of Provectus' pivotal melanoma Phase 3 trial on its ClinicalTrial.gov's webpage -- when an additional clinical trial site (Salt Lake City, UT's Huntsman Cancer Institute) was added on January 5th, see Updated Clinical Trial Information (January 6, 2016) below -- management's guidance regarding the timing of the trial's interim assessment of efficacy and safety remained unchanged. As management has previously guided, the assessment would (will) occur about halfway between the study start date and the estimated primary completion date.
The trial's .gov webpage of course notes the interim assessment will be performed by the study's independent review committee (IRC) when 50% of the events required for the primary endpoint (progression-free survival [PFS]) have occurred.
The timing of an events-based trigger (i.e., when 50% of the events required for the primary endpoint have occurred) is unchanged because the timing of a time-based condition (i.e., halfway between the study start date and the estimated primary completion date) is unchanged. Yeah, that's the ticket. I jest of course because I believe it makes sense if one assumes the "real" trigger is a prescribed time one*.
I don't believe the pivotal Phase 3 trial should be stopped early, although patients in the control arm should be immediately conveyed to the treatment arm once statistically significant separation of the PFS curves is deemed achieved by the study's independent data monitoring committee. The only way to fully prove one of the trial's two hypotheses is to continue measuring overall survival of patients, irrespective of whether they started out in the treatment arm or crossed over into it. The trial needs to establish that, for Stage III patients, treating all disease with PV-10 can forestall or prevent the spread of melanoma to Stage IV.
Halfway between the study start date and the estimated primary completion date is June/July 2016.
* See July 23, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part IV or August 4, 2015 blog post Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part VI.
Updated (1/8/16): From life sciences venture capitalist Bruce Booth's blog post Sentiments Likely To Be Overheard At #JPM16.
Makes sense (January 7, 2016)
Updated below, again.
Intralesional agent for oncology experience.
Updated (1/7/16): It make sense to highlight the experience of clinical trial investigators and/or their sites (and other investigators at said sites) with advanced (i.e., in late-stage clinical trials) intralesional (IL) agents. These drugs or compounds currently would be/are (all for melanoma):
Disease: Where Will it Fit in?.
Tender offer for Provectus' non-tradable warrants. The construct of and rationale behind the tender offer makes sense for Provectus and existing shareholders. My "grade" would be directly related to the tender's success, i.e., the higher the number of warrants exercised, the higher the grade. Thus, the key to this success will be Network 1 Financial's (Damon Testaverde and Bill Hemming's) ability to convince their clients of the value proposition of exercising their non-tradable warrants.
If I was a client of theirs, and knowing Provectus' CTO Dr. Eric Wachter, PhD, CFO/COO Peter Culpepper, and board of directors member Jan Koe's already have signaled their intention to exercise 100% of their ~1.3 million non-tradable warrants for a ~$960K investment in the company, I'd want to know what amount of warrants and what proportion of warrant holdings Testaverde, Hemming and the firm intend to exercise before making my decision. Going by Provectus' SEC filings, it would appear Network 1 owns ~4.9 million warrants, or a potential ~$3.7 million investment in the company. See Moneyness (January 2, 2016) below.
A co-development deal for the combination of PV-10 and [fill in the blank]. At this point, it makes sense a co-development "deal" between Provectus and, say, Merck & Co. merely means the latter supplies its drug pembrolizumab (Keytruda) at no cost to the former for it to conduct early-stage clinical trials combining PV-10 and the checkpoint inhibitor in indications besides melanoma. I suppose the question for me is what indications.
Updated (1/7/16): S-4. It makes sense to read the registration statement. Of note, perhaps:
Updated below, again.
PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma:MD Anderson, Huntsman Cancer Institute, and Sharp Memorial Hospital (all not yet recruiting)
PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma: MD Anderson, and Princess Alexandra Hospital (both not yet recruiting)
Updated (1/6/16): My error: Phase 3 sites above that are struck out already were on the ClinicalTrials.gov site. A table of the pivotal melanoma (Stage III) Phase 3 trial and related qualitative interview study is below.
Updated (1/6/16): A table of the melanoma (Stage IV disease) combination therapy Phase 1b trial is below.
For both melanoma trials (as well as for the expanded liver Phase 1 trial), Provectus' compassionate use program (CUP)/expanded access sites play an important role.
Show me the moneyness (January 5, 2016)
My goal for the following blog news item is to speak accurately but precisely. The below ignores transactions costs and the realities of supply & demand (bid-offer) when discussing how and how much the tradable warrant price (PVCT.WS) might move up with an increasing share price (PVCT).
The basic rationale for the recently announced tender offer -- see Love Me Tender under Cheers (January 1, 2016) below -- is to raise money, and to do it, at least for now, from "friends & family" (i.e., mostly Network 1 clients, and perhaps the firm itself that owns a large number of non-tradable warrants). Duh. The whys of fundraising and fundraising by February 15th probably are open to speculation. I'd love to know, for example, how many of the firm's warrants Network 1 intends to or will or eventually does exercise.
"Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma" (January 4, 2016)
Updated below.
Previous related blog posts and news items, all on the blog's Archived News IV page:
A follow-up to the table of whom held what warrants issued issued (as a result of related fund raising) between January 6, 2011 and November 1, 2015 -- see Moneyness (January 2, 2016) below -- is a table of the fund raising dollars. While roughly 85% of the warrants involved in the recently announced tender offer are held by Network 1 Financial's clients and the firm, Network 1 was responsible for approximately 70% of the money raised during the period in question.
When you combine the non-tradable and tradable warrant totals, so as to adjust or account for the already trading warrants from the Maxim Group PVCT.WS public offering, Network 1 and Maxim's warrant "responsibility" percentages are about 65% and 35%, respectively.
Network 1 and Maxim touch points with Provectus over the years might include:
Publicly traded warrants is no new things to Provectus. The failed 2012 PVCTP "IPO" was a warrant-based fundraising effort that featured Maxim as the sole book runner. As I've previously noted, the current tender offer is akin to offering a slightly used version ("used" by about 7-8 months when all is said and done) of the June 2015 Maxim-led public offering. At the time of the June offering I recall hearing Network 1 was extremely disappointed (my words) Provectus' CFO/COO Peter Culpepper elected to raise money via Maxim. My sense/understanding of the situation was that Peter did not believe Network 1 could raise the amount of dollars he needed, and (I presume but did not hear this) from whom he needed (i.e., institutional investors). It would appear to me Network 1's client base is primarily higher, high and ultra high net worth individuals and families, and not institutional investors and funds like, say, Maxim.
I also recall, at the time, a discussion about eventually making the existing non-tradable warrants available for trading. I had wondered how one would conform the different features of the non-tradable warrants (i.e., different exercise prices and expirations) to the tradable warrants (a $0.85 exercise price, a June 19, 2020 expiration), but didn't really grasp or focus on a possible approach. Now I know.
It would seem former/current (?) Provectus strategic advisory board (SAB) members and current/former Boehringer Ingelheim folks Dr. Joseph Chalil, MD and Christopher Kaplan are a package deal. They were named to the company's SAB in March 2014 and September 2014, respectively. In 2015 they were named to the SABs of Medifocus in June (MFS.V, a market cap of ~$7 million) and Oxysure in September/October (OXYS, OTC, ~$7 million). Medifocus was/is a client of Porter, LeVay & Rose, which also is Provectus' investor relations firm.
In July 2015, Provectus and Boehringer Ingelheim (BI) China made announcements of their entering into/signing a letter of intent: Provectus Biopharmaceuticals Signs Letter of Intent with Boehringer Ingelheim (China) to Collaborate in Bringing PV-10 to Market in China (Provectus' PR on July 2nd) and Boehringer Ingelheim and Provectus biopharmaceutical company signed a letter of intent - the two sides will cooperate to promote melanoma and liver cancer research new drugs listed in the Chinese mainland, Hong Kong and Taiwan (BI's PR, translated into English, on July 3rd).
On July 10th, Jialing Dai wrote a LinkedIn article entitled Boehringer's Ambition In Liver Cancer. The article's byline read: "Eye On Oncology Market: Boehringer Ingelheim In-Licenses China Rights For A Provectus Late-Stage Oncology Compound." The article was edited by Howard Fields. Dai describes his some of his work as "PharmaDJ is online portal in English to provide tailored services and intelligence of China market for pharma executives. our goal is to build up a bridge to China market for foreign companies doing business in China." In his article Dai wrote:
What did Provectus mean when the company wrote in its July LOI PR (see my underlined emphasis)?
Moneyness (January 2, 2016)
Updated below.
The concept of moneyness when considering financial derivatives (e.g., forwards, swaps, puts, calls, etc.) means the amount or lack of profit a derivative security has relative to the then price of its underlying security.
Consider, for example, some option like the AAPL July 15, 2016 $115 calls (underlying security, expiration date, strike price, derivative) -- see this link. Apple's share price closed on Friday at $105.26. Such a call is out-of-the-money (OTM) because the share price < the strike price. Should the share price rise above $115 (plus the transaction cost of purchasing the call), the call becomes in-the-money (ITM). Illustratively, at a share price of exactly $115, the call option is at-the-money (ATM). The strike price is the same thing as the exercise price or the trigger [price].
Allow me to analogize the tender for the company's approximately 60 million non-tradable warrants to a derivative:
From an analysis of moneyness perspective, I don't believe management has sufficient credibility to qualitatively facilitate the tender becoming ITM -- by this I mean, I would not exercise a non-tradable warrant (if I owned one) based on projection, speculation or the future.
As a result, I would imagine the tender actually has to become ITM from a quantitative perspective; that is, the price of a common share (and, thus, the tradable warrant, which should move in tandem with the stock) has to rise above the tender's strike price before its expiration. If one assume's management is given no benefit of the doubt by the capital markets, then the share price only should move because of news (e.g., pre-clinical and/or clinical development, regulatory, business development, corporate development, media, etc.), and that news would have to materialize before February 15th.
If the share price does not rise materially before that date -- that is, if no news revealing or signaling a sustainable, long-term measure of business success for Provectus -- then the tender would remain OTM. A warrant holder then/thus would not tender their non-tradable warrants.
Most of the non-tradable warrants are held by Network 1 Financial's clients and the firm itself. See the table below.
A successful tender -- let's say northwards of at least 20% (~12 million warrants, $9 million) and more likely much more participation -- could provide the following benefits (in no particular order, and among other things):
Love Me Tender. Provectus made a tender offer to shareholders owning non-tradable warrants. SEC filings made on December 31, 2015 are here (S-4) and here (tender offer). In this offer, such warrant holders are offered to (i) exercise their non-tradable warrants for cash -- irrespective of the warrant's original exercise price -- at a "tender exercise price" of $0.75 and (ii) receive a tradable warrant (one-for-one). These tradable warrants have the NYSE ticker PVCT.WS, an exercise price of $0.85, convert into one share of Provectus common stock, and an approximately 4.5-year expiration of June 19, 2020. The tender offer is open until February 15th.
In other words, if you own a non-tradable warrant, you may exercise it for $0.75, and receive a tradable warrant.
Takeaways:
"Raw" warrant data as of 12/31/14 is below:
I can't yet figure out my discrepancy between the number of non-tradable warrants denoted by tender documents note (59,861,601) and my calculation of the same figure using issued and forfeited warrant data from the 10-K and 10-Qs. See below:
Fully diluted shares outstanding through 12/31/15 could be ~275 million. See below:
If all non-tradable warrants are exercised, net proceeds to Provectus could be $42 million. See below:
If all non-tradable warrants are exercised and replacement tradable warrants are issued for them, dilution would be ~22% (at the time of the transaction's completion); however, dilution would be notably reduced should the subsequently issued tradable warrants be cashlessly exercised. See below for an example (e.g., 22% vs 14%).
Take for example the holders of the non-tradable $1.00 exercise price warrants. Preliminarily (because my calculations could be incorrect), the value proposition is the potential for an added 75% return from tendering. See below:
For the non-tradable $3.00 exercise price warrant holders, the potential benefit is an added 275% return from tendering. See below:
A quick 'n dirty summary overview of the potential risk-return decision for non-tradable warrant holders is below in two parts:
The existing non-tradable warrants, one could argue, are effectively worthless.
It would Eric and Peter may end buying nearly $960K of common stock through stock option and non-tradable warrant exercises. See below in two parts:
Below expectations. Eric set several expectations on the company's November 5th 3Q15 business update conference call, most of which he missed {underlined emphasis is mine}:
- Is my math wrong?
- Of the four patients of the initial six enrolled and treated in Provectus' liver Phase 1 trial, how may are alive and for how long?
* Armageddon (1998)
 |
| Click to enlarge. |
In the news item I noted HMGB1 plays a key role in immunogenic cell death, and referenced an article by Gebremeskel et al. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: Impact on clinical studies and considerations for combined therapies., Oncotarget. 2015 Oct 14. I marked up a figure in the article in purple, with the mark-up related to HMGB1 release.
 |
| Click to enlarge. Blue and purple markups are mine. Image source is the article immediately above. |
Moffitt Cancer Center and the University of Illinois at Chicago independently, and independently from each other, reproduced Provectus' original work, which first demonstrated PV-10’s two-prong approach to successfully fighting cancer in multiple indications: Tumor ablation, the local effect of destroying injected tumors; a tumor-specific immune response, the systemic effect of destroying non-injected tumors; tumor-specific IFN-gamma/γ production; and multi-indication viability in at least melanoma, breast cancer and colorectal cancer.
Both medical research organizations now have an/some understanding of Rose Bengal/PV-10's mechanism of immune action.
"PV-10 Induces Potent Immunogenic Apoptosis in Colon Cancer Cells" (January 19, 2016)
Updated below, again.
H/t InvestorVillage poster Juggernaut: The University of Illinois at Chicago (UIC) appears to have submitted a PV-10 (Rose Bengal) abstract to the 11th Annual Academic Surgical Congress, which will take place on February 2-4 in Jacksonville, Florida.
 |
| Click to enlarge. Image source |
Abstract introduction:
We have previously demonstrated that human and murine colon cancer cells undergo near complete cell death in vitro and in vivo upon direct exposure to PV-10 (10% rose bengal disodium), a synthetic dye currently in clinical trails for intralesional therapy of in-transit melanoma. Occasional bystander responses have raised the possibility that PV-10 induced cell death can generate an anti-tumor immune response. The mechanism of PV-10 cell death in not known, and it is critical to determine, if rose bengal disodium is to be used as an immunotherapeutic strategy for solid tumors.Conclusion:
Treatment of colon cancer cells with PV-10 induced cell cycle arrest, apoptosis, autophagy, and significant ER stress; consistent with immunogenic apoptosis. In order for cytotoxic agents to act as potential immunotherapeutic strategies in the treatment of solid tumors, immunogenic cell death targeting the endoplasmic reticulum (ER), leading to ER stress may be critical. Therefore, based on these results, further evaluation of PV-10 as a potential agent to stimulate immunologic cell death in solid tumors is warranted.Updated (1/19/16): Takeaways, in not particular order:
- The above is the third piece of published/presented work by UIC's College of Medicine's Department of Surgical Oncology's Maker Laboratory.
In March 2015 lab members published/presented "Intralesional Injection of Rose Bengal Induces an Anti-tumor Immune Response and Potent Tumor Regressions in a Murine Model of Colon Cancer" at the 2015 annual meeting of the Society of Surgical Oncology.
In September they published/presented a review paper on PV-10 tumor ablation and immune stimulation entitled "The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses."
- It is a reminder of Dr. Ajay Maker, MD's background, which includes:
"...was accepted into the post-doctoral surgical oncology fellowship program under Dr. Steven Rosenberg in the Surgery Branch of the National Cancer Institute at the National Institutes of Health (NCI/NIH). Thereafter, he went on to complete a surgical oncology fellowship at the Memorial Sloan-Kettering Cancer Center...His research has been funded by foundations and pharmaceutical companies; and he currently is the principal investigator on a multi-year federal research grant from the National Cancer Institute/National Institutes of Health to study the immunobiology of GI cancers."
- B. Prabhakar, also a member of the lab, has co-authored all three pieces of work. His background importantly and interestingly includes:
"...as Head, Department of Microbiology and Immunology in the College of Medicine...Dr. Prabhakar has been funded by the NIH continuously for the past twenty years. He has nearly 200 publications to his credit in journals including Science, Nature, New England Journal of Medicine, PNAS, and the Journal of Clinical Investigation."
- "The mechanism of PV-10 cell death in not known, and it is critical to determine, if rose bengal disodium is to be used as an immunotherapeutic strategy for solid tumors."
While Provectus' Chairman and CEO Dr. Craig Dees, PhD has said for many years that knowledge or understanding of PV-10's was not necessary for FDA drug approval, the drug compound's transition from tumor ablator to tumor ablator & immune stimulator more than likely requires explanation to some, such as Big Pharma.
- "...PV-10 as a potential agent to stimulate immunologic cell death in solid tumors..."
Immunologic cell death: "First, antigens released by dying...tumor cells can be taken up, processed, and presented by antigen-presenting cells (APCs) in the microenvironment and draining lymph nodes. Second, dying tumor cells release a specific “danger” signal that acts upon DCs to stimulate antigen processing and presentation to T cells." (Illustrative example taken from Combining radiation and immunotherapy for synergistic antitumor therapy, Ferrara et al.)
- "In order for cytotoxic agents to act as potential immunotherapeutic strategies in the treatment of solid tumors, immunogenic cell death targeting the endoplasmic reticulum (ER), leading to ER stress may be critical."
Immunogenic cell death: "Immunogenic cell death or immunogenic apoptosis is a form of cell death caused by some cytostatic agents such as anthracyclines, oxaliplatin and bortezomib, or radiotherapy and photodynamic therapy (PDT). Unlike normal apoptosis, which is mostly nonimmunogenic or even tolerogenic, immunogenic apoptosis of cancer cells can induce an effective antitumour immune response through activation of dendritic cells (DCs) and consequent activation of specific T cell response. Most of the agents inducing immunogenic cell death are targeting Endoplasmic reticulum (ER), leading to ER stress and production of reactive oxygen species (ROS). Both ER stress and ROS production are key players of intracellular signaling pathways that govern ICD. ICD is characterized by secretion of damage-associated molecular patterns (DAMPs).There are three most important DAMPs which are exposed to the cell surface during ICD. Calreticulin (CRT), one of the DAMP molecules, which is normally in the lumen of endoplasmic reticulum (ER), is translocated after the induction of immunogenic apoptosis to the surface of dying cell where it functions as an “eat me” signal for professional phagocytes. Other important surface exposed DAMPs are heat-shock proteins (HSPs), namely HSP70 and HSP90, which are under stress condition also translocated to the plasma membrane. On the cell surface they have an immunostimulatory effect, based on their interaction with number of antigen-presenting cell (APC) surface receptors like CD91 and CD40 and also facilitate crosspresentation of antigens derived from tumour cells on MHC class I molecule, which than leads to the CD8+ T cell response. Other important DAMPs, characteristic for ICD are secreted amphoterin (HMGB1) and ATP. HMGB1 is considered to be late apoptotic marker and its release to the extracellular space seems to be required for the optimal release and presentation of tumour antigens to dendritic cells. It binds to several pattern recognition receptors (PRRs) such as Toll-like receptor (TLR) 2 and 4, which are expressed on APCs. The most recently found DAMP released during immunogenic cell death is ATP, which functions as a “find-me” signal for monocytes when secreted and induces their attraction to the site of apoptosis." (Wikipedia Immunogenic cell death entry)Updated (2/3/16): Provectus issued a press release and made an associated 8-K filing today regarding UIC's ASC 2016 presentation, Data on Immunology Effects of Provectus Biopharmaceuticals PV-10 in Colon Cancer Presented at 11th Annual ASC Meeting. Of note was the company's CTO Dr. Eric Wachter, PhD's quote therein, with my underlined emphasis:
"The work reported in Dr. Kunda's presentation further expands our understanding of the mechanism of action of PV-10 as an ablative immunotherapy for solid tumors, and parallels immunologic signaling noted upon ablation of melanoma with PV-10."Moffitt Cancer Center previously demonstrated immunologic signaling upon ablation of melanoma in pre-clinical models, and in melanoma patients. Reproducibility, the hallmark of Western Science...
Takeaway:
- Most of the time I'm trying (just like you) to read perceived or actual tea leaves. Of course, tea leaves may just be leaves of tea.
Provectus' CFO/COO Peter Culpepper tweeted Yahoo Finance!'s edited transcript of the company's November 5, 2015 3Q15 business update conference call. The Yahoo transcript was published January 15th. Seeking Alpha published its transcript version on November 6th.
 |
| Click to enlarge. Image source. Purple emphasis is mine. |
Keytruda and pembrolizumab references (14 of them) on the November 5th call were (bolded and underlined emphasis is mine):
- "Let's start with a discussion of these pillars and focus areas with an update on our clinical trials. These include our Phase III trial for PV-10 as a treatment for Stage III melanoma; our Phase Ib/II trial of PV-10 in combination with pembrolizumab, an anti-PD-1 drug approved by the FDA end marketed by Merck as Keytruda, as a treatment for Stage IV melanoma; our ongoing studies on PV-10's use in treating cancers of the liver; and our upcoming Phase Ib/II trial in treating primary liver cancer, and our work with other solid tumor indications." — Peter
- "In this specific instance, we have completed development of the protocol for Phase Ib/II testing of PV-10 in combination with Merck's Keytruda in patients with Stage IV melanoma, and we have begun recruitment of patients. Keytruda is an immune checkpoint inhibitor approved for patients -- treatment of patients -- with advanced or unresectable melanoma." — Peter
- "Put simply, they may work better together especially for late-stage patients. And this Phase Ib/II study will help us prepare for potential marketing of PV-10 as part of a combination therapy with Keytruda." — Peter
- "When we announced the joint patent co-owned with Pfizer in August, it specifically covered the use of PV-10 to treat melanoma in liver cancers in combination with systemic inhibitors of immune system down regulation such as anti-CTLA-4, PD-1 and PDL-1 antibodies, along with enhancers of immune system up regulation such as IL-2 and interferon gamma. In other words, the Keytruda work we have begun is patent-protected." — Peter
- "As Pete noted, we selected the anti-PD-1 drug pembrolizumab, also known as Keytruda, as the checkpoint inhibitor for this study. This class of drugs has been shown to work favorably with PV-10 in mouse models of melanoma as presented by our colleagues at Moffett last November at the annual meeting at the Society for Immunotherapy of Cancer. And, as anticipated in our joint patent with Pfizer, the two drugs have largely unrelated or orthogonal side effect profiles. These factors provide justification for conducting the study. Also, since pembrolizumab is standard of care for the study's patient population, it is standard practice to conduct these kinds of studies in an add-on mode where all patients receive standard of care." — Provectus CTO Dr. Eric Wachter, PhD
- "Since pembrolizumab is licensed in the US and Australia, we were able to commerce the study without assistance of a partner. This was designed to facilitate -- this study was designed to facilitate use with other drugs to enable a similar testing in a straightforward manner. Thus, if ongoing negotiations with prospective corporate partners leads to interest in testing PV-10 with a different checkpoint inhibitor, or even in other class of systemic drug, the design can readily accommodate such interests." — Eric
- That being said, in just the category of drugs that function based on controlling immune checkpoint processes with T cells, there are three classes of drugs that are either commercial or soon to be commercial, the anti-CTL-4 drugs, which is the first generation, epitomized by ipilimumab; the anti-PD-1 drugs, which are epitomized by pembrolizumab and nivolumab. And then, following along these, I think the story next year will be the anti-PDL-1 drugs, certainly in some indications. Those all attack immune checkpoints that are involved in the function of T cells. What we have definitively seen in the work that Moffitt has reported is that PV-10 elicits a T cell response, and the translation of that into concrete results is the launching of our combination protocol using PV-10 in combination with that T cell-based drug pembrolizumab, the anti-PD-1 drug -- the direct zenith of that work reported, as you recall, in 2002. — Eric
- From a CFO/COO perspective, referring to the press release we did September 23rd, we did say that the study we are doing, the Ib/II combination study, PV-10 with pembrolizumab, is scientifically and commercially important. So when we are going back to the prior question of Joe Leo, the reason this is so critical for the industry is, we know we have the joint patent with Pfizer. That's the first key step. We also now know that we have this Ib/II combination study that has commenced. We're treating patients with PV-10 in combination with pembrolizumab. That's the second step. And the third is the immune mechanism of action clinical study, which you and Eric were just discussing, that's been underway at Moffitt Cancer Center and obviously completed recruitment. — Peter
H/t regular hatter AL:
 |
| Click to enlarge. Image source |
| Click to enlarge. |
Updated below.
 |
| Click to enlarge. Image source. Colored edits above to the original tweet's illustration are mine. |
Provectus’ outsiders have spent the well over 15 years [5] trying to convince the FDA, the medical community, Big Pharma, healthcare-focused Wall Street, biotechnology investors, and the media of the veracity of their claims about PV-10 and its potential groundbreaking therapeutic benefits for treating solid tumor cancer.
Sometimes I miss the mark in trying to communicate the kernel of the thought (the takeaway, as it were) that sparks a blog post or news item. In this case, for Time, ignore @JohnTuckerPhD's goal for his tweet above and a subsequent one that relate to antibody-based drug development programs that purportedly have intracellular ("located or occurring within a cell or cells") drug target locations.
The kernel of my thought for this news item was at least two-fold. First, I wanted to remind myself that most folks (particularly chemists) who yearn for the day when a small molecule rises up and provides robust immunotherapeutic benefits to cancer patients don't know (understandable), don't understand (less understandable, but not particularly egregious) or dismiss the fact (egregious) that Rose Bengal is a small molecule. The upshot of Rose Bengal's small molecule-ness is that part of the benefit a patient derives is from PV-10/Rose Bengal as a chemical cancer therapy, and not a biological therapy like anti-CTLA-4 (Yervoy), anti-PD-1 (Keytruda, Opdivo) and anti-PD-L1 approved drugs and investigational agents. See December 20, 2015 blog post An incomplete thought.
 |
| Click to enlarge. |
[1] Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie, 1887; 238: 318-338
[2] The Rose Bengal test for liver function, Delprat, G.D. et al., The Journal of Laboratory and Clinical Medicine, Volume 16, Issue 9, 923-925
[3] Induction of thyroid tumors in (C57BL/6N x C3H/N) F1 mice by oral administration of 9-3',4',5',6'-tetrachloro-o-carboxy phenyl-6-hydroxy-2,4,5,7-tetraiodo-3-isoxanthone sodium (Food Red 105, rose bengal B), Ito et al., J Natl Cancer Inst. 1986 Jul;77(1):277-81
[4] American Society of Clinical Oncology, 2010 Annual Meeting and Rose Bengal: From a Wool Dye to a Cancer Therapy, Alexander, W., Pharmacy & Therapeutics. 2010 Aug; 35(8): 469-474, 478
[5] Provectus' Chairman and CEO Dr. Craig Dees, PhD starts counting at the inception of Provectus in 2002 (which is going on about 14 years). I don't. Rather, I start counting when they re-discovered Rose Bengal in 1998 as an antineoplastic candidate (which elongates the story to about 18 years).
Intracellular drug target locations & small molecules? Ask Genentech.
 |
| Image source |
Updated below, again.
* Perfect is the enemy of good (Wikipedia entry).
I suppose there might've been at least two reactions to Provectus' January 13th press release, Confirms First Patients Dosed in Trials of PV-10 for Melanoma. One, yawn. Two, the company began dosing in 2016. Three, Provectus confirmed patients already were/had been treated (in both melanoma trials).
In fact, one entity/individual had the second and third reactions, in that order. BioPharmCatalyst is a website by a random person on the Internet (kind of like this blog and me) tabulating and collating FDA approval, PDUFA and clinical trial catalysts. After the PR came out BioPharmCatalyst or @crusadernz tweeted:
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
To be clear, I like and embrace Eric's approach to the company's clinical development program's process. He is the right person in the right place at the right time, both among Provectus' principals, and from an industry perspective in regards to intralesional (IL) agents and IL agent experience in oncology. I draw the distinction between developed expertise and experience in the pursuit of generating meaningful data, and the communication of it. I think Eric places different levels of importance on things than other members of management do, and certainly most investors do.
One message might've been that Provectus has been treating patients in its pivotal Phase 3 trial (for Stage III melanoma). Although it's spilt milk/water under the bridge by now, management clearly was being reactive in addressing the topic of whether the first patient, and patients in generally, already was/were treated. Other than acquiescing to internal and external pressures, what was Eric's intellectually consistent rationale to put out this PR 9 months late?
I thought a more important message might have been in regards to the Phase 1b study (for Stage IV melanoma patients). Here, I believe, Eric has seen or been told of preliminary data from this clinical trial. Those very early results, building on pre-clinical combination work undertaken by both Provectus (SITC 2012, AACR 2013) and Moffitt Cancer Center (SITC 2014), may well have informed his statements and comments in the PR -- that the effect size of adding pembrolizumab to PV-10 is potentially much larger (and perhaps much larger than expected) than pembrolizumab alone in this patient population. These comments were:
"A total of an estimated 120 subjects will be randomized in a 1:1 ratio to the two treatment arms (i.e., PV-10 + pembrolizumab or pembrolizumab alone) in the Phase 2 portion of the study. This number of subjects may be modified based on emerging evidence of preliminary efficacy and effect size from the Phase 1b portion of the study."As I wrote under "FPFV" (January 12, 2016) below, in Provectus' October 2015 press release about the melanoma combination trial, Announces Initiation of Phase 1b/2 Clinical Trial to Study PV-10 in Combination with Immune Check Point Inhibitor Pembrolizumab, Eric noted: "We will use an adaptive design for powering Phase 2 based on preliminary results from Phase 1, and estimate this portion of the study to require at least 120 subjects, with a primary endpoint of PFS and key secondary endpoint of ORR. In regards to the number of patients required for a Phase 2 trial, I believe a floor of 120 subjects from October 2015 may have changed to a ceiling of 120.
New SEC filing. Provectus' amended S-4 (for the tender offer related to non-tradable warrants with varying exercise prices) notes the following primary changes (on the right, when compared to the initial S-4 filed on December 31st) (I used Text Compare!):
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated (1/14/16): Management's guidance in regards to an interim assessment of efficacy and safety (i.e., interim data readout) of Provectus' pivotal melanoma Phase 3 trial (underlined emphasis below is mine):
"We have an estimated primary completion date of September 2017 and an estimated study completion date of October 2017. An interim assessment of efficacy and safety will be performed by the independent data monitoring committee when 50 percent of the events required for the primary endpoint have occurred. Therefore, meaningful clinical data are potentially available via an interim analysis on a shorter timeline possibly around this time next year." — Peter, August 6, 2015 2Q15 business update conference callH/t InvestorVillage poster leave_the_gun: Reiterated guidance (bolded and underlined emphasis below is mine):
"Our estimated primary completion date is September 2017, and an estimated study completion date of October 2017. When 50 percent of the events required for the primary endpoint have occurred, the Independent Data Monitoring Committee will report an interim assessment of efficacy and safety. So, meaningful clinical data could come as early as the middle of next year, which is halfway through the study, as documented on clinicaltrials.gov. I stretch the word could, and we will continue to make every effort possible to keep our stockholders and the market updated." — Peter, November 5, 2015 3Q15 business update conference callUpdated (1/14/16): There was nothing new in the company's Biotech Showcase 2016 presentation in San Francisco on Wednesday, January 13th. The compassionate use program (CUP) slide was updated to reflect information discussed by Eric on the November 5, 2015 3Q15 business update conference call -- that CY 2014 CUP-treated patients numbered 40 (given a cumulative number through CY 2013 of 100):
"With regard to other activity in melanoma, we expect to provide an update on the status of our expanded access protocol by the end of the calendar year. And I'll note for now that approximately 140 patients have received PV-10 under this program."Although Peter continues to stick by previous management guidance (see above) of a mid-2016 pivotal melanoma Phase 3 trial interim data readout, based of course on Eric's guidance (as head of and fully/solely responsible for Provectus' clinical development program), a potential risk is that Eric's timing guidance to him is wrong. I don't think there is much doubt about the outcome of efficacy and safety. Enrollment rate strongly influences, if not entirely determines, timing. Of course, timing may boil down to a minimum patient number threshold to achieve statistically significant progression-free survival curve separation between the trial's treatment and control arms. Timing also may boil down to whether there indeed is an unspoken prescribed time-based trigger for the interim assessment of efficacy and safety, in addition to the spoken prescribed event-based trigger.
"FPFV" (January 12, 2016)
Updated below, again.
Provectus issued a press release and made a related 8-K filing today regarding patient enrollment in its two melanoma studies and trials, Confirms First Patients Dosed in Trials of PV-10 for Melanoma. Of note in the PR (from top to bottom, quotations are taken from the release):
- Phase 3 trial. "The primary outcome measure of PFS is assessed every 12 weeks up to 18 months using RECIST 1.1 criteria."
- Does this mean Provectus looks ("peeks," statistically speaking) at the data every 3 months (12 weeks)?
- For the purposes of measuring progression-free survival (PFS), the pivotal melanoma Phase 3 trial's primary endpoints, PFS (via appropriate measurement of tumor regression or progression) is assessed every 12 weeks (3 months). Patients, however, are clinically assessed at the end of each cycle, which is 28 days (4 weeks or 1 month), which may then facilitate crossover (from the control arm to the treatment arm) if the he or she is deemed to progress. As a result, progression of a patient in the control arm, and thus "failure," might occur well prior to 12 weeks when an assessment of progression via tumor measurement is made.
 |
| Click to enlarge. Image source |
- "We are currently finalizing amendments to the protocol that will refine the eligible patient population, consistent with a trial to be expanded this year beyond our historic base in the U.S. and Australia, and to afford additional flexibility in choice of comparator to address the changing treatment options available to patients globally."
- "[F]inalizing amendments" may refer to the loosening of inclusion criterion no. 8, "Failed, did not tolerate, or not a candidate for (due to co-morbidities, pre-existing autoimmune disease or drug unavailability) treatment with ipilimumab or another immune checkpoint inhibitor."
- "[A]dditional flexibility in choice of comparator" should refer to the addition of intralesional agent Imylgic (T-Vec), which was approved for patients with advanced melanoma in 2015.
- If there is a delay in the estimated primary completion date of the trial as a result of perceived slower than expected patient enrollment, and thus the interim readout (i.e., halfway between the study start and estimated primary completion dates), I would imagine (and believe) Provectus' CTO Dr. Eric Wachter, PhD would make this change when he files the protocol amendments on ClinicalTrials.gov. See No change (January 8, 2016) below.
- If there is no delay (my sense is that enrollment should increase with the planned changes to the protocol), he would not change the estimated primary complete date, and thereby signal no change to the June/July 2016 timing of the interim data readout.
- Updated (1/12/16): "Additional sites are in process of being added in the coming weeks."
- In Provectus' April 2015 press release about the start of the trial, Opens Patient Enrollment; Begins Phase 3 International FDA Comparative Clinical Trial of PV-10 for Melanoma, management guided essentially the same thing (i.e., "coming weeks") -- "The Company is seeking 225 patients and enrollment has begun at St. Luke's University Hospital and Health Network, Bethlehem, PA, the first study site to be opened, with additional sites to be added in the coming weeks and months." Underlined emphasis is mine. Why would anyone believe Eric on timing again?
- Phase 1b/2 trial. "The expected completion date is in 2016 for the Phase 1b portion of the study."
- The above may lend credence to the speculation Provectus is planning to present interim data at ASCO 2016.
- Updated (1/12/16): "A total of an estimated 120 subjects will be randomized in a 1:1 ratio to the two treatment arms (i.e., PV-10 + pembrolizumab or pembrolizumab alone) in the Phase 2 portion of the study. This number of subjects may be modified based on emerging evidence of preliminary efficacy and effect size from the Phase 1b portion of the study." Bolded and underlined emphasis is mine.
- In Provectus' October 2015 press release about the melanoma combination trial, Announces Initiation of Phase 1b/2 Clinical Trial to Study PV-10 in Combination with Immune Check Point Inhibitor Pembrolizumab, Eric noted: "We will use an adaptive design for powering Phase 2 based on preliminary results from Phase 1, and estimate this portion of the study to require at least 120 subjects, with a primary endpoint of PFS and key secondary endpoint of ORR." In regards to the number of patients required for a Phase 2 trial, has "at least" from October 2015 potentially changed to a lower figure (i.e., "[t]his number of subjects may be modified")?
- It strikes me that Eric may be hoping to set up the Phase 2 trial (presumably with FDA input and/or acquiescence and/or acceptance) as a pivotal study.
E/n: My language above assumes, very roughly, 7-day weeks and 4-week months.
Lawsuit "update." InvestorVilllage poster Area51 has posted updates on Provectus' lawsuits.* It appears the source of this information is the TNED (Tennessee Eastern District) PACER (Public Access to Court Records) website. Area51's last update on December 20, 2015 noted no items added to the court docket since November 4th. As of this writing, there were no additions to the docket.
| Click to enlarge. |
This does not mean there has been no or any progress between the parties to resolve the matter.
Updated (1/12/16): * See, for example, Lawsuit Update (November 4, 2015), Friday (October 2, 2015) and Tuesday (September 1, 2015) on the blog's Archived News IV page.
Updated (1/12/16): * See, for example, Lawsuit Update (November 4, 2015), Friday (October 2, 2015) and Tuesday (September 1, 2015) on the blog's Archived News IV page.
Oz (January 11, 2016)
As of this writing, on Provectus' ClinicalTrials.gov webpage, Princess Alexandra Hospital of Brisbane (Queensland), Australia is listed as "Not Yet Recruiting" for both PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma and PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma.
On Australia's clinical trials registry website, Provectus' studies above are listed as Recruiting for Princess Alexandra Hospital.
Is the Australian site recruiting patients? It is possible the Recruiting reference refers to the sites already recruiting (and the US clinical trials registry), and not specifically to Princess Alexandra.
In November 2010 Provectus issued press release Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia in which the company noted:
The hatter is referring to the below (with the purple emphasis being mine).
We're all trying to figure out what is going in Australia in regards to PV-10.
On Australia's clinical trials registry website, Provectus' studies above are listed as Recruiting for Princess Alexandra Hospital.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
In November 2010 Provectus issued press release Meets with the Therapeutic Goods Administration to Review Path for Approval of PV-10 in Australia in which the company noted:
"The proposed primary endpoint of progression free survival, which Provectus proposed to the U.S. Food and Drug Administration (FDA) earlier this year in its first end-of-Phase-2 meeting with FDA, was deemed appropriate for assessment of efficacy in light of established European Medicines Agency (EMEA) standards adopted by TGA. Use of interim data from the first half of Phase 3 study subjects, in conjunction with safety data collected in earlier studies of PV-10 for melanoma, was discussed to allow early evaluation for marketing approval for metastatic melanoma, and TGA agreed that these data should be sufficient for this review if the analysis confirmed efficacy."In the company's April 2012 annual CEO letter management wrote:
"As you may recall, too, the Australian Therapeutic Goods Administration ("TGA") has agreed in November 2010 to the same primary endpoint of progression free survival that has been proposed to the FDA. In our meetings with TGA we discussed the use of interim data from the first half of Phase 3 study subjects, in conjunction with safety data collected in earlier studies of PV-10 for melanoma, to allow early evaluation for marketing approval in Australia for metastatic melanoma. TGA agreed that these data should be sufficient for this review if the analysis confirmed efficacy."One regular blog hat tipper (hatter) wrote in regards to Australia and another Aussie trial being run at the same Princess Alexandra hospital using a different compound, Topical Imiquimod or Diphenylcyclopropenone for the Management of Cutaneous In Transit Melanoma Metastases – A Phase II Single Centre Prospective Randomised Pilot Study: "The Australia study involving topical imiquimod had a start date of February 1, 2016 and excluded patients treating with PV-10 within 12 weeks of starting the trial. I think the Aussies are anticipating PV-10 will be approved there in 2016." Maybe. Maybe not.
The hatter is referring to the below (with the purple emphasis being mine).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
MIT > Wisconsin (January 11, 2016)
Underlined emphasis below is mine. In an August 3rd article by Forbes.com's Matthew Herper entitled Lexicon's Quest For A New Blockbuster, he quotes Brent Stockwell, a scientist at the MIT-affiliated Whitehead Institute for Biomedical Research (who now appears to be at Columbia University):
"Most drugs are really German dyes." — Rose Bengal, the active ingredient in PV-10 is a water soluble German industrial dye (Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie 1887; 238:318–338)
"...immunomodulators need to be delivered to the right cells in the right tissue microenvironments so that the total dose required is minimized..." — Rose Bengal has been used as a stain for visualizing corneal ulcers and a marker for impaired liver function (Norn MS. Acta Ophthalmol 1970;48(3):546-559 and Delprat GD. Arch Int Med 1923; 32(3):401–410, respectively)
"In a sense, the pharmaceutical industry has been working with a surprisingly repetitive set of compounds. “Most drugs are really German dyes. That’s because they’re based on what has worked before–mostly compounds that evolved from drugs developed at dye companies like F. Bayer & Co. a century ago."
In a Nature Reviews Materials comment published today entitled Materializing the future of vaccines and immunotherapy, MIT professor Darrell Irvine (Department of Biological Engineering and Department of Materials Science & Engineering) wrote:
How can we do better? There are at least two objectives to be met: immunomodulators need to be delivered to the right cells in the right tissue microenvironments so that the total dose required is minimized, and methods to minimize systemic exposure or dissemination of these potent therapeutics must be developed. Engineered materials provide numerous strategies to achieve these goals, through the use of implantable scaffolds or hydrogels that locally provide immunoregulatory cues, nanoparticles that can be injected intratumorally and remain trapped in the local tissue, or particles that are administered systemically and accumulate in tumours with systemic exposure times that are much shorter than those for free biologics such as antibodies...Traditional pharmaceutical companies do not have an established infrastructure to develop these complex products, but I suspect it will take only one compelling, successful example in humans to drive this field forward.
 |
| Image source |
"...immunomodulators need to be delivered to the right cells in the right tissue microenvironments so that the total dose required is minimized..." — Rose Bengal has been used as a stain for visualizing corneal ulcers and a marker for impaired liver function (Norn MS. Acta Ophthalmol 1970;48(3):546-559 and Delprat GD. Arch Int Med 1923; 32(3):401–410, respectively)
"...injected intratumorally and remain trapped in the local tissue..." — PV-10 is administered intralesionally or intratumorally
"...accumulate in tumours with systemic exposure times that are much shorter..." — The half-life of PV-10, from a pharmacokinetic perspective, is measured in hours (for, thus far, melanoma and liver) (ASCO 2010 and ESMO World GI Congress 2015, respectively)
H/t @bradpalm1's tweet:
A key aspect of PV-10's clinical value proposition is the local effect of tumor ablation upon injection (the first of its dual mechanisms of action) that produces the systemic effect of a tumor-specific immune response and eventually anti-tumor immunity (the second mechanism).
Moffitt Cancer Center's SITC 2014 poster "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma" described PV-10's synergy with anti-CTLA4, PD-1 and PD-L1 agents.
 |
| Click to enlarge. |
He is referencing a January 6, 2016 transcript entitled Radiation Therapy and Abscopal Effect in Melanoma Treatment of an OncLive panel. Underlined emphasis below is mine.
Dr. Keith Flaherty, MD of Massachusetts General Hospital sets the scene by saying:
I wanted to focus a little bit on radiation therapy, pivoting a little bit from this injectable-lesion concept to radiation as an adjunct to systemic therapy. This has been particularly popular in terms of immune therapy. Jason, what’s your current take on emerging data and current clinical practice considerations in terms of weaving radiation therapy into a patient’s management?
Dr. Jason Luke, MD of University of Chicago Medicine replies:
I think it’s a two-fold, two-pronged approach. One is we have highly effective therapies now, but directed radiation to a lesion that’s symptomatic is also highly effective. So the question becomes: is that palliative approach useful and is there more beyond that? Because, as you’re alluding to, there’s pretty good preclinical data suggesting that you can actually get some distant effects with radiation with upregulation of other immune molecules like PD-L1.
Perhaps that has the potential to augment the activity of other immunotherapy. In my practice, I don’t necessarily go around looking for lesions that I want to radiate. But if it comes up that a patient has a symptomatic lesion, I certainly don’t hesitate to say I’m going to add radiation to that lesion, both palliatively, as well as hopefully, to sort of amplify an immune response that’s already ongoing.
Dr. Jeffrey Weber, MD, PhD of NYU Langone Medical Center notes during the panel:
There’s a long history to the so-called abscopal effect. I guess you could define it as a scenario where you deliver a local therapy that causes local destruction of tumor, and at some point, subsequently, there’s a systemic effect where you have regression of distant disease...
There was a 2012 article in the New England Journal of Medicine, where Michael Postow and Jedd Wolchok’s group published a very interesting case of a person who had obvious progression in multiple lesions on ipilimumab, in a clinical trial—I forget the dose, I think it was probably around 1 or 3 mgs/kg—who then had a growing painful pleural-based lesion that was posterior, so it was invading into the ribs...
...There was a prostate study with ipilimumab and radiation, and it wasn’t obvious that there was any abscopal effect at all because the ipilimumab didn’t really add to the effect of the radiation. You figure if ipilimumab with radiation together were included, you would get some abscopal effect, which promote the activity of ipilimumab in prostate cancer, which you really didn’t see in that randomized study.Another way of referring to the abscopal effect is to call it the bystander effect, which has been used in the context of PV-10. The OncLive panel (at least Dr. Weber) does not label the combination of radiation (radiotherapy) and immune checkpoint inhibitors as synergistic.
A key aspect of PV-10's clinical value proposition is the local effect of tumor ablation upon injection (the first of its dual mechanisms of action) that produces the systemic effect of a tumor-specific immune response and eventually anti-tumor immunity (the second mechanism).
Moffitt Cancer Center's SITC 2014 poster "Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma" described PV-10's synergy with anti-CTLA4, PD-1 and PD-L1 agents.
Provectus management has said the preponderance of non-clinical data support the concept of combining PV-10 with a drug like pembro (Keytruda). The former elicits a functional anti-tumor T cell response in patients, while the latter increases the anti-tumor function of T cells.
In the case of the OncLive panel, radiation/radiotherapy has the potential to elicit a functional anti-tumor T cell response in patients, but it would appear the checkpoint inhibitors cannot increase the anti-tumor function of T cells as a result (for this pairing of radiation and checkpoint inhibitors).
Recall Foote et al.'s initial work "A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series," Melanoma Research, 20:48, Jan 2010 that discussed three situations where radiotherapy was combined with PV-10 (first PV-10, second radiotherapy). Also recall Tan et al's observation "Novel use of Rose Bengal (PV-10) in two cases of refractory scalp sarcoma," ANZ Journal of Surgery, 83:1-2:93, Jan 2013 that also discussed the combination of radiotherapy and PV-10 (first radiotherapy, second PV-10).
Foote is conducting a much larger trial of 25 patients to explore the clinical benefits of combining PV-10 and radiotherapy. See Below expectations under Cheers (January 1, 2016) below.
Promise (January 9, 2016)
Updated below.
H/t AL, a shareholder, blog reader and regular hatter for this news item.
In an article entitled International Symposium on Melanoma May Set Foundations for Clinical Decision Making by Sandra Kear and published online in TargetedOncology on January 5th, Dr. Omid Hamid, MD*, co-chair of the 12th Annual International Symposium on Melanoma and Other Cutaneous Malignancies (February 20th) notes:
 |
| Click to enlarge. |
Bristol-Myers Squibb, GlaxoSmithKline, Roche, Merck, Incyte, Eisai, MedImmune, Merck-Serano, Pfizer, Novartis, Immunocore; Consultant: Bristol-Myers Squibb, Roche, Genentech, Amgen, Merck; Speakers Bureau: Bristol-Myers Squibb, Genentech, GlaxoSmithKline* Chief, Translational Research and Immunotherapy, Director, Melanoma Therapeutics, The Angeles Clinic and Research Institute, Los Angeles, CA
Updated (1/11/16): Dr. Hamid noted investigational strategies in late-stage development with the promise of changing melanoma treatment that included IDO inhibitors (and PV-10). Note the below from an article by FierceBiotech's John Carroll today entitled Merck buys out IOmet, adding new immuno-oncology tech:
"Merck has snagged Edinburgh-based IOmet for an undisclosed sum, adding a preclinical pipeline of therapies that target the IDO/TDO pathways that may help spur an immune system attack on cancer...IDO/TDO are two pathways that have attracted considerable attention from pharma companies zeroing in on immunotherapy. Roche struck a deal with India's Curadev last April, paying $25 million up front and offering $530 million in milestones for access to its IDO/TDO work. Roche ($RHHBY) is considered just one step behind Merck and Bristol-Myers Squibb ($BMY) with the PD-L1 checkpoint program for atezolizumab. Incyte ($INCY) has already paired with Merck's Keytruda--and other checkpoints--on its IDO1 drug epacadostat (INCB24360)."
Updated below.
 |
| Image source |
The trial's .gov webpage of course notes the interim assessment will be performed by the study's independent review committee (IRC) when 50% of the events required for the primary endpoint (progression-free survival [PFS]) have occurred.
 |
| Image source |
I don't believe the pivotal Phase 3 trial should be stopped early, although patients in the control arm should be immediately conveyed to the treatment arm once statistically significant separation of the PFS curves is deemed achieved by the study's independent data monitoring committee. The only way to fully prove one of the trial's two hypotheses is to continue measuring overall survival of patients, irrespective of whether they started out in the treatment arm or crossed over into it. The trial needs to establish that, for Stage III patients, treating all disease with PV-10 can forestall or prevent the spread of melanoma to Stage IV.
Halfway between the study start date and the estimated primary completion date is June/July 2016.
 |
| Click to enlarge. |
Updated (1/8/16): From life sciences venture capitalist Bruce Booth's blog post Sentiments Likely To Be Overheard At #JPM16.
| Click to enlarge. |
Updated below, again.
Intralesional agent for oncology experience.
 |
| Click to enlarge. |
- Amgen's Imylgic (T-Vec) (formerly called OncoVEX), which was approved in October 2015,
- Provectus' PV-10, which currently is in Phase 3 as of April 2015, and
- Vical's Allovectin-7, which failed its pivotal Phase 3 trial in August 2013.
Disease: Where Will it Fit in?.
 |
| Click to enlarge. |
If I was a client of theirs, and knowing Provectus' CTO Dr. Eric Wachter, PhD, CFO/COO Peter Culpepper, and board of directors member Jan Koe's already have signaled their intention to exercise 100% of their ~1.3 million non-tradable warrants for a ~$960K investment in the company, I'd want to know what amount of warrants and what proportion of warrant holdings Testaverde, Hemming and the firm intend to exercise before making my decision. Going by Provectus' SEC filings, it would appear Network 1 owns ~4.9 million warrants, or a potential ~$3.7 million investment in the company. See Moneyness (January 2, 2016) below.
A co-development deal for the combination of PV-10 and [fill in the blank]. At this point, it makes sense a co-development "deal" between Provectus and, say, Merck & Co. merely means the latter supplies its drug pembrolizumab (Keytruda) at no cost to the former for it to conduct early-stage clinical trials combining PV-10 and the checkpoint inhibitor in indications besides melanoma. I suppose the question for me is what indications.
Updated (1/7/16): S-4. It makes sense to read the registration statement. Of note, perhaps:
- The company appears to have issued ~1.5 million warrants ($1 exercise price) -- to consultants in exchange for services, others?
| Click to enlarge. |
- What does this mean, exactly? (breast cancer, p. 21): "Assessing further development in 2013, 2014 and 2015 in conjunction with Moffitt Cancer Center research."
- The expanded Phase 1 liver trial continues, I presume (liver metastasis, p. 21): "Expanded Phase 1 trial, currently in progress, is expected to continue in 2016."
- The tender's background (pp. 22-23): "Since 2011, the Company has issued the Existing Warrants in transactions exempt from the registration requirements of the Securities Act. After the issuance of the June 2015 Warrants in the Company’s June 2015 public offering, the Company became interested in having the Existing Warrants listed on the NYSE MKT and simplifying its capitalization structure by consolidating its classes of outstanding warrants. The Company completed the June 2015 Offering of Common Stock and June 2015 Warrants on June 24, 2015, underwritten by Maxim. Following the June 2015 Offering, the Company believed there was investor interest in purchasing the June 2015 Warrants. In July, August and September 2015, the Company held a series of discussions with its advisors, including Maxim, regarding the idea of exchanging some or all of its outstanding warrants issued in one or more private placements for June 2015 Warrants. As a result of these discussions, the Company determined that it would be desirable to have these warrant holders exchange their warrants for warrants that were the same class as the June 2015 Warrants and cash in order to simplify its warrant structure and raise capital. In October 2015, the Company and its advisors evaluated the merits of the Offer, including the potential for listing the new warrants, the Replacement Warrants, on the NYSE MKT. On October 14, 2015, the Company met with Maxim and decided to formally pursue the Offer. On December 10, 2015, the Company held a meeting of its Board where the Board discussed the Offer and its intended purposes and reasons for entering into such Offer. The Board acted by unanimous written consent to approve a Letter of Engagement between the Company and Maxim on December 14, 2105, at which time Maxim was formally engaged as the Company’s advisor and dealer manager for the Offer. On December 31, 2015, Maxim entered into an agreement with Network 1, pursuant to which Network 1 agreed to act as co-dealer manager for the Offer. On December 30, 2015, the Board acted by unanimous written consent to approve the final terms of the Offer and to enter into the transactions contemplated thereby.
- One purpose of the tender (p. 23): "The purpose is also to simplify the Company’s outstanding warrants by making the terms of the Company’s outstanding warrants the same so investors can more easily understand its capital structure."
 |
| Click to enlarge |
- The apparent implied volatility for warrant pricing calculations (p. 34)" "The value of 59,861,601 Replacement Warrants to be issued as an inducement in the Exchange Offer based on the 59,861,601 Existing Warrants outstanding prior to December 28, 2015, using a Black-Scholes model assuming a stock price of $0.39, resulting in an estimated expense of approximately $5,434,000. The fair value of the modified Existing Warrants was determined to be zero as of December 28, 2015 using a Black-Scholes model." I've been a little lazy with my option pricing gymnastics below. The above could suggest a premium of ~$0.09 ($5,434,000 ÷ 59,861,601), which could mean a vol of 50-55%. See the worthless online calculator to the right.
Updated below, again.
PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma:
PV-10 in Combination With Pembrolizumab for Treatment of Metastatic Melanoma: MD Anderson, and Princess Alexandra Hospital (both not yet recruiting)
Updated (1/6/16): My error: Phase 3 sites above that are struck out already were on the ClinicalTrials.gov site. A table of the pivotal melanoma (Stage III) Phase 3 trial and related qualitative interview study is below.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source. Reddish edits (white background) are mine. |
My goal for the following blog news item is to speak accurately but precisely. The below ignores transactions costs and the realities of supply & demand (bid-offer) when discussing how and how much the tradable warrant price (PVCT.WS) might move up with an increasing share price (PVCT).
 |
| Image source |
As I wrote in Moneyness (January 2, 2016) below, I contended that Provectus' tender offer is out-of-the-money (OTM), and that if the share price does not rise materially before that date (that is, if no news revealing or signaling a sustainable, long-term measure of business success for Provectus), then the tender would remain OTM. A warrant holder then/thus would not tender their non-tradable warrants.
I did a quick 'n dirty analysis of the potential breakeven for the tender offer -- i.e., an exercised non-tradable warrant turning into a common share with a cost basis of $0.75, and a tradable warrant priced by market supply & demand. I used rough as starting points a share price of $0.35 and a warrant price of $0.20. I used this online Black-Scholes option pricing model calculator with the assumptions noted in the table below. I added the increase in the warrant price for every 5 cents increase in the share price to the $0.20 starting point.
Roughly speaking, the tender offer only begins to approach contemplation as attractive north of a share price of $0.50, which may drag the warrant price up to $0.25, for a total of $0.75, the cost of the tender.
In truth, and reality, the share price more than likely would have to increase much, much more, and the news flow that presumably underlaid said increase would have to be meaningful and thoughtful and promising and attractive and point to a sustainable long-term positive story for PV-10 and Provectus so as to start cleansing management's soiled credibility as public company managers.
In truth, and reality, the share price more than likely would have to increase much, much more, and the news flow that presumably underlaid said increase would have to be meaningful and thoughtful and promising and attractive and point to a sustainable long-term positive story for PV-10 and Provectus so as to start cleansing management's soiled credibility as public company managers.
 |
| Click to enlarge. |
 |
| Click to enlarge. |
Updated below.
Previous related blog posts and news items, all on the blog's Archived News IV page:
- Prom (December 11, 2015),
- Patient-reported outcomes (PROs) (November 4, 2015),
- Recruiting (September 2, 2015),
- Assessing Provectus' Pivotal Melanoma Phase 3 Trial, Part V (July 24, 2015),
- Qualitative Interviews with people with Locally Advanced Cutaneous Melanoma (July 23, 2015), and
- New Moffitt Cancer Center study related to PV-10? (July 21, 2015).
In addition to Moffitt Cancer Center (Tampa, FL) and Gabrail Cancer Center (Canton, Ohio), Huntsman Cancer Institute (Salt Lake City, UT) appears to be conducting a clinical study entitled "Qualitative Interviews with People with Locally Advanced Cutaneous Melanoma." See a screenshot from Huntsman's website for melanoma trials. Note the principal investigator is Dr. Robert Andtbacka, MD, who also has been an investigator for Amgen's intralesional agent T-Vec (Imylgic).
 |
| Click to enlarge. Purple emphasis is mine. |
Provectus' pivotal melanoma Phase 3 trial is entitled "PV-10 Intralesional Injection vs Systemic Chemotherapy for Treatment of Locally Advanced Cutaneous Melanoma" {underlined emphasis is mine}, which is the only trial the pulls up on ClinicalTrials.gov when you search using the following phrase "Locally Advanced Cutaneous Melanoma."
Updated (1/4/15): The addition of the Huntsman study interests me because I think it is related to the PRO component of the pivotal melanoma Phase 3 trial, and eventually to the drug labeling claim(s) of PV-10 for the patient population when in commerce.
The Engelberg Center for Health Care Reform at the Brookings Institution noted in its July 2014 discussion guide Enhancing the Development and Use of Patient-Reported Outcomes in Drug
Development:
Of note about T-Vec:
Was he referring to PV-10?
Network 1, Maxim, China, etc. (January 3, 2016)Updated (1/4/15): The addition of the Huntsman study interests me because I think it is related to the PRO component of the pivotal melanoma Phase 3 trial, and eventually to the drug labeling claim(s) of PV-10 for the patient population when in commerce.
The Engelberg Center for Health Care Reform at the Brookings Institution noted in its July 2014 discussion guide Enhancing the Development and Use of Patient-Reported Outcomes in Drug
Development:
"Sponsors routinely use PRO instruments to collect a range of health-related quality of life data in pivotal clinical trials prior to approval. However, this information is typically gathered on an exploratory basis, or is intended to demonstrate the benefit of a new treatment to payers making coverage and reimbursement decisions, rather than to support labeling claims. As a result, these measures may lack analytical or statistical validity, or may not be included in publications on trials results."And:
"The use of PRO instruments in clinical trials has grown in recent years. A 2009 analysis of ClinicalTrials.gov found that 14% of the interventional clinical trials registered between 2004 and 2007 used some sort of PRO measure, compared to 4.2% from the period between 1980 and 1997. However, while their value is widely recognized, their use is often inconsistent, and some have argued that they are underutilized in many disease areas that would benefit from an increased focus on how patients feel or function in relation to their disease, such as in cancer, cardiovascular disease, and diabetes. Of particular concern is the relative lack of PROs being used to support labeling claims. A recent review observed that the number of PRO claims approved by the FDA for inclusion in drug labeling fell slightly over the last several years. Whereas 30% of drug approvals granted between 1997 and 2002 included a PRO claim, between 2006 and 2010 this rate fell to 24% of drugs approved. Labeling claims constitute the portion of a drug label that manufacturers can legally use to promote their products, and as such are considered to be of primary importance, both to manufacturers seeking to distinguish their products in the market and to clinicians seeking information to support their prescribing choices."Off-topic Item. H/t a shareholder and regular hatter who pointed me to an article published December 28th entitled Practical Application of T-VEC in Melanoma Treatment, which was an interview of Dr. Keith Flaherty, MD, Massachusetts General Hospital, Dr. Rene Gonzalez, MD, University of Colorado Melanoma Research Clinics, Dr. Jason Luke, MD, University of Chicago Medicine, and Dr. Jeffrey Weber, MD, PhD, NYU Langone Medical Center.
Of note about T-Vec:
| Click to enlarge. |
| Click to enlarge. |
 |
| Click to enlarge. |
A follow-up to the table of whom held what warrants issued issued (as a result of related fund raising) between January 6, 2011 and November 1, 2015 -- see Moneyness (January 2, 2016) below -- is a table of the fund raising dollars. While roughly 85% of the warrants involved in the recently announced tender offer are held by Network 1 Financial's clients and the firm, Network 1 was responsible for approximately 70% of the money raised during the period in question.
 |
| Click to enlarge. |
Network 1 and Maxim touch points with Provectus over the years might include:
 |
| Click to enlarge. |
I also recall, at the time, a discussion about eventually making the existing non-tradable warrants available for trading. I had wondered how one would conform the different features of the non-tradable warrants (i.e., different exercise prices and expirations) to the tradable warrants (a $0.85 exercise price, a June 19, 2020 expiration), but didn't really grasp or focus on a possible approach. Now I know.
It would seem former/current (?) Provectus strategic advisory board (SAB) members and current/former Boehringer Ingelheim folks Dr. Joseph Chalil, MD and Christopher Kaplan are a package deal. They were named to the company's SAB in March 2014 and September 2014, respectively. In 2015 they were named to the SABs of Medifocus in June (MFS.V, a market cap of ~$7 million) and Oxysure in September/October (OXYS, OTC, ~$7 million). Medifocus was/is a client of Porter, LeVay & Rose, which also is Provectus' investor relations firm.
In July 2015, Provectus and Boehringer Ingelheim (BI) China made announcements of their entering into/signing a letter of intent: Provectus Biopharmaceuticals Signs Letter of Intent with Boehringer Ingelheim (China) to Collaborate in Bringing PV-10 to Market in China (Provectus' PR on July 2nd) and Boehringer Ingelheim and Provectus biopharmaceutical company signed a letter of intent - the two sides will cooperate to promote melanoma and liver cancer research new drugs listed in the Chinese mainland, Hong Kong and Taiwan (BI's PR, translated into English, on July 3rd).
On July 10th, Jialing Dai wrote a LinkedIn article entitled Boehringer's Ambition In Liver Cancer. The article's byline read: "Eye On Oncology Market: Boehringer Ingelheim In-Licenses China Rights For A Provectus Late-Stage Oncology Compound." The article was edited by Howard Fields. Dai describes his some of his work as "PharmaDJ is online portal in English to provide tailored services and intelligence of China market for pharma executives. our goal is to build up a bridge to China market for foreign companies doing business in China." In his article Dai wrote:
"PV-10, an injectable formulation of rose bengal, also known as Provecta, is the leading compound of the Knoxville, Tennessee-based company. The firm received orphan-drug designation from the U.S. FDA for treating of hepatocellular carcinoma (HCC) in 2011 and melanoma in 2007. Currently, the drug is in a U.S. Phase III clinical trial for melanoma and Phase I trial for HCC.
“We need to wait on the outcomes of these studies before deciding to file or not with CFDA, but we certainly have an interest in developing this drug in HCC in China, as there is a large unmet medical need,” BI China told PharmaDJ in an interview." {Underlined emphasis is mine}Dai also noted in his article the below:
"The German pharma giant doesn't have a footprint in the China oncology market so far. However, BI is intent on building its oncology platform in the country, where it filed an approval application in 2013 for its first oncology product, Giotrif (afatinib).
“We are now awaiting CFDA’s decision regarding our application, but hope to have a positive opinion from CFDA very soon so that we can provide Giotrif to NSCLC patients in China,” BI said. Giotrif was approved in the U.S. and Europe in 2013." {Underlined emphasis is mine}In December Sanofi and BI announced "...they were in “exclusive negotiations” on a potential asset swap that would make Sanofi one of the world’s largest manufacturers of nonprescription medicines. Under the terms of the deal, Sanofi would send its animal health business to Boehringer and Sanofi would receive 4.7 billion euros, or about $5.2 billion, in cash and Boehringer’s consumer health care business, excluding its operations in China."{Underlined emphasis is mine}
What did Provectus mean when the company wrote in its July LOI PR (see my underlined emphasis)?
"Under the terms of the LOI, Boehringer will provide certain commercially reasonable support in the aspects of product registration with the China Food and Drug Administration ("CFDA"), communication preparation, market intelligence and other assistance to Provectus in China to the extent that is within Boehringer's approved business scope and permissible by Chinese laws.
In return, Provectus will grant Boehringer the first priority to be the exclusive collaborator of Provectus in China for PV-10 in the event that PV-10 is successfully registered and approved by the CFDA. The exclusive collaboration may take the form of exclusive distribution and promotion, exclusive licensing or other agreement, subject to both parties' mutual agreement. At the appropriate time, Provectus and Boehringer will enter into a definitive agreement, including a non-compete provision, for PV-10 to be exclusively developed, distributed and promoted through the collaboration within China, although there can be no assurance that the parties will enter into a definitive agreement."One could imagine "registered" may refer to a filing of a Investigational New Drug (IND) application in China. Does "approved by the CFDA" refer to drug approval, or the approval of a clinical trial to be conducted in the country like the below?
- December 16, 2015, Celsion Receives CFDA Approval to Conduct the OPTIMA Study in China,
- December 10, 2015, Sinovac Obtains Clinical Trial Approval for Sabin-IPV Candidate, and
- March 30, 2015, CASI receives CFDA approval for ENMD-2076 Phase 2 clinical trial in ovarian clear cell carcinoma.
Moneyness (January 2, 2016)
Updated below.
The concept of moneyness when considering financial derivatives (e.g., forwards, swaps, puts, calls, etc.) means the amount or lack of profit a derivative security has relative to the then price of its underlying security.
Consider, for example, some option like the AAPL July 15, 2016 $115 calls (underlying security, expiration date, strike price, derivative) -- see this link. Apple's share price closed on Friday at $105.26. Such a call is out-of-the-money (OTM) because the share price < the strike price. Should the share price rise above $115 (plus the transaction cost of purchasing the call), the call becomes in-the-money (ITM). Illustratively, at a share price of exactly $115, the call option is at-the-money (ATM). The strike price is the same thing as the exercise price or the trigger [price].
 |
| Click to enlarge. Image source |
- Let's say it's a call option, where up/to the right is good, and down/to the left is bad,
- The "strike price" is something like (i) the combination of the common share and tradable warrant price that makes the return on investment from tendering better than the ROI from just holding onto your non-tradable warrant, (ii) $0.75, just to make the math "easy," or (iii) some share price less than a $1. I often think and write overly analytically, and
- The "expiration date" is February 15th, the date the tender closes (unless the offer is extended).
From an analysis of moneyness perspective, I don't believe management has sufficient credibility to qualitatively facilitate the tender becoming ITM -- by this I mean, I would not exercise a non-tradable warrant (if I owned one) based on projection, speculation or the future.
As a result, I would imagine the tender actually has to become ITM from a quantitative perspective; that is, the price of a common share (and, thus, the tradable warrant, which should move in tandem with the stock) has to rise above the tender's strike price before its expiration. If one assume's management is given no benefit of the doubt by the capital markets, then the share price only should move because of news (e.g., pre-clinical and/or clinical development, regulatory, business development, corporate development, media, etc.), and that news would have to materialize before February 15th.
If the share price does not rise materially before that date -- that is, if no news revealing or signaling a sustainable, long-term measure of business success for Provectus -- then the tender would remain OTM. A warrant holder then/thus would not tender their non-tradable warrants.
Most of the non-tradable warrants are held by Network 1 Financial's clients and the firm itself. See the table below.
 |
| Click to enlarge. |
- A larger company cash balance,
- A cleaner capital structure,
- A deeper tradable warrant market,
- Resources to advance more indications into the clinic (i.e., Phase 1b studies), and
- A go-pound-sand position to prospective licensees and/or acquirer if deal structure and valuation discussions are inadequate.
Network 1 probably has some sense of the prospects of the tender becoming ITM (at least certainly hope that it does, but Missouri-type hope) and their own assessment of the amount of moneyness (in no small part for their clients and themselves to gauge and then achieve some measure of monetization).
Updated (1/2/15): TLC. Adding to the calculation of the tender, from the company's perspective as well as from the potential tendering warrant holders, is the fact that some non-tradable warrants have a cashless exercise feature and some do not.
Offering warrants as part of an equity investment obviously is "juice" or an inducement for prospective investors. The more warrants (the greater the warrant coverage) the better. From the company seeking investment and offering a warrant-laced equity investment structure, warrants -- when and if exercised -- can provide working capital. Duh. If the warrants possess a cashless exercise feature, warrant exercises result in some fraction of underlying security but no cash to the issuer. In a way, good for the investor because no upfront cash is necessary to exercise, bad for the company seeking capital (dilution, no money).
If the warrants have a cash exercise feature, while dilution still occurs of course, the company gets the cash it perhaps needs or desires. If the investors owning the warrants do not have sufficient cash to exercise them, the warrants would be forfeited. In a way, bad for the investor (no additional shares) and bad for the company (no money), or good for the investor (additional shares) and presumably net good for the company (dilution, money).
As I've previously noted, the tender offer is akin to offering a slightly used version ("used" by about 7-8 months when all is said and done) of the June 2015 Maxim-led public offering. A good feature of the offering is the condition of exercising one's warrant for cash in order to obtain the replacement warrant. Hopefully, this is good for the investor (additional shares) and net good for the company (dilution, money).
TIM. An interesting article appeared in this project's Twitter feed via @MaverickNY, Study details a link between inflammation and cancer:
Cheers (January 1, 2016)Updated (1/2/15): TLC. Adding to the calculation of the tender, from the company's perspective as well as from the potential tendering warrant holders, is the fact that some non-tradable warrants have a cashless exercise feature and some do not.
Offering warrants as part of an equity investment obviously is "juice" or an inducement for prospective investors. The more warrants (the greater the warrant coverage) the better. From the company seeking investment and offering a warrant-laced equity investment structure, warrants -- when and if exercised -- can provide working capital. Duh. If the warrants possess a cashless exercise feature, warrant exercises result in some fraction of underlying security but no cash to the issuer. In a way, good for the investor because no upfront cash is necessary to exercise, bad for the company seeking capital (dilution, no money).
 |
| Click to enlarge. Image source |
As I've previously noted, the tender offer is akin to offering a slightly used version ("used" by about 7-8 months when all is said and done) of the June 2015 Maxim-led public offering. A good feature of the offering is the condition of exercising one's warrant for cash in order to obtain the replacement warrant. Hopefully, this is good for the investor (additional shares) and net good for the company (dilution, money).
TIM. An interesting article appeared in this project's Twitter feed via @MaverickNY, Study details a link between inflammation and cancer:
"A new study from MIT reveals one reason why people who suffer from chronic inflammatory diseases such as colitis have a higher risk of mutations that cause cancer. The researchers also found that exposure to DNA-damaging chemicals after a bout of inflammation boosts these mutations even more, further increasing cancer risk.
The findings confirm a longstanding theory about why inflammation and cancer are linked, and offer possible ways to help prevent and treat cancer, says Bevin Engelward, an MIT professor of biological engineering and senior author of a Jan. 15 PLoS Genetics paper describing the findings." {Underlined emphasis is mine}If I, um, play scientist (while at the same time joining the chorus of "who the heck knows how Rose Bengal works)...at SITC 2015 Moffitt Cancer Center observed:
"IL PV-10 led to the necrosis of melanoma cells and release of HMGB1 to activate DCs and elicit a systemic anti-tumor immune response."DNA damage and promotion (cell growth) may influence cancer. Wikipedia's HMGB1 entry notes under Role in inflammation:
"HMGB1 is secreted by immune cells (like macrophages, monocytes and dendritic cells) through leaderless secretory pathway. Activated macrophages and monocytes secrete HMGB1 as a cytokine mediator of Inflammation. Antibodies that neutralize HMGB1 confer protection against damage and tissue injury during arthritis, colitis, ischemia, sepsis, endotoxemia, and systemic lupus erythematosus. The mechanism of inflammation and damage is binding to TLR4, which mediates HMGB1-dependent activation of macrophage cytokine release. This positions HMGB1 at the intersection of sterile and infectious inflammatory responses." {Underlined emphasis is mine}Randomly, via search terms of HMGB1 and inflammation, HMGB1 and RAGE in inflammation and cancer, Sims et al., Annu Rev Immunol. 2010;28:367-88:
The immune system has evolved to respond not only to pathogens, but also to signals released from dying cells. Cell death through necrosis induces inflammation, whereas apoptotic cell death provides an important signal for tolerance induction. High mobility group box 1 (HMGB1) is a DNA-binding nuclear protein, released actively following cytokine stimulation as well as passively during cell death; it is the prototypic damage-associated molecular pattern (DAMP) molecule and has been implicated in several inflammatory disorders. HMGB1 can associate with other molecules, including TLR ligands and cytokines, and activates cells through the differential engagement of multiple surface receptors including TLR2, TLR4, and RAGE. RAGE is a multiligand receptor that binds structurally diverse molecules, including not only HMGB1, but also S100 family members and amyloid-beta. RAGE activation has been implicated in sterile inflammation as well as in cancer, diabetes, and Alzheimer's disease. While HMGB1 through interactions with TLRs may also be important, this review focuses on the role of the HMGB1-RAGE axis in inflammation and cancer. {Underlined emphasis is mine}
 |
| Image source |
In other words, if you own a non-tradable warrant, you may exercise it for $0.75, and receive a tradable warrant.
Takeaways:
- In no particular order, the rationale for the tender/deal structure seems to be (i) doing a good one for some long-time existing shareholders/warrant holders (the higher the exercise price of their existing non-tradable warrants, the larger the do), (ii) enabling a "least toxic financing as possible," and (iii) deepening the market for tradable warrants, and hoping to prevent PVCT.WS's price from dictating the price of PVCT.
- Dilution would derive from the issuance of replacement, tradable warrants that a non-tradable warrant holder would receive in exchange for exercising his, her or its existing, non-tradable warrants. No non-tradable warrant exercises, no dilution. If all non-tradable warrants (~60 million) are exercised, dilution caused by this transaction (at the time of the transaction) would be ~22%.
- The yin of dilution has the yang of cash infusion. No non-tradable warrant exercises, no dilution and no cash. If all non-tradable warrants (~60 million) are exercised, 22% dilution is caused and Provectus would receive gross proceeds of $45 million.
- According to the S-4, three officers and directors -- CTO Dr. Eric Wachter, PhD, CFO/COO Peter Culpepper, and board of directors member Jan Koe -- own non-tradable warrants. All three of them intend to exercise all of the non-tradable warrants they own (~1.3 million), potentially incurring a cumulative dollar expense of ~$960K.
"Raw" warrant data as of 12/31/14 is below:
 |
| Click to enlarge. Data source |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. Image source. Purple emphasis is mine. |
 |
| Click to enlarge. |
- Pivotal melanoma Phase 3 trial: "We expect to submit the necessary protocol amendment to support use of Imlygic to FDA before year’s end." MISSED
- Melanoma Phase 1b combination study: "We've already opened the first center in the study, and we're very optimistic that we can open several additional centers by the end of the calendar year." MISSED [f/n 1]
- Compassionate use program: "With regard to other activity in melanoma, we expect to provide an update on the status of our expanded access protocol by the end of the calendar year. And I'll note for now that approximately 140 patients have received PV-10 under this program." MISSED
- PV-10 + Radiation therapy: "We also continued to work with the team in Australia conducting the investigator initiated trial of PV-10 in combination with radiation to report ongoing results in a suitable venue." MISSED [f/n 2]
- Mechanism of action work: "We are also continuing to sponsor mechanism studies at Moffitt and the University of Illinois at Chicago, and anticipate further data from both groups to be released in coming months." The benefit of the doubt pushes this expectation into at least very early-2016.
[f/n 1] Eric also said on the call: "We don’t add sites to this listing until opening is assured, and we don’t open sites without listing them on the website."
[f/n 2] On the company's March 12th 1Q15 call Eric said: "We have had discussions over the last several months with the investigator about ways to get data from that study available publicly and we anticipate that sometime in the coming months, that is sometime this year, that there will be some presentation of interim data from that study."
Popularity contest. The below was a funny exchange on Twitter.
It spurred me, however, to tabulate the below:
Small molecule. A small molecule is defined as one less than 900 g/mol or Daltons. Very small molecules like dimethyl fumarate (aka Biogen's Tecfidera for multiple sclerosis) weighs less than 150 g/mol. Rose Bengal weighs less than 1,000 g/mol. Biologics "can be as heavy as 150,000 g/mol" because they "are essentially copies or optimized versions of endogenous human proteins." I'm looking forward to In The Pipeline's Dr. Derek Lowe, PhD's chemistry-based and -focused comments on Rose Bengal (e.g., from its origin to Provectus' intellectual property to its diagnostic features to its therapeutic ones to its pricing).
Valuation. The title of this blog, Connecting the Dots, initially derived from my efforts to try to narrow the information gap between those with more information and those with less (which isn't to say having more information is equivalent to having more knowledge, but having more information at a minimum is better than less because it makes mosaic theory analysis easier to conduct). The inspiration of the blog's title eventually evolved as I better understood who had/has the most information, and of course the most knowledge (Eric). But I digress...
It would appear 13 types of solid tumor cancers have been successfully clinically treated with and presumably completely ablated (i.e., local response/efficacy) by Rose Bengal/PV-10. More than 30 solid tumor indications (presentations?) have been pre-clinically and clinically treated. It's not clear to me yet how many clinical and total indications are attributed to successful bystander effects (i.e., systemic response/efficacy).
As it relates to successful ablation upon injection (i.e., 13), think about Eric's agnosticism reference over the summer: "The study suggests that PV-10 has moved beyond just melanoma and may be agnostic to tumour type." See the following news items on the blog's Archived News IV page:
Popularity contest. The below was a funny exchange on Twitter.
 |
| Click to enlarge. Image source |
 |
| Click to enlarge. |
Valuation. The title of this blog, Connecting the Dots, initially derived from my efforts to try to narrow the information gap between those with more information and those with less (which isn't to say having more information is equivalent to having more knowledge, but having more information at a minimum is better than less because it makes mosaic theory analysis easier to conduct). The inspiration of the blog's title eventually evolved as I better understood who had/has the most information, and of course the most knowledge (Eric). But I digress...
It would appear 13 types of solid tumor cancers have been successfully clinically treated with and presumably completely ablated (i.e., local response/efficacy) by Rose Bengal/PV-10. More than 30 solid tumor indications (presentations?) have been pre-clinically and clinically treated. It's not clear to me yet how many clinical and total indications are attributed to successful bystander effects (i.e., systemic response/efficacy).
As it relates to successful ablation upon injection (i.e., 13), think about Eric's agnosticism reference over the summer: "The study suggests that PV-10 has moved beyond just melanoma and may be agnostic to tumour type." See the following news items on the blog's Archived News IV page:
- Radiation (September 22, 2015),
- Not that good (August 27, 2015),
- Comfort Level (July 7, 2015), and
- Thoughts about Barcelona (July 2, 2015).
- -13% for # of unique visitors (1,692 v. 1,945),
- -11% for # of page views (23,313 v. 26,326),
- -16% for # of visits (7,047 v. 8,377),
- -6% for # of U.S. cities from where visitors came (549 v. 584),
- -49% for # of world cities (84 v. 164), and
- -12% for # of countries (44 v. 50).
 |
| Click to enlarge. |
 |
| Click to enlarge. |
 |
| Click to enlarge. |













No comments:
Post a Comment